Chapter 46 Uterine Leiomyomas
PREVALENCE
Uterine leiomyomas (also known as fibroids or myomas) are the most common benign pelvic tumors found in women. As many as 50% of reproductive-age women will have clinically apparent uterine leiomyomas, and 25% of women have symptomatic leiomyomas. On pathologic examination, 77% of hysterectomy specimens contain one or more uterine leiomyomas.1
Although benign, uterine leiomyomas are associated with significant morbidity, including abnormal uterine bleeding, chronic pelvic pain, impaired fertility, and recurrent pregnancy loss. In the United States, leiomyomas are cited as the primary indication for hysterectomy in more than 250,000 cases per year and account for over $5 billion in healthcare costs annually.2
CLASSIFICATION
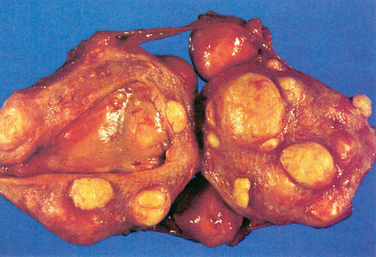
Uterine leiomyomas are typically classified into subgroups by their position relative to the layers of the uterus. Subserosal myomas occur near the serosal surface of the uterus. They may have either a broad or pedunculated base and can extend between the folds of the broad ligament. Intramural myomas originate within the myometrium and may enlarge enough to distort the uterine cavity or the serosal surface. Submucosal myomas develop just below the endometrium and with progression protrude into the uterine cavity. These can also be either pedunculated or broad-based. Cervical myomas, on the other hand, are derived from cells in the cervix as opposed to the corpus of the uterus (Fig. 46-1).
CLINICAL IMPACT
Abnormal Uterine Bleeding
Abnormal uterine bleeding is the most common symptom reported by women with leiomyomas. The most common types of leiomyomas associated with heavy bleeding are intramural or submucosal myomas. The typical bleeding patterns are menorrhagia and hypermenorrhea. Intermenstrual bleeding can represent an intracavitary myoma or specific endometrial pathology. Therefore, these patients must always undergo a more detailed evaluation of the uterine cavity. Very heavy vaginal bleeding can lead to problems such as iron deficiency anemia, which can be severe enough to require blood transfusions, and the necessity for frequent changes of sanitary protection can cause significant distress in work or social situations.3
Chronic Pelvic Pain
Pelvic pain or pressure, the second most common complaint in women with leiomyomas, is frequently described as analogous to the discomfort associated with uterine growth during pregnancy. The pain can occur both during and between bleeding episodes. Posterior leiomyomas may give rise to low back pain, whereas anterior leiomyomas may compress the bladder. Leiomyomas that become large enough to fill the pelvis may potentially interfere with voiding or defecation or may cause dyspareunia. Very large leiomyomas can on occasion outgrow their blood supply, leading to tissue ischemia and necrosis clinically manifested as acute, severe pelvic pain. Pedunculated leiomyomas can suffer torsion, also causing ischemia and acute pain. During pregnancy, leiomyomas have been known to undergo red degeneration, where hemorrhage occurs within the myoma, also leading to acute pain.3
Reproductive Function
Yet the indirect evidence is substantial. In one review, pregnancy rates among women with leiomyomas distorting and not distorting the uterine cavity were 9% and 35%, respectively, as compared to 40% among controls with no leiomyomas.4 Furthermore, the multiple reports of successful pregnancies among infertile women after myomectomy strongly suggest a connection.5–7
Although exact physiologic mechanisms for reproductive dysfunction are unclear, many plausible theories exist. There is a potential for reduced fecundity if a myoma occurs in the cornual region of the uterus, causing mechanical occlusion of a fallopian tube.3 It is possible that large leiomyomas may impair the rhythmic uterine contractions that facilitate sperm motility.8 It has further been documented that endometrial histology varies in relation to the location of the leiomyoma. Atrophy, as well as alterations in the vascular blood flow produced by sumbucosal leiomyomas, may impede implantation of an embryo, prevent delivery of hormones or growth factors involved in implantation, or interfere with the normal immune response to pregnancy.9–11 Submucosal leiomyomas that distort the uterine cavity are associated with first-trimester pregnancy loss, preterm delivery, abnormal presentation in labor, and postpartum hemorrhage.12
In regard to the effectiveness of assisted reproductive technology (ART), leiomyomas are generally thought to reduce the effectiveness of ART procedures. Early evidence demonstrated that both pregnancy and implantation rates were significantly lower in patients with intramural or submucosal leiomyomas.13,14 In one study, the presence of an intramural leiomyoma decreased the chances of an ongoing pregnancy after in vitro fertilization by 50%.15 The latest evidence suggests that patients with subserosal leiomyomas have ART outcomes consistent with patients lacking leiomyomas.4,16,17
EPIDEMIOLOGY OF UTERINE LEIOMYOMAS
The diagnosis of uterine leiomyomas increases with age throughout the reproductive years, with the highest prevalence occurring in the fifth decade of a woman’s life. The most common types of leiomyomas associated with heavy bleeding are intramural or submucosal myomas; these tend to be diagnosed at an earlier age and to result in more severe disease in African American women. (larger leiomyomas and greater incidence of anemia) as compared to white women.18,19
Nulliparous women have higher rates of leiomyomas than multiparous women, and the risk of developing leiomyomas decreases consistently with each subsequent term birth.20 Early age at menarche is associated with a twofold to threefold increased risk of developing leiomyomas.21
Leiomyomas clearly demonstrate their hormonal responsiveness in the fact that they form after puberty, have the potential to enlarge during pregnancy, and regress after menopause. However, studies of exogenous hormone treatments, including oral contraceptives and hormone replacement therapy, reveal conflicting data; no clear association can be inferred.22
Twin and family studies suggest a familial predisposition to developing leiomyomas, although further research in the genetics of leiomyomas has yet to be done.22 These studies are hampered by the extremely high incidence of leiomyoma formation in the general population.
