Chapter 19 Variants of Preexcitation
Variants of Preexcitation (Atypical Bypass Tracts)
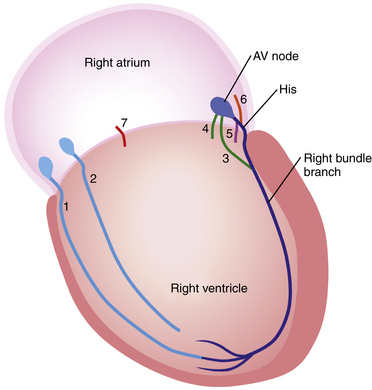
A working definition of an atypical bypass tract (BT) is a conduction pathway that bypasses all or part of the normal conduction system but is not a rapidly conducting pathway connecting atrium and ventricle near the mitral or tricuspid annulus. Thus, pathways that connect the atrium to the His bundle (HB), the atrioventricular node (AVN) to the His-Purkinje system (HPS) or the ventricle, or the HPS to the ventricle fit into this designation (Fig. 19-1).
“Mahaim Fibers”
In 1937, during pathological examination of the heart, Mahaim and Benatt identified islands of conducting tissue extending from the HB into the ventricular myocardium. These fibers were called Mahaim fibers or fasciculoventricular fibers.1–3 This description was subsequently expanded to include connections between the AVN and the ventricular myocardium (nodoventricular fibers). Later, it was recognized that BTs could arise from the AVN and insert into the right bundle branch (RB; nodofascicular fibers).2,3 This classification for Mahaim fibers persisted until evidence suggested that the anatomical substrate of tachycardias with characteristics previously attributed to nodoventricular and nodofascicular fibers is actually atrioventricular (AV) and atriofascicular BTs with decremental conduction properties (i.e., conduction slows at faster heart rates) (see Fig. 19-1). Although these BTs are sometimes collectively referred to as “Mahaim fibers,” the use of this term is discouraged because it is more illuminating to name the precise BT according to its connections. In this chapter, these BTs are referred to as atypical BTs to differentiate them from the more common (typical) rapidly conducting AV BTs that result in the Wolff-Parkinson-White (WPW) syndrome.4
“Mahaim Tachycardia”
The term Mahaim tachycardia is used to describe the typical constellation of electrophysiological (EP) features that characterize the unusual form of reentrant tachycardia using an atypical BT, without implication about the underlying anatomical cause. It should be noted that, because the term was originally applied to an anatomical finding and subsequently (incorrectly) applied to physiology that matched what would be expected from this anatomy, it has given rise to more confusion than understanding. Hence, the use of the term Mahaim tachycardia should generally be discouraged; instead, one should simply describe the physiological characteristics of the tachyarrhythmia.5
Types of Atypical Bypass Tracts
Long Decrementally Conducting Atrioventricular and Atriofascicular Bypass Tracts
These BTs comprise the majority (80%) of atypical BTs; their atrial insertion site is in the right atrial (RA) free wall.6,7 These BTs tend (84%) to cross the tricuspid annulus in the lateral, anterolateral, or anterior region. They extend along the right ventricular (RV) free wall to the region where the moderator band usually inserts at the apical third of the RV free wall, inserting into the distal part of the RB (atriofascicular BT) or into the ventricular myocardium close to the RB (long decrementally conducting AV BT). These BTs are functionally similar to the normal AV junction, with an AVN-like structure leading to a His bundle (HB)–like structure. In essence, those BTs function as an auxiliary conduction system parallel to the normal conduction system (AVN–HPS). Similar to the normal AVN, these BTs demonstrate decremental conduction (related to the slow rate of recovery of excitability) and Wenckebach-type block in response to rapid atrial pacing and are sensitive to adenosine. The conduction delay in these BTs has been localized to the intraatrial portion of the BT (the AVN-like portion), whereas the interval from the inscription of the BT potential at the tricuspid annulus and the onset of ventricular activation (BT-V interval) remains constant.4–6,8,9
Short Decrementally Conducting Atrioventricular Bypass Tracts
These BTs are analogous to decrementally conducting concealed BTs responsible for the permanent form of junctional reciprocating tachycardia (PJRT; see Chap. 18) in that they bridge the AV rings and insert proximally into ventricular myocardium in close proximity to the AV annulus.7,10 These BTs primarily arise from the RA free wall, but can also arise from the posterior or septal region. Left-sided BTs with decremental conduction characteristics have rarely been described. Although these BTs demonstrate decremental conduction and Wenckebach-type block in response to rapid atrial pacing, they do not consistently appear to be responsive to adenosine, which suggests that their structure is not composed of AVN-like tissue.10
Nodoventricular and Nodofascicular Bypass Tracts
Nodoventricular BTs arise in the normal AVN and insert into ventricular myocardium near the AV junction.7 Nodofascicular BTs arise in the normal AVN and insert into the RB. These BTs are sensitive to adenosine, probably because of their AVN connection.5
Arrhythmias Associated with Atypical Bypass Tracts
Atypical BTs in patients with clinical arrhythmias have the following characteristics: (1) unidirectional (anterograde-only) conduction (with rare exceptions); (2) long conduction times; and (3) decremental conduction (i.e., cycle length [CL]-dependent slowing of conduction).
Atypical BTs comprise 3% to 5% of all BTs. The incidence is slightly higher (6%) in patients presenting with supraventricular tachycardia (SVT) with a left bundle branch block (LBBB) morphology.7 Multiple BTs occur in 10% of patients with atypical BTs. In some cases, a typical, rapidly conducting AV BT can mask the presence of an atypical BT, which only becomes apparent after ablation of the typical BT. Dual AVN pathways or multiple BTs occur in 40% of patients with atypical BTs. Atypical BTs can also be associated with Ebstein anomaly.
