Chapter 3 Uvea
Iris
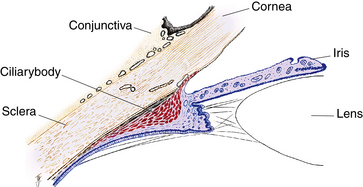
The iris is a thin, circular structure located anterior to the lens, often compared with a diaphragm of an optical system. The center aperture, the pupil, actually is located slightly nasal and inferior to the iris center.1 Pupil size regulates retinal illumination. The diameter can vary from 1 mm to 9 mm depending on lighting conditions. The pupil is very small (miotic) in brightly lit conditions and fairly large (mydriatic) in dim illumination. The average diameter of the iris is 12 mm, and its thickness varies. It is thickest in the region of the collarette, a circular ridge approximately 1.5 mm from the pupillary margin. This slightly raised jagged ridge was the attachment site for the fetal pupillary membrane during embryologic development.1,2 The collarette divides the iris into the pupillary zone, which encircles the pupil, and the ciliary zone, which extends from the collarette to the iris root (Figure 3-1). The color of these two zones often differs.
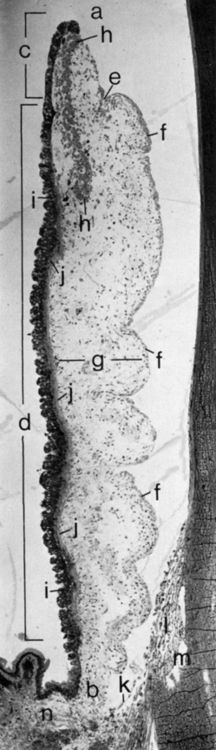
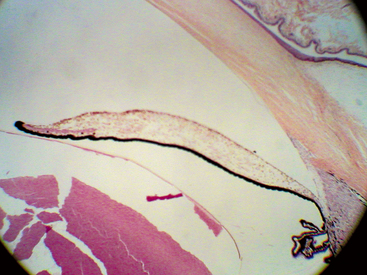
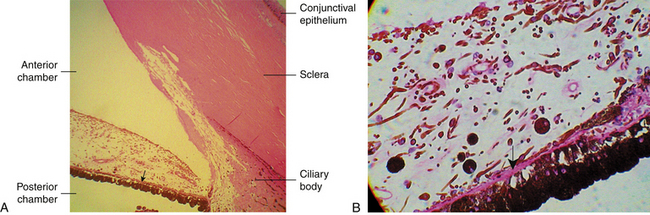
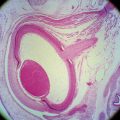
FIGURE 3-1 Light micrograph of the iris and anterior chamber.
(From Hogan MJ, Alvarado JA, Weddell JE: Histology of the human eye, Philadelphia, 1971, Saunders.)
The pupillary margin of the iris rests on the anterior surface of the lens and, in profile, the iris has a truncated cone shape such that the pupillary margin lies anterior to its peripheral termination, the iris root (Figure 3-2). The root, approximately 0.5 mm thick, is the thinnest part of the iris and joins the iris to the anterior aspect of the ciliary body (Figure 3-3).1 The iris divides the anterior segment of the globe into anterior and posterior chambers, and the pupil allows the aqueous humor to flow from the posterior into the anterior chamber with no resistance.
Histologic Features of Iris
Anterior Border Layer
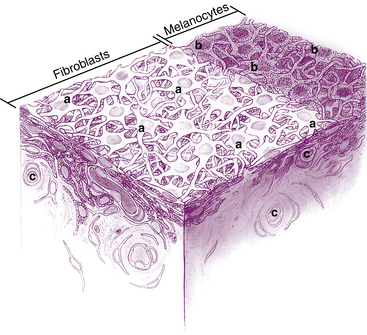
The surface layer of the iris, the anterior border layer, is a thin condensation of the stroma. In fact, some do not consider this to be a separate layer. It is composed of fibroblasts and pigmented melanocytes. The highly branching processes of the cells interweave to form a meshwork in which the fibroblasts are on the surface and the melanocytes are located below1,2 (Figure 3-4). The thickness of the melanocyte layer may vary throughout the iris, with accumulations of melanocytes forming elevated frecklelike masses, evident in the anterior border layer. The density and arrangement of the meshwork differ among irises and are contributing factors in iris color.
Iris Stroma and Sphincter Muscle
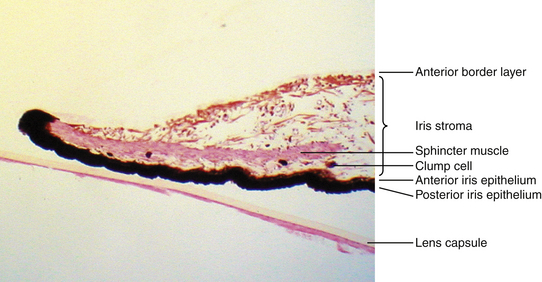
The connective tissue stroma is composed of pigmented and nonpigmented cells, collagen fibrils, and extensive ground substance. The pigmented cells include melanocytes and clump cells, whereas the nonpigmented cells are fibroblasts, lymphocytes, macrophages, and mast cells.1 Although melanocytes and fibroblasts have many branching processes, the cells are widely spaced in the stroma, so their branches do not form a meshwork. Clump cells are large, round, darkly pigmented cells and are likely “altered macrophages” and are scavengers of free pigment within the iris.1,3 Clump cells usually are located in the pupillary portion of the stroma, often near the sphincter muscle (Figure 3-5). The collagen fibrils are arranged in radial columns (trabeculae) that are seen easily as white fibers in light-colored irises.3
The iris arteries are branches of a circular vessel, the major circle of the iris, located in the ciliary body near the iris root. The iris vessels usually follow a radial course from the iris root to the pupil margin. These vessels were historically thought to have an especially thick tunica adventitia and have been called “thick-walled blood vessels.”1–3 Improved histologic staining has shown, however, that the bundles of collagen fibrils encircling the vessels are continuous with the collagen network of the stroma and not part of the actual vessel wall. This fibril network anchors the vessels in place and protects them from kinking and compression during the extensive iris movement that occurs with miosis and mydriasis.4 An incomplete circular vessel, the minor circle of the iris, is located in the iris stroma inferior to the collarette and is a remnant of embryologic development. The iris capillaries are not fenestrated and form part of the blood-aqueous barrier.1 The iris stroma is continuous with the stroma of the ciliary body.
The sphincter muscle lies within the stroma (see Figure 3-5) and is composed of smooth-muscle cells joined by tight junctions.1 As its name implies, the sphincter is a circular muscle 0.75 to 1 mm wide, encircling the pupil and located in the pupillary zone of the stroma1,2 (Figure 3-6). The sphincter muscle is anchored firmly to adjacent stroma and retains its function even if severed radially.1 Contraction of the sphincter causes the pupil to constrict in miosis. The muscle is innervated by the parasympathetic system.
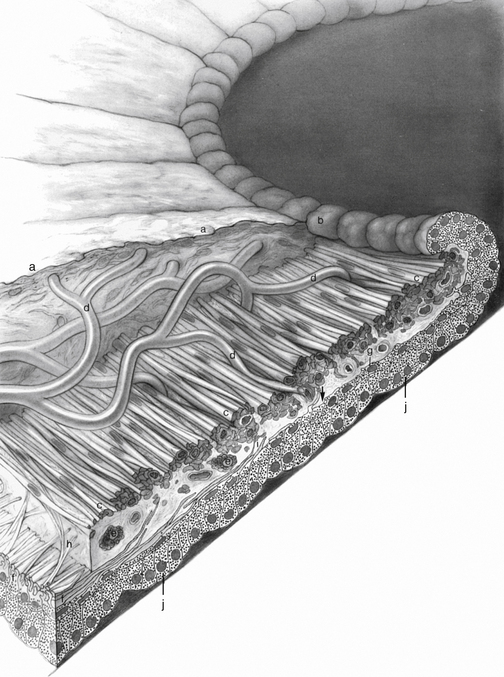
FIGURE 3-6 Pupillary portion of the iris.
