Chapter 91B Vascular reconstruction techniques
Overview
Improvements in our understanding of the anatomy and function of the liver, expertise stemming from liver transplantation, and the advances of hepatic imaging have allowed extension of the surgical limits in the area of hepatic resection. Extensive liver resection for malignant tumors, which requires a sufficient functional liver remnant volume for patient recovery, may be performed with concomitant vascular reconstruction to ensure oncologically adequate resection and adequate liver function (see Chapter 94). Parenchymal resection can be performed with caval, portal, and, exceptionally, arterial resection to improve resectability of many tumor types. Hepatic vein (HV) reconstruction has been more recently emphasized for improving postoperative liver function and regeneration.
Vena Cava and Hepatic Vein Resection
Complete Obstruction of the Inferior Vena Cava
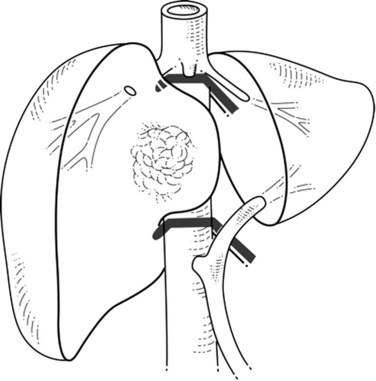
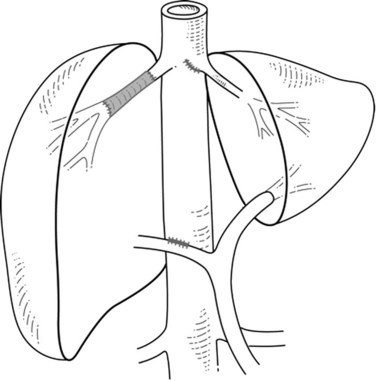
When liver tumor is associated with complete inferior vena cava (IVC) obstruction, collateral circulation usually provides sufficient venous drainage (Fig. 91B.1), therefore obstruction symptoms, such as edema of the lower extremity, are uncommon. Leiomyosarcoma of the retrohepatic IVC is the main etiology of a complete obstruction of the IVC. In this situation, liver resection with concomitant IVC resection is appropriate, providing preservation of the collaterals can be performed without restoration of caval flow. IVC replacement is considered in patients who have poor development of collateral circulation and in those who require wide retroperitoneal resection for lymph node dissection, which can disrupt preexisting venous channels and reduce collateral venous return (Beck & Lalka, 1998; Yoshidome et al, 2005).
FIGURE 91B.1 Complete obstruction of the inferior vena cava with development of collateral circulation.
After IVC resection, lower-limb venous thrombosis occurs in about 25% of patients, and reduction of the collateral circulation may increase this risk (Mingoli et al, 1991). After caval flow interruption, postoperative renal dysfunction occurs in 0% to 40% (Daylami et al, 2010). Because the left renal vein has multiple collateral venous developments—gonadal, adrenal, and lumbar—ligation of the left renal vein is not deleterious (Griffin & Sterchi, 1987); however, the right renal vein has few collateral venous systems, and its ligation can be harmful and often necessitates reimplantation into the IVC or into the left renal vein.
Partial Obstruction of the Inferior Vena Cava
Colorectal liver metastasis and intrahepatic cholangiocarcinoma (see Chapters 50A and 81A), which are best treated by R0 resection, may involve the IVC. Preoperative morphologic assessments are generally inaccurate to differentiate malignant infiltration of the vena cava wall from a simple tumoral adhesion; therefore concomitant hepatic and IVC/HV resection should be considered when tumor involvement of the IVC or the venous confluence is suspected. Invasion of the vena wall is found in 22% to 72% of patients and can be assessed intraoperatively by palpation, ultrasonography, or even by intravascular ultrasonography (Azoulay et al, 2006; Hemming et al, 2002; Kaneko et al, 1996; Kikumori et al, 2003). Experience in living donor liver transplantation (LDLT) has shown that congestion of the remnant liver territory is underestimated (Scatton et al, 2008). Impairment of venous outflow of the remnant liver has deleterious consequences on postoperative liver function and regeneration, emphasizing the need for reconstruction of the corresponding draining HV, especially when the volume of the remnant liver is marginal (Nakamura et al, 1990). In addition, maintaining the normal anatomy of the hepatic veins in the remnant liver may be beneficial to preserve the possibility of repeat hepatectomy for tumor recurrence, particularly in patients resected for colorectal liver metastasis (Pessaux et al, 2006).