According to some studies, an increase in body mass index (BMI) has been found to increase the risk for uterine leiomyomas by a factor of 2 to 3, and the evidence suggests that adult-onset obesity rather than excessive weight in childhood confers this risk. However, other studies have not observed similar associations with increased BMI.21
The majority of epidemiologic studies find that cigarette smokers are at a 20% to 50% reduced risk for the development of uterine leiomyomas through an unclear mechanism, and that the inverse association was independent of BMI. It is unclear whether this relationship varies as a function of pack-years. No clear relationship has been shown between leiomyomas and specific dietary factors or physical activity.21
PATHOLOGY AND PATHOPHYSIOLOGY
Genetics
Leiomyomas are defined as monoclonal proliferations of benign smooth muscle.23 Each monoclonal myoma may be associated with various chromosomal translocations, duplications, and deletions.24 Many but not all myomas contain nonrandom cytogenetic abnormalities while the myometrium has a normal karyotype—typically these involve chromosomes 7, 12, and 14. Most of the mutations occur in genes involved in cellular growth or responsible for architectural transcription.
Two hereditary disorders have been reported in which uterine leiomyomas are part of a syndrome complex that demonstrates the potential genetic contribution to myoma formation. The first is hereditary leiomyomatosis and renal cell cancer complex. This is an autosomal dominant syndrome with smooth muscle tumors of the uterus, skin, and kidney. The second is a syndrome of pulmonary leiomyomatosis and lymphangiomyomatosis (LAM) that is the result of mutations in one of the two genes responsible for tuberous sclerosis, a syndrome that results in multiple hamartomas.
Pathology
Grossly, myomas usually appear as discrete, round masses that are lighter in color than the surrounding myometrium, with a glistening, pearly white appearance. Histologic features include smooth muscle fibers that form interlacing bundles, with fibrous tissue in between the bundles. A more detailed description is found in Chapter 8.
Endocrinology
Tumor initiators and yet-to-be-determined genetic factors are involved in key somatic mutations that facilitate the progression of a normal myocyte into a leiomyocyte responsive to estrogen and progesterone. Estrogen receptors, progesterone receptors, and epidermal growth factor receptors (EGFR) are integral in the development of myomas.25 Studies have shown that, in comparison with the normal myometrium, myomas have an increased concentration of estrogen receptors and progesterone receptors.26,27
Aromatase p450 is overexpressed by leiomyomas.28,29 Therefore, in addition to circulating estrogen acting on the ER, the local conversion of circulating androgens to estrogens may be important in potentiating the actions of estrogen in the leiomyocyte (Fig. 46-2).30
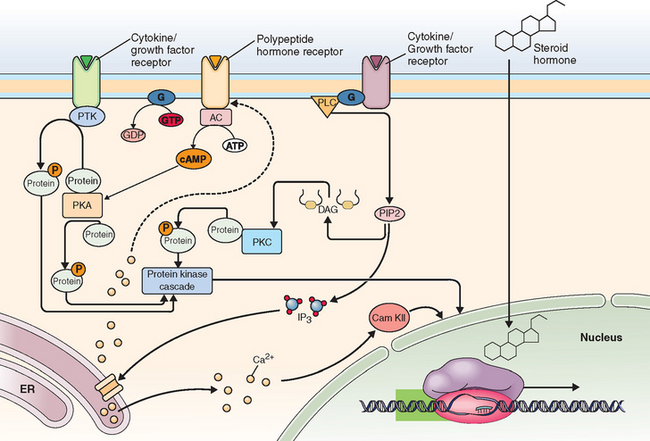
Figure 46-2 Sex steroid hormone action. Estrogen and progesterone exert action through binding of specific receptors, which then bind to DNA at specific response elements. Binding of estrogen and progesterone at a variety of genes has different effects in various cells. Figure provided to the public via the internet by Fisher Scientific, Inc. (www.fishersci.com).
Traditionally, estrogen was thought to be the primary hormonal mediator of myoma growth. Although progestins have been applied for the treatment of bleeding from symptomatic myomas, recent studies have shown that progesterone may play a much greater role as a mediator of myoma growth than previously thought.31 The antiprogestin RU486 (mifepristone) has been shown to decrease the size of myomas,32,33 and another study showed that myomas in the secretory phase have increased mitotic counts compared to those in the proliferative phase.34
Growth of neoplastic tumors is the result of accelerated cellular proliferation, which outpaces the inhibitory effect of apoptosis. Apoptosis has been shown to be inhibited in uterine leiomyomas. Progesterone has been shown to increase the antiapoptotic protein, bcl-2.35 Therefore, the stimulation of myoma expansion may be a function of the suppression of apoptosis by progesterone. It has been observed in vitro that the addition of progesterone to cultured leiomyoma cells increased the expression of bcl-2 when compared to controls.35 Normal myometrium did not express increased levels of bcl-2 in the presence of progesterone.
The complex process of apoptosis involves not only the bcl-2 family, but Fas/FasL and Rb-1.36 Martel and coworkers have described the various apoptotic pathways deficient in leiomyomas and potential corresponding targets for therapy of myomas. The role of apoptosis in the pathogenesis of myomas is a promising area for future research, with a great potential for clinical application.
The synergistic interplay between estrogen and progesterone signaling in the pathophysiology of myoma growth has been observed as well. The increase in progesterone receptors as a result of increased estrogen has been well established. An in vitro study showed that progesterone up-regulates the expression of EGF, and estrogen also increases the expression of EGFR.25
DIAGNOSTIC IMAGING AND LEIOMYOMAS
Ultrasound
Traditional ultrasound is a cost-effective technology for assessing uterine leiomyomas (see Chapter 30 for details). The transvaginal approach is more accurate than abdominal ultrasound. However, abdominal ultrasound may be a useful adjunct to transvaginal ultrasound, if a large uterine size warrants such an approach.22 The presence of myomas may be detected by ultrasound, as uterine enlargement or a nodular contour of the uterus. They may also appear as discrete, focal masses within the myometrium.37,38 Myomas can appear hypoechoic or heterogeneous when compared with the appearance of the myometrium on ultrasound, and they may be characterized by calcification and posterior shadowing.37,39 Sagittal and axial views aid in providing information on the location and size of myomas.