Supraventricular Tachycardias Requiring An Atrioventricular Bypass Tract for Initiation and Maintenance
Antidromic atrioventricular reentrant tachycardia (AVRT) can use the atypical BT anterogradely and the HPS-AVN retrogradely. Antidromic AVRT can also use the atypical BT anterogradely and a second AV BT retrogradely. In the latter case, the AVN can participate as an innocent bystander mediating anterograde or retrograde fusion. Because these atypical BTs almost always conduct anterogradely only, they cannot mediate orthodromic AVRT, but can mediate antidromic AVRT or can be an innocent bystander during other SVTs (e.g., atrioventricular nodal reentrant tachycardia [AVNRT]). However, they can coexist with typical rapidly conducting AV BTs. AVRTs using a nodoventricular or nodofascicular BT as the anterograde limb generally use a second AV BT as the retrograde limb of the reentrant circuit.5 Right free wall atriofascicular BTs capable of both anterograde and retrograde conduction that participate in both antidromic and orthodromic AVRT have rarely been reported.11
Electrocardiographic Features
Normal Sinus Rhythm
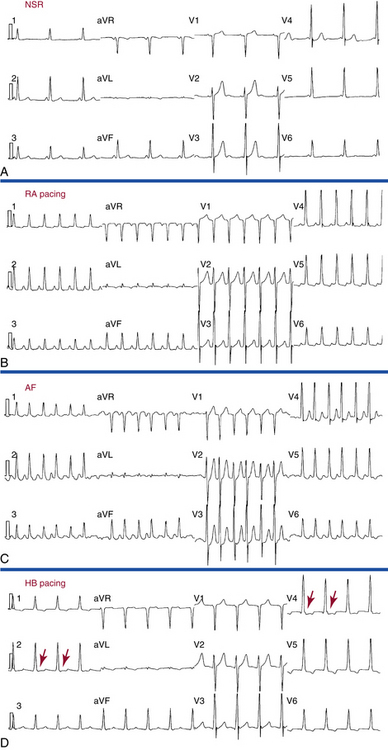
During normal sinus rhythm (NSR), the ECG shows normal QRS or minimal preexcitation in most patients with atypical BTs. Subtle preexcitation can be suspected by the absence of the normal septal forces (small q waves) in leads I, aVL, V5, and V6 and the presence of an rS complex in lead III in the setting of a narrow QRS.12 The degree of preexcitation depends on the relative conduction time over the AVN and BT. Maneuvers that prolong conduction over the AVN (e.g., atrial pacing, vagal maneuvers, or drugs) to a greater degree than BT conduction will increase the degree of preexcitation. Because atypical BTs exhibit decremental conduction, increasing the atrial pacing rate results in prolongation of the P-delta interval. In contrast, in the setting of typical rapidly conducting AV BTs, the P-delta interval remains relatively constant regardless of the degree of preexcitation; whereas prolonging the AVN conduction time results in more preexcitation. The P-delta interval remains constant or exhibits mild prolongation because conduction over the typical BT displays less decrement than does the AVN.5,7,12–14
Preexcited Qrs Morphology
For atriofascicular and nodofascicular BTs, the QRS is relatively narrow (133 ± 10 milliseconds), and its morphology is classic for typical LBBB with a QRS axis between 0 and –75 degrees and a late precordial R/S transition zone (at V4 or V5, and sometimes V6). However, for long decrementally conducting AV BTs, the QRS is relatively wider (166 ± 26 milliseconds) and the LBBB pattern is less typical (with broad initial R in V1). The QRS is even wider and the LBBB pattern is less typical with nodoventricular and decrementally conducting short AV BTs than that with atriofascicular or long decrementally conducting AV BTs.5,12,14
Supraventricular Tachycardias
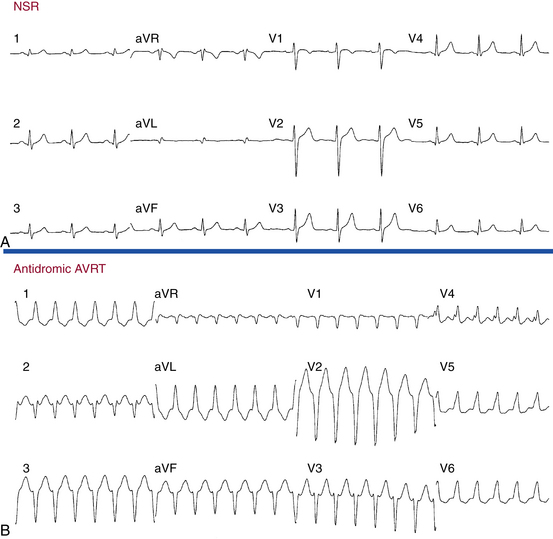
Arrhythmias associated with atypical BTs are associated with an LBBB pattern and, most often, in the setting of long decrementally conducting AV and atriofascicular BTs, left axis deviation on the surface ECG (Fig. 19-2). There are several ECG features that suggest (although are not diagnostic of) atypical BTs as the cause of an SVT with LBBB pattern. These include (1) QRS axis between 0 and –75 degrees, (2) QRS duration of 150 milliseconds or less, (3) R wave in lead I, (4) rS complex in lead V1, and (5) precordial R wave transition in lead V4 or later.5,12,14
Electrophysiological Testing
Baseline Observations During Normal Sinus Rhythm
Atrial Pacing and Atrial Extrastimulation during Normal Sinus Rhythm
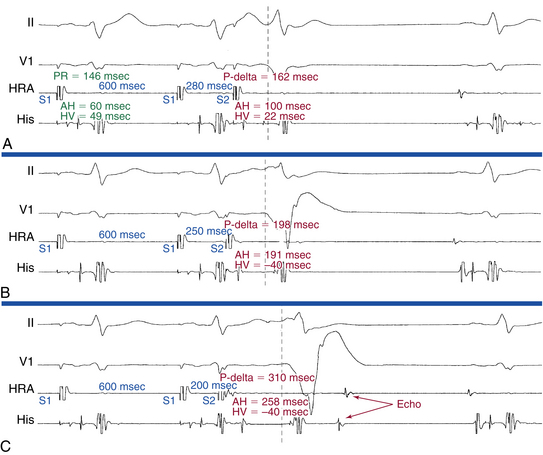
Progressively shorter atrial pacing CLs or atrial extrastimulus (AES) coupling intervals produce decremental conduction in both the atypical BT and, to a greater degree, the AVN (Fig. 19-3).12 Consequently, the atrial–His bundle (AH) interval increases, the QRS morphology gradually shifts to a more preexcited LBBB morphology, and the AV (A-delta) interval increases. However, the AV (A-delta) interval increases to a lesser degree than the AH interval. This is in contrast to the setting of typical rapidly conducting AV BTs, in which the AV (A-delta) interval remains constant despite prolongation of the AH interval and exaggeration of the degree of preexcitation, because the A-delta interval represents conduction time over the BT. Typical AV BTs maintain constant conduction time during different pacing rates and AES coupling intervals—that is, nondecremental conduction.5
With progressively shorter atrial pacing CLs or AES coupling intervals, the HV interval decreases as the His potential becomes progressively inscribed into the QRS (usually within the first 5 to 25 milliseconds after the onset of the QRS). The His potential eventually becomes activated retrogradely as the wavefront travels anterogradely down the BT and then retrogradely up the RB to the HB (see Fig. 19-3). When the His potential is lost within the QRS, it is unclear whether anterograde AV conduction continues to propagate over the HB or block has occurred.7
At the point of maximal preexcitation, the AV (A-delta) interval continues to prolong with more rapid pacing because of the decremental conduction properties of the BT, and the His potential–QRS relationship remains unaltered because the HB is activated retrogradely until block in the BT occurs. The fixed ventricular–His bundle (VH) interval, despite shorter pacing CLs or AES coupling intervals, suggests that the BT inserts into or near the distal RB at the anterior free wall of the RV with retrograde conduction to the HB. Whenever the VH interval is less than 20 milliseconds, insertion into the RB (i.e., atriofascicular or nodofascicular BT) is likely. On the other hand, with long decrementally conducting AV BTs, which insert into the ventricular myocardium close to the RB, the VH interval approximates the HV interval minus the duration of the His potential (because the His potential is activated retrogradely).5,7
For short decrementally conducting BTs, the HB is activated anterogradely, and retrograde conduction to the HB is only seen following AV block or during antidromic AVRT. Decremental conduction (progressive prolongation of the AV interval) and Wenckebach-type block develop in the BT. The conduction delay in these BTs is localized to the intraatrial portion of the BT; the interval from the inscription of the BT potential at the tricuspid annulus to the onset of ventricular activation (BT-V interval) remains constant.5
Effects of Adenosine
Adenosine produces conduction delay in most atypical BTs except for short decrementally conducting AV BTs. The conduction delay has been localized to the intraatrial portion of the BT; the interval from the inscription of the BT potential at the tricuspid annulus and the onset of ventricular activation remains constant.7,13 When adenosine administration slows AVN conduction, an increase in the degree of preexcitation is noted in all types of BTs (as long as adenosine does not block the BT).
