Chapter 124 Vascularized Bone Grafts in Spine Surgery
Bone grafting has had an important role in surgery since Barth first introduced bone-grafting techniques in the late 19th century.1 Bone grafts typically have been used in the treatment of fracture nonunions, arthrodesis of joints, the filling of bone cavities, replacement of bone lost due to infection, trauma, tumor, augmentation of fracture healing, and spinal fusion. The different types of bone grafts used today include autogenous cancellous, nonvascularized autogenous cortical, vascularized autogenous cortical, allogeneic cancellous, allogeneic cortical, allogeneic demineralized bone matrix, and allogeneic inductive proteins.
Bone Grafts in Spine Surgery
Albee first described utilizing a bone graft for spinal fusion in 1911 as a treatment for Pott disease.2 Many advances have been made since that time, and fusion is now the standard treatment for a variety of spinal disorders. Achieving a proper fusion involves two key components: (1) preparation of the site to be fused and (2) stimulation of bone formation with the use of a bone graft. The most effective graft material currently available is autologous cancellous bone. This graft has a large surface area that allows for vascularization of the graft and incorporation with the host bone. In cases in which the fusion must span several segments, the amount of autogenous cancellous bone that is available may not be sufficient.
Autologous cortical bone is another commonly used graft in spine surgery. Unfortunately, this graft has fewer osteoblasts that survive and is associated with a slower rate of revascularization. This slower revascularization results in a slower rate of incorporation of the graft, thus limiting its use.3 The advantage of a cortical graft, however, is that it can provide immediate structural support and that the graft is available in larger sizes. Over time, during a process called creeping substitution, the strength of the graft decreases. During this process, the avascular nature of the graft causes resorption by osteoclasts, while new bone is laid down by osteogenic cells originating from the recipient bed rather than the graft, a phenomenon first observed and described by Phemister in 1914.4 This is why a cortical graft (such as a strut graft used in the treatment of kyphosis) may take up to 2 years to incorporate completely. During the process of creeping substitution, the bone graft is found to be weakest at 6 months, increasing the risk of fracture at the graft site.5 By retaining its vascular supply and viability of the osteocytes, a vascularized bone graft provides a mechanically stronger support than a nonvascularized graft.
Vascularized Bone Grafts
The use of vascularized bone grafts parallels the developments associated with the history of vascular surgery. The beginnings of vascular surgery can be traced back to Carrel’s classic paper published in 1908, “Results of the Transplantation of Blood Vessels, Organs and Limbs,”6 in which he describes a technique whereby blood vessels can be anastomosed. Various tools for anastomosing small vessels were designed and tested in the years following that publication. Androsov designed the first vascular stapling machine,7 Jacobsen and Suarez demonstrated the utility of the microscope in the operating room,8 and Buncke and Schulz improved microsurgical instrumentation and performed much of the early experimental work in the field.9 Strauch et al. used a canine model to transpose a rib to the mandible on its internal mammary pedicle in 1971,10 and in 1973 a free vascularized rib graft was performed in a dog by McCullough and Fredrickson.11 The first free skin flap using microvascular anastomoses was reported by Taylor in 1973,12 and in 1975, Taylor transferred a fibula to a tibial defect as the first free vascularized bone graft in a human.13
The vascularized bone graft traditionally has been used in refractory nonunions or in areas where there is a large segmental defect. The need to use a more structurally supportive bone graft for kyphosis surgery resulted in the use of the first vascularized bone graft in spine surgery. Surgery for severe kyphosis secondary to infection, trauma, or deformity requires spanning multiple levels. When a nonvascular rib or fibula was used to span such a defect, the length of the graft and the slow rate of incorporation resulted in a high rate of nonunion. Bradford encountered fatigue fractures in 4 of 23 patients when a nonvascularized fibula was used for spinal kyphosis surgery.14 These results encouraged spine surgeons to seek out alternatives to traditional bone grafts. In two separate reports, Rose et al. and Bradford described successful techniques of using a rib graft with a vascular pedicle.15,16 Because the cross-sectional area of the rib was too small and could not provide the structural support needed for certain areas of spinal fusion, surgeons began to explore the use of the fibula as a vascularized graft.17,18 Currently, the rib, fibula, and iliac crest are used as primary donor sites for a vascularized graft.