Additional information regarding intracavitary masses, such as submucous myomas, may be obtained by means of saline infusion sonohysterography. This imaging technique consists of real-time transvaginal ultrasound during which sterile saline solution is injected into the uterine cavity. The saline is injected transcervically via a small-caliber catheter. As the uterine cavity is distended by the saline solution, intracavitary masses may be visualized as echogenic structures against the echolucent background of the distension media.40 Intramural myomas within close proximity of the endometrial cavity may also be assessed by sonohysterography. In addition, entities such as endometrial polyps and uterine anomalies such as adhesions may also be detected. Sonohysterography can be used not only to diagnose submucous myomas, but also to assess the potential access to surgical intervention.41
Three-dimensional ultrasound42 and color Doppler ultrasound43 are increasingly being applied to the evaluation of myomas for imaging. Color Doppler ultrasound highlights vascular flow, which is usually increased at the periphery of myomas and decreased centrally.42,43
Hysterosalpingography
HSG is a screening test for intracavitary anatomic defects and entails injection of iodine contrast dye transcervically, via a catheter, into the uterine cavity with radiologic assessment under fluoroscopy (see Chapter 29). HSG is performed in the follicular phase of the menstrual cycle to avoid interfering with ovulation or a potential pregnancy. Because the HSG instillation medium contains iodine, an iodine-allergic patient requires premedication with glucocorticoids and antihistamines before the procedure.44
Hysterosalpingography allows visualization of submucous myomas as the uterine cavity is distended by the contrast medium. The size and contour of the uterus may be altered by submucous myomas. Intramural myomas may enlarge the uterine cavity in a globular manner, and fundal myomas may enlarge the space between the cornuae. Subserosal myomas are not typically noted on HSG; however, if large enough, they may be detected as a mass effect on the uterine cavity.23 In cases where a submucous myoma must be differentiated from an endometrial polyp on HSG, hysteroscopy or sonohysterography play roles as complementary, potentially confirmatory adjuncts.
Magnetic Resonance Imaging
Disadvantages of MRI include cost, limited availability, and an inability to perform the procedure in patients with morbid obesity or claustrophobia. Traditionally, cost had been more of a disadvantage in the past; however, as the expense of MRI decreases, it is more commonly employed in clinical and presurgical evaluation. MRI is contraindicated in patients with pacemakers, defibrillators, metallic foreign bodies, and in rare cases of allergy to gadolinium.45
In T2-weighted images, the endometrial stripe is visualized as a central, high signal; the junctional zone is a low signal; and the myometrial areas are an intermediate signal.40 Leiomyomas are represented by variable signal density. Most of the time, they appear as hypodense, well-demarcated masses; however, increased cellularity47 and degeneration may be seen as high signal intensity.46
There is less distinction of the endometrial lining, junctional zone, and myometrium in T1-weighted images. These components are usually homogeneous and, consequently, obscured in appearance. Fatty or hemorrhagic degeneration may be represented by a high signal intensity.46 A detailed description can be found in Chapter 31.
MEDICAL TREATMENT OF LEIOMYOMAS
Gonadotropin-Releasing Hormone Agonists
Gonadotropin-releasing hormone (GnRH) agonists are an effective means of medically treating patients with symptomatic leiomyomas. After producing an initial flare of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), GnRH agonists down-regulate the hypothalamic-pituitary-ovarian axis via action on pituitary receptors. The flare effect is due to an initial stimulation of FSH and LH due to the binding of pituitary receptors, after which these receptors are desensitized, with a subsequent decrease in FSH and LH secretion.49 This results in decreased estrogen production.
Gonadotropin-releasing hormone agonists have been shown to directly inhibit local aromatase p450 expression in leiomyoma cells,50 thereby presumably resulting in decreased local conversion of circulating androgens to estrogens within the leiomyocyte. Several studies have concluded that GnRH agonists can directly induce apoptosis and also suppress the cellular proliferation of myomas, presumably via action on peripheral GnRH receptors.
Maximum reduction of the mean uterine volume occurs within 3 months of GnRH agonist administration. The decrease in volume is usually in the range of 40% to 80%. However, after the discontinuation of GnRH agonists, myomas will rapidly grow back to their pretreatment size, usually in the span of several months.51
Advantages of GnRH agonists include their use in the perimenopausal transition with add-back therapy for the goal of avoiding hysterectomy. Additionally, laparoscopic myomectomy may be made more feasible with GnRH agonist pretreatment, and GnRH agonists can also be beneficial in a patient who is to undergo hysterectomy to facilitate a vaginal approach rather than an abdominal incision. In a randomized clinical trial comparing the study group (patients receiving GnRH agonist and iron) to a control group (iron alone), preoperative hematologic parameters were improved.52
Although decreased tumor bulk and a decrease in associated symptoms are attained, the potential for unwanted long-term side effects exists; therefore, treatment with GnRH agonist is recommended for no more than 6 months. Common side effects include hot flashes, vaginal dryness, headache, and mood swings. Most importantly, in terms of bone health status, there is a recognized decrease in bone mineral density during therapy.53
Although add-back doses of steroidal hormones can be used with the aim of decreasing this bone loss, the long-term use of GnRH agonists with add-back is impractical and not recommended, especially in younger patients. Add-back therapy with progestins alone will impede the effectiveness of the GnRH agonist in reducing uterine size. In a randomized crossover study, patients were assigned to GnRH agonist alone versus GnRH agonist and a progestin. Uterine volume decreased to 73% of baseline if the agonist was used alone. When the progestin was added the uterine volume increased toward baseline. If the progestin was introduced when the GnRH agonist was started, the uterine volume did not decrease. This effect is attributed to the direct effect of progestins on the leiomyoma.54
Selective Estrogen Receptor Modulators
Selective estrogen receptor modulators (SERMs), such as tamoxifen and raloxifene, are compounds that bind to the estrogen receptor and confer an agonist or antagonist effect, depending on tissue specificity. They have been applied to the treatment and prevention of estrogen-responsive breast cancer. Tamoxifen, a triphenylethylene, has antagonist activity in the breast and displays desirable agonist activity in bone and the cardiovascular system as well as mild agonist activity in endometrial tissue.55 Raloxifene, a benzothiophene, has a similar profile and the added benefit of not acting as an agonist in the endometrium.56
In animal models, SERMs have been shown to be effective in decreasing the growth of myomas. Eker rats are a rat strain with a tuberous sclerosis gene defect that can spontaneously develop leiomyomas. In studies, the administration of SERMs was associated with the inhibition of leiomyoma formation in Eker rats.57,58 Guinea pigs require long-term exposure to estrogen for leiomyoma formation to occur. Two groups of oophorectomized guinea pigs, an estrogen-only group and an estrogen plus raloxifene group, were compared for myoma formation. A decrease in the size of the induced myomas was observed in the estrogen plus raloxifene group.59
In humans, raloxifene appears effective in decreasing the size of myomas in postmenopausal women,60 but beneficial effects were not significant in premenopausal women.61 A recent study has demonstrated that a combination of raloxifene and GnRH agonist is more effective in reducing leiomyoma volume62 and preventing a decrease in bone mineral density63 than the use of GnRH agonists alone.