Induction of Tachycardia
Initiation by Atrial Extrastimulation or Atrial Pacing
As noted, progressively shorter atrial pacing CLs (especially from the RA) result in progressive AV (A-delta) interval prolongation and a greater degree of preexcitation until maximal. Often, once maximal preexcitation has been achieved, cessation of pacing is followed by preexcited SVT. Progressively shorter AES coupling intervals similarly result in progressive AV (A-delta) interval prolongation and a greater degree of preexcitation until maximal. When anterograde AVN conduction fails but conduction persists over the BT, the HPS-AVN can be activated retrogradely to initiate antidromic AVRT.7
The sudden appearance of preexcitation associated with a “jump” from the fast to the slow AVN pathway with a His potential inscribed before ventricular activation or with a VH interval of less than 10 milliseconds strongly favors AVNRT. Although a slowly conducting atriofascicular BT that becomes manifest with a jump to the slow AVN pathway cannot be excluded, a consistent pattern of dual pathway dependence and an HV relationship too short to be retrograde from the distal RB would be unlikely.7 Induction of AVNRT with AES is almost always associated with a dual pathway response, which may not be seen if the impulse conducts anterogradely over the BT and captures the HB before it is activated by the impulse traversing the slow AVN pathway anterogradely. In other cases, a jump can be seen so that the anterograde His potential follows the QRS with a typical AVN echo to initiate SVT, analogous to 1:2 conduction initiating antidromic AVRT.
Initiation by Ventricular Extrastimulation or Ventricular Pacing
Ventricular pacing can initiate SVT in 85% of cases. Initiation is almost always associated with retrograde conduction up a relatively fast AVN pathway, followed by anterograde conduction down a slow pathway, which is associated with preexcitation. The anterograde slow pathway can be a BT (i.e., antidromic AVRT) or a slow AVN pathway (i.e., AVNRT with an innocent bystander BT). During induction of the SVT by ventricular pacing at a CL similar to the tachycardia CL or by a VES that advances the His potential by a coupling interval similar to the H-H interval during the SVT, the His bundle–atrial (HA) interval following the ventricular stimulus is compared with that during the SVT—an HA interval that is longer with ventricular pacing or VES initiating the SVT than that during the SVT suggests AVNRT. This occurs despite the fact that the H-H interval of the VES (i.e., the interval between the His potential activated anterogradely by the last sinus beat to the His potential activated retrogradely by the VES initiating the SVT) exceeds the H-H interval during the SVT. Because the AVN usually exhibits greater decremental conduction with repetitive engagement of impulses than in response to a single impulse at a similar coupling interval, the more prolonged the HA with the initiating ventricular stimulus, the more likely the SVT is AVNRT. If the SVT uses the BT for anterograde conduction, the HA interval during ventricular pacing or the VES initiating the SVT, at a comparable coupling interval as the tachycardia CL, should have the same HA interval as during the SVT.
Tachycardia Features
Antidromic Atrioventricular Reentrant Tachycardia Using an Atypical Bypass Tract Anterogradely
Ventricular–His Bundle Interval
For atriofascicular and nodofascicular BTs, the VH interval is short (16 ± 5 milliseconds), much shorter than the nonpreexcited HV interval and also shorter than the VH interval during ventricular pacing, because the BT inserts into the RB, hence, the HB and ventricle are activated in parallel, not in sequence. Conduction time to the distal RB is short (V-RB = 3 ± 5 milliseconds).13 For long decrementally conducting AV BTs, the VH interval is short (37 ± 9 milliseconds) but longer than that of atriofascicular BTs because the ventricle and HB are activated in sequence, not in parallel. However, the VH interval is still shorter than the nonpreexcited HV interval. In this setting, the VH interval approximates the HV interval minus the duration of the His potential, because the BT inserts close to the RB and the His potential is activated retrogradely. In the presence of long decrementally conducting AV BTs, conduction time to the distal RB (V-RB = 25 ± 6 milliseconds) is longer than that for atriofascicular BTs. During antidromic AVRT using a nodoventricular or a short decrementally conducting AV BT, intermediate VH intervals are observed, whereby the His potential is inscribed within the QRS. The VH interval during the AVRT is longer than the nonpreexcited HV interval and than the VH interval during RV apical pacing, exceeding it by the time it takes the impulse to travel from the ventricular insertion site of the BT at the RV base to the distal RB (i.e., because of the long V-RB interval).7,13 When antidromic AVRT occurs in the presence of retrograde right bundle branch block (RBBB), the VH interval is long (the His potential is inscribed after the QRS and the VH interval is longer than the nonpreexcited HV interval). Retrograde block over the RB results in anterograde conduction over the distal RB (in the setting of atriofascicular BTs) or RV and transseptal impulse propagation with subsequent retrograde conduction over the left bundle branch (LB) into the HB and AVN. This results in an antidromic AVRT with a macroreentrant circuit incorporating the LB retrogradely and either an atriofascicular, a long decrementally conducting, or a short decrementally conducting AV BT anterogradely.7,13
Atrial-Ventricular Relationship
For atriofascicular BTs, long decrementally conducting AV BTs, and short decrementally conducting AV BTs, a 1:1 A-V relationship is a prerequisite for the maintenance of antidromic AVRT, because parts of both the RA and the RV are incorporated in the reentrant circuit. However, in the setting of nodofascicular and nodoventricular BTs, the atrium is neither part of nor required for the reentrant circuit, and AV block or dissociation can (although rarely) be present without disrupting the SVT.7,13
Changes in Tachycardia Cycle Length
