Chapter 194 Vascular and Soft Tissue Complications
Complications at the Craniovertebral Junction
The simplest and most direct ventral access to the craniovertebral junction is the transoral/transpharyngeal approach. Many different variations of this operation have been described, including combined resection of the hard palate, median labiomandibular glossotomy, maxillectomy, and LeFort osteotomies to expand the exposure from the midclivus to the C3 level. The main limitation of these procedures is the inevitable contamination of the wound by mouth flora. Additionally, the location of the vertebral arteries limits the lateral extent of these procedures.1 By reserving these approaches for extradural pathologic conditions and by using perioperative antibiotics, many of the septic complications that were initially encountered with this operation have been overcome. Airway management in transoral procedures demands special attention. Significant tongue swelling is often encountered, and this can easily lead to obstruction of the oropharynx. In cases of major resections or those in which the patient has any preoperative difficulty with swallowing or aspiration, a tracheotomy is routinely performed. In more limited operations at the C1-2 level and without concurrent lower cranial neuropathy, the patient may be left intubated for 48 to 72 hours postoperatively or until glossal swelling has abated. Periodic relaxation of the intraoral retractors during surgery may mitigate the problem. Additionally, precautions should be taken to ensure that the tongue is not trapped between the retractor blade and the lower teeth. Steroids are often invoked as well, but they are no substitute for controlled extubation in an intensive care unit setting by someone who is skilled in airway management. Close observation with a bedside tracheostomy setup is mandatory. Although intradural procedures and bone grafting can be successfully performed through this route, these maneuvers carry a heightened risk and can be the source of significant morbidity. A layered, tensionless reapproximation of the dorsal pharyngeal musculature and mucosa with resorbable sutures is important, especially if the dura mater has been violated. In this case, reinforcement of the dural repair with a fascial graft and fibrin glue and placement of a spine drain postoperatively are advised. If bone grafts or reconstructive cages have been inserted, they should have a low profile, without protrusion into the pharynx and resultant compromise of the soft tissue closure. Because the retropharyngeal soft tissues are well vascularized, surgeons tend to use electrocautery to divide and reflect these structures off the bone. This can result in significant retraction of the wound margins, which becomes most apparent at the time of closure. Infiltration of the retropharyngeal tissues with a dilute epinephrine solution before sharp incision and blunt reflection with the use of bipolar cautery for direct hemostasis minimizes this problem. If a primary closure cannot be obtained (or if one should subsequently break down), satisfactory repair can usually be achieved with either a pharyngeal or a septal flap reconstruction. When the soft palate has been divided, a similar degree of attention should be devoted to the tensionless anatomic reapproximation of its edges, so that a cleft or fistula does not result. In the immediate postoperative period, oral feedings should be avoided for the first 5 days to minimize risk of fascial dehiscence.
Vertebral artery injury is always a theoretical risk during these procedures. At the arch of C1, the vertebral arteries are located approximately 24 mm laterally from the midline; at the level of the foramen magnum and the level of the C2-3 disc space, the arteries are approximately 11 mm from the midline. Pathology such as rotatory subluxation can significantly distort the relationship of these structures to the midline. Identification of the midline structures such as the anterior tubercle of C1 and the pharyngeal tubercle on the clivus are the most important steps in establishing orientation for these approaches. Furthermore, the anatomic midline can be identified by the symmetry of the anterior longitudinal ligament and longus colli muscles.1 Fluoroscopy is also useful in establishing the midline, as may be intraoperative neuronavigation. Regarding management of vertebral artery injury, please see the next section.
Complications in the Subaxial Spine
The ventrolateral approach to the subaxial spine as popularized by Robinson and Smith2 is among the most commonly performed spine surgeries. The esophagus, larynx, and trachea are mobilized medially as a unit, and the carotid sheath is retracted laterally. The incidence of clinically significant injuries to these structures is low during primary surgeries. When an injury does occur, sharp-toothed retractors are often implicated. Hand-held blunt retractors are used exclusively until the musculus longus colli have been reflected off the vertebral bodies ventrolaterally to create two soft tissue leaves into which a self-retaining retractor system can be anchored. Great care is taken with the initial placement of retractors, because this permits safe, stable, and sustained exposure that sets the stage for the remainder of the case. Toothed blades are inserted accurately under the musculus longus colli under direct vision. Proper engagement of the muscles usually requires the use of asymmetrical blade lengths, the medial blade being a bit longer.
Recurrent Laryngeal Nerve Injury
Vocal cord paresis is a complication in anterior cervical surgery that is probably underappreciated. In patients who have undergone the anterior cervical approach to the spine for discectomy or corpectomy, the reported rate of injury varies from 0% to 16%.3 Nevertheless, a prospective study looking at 120 patients undergoing anterior cervical spine surgery who underwent preoperative and postoperative laryngoscopy revealed a clinically symptomatic recurrent laryngeal nerve palsy rate of 8.3%, and the incidence of recurrent laryngeal nerve palsy not associated with hoarseness (i.e., clinically unapparent without laryngoscopy) was 15.9% (overall incidence, 24.2%). At 3-month follow-up evaluation, the rate had decreased to 2.5% in cases with hoarseness and 10.8% without hoarseness.4 Most of these are blunt injuries, believed to have resulted from retractor pressure against the recurrent laryngeal nerve within the tracheoesophageal groove. The left recurrent laryngeal nerve has a longer course, swinging around the aortic arch before ascending in the relatively protected cleft between the trachea and esophagus, whereas the right recurrent laryngeal nerve loops around the subclavian artery, and thus has a correspondingly shorter course. The extra length of nerve available on the left allegedly renders it less vulnerable to stretch injury than its counterpart on the right is, but the evidence for this is scant. A recent prospective study assessed 242 patients undergoing anterior cervical spine surgery postoperatively with laryngoscopy. All patients underwent a left-sided approach, but one group (149 patients) was operated on with an additional reduction of endotracheal cuff pressure to below 20 mmHg. Ninety-three patients underwent a left-sided approach without reduction in cuff pressure. In the group with the left-sided approach and the low cuff pressures, the total rate of persisting (at 3 months) symptomatic and asymptomatic recurrent laryngeal nerve palsy was 1.3%. In the group with the left-sided approach without the reduced cuff pressures, the total rate of persisting (at 3 months) symptomatic and asymptomatic recurrent laryngeal nerve palsy was 6.5%. The authors noted that this compared favorably with their historic data, in which they noted a total rate of persisting recurrent laryngeal nerve palsy of 13.3% in patients undergoing the right-sided approach without reduction of cuff pressure.5
In cases of suspected vocal fold motion impairment, an otorhinolaryngology consult should be obtained. Initial evaluation should include a detailed history with the patient relating symptoms and their time of onset, followed by a physical examination, in which particularly close attention is paid to the neck, and a neurologic examination of the lower cranial nerves.6 This should be followed by visualization of the vocal cords with a fiberoptic laryngoscope.7 Also very valuable is a videofluoroscopic swallowing evaluation. This can show signs of pharyngeal plexus injury such as disruption of velar movement, cricopharyngeal spasm, and other patterns of swallowing dysfunction.6 If there is an immobile vocal cord, a useful examination tool is laryngeal electromyography. Laryngeal electromyography can help in determining the site of a peripheral vagal lesion (high cervical vs. low cervical), as it allows separate testing of laryngeal muscles supplied by the recurrent laryngeal and superior laryngeal nerves. Laryngeal electromyography also allows for differentiation between vocal cord paralysis and vocal cord fixation.8