Many studies report the technical aspects of vascular resection with hepatectomy, but few report the long-term results with survival rate. Venous involvement is a poor prognostic factor, but given the poor results of nonsurgical treatment, aggressive surgical resection that includes major vascular resection offers the best results in selected patients (Aoki et al, 2004; Miyazaki et al, 1999).
Hepatocellular carcinoma (HCC) has a strong propensity for vascular invasion and direct intravascular extension (see Chapter 80). Autopsy series report IVC involvement from 9% to 26% and right atrium involvement from 2.4% to 6.3% (Sung et al, 2008). Most patients are seen initially with symptoms of portal hypertension or right-sided heart failure (diuretic-resistant lower extremity edema). Efficient treatment requires liver parenchymal resection associated with vascular resection and thrombectomy under vascular exclusion (see Chapter 91A). This procedure, which requires expertise in both vascular and liver surgery, usually includes simultaneous hepatectomy and thrombectomy under vascular exclusion (Sung et al, 2008). Major bleeding and difficult vascular reconstruction are associated with the risk of thrombus migration into pulmonary arteries (Shirabe et al, 2001). When tumor thrombus extends to the right atrium, liver resection associated with cardiopulmonary bypass should be considered.
Resection
According to the extent and location of the IVC involvement, various approaches, including thoracoabdominal access, have been described (Torzilli et al, 2007). Because complete mobilization of the liver could be hazardous, an anterior approach to the IVC can be performed (Liu et al, 2000). After extrahepatic control of the IVC below and in the upper liver, the anterior approach includes initial liver resection toward the IVC.
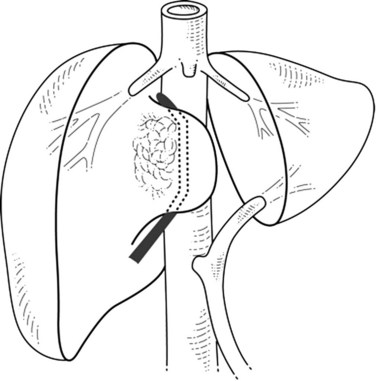
If the portion of the IVC involved by the tumor is below the hepatic veins, the hepatic parenchyma is divided using intermittent inflow occlusion if necessary (Belghiti et al, 1999). Once the IVC is exposed, portal inflow occlusion is released if used, the patient is volume loaded, and clamps are placed above and below the area of tumor involvement (Fig. 91B.2; see Chapter 91A). This caval clamping allows resection of the portion of the liver involved by tumor with the portion of vena cava involved by tumor, followed by partial or total reconstruction of the IVC. Positioning the clamps below the hepatic veins allows perfusion of the remnant liver and minimizes ischemic time.
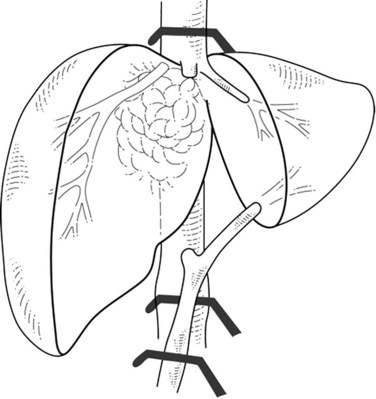
In cases of lateral IVC involvement, a single clamp may be applied tangentially to the IVC to preserve a vena caval flow (Fig. 91B.3), but this procedure is not applicable when the tumor involves the whole hepatocaval confluence. In such cases, are two different approaches may be applied. The first one includes a total vascular exclusion of the liver with subsequent infrahepatic IVC, pedicular, and suprahepatic IVC clamping (Fig. 91B.4; see Chapter 91A). Total vascular exclusion induces a decrease of 40% to 50% in the cardiac index and may be not well tolerated in some patients (Azoulay et al, 2006; Hemming et al, 2004). The second one includes use of an active venovenous bypass between the portal and axillary vein and between IVC and axillary vein can also be used (Azoulay et al, 2006; Hemming et al, 2004). When a biopump is used, in situ hypothermic perfusion of the liver parenchyma can be added to prevent postoperative liver failure as a result of prolonged ischemia (Azoulay et al, 2006; Hemming et al, 2004; see Chapter 94).