Selective Progesterone Receptor Modulators
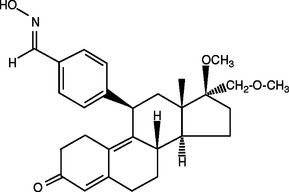
This class of compounds has either agonist or antagonist effects on the progesterone receptor, based on tissue specificity.64 Asoprisnil is a recently developed selective progesterone receptor modulator that, along with its major metabolite, J912, has high affinity for the progesterone receptor, moderately binds the growth hormone receptor, and has a very low binding potential for androgen receptors (Fig. 46-3). Asoprisnil has virtually no affinity for estrogen recptors or mineralocorticoid receptors.65 It differs from the long-term effect of progesterone on the endometrium in that amenorrhea is rapidly established without breakthrough bleeding.66 As it is studied and tested further in clinical trials, there may be a practical use for asoprisnil and other newly developed compounds in this class in the treatment of leiomyomas, especially in women with menorrhagia who are interested in avoiding surgery or maintaining future fertility.
SURGICAL THERAPY OF LEIOMYOMAS
Surgical treatment is the mainstream therapy for leiomyomas. Hysterectomy represents the only definitive curative therapy; however, myomectomy, endometrial ablation, and myolysis are being used with increasing frequency as alternative therapeutic procedures. Indications for surgical intervention include failure to respond to medical treatment, worsening vaginal bleeding, suspicion of malignancy, or treatment of recurrent pregnancy loss. In postmenopausal women with an enlarging pelvic mass and abnormal bleeding, surgery should be strongly considered. In this population, the incidence of a sarcoma is still uncommon but is higher than the incidence found in the premenopausal population, about 1% to 2%.67
Hysterectomy
Hysterectomy has been described as the definitive management for symptomatic leiomyomata. When employed, it is highly effective for the carefully selected patient, with symptomatic leiomyomata, who does not desire future fertility. Among hysterectomies performed abdominally versus vaginally for uterine leiomyomata, the abdominal route has been chosen approximately 75% of the time based on data from the late 1980s and early 1990s.68
Vaginal hysterectomy is associated with a lower complication rate and decreased need for blood transfusion. Another advantage of vaginal hysterectomy is the association with shorter operating times.69 Myoma size and location, total uterine size, and the surgeon’s skill are factors that may determine the feasibility of vaginal hysterectomy.
Laparoscopy-assisted vaginal hysterectomy, total laparoscopic hysterectomy, and laparoscopic supracervical hysterectomy are minimally invasive surgical methods that are associated with decreased postoperative pain and recovery time in comparison to vaginal and abdominal hysterectomy.70 These surgical modalities may incur increased hospital costs in terms of equipment and operating room time, but they have the recognized advantages listed above. In addition, these options provide the ability to assess the pelvis if the patient also complains of pelvic pain.
Myomectomy
Hysterectomy has long been considered the definitive treatment for symptomatic uterine leiomyomas. Yet as more and more women delay childbearing, the incidence of uterine leiomyomas among patients suffering with infertility increases and hysterectomy becomes an unacceptable management option. Therefore, abdominal, laparoscopic, and hysteroscopic myomectomies have become increasingly common treatment modalities for women with leiomyomas and infertility. Hysteroscopic myomectomy is covered in detail in Chapter 42.
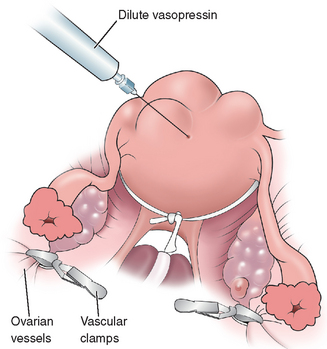
The surgeon must be diligent regarding the orientation of the uterus, especially during the repair, to preserve the integrity of the endometrial cavity. A myomectomy can be performed through several types of abdominal incisions, depending on the size of the uterus. Several techniques have been described to decrease blood loss with an abdominal myomectomy, including the use of tourniquets around the lower segment of the uterus to occlude the uterine arteries and the use of dilute vasopressin (Fig. 46-4).