As a rule, functional recovery occurs over a period of weeks to months. Nevertheless, injury may be permanent or slow to heal, and surgical intervention may be required. Various options exist for the treatment of unilateral vocal fold paresis and paralysis. These include injection laryngoplasty, medialization laryngoplasty, arytenoid adduction, and nerve-muscle transfer. Injection laryngoplasty may be done with hemostatic gelatin (Gelfoam), fat, collagen, or Teflon (El Du Pont de Nemours & Co., Inc., Wilmington, DE). Medialization laryngoplasty is usually done with silicone elastomer (Silastic) or hydroxylapatite. More recently, novel materials such as titanium, GORE-TEX (W. L. Gore and Associates, Inc., Flagstaff, AZ), and polylactic/polyglycolic acid have been used. Arytenoid adduction uses a permanent suture to relocate the arytenoid into a more physiologically sound position.7,9
Esophageal Injury
Transient swallowing disorders are commonly recorded after even uncomplicated primary anterior cervical surgeries. This may be seen in up to 80% of patients who undergo anterior cervical spine surgery.10 Symptoms usually resolve within a few weeks but may persist in up to 10% of patients, although only rarely at a level that is functionally disabling.11 Refractory cases should be evaluated with videofluoroscopic swallowing evaluation (modified barium swallow) to determine the integrity of the swallowing mechanism and whether the patient will safely tolerate oral intake. This then can expedite appropriate swallowing therapy and an appropriate route for nutrition.12,13 Though many patients will do well, recovery, presumably through reinnervation of pharyngeal musculature, does not always occur.14 The speech pathologist can be of tremendous help here in determining the patient’s potential for recovery. If the dysphagia is related to cricopharyngeal spasm and lasts several months and follow-up study does not show improvement with conservative therapy, surgical intervention such as cricopharyngeal myotomy may be considered.6
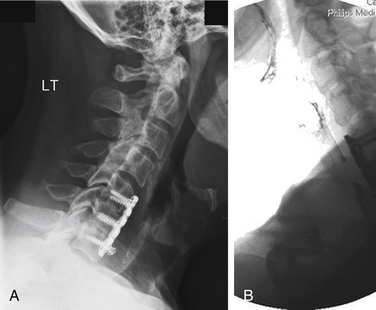
Esophageal perforation is also encountered as a delayed complication associated with ventral graft extrusion and hardware failure. This occurs most commonly because of infection, poor carpentry, a technical error in the method of instrumentation, or application of instrumentation in softened osteoporotic bone. This can also occur in the setting of pseudarthrosis (Fig. 194-1). When screws are observed to back out in follow-up radiographs, their elective removal should be considered. There are now abundant reports of esophageal injury secondary to screw migration.15–20 Screw heads should be flush with the plate to minimize their profile and allow their locking mechanism to function properly to prevent backout. Plate length must be selected carefully so that there is no overhang over adjacent disc spaces, and unfused segments should not be instrumented, because these circumstances promote hardware loosening. If the quality of the bone stock is poor, bicortical screw fixation should be used. If this is not feasible, posterior segmental instrumentation should be performed.
Esophageal Repair
Esophageal perforation may be apparent intraoperatively, but more often it presents postoperatively with deep wound infection, severe dysphagia, and mediastinitis.19,20 Perforation related to hardware failure might not occur until years after the operative procedure.21,22 Intraoperative tears may be either partial or full thickness. A partial-thickness injury to the esophagus is readily repaired with resorbable sutures and should not cause modification of the primary procedure. To ensure that a transmural injury has not occurred, the surgeon may instill indigo carmine dye into the hypopharynx and monitor for dye egress within the wound. Transmural injuries are repaired primarily, again with the surgeon observing the principles of a layered, tensionless closure using resorbable sutures. The wound is irrigated copiously with antibiotic-containing solution, and systemic antibiotic coverage is broadened to include anaerobic organisms. Assuming an absence of gross contamination and a satisfactory repair, the surgeon can proceed with the intended decompression and fusion in most instances. In these circumstances, any form of spine instrumentation should be used with caution. The patient is fed through a Silastic feeding tube during the first postoperative week. Intravenous antibiotics are continued for 2 to 6 weeks postoperatively. A further course of oral antibiotics thereafter is discretionary.
Delayed perforation may present with similar though less fulminant symptoms or with spinal osteomyelitis. In most cases, the original site of injury will have sealed over, although this should be evaluated with a swallowing study using a water-soluble contrast agent. Subsequent procedures depend on several factors. Any sign of contrast extravasation mandates operative repair; any deep abscess requires drainage. Grossly infected or collapsed bone grafts or vertebrae should be thoroughly debrided and regrafted after a vigorous washout. Long-term (6 weeks), organism-specific intravenous antibiotics should be administered. In less fulminant infections with good anatomic and neurologic preservation, a more conservative approach with drainage of superficial pus and administration of systemic antibiotics may be elected initially. Close clinical and radiographic follow-up is extremely important. If the patient is without signs of deep infection, nonoperative management may be a viable option, even in the presence of spine instrumentation. The erythrocyte sedimentation rate and C-reactive protein level are useful laboratory parameters to monitor. Clinical or radiographic progression would then mandate operative management.
Laryngotracheal Injuries
Sore throat and hoarseness are common and mostly transient complaints after anterior cervical surgery. Some researchers have sought to relate this phenomenon to increased pressure exerted on the laryngotracheal lumen by the endotracheal tube cuff following insertion of deep retractors. Venting enough air from the cuff to create a small air leak around the endotracheal tube may alleviate at least some of this problem.23
Carotid and Vertebral Artery Injury
Vertebral artery injury is an unusual complication for cervical spine surgery, with an overall estimated incidence of 0.14%.24 The vertebral artery is not routinely encountered in routine anterior cervical approaches. It may be injured by lateral exploration of the neural foramen in pursuit of uncovertebral joint osteophytes. This type of injury is usually minor and is controlled with small amounts of hemostatic packing. Most commonly, the vertebral artery is injured in anterior cervical spine surgery in the V2 segment (extending from the C6-1 transverse foramina) by using the drill off the midline, excessive lateral foraminal decompression/bone disc removal, or pathologic softening of the bone of the lateral part of the spinal canal caused by infection or tumor. Additionally, the artery runs between the transverse process of C7 and the longus colli musculature. Thus, extensive lateral dissection at C7 should be avoided.25 More significant injury to the vertebral artery can result during cervical corpectomy if the decompression is taken too far laterally.26 These injuries are usually incurred by overly aggressive drilling. They can be avoided if all dissection is performed under magnification and if the drill is not permitted to penetrate the deep bony cortex. The vertebra is “eggshelled out” by the drill, leaving only a thin bony cortex to be avulsed with a fine curet or thin-footed Kerrison rongeur. The ventral aspect of the transverse processes of C3-6 is also marked by a small bony tubercle that alerts the operator to the laterality of the exposure. More often, however, the point of injury occurs on the medial side of the artery, where the drill has broken through the vertebral cortex.