Changes in the rate of antidromic AVRT using an atriofascicular or a long AV BT as the anterograde limb of the circuit can occur secondary to changes in the VA conduction time, due to either retrograde RBBB or shift of retrograde conduction over a fast AVN pathway to conduction over a slow AVN pathway. The prolongation of the tachycardia CL will be in the VH interval in the former setting, but in the HA interval in the latter setting. Additionally, VA conduction over the HB-AVN axis changing into VA conduction over a second BT can alter the tachycardia CL. When this occurs, the change in the tachycardia CL will depend on the location of the ventricular end of the second BT and the conduction properties of that BT. Therefore, there may be a shortening or a prolongation of the CL. The behavior of the V-RB and VH interval in that situation will depend on where the block is located in the bundle branch–HB-AVN axis.15
Preexcited Atrioventricular Nodal Reentrant Tachycardia Using an Atypical Bypass Tract as an Innocent Bystander
The site of earliest ventricular activation depends on the type of the atypical BT mediating preexcitation (see previous discussion). A positive HV interval or VH interval of 10 milliseconds or less (especially when the HA interval is 50 milliseconds or less) is characteristic of AVNRT, because the HB is usually activated anterogradely. However, the HB can become activated retrogradely when conduction over the anterograde slow AVN pathway is very slow; then the VH interval will depend on the type of the atypical BT mediating preexcitation (see earlier).7,13
Diagnostic Maneuvers During Tachycardia
Atrial Extrastimulation and Atrial Pacing during Tachycardia
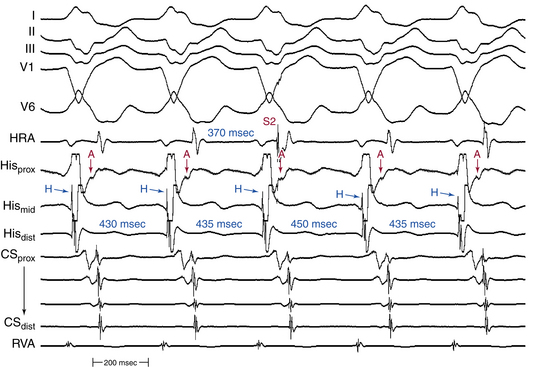
To prove the presence of a BT and its participation in the SVT, a late-coupled AES is delivered from the lateral RA (close to the BT) when the AV junctional portion of the atrium is refractory, so that the AES does not penetrate the AVN, as indicated by the lack of advancement of local atrial activation in the HB or coronary sinus ostium (CS os) recording. Therefore, such an AES cannot conduct to the ventricle over the AVN; this maneuver is analogous to the introduction of VES when the HB is refractory during orthodromic AVRT. If this AES advances (or delays) the timing of the next ventricular activation, it indicates that an anterogradely conducting AV or atriofascicular BT is present, and excludes nodoventricular and nodofascicular BTs. If the AES advances (or delays) the timing of the next ventricular activation and the advanced (or delayed) QRS morphology is identical to that during the SVT, this proves that the AV or atriofascicular BT also mediates preexcitation during the SVT, either as an integral part of the SVT circuit or as an innocent bystander. On the other hand, if the AES advances the timing of both the next ventricular activation and subsequent atrial activation, it proves that the SVT is an antidromic AVRT using an AV or atriofascicular BT anterogradely, and excludes preexcited AVNRT (Fig. 19-4). Advancement of both ventricular and atrial activation by such an AES requires anterograde conduction over the BT followed by retrograde conduction over the AVN. This can occur during antidromic AVRT but not in AVNRT, because in the setting of AVNRT the HB would be refractory because of anterograde activation by the time the advanced ventricular impulse invades the HPS retrogradely, with subsequent failure of the advanced ventricular activation to penetrate the HPS-AVN and affect the timing of subsequent atrial activation.7,13
During entrainment of the SVT by atrial pacing at a CL slightly shorter than the tachycardia CL, the presence of a fixed short VH interval suggests antidromic AVRT, but does not exclude AVNRT. Atrial pacing can usually terminate the SVT; whereby anterograde block is always produced in the AVN, with or without block in the BT. A short-coupled AES can block in the BT, terminating the SVT (in the setting of antidromic AVRT) or changing the SVT to a narrow QRS complex SVT at the same CL and same HA interval (in the setting of preexcited AVNRT).
Differential Diagnosis
The goals of programmed electrical stimulation during SVT are evaluation of the relationship among the His potential, the QRS, and the VH interval during atrial pacing and during SVT and differentiation between the different types of atypical BTs (Table 19-1). In addition, exclusion of a separate BT is necessary, especially if a rapid and fixed VA interval exists during incremental rate ventricular pacing. Furthermore, it is important to differentiate between antidromic AVRT using the BT anterogradely and preexcited AVNRT, in which the BT is an innocent bystander (Table 19-2).
TABLE 19-1 Differentiation among Different Types of Atypical Bypass Tracts
• Atriofascicular BTs: The earliest ventricular activation occurs at or near the RV apex.
• Long decrementally conducting AV BTs: The earliest ventricular activation occurs at or near the RV apex.
• Nodofascicular BTs: The earliest ventricular activation occurs at or near the RV apex.
• Nodoventricular BTs: The earliest ventricular activation occurs adjacent to the tricuspid annulus.
• Short decrementally conducting AV BTs: The earliest ventricular activation occurs adjacent to the tricuspid annulus.
• Atriofascicular BTs: Preexcitation increases when atrial stimulation is performed closer to the atrial insertion site.
• Long decrementally conducting AV BTs: Preexcitation increases when atrial stimulation is performed closer to the atrial insertion site.
• Nodofascicular BTs: The degree of preexcitation is not influenced by the site of atrial stimulation.
• Nodoventricular BTs: The degree of preexcitation is not influenced by the site of atrial stimulation.
• Short decrementally conducting AV BTs: Preexcitation increases when atrial stimulation is performed closer to the atrial insertion site.
• Atriofascicular BTs: The AES can advance or delay the next ventricular activation.
• Long decrementally conducting AV BTs: The AES can advance or delay the next ventricular activation.
• Nodofascicular BTs: The AES cannot advance the next ventricular activation.
• Nodoventricular BTs: The AES cannot advance the next ventricular activation.
• Short decrementally conducting AV BTs: The AES can advance or delay the next ventricular activation.