(From Hogan MJ, Alvarado JA, Weddell JE: Histology of the human eye, Philadelphia, 1971, Saunders.)
Anterior Epithelium and Dilator Muscle
Posterior to the stroma are two layers of epithelium. The first of these, the epithelial layer lying nearest to the stroma, is the anterior iris epithelium, which is composed of the unique myoepithelial cell. The apical portion is pigmented cuboidal epithelium joined by tight junctions and desmosomes, whereas the basal portion is composed of elongated, contractile, smooth muscle processes (Figure 3-7). The muscle fibers extend into the stroma, forming three to five layers of dilator muscle fibers joined by tight junctions (Figure 3-8).
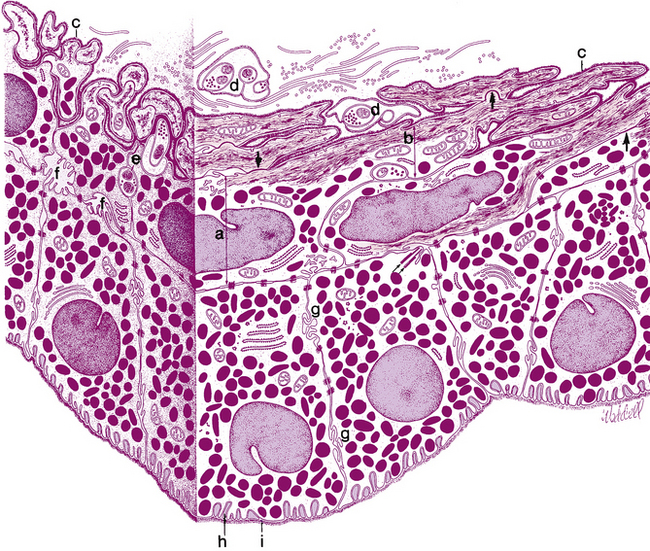
FIGURE 3-7 Posterior epithelial layers.
(From Hogan MJ, Alvarado JA, Weddell JE: Histology of the human eye, Philadelphia, 1971, Saunders.)
The dilator muscle is present from the iris root to a point in the stroma below the midpoint of the sphincter.1 The stroma separating the sphincter and dilator muscles is a particularly dense band of connective tissue. Near the termination of the dilator muscle, small projections insert into the stroma or, more accurately, into the sphincter1,2 (see Figure 3-6). Because the fibers are arranged radially, contraction of the dilator muscle pulls the pupillary portion toward the root, thereby enlarging the pupil in mydriasis. The dilator is sympathetically innervated.
Posterior Epithelium
The second epithelial layer posterior to the stroma is the posterior iris epithelium, a single layer of heavily pigmented, approximately columnar cells joined by tight junctions and desmosomes.2,3 In the periphery, the posterior iris epithelium begins to lose its pigment as it continues into the ciliary body as the nonpigmented epithelium. A thin basement membrane covers the basal aspect of this cellular layer, which lines the posterior chamber.
The anterior and posterior iris epithelial layers are positioned apex to apex, a result of events during embryologic development. Apical microvilli extend from both surfaces, and desmosomes join the two apical surfaces. The epithelial cells curl around from the posterior iris to the anterior surface at the pupillary margin, forming the pigmented pupillary ruff (or frill), which encircles the pupil; this normally has a serrated appearance (see Figure 3-6).
Clinical Comment: Iris Synechiae
IRIS SYNECHIA is an abnormal attachment between the iris surface and another structure. In a posterior synechia, the posterior iris surface is adherent to the anterior lens surface. In an anterior synechia, the anterior iris surface is adherent to the corneal endothelium or the trabecular meshwork. Synechiae can occur as a result of a sharp blow to the head or a whiplash-type movement that brings the two structures forcefully together. Alternatively, cells and debris from a uveal infection that are circulating in the aqueous humor can make the surfaces “sticky” and so cause synechiae.5
Anterior Iris Surface
Thin, radial, collagenous columns or trabeculae are evident in lightly pigmented irises. Thicker, radially oriented, branching trabeculae encircle depressions or openings in the surface called crypts.3 Crypts are located on both sides of the collarette (Fuchs’ crypts) and near the root (peripheral crypts). They allow the aqueous quick exit and entrance into spaces in the iris stroma as the volume of the iris changes with iris dilation and contraction.
Circular contraction folds, evident on the anterior surface of the ciliary zone, result from tissue moving toward the iris root during pupillary dilation. Figure 3-9 shows the topography of the anterior and posterior iris surfaces.
Posterior Iris Surface
The posterior surface of the iris is fairly smooth, but when viewed with magnification, small circular furrows are evident near the pupil. Radial contraction furrows (of Schwalbe) are located in the pupillary zone, and the deeper structural furrows (of Schwalbe) run throughout the ciliary zone and continue into the ciliary body as the valleys between the ciliary processes.1–3 Also found on the posterior surface are circular contraction folds similar to those seen on the anterior surface.
Iris Color
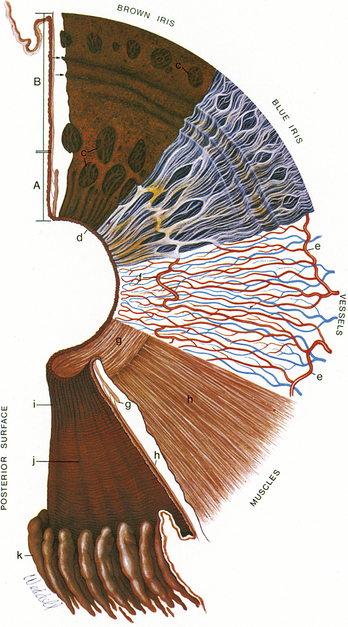
It was once thought that iris color depended on the arrangement and density of connective tissue components in the anterior border layer and stroma, the number of melanocytes, and the size and density of melanin granules within the melanocytes.6 Studies in which melanocyte counts have been done between irises of various colors and from different races have shown that the number of melanocytes is fairly constant.7,8 Color seems to be determined by the number of melanin granules within the melanocytes and the area they occupy.6 The type of melanin present and the arrangement of the connective tissue components can also affect the transmission and reflection of light contributing to iris color.9 An iris appears blue for the same reason that the sky is blue; the wavelength seen results from light scatter caused by the arrangement and density of the connective tissue components. Other iris colors are caused by the amount of light absorption, which depends on the pigment density within the melanocytes. If the iris is heavily pigmented, the anterior surface appears brown and smooth, even velvety, whereas in a lighter iris, the collagen trabeculae are evident and the color ranges from grays to blues to greens depending on the density of pigment and collagen. A freckle or a nevus is an area of hyperpigmentation, an accumulation of melanocytes, and frequently is seen in the anterior border layer. In all colored irises, the two epithelial layers are heavily pigmented. Only in the albino iris do the epithelial layers lack pigment.3
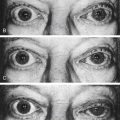
Clinical Comment: Pigmentary Dispersion Syndrome
IN PIGMENTARY DISPERSION SYNDROME pigment granules are shed from the posterior iris surface and are dispersed into the anterior chamber. They can be deposited on the iris, lens, or corneal endothelium or in the trabecular meshwork, where they might compromise aqueous outflow.4 Significant pigment loss will be evident on transillumination of the iris when the red fundus reflex shows through in the depigmented areas (Figure 3-10).