Vertebral artery injury with posterior cervical surgery has been most commonly associated with C1-2 transarticular screw insertion with a reported incidence of 1.3%.24 The artery may be injured if the screw trajectory is too low or too lateral. C1 lateral mass and C2 pedicle constructs may reduce the risk of vertebral artery injury. Nevertheless, lateral perforation of the C2 pedicle may result in vertebral artery injury. Also, too far lateral exposure of the posterior or superior ring of C1 may predispose to vertebral artery injury.27 Subaxial lateral mass screw insertion may result in vertebral artery injury, but such an injury is most unusual.28
When a vertebral artery injury occurs intraoperatively, there is usually sudden, nonpulsatile, copious bright red bleeding, although it may appear dark because of injury to the surrounding venous plexus. Injury is dangerous, as hemorrhage may be massive and cerebral ischemia/embolic phenomena may result.25
Strategies to manage a vertebral artery intraoperatively include tamponade, repair, and ligation. Tamponade includes use of hemostatic agents such as Gelfoam and oxidized cellulose (Surgicel).25 Should this fail to control hemorrhage, the surgeon must enlarge the exposure, including deliberate resection of the ventral lip of the transverse process to uncover more of the artery proximally and distally as localized pressure is applied over a cottonoid at the point of hemorrhage. The surgeon must then weigh the options of vertebral ligation versus repair. Most patients, especially youths, tolerate unilateral vertebral ligation well.26 However, a small number of patients will have an isolated vertebral artery terminating in the posterior inferior cerebellar artery or a compromised contralateral vertebral artery. Ligation of a vertebral artery in these circumstances could result in cerebellar or brainstem infarction. Because the status of the vertebral artery anatomy might not be known preoperatively, significant effort should be made to preserve vascular patency whenever possible. In this setting, intraoperative angiography should be considered.25 If injury occurs at C1-2 during transarticular screw insertion, many surgeons advocated placement of a screw into the drilled hole to reduce bleeding.24,25
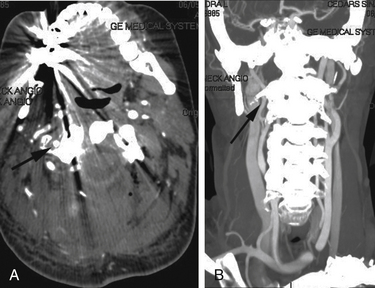
Postoperative management remains controversial. Some surgeons advocate that the patient be observed and any postoperative intervention be dictated by the patient’s clinical course. Others recommend that a postoperative angiogram be obtained to rule out significant injury, stenosis, pseudoaneurysm, or arteriovenous (AV) fistula (Fig. 194-2). This may allow for endovascular intervention or vessel sacrifice, if need be. Additionally, the postoperative neurologic examination should be followed as anticoagulation and antiplatelet therapy may be needed to prevent vertebrobasilar thromboembolism. Complications of vertebral artery injury include AV fistulae, late-onset hemorrhage, pseudoaneurysm and thrombosis with embolic incidents, cerebral ischemia, stroke, and even death. The vascular complications might occur days to years later. Thus, after identifying an injury, serial imaging with magnetic resonance angiography or CT angiography should be considered to rule out development of a pseudoaneurysm.25
Studying preoperative CT and MRI scans and noting the possibility of ectatic, tortuous, or aberrant vasculature can reduce the risk of vertebral artery injury. Additionally, these studies are useful for tumor surgery.25
Vascular Injuries
One of the principal concerns during the conduct of any transthoracic procedure is the avoidance of injury to major arterial and venous structures. The aortic arch is usually located at the T4 level. Rostral to this, the esophagus and the trachea lie immediately ventral to the spine; however, they are tethered by the brachiocephalic trunk and cannot be readily mobilized. Below the level of the arch, the descending aorta lies closely applied to the lateral aspect of the thoracic vertebrae on the left side. The thoracic duct swings dorsal to the esophagus to lie nearly in the midline, interposed between the esophagus and spine. Paired azygos veins flank the spine and anastomose extensively with each other and with the vena cava. Crossing transversely at each midvertebral body level are paired segmental arteries and veins. These are branches of the aorta and azygos systems, respectively, and they divide into an intercostal vessel and a radiculomedullary branch at the level of the neural foramen.
Oskouian and Johnson reviewed an institutional experience of 207 patients who underwent anterior reconstructive procedures in the thoracic and lumbar spine. Direct vascular injuries were identified in seven patients, including one thoracic aortic dissection ascribed to retraction, one torn intercostal artery, and five venous injuries.29 Injuries that are recognized intraoperatively obviously mandate immediate repair. The exact technique that is required depends on the size and anatomy of the injury. Branch avulsions are simply ligated. Small tears in the aorta proper or the vena cava are oversewn directly with fine vascular suture technique. Larger and more complex tears require placement of vascular clamps proximally and distally to isolate the injured segment for repair. Intraoperative consultation with an experienced cardiothoracic or vascular surgeon is appropriately sought. In cases of reoperation, prior infection, or irradiation, the possibility of scarification or friability of tissues in the mediastinum may be anticipated. These dissections can be tedious, and occasionally, it may be advisable to place umbilical tapes about the aorta preemptively or to prepare sites for cross-clamping in cases that are deemed high risk.
Splenic and Vascular Injury
Splenic injury has rarely been reported in the literature and should not present a problem as long as it is recognized promptly.30 Recent abdominal trauma, splenomegaly, and venous hypertension may be predisposing factors. Presumably, these injuries are most often caused by overzealous mobilization or retraction. General surgical self-retaining retractor systems provide a significant advantage over hand-held instruments. The smooth blades that are used with these systems can be placed accurately and maintained without the trauma of repeated readjustments that are the inevitable outgrowth of the fatigue of prolonged manual retraction. A large moistened laparotomy pad is placed under the retractor to minimize the risk of injury. If a splenic injury occurs, consultation with a general surgeon should be sought. Brisk bleeding from the hilum necessitates splenectomy. However, in many cases of lesser injury, the spleen can be salvaged. When it is feasible, splenorrhaphy is preferable to splenectomy because of the increased incidence of overwhelming sepsis in patients who have been splenectomized. Surgeons should bear in mind the possibility of an occult splenic injury in cases of otherwise unexplained hypotension intraoperatively or postoperatively.