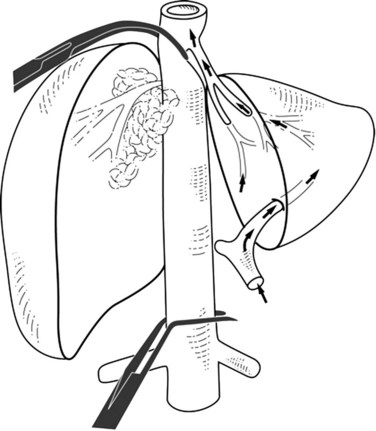
Extrahepatic control of the IVC can be attained without using total hepatic vascular exclusion (Varma et al, 2007). The suprahepatic IVC is taped just below the diaphragm, the common trunk of the left and middle hepatic veins is dissected, and a previously deployed suprahepatic tape is rotated to the right side between the vena cava and common trunk; this isolates the right hepatocaval confluence and juxtahepatic vena cava, leaving the common trunk and the rest of IVC free. The infrahepatic IVC is clamped, and concerning the suprahepatic IVC, a clamp is placed obliquely to occlude the right hepatocaval confluence and juxtahepatic vena cava (Fig. 91B.5). When right the hepatocaval junction is uninvolved, this procedure can be used in patients with left-sided neoplasms by reversing the caval tape to the left side (Maeba et al, 2001). The remnant liver receives complete inflow with uninterrupted outflow through the uninvolved hepatocaval confluence, the patency of which is maintained. This technique may also be used when synchronous involvement of the IVC and any one of the hepatocaval confluences is apparent, with at least one HV being free of tumor. This procedure has the potential disadvantage of incomplete vascular control during parenchymal transection in the presence of large veins from the caudate lobe, which leads to backflow venous bleeding. This problem can be overcome by associated resection of segment I.
Advances in liver transplantation techniques have allowed expansion of the ex vivo procedure in selected resections (see Chapter 94). This challenging technical procedure, described by Pichlmayr and colleagues (1988), is seldom performed (Hemming & Cattrall, 1999; Miyazaki et al, 2007b; Yanaga et al, 1993). Although a biopump ensures venous return to the supradiaphragmatic venous system, the liver is completely removed and perfused with preservation solution. Bloodless transection of hepatic parenchyma can then be performed, allowing complex reconstruction of hepatic veins or portal structures before reimplantation of the liver. A simplified technique was proposed with preservation of the continuity of the portal triad (Sauvanet et al, 1994). This technical modification reduces the duration of the anhepatic phase and avoids the risks of artery and biliary reconstruction.
Reconstruction
Hepatic Vein
Autologous vein graft using the external iliac, superficial femoral, saphenous, ovarian, portal, and left renal vein has been used for hepatic vein reconstruction (Nakamura et al, 1990, 1993; Ohwada et al, 1998; Takayama et al, 1996). The quality of the anastomosis itself and the blood flow volume through the graft are a concern in the long-term patency after a hepatic vein reconstruction. The selection of an optimal graft size is also important. Hepatic reconstruction using autologous vein grafts obtained from the upper abdominal cavity could be performed without requiring an additional skin incision. In addition, the portal vein or uninvolved hepatic vein from the resected specimen could be used (Hemming & Cattral, 1999), provided it is not involved with tumor; however, it has been reported that interposition grafts are complicated by thromboses in about 25% of patients (Nakamura et al, 1993). Venous graft must be kept as short as possible to avoid kinking, a risk factor for occurrence of thrombosis; however, grafts are not always necessary (Hemming et al, 2002; Nakamura et al, 1997). Even if cut flush with the liver surface, an HV can be anastomosed without an extension graft to either the origin of the same HV or, if necessary, lower down on the IVC. It is necessary to fashion a long, isolated segment of the HV (Oshita et al, 2009).
The advantage of expanded polytetrafluoroethylene (ePTFE) graft is the range of lengths and diameters that can be easily obtained (Fig. 91B.6). Potential disadvantages include long-term stricture and thrombosis. A synthetic graft carries a high risk of infection in cases of hepatic resection, especially with concomitant bilioenteric anastomosis or in cases with high risk of postoperative bile leakage, such as after segment VIII resection or after a resection close to the portal bifurcation (Yamashita et al, 2001).
Vena Cava
Repair of the resected IVC may be performed by three different modalities: 1) primary closure, 2) patch repair, and 3) prosthetic replacement, depending on the extent of the defect. In the literature, infection of prosthetic caval grafts has been reported (Bower et al, 2000; Yoshidome et al, 2005). An omental wrap has been described to protect prosthetic caval grafts from infection (Hemming et al, 2004). The second problem is the long-term patency of the prosthetic graft. The ringed, reinforced ePTFE graft is currently recommended, because it provides the best results, given the length of the missing segment and the need for strength to resist compression in the abdomen to prevent graft collapse. It remains to be demonstrated whether anticoagulation is required. Some authors (Hardwigsen et al, 2001) have proposed an arterialization of the graft to enhance its patency between either the remaining left renal vessels or the right renal artery and homolateral gonadic vein.