Clinical Comment: Heterochromia
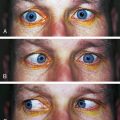
HETEROCHROMIA of the iris is a condition in which one iris differs in color from the other or portions of one iris differ in color from the rest of the iris. This can be congenital or a sign of uveal inflammation. If congenital, a disruption of the sympathetic innervation may be suspected.4,10 A history regarding iris coloration should be elicited.
Ciliary Body
When viewed from the front of the eye, the ciliary body is a ring-shaped structure. Its width is approximately 5.9 mm on the nasal side and 6.7 mm on the temporal side.2 The posterior area of the ciliary body, which terminates at the ora serrata, appears fairly flat, but the anterior ciliary body contains numerous folds or processes that extend into the posterior chamber. In sagittal section, the ciliary body has a triangular shape, the base of which is located anteriorly; one corner of the base lies at the scleral spur, the iris root extends from the approximate center of the base, and portions of the base border both the anterior and posterior chambers. The outer side of the triangle lies against the sclera, and the inner side lines the posterior chamber and a small portion of the vitreous cavity (Figure 3-11). The apex is located at the ora serrata.
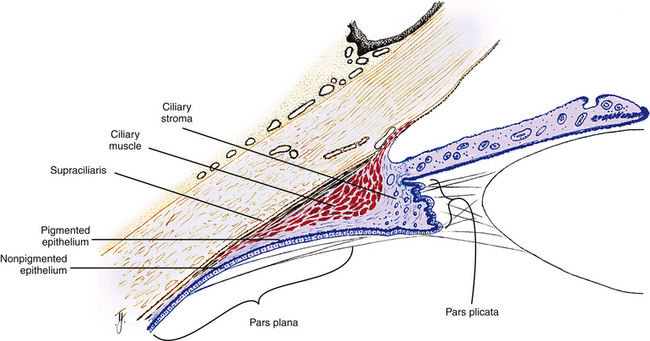
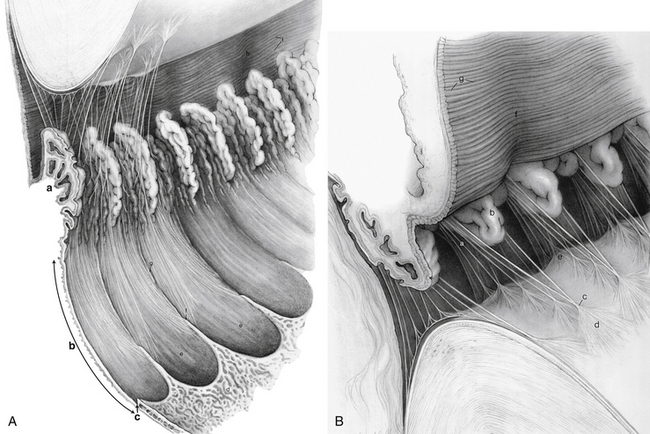
The ciliary body can be divided into two parts: the pars plicata (corona ciliaris) and the pars plana (orbicularis ciliaris). The pars plicata is the wider, anterior portion containing the ciliary processes (see Figure 3-10). Approximately 70 to 80 ciliary processes extend into the posterior chamber, and the regions between them are called valleys of Kuhnt. A ciliary process measures approximately 2 mm in length, 0.5 mm in width, and 1 mm in height, but there are significant variations in all measurements.1
The pars plana is the flatter region of the ciliary body. It extends from the posterior of the pars plicata to the ora serrata, which is the transition between ciliary body and choroid. The ora serrata has a serrated pattern, the forward-pointing apices of which are called teeth or dentate processes. The rounded portions that lie between the teeth are called oral bays (Figure 3-12, A). The dentate processes are elongations of retinal tissue into the region of the pars plana.
The zonule fibers course from the ciliary body to the lens. Some of these fibers insert into the internal limiting membrane of the pars plana region and travel forward through the valleys between the ciliary processes. Some attach to the internal limiting membrane of the valleys of the pars plicata (Figure 3-12, B). The ciliary body is attached to the vitreous base, which extends forward approximately 2 mm over the posterior pars plana.1
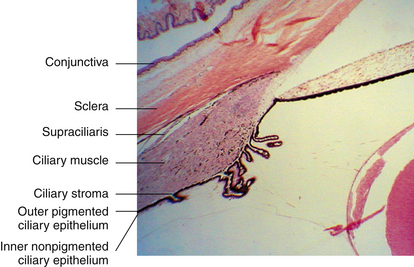
Supraciliaris (Supraciliary Lamina)
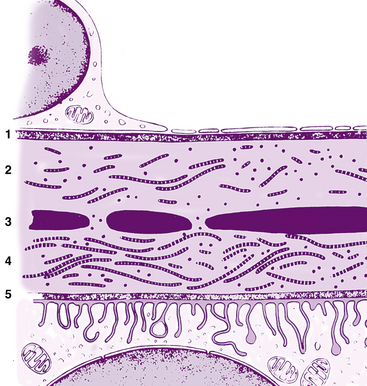
The supraciliaris is the outermost layer of the ciliary body adjacent to the sclera. Its loose connective tissue is arranged in ribbonlike layers containing pigmented melanocytes, fibroblasts, and collagen bands1 (Figure 3-13). The arrangement of these bands allows the ciliary body to slide against the sclera without detaching from or stretching the tissue. The arrangement of the supraciliaris allows for the accumulation of fluid within its spaces, which may cause a displacement of the ciliary body from the sclera. Damage to the layer caused by trauma may result in a ciliary body detachment.
Ciliary Muscle
The ciliary muscle is composed of smooth muscle fibers oriented in longitudinal, radial, and circular directions. Interweaving occurs between fiber bundles and from layer to layer, such that various amounts of connective tissue are found among the muscle bundles.1 The longitudinal muscle fibers (of Brücke) lie adjacent to the supraciliaris and parallel to the sclera. Each muscle bundle resembles a long narrow V, the base of which is at the scleral spur, whereas the apex is in the choroid. The tendon of origin attaches the muscle fibers to the scleral spur and to adjacent trabecular meshwork sheets. The insertion of the longitudinal ciliary muscle is in the anterior one third of the choroid in the form of stellate-shaped terminations or “muscle stars.”1,2 Inner to the longitudinal muscle fibers, the radial fibers form wider, shorter interdigitating Vs that originate at the scleral spur and insert into the connective tissue near the base of the ciliary processes.1 This layer is a transition from the longitudinally oriented fibers to the circular fibers.
The innermost region of ciliary muscle, (Müller’s) annular muscle, is formed of circular muscle bundles with a sphincter type of action. These fibers are located near the major circle of the iris. Figure 3-14 shows the relationship between these regions of the ciliary muscle and surrounding structures.
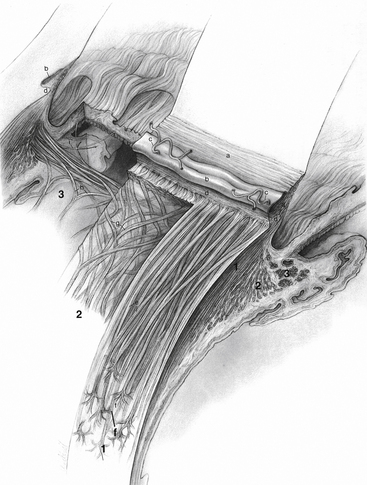
FIGURE 3-14 Ciliary body, including the ciliary muscle and its components.