Indications and Principles
• Bone graft greater than 5 cm needed19
• Strut graft that will be more than 4 cm from the anterior border of the spine and thus more prone to fracture
• A pseudarthrosis after a nonvascularized bone graft
• An area of the spine that will require radiation postoperatively, as in the setting of a malignancy
• Cases of infection where placing instrumentation or avascular bone would propagate the infection
• Surgeries in which fusion is expected to be difficult to achieve, as with operations for neurofibromatosis
Surgical Technique
Fibula
The procedure that follows is described by Vail and Urbaniak using an extraperiosteal dissection.20 This procedure also has been described by Gore et al. in a subperiosteal plane.21 Vail and Urbaniak report that the extraperiosteal dissection leads to decreased complaints of pain.
Iliac Crest
Grafting of the iliac crest is based on the fact that the most reliable pedicle is the deep circumflex iliac artery. This artery has been shown to supply the majority of the bone.22 The following technique has been described by Mezera and Weiland and is based on the original work by Taylor.22,23
The patient is placed supine on the operating table with a small bump under the donor hip. An incision is made from the femoral artery to a point 10 cm posterior to the anterior superior iliac spine (ASIS). The external oblique muscle is exposed and incised in line with its fibers, to a point 3 cm superior to the iliac crest. The incision is curved toward the ASIS and parallel to the inguinal ligament so that the inguinal canal can be entered. The spermatic cord or round ligament is identified and retracted upward and medially. The fascia at this point is incised, and the deep circumflex iliac artery and vein are identified. The vessels are traced laterally, dividing the transversalis fascia, internal oblique, and transversus abdominis from the inguinal ligament. The ascending branch of the deep circumflex iliac artery will become easier to identify as the ASIS is approached. Its origin from the superficial circumflex iliac artery can be identified medially by incising the internal oblique muscle 3 cm above and behind the ASIS.
Rib
As first described by Bradford, a vascularized rib graft should be planned so that the rib removed will be long enough to span the defect. The rib to be used should be two to three segments below the rostral vertebrae.24 The patient is placed in the lateral decubitus position. A skin incision is made over the level of the rib. The intercostal musculature is cut 0.5 to 1 cm above the rib. The rib is divided at the costochondral junction, and the intercostal musculature is then divided inferiorly to the rib, distally to proximally. A wide margin is left to avoid dividing the vascular complex. Chest retractors are placed into the wound, and the intercostal vasculature is identified. Dissection is carried out dorsally, and the rib is divided at the rib transverse process junction. At this point the rib can be mobilized along with its vascular complex. The vessels are dissected and then mobilized to the junction of the intervertebral foramen. A centimeter of rib should be dissected subperiosteally so that bone-to-bone contact can be made with the adjacent vertebra. The rib is then rotated and mobilized to span the vertebra above and below. Incising the periosteum 1 cm over the rib can test circulation to the graft. If brisk bleeding is encountered, then an intact vascular pedicle is confirmed. The chest can now be closed in the usual fashion.
Results and Complications
Results
As first reported in 1980 by Bradford, a vascularized rib graft is suitable for areas in the spine that would require strong biomechanical support.24 This typically occurs when a large defect requires spanning. By avoiding the process of creeping substitution, the vascularized bone graft prevents microfracture and pseudarthrosis, which are commonly observed when a large nonvascularized graft is used. Most of the cases reported in the literature have used the graft in areas where mechanical support is essential, while awaiting bone graft incorporation.
Vascularized bone grafts also are useful in the setting of malignancy in cases where radiation therapy is to be employed. Often a subtherapeutic dose of radiation therapy must be applied at the site of bone fusion to minimize the occurrence of a nonunion of the bone graft.25,26 Because of its greater number of available viable osteocytes and osteoblasts, along with its relative diminished requirement of ingrowth and neovascularization, a vascularized graft may better tolerate the deleterious effects of the radiation.27,28
The superior mechanical stability afforded by a vascularized graft also is useful in the setting of infection. When nonoperative therapy has failed to eradicate a spinal infection, operative intervention requires decompression of the infected bone along with fusion for stabilization. Using instrumentation or long segments of nonvascularized bone can provide a nidus for the infection. In these cases, a vascularized bone graft has been successful in aiding the speed and stability of the fusion. Table 124-1 presents a summary of different spinal uses of vascularized bone grafts.