Methods employed to minimize postoperative adhesion formation include the use of permanent or absorbable barriers and good surgical technique, minimizing trauma to the tissues, use of nonreactive suture material, and the avoidance of tissue desiccation or aggressive cautery. Incisions placed on the anterior aspect of the uterus seem to be associated with fewer adhesions than incisions placed in the posterior part of the uterus. Due to the potential risk of uterine rupture, a trial of labor after myomectomy is not recommended by the American College of Obstetricians and Gynecologists.71
Laparoscopic Myomectomy
Several studies have shown advantages of the laparoscopic approach to myomectomy. Mais and colleagues randomized 20 patients undergoing myomectomy to laparoscopy and 20 patients to laparotomy. The laparoscopy group had lower postoperative pain as well as a greater number of patients who were analgesia-free on postoperative day 2, discharged home by postoperative day 3, and fully recovered on postoperative day 15.72 Among a group of 131 patients randomly assigned to myomectomy via laparoscopy or laparotomy, Seracchioli and colleagues found that laparoscopic myomectomy is associated with lower intraoperative blood loss and shorter postoperative length of stay in the hospital. Moreover, no significant difference in subsequent fecundability, spontaneous miscarriage rate, preterm delivery rate, or cesarean section rate was found between the groups. A lower incidence of postoperative febrile morbidity was yet another advantage found in this study.67
Adhesion formation was evaluated in a retrospective study of 28 patients who underwent laparoscopy or laparotomy for myomectomy followed by a second-look laparoscopy.73 A lower incidence of adhesion formation was noted in patients that initially underwent a laparoscopic approach to their myomectomy. Similar observations were made in two other studies.74,75
Disadvantages of laparoscopic myomectomy include the lack of opportunity to palpate the uterus intraoperatively, resulting in the potential for a higher rate of subsequent recurrence of myomas,76,77 a finding that is supported by several studies and refuted by others.78 An additional disadvantage is operator-dependent and involves the technical difficulty in repairing the enucleated leiomyomas, with a potential risk for uterine rupture during future pregnancies if improperly closed.
Technical Considerations
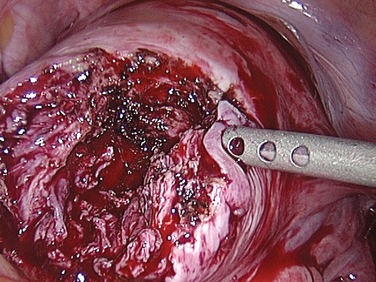
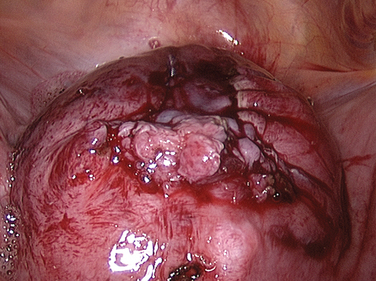
Laparoscopic myomectomy is usually performed with the standard three to four ports. Dilute vasopressin, although not approved by the U.S. Food and Drug Administration (FDA) for this indication, is diluted 20 units in 100 mL of normal saline solution and injected into the myoma. An incision is then made into the myoma and enucleated bluntly (Figs. 46-5 and 46-6). After enucleation, the defect (Fig. 46-7) is closed in two or three layers, typically a deep layer and then a seromuscular layer. Intracorporeal or extracorporeal knot tying are both acceptable. This step is the most critical part of a laparoscopic myomectomy, and the defect should be closed tightly (Fig. 46-8).
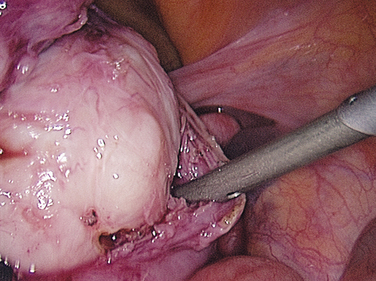
Figure 46-5 Dissection of the myoma bed is usually accomplished bluntly, as in open cases.
(Courtesy of Dr. T. Falcone.)
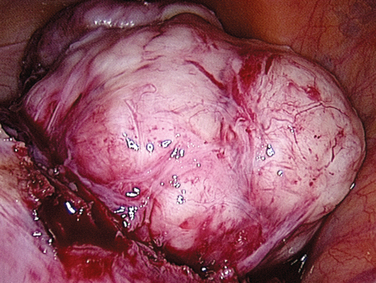
Figure 46-6 The myoma has been almost completely enucleated. Vessels at the base of the myoma should be cauterized.
(Courtesy of Dr. T. Falcone.)
UTERINE ARTERY/LEIOMYOMA EMBOLIZATION
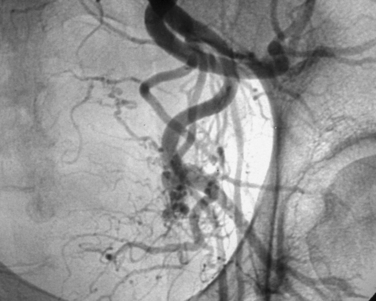
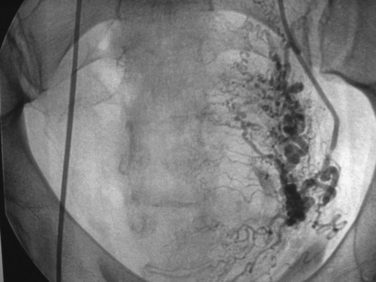
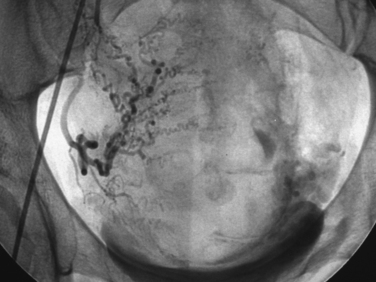
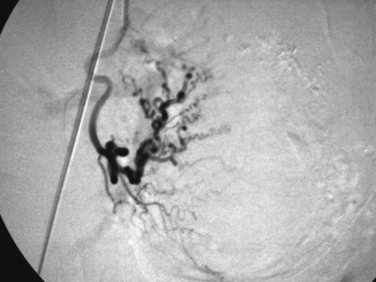
Uterine artery embolization (UAE) or uterine fibroid embolization (UFE) is a minimally invasive alternative to hysterectomy and myomectomy that has been applied to the treatment of symptomatic leiomyomas (Figs. 46-9 to 46-12). UAE was initially developed for the control of pelvic hemorrhage and has been utilized for the primary treatment of leiomyomas since 1995.79 It has been associated with positive clinical outcomes and a high rate of patient satisfaction. Success rates of over 90% have been reported.80,81 Decreases in the size of the uterus and dominant leiomyomas of 45% (from baseline volumes) have been reported.25
Pre-embolization imaging with MRI aids in precisely locating the position of leiomyomas before the procedure. Embolization of the uterine arteries with polyvinyl alcohol or tris-acryl gelatin microspheres involves femoral artery catheterization under fluoroscopic guidance. Typically, UFE is performed with the patient under conscious sedation.31
Postembolization follow-up consists of clinical evaluation as well as a follow-up MRI to assess and monitor the final resulting volume of leiomyomas and the uterus. The degeneration and devascularization of leiomyomas can be visualized on MRI as increased signals on T1– and decreased signals on T2-weighted images.82–84 After UFE, the size of leiomyomas can continue to decrease for 1 year or more. The high rate of patient satisfaction80 is due to the improvements in menorrhagia and pressure symptoms as well as pain relief experienced by a high percentage of women undergoing UFE.