Ureteral Injury
The ureters lie in loose areolar tissue on the psoas muscles. They cross ventrally over the iliac arteries to descend into the pelvis. They can be distinguished by observing peristalsis after a momentary pinch with an atraumatic forceps. The risk of ureteral injury in primary surgeries is exceedingly small. However, if normal tissue planes have been obscured by previous surgery, retroperitoneal fibrosis, tumor, or irradiation, dissection must proceed with utmost caution. During reoperations, sponge and Kittner dissection is advantageous. Intraoperative identification of the ureter may be facilitated by retrograde placement of a stent at the outset of the procedure. If a ureteral injury is incurred, immediate repair is undertaken. The exact nature of the repair is dictated by the type of injury. Clean lacerations may be simply reanastomosed end to end, whereas a segmental injury, as may be caused by a drill or rongeur, requires segmental resection and reanastomosis with ureteral mobilization to allow for a tensionless repair. In either case, the reconstruction should be accomplished over a stent that can be subsequently retrieved cystoscopically. Injuries over segments that are too lengthy to permit primary repair are uncommon; however, they demand more sophisticated reconstructive techniques such as appendiceal interposition or ureteroureteral anastomosis. Obviously, the input of a urologic surgeon should be sought. Not all ureteral injuries are recognized during surgery. The postoperative development of hydronephrosis, a urinoma, or a retroperitoneal abscess should prompt investigation of the upper urinary tract by CT scan with contrast injection with delayed scans.31 Two instances of delayed ureteral obstruction attributed to retroperitoneal fibrosis after scoliosis surgery with Dwyer instrumentation have been reported.32,33
Vascular Injury
Arterial anatomy is more consistent than is venous anatomy. The level of the aortic bifurcation can be anticipated at L4; therefore, a direct ventral approach to the lumbosacral junction necessitates dissection within the limbs of the bifurcation. Typically, the right iliac artery has to be mobilized to uncover the L4-5 disc, and great care must be taken with insertion and removal of interbody retractors to minimize the possibility of blunt or sharp vascular injury. It is wise to confirm the presence of the femoral and pedal pulses periodically throughout the surgery. Loss of pulses should prompt an intraoperative angiogram and any indicated repair.
The venous anatomy is the more problematic aspect of these exposures. The level of confluence of the iliac veins to form the vena cava is at the L4-5 disc level in approximately 70% to 80% of patients. A more caudally situated confluence is often associated with unusually large common iliac veins. They may then directly overlie the L5 body or the L5-S1 disc space, sometimes precluding ventral access at this level entirely. These anatomic variations may be discerned in close inspection of the preoperative MRI or CT examinations.34 In patients who are more typical, the left common iliac vein requires the greatest mobilization. Depending on the scope and level of the surgery, this vein may have to be retracted medially, laterally, or in both directions. In inflammatory or degenerative conditions, the left common iliac vein can easily become scarred down in the soft tissues immediately ventral to the disc. Successful collapse and mediolateral retraction are possible in this case only if the vein is carefully dissected longitudinally along its axis. The middle sacral vessels originate from either the common or the internal iliac vessels, and they overlie the L5-S1 disc space. They often can be retracted with ease, although no harm attends their sacrifice. The surgeon should take care to clearly identify all major vascular structures in the field before incising the disc space. The use of monopolar cautery over the disc space should be minimized to spare the hypogastric (sympathetic) plexus. Bipolar cautery is used to secure smaller vessels, and clips or ligatures are used to occlude larger vessels. Injuries to the common iliac veins or the vena cava are encountered in 1.9% to 15% of transabdominal approaches to the lumbosacral junction.35–41 Additionally, complication rates for revision anterior lumbar surgery may be three to five times higher than those reported for primary exposures.42 If a major vein is injured, venorrhaphy is preferred over ligation if at all possible. Hemorrhage is initially controlled by direct pressure at the point of injury until the vessel can be isolated proximally and distally. Particularly challenging are “backside” repairs brought on through avulsion of unseen tributaries into the dorsal wall of the common iliac veins or vena cava during blunt dissection. The largest and anatomically most consistent of these tributaries are the lateral lumbar veins and the iliolumbar vein. Because the inferior vena cava lies slightly to the right of midline, the most efficient strategy is to mobilize it farther rightward if it is in the way. Special effort must be taken to clearly identify, doubly ligate, and divide the lumbar and iliolumbar veins on the left side before retracting the inferior vena cava in this manner, in case the veins are avulsed or the vena cava is ruptured. Regrettably, efforts to graft veins using either autologous or prosthetic materials have been fraught with a high incidence of occlusion.
Lymphocele after the Anterior Approach to the Spine
Lymphocele is a complication after anterior lumbar spine surgery that may occur as often as 1% of the time after retroperitoneal approaches.43 They may relate to surgical disruption of the lymphatics surrounding the iliac artery and vein. In performing retroperitoneal approaches to the lumbosacral spine, fluid may be sequestered in the retroperitoneal space and subsequently compress the peritoneum. Clinical presentation includes abdominal mass, wound drainage, fever, and weight gain. CT scanning is used for the diagnosis of an uncomplicated lymphocele that shows a low-attenuation mass. The differential for such a mass includes a complicated lymphocele (infected), urinoma, and cerebrospinal fluid collection. Though conservative treatment options exist, typical treatment involves drainage, open or laparoscopic marsupialization of the cyst to the peritoneal cavity, or sclerotherapy.43–46
Complications of Minimally Invasive Thoracolumbar Discectomy and Fusion
Minimally invasive spine procedures have grown in popularity. Theoretical benefits include less tissue trauma, less blood loss, and possibly reduced medical complications.47,48 This is most apparent in minimally invasive deformity correction and fusion. Recently, the transsacral approach for discectomy and fusion and the transpsoas approach have increased in popularity. However, these access corridors have a unique set of complications associated with them, somewhat different than those of traditional anterior lumbar surgery.
The transsacral approach has been used for L5-S1 discectomy and interbody fusion but also, more recently, for L4-5 and L5-S1 discectomy and fusion.47,49–51 This approach relies on a normal presacral space, which exists between the anterior sacral margin and the mesorectum.52,53 The complication rate with the technique has been noted to be low. A retrospective analysis of complications noted, per the U.S. Food and Drug Administration medical device reported data (in 5300 cases of transsacral fusion), that this technique was associated with a 0.7% complication rate. A 0.47% bowel injury rate was noted.54
If a bowel injury is suspected after such a procedure, rigid proctosigmoidoscopy, flexible sigmoidoscopy, or Gastrografin (Bayer AG, Germany) enema can be performed to identify injury. Alternatively, if a patient presents in a delayed manner, CT of the abdomen and pelvis with rectal Gastrografin contrast should be performed. Venous bleeding tends to tamponade in the presacral space. Arterial bleeding is unusual in this situation. For a stable hematoma, observation is recommended. Any expanding pelvic hematoma requires emergency resuscitation and angiography/embolization.55
The transpsoas approach to the lumbar spine is a strictly retroperitoneal approach to the disc space. Complications commonly seen, such as thigh paresthesias or hip flexor and quadriceps weakness, are typically related to the neural structures that have a close relationship to this muscle. Nevertheless, retroperitoneal structures such as the ureter and kidney can be injured, in addition to bowel and vasculature, although the rates of these injuries are probably significantly lower than those in traditional abdominal approaches.56–58 If at any point there is excessive bleeding from this approach, the exposure can be readily converted to a laparotomy and/or thoracotomy.