(From Hogan MJ, Alvarado JA, Weddell JE: Histology of the human eye, Philadelphia, 1971, Saunders.)
The ciliary muscle is dually innervated by the autonomic nervous system. Parasympathetic stimulation activates the muscle for contraction, whereas sympathetic innervation likely has an inhibitory effect that is a function of the level of parasympathetic activity.11–13
Ciliary Stroma
The highly vascularized, loose connective tissue stroma of the ciliary body lies between the muscle and the epithelial layers and forms the core of each of the ciliary processes; it is continuous with the connective tissue that separates the bundles of ciliary muscle. Anteriorly, the stroma is continuous with iris stroma. It thins in the pars plana, where it continues posteriorly as choroidal stroma. The major arterial circle of the iris is located in the ciliary stroma anterior to the circular muscle and near the iris root. This circular artery is formed by the anastomosis of the long posterior ciliary arteries and the anterior ciliary arteries. The stromal capillaries are large and fenestrated, particularly in the ciliary processes, and most are located near the pigmented epithelium.1
Ciliary Epithelium
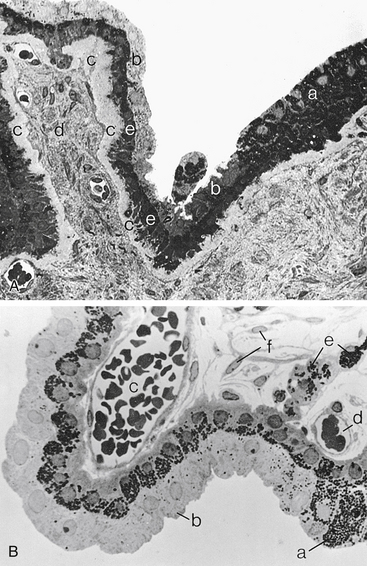
Two layers of epithelium, positioned apex to apex, cover the ciliary body and line the posterior chamber and part of the vitreous chamber. The two epithelial layers are positioned apex to apex because of invagination of the neural ectoderm in forming the optic cup (see Chapter 7). Intercellular junctions, desmosomes, and tight junctions, connect the two layers.1 Gap junctions between the apical surfaces provide a means of cellular communication between the layers and are important in the formation of aqueous.14–17 Both epithelial layers contain cellular components characteristic of cells actively involved in secretion.18
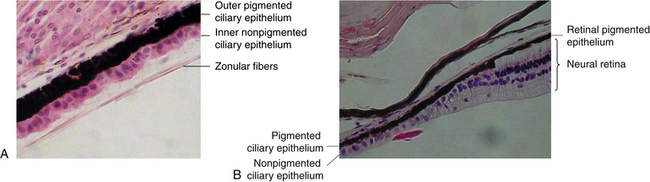
The outer layer (i.e., the one next to the stroma) is pigmented and cuboidal, and the cells are joined by desmosomes and gap junctions.1,14,15,19 Anteriorly, the pigmented ciliary epithelium is continuous with the anterior iris epithelium (Figure 3-15). Posteriorly, it is continuous with the retinal pigment epithelium (RPE) (Figure 3-16). A basement membrane attaches the pigment epithelium to the stroma. This basement membrane is continuous anteriorly with the basement membrane of the anterior iris epithelium and posteriorly with the inner basement membrane portion of Bruch’s membrane of the choroid.1
The inner epithelial layer (i.e., the layer lining the posterior chamber) is nonpigmented and is composed of columnar cells in the pars plana and cuboidal cells in the pars plicata.1 The lateral walls of the cells contain extensive interdigitations and are joined, near their apices, by desmosomes, gap junctions, and zonula occludens, which form one site of the blood-aqueous barrier.3,12–16,19–22
The nonpigmented ciliary epithelium is continuous anteriorly with the posterior iris epithelium (see Figure 3-15). It continues posteriorly at the ora serrata, where it undergoes significant transformation, becoming neural retina (see Figure 3-16). The metabolically active nonpigmented epithelial cells are involved in active secretion of aqueous humor components and serve as a diffusion barrier between blood and aqueous.22 The nonpigmented cells have a greater number of mitochondria than the pigmented cells and thus a higher degree of metabolic activity, with a significant role in the active secretion of aqueous humor components.
The basal and basolateral aspects of the nonpigmented cell have numerous invaginations, providing an extensive surface area adjacent to the posterior chamber.23 The basement membrane covering the nonpigmented epithelium, the internal limiting membrane of the ciliary body, lines the posterior chamber, extends into the invaginations, and is continuous with the internal limiting membrane of the retina.1,3 The internal limiting membrane in the pars plana region is the attachment site for the zonular fibers and the fibers of the vitreous base.
Choroid
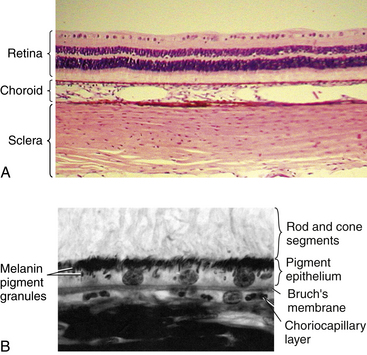
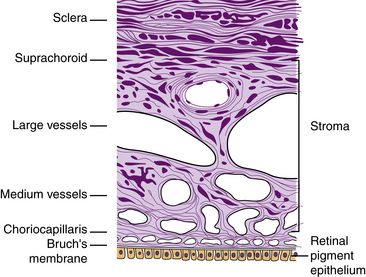
The choroid extends from the ora serrata to the optic nerve and is located between the sclera and the retina, providing nutrients to outer retinal layers (Figure 3-17). It consists primarily of blood vessels. However, a thin connective tissue layer lies on each side of the stromal vessel layer.
Suprachoroid Lamina (Lamina Fusca)
Thin, pigmented, ribbonlike branching bands of connective tissue—the suprachoroid lamina or lamina fusca—traverse a potential space (the suprachoroidal space or “perichoroidal” space) between the sclera and the choroidal vessels.1,3 This layer contains components from both sclera (collagen bands and fibroblasts) and choroidal stroma (melanocytes) (Figure 3-18).1 If the choroid separates from the sclera, part of the suprachoroid will adhere to the sclera and part will remain attached to the choroid.2 The looseness of the tissue allows the vascular net to swell without causing detachment. The suprachoroidal space carries the long posterior ciliary arteries and nerves from posterior to anterior globe.