• Atriofascicular BTs: The VH interval is short (VH interval = 16 ± 5 msec and V-RB interval = 3 ± 5 msec; VH interval < HV interval and < VH interval during RV pacing).
• Long decrementally conducting AV BTs: The VH interval is short but longer than that with atriofascicular BTs (VH interval = 37 ± 9 msec and V-RB interval = 25 ± 6 msec).
• Nodofascicular BTs: The VH interval is short, as for atriofascicular BTs.
• Nodoventricular BTs: The VH interval is intermediate (His potential is inscribed in the QRS, VH interval ≥ HV interval; VH interval > HV interval and > VH interval during RV pacing).
• Short decrementally conducting AV BTs: The VH interval is intermediate, as for nodoventricular BTs.
• Antidromic AVRT in presence of retrograde RBBB: The VH interval is long (His potential is inscribed after the QRS, VH interval > HV interval).
• Adenosine produces conduction delay in most atypical BTs (except for short decrementally conducting BTs).
• When adenosine administration slows AVN conduction but not the BT, an increase in the degree of preexcitation is noted in all types of BTs except for fasciculoventricular BTs, whereby the degrees of preexcitation and HV interval remain fixed.
AES = atrial extrastimulation; AV = atrioventricular; AVRT = atrioventricular reentrant tachycardia; HV = His bundle–ventricular; LBBB = left bundle branch block; RA = right atrium; RBBB = right bundle branch block; RV = right ventricle; SVT = supraventricular tachycardia; VA = ventriculoatrial; VH = ventricular–His bundle; V-RB = ventricular–right bundle branch.
TABLE 19-2 Differentiation between Antidromic AVRT and Preexcited AVNRT Using an Atypical BT
AES = atrial extrastimulation; AV = atrioventricular; AVNRT = atrioventricular nodal reentrant tachycardia; AVRT = atrioventricular reentrant tachycardia; BT = bypass tract; CL = cycle length; HA = His bundle–atrial; NSR = normal sinus rhythm; RA = right atrium; RBBB = right bundle branch block; RV = right ventricle; SVT = supraventricular tachycardia; VA = ventriculoatrial; VES = ventricular extrastimulation; VH = ventricular–His bundle.
Localization of the Bypass Tract
Mapping of the ventricular insertion site of atriofascicular and long decrementally conducting AV BTs is difficult because of the long intracardiac course and distal insertion of these BTs, which shows extensive arborization over a wide area of ventricular muscle, with a diameter of up to 0.5 to 2 cm. A propensity to temporary loss of conduction of the atypical BT because of catheter trauma further complicates ventricular mapping.5
Mapping of the atrial insertion site can be performed by (1) P-delta interval mapping by stimulation at different atrial sites, (2) recording of the BT potential at the tricuspid annulus, and (3) AES from the RA during antidromic AVRT.8
Careful mapping of the tricuspid annulus and the anterior free wall of the RV typically demonstrates discrete potentials with complexes comparable to those recorded at the AV junction. The BT potential is analogous to the His potential. Atrial pacing, AES, and adenosine produce delay proximal to BT potential with a constant BT potential to QRS (BT-V) interval. Faster atrial pacing produces Wenckebach block proximal to the BT potential.13
Mapping the Atrial Insertion Site
Mapping the Shortest Atrial Stimulus to Delta (S-V) Interval
The mapping catheter is advanced from site to site along the atrial aspect of the tricuspid annulus while pacing from its distal tip. The resulting interval between the stimulus and the onset of the delta wave (S-V interval) should decrease progressively as the BT atrial insertion site is approached, and increase as it is passed. Thus, the atrial pacing site associated with the shortest S-V interval is the site closest to the BT atrial insertion site. It is essential that pacing at different sites be performed at a constant CL to avoid rate-dependent conduction slowing in the BT as a reason for changing the S-V interval. This method is rarely used because of several limitations: (1) a constant distance of the mapping-pacing catheter from the tricuspid annulus must be maintained to reduce the influence of the time spent traversing intervening atrial tissue; (2) catheter manipulation during pacing can result in initiation of tachycardia, which must then be terminated to continue mapping; (3) optimal sites can be overlooked if they cannot be consistently paced because of unstable catheter contact; and (4) this method cannot be applied in the setting of incessant tachycardia or when AF is present.13
Atrial Extrastimulation Mapping during Supraventricular Tachycardias
The BT atrial insertion site is close to the site from which the longest coupled AES delivered during preexcited tachycardia advances the timing of the next ventricular activation. Alternatively, the BT atrial insertion site is close to the site from which the greatest amount of advancement of the next ventricular activation occurs when using a fixed AES coupling interval. This method is rarely used because of several limitations: (1) it is time-consuming, and cannot be used if the SVT is difficult to initiate or nonsustained; (2) it is not particularly useful in cases of true nodofascicular BTs; (3) a constant distance of the mapping-pacing catheter from the tricuspid annulus must be maintained to reduce the influence of the time spent traversing intervening atrial tissue; and (4) optimal sites can be overlooked if they cannot be consistently paced because of unstable catheter contact.13
Mapping the Ventricular Insertion Site
Mapping the Distal Fascicular Insertion Site (for Atriofascicular Bypass Tracts)
The distal insertion site of atriofascicular BTs can be localized by careful mapping along the lateral RV wall toward the apex, seeking the earliest site of ventricular activation during anterograde BT conduction. A distal RB recording is usually present at this site. This method can be used to map any rhythm during which consistent preexcitation is present (atrial pacing, AF, and preexcited SVT). However, seeking the distal insertion is less precise because a distal RB recording may be localized, but not the portion into which the atriofascicular fiber inserts. It is most useful if the course of the atriofascicular BT can be traced from the tricuspid annulus to its insertion into the RB. Additionally, ablation at the distal site offers no advantage unless catheter stability is better at that location as opposed to the tricuspid annulus. If the RB is ablated rather than the BT, RBBB will result. Not only will this fail to eliminate the BT function, but it can also facilitate induction and maintenance of the SVT by increasing the reentrant circuit size.8,13
Mapping the Distal Ventricular Insertion Site (for Slowly Conducting Atrioventricular Bypass Tracts)
Mapping is performed in the same fashion as for typical rapidly conducting AV BTs—seeking the ventricular site with the earliest unipolar or bipolar ventricular electrogram recording. However, there is evidence that a variable degree of arborization of the distal insertion site occurs in some patients. This feature makes the ventricular insertion site a less attractive ablation target because of the potential of requiring ablation of a relatively large amount of ventricular myocardium to be effective.13