| Authors | Indication | Graft Used |
|---|---|---|
| Bradford DS et al.14 | Severe kyphosis | Rib |
| Meyers AM et al.36 | Salvage reconstruction in severe spondylolisthesis | Fibula |
| Nakamura H et al.37 | Anterior thoracic and lumbar fusion | Folded rib graft |
| Freidberg SR et al.38 | Replacement of resected cervical vertebral bodies | Fibula |
| Govender S et al.39 | Tuberculosis kyphosis | Rib |
| Wright NM et al.40 | Anterior decompression and fusion in the setting of radiation therapy for cervical chordoma | Fibula |
| Asazuma T et al.41 | Cervical kyphosis due to neurofibromatosis | Fibula |
| Wuisman PIJM et al.42 | Thoracolumbar scoliotic deformity in the setting of osteogenesis imperfecta | Fibula |
Complications
Most complications involving vascularized bone grafts in spine surgery are related to donor site morbidities. In 1996, Vail and Urbaniak reported on complications of harvesting vascularized fibular grafts.29 They noted that pain and motor weakness were the most prevalent complications. Sensory deficits also were noted, along with rare cases of skin breakdown secondary to loss of vascular supply. In a separate report, a functional iatrogenic valgus of the ankle joint has been reported when resection of the fibula ended too distally.30 Other morbidities related to fibula graft harvesting include transient peroneal nerve palsy, flexor hallucis longus contracture, compartment syndrome, and fracture of the ipsilateral tibia.31
Persistent pain following removal of the iliac crest has been reported with chronic disability when a large graft is harvested.32–34 Infection, hematoma, and fracture of the anterior superior iliac spine also have been reported.35
Case Presentation
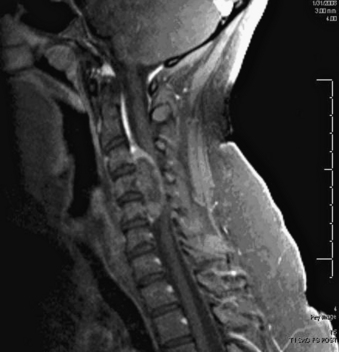
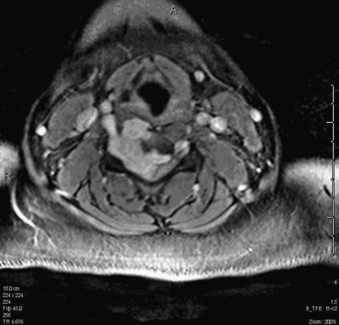
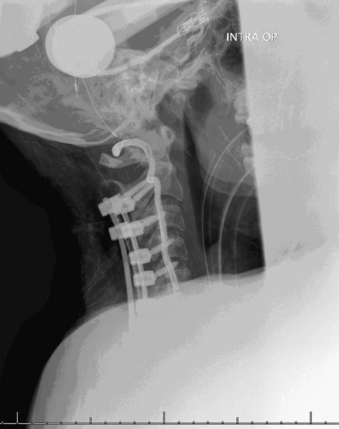
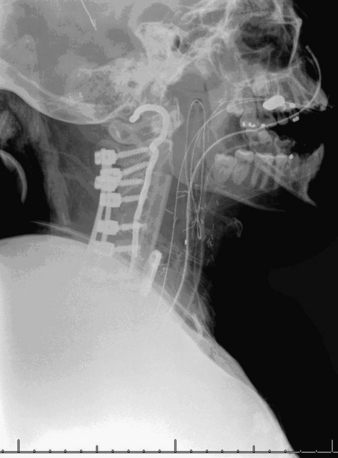
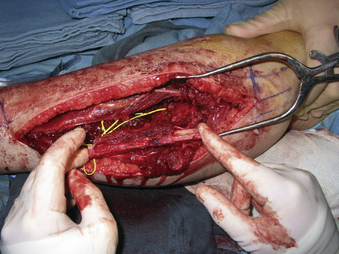
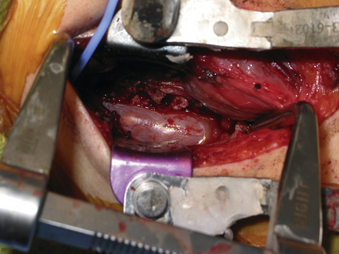
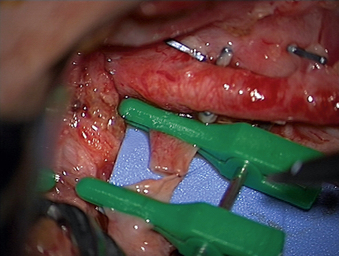
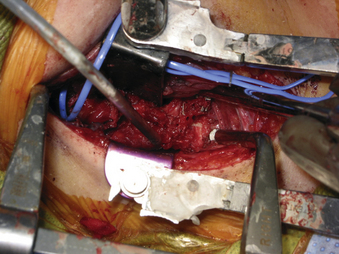
A 24-year-old woman presented 3 months postpartum with right upper extremity weakness and a clumsy weak hand. Her examination was consistent with myelopathy with upper extremity strength at 2/5 motor strength. An MRI demonstrated a large epidural tumor from C3 to C6 exiting through the neural foramen, causing bony destruction of predominantly the C4 vertebra, as well as the C3 and C5 vertebrae (Figs. 124-1 and 124-2). A biopsy had been obtained and was suggestive of a chordoma. The patient underwent a two-staged en bloc tumor resection and reconstruction. The first stage involved a posterior C3-6 laminectomy and reconstruction from C2-T1 with disconnection of dorsal elements and tumor resection (Fig. 124-3). This was followed the next day with a C3-5 corpectomy and fusion from C2-6 with a vascularized fibula bone graft and cervical interference plate, placed ventrally, at C6 (Figs. 124-4 to 124-8).