Portal Vein Resection
Complete Obstruction
The complete obstruction of the portal vein with cavernous transformation remains a contraindication for the resection. In cases of HCC involving portal veins, the liver Cancer Study Group of Japan classifies the tumor invasion of the first branch of the portal vein and tumor in the main portal trunk or the opposite-side portal branch into two groups, vp3 and vp4 (Liver Cancer Study Group of Japan, 2003), because there might be a crucial difference between both groups in terms of therapeutic and prognostic aspects. Tumor thrombus of vp3 can be completely removed by a major hepatectomy with the division of the ipsilateral portal branch near the bifurcation, but vp4 tumor thrombus must be removed by a thrombectomy or via an en bloc combined resection and reconstruction of the main portal vein with the hepatectomy.
The difference in outcome between thrombectomy and partial portal vein resection is clear: Konishi and colleagues (2001) reported a 2-year survival rate of 34% with a 0% postoperative mortality rate following thrombectomy. Wu and colleagues (2000) reported a 2-year survival rate of 48% with 0% postoperative mortality following hepatectomy with combined partial portal vein resection. These results were confirmed by Inoue and colleagues (2009), who reported no survival difference in patients with macroscopic portal tumor thrombus undergoing a thrombectomy and a portal vein resection. Tumor thrombus derived from HCC grows expansively and rarely infiltrates the portal wall. It is logical to assume that if the tumor thrombus does not infiltrate into the portal venous wall, thrombectomy is a viable treatment option.
Partial Obstruction
Hilar or intrahepatic cholangiocarcinoma and liver metastases could involve the portal bifurcation (see Chapter 50A, Chapter 50B, Chapter 50C, Chapter 50D ). An en bloc portal vein resection may be necessary to achieve local tumor clearance and to increase resectability. In cases of hilar cholangiocarcinoma, infiltration or perivascular involvement after portal vein resections are present only in 22% and 13%, respectively (Neuhaus et al, 1999). No-touch techniques are generally used in oncologic surgery to prevent dissemination of tumor cells. Right-sided liver resections follow this principle, all the more so if the resection line is extended to the left portion of the hepatoduodenal ligament by portal vein resection. It is unnecessary to strip the right portal vein branch of the hepatic duct bifurcation, and the portal bifurcation can be left undisturbed as well, which further decreases the required extent of hepatoduodenal dissection. Recent studies have reported improved postoperative outcomes similar to those in patients without portal vein resection with a mortality rate of less than 10% (Bachelier et al, 2011; Kondo et al, 2003; Miyazaki et al, 2007a; Neuhaus et al, 1999). Neuhaus and colleagues (1999) reported a 5-year survival rate of 65% for patients who underwent hepatectomy with en bloc portal vein resection.
Resection (See Chapters 50B and 90B)
Some authors have reported portal vein resection and reconstruction before hepatic dissection (Kondo et al, 2003). The risk of this technique is that surgical maneuvers after portal vein reconstruction might produce tension on the portal anastomosis, leading to its disruption.
Arterial Resection
Indication
Hilar cholangiocarcinoma and advanced gallbladder cancer (see Chapters 49 and 50B) have the propensity to invade extensively, not only along the bile duct but also into adjacent organs via lymphatics and perineural spaces. Regions of the portal vein and right hepatic artery bifurcations are often involved, because these vessels run just behind the bile duct and to the left of the gallbladder neck. Hepatic artery resection is less common, because the reconstruction is more complicated, and microvascular techniques are required. Furthermore, this level of invasion is often associated with advanced disease that is not amenable to resection, particularly in cases of gallbladder cancer; however, hepatectomy with combined portal vein/hepatic artery resection and reconstruction has been reported (Miyazaki et al, 2007a; Shimada et al, 2003). Hepatic artery resection may result in a higher postoperative mortality rate than portal vein resection, because hepatic artery resection has been combined with portal vein resection in most patients: the mortality rate could reach 33% to 56% (Gerhards et al, 2000; Miyazaki et al, 2007a, 2007b).