Complications of Posterior Lumbar Discectomy
Lumbar discectomy is among the most commonly performed spine operations. Complications are few and usually relate to infection, instability, cerebrospinal fluid leak, and neurologic events. Although vascular injuries are uncommon, the potential for bowel, ureteral, and catastrophic vascular injury is now well documented. The shared mechanism of these injuries is violation of the anulus ventrally with some type of biting instrument. A review of the literature in English since 1965 identified 98 cases of vascular injury associated with posterior lumbar discectomy and estimated an incidence of one to five events per 10,000 procedures.59 Goodkin and Laska noted a rate of symptomatic anular perforation (with vascular or viscus injury) of 1.6 to 6 per 10,000 cases.60 Nevertheless, in another series, the incidence was noted at 17 per 10,000.61 It is likely that these complications are considerably more common than is generally appreciated.
Bleeding from the disc space that is not caused by epidural or bone bleeding; findings of fat, viscera, or vessel wall in the specimen; and unexplained hypotension during surgery should lead the surgeon to suspect a vascular or visceral injury. Should this occur, a general or vascular surgeon should be immediately consulted, the wound should be packed or closed, and laparotomy or additional appropriate testing should be performed. Delay of treatment after injury was associated with a 40% mortality rate, while early operation was associated with a 24% mortality rate in one series.60
Vascular Injury
Vascular injury during posterior lumbar surgery may take several forms. The most dramatic are lacerations or partial wall avulsions in large-caliber retroperitoneal arteries or veins that result in massive, acute retroperitoneal hemorrhage. Bleeding is manifested through the disc space itself in fewer than half of these cases. Often, the injury is first suspected because of unexplained hypotension and tachycardia, either in the operating room or during postanesthetic recovery.59,62,63 Lesser injuries may tamponade themselves in a contained retroperitoneal hematoma, later giving rise to a pseudoaneurysm, which can present with delayed rupture or distal embolization. Venous injuries are probably more common, but their diagnosis is less obvious. They may be a source of retroperitoneal hemorrhage, venous thrombosis, leg swelling, and pulmonary embolism. The presence of an expanding lower abdominal mass, unexplained anemia, an unexpected degree of back or leg pain, and lower-extremity swelling or thromboembolism should prompt consideration of a vascular injury. If an artery and a vein are injured in proximity to each other, the potential for the development of an AV fistula exists.59,63 These fistulas are recognized by the presence of a thrill, symptoms of limb claudication, and cardiac overload secondary to a left-to-right shunt. Although AV fistulas may present in the first days postoperatively, full symptomatic maturation is more typically delayed by weeks to months. Violation of the ventral anulus with curets and rongeurs may be a much more common occurrence than is generally realized. Three of 25 patients undergoing follow-up lumbar discography were shown to have new, but asymptomatic, ventral anular defects at the operated levels.64 The L4-5 and L5-S1 levels have been implicated with equal frequency. The anatomy of the aortic bifurcation and the more variable confluence of the common iliac veins have been reviewed. Because the aorta usually terminates opposite the L4 vertebra, aortic injury per se is rare. The common iliac veins usually join at the level of the L4-5 disc space to the right of midline to form the inferior vena cava. Injuries on the lateral aspect of the disc on the right side are expected to involve the terminal inferior vena cava (at L4-5) or the proximal right common iliac vein. In paramedian injuries, the right common iliac artery and the right or left common iliac vein are vulnerable. With left lateral injuries, the left common iliac artery is at risk. With rostral bifurcation of the common iliac arteries, occasional instances of isolated internal iliac injury have been reported during L5-S1 discectomy. The likelihood of breach of the ventral anulus is increased by the vigor with which disc space evacuation is pursued. Prevention of such an injury involves the surgeon’s consciously attempting to avoid breach of the anterior longitudinal ligament. Nevertheless, this often occurs without appreciation of the surgeon. Other risk factors for anterior longitudinal ligament violation may also be present, such as preexisting fissures and peridiscal fibrosis.60
The diameter of the targeted disc space along the projected axis of the discectomy can be accurately measured from preoperative MRI or CT studies acquired in axial planes oriented parallel to the disc space. Ordinarily, this varies from 37 to 48 mm from L3 to the sacrum and is somewhat larger in men than in women.61 The shafts of instruments that are to be introduced into the disc space may then be appropriately marked with a small adhesive strip as a caution against overly deep insertion. The disc rongeur should enter the disc space with the jaws in a closed position to discourage engagement of the thecal sac or a nerve root; however, once within the disc space, the jaws are opened while the rongeur is advanced to its working depth. This distributes any applied forces over two contact points and makes unintended breach of the ventral anulus more difficult. The management of vascular complications is context specific. In the face of acute hemodynamic instability, the prone patient must be quickly returned to the supine position to aid resuscitation and enable exploratory laparotomy. Swift diagnosis and appropriate action are of paramount importance if a catastrophic outcome is to be avoided. Skin edges can be swiftly reapproximated with a whipstitch closure or staples while the anesthetic is lightened and volume resuscitation is begun. The assistance of a general or vascular surgeon should be sought immediately. The area of injury will lie beneath a bulging retroperitoneal hematoma. Arterial hemorrhage may be controlled with manual compression of the distal aorta until desired personnel and equipment can be mobilized. The retroperitoneal hematoma must be explored carefully to delineate the site of injury precisely and to obtain proximal and distal control. It may then be repaired primarily or grafted according to circumstances. Although retroperitoneal hematoma remains an uncommon complication of lumbar discectomy, mortality approaches 50%, in part because of delayed diagnosis. Only through early recognition of such injuries and prompt institution of corrective action can these patients be salvaged. Less calamitous vascular injuries present in a delayed fashion in hemodynamically stable individuals.63 Typically, such injuries are initially evaluated by an abdominal CT scan. If a retroperitoneal mass is defined adjacent to a discectomy site, it should be further evaluated angiographically. In some institutions, magnetic resonance angiography and CT angiography can obviate the need for invasive examination in select settings. Color flow Doppler studies may also be appropriate in patients with significant lower-limb swelling that is suggestive of proximal venous injury or thrombosis. Arterial injuries such as AV fistulas or pseudoaneurysms are repaired or reconstructed as open procedures, usually on an elective basis. Evolving reconstructive endovascular techniques may soon allow for percutaneous management of some of these conditions. Partial or complete thrombosis of an iliac vein or inferior vena cava may require either systemic anticoagulation or placement of an infrarenal caval filter, depending on the circumstances.