Mapping the Bypass Tract Potential
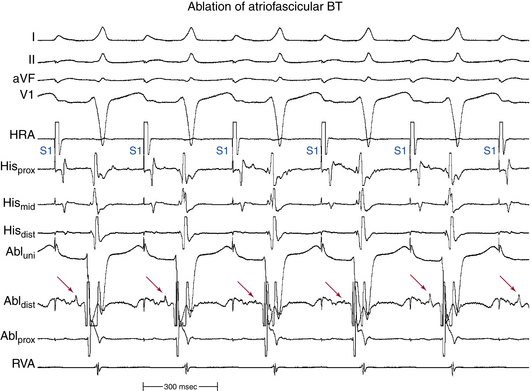
Direct recording of the BT potential at the tricuspid annulus is the most precise and preferred method of localizing the atypical BT. The BT potential is usually a low-amplitude, high-frequency recording made at the tricuspid annulus, which resembles a His potential (Fig. 19-5). Only the annular and subannular portions of the BT have been successfully recorded; attempts to record potentials from the atrial portion (corresponding to nodal-like tissue) have been unsuccessful. Distinct atrial, BT, and ventricular potentials can be found at the BT atrial insertion site. Recording a BT potential can be successful during NSR, atrial pacing, or preexcited SVT. However, recording of a BT potential along the tricuspid annulus may not be successful in up to 48% of cases. Furthermore, because of the low amplitude of the BT potential, it is difficult or impossible to record it during AF. Additionally, this technique presents the risk of producing mechanical block in the BT. Nevertheless, this method is less time-consuming and more precise than the previous ones.8,13
Mapping Sites of Mechanically Induced Loss of Preexcitation
Atypical BTs are particularly sensitive to mechanical trauma, and catheter manipulation along the tricuspid annulus during mapping of the BT may result in loss of BT function, even as a result of gentle pressure from the catheter tip. When mapping is performed during preexcited atrial pacing or SVT, damage to the BT is indicated by a sudden, transient loss of preexcitation. This phenomenon can be used to localize the BT precisely (bump mapping). Conduction block typically occurs while a BT potential is still recorded; thus, conduction is interrupted within the ventricular course of the BT. Block usually lasts from a few beats to a few minutes but can last for hours, after which preexcitation resumes. This method can be used during any consistently preexcited rhythm (atrial pacing, AF, and antidromic AVRT). However, if it occurs during antidromic AVRT, termination of the tachycardia may result in catheter displacement and loss of the exact location of the BT. Additionally, interruption of BT conduction can occur as the catheter is passing the area, and where the catheter comes to rest may not be the same site as where loss of BT function occurred; in this situation, the target cannot be relocated until BT conduction resumes. Delivery of radiofrequency (RF) energy at a site at which catheter pressure caused loss of preexcitation may successfully eliminate conduction in the BT; however, it is best to wait to deliver energy until preexcitation resumes, because of the possibility that the catheter position may have changed. Electroanatomical mapping (e.g., CARTO, NavX) can help tag sites of interest, facilitating precise relocation of the ablation catheter to these sites if it has been determined that they are a good ablation target. Atriofascicular BTs are more susceptible to mechanically induced block, probably suggesting that these BTs are composed of thinner strands or are located closer to the endocardium.13
Ablation
Target of Ablation
Direct recording of the BT potential at the tricuspid annulus is the most precise and preferred method of localizing the BT and serves as the target of ablation (see Fig. 19-5).7,13 Ablation at the ventricular insertion site of the BT offers no advantage over targeting the atrial insertion site because there is evidence that a variable degree of arborization of the distal insertion site occurs in some patients, with the potential of requiring ablation of a relatively large amount of ventricular myocardium to be effective.5,8
However, when a BT potential along the tricuspid annulus cannot be recorded, ablation of the distal insertion sites of atriofascicular BTs becomes an alternative and has been found to be highly effective, but is commonly (57%) associated with the development of RBBB. Ablation of the RB can carry a proarrhythmic effect and facilitate induction of the SVT or cause incessant tachycardia; however, this is not a concern as long as the BT itself is also successfully ablated.8,13
Ablation Technique
Once an appropriate target site for ablation has been identified, RF energy may be applied during NSR, atrial pacing, or antidromic AVRT. Atrial pacing is preferred to ensure that adequate preexcitation is evident (unlike NSR) to be able to assess the efficacy of ablation, and that the rhythm remains the same after elimination of preexcitation (unlike antidromic AVRT) to prevent catheter dislodgment.5,8
The use of long curved sheaths can help achieve good catheter positioning and stability along the tricuspid annulus. Typical RF settings consist of a maximal power of 50 W and a maximal temperature of 55°C to 60°C, continued for 30 to 60 seconds after elimination of the BT function (i.e., after disappearance of preexcitation). During RF energy delivery, an accelerated preexcited rhythm is often observed. This so-called Mahaim automatic tachycardia is analogous to the accelerated junctional rhythm observed during AVN modification, and is presumably secondary to heating-related automaticity of nodal-like tissue of the BT. Accelerated automatic beats during RF ablation have been considered a marker of a successful result, and RF energy delivery should be continued for a sufficient duration after termination of this rhythm.13 Heat-induced automaticity during RF ablation is observed less commonly in short decrementally conducting AV BTs as compared with atriofascicular BTs (50% versus 91%).16,17
Endpoints of Ablation
Complete loss of BT function, not just noninducibility of tachycardias, is an essential endpoint. BT block is confirmed by demonstrating loss of preexcitation with atrial pacing and AES and noninducibility of AVRT. Atrial stimulation should be performed at sites and rates that were associated with preexcitation before ablation.13
Fasciculoventricular Bypass Tracts
General Considerations
Fasciculoventricular BTs are the rarest form of preexcitation (1.2% to 5.1% of atypical BTs). They connect the HB to ventricular myocardium in the anteroseptal location. These fibers do not give rise to any reentrant tachycardia, and appear to be only an ECG and EP curiosity. Even during AF and AFL, a rapid ventricular response is not expected in the presence of a normal AVN proximal to the BT. However, it is important to distinguish fasciculoventricular BTs from superoparaseptal BTs to avoid unnecessary invasive EP procedures and potential harm to the AVN-HB if such a BT is mistakenly targeted by ablation, because this form of preexcitation does not require treatment.7
Electrocardiographic Features
In patients with fasciculoventricular BTs, preexcitation is always present during NSR. The ECG preexcitation pattern can mimic that of manifest WPW pattern, especially that of superoparaseptal AV BTs, with a normal frontal plane axis between 0 and 75 degrees and precordial RS transitional zone in leads V2-V3. With fasciculoventricular BTs, the PR interval is normal despite the presence of preexcitation (Fig. 19-6). This is in contrast to superoparaseptal AV BTs, which result in the WPW pattern with significant shortening of the PR interval because of the relative proximity of the BT location to the sinus node.