The survival rate is very poor after hepatic artery resection. Miyazaki and colleagues (2007b) reported 1- and 3-year survival rates of 11% and 0%, respectively, similar to unresectable patients, with survival rates of 15% and 0% at 1 and 2 years. A surgical team reported controversial results, with acceptable mortality, by an experienced surgeon (2%) and acceptable long-term survival in selected patients (30.3% at 5 years) (Nagino et al, 2010). The authors explained these good results by the fact that arterial vascular reconstruction was performed by plastic or vascular surgeons under a surgical microscope, they had previous experience with portal resection, and they practiced meticulous perioperative management.
Reconstruction
The hepatic artery can be reconstructed in an end-to-end fashion in most patients, and the use of the gastroduodenal artery has been reported as a conduit. The anastomosis may be difficult or impossible to perform, when the arterial stump is small or when there are multiple vessels. Experimental studies using dogs subjected to hepatobiliary dearterialization demonstrated that arterioportal shunting reduced acute hypoxic hepatic injury and improved hypoxia in the bile duct, permitting successful choledochojejunostomy (Munemura, 1995), enhancing hepatic regeneration, and resulting in improved survival (Shimizu et al, 2000). In the literature, few clinical cases report the feasibility and safety of arterioportal shunting as an alternative procedure (Kondo et al, 2004; Young et al, 2008). Hepatopetal arterial collaterals develop within 1 month, and the shunt could be closed when still patent to prevent portal hypertension (Kondo et al, 2004).
Aoki T, et al. Hepatic resection with reconstruction of the inferior vena cava or hepatic venous confluence for metastatic liver tumor from colorectal cancer. J Am Coll Surg. 2004;198:366-372.
Azoulay D, et al. Combined liver resection and reconstruction of the supra-renal vena cava: the Paul Brousse Experience. Ann Surg. 2006;244:80-88.
Bachelier P, et al. Risk factors for liver failure and mortality after hepatectomy associated with portal vein resection. Ann Surg. 2011;253:173-179.
Beck SD, Lalka SG. Long-term results after inferior vena caval resection during retroperitoneal lymphadenectomy for metastatic germ cell cancer. J Vasc Surg. 1998;28:808-814.
Belghiti J, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369-375.
Bower TC, et al. Replacement of the inferior vena cava for malignancy: an update. J Vasc Surg. 2000;31:270-281.
Daylami R, et al. Inferior vena cava leiomyosarcoma: is reconstruction necessary after resection? J Am Coll Surg. 2010;210:185-190.
Gerhards MF, et al. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma: a single center experience. Surgery. 2000;127:395-404.
Griffin AS, Sterchi JM. Primary leiomyosarcoma of the inferior vena cava: a case report and review of the literature. J Surg Oncol. 1987;34:53-60.
Hardwigsen J, et al. Resection of the inferior vena cava for neoplasms with or without prosthetic replacement: a 14-patient series. Ann Surg. 2001;233:242-249.
Hemming AW, Cattral MS. Ex vivo liver resection with replacement of the inferior vena cava and hepatic vein replacement by transposition of the portal vein. J Am Coll Surg. 1999;189:523-526.
Hemming AW, et al. Hepatic vein reconstruction for resection of hepatic tumors. Ann Surg. 2002;235:850-858.
Hemming AW, et al. Combined resection of the liver and inferior vena cava for hepatic malignancy. Ann Surg. 2004;239:712-721.
Inoue Y, et al. Is there any difference in survival according to the portal tumor thrombectomy method in patients with hepatocellular carcinoma? Surgery. 2009;145:9-19.
Kaneko T, et al. Intracaval endovascular ultrasonography for preoperative assessment of retrohepatic inferior vena cava infiltration by malignant tumors. Hepatology. 1996;24:1121-1127.
Kikumori T, et al. Intracaval endovascular ultrasonography for large adrenal and retroperitoneal tumors. Surgery. 2003;134:989-993.
Kondo S, et al. Portal vein resection and reconstruction prior to hepatic dissection during right hepatectomy and caudate lobectomy for hepatobiliary cancer. Br J Surg. 2003;90:694-697.
Kondo S, et al. Arterioportal shunting as an alternative to microvascular reconstruction after hepatic artery resection. Br J Surg. 2004;91:248-251.
Konishi M, et al. Surgical treatment of hepatocellular carcinoma with direct removal of the tumor thrombus in the main portal vein. Hepatogastroenterology. 2001;48:1421-1424.
Liu CL, et al. Anterior approach for major right hepatic resection for large hepatocellular carcinoma. Ann Surg. 2000;232:25-31.
Liver Cancer Study Group of Japan. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 2nd ed. Tokyo: Kanehara; 2003.