Other Visceral Injury
Ureteral injury after lumbar discectomy appears to be an even rarer complication than vascular injury.65 The ureters are located more laterally in the retroperitoneum than are the great vessels, lying in the cleft between the psoas muscles and the spine, within a bed of periureteral fat. Therefore, they are somewhat more mobile than the great vessels and are less vulnerable to injury. In cases in which the ureter is not recognized as part of the operative specimen, diagnosis is usually delayed by several weeks and is announced by abdominal pain, distention, hematuria, and urinary or systemic sepsis. A urinoma may be visualized sonographically or on abdominal CT. Abdominal CT with contrast injection and delayed scans is the gold standard for staging such injuries.31 It is postulated that an aberrantly medial course of the ureters or a thin body habitus allowing compression of the retroperitoneal structures against the spine in the prone position is a factor that contributes to this unusual complication. Treatment includes proximal diversion by nephrostomy followed by a definitive urologic reconstruction according to principles already discussed.66 The possibility of concomitant vascular injury must be excluded by vascular imaging studies and through thorough intraoperative assessment. Another rare but serious complication resulting from ventral penetration of the disc space is bowel perforation.67,68 Most were injuries to the ileum after L5-S1 discectomy. The mesenteric root attaches to the retroperitoneum obliquely, crossing the midline at the L5-S1 interspace as it courses toward the right sacroiliac joint. Because the great vessels bifurcate above this level, it is postulated that a narrow window is thereby created in the midline through which instruments may be passed into the mesentery. It has also been postulated that air-filled loops of bowel tend to float upward and apply themselves ventrally against the spine in the prone position. Intraoperative diagnosis is obvious if the rongeur retrieves intestinal mucosa. Otherwise, in the early postoperative period, patients have an acute abdomen or, in a delayed fashion, chronic wound infections attributable to intestinal flora. Patients with a bowel injury may also present with discitis.60 Treatment is usually surgical, especially when a walled-off abscess is suspected.
Instrumentation Complications
The use of instrumentation has introduced a new level of complexity and complications to spine surgery. Apart from issues related to biomechanical failure, the various forms of dorsal instrumentation, including assorted types of pedicle screws, claws, hooks, and sublaminar wires, mainly pose a threat of neurologic injury or cerebrospinal fluid leak. Placement of transarticular C1-2 screws must be technically exact to avoid vertebral artery injury. Consideration of placing C1-2 instrumentation first on the side with the safer trajectory (considering the artery) should be made. For transarticular screws, the safest trajectory involves placing the screw through the most dorsal and medial part of the isthmus.25 If an initial C1-2 screw placement results in arterial injury, a pars screw may be a preferable option on the contralateral side rather than risking bilateral vertebral artery occlusion. It is conceivable that overly long pedicle screws could penetrate far enough through the vertebral cortex ventrally to transfix the esophagus or great vessels.
Bicortical screw fixation at the S1 level raises concern for internal iliac vein or lumbosacral trunk injury. Both of these structures lie in close apposition to the sacrum just medial to the sacroiliac joint. On the basis of anatomic studies in cadavers, the potential for injury to these structures seems to be increased with screws that are placed laterally.69 Screws that are directed medially through the S1 pedicles to engage the ventral sacral cortex have only a remote chance of causing visceral injury. Major vascular structures are located more laterally, and the sigmoid colon is still attached on a short mesentery at this level. It is possible that the preparatory drilling, probing, and tapping of the pilot holes are more likely to produce injury than is the placement of the screw itself. These maneuvers must be performed with extreme caution to minimize the risk of overdrilling or overtapping of the holes, which could easily puncture a vessel or viscus. Clinically relevant injuries appear to be extremely uncommon thus far, but continued strict attention to the technical details of instrumentation insertion is mandatory if these untoward events are to be avoided. Presumably, the expanding use of image-guidance technologies will add an extra margin of safety to these endeavors. Ventral thoracic and thoracolumbar spine instrumentation has demonstrated its potential to cause serious vascular complications. The Dunn device was withdrawn from clinical use after it was associated with the development of abdominal aneurysms in three cases. Whether this was caused by the device’s design or by the method of its application in these particular individuals remains unclear. Contemporary ventral fixation systems for use in the thoracic and lumbar spine, using rod or plate constructs, have been free of vascular complications thus far. This is attributed to design parameters that emphasize a low profile and that demand an exactly lateral application on the vertebral bodies with an orthogonal screw trajectory dorsal to all major vessels. If care is taken to apply the instrumentation as intended, with screws of appropriate length, the potential for significant vascular or soft tissue injury should be exceedingly small. Aortic injury at the T6 level may result from screw penetration during ventral spine fixation.70 Delayed aortic rupture has been attributed to erosion by a mesh cage placed for ventral reconstruction in the thoracic spine.71 Complications such as these underscore the need for careful correlation of the field as viewed intraoperatively with preoperative and intraoperative imaging to reconfirm that spine instrumentation is applied as intended. Such cross-checking is especially advisable in cases of deformity correction, in which normal anatomic relations can be highly distorted. The use of screws with a blunt tip and a tapered run out to the threads may mitigate the possibility of engagement of a major vessel. Above all, surgeons must adhere to proper operative technique and pay close attention to screw length and trajectory. The width of the vertebral body and the exact location of the great vessels relative to the projected screw path can be ascertained through inspection of the axial CT or MRI scans at the levels to be instrumented.
Summary
Contemporary surgical techniques enable both ventral and dorsal exposure of the entire spine from occiput to sacrum. This allows any pathologic condition to be addressed in an anatomically appropriate fashion. As a group, the ventral approaches are more likely to have soft tissue or vascular complications because of the need to reflect these structures that overlie the spine. This inevitably subjects the patient to heightened risks of exposure-related complications compared with the simpler midline dorsal approaches.72 This risk is justified only if it is kept acceptably low and if patient outcomes are significantly improved in relation to the natural history of the disease and to safer but possibly less definitive procedures. Sound case selection and excellent surgical technique are the keys to avoiding or minimizing these complications.
Baron E.M., Soliman A.M., Gaughan J.P., et al. Dysphagia, hoarseness, and unilateral true vocal fold motion impairment following anterior cervical diskectomy and fusion. Ann Otol Rhinol Laryngol. 2003;112:921.
Faciszewski T., Winter R.B., Lonstein J.E., et al. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults: a review of 1223 procedures. Spine (Phila Pa 1976). 1995;20:1592.
Goodkin R., Laska L.L. Vascular and visceral injuries associated with lumbar disc surgery: medicolegal implications. Surg Neurol. 1998;49:358.
Jung A., Schramm J. How to reduce recurrent laryngeal nerve palsy in anterior cervical spine surgery: a prospective observational study. Neurosurgery. 2010;67:10.