Choroidal Stroma
The choroidal stroma is a pigmented, vascularized, loose connective tissue layer containing melanocytes, fibroblasts, macrophages, lymphocytes, and mast cells. Collagen fibrils are arranged circularly around the vessels, which are branches of the short posterior ciliary arteries. These vessels are organized into tiers, those with larger lumina occupying the outer layer (Haller’s layer). They branch as they pass inward, forming the medium-sized vessels (Sattler’s layer), which continue branching to form a capillary bed (Figure 3-19). The venules join to become veins that gather in a characteristic vortex pattern in each quadrant of the eye and exit the choroid as four (occasionally five) large vortex veins. Choroidal veins contain no valves.2
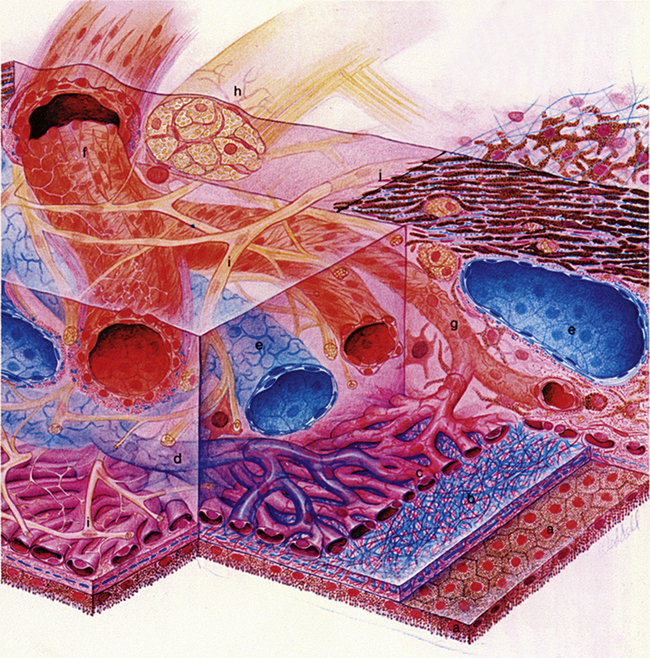
FIGURE 3-19 Drawing of choroidal blood supply and innervation and Bruch’s membrane.
(From Hogan MJ, Alvarado JA, Weddell JE: Histology of the human eye, Philadelphia, 1971, Saunders.)
The choroidal vessels are innervated by the autonomic nervous system. Sympathetic stimulation causes vasoconstriction and decreased choroidal blood flow; parasympathetic stimulation causes a nitrous oxide responsive vasodilation, resulting in increased choroidal blood flow.20
Choriocapillaris
The specialized capillary bed is called the choriocapillaris (lamina choroidocapillaris). It forms a single layer of anastomosing, fenestrated capillaries having wide lumina (see Figure 3-19) with most of the fenestrations facing toward the retina.24 In each, the lumen is approximately three to four times that of ordinary capillaries, such that two or three red blood cells can pass through the capillary abreast, whereas in ordinary capillaries the cells usually course single file.1 The cell membrane is reduced to a single layer at the fenestrations, facilitating the movement of material through the vessel walls.25 Occasional pericytes (Rouget cells), which may have a contractile function, are found around the capillary wall.1,3 Pericytes have the ability to alter local blood flow.26 The choriocapillaris is densest in the macular area, where it is the sole blood supply for a small region of the retina. The choriocapillaris is unique to the choroid and does not continue into the ciliary body.
Bruch’s Membrane (Basal Lamina)
The innermost layer of the choroid, Bruch’s membrane, fuses with the retina. It runs from the optic nerve to the ora serrata, where it undergoes some modification before continuing into the ciliary body.1 Bruch’s membrane (or the basal lamina) is a multilaminated sheet containing a center layer of elastic fibers.27 As seen through an electron microscope, the membrane components, from outer to inner, are the (1) interrupted basement membrane of the choriocapillaris, (2) outer collagenous zone, (3) elastic layer, (4) inner collagenous zone, and (5) basement membrane of the RPE cells1,2 (Figure 3-20). Fine filaments from the basement membrane of the RPE merge with the fibrils of the inner collagenous zone, contributing to the tight adhesion between choroid and the outer, pigmented layer of the retina.
Functions of Uvea
Functions of Ciliary Body
Clinical Comment: Accommodation
Ciliary muscle contraction can change the configuration of the trabecular meshwork because some of the longitudinal fibers are attached to trabecular meshwork sheets. This altered configuration can facilitate aqueous movement through the anterior chamber angle structures.28 Accommodation has been found to cause a decrease in IOP29 (accommodation is discussed further in Chapter 5).
Clinical Comment: Presbyopia
PRESBYOPIA is the loss of the ability to accommodate; it a normal age-related change and the subject of continuing research. In rhesus monkeys, the tendon that attaches the ciliary muscle to the scleral spur shows extensive age-related structural changes: It thickens with age and becomes surrounded by a dense layer of collagen, thus losing its elasticity. This loss of elasticity restricts muscle movement and hampers accommodation.30 A similar mechanism may be one component of human presbyopia; however, other changes are likely involved including changes involving the lens itself (see Chapter 5).
Aqueous Production
The ciliary body capillaries and the ciliary epithelial layers are significant factors in the production and secretion of aqueous. The stroma within the ciliary processes contains a dense network of fenestrated capillaries, and the number and shape of the processes provides a large surface area for secretion into the posterior chamber. Three mechanisms contribute to production and secretion: diffusion, ultrafiltration, and active secretion.31 Diffusion occurs when an uneven distribution of molecules exists across a membrane and the molecules move from the higher concentration to the lower concentration. Ultrafiltration occurs as bulk flow across a semipermeable membrane augmented by a hydrostatic pressure. In active secretion molecules are transported across the membrane against a concentration gradient in an energy-utilizing process; active secretion likely accounts for 80% to 90% of aqueous production.15
Pressure on the ciliary stroma side of the two-layered epithelia is estimated at greater than 15 mm Hg, providing a driving force that moves solution minus macromolecules across the zonula occludens barrier of the nonpigmented epithelium, thus producing an ionic concentration comparable to blood plasma. An oncotic gradient of approximately 14 mm Hg could move water across the semipermeable membrane of the nonpigmented epithelium from a solution with low concentration of macromolecules (the aqueous) into a higher concentration of macromolecules (blood plasma), counteracting the ultrafiltration process. As these forces are in the opposite direction, it is unclear how great a factor ultrafiltration actually is in aqueous production because it might be offset by water movement back into the ciliary processes.23
As molecules exit the blood through the walls of the ciliary capillaries, they move through the stroma and the epithelia. The model of ion movement through these cells is still theoretical; transport mechanisms have been identified but the regulation of those mechanisms is not clear.23,31–33 The two layers of epithelium are thought to function together as a syncytium due to the extensive gap junctions joining the cells within each layer as well those between the two layers.
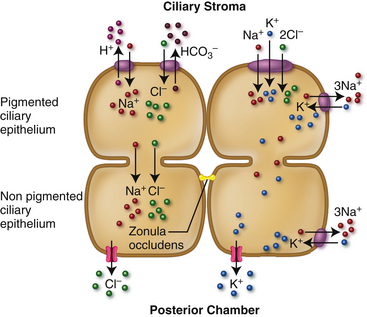
The driving force for secretion of the fluid is produced by transepithelial ionic transport across this bilayered ciliary epithelium.34 Ions enter the basolateral pigmented ciliary epithelium (PE); (Na+ and Cl− likely enter by Na+/H+and Cl−/HCO3− exchangers and the Na+/K+/2Cl− cotransporter), then diffuse through the apical membrane into the extracellular fluid, as well as directly into the nonpigmented ciliary epithelium (NPE) through gap junctions.23,34 The active ATPase pump within the NPE cell accounts for a relatively low concentration of Na+ in the NPE, creating a driving force for Na+ movement. The Na+/H+ exchange and the Na+/K+/2Cl− transporter maintain the cycle of Na+ passage.
Ions exit the basolateral membrane of the NPE through ionic pumps, ion channels, and cotransporters into the posterior chamber; the electrical voltage change due to movement of ions can drive increased movement of Na+ and Cl− and thus shift water. Potassium ions that were brought into the cell by Na+/K+ ATPase pumps are kept circulating by K+ channels in the basolateral membrane.34 The coordination of ion pumps, channels, and cotransporters in these two epithelia, as well as aquaporins in the NPE that facilitate water movement, produce the substance secreted into the posterior chamber as aqueous humor.23,35 The current belief is that the movement of Na+ and Cl− primarily drives secretion into the posterior chamber with bicarbonate ions having an indirect role by moderating Cl− flux.23 Figure 3-21 shows an abreviated schematic of ion flow.