It is important to note that successful pregnancies have occurred after this procedure.85,86 There is a recognized risk of inducing premature ovarian failure in older women. Several studies have shown an increased risk of intrauterine growth restriction and placentation problems in pregnancies after UFE. This procedure is not considered the treatment of choice for symptomatic leiomyomas in patients who desire future fertility.
Complications of UFE include angiographic-related problems,31 allergic reactions,87 perforation of the uterus,88 and infection.83,87 If the collateral blood supply to the ovary is embolized, infertility, amenorrhea, and premature menopause are potential risks.81,89 Sciatic nerve injury leading to buttock claudication is a recognized potential complication of UFE.88 Deep venous thrombosis and pulmonary embolus are rare risks, as is death.88,90,91 The incidence of death with UFE is reported to be 3/10,000 compared with the 1/1000 rate from hysterectomy.92
Postembolization syndrome is a relatively common occurrence and consists of nausea, vomiting, pain, and a temporary increased white blood cell count after the procedure. This syndrome affects most patients to some extent in the first 48 hours after the procedure, but is severe in approximately 15% of those who undergo UFE.93
HIGH-INTENSITY MRI-GUIDED FOCUSED ULTRASOUND THERAPY
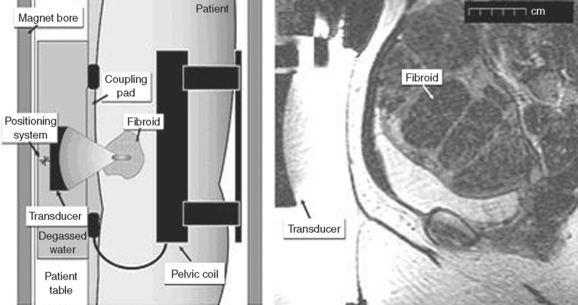
Recent advances in the developing field of therapeutic ultrasound have led to the development of high-intensity focused ultrasound surgery (HIFU). HIFU unites two technologies, therapeutic ultrasound and diagnostic MRI, and provides a combined noninvasive procedure that aims to destroy leiomyoma tissue in a precise and controlled fashion. By placing an ultrasound transducer on the abdomen of a patient and focusing the ultrasound energy at a specific, controllable depth and position, leiomyoma tissue is destroyed within the focal zone (Fig. 46-13). The therapeutic ultrasound effect is monitored by MRI, which precisely records the temperature elevation from the heat generated over time. Once the temperature reaches 57°C for 1 second, tissue is rapidly destroyed within the focal zone. Tissue within 2 to 3 mm of the focal zone is unaffected due to the very precise demarcation between normal and destroyed tissue.
Magnetic resonance imaging-guided focused ultrasound surgery as a therapy for leiomyoma treatment received FDA approval in October 2004. The ExAblate System (InSightec, Dallas) is the first medical device approved for the treatment of leiomyomas as its primary indication. General patient selection criteria include leiomyomas between 4 and 10 cm, maximum depth of subcutaneous tissue to the leiomyoma less than 12 cm, completion of childbearing, premenopausal status, and leiomyomas that can be clearly visualized on MRI. Based on results of a 6-month follow-up study, the mean leiomyoma reduction in volume after HIFU was 13.5 cm3, and the mean volume of nonperfused tissue was 51.2 cm3. Furthermore, 79.3% of patients reported a greater than 10-point reduction in symptom scores and improvement in quality of life measures on the questionnaire used in the study.94 Adverse events included minor skin burns in 4% of patients, worsening menorrhagia in 4% of patients, hospitalization for nausea in only 1% of patients, and nontargeted sonication of the uterine serosa in 1% of patients.94 There are no randomized clinical trials that have compared this technology with surgical or other radiologic treatment.