Oskouian R.J.Jr., Johnson J.P. Vascular complications in anterior thoracolumbar spinal reconstruction. J Neurosurg. 2002;96:1.
Peng C.W., Chou B.T., Bendo J.A., et al. Vertebral artery injury in cervical spine surgery: anatomical considerations, management, and preventive measures. Spine J. 2009;9:70.
Tormenti M.J., Maserati M.B., Bonfield C.M., et al. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg Focus. 2010;28:E7.
1. Baron E.M., Choi D., Harrop J.S., et al. Anterior odontoid resection. In: Vaccaro A.R., Baron E.M., editors. Atlas of spinal surgery. Philadelphia: Elsevier; 2007:25.
2. Robinson R., Smith G. Anterolateral cervical disc removal and interbody fusion for cervical disk syndrome. Bull Johns Hopkins Hosp. 1955;96:223.
3. Baron E.M., Soliman A.M., Gaughan J.P., et al. Dysphagia, hoarseness, and unilateral true vocal fold motion impairment following anterior cervical diskectomy and fusion. Ann Otol Rhinol Laryngol. 2003;112:921.
4. Jung A., Schramm J., Lehnerdt K., et al. Recurrent laryngeal nerve palsy during anterior cervical spine surgery: a prospective study. J Neurosurg Spine. 2005;2:123.
5. Jung A., Schramm J. How to reduce recurrent laryngeal nerve palsy in anterior cervical spine surgery: a prospective observational study. Neurosurgery. 2010;67:10.
6. Rontal E., Rontal M. The immobile cord. In: Cummings C., editor. Otolaryngology: head and neck surgery. St. Louis: Mosby; 1986:2055.
7. Benninger M., Schwimmer C., et al. Functional neurophysiology and voice disorders. In: Rubin J., Sataloff R., Korovin G., editors. Diagnosis and treatment of voice disorders. New York: Igaku-Shoin; 1995:105.
8. Woo P. Laryngeal electromyography is a cost-effective clinically useful tool in the evaluation of vocal fold function [see comments]. Arch Otolaryngol Head Neck Surg. 1998;124:472.
9. Flint P., Cummings C., et al. Phonosurgical procedures. In: Cummings C., Fredrickson J., Harker L., editors. Otolaryngology: head and neck surgery. ed 3. St. Louis: Mosby; 1998:2073.
10. Cloward R. New method of diagnosis and treatment of cervical disc disease. Clin Neurosurg. 1962;8:93.
11. Stewart M., Johnston R.A., Stewart I., et al. Swallowing performance following anterior cervical spine surgery. Br J Neurosurg. 1995;9:605.
12. Daniels S.K., Mahoney M.C., Lyons G.D. Persistent dysphagia and dysphonia following cervical spine surgery. Ear Nose Throat J. 1998;77:470.
13. Welsh L.W., Welsh J.J., Chinnici J.C. Dysphagia due to cervical spine surgery. Ann Otol Rhinol Laryngol. 1987;96:112.
14. Buchholz D.W. Dysphagia following anterior cervical spine surgery. Dysphagia. 1997;12:9.
15. Geyer T.E., Foy M.A. Oral extrusion of a screw after anterior cervical spine plating. Spine (Phila Pa 1976). 2001;26:1814.
16. Smith M.D., Bolesta M.J. Esophageal perforation after anterior cervical plate fixation: a report of two cases. J Spinal Disord. 1992;5:357.
17. Finiels P.J., Hernandez G., Sabatier P., et al. Delayed esophageal perforation after cervical osteosynthesis. Case illustration. J Neurosurg. 2000;92:123.
18. Burger R., Tonn J.C., Vince G.H., et al. Median corpectomy in cervical spondylotic multisegmental stenosis. Zentralbl Neurochir. 1996;57:62.
19. Newhouse K.E., Lindsey R.W., Clark C.R., et al. Esophageal perforation following anterior cervical spine surgery. Spine (Phila Pa 1976). 1989;14:1051.
20. Gaudinez R.F., English G.M., Gebhard J.S., et al. Esophageal perforations after anterior cervical surgery. J Spinal Disord. 2000;13:77.
21. Sahjpaul R.L. Esophageal perforation from anterior cervical screw migration. Surg Neurol. 2007;68:205.
22. Gazzeri R., Tamorri M., Faiola A., et al. Delayed migration of a screw into the gastrointestinal tract after anterior cervical spine plating. Spine (Phila Pa 1976). 2008;33:E268.
23. Ratnaraj J., Todorov A., McHugh T., et al. Effects of decreasing endotracheal tube cuff pressures during neck retraction for anterior cervical spine surgery. J Neurosurg. 2002;97:176.
24. Neo M., Fujibayashi S., Miyata M., et al. Vertebral artery injury during cervical spine surgery: a survey of more than 5600 operations. Spine (Phila Pa 1976). 2008;33:779.
25. Peng C.W., Chou B.T., Bendo J.A., et al. Vertebral artery injury in cervical spine surgery: anatomical considerations, management, and preventive measures. Spine J. 2009;9:70.
26. Pfeifer B.A., Freidberg S.R., Jewell E.R. Repair of injured vertebral artery in anterior cervical procedures. Spine (Phila Pa 1976). 1994;19:1471.
27. Ebraheim N.A., Xu R., Ahmad M., et al. The quantitative anatomy of the vertebral artery groove of the atlas and its relation to the posterior atlantoaxial approach. Spine (Phila Pa 1976). 1998;23:320.
28. Cho K.H., Shin Y.S., Yoon S.H., et al. Poor surgical technique in cervical plating leading to vertebral artery injury and brain stem infarction: case report. Surg Neurol. 2005;64:221.
29. Oskouian R.J.Jr., Johnson J.P. Vascular complications in anterior thoracolumbar spinal reconstruction. J Neurosurg. 2002;96:1.
30. Hodge W.A., DeWald R.L. Splenic injury complicating the anterior thoracoabdominal surgical approach for scoliosis: a report of two cases. J Bone Joint Surg [Am]. 1983;65:396.
31. Asali M.G., Romanowsky I., Kaneti J. [External ureteral injuries]. Harefuah. 2007;146:686.
32. Cleveland R.H., Gilsanz V., Lebowitz R.L., et al. Hydronephrosis from retroperitoneal fibrosis after anterior spine fusion: a case report. J Bone Joint Surg [Am]. 1978;60:996.
33. Silber I., McMaster W. Retroperitoneal fibrosis with hydronephrosis as a complication of the Dwyer procedure. J Pediatr Surg. 1977;12:255.
34. Kleeman T.J., Michael Ahn U., Clutterbuck W.B., et al. Laparoscopic anterior lumbar interbody fusion at L4-L5: an anatomic evaluation and approach classification. Spine (Phila Pa 1976). 2002;27:1390.
35. Rajaraman V., Vingan R., Roth P., et al. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg. 1999;91:60.
36. Brau S.A., Delamarter R.B., Schiffman M.L., et al. Vascular injury during anterior lumbar surgery. Spine J. 2004;4:409.