Function and Rate of Production
Approximately 2.5 μl of aqueous is produced per minute.15 Aqueous production follows the circadian rhythm with a higher rate during the day, that rate is decreased by about 50% during the night.23 It is unclear whether the fluctuation of IOP coincides with this production cycle. During sleep circulating epinephrine is decreased which may in part account for the reduction in production but is unlikely the sole factor for the circadian cycle.36
Although the ultrafiltration process can be influenced by changes in IOP the effect on the rate of formation is slight.15 Since active secretion is the primary mechanism for aqueous formation moderate changes in blood pressure have little effect on the rate of formation.31 Autonomic nerves located within the ciliary body can influence aqueous production by acting on the blood vessels, dilating them and increasing blood volume or decreasing volume by constricting the vessels. Although no anatomic evidence has been found identifying autonomic innervation to the epithelia, animal studies have found some alteration in production volumes in response to manipulations of the autonomic signal. Further information on the effect of aqueous production on intraocular pressure and drug treatments that reduce aqueous production will be found in Chapter 6.
Vitreous Production
Investigators recently have suggested that similar processes may occur in the epithelium of the pars plana region and have a significant role in the production and secretion of various connective tissue macromolecules located in the vitreous body.37
Blood-Aqueous Barrier
The blood-aqueous barrier selectively controls the secreted substance—aqueous humor. The fenestrated ciliary body capillaries permit large molecules to exit the blood. However, the tight zonular junctions of the nonpigmented epithelium prevent the molecules from passing between the cells, forcing them instead to pass through the cell to enter the posterior chamber. One of the substances thus controlled is protein. The protein content of aqueous humor is very small compared with that of blood.38 Proteins pass easily out of the ciliary vessels through the fenestrations but do not pass into the posterior chamber because of the tight junction barrier of the nonpigmented epithelium.15,39–41
The iris is freely permeated by the aqueous humor, which readily enters the stroma through the surface crypts.1 To prevent large molecules from leaking out of the iris blood vessels and altering the content of the aqueous fluid, the iris capillaries have no fenestrations, and their endothelial cells maintain the barrier function through their zonula occludens junctions.21,41–43
Clinical Comment: Tyndall Phenomenon
Clinical examination of the aqueous with the biomicroscope is accomplished by focusing a conical beam within the anterior chamber and with high magnification and a dark room watching for movement within the beam. In normal aqueous the beam will be invisible, the out-of-focus cornea and lens will be visible in the reflected light, but the aqueous will be dark or “optically empty.” If there are particles in the pathway of the beam, light will be reflected and scattered producing the Tyndall phenomenon, making the beam visible within the aqueous.
Cells and flare in the anterior chamber can be indicative of uveal inflammation or infection. A disruption of the zonula occludens between the nonpigmented ciliary cells causes a breakdown of the blood-aqueous barrier and can occur in inflammatory conditions, allowing immune factors and leucocytes into the eye to fight invading microbes, causing cells and flare.35 This accumulation of material usually appears whitish and if there is significant amount it may settle in the inferior anterior chamber, forming an hypopyon.
Functions of Choroid
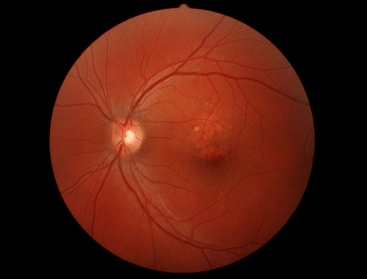
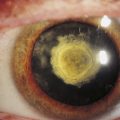
With aging, excessive basement membrane (basal lamina) material is deposited in the collagenous zones of Bruch’s membrane.44–46 These deposits in the inner collagenous zone, called drusen, can be seen as small, pinhead-sized, yellow-white spots in the fundus (Figure 3-22). The drusen, which contain cellular fragments and an accumulation of basal laminar material, are located outer to the RPE basement membrane and displace the retina inward.47
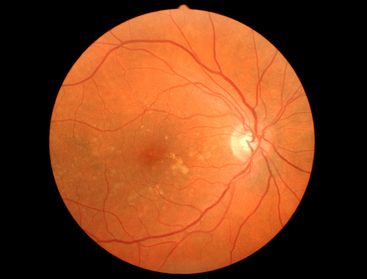
FIGURE 3-22 Fundus photo showing right eye of 49-year-old with scattered retinal drusen.
(Courtesy Fraser Horn, OD, Pacific University Family Vision Center, Forest Grove, Ore.)
Clinical Comment: Age-Related Macular Degeneration
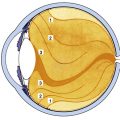
Degenerative processes involving the choroid-retina interface in the macular area often are manifested as age-related macular degeneration (AMD). AMD is the most common cause of blindness in Western countries.48 This disease is multifaceted in origin and can involve the presence of multiple or confluent drusen, a thick layer of basal laminar deposit, detachment or atrophy of the RPE, subsequent formation of disciform scars, (Figure 3-23) loss of photoreceptors, and neovascularization.47,49
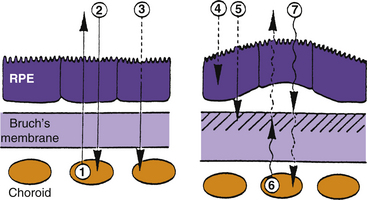
Metabolites from the choriocapillaris and waste products from the retina must pass through Bruch’s membrane. With age, phospholipids accumulate in this membrane, probably because of defective mechanisms in the dephosphorylation process.45,50–53 Free radicals resulting from oxidative stress have been implicated in these cellular metabolic changes.54 The accumulation of lipids in Bruch’s membrane with increasing age appears to be greater in the central fundus than in the periphery.55 Bruch’s membrane becomes hydrophobic and presents a barrier to water movement, thereby inhibiting the passage of metabolites.56 If water accumulates between the RPE and Bruch’s membrane, displacement and detachment may occur.52 This process is represented diagrammatically in Figure 3-24.
Loss of nutrients to the highly metabolic retina can cause (1) atrophy of the RPE, followed by loss of photoreceptors,53,57 or (2) development of a neovascular membrane in an attempt to compensate for the loss of nutrients.54,56 The new vessels branch from the choriocapillaris and can remain beneath the RPE or can penetrate Bruch’s membrane and enter the retina.54 However, these vessels are fragile, leak, and tend to hemorrhage into retinal tissue.52,58 Visual loss in AMD results from detachment of the RPE resulting from water accumulation, atrophy of the RPE and photoreceptors, or the presence of a subretinal neovascular membrane.45,50
Risk factors associated with AMD include genetics; age; lighter pigmentation of skin, hair, or iris; skin sensitivity to sun; smoking; sun exposure; and cataract surgery.54,56,59–63 Although these risk factors have been elucidated, their role in AMD is still not seen as causative. The complex interplay among these factors, however, implicates many if not all of them in AMD.
No definitive treatment for AMD exists as yet, but supplementation with antioxidants or minerals (e.g., high doses of vitamins C and E, beta carotene, zinc, lutein, and zeaxanthin) may provide some protective effect or slow the progression to advanced disease in patients with mild AMD.64,65 However, others question the effectiveness of such supplements in AMD.66–70 Surgical and drug delivery treatments have been identified to be successful in slowing or eliminating the progression of AMD. Clinical trials continue to seek improved treatment modalities.