Several ECG findings in lead V1 favor fasciculoventricular BTs as the cause of preexcitation, including: (1) PR interval greater than 110 milliseconds; (2) R wave width less than 35 milliseconds; (3) S wave amplitude less than 20 mm; (4) flat or negative delta wave; and (5) notching in the descending limb of the S wave (see Fig. 19-6).18
Electrophysiological Testing
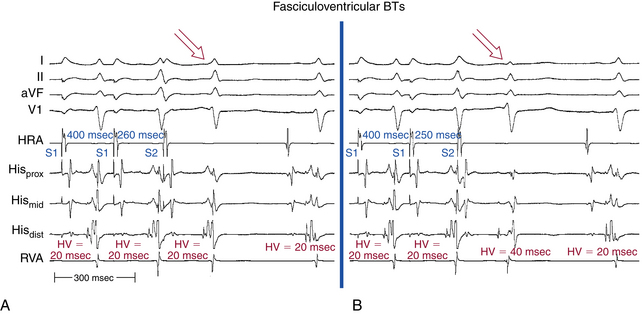
With fasciculoventricular BTs, preexcitation is present during NSR with a normal AH interval and short HV interval. The earliest ventricular activation occurs at the HB region (Table 19-3).
HB = His bundle; HV = His bundle–ventricular; NSR = normal sinus rhythm.
Progressively shorter atrial pacing CLs or AES coupling intervals produce progressive prolongation of the PR and AH intervals but with a fixed degree of preexcitation, a constant and short HV interval, and a fixed relationship between the His potential and RB potential (Fig. 19-7). AVN Wenckebach block can develop, which is then associated with a fixed degree of preexcitation and a constant, short HV interval. The loss of AV conduction is associated with loss of preexcitation. AES can result in block in the fasciculoventricular BT, producing a sudden loss of preexcitation and prolongation of the HV interval to normal values (see Fig. 19-7).
HB pacing normalizes the HV interval and eliminates preexcitation in all types of BTs except for fasciculoventricular BTs, in which a fixed degree of preexcitation and a constant short HV interval remain unchanged during HB pacing (see Fig. 19-6).
When adenosine administration slows AVN conduction, an increase in the degree of preexcitation is noted in all types of BTs, as long as adenosine does not block the BT, except for fasciculoventricular BTs, in which the degree of preexcitation and HV interval remain fixed. Moreover, adenosine administration can be associated with complete AV block and junctional escape beats and, in the setting of fasciculoventricular BTs, these beats are associated with the same degree of preexcitation and the same HV interval as during NSR, even when these ectopic beats are associated with retrograde VA block and no atrial depolarization.
Atrio-Hisian Bypass Tracts
General Considerations
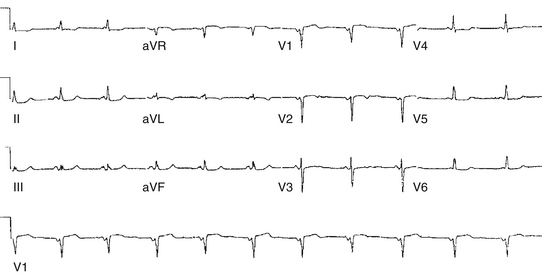
Patients with palpitations who had a short PR interval but normal QRS complex in the resting ECG were first described in 1938 and then further evaluated by Lown and colleagues in 1952.19 The latter report consisted of a retrospective examination of 13,500 ECGs and identified short PR intervals in a mixed group of 200 subjects, most of whom had a normal QRS complex (Fig. 19-8). The authors described a “syndrome” characterized by short PR interval, narrow QRS complex, and recurrent paroxysmal SVTs. They initially ascribed this syndrome to the presence of an AV nodal BT.7,19 A variety of explanations were later offered to account for the short PR interval.
Although the incidence of palpitations was significantly higher in these patients with short PR intervals when compared with a control group with a normal PR interval (17% versus 0.5%), most contemporary electrophysiologists do not consider the “Lown-Ganong-Levine syndrome” to be a recognized syndrome consisting of a single entity but an electrocardiographic description. It probably represents one end of the normal spectrum of AVN conduction properties. Therefore, the use of the term is inappropriate and should be discouraged and the mechanism responsible for the short PR interval should be used instead. The persistence of the term probably relates to the appealing parallel with the initials of the WPW syndrome.
The short PR interval can have different mechanisms: (1) enhanced AVN conduction (perhaps using specialized intranodal fibers), which is believed responsible for most cases of short PR interval and is secondary to an anatomically small AVN, enhanced sympathetic tone, or a variant of normal; (2) atrio-Hisian BT (rare), in which case AF or AFL with a rapid ventricular response is the presenting arrhythmia; (3) ectopic atrial rhythm with differential input into the AVN; and (4) isorhythmic AV dissociation, whereby the short PR interval is not caused by a conducted P wave.7
Supraventricular Tachycardias in Patients with Short PR Intervals
Patients with Enhanced Atrioventricular Node Conduction
The mechanism(s) of SVTs in patients with enhanced AVN conduction does not appear to differ significantly from those in patients with normal PR intervals (AVNRT being the most common, followed by orthodromic AVRT). The tachycardia CL of AVNRT occurring in patients with short PR intervals is not different from that in patients with normal PR intervals. This is expected, because the tachycardia CL of AVNRT is determined by conduction over the slow AVN pathway, which is similar in these patients and those with a normal PR interval. In contrast, the tachycardia CL of orthodromic AVRT tends to be much shorter in these patients compared with patients with normal PR intervals, which is expected because the circuit of orthodromic AVRT uses the fast AVN pathway for anterograde conduction. In fact, in any SVT with a tachycardia CL shorter than 250 milliseconds enhanced AVN conduction and orthodromic AVRT should be suspected. In such patients, bundle branch block (BBB) is common during SVT, resulting in wide complex tachycardia.7
Patients with Atrio-Hisian Bypass Tracts
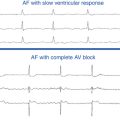
These patients primarily present with AF and AFL with a rapid ventricular response, and do not develop reentrant SVTs using the AV junction as one limb.7 Rapid ventricular response during AF depends on refractoriness of the tissue responsible for AV conduction (i.e., AVN or BT) and not on the site of insertion for that tissue.
Electrophysiological Testing
Baseline Observations During Normal Sinus Rhythm
Enhanced AVN conduction is characterized by a short AH interval (<60 milliseconds) and normal HV interval.7 In rare cases in which the AVN is completely bypassed by an atrio-Hisian BT, the HV interval is short. The HV interval in these cases is artifactually short because the proximal HB and the ventricle are activated in parallel since the atrio-Hisian BT inserts into the distal HB (i.e., the proximal HB is activated retrogradely).