Freidberg S.R., Gumley G.J., Pfeifer B.A., et al. Vascularized fibular graft to replace resected cervical vertebral bodies. J Neurosurg. 1989;71:283.
Govender S., Suresh Kumar K.P., Med P.C. Long term follow-up assessment of vascularized rib pedicle graft for tuberculosis kyphosis. J Pediatr Orthop. 2001;21:281.
Kaneda K., Kurakami C., Minami A. Free vascularized fibular strut graft in the treatment of kyphosis. Spine (Phila Pa 1976). 1988;13:1273.
Minami A., Kaneda K., Satoh S., et al. Free vascularised fibular strut graft for anterior spinal fusion. J Bone Joint Surg [Br]. 1997;79(1):43.
Nakamura H., Yamano Y., Seki M., et al. Use of folded vascularized rib graft in anterior fusion after treatment of thoracic and upper lumbar lesions. J Neurosurg. 2001;94(Suppl 2):323.
Wuisman P.I., Jiya T.U., Van Dijk M., et al. Free vascularized bone graft in spinal surgery: indications and outcome in eight cases. Eur Spine J. 1999;8:296.
1. Barth H. Histologische Untersuchchungen über knochen Transplantation. Beitr Pathol Anat Allg Pathol. 1895;17:65.
2. Albee F.H. Transplantation of a portion of the tibia into the spine for Pott’s disease. JAMA. 1911;57:885.
3. Motoki D.S., Mulliken J.B. The healing of bone cartilage. Clin Plast Surg. 1990;17:527.
4. Phemister D.B. The fate of transplanted bone and regenerative power of its constituents. Surg Gynecol Obstet. 1914;19:303.
5. Cervansky J., Skovring B., Moor D. Use of fibular bone grafts in reconstructive surgery. Chir Narzadow Ruchu Ortop Pol. 1962;27:297.
6. Carrell A. Results of the transplantation of blood vessels, organs and limbs. JAMA. 1908;51:1661.
7. Androsov P.I. New methods of surgical treatment of blood vessel lesions. Arch Surg. 1956;73:902.
8. Jacobsen J.H., Suarez E.L. Microsurgery in anastomosis of small vessels. Surg Forum. 1960;11:243.
9. Buncke H.J., Schulz W.P. Experimental digital amputation and reimplantation. Plast Reconstr Surg. 1965;36:62.
10. Strauch B., Bloomberg A.E., Lewin M.L. An experimental approach to mandibular replacement: island vascular composite grafts. Br J Plast Surg. 1971;24:334.
11. McCullough D.W., Fredrickson J.M. Neovascularized rib grafts to reconstruct mandibular defects. Can J Otolaryngol. 1973;2:96.
12. Taylor G.I., Daniel R.K. The free flap: composite tissue transfer by vascular anastomoses. Aust N Z J Surg. 1973;43:1.
13. Taylor G.I., Miller G.D., Ham F.J. The free vascularized bone graft. A clinical extension of microvascular techniques. Plast Reconstr Surg. 1975;55:533.
14. Bradford D.S., Winter R.B., Lonstein J.E., et al. Technique of anterior spinal surgery for the management of kyphosis. Clin Orthop Relat Res. 1977;128:129.
15. Rose G.K., Owen R., Sanderson J.M. Transposition of rib with blood supply for stabilization of spinal kyphosis. J Bone Joint Surg [Br]. 1975;57:112.
16. Bradford D.S. Anterior vascular pedicle bone grafting for the treatment of kyphosis. Spine. 1980;5:318.
17. Kaneda K., Kurakami C., Minami A. Free vascularized fibular strut graft in the treatment of kyphosis. Spine (Phila Pa 1976). 1988;13:1273.