Maeba T, et al. Retrohepatic vena cava replacement of hepatic malignancies without using total hepatic vascular exclusion or extracorporeal bypass. Hepatogastroenterology. 2001;48:1455-1460.
Mingoli A, et al. Leiomyosarcoma of the inferior vena cava: analysis and search of world literature on 141 patients and report of three new cases. J Vasc Surg. 1991;14:688-699.
Miyazaki M, et al. Aggressive surgical resection for hepatic metastases involving the inferior vena cava. Am J Surg. 1999;177:294-298.
Miyazaki M, et al. Combined vascular resection in operative resection for hilar cholangiocarcinoma: does it work or not? Surgery. 2007;141:581-588.
Miyazaki M, et al. Recent advance in the treatment of hilar cholangiocarcinoma: hepatectomy with vascular resection. J Hepatobiliary Pancreat Surg. 2007;14:463-468.
Munemura T. An experimental study of blood supply to the bile duct under arterio-portal shunting. Hokkaido Igaku Zasshi. 1995;70:591-608.
Nagino M, et al. Hepatectomy with simultaneous resection of the portal vein and hepatic artery for advanced perihilar cholangiocarcinoma: an audit of the 50 consecutive cases. Ann Surg. 2010;252:115-123.
Nakamura S, et al. Hepatic vein reconstruction for preserving remnant liver function. Arch Surg. 1990;125:1455-1459.
Nakamura S, et al. Significance of hepatic vein reconstruction in hepatectomy. Surgery. 1993;114:59-64.
Nakamura S, et al. Direct hepatic vein anastomosis during hepatectomy for colorectal liver metastases. Am J Surg. 1997;174:331-333.
Neuhaus P, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808-818. discussion 819
Ohwada S, et al. Hepatic vein reconstruction at inferior vena cava confluence using left renal vein graft. Hepatogastroenterology. 1998;45:1833-1836.
Oshita A, et al. A new technique for reconstruction of the middle hepatic vein without graft interposition: “the digging technique.”. Hepatogastroenterology. 2009;56:1507-1510.
Pessaux P, et al. Repeat hepatectomy for recurrent colorectal liver metastases. J Surg Oncol. 2006;93:1-7.
Pichlmayr R, et al. Ex-situ Operation an der Leber: eine neue Möglichkeit in der Leberchirurgie. Langenbecks Arch Chir. 1988;373:122-126.
Sauvanet A, et al. A simplified technique of ex situ hepatic surgical treatment. J Am Coll Surg. 1994;178:79-82.
Scatton O, et al. Impact of localized congestion related to venous deprivation after hepatectomy. Surgery. 2008;143:483-489.
Shimada H, et al. Hepatic resection combined portal vein or hepatic artery reconstruction for advanced carcinoma of the hilar bile duct and gallbladder. World J Surg. 2003;27:1137-1142.
Shimizu Y, et al. Beneficial effects of arterialisation of the portal vein on extended hepatectomy. Br J Surg. 2000;87:784-789.
Shirabe K, et al. Thrombectomy before hepatic resection for hepatocellular carcinoma with a tumor thrombus extending to the inferior vena cava. Int Surg. 2001;86:141-143.
Sung AD, et al. Hepatocellular carcinoma with intracavitary cardiac involvement: a case report and review of the literature. Am J Cardiol. 2008;102:643-645.
Takayama T, et al. Reconstruction of a single remnant hepatic vein. Br J Surg. 1996;83:762-763.
Torzilli G, et al. Salvage hepatic resection after incomplete interstitial therapy for primary and secondary liver tumours. Br J Surg. 2007;94:208-213.
Varma D, et al. Isolated total caval clamping with “preserved remnant liver perfusion” for combined hepatic and vena caval resection in tumors involving vena cava. Surgery. 2007;141:112-116.
Wu CC, et al. An appraisal of liver and portal vein resection for hepatocellular carcinoma with tumor thrombi extending to portal bifurcation. Arch Surg. 2000;135:1273-1279.
Yamashita Y, et al. Bile leakage after hepatic resection. Ann Surg. 2001;233:45-50.
Yanaga K, et al. Extracorporeal hepatic resection for previously unresectable neoplasms. Surgery. 1993;113:637-643.
Yoshidome H, et al. Should the inferior vena cava be reconstructed after resection for malignant tumors? Am J Surg. 2005;189:419-424.
Young AL, et al. Portal vein arterialization as a salvage procedure during left hepatic trisectionectomy for hilar cholangiocarcinoma. J Am Coll Surg. 2008;207:e1-e6.