Atrial Pacing and Atrial Extrastimulation
Patients with Enhanced Atrioventricular Node Conduction
The AH interval prolongs with progressively shorter atrial pacing CLs and AES coupling intervals.7 The prolongation in the AH interval is smooth, continuous, and blunted, with a maximal increase in the AH interval of 100 milliseconds or less during pacing at a CL of 300 milliseconds compared with the value measured during NSR. The maximal AH interval (at any pacing rate) is rarely longer than 200 milliseconds and 1:1 conduction typically is maintained to pacing rates greater than 200 beats/min.
The AH interval response can be characteristic of dual AVN physiology, with an initial blunted small prolongation in the AH interval followed by a significant jump at a critical pacing CL or AES coupling interval, while maintaining 1:1 conduction at a pacing rate greater than 200 beats/min. In such patients, the maximal AH interval can be greater than 200 milliseconds, and the maximal prolongation in the AH interval can be greater than 100 milliseconds.7 Atrial pacing from the CS is associated with shorter AH intervals, shorter Wenckebach CLs, and shorter AVN effective refractory periods, suggesting a preferential input into the AVN.
Patients with Atrio-Hisian Bypass Tracts
The AH interval remains short with no or minimal prolongation in response to progressively shorter atrial pacing CLs and AES coupling intervals. Block in the BT, which can usually be achieved by antiarrhythmic agents or occasionally by the induction of AF, is associated with a simultaneous increase in the PR and AH intervals and normalization of the HV interval.7
Ventricular Pacing and Ventricular Extrastimulation
Patients with Enhanced Atrioventricular Node Conduction
Retrograde AVN conduction is extremely rapid in these patients. In general, the HA interval is shorter than the AH interval at comparable pacing CLs. The HA interval typically remains relatively short with little prolongation in response to progressively shorter ventricular pacing CLs and VES coupling intervals.7 A concealed AV BT mediating retrograde VA conduction should be excluded, which can be achieved by a variety of pacing maneuvers (see Chap. 18).
Response to Pharmacological and Physiological Maneuvers
Patients with Enhanced Atrioventricular Node Conduction
The AH interval prolongs in response to beta blockers, verapamil, digoxin, carotid sinus massage, and vagal maneuvers. Complete autonomic blockade results in increases in the AH interval and AVN functional refractory period, but a minimal change in the AVN effective refractory period (suggesting sympathetic tone predominance in these patients, in contrast to individuals with normal AVN conduction in whom vagal tone predominates).7
1. Mahaim I., Benatt A. Nouvelles recherches sur les connections superieures de la branche du faisceau de His-Tawara avec cloison interventriculaire. Cardiologia. 1937;1:61.
2. Mahaim I., Winston M.R. Recherches d’anatomie comparee et de pathologie experimentale sur les connexions hautes des His-Tawara. Cardiologia. 1941;33:651-653.
3. Mahaim I. Kent’s fibers and the A-V paraspecific conduction through the upper connections of the bundle of His-Tawara. Am Heart J. 1947;33:651-653.
4. Benditt D.G., Lu F. Atriofascicular pathways: fuzzy nomenclature or merely wishful thinking? J Cardiovasc Electrophysiol. 2006;17:261-265.
5. Miller J., Olgin J.E. Catheter ablation of free-wall accessory pathways and “Mahaim” fibers. In: Zipes D.P., Haissaguerre M., editors. Catheter ablation of arrhythmias. Armonk, NY: Futura; 2002:277-303.
6. Sternick E.B., Lokhandwala Y., Timmermans C., et al. The atrioventricular interval during pre-excited tachycardia: a simple way to distinguish between decrementally or rapidly conducting accessory pathways. Heart Rhythm. 2009;6:1351-1358.
7. Josephson M.E. Preexcitation syndromes. In: Josephson M.E., editor. Clinical cardiac electrophysiology. ed 4. Philadelphia: Lippincott Williams & Wilkins; 2008:339-445.
8. Kothari S., Gupta A.K., Lokhandwala Y.Y., et al. Atriofascicular pathways: where to ablate? Pacing Clin Electrophysiol. 2006;29:1226-1233.
9. Davidson N.C., Morton J.B., Sanders P., Kalman J. Latent Mahaim fiber as a cause of antidromic reciprocating tachycardia: recognition and successful radiofrequency ablation. J Cardiovasc Electrophysiol. 2002;13:74-78.
10. Sternick E.B., Fagundes M.L., Cruz F.E., et al. Short atrioventricular Mahaim fibers: observations on their clinical, electrocardiographic, and electrophysiologic profile. J Cardiovasc Electrophysiol. 2005;16:127-134.
11. Kalbfleisch S., Bowman K., Augostini R. A single Mahaim fiber causing both antidromic and orthodromic reciprocating tachycardia. J Cardiovasc Electrophysiol. 2008;19:740-742.
12. Sternick E.B., Timmermans C., Sosa E., et al. The electrocardiogram during sinus rhythm and tachycardia in patients with Mahaim fibers: the importance of an “rS” pattern in lead III. J Am Coll Cardiol. 2004;44:1626-1635.
13. Miller J.M., Rothman S.A., Hsia H.H., Buxton A.E. Ablation of Mahaim fibers. In: Huang S.K.S., Wilber D.J., editors. Radiofrequency catheter ablation of cardiac arrhythmias: basic concepts and clinical applications. Armonk, NY: Futura; 2000:559-578.
14. Bogun F., Krishnan S., Siddiqui M., et al. Electrogram characteristics in postinfarction ventricular tachycardia: effect of infarct age. J Am Coll Cardiol. 2005;46:667-674.
15. Sternick E.B., Rodriguez L.M., Timmermans C., et al. Effects of right bundle branch block on the antidromic circus movement tachycardia in patients with presumed atriofascicular pathways. J Cardiovasc Electrophysiol. 2006;17:256-260.
16. Sternick E.B., Gerken L.M., Vrandecic M. Appraisal of “Mahaim” automatic tachycardia. J Cardiovasc Electrophysiol. 2002;13:244-249.
17. Sternick E.B., Sosa E.A., Timmermans C., et al. Automaticity in Mahaim fibers. J Cardiovasc Electrophysiol. 2004;15:738-744.
18. Oh S., Choi Y.S., Choi E.K., et al. Electrocardiographic characteristics of fasciculoventricular pathways. Pacing Clin Electrophysiol. 2005;28:25-28.
19. Lown B., Ganong W.F., Levine S.A. The syndrome of short P-R interval, normal QRS complex and paroxysmal rapid heart action. Circulation. 1952;5:693-706.