CRYOMYOLYSIS
Cryomyolysis of uterine leiomyomas has been performed in the past by laparoscopy. In recent years, MRI-guided cryomyolysis has been devised as a less invasive approach. Cryomyolysis involves placement of a 2-cm diameter cryoprobe directly into a uterine leiomyoma. After the cryoprobes are advanced and placed into position, cryomyolysis is then performed by the instillation of cryogenic media. Laparoscopic cryomyolysis for women with leiomyomas as well as a combination of abnormal uterine bleeding, pelvic pain/pressure, and/or urinary frequency was shown to be effective in a study of 20 patients.95
A report of MRI-guided cryomyolysis used to treat leiomyomas in 10 patients showed MRI evidence of marked uterine volume reduction 48 to 334 days after the procedure. The mean volume reduction was 65%. All patients reported improvement of symptoms, whether they were due to bleeding or pressure. One patient had uterine bleeding for 2 months after the procedure, with subsequent spontaneous resolution. Another patient had a residual submucosal leiomyoma that had to be resected hysteroscopically at a later date. Complications included a patient with laceration of a serosal vessel covering a leiomyoma, which required a laparotomy and open myomectomy for repair. Another complication was peroneal nerve involvement and a mild footdrop that resolved over several months. Nausea and mild abdominal discomfort that was relieved by NSAIDs were reported as minor complications.96
Another study of 14 women evaluated the efficacy of 2 months of pretreatment with GnRH agonist before laparoscopically directed cryomyolysis.97 The GnRH agonist was discontinued immediately before the procedure. Four months after cryomyolysis, the follow-up MRI showed a mean volume decrease of 10% among the frozen leiomyomas, whereas other uterine tissue returned to their size before GnRH agonist treatment.
LAPAROSCOPIC UTERINE ARTERY LIGATION
Laparoscopic uterine artery ligation is yet another potential option for women who opt to preserve their uteri. A study comparing this technique to UAE suggests that the uterine volume was slightly reduced at 3 months and stabilized at 6 months, with an average volume reduction of 58.5%.98 The authors concluded that both laparoscopic uterine artery ligation and UAE are reasonable alternatives to hysterectomy.
CONCLUSION
Approximately $5 billion is currently spent annually in the diagnosis and treatment of uterine leiomyomas. This has resulted in a recent commitment to expanded public and industrial research funding for the development of innovative treatment modalities.
1 Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94:435-438.
2 Lepine LA, Hillis SD, Marchbanks PA, et al. Hysterectomy surveillance—United States, 1980–1993. MMWR. 1997;46:1-15.
3 Stovall DW. Clinical symptomatology of uterine leiomyomas. Clin Obstet Gynecol. 2001;44:364-371.
4 Donnez J, Jadoul P. What are the implications of myomas on fertility? A need for debate? Hum Reprod. 2002;17:1424-1430.
5 Bulletti C, De Zieger D, Polli V, Flamigni C. The role of leiomyomas in infertility. J Am Assoc Gynecol Laparosc. 1999;6:441-445.
6 Vercellini P, Maddalena S, De Giorgi O, et al. Determinants of reproductive outcome after abdominal myomectomy for infertility. Fertil Steril. 1999;72:109-114.
7 Dubuisson J-B, Chapron C, Chalet X, et al. Infertility after laparoscopic myomectomy of large intramural myomas: Preliminary results. Hum Reprod. 1996;11:518-522.
8 Coutinho EM, Maia HS. The contractile response of the human uterus, fallopian tunes and ovary to prostaglandins in vivo. Fertil Steril. 1971;22:539-543.
9 Deligdish L, Loewenthal M. Endometrial changes associated with myomata of the uterus. J Clin Pathol. 1970;23:676-679.
10 Farrer-Brown G, Beilby JO, Tarbit MH. Venous changes in the endometrium of myomatous uteri. Obstet Gynecol. 1971;38:743-746.
11 Ng EHY, Ho PC. Doppler ultrasound examination of uterine arteries on the day of oocyte retrieval in patients with uterine fibroids undergoing IVF. Hum Reprod. 2002;17:765-770.
12 Katz VL, Dotters DJ, Droegemueller W. Complications of uterine leiomyomas in pregnancy. Obstet Gynecol. 1989;73:593-596.
13 Eldar-Geva T, Meagher S, Healy DL, et al. Effects of intramural, subserosal and submucosal uterine fibroids on the outcome of assisted reproduction technology treatment. Fertil Steril. 1998;70:687-691.
14 Stovall DW, Parrish SB, Van Voorhis BJ, et al. Uterine leiomyomas reduce the efficacy of assisted reproduction cycles: Results of a matched follow-up study. Hum Reprod. 1998;13:192-197.
15 Hart R, Khalaf Y, Yeong CT, et al. A prospective controlled study of the effect of intramural uterine fibroids on the outcome of assisted conception. Hum Reprod. 2001;16:2411-2417.
16 Oliveira FG, Abdelmassih VG, Diamond MP, et al. Impact of subserosal and intramural uterine fibroids that do not distort the endometrial cavity on the outcome of in vitro fertilization–intracytoplasmic sperm injection. Fertil Steril. 2004;81:582-587.
17 Yarali H, Bukulmez O. The effect of intramural and subserous uterine fibroids on implantation and clinical pregnancy rates in patients having intracytoplasmic sperm injection. Arch Gynecol Obstet. 2002;266:30-33.
18 Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973.
19 Kjerulff HK, Langenberg P, Seidman JD, et al. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J Reprod Med. 1996;41:483-490.
20 Ross RL, Pike MC, Vessey MP, et al. Risk factors for uterine fibroids: Reduced risk associated with oral contraceptives. BMJ (Clin Res Ed). 1986;293:359-362.
21 Treloar SA, Martin NG, Dennerstein L, et al. Pathways to hysterectomy: Insights from longitudinal twin research. Am J Obstet Gynecol. 1992;167:82-88.
22 Leibman AJ, Kruse B, McSweeney MB. Transvaginal sonography: Comparison with transabdominal sonography in the diagnosis of pelvic masses. Am J Radiol. 1988;151:89-92.
23 Stewart EA. Uterine fibroids. Lancet. 2001;357:293-298.
24 Catherino W, Salama A, Potlog-Nahari C, et al. Gene expression studies in leiomyomata: New directions for research. Semin Reprod Med. 2004;22:83-90.
25 Shimomura Y, Matsuo H, Samoto T, Maruo T. Upregulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J Clin Endocrinol Metab. 1998;83:2192-2198.
26 Brandon DD, Betheq CL, Strawn EY, et al. Progesterone receptor messenger ribonucleic acid and protein are over expressed in human uterine leiomyomas. Am J Obstet Gynecol. 1993;169:78-85.