37. Sasso R.C., Best N.M., Mummaneni P.V., et al. Analysis of operative complications in a series of 471 anterior lumbar interbody fusion procedures. Spine (Phila Pa 1976). 2005;30:670.
38. Garg J., Woo K., Hirsch J., et al. Vascular complications of exposure for anterior lumbar interbody fusion. J Vasc Surg. 2010;51:946.
39. Baker J.K., Reardon P.R., Reardon M.J., et al. Vascular injury in anterior lumbar surgery. Spine. 1993;18:2227.
40. Kaiser M.G., Haid R.W.Jr., Subach B.R., et al. Comparison of the mini-open versus laparoscopic approach for anterior lumbar interbody fusion: a retrospective review. Neurosurgery. 2002;51:97.
41. Zdeblick T.A., David S.M. A prospective comparison of surgical approach for anterior L4-L5 fusion: laparoscopic versus mini anterior lumbar interbody fusion. Spine. 2000;25:2682.
42. Schwender J.D., Casnellie M.T., Perra J.H., et al. Perioperative complications in revision anterior lumbar spine surgery: incidence and risk factors. Spine (Phila Pa 1976). 2009;34:87.
43. Jagannathan J., Anton T., Baweja H., et al. Evaluation and management of abdominal lymphoceles after anterior lumbar spine surgery. Spine (Phila Pa 1976). 2008;33:E852.
44. Patel A.A., Spiker W.R., Daubs M.D., et al. Retroperitoneal lymphocele after anterior spinal surgery. Spine (Phila Pa 1976). 2008;33:E648.
45. Schizas C., Foko’o N., Matter M., et al. Lymphocoele: a rare and little known complication of anterior lumbar surgery. Eur Spine J. 2009;18(Suppl 2):228.
46. Thaler M., Achatz W., Liebensteiner M., et al. Retroperitoneal lymphatic cyst formation after anterior lumbar interbody fusion: a report of 3 cases. J Spinal Disord Tech. 2010;23:146.
47. Anand N., Baron E.M., Thaiyananthan G., et al. Minimally invasive multilevel percutaneous correction and fusion for adult lumbar degenerative scoliosis: a technique and feasibility study. J Spinal Disord Tech. 2008;21:459.
48. Park Y., Ha J.W. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine. 2007;32:537.
49. Aryan H.E., Newman C.B., Gold J.J., et al. Percutaneous axial lumbar interbody fusion (AxiaLIF) of the L5-S1 segment: initial clinical and radiographic experience. Minim Invasive Neurosurg. 2008;51:225.
50. Erkan S., Wu C., Mehbod A.A., et al. Biomechanical evaluation of a new AxiaLIF technique for two-level lumbar fusion. Eur Spine J. 2009;18:807.
51. Marotta N., Cosar M., Pimenta L., et al. A novel minimally invasive presacral approach and instrumentation technique for anterior L5-S1 intervertebral discectomy and fusion: technical description and case presentations. Neurosurg Focus. 2006;20:E9.
52. Cragg A., Carl A., Casteneda F., et al. New percutaneous access method for minimally invasive anterior lumbosacral surgery. J Spinal Disord Tech. 2004;17:21.
53. Yuan P.S., Day T.F., Albert T.J., et al. Anatomy of the percutaneous presacral space for a novel fusion technique. J Spinal Disord Tech. 2006;19:237.
54. Anand N., Baron E.M., Rosemann R., et al. Safety and Complication Profile of Percutaneous Lumbosacral Interbody Fusion. Congress of Neurological Surgeons. New Orleans: LA; 2009.
55. Lee S, Rivadeneira D, Härtl R: Best practices in avoidance, detection and treatment of colorectal perforations during AxiaLIF surgery. Wilmington, NC, 2009, Tran S1
56. Anand N., Rosemann R., Khalsa B., et al. Mid-term to long-term clinical and functional outcomes of minimally invasive correction and fusion for adults with scoliosis. Neurosurg Focus. 2010;28:E6.
57. Baron E.M., Anand N., Vaccaro A.R. Complications of extreme lateral interbody fusion (XLIF). In: Goodrich J.A., Volcan I., editors. eXtreme lateral interbody fusion (XLIF). St. Louis: Quality Medical; 2008:117.
58. Tormenti M.J., Maserati M.B., Bonfield C.M., et al. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg Focus. 2010;28:E7.
59. Papadoulas S., Konstantinou D., Kourea H.P., et al. Vascular injury complicating lumbar disc surgery: a systematic review. Eur J Vasc Endovasc Surg. 2002;24:189.
60. Goodkin R., Laska L.L. Vascular and visceral injuries associated with lumbar disc surgery: medicolegal implications. Surg Neurol. 1998;49:358.
61. Anda S., Aakhus S., Skaanes K.O., et al. Anterior perforations in lumbar discectomies. A report of four cases of vascular complications and a CT study of the prevertebral lumbar anatomy. Spine (Phila Pa 1976). 1991;16:54.
62. Raptis S., Quigley F., Barker S. Vascular complication of elective lower lumbar disc surgery. Aust N Z J Surg. 1994;64:216.
63. Brewster D.C., May A.R., Darling R.C., et al. Variable manifestations of vascular injury during lumbar disk surgery. Arch Surg. 1979;114:1026.
64. Solonen K.A. Perforation of the anterior annulus fibrosus during operation for prolapsed disc. Ann Chir Gynaecol Fenn. 1975;64:385.
65. Krone A., Heller V., Osterhage H.R. Ureteral injury in lumbar disc surgery. Acta Neurochir (Wien). 1985;78:108.
66. Noyes D.T., Morrisseau P.M. Ureteral transection secondary to lumbar disk surgery. Urology. 1982;19:651.
67. Shaw E.D., Scarborough J.T., Beals R.K. Bowel injury as a complication of lumbar discectomy: a case report and review of the literature. J Bone Joint Surg Am. 1981;63:478.
68. Smith E.B., DeBord J.R., Hanigan W.C. Intestinal injury after lumbar discectomy. Surg Gynecol Obstet. 1991;173:22.
69. Mirkovic S., Abibtol J.J., Steinman J., et al. Anatomic consideration for sacral screw placement. Spine (Phila Pa 1976). 1991;16:S289.
70. Matsuzaki H., Tokuhashi Y., Wakabayashi K., et al. Penetration of a screw into the thoracic aorta in anterior spinal instrumentation: a case report. Spine (Phila Pa 1976). 1993;18:2327.
71. Floch N.R., Harvey J.C., Beattie E.J.Jr. Aortoesophageal fistula after reconstruction of the thoracic spine. Ann Thorac Surg. 1995;60:191.
72. Faciszewski T., Winter R.B., Lonstein J.E., et al. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults: a review of 1223 procedures. Spine (Phila Pa 1976). 1995;20:1592.