Blood Supply to Uveal Tract
The short posterior ciliary arteries enter the globe in a circle around the optic nerve, and their branches form the choroidal vessels. The long posterior ciliary arteries and the anterior ciliary arteries join to form the major circle of the iris, which supplies vessels to the iris and ciliary body. The venous return for most of the uvea is through the vortex veins (see Chapter 11 for further information on the blood supply).
Uveal Innervation
Sensory innervation of the uvea is provided through the nasociliary branch from the trigeminal nerve. Sympathetic fibers from the superior cervical ganglion via the ophthalmic and short ciliary nerves innervate the choroidal blood vessels, and sympathetic fibers from the superior cervical ganglion via the long ciliary nerves innervate the iris dilator and ciliary muscles. Parasympathetic fibers from the ciliary ganglion innervate the ciliary muscle, the iris sphincter muscle, and the choroidal vessels.
Aging Changes In Uvea
Iris
With age, loss of pigment from the epithelium is evident at the pupillary margin and on transillumination. Pigment deposition may be seen on the iris surface, anterior lens surface, posterior cornea, and trabecular meshwork. The dilator muscle becomes atrophic, and the sphincter muscle becomes sclerotic, making it more difficult to dilate the older pupil pharmacologically.71
Ciliary Body
Although the amount of connective tissue within the layer of ciliary muscle increases with age,72 there is no significant correlation between loss of ciliary muscle contractile ability and age.72,73 Ciliary muscle contraction does not diminish with age.74 The formation of aqueous decreases with age and by age 80 is approximately 25% of what it was; however, the volume of the anterior chamber decreases by about 40%.36
Choroid
The changes that occur in AMD occur throughout the choroid with advancing age. However, only those occurring in the macula significantly affect visual acuity and the patient’s daily life. Bruch’s membrane increases in thickness with age. Various substances and particles accumulate, decreasing the membrane’s permeability to serum proteins, and its capacity to facilitate metabolite exchange with the retina decreases.75–78 Calcification and deposits in the inner collagenous layer are responsible for the clinical appearance of drusen, which increase in number with age.56 As lysosomal activity in the RPE decreases with age, atypical material (including lipofuscin) may accumulate in Bruch’s membrane.54
The choriocapillaris decreases in density and diameter, resulting in a decrease in choroidal blood flow, and choroidal thickness decreases in the normal-aging macula.54,79
1. Hogan M.J., Alvarado J.A., Weddell J.E., editors. Histology of the human eye. Philadelphia: Saunders, 1971. p 202
2. Warwick R. Wolff’s anatomy of the eye and orbit, ed 7. Philadelphia: Saunders; 1976. p 61
3. Fine B.S., Yanoff M. Ocular histology, Hagerstown, Md. Harper & Row; 1979. p 195
4. Loewenfeld I.E. The pupil: anatomy, physiology, and clinical applications. Boston: Butterworth-Heinemann; 1999.
5. Bartlett J.D., Jaanus S.D. Clinical ocular pharmacology, ed 3. Boston: Butterworth-Heinemann; 1995. pp 776–915
6. Imesch P.D., Bindley C.D., Khademian Z., et al. Melanocytes and iris color. Electron microscopic findings. Arch Ophthalmol. 1996;114:443.
7. Wilkerson C.L., Syed N.A., Fisher M.R., et al. Melanocytes and iris color. Light microscopic findings. Arch Ophthalmol. 1996;114:437-442.
8. Albert D.M., Green W.R., Zimbri M.L., et al. Iris melanocyte numbers in Asian, African American, and Caucasian irides. Trans Am Ophthalmol Soc. 2003;101:217-222.
9. Wakamatsu K., Hu D.N., McCormick S.A., et al. Characterization of melanin in human iridal and choroidal melanocytes from eye with various colored irides. Pigment Cell Melanoma Res. 2007;21:97-105.
10. Catania L.J. Primary care of the anterior segment. East Norwalk, Conn: Appleton & Lange; 1988. p 211
11. Gilmartin B. A review of the role of sympathetic innervation of the ciliary muscle in ocular accommodation. Ophthalmic Physiol Opt. 1986;6(1):23.
12. Rosenfield M., Gilmartin B. Oculomotor consequences of beta-adrenoreceptor antagonism during sustained near vision. Ophthalmic Physiol Opt. 1987;7(2):127.
13. Gilmartin B., Bullimore M.A., Rosenfield M., et al. Pharmacological effects on accommodative adaptation. Optom Vis Sci. 1992;69(4):276.
14. Raviola G., Raviola E. Intercellular junctions in the ciliary epithelium. Invest Ophthalmol Vis Sci. 1978;17:958.
15. Gabelt B.T. Kaufman PL: Aqueous humor dynamics. In: Kaufman PL, Alm A., editors. Adler’s physiology of the eye. ed 10. St Louis: Mosby; 2003:237.
16. Raviola G. The structural basis of the blood-ocular barriers. Exp Eye Res. 1977;25(suppl):27.
17. Takats K., Kasahara T., Kasahara M., et al. Ultracytochemical localization of the erythrocyte/HepG2-type glucose transporter (GLUT1) in the ciliary body and iris of the rat eye. Invest Ophthalmol Vis Sci. 1991;32(5):1659.
18. Eichhorn M., Inada K., Lütjen-Drecoll E. Human ciliary body in organ culture. Curr Eye Res. 1991;19(4):277.
19. Raviola G. The fine structure of the ciliary zonule and ciliary epithelium. Invest Ophthalmol. 1971;10(11):851.
20. Noske W., Stamm C.C., Hirsch M. Tight junctions of the human ciliary epithelium: regional morphology and implications on transepithelial resistance. Exp Eye Res. 1994;59:141.
21. Sonsino J., Gong H., Wu P., et al. Co-localization of junction-associated proteins of the human blood—aqueous barrier: occludin, ZO-1 and F-actin. Exp Eye Res. 2002;71:123. (abstract)
22. Shiose Y. Electron microscopic studies on blood-retinal and blood-aqueous barriers. Jpn J Ophthalmol. 1970;14:73. (abstract)
23. Delamere N.A. Ciliary body and ciliary epithelium. In: Fischbarg J., editor. The biology of the eye. Elsevier; 2006:127-148.
24. Spitzmas M., Reale E. Fracture faces of fenestrations and junctions of endothelial cells in human choroidal vessels. Invest Ophthalmol. 1975;14:98-107.
25. Bernstein M.H., Hollenberg M.J. Fine structure of the choriocapillaris and retinal capillaries. Invest Ophthalmol. 1965;4(6):1016.
26. Chakravarthy U., Gardiner T.A. Endothelium-derived agents in pericyte function/dysfunction. Prog Retin Eye Res. 1999;18(4):511.
27. Alexander R.A., Garner A. Elastic and precursor fibres in the normal human eye. Exp Eye Res. 1983;36:305.
28. Ebersberger A., Flugel C., Lütjen-Drecoll E. Ultrastructural and enzyme histochemical studies of regional structural differences within the ciliary muscle in various species. Klin Monbl Augenheilkd. 1993;203(1):53. (abstract)
29. Mauger R.R., Likens C.P., Applebaum M. Effects of accommodation and repeated applanation tonometry on intraocular pressure. Am J Optom Physiol Opt. 1984;61(1):28.
30. Tamm E., Lütjen-Drecoll E., Jungkunz .W., et al. Posterior attachment of ciliary muscle in young, accommodating, old, presbyopic monkeys. Invest Ophthalmol Vis Sci. 1991;32:1678.