18. Minami A., Kaneda K., Satoh S., et al. Free vascularised fibular strut graft for anterior spinal fusion. J Bone Joint Surg [Br]. 1997;79:43.
19. Honma T., Yoshizu T. Vascularized fibular bone graft in spinal surgery. Monthly Book Orthopaedics. 1992;5:71.
20. Vail T.P., Urbaniak J.R. Donor-site morbidity with use of vascularized autogenous fibular grafts. J Bone Joint Surg [Am]. 1996;78(2):204.
21. Gore D.R., Gardner G., Sepic S., et al. Function following partial fibulectomy. Clin Orthop Relat Res. 1987;220:206.
22. Taylor G.I., Townsend P., Corlett R. Superiority of the deep circumflex iliac vessels as the supply for free groin flaps: experimental work. Plast Reconstruct Surg. 1979;64:595.
23. Mezera K.K., Weiland A.J. Vascularized bone grafts. In: Chapman M.W., Szabo R.M., editors. Operative orthopaedics. Lippincott Williams & Wilkins; 2001:1209-1223.
24. Bradford D.S. Anterior vascular pedicle bone grafting for the treatment of kyphosis. Spine (Phila Pa 1976). 1980;5(4):318.
25. Harrington K.D. Anterior cord decompression and spinal stabilization for patients with metastatic lesions of the spine. J Neurosurg. 1984;61:107.
26. Harrington K.D. The use of methylmethacrylate for vertebral body replacement and anterior stabilization of pathological fracture-dislocation of the spine due to metastatic disease. J Bone Joint Surg [Am]. 1981;63:36.
27. Goldberg V., Shaffer J.W., Field G., et al. Biology of vascularized bone grafts. Orthop Clin North Am. 1987;18:197.
28. Shaffer J.W., Field G.A., Goldberg V.M., et al. Fate of vascularized and nonvascularized autografts. Clin Orthop Relat Res. 1985;197:32.
29. Vail T.P., Urbaniak J.R. Donor-Site Morbidity with use of vascularized autogenous fibular grafts. J Bone Joint Surg [Am]. 1996;78(2):204.
30. Hsu L.C., Yau A.C., O’Brien J.P., et al. Valgus deformity of the ankle resulting from fibular resection for a graft in subtalar fusion in children. J Bone Joint Surg [Am]. 1972;54:585.
31. Han C.S., Wood M.B., Bishop A.T., et al. Vascularized bone transfer. J Bone Joint Surg [Am]. 1992;74:1441.
32. Banwart J.C., Asher M.A., Hassanein R.S. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine (Phila Pa 1976). 1995;20:1050.
33. Kreibich D.N., Scott I.R., Wells J.M., et al. Donor site morbidity at the iliac crest: comparison of percutaneous and open methods. J Bone Joint Surg [Br]. 1994;76:847.
34. Summers B.N., Eisenstein S.M. Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg [Br]. 1989;71:677.
35. Kurz L.T., Garfin S.R., Booth R.E.Jr. Harvesting autogenous iliac crest bone grafting. A review of complications and techniques. Spine (Phila Pa 1976). 1989;14:1324.
36. Meyers A.M., Noonan K.J., Mih A.D., et al. Salvage reconstruction with vascularized fibular strut graft fusion using posterior approach in the treatment of severe spondylolisthesis. Spine (Phila Pa 1976). 1820;26(16):2001.
37. Nakamura H., Yamano Y., Seki M., et al. Use of folded vascularized rib graft in anterior fusion after treatment of thoracic and upper lumbar lesions. J Neurosurg. 2001;94(Suppl 2):323.
38. Freidberg S.R., Gumley G.J., Pfeifer B.A., et al. Vascularized fibular graft to replace resected cervical vertebral bodies. J Neurosurg. 1989;71:283.
39. Govender S., Suresh Kumar K.P., Med P.C. Long term follow-up assessment of vascularized rib pedicle graft for tuberculosis kyphosis. J Pediatr Orthop. 2001;21:281.
40. Wright N.M., Kaufman B.A., Haughey B.H., et al. Complex cervical spine neoplastic disease: reconstruction after surgery by using a vascularized fibular strut graft. J Neurosurg. 1999;90(Suppl 1):133.
41. Asazuma T., Yamagishi M., Nemoto K., et al. Spinal fusion using a vascularized fibular bone graft for a patient with cervical kyphosis due to neurofibromatosis. J Spinal Disord. 1997;10(6):537.
42. Wuisman P.I., Jiya T.U., Van Dijk M., et al. Free vascularized bone graft in spinal surgery: indications and outcome in eight cases. Eur Spine J. 1999;8:296.