27 Brandon DD, Erickson TE, Keenan EJ, et al. Estrogen receptor gene expression in human uterine leiomyomata. J Clin Endocrinol Metab. 1995;80:1876-1881.
28 Folkerd EJ, Newton CJ, Davidson K, et al. Aromatase activity in uterine leiomyomata. J Steroid Biochem. 1984;20:1195-1200.
29 Bulun SE, Simpson ER, Word RA. Expression of the CYP19 gene and its product aromatase cytochrome p450 in human uterine leiomyoma tissues and cells in culture. J Clin Endocrinol Metab. 1994;78:736-743.
30 Shozu M, Sumitani H, Segawa T, et al. Over expression of aromatase p450 in leiomyoma tissue is driven primarily through promoter 14 of the aromatase p450 gene (CYP19). J Clin Endocrinol Metab. 2002;87:2540-2548.
31 Rein MS, Barbieri RL, Friedman AJ. Progesterone: A critical role in the pathogenesis of uterine myomas. Am J Obstet Gynecol. 1995;172:14-18.
32 Kettel LM, Murphy A, Morales AJ, Yen SS. Clinical efficacy of the antiprogesterone RU486 in the treatment of endometriosis and uterine fibroids. Hum Reprod. 1994;9:116-120.
33 Murphy AA, Kettel L, Morales AJ, et al. Regression of uterine leiomyomata in response to the antiprogesterone RU486. J Clin Endocrinol Metab. 1993;76:513-517.
34 Kawaguchi K, Fujii S, Konishi I, et al. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol. 1989;160:637-641.
35 Matsuo H, Maruo T, Samoto T. Increased expression of Bcl-2 protein in human uterine leiomyoma and its upregulation by progesterone. J Clin Endocrinol Metab. 1997;82:293-299.
36 Martel KM, Ko AC, Christman GM, Stribley JM. Apoptosis in human uterine leiomyomas. Semin Reprod Med. 2004;22:91-103.
37 Karasick S, Lev-Toaff AS, Toaff ME. Imaging of uterine leiomyomas. Am J Radiol. 1992;158:799-805.
38 Zawin M, McCarthy S, Scoutt LM, Comite F. High-field MRI and US evaluation of the pelvis in women with leiomyomas. Magnet Reson Imag. 1990;8:371-376.
39 Weintraub JL, Romano WJ, Kirsch MJ, et al. Uterine artery embolization: Sonographic imaging findings. J Ultrasound Med. 2002;21:633-637.
40 ACOG Technology Assessment. Saline infusion sonography. Obstet Gynecol. 2003;102:659-662.
41 Wamsteker K, Emanuel MH, de Kruif JH. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: Results regarding the degree of intramural extension. Obstet Gynecol. 1993;82:736-740.
42 Jurkovic D. Three-dimensional ultrasound in gynecology: A critical evaluation. Ultrasound Obstet Gynecol. 2002;19:109-117.
43 Fleischer AC. Color Doppler sonography of uterine disorders. Ultrasound Q. 2003;19:179-183.
44 Baramki TA. Hysterosalpingography. Fertil Steril. 2005;83:1595-1606.
45 Mueller GC, Gomez J, Carlos RC. Diagnostic imaging and vascular embolization for uterine leiomyomas. Semin Reprod Med. 2004;22:131-142.
46 Lee JK, Gersell DJ, Balfe DM, et al. The uterus: In vitro MR-anatomic correlation of normal and abnormal specimens. Radiology. 1985;157:175-179.
47 Yamashita Y, Torashima M, Takahashi M, et al. Hyperintense uterine leiomyoma at T2-weighted MR imaging: Differentiation with dynamic enhanced MR imaging and clinical implications. Radiology. 1993;89:721-725.
48 Okizuka H, Sagimura K, Takemori M, et al. MR detection of degenerating uterine leiomyomas. J Comput Assist Tomogr. 1993;17:760-766.
49 Friedman AJ, Label SM, Rein MS, Barbieri RL. Efficacy and safety considerations in women with uterine leiomyomas treated with gonadotropin-releasing hormone agonists: The estrogen threshold hypothesis. Am J Obstet Gynecol. 1990;163:1114-1119.
50 Shozu M, S.H., Segawa T, et al. Inhibition of in situ expression of aromatase p450 in leiomyoma of the uterus by leuprolide acetate. J Clin Endocrinol Metab. 2001;86:5405-5411.
51 De Leo V, Morgante G, La Marca A, et al. A benefit-risk assessment of medical treatment for uterine leiomyomas. Drug Safety. 2002;25:759-779.
52 Stovall TG, Muneyyirici-Delale O, Summitt RL, Scialli AR. GnRH agonist and iron versus placebo and iron in the anemic patient before surgery for leiomyomas: A randomized controlled trial. Leuprolide Acetate Study Group. Obstet Gynecol. 1995;86:65-71.
53 Dawood M, Lewis V, Ramos J. Cortical and trabecular bone mineral content in women with endometriosis: Effect of gonadotropin-releasing hormone agonist and danazol. Fertil Steril. 1998;52:21-26.
54 Carr BR, Marshburn PB, Weatherall PT, et al. An evaluation of the effect of gonadotropin releasing hormone analog and medroxyprogesterone acetate on uterine leiomyoma volume by MRI: A prospective randomized double blind placebo controlled cross over trial. J Clinic Endocrinol Metab. 1993;76:1217-1223.
55 Guetta V, Lush RM, Figg WD, et al. Effects of the antiestrogen tamoxifen on low-density lipoprotein concentrations and oxidation in postmenopausal women. Am J Cardiol. 2005;76:1072-1073.
56 Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. NEJM. 1997;337:1641-1647.
57 Fuchs-Young R, Howe S, Hale L, et al. Inhibition of estrogen-stimulated growth of uterine leiomyomas by selective estrogen receptor modulators. Mol Carcinog. 1996;17:151-159.
58 Walker CL, Burroughs K, Davis B, et al. Preclinical