31. Bill A. The role of ciliary blood flow and ultrafiltration in aqueous humor formation. Exp Eye Res. 1973;16:287-298.
32. Candia O.A., Alvarez L.J. Fluid transport phenomena in ocular epithelia. Prog Retin Eye Res. 2008;27:197-212.
33. Macri F.J., Cevario S.J. The formation and inhibition of aqueous humor production. A proposed mechanism of action. Arch Ophthalmol. 1978;96:1664-1667.
34. Do C.W., Civan M.M. Species variation in biology and physiology of the ciliary epithelium: similarities and differences. Exp Eye Res. 2009;88:631-640.
35. Levin M.H., Verkman A.S. Aquaporins and CFTR in ocular epithelial fluid transport. J Membr Biol. 210, 2006. 205-115
36. Mc Laren J.W. Measurement of aqueous humor flow. Exp Eye Res. 2009;88:641-647.
37. Bishop P.N., Takanosu M., Le Goff M., et al. The role of the posterior ciliary body in the biosynthesis of vitreous humour. Eye. 2002;16:454.
38. Krause U., Raunio V. Proteins of the normal human aqueous humour. Ophthalmologica. 1969;159:178.
39. Smith R.S. Ultrastructural studies of the blood-aqueous barrier. I. Transport of an electron-dense tracer in the iris and ciliary body of the mouse. Am J Ophthalmol. 1971;71:1066.
40. Varma S.D., Reddy V.N. Phospholipid composition of aqueous humor plasma and lens in normal and alloxan diabetic rabbits. Exp Eye Res. 1972;13:120.
41. Cunha-Vaz J.G. The blood-ocular barriers: past, present, and future. Doc Ophthalmol. 1997;93:149.
42. Waitzman M.B., Jackson R.T. Effects of topically administered ouabain on aqueous humor dynamics. Exp Eye Res. 1965;4:135.
43. Schlingemann R.O., Hofman P., Klooster J., et al. Ciliary muscle capillaries have blood-tissue barrier characteristics. Exp Eye Res. 1998;66:747.
44. Sarks S.H. Aging and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976;60:324.
45. van der Schaft T.L., Mooy C.M., de Bruijn W.C., et al. Histologic features of the early stages of age-related macular degeneration. A statistical analysis. Ophthalmology. 1992;99:278.
46. Van der Schaft T.L., Mooy C.M., de Bruijn W.C., et al. Immunohistochemical light and electron microscopy of basal laminar deposit. Graefes Arch Clin Exp Ophthalmol. 1994;232(1):40.
47. Ramrattan R.S., van der Schaft T.L., Mooy C.M., et al. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35(6):2857.
48. Leibowitz H.M., Krueger D.E., Maunder L.R., et al. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol. 1980;24:335.
49. Bressler N.M., Bressler S.B., Fine S.L. Age-related macular degeneration. Surv Ophthalmol. 1988;32:375.
50. Sheraidah G., Steinmetz R., Maguire J., et al. Correlation between lipids extracted from Bruch’s membrane and age. Ophthalmology. 1993;100(1):47.
51. Pauleikhoff D. Drusen in Bruch’s membrane. Their significance for the pathogenesis and therapy of age-associated macular degeneration. Ophthalmologe. 1992;89(5):363. (abstract)
52. Pauleikhoff D., Harper C.A., Marshall J., et al. Aging changes in Bruch’s membrane. A histochemical and morphologic study. Ophthalmology. 1990;97(2):171.
53. Zarbin M.A. Age-related macular degeneration: review of pathogenesis. Eur J Ophthalmol. 1998;8(4):199.
54. Ambati J., Ambati B.K., Yoo S.H., et al. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48(3):257.
55. Holz F.G., Sheraidah G., Pauleikhoff D., et al. Analysis of lipid deposits extracted from human macular and peripheral Bruch’s membrane. Arch Ophthalmol. 1994;112(3):402.
56. Guymer R., Luthert P., Bird A. Changes in Bruch’s membrane and related structures with age. Prog Retin Eye Res. 1999;18(1):59.
57. Curcio C.A., Millican C.L., Bailey T., et al. Accumulation of cholesterol with age in human Bruch’s membrane. Invest Ophthalmol Vis Sci. 2001;42:265.
58. Bailey R.N. The surgical removal of a subfoveal choroidal neovascular membrane: an alternative treatment to laser photocoagulation. J Am Optom Assoc. 1993;64(2):104.
59. Cruickshanks K.J., Klein R., Klein B.E., et al. Sunlight and the 5-year incidence of early age-related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol. 2001;119(2):246.
60. McCarty C.A., Mukesh B.N., Fu C.L., et al. Risk factors for age-related maculopathy: the Visual Impairment Project. Arch Ophthalmol. 2001;119(10):1455.
61. Klein R., Klein B.E., Jensen S.C., et al. The relationship of ocular factors to the incidence and progression of age-related maculopathy. Arch Ophthalmol. 1998;116(4):506.
62. Mitchell P., Smith W., Wang J.J. Iris color, skin sun sensitivity, and age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 1998;105(8):1359.
63. Young R.W. The family of sunlight-related eye diseases. Optom Vis Sci. 1994;71(2):125.
64. Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417.
65. Gale C.R., Hall N.F., Phillips D.I., et al. Lutein and zeaxanthin status and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2003;44:2461.
66. Smith W., Mitchell P., Webb K., et al. Dietary antioxidants and age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 1999;106(4):761.
67. Flood V., Smith W., Wang J.J. Dietary antioxidant intake and incidence of early age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 2002;109(12):2272.
68. Kuzniarz M., Mitchell P., Flood V.M., et al. Use of vitamin and zinc supplements and age-related maculopathy: the Blue Mountains Eye Study. Ophthalmic Epidemiol. 2002;9(4):283.
69. Bone R.A., Landrum J.T., Guerra L.H., et al. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J Nutr. 2003;133(4):922.
70. Klein R., Klein B.E., Tomany S.C., et al. Ten-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2002;109:1767.
71. Oates D.C., Belcher C.D. Aging changes in trabecular meshwork, iris, and ciliary body. In: Albert D.M., Jakobiec F.A., editors. Principles and practice of ophthalmology. Philadelphia: Saunders; 1994:697.
72. Pardue M.T., Sivak J.G. Age-related changes in human ciliary muscle. Optom Vis Sci. 2000;77(4):204.
73. Strenk S.A., Semmlow J.L., Strenk L.M., et al. Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study. Invest Ophthalmol Vis Sci. 1999;40(6):1162.
74. Strenk S.A., Strenk L.M., Guo S. Magnetic resonance imaging of aging, accommodating, phakic, and pseudophakic ciliary muscle diameters. J Cataract Refract Surg. 2006;32:1792-1798.
75. Moore D.J., Clover G.M. The effect of age on the macromolecular permeability of human Bruch’s membrane. Invest Ophthalmol Vis Sci. 2001;42:2970.
76. Moore D.J., Hussain A.A., Marshall J. Age-related variation in the hydraulic conductivity of Bruch’s membrane. Invest Ophthalmol Vis Sci. 1995;36(7):1290.
77. Ruberti J.W., Curcio C.A., Millican C.L., et al. Quick-freeze/deep-etch visualization of age-related lipid accumulation in Bruch’s membrane. Invest Ophthalmol Vis Sci. 2003;44:1753.
78. Huang J.D., Presley J.B., Chimento M.F., et al. Age-related changes in human macular Bruch’s membrane as seen by quick-freeze-deep-etch. Exp Eye Res. 2007;85:202-218.
79. Ramrattan R.S., van der Schaft T.L., Mooy C.M., et al. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35(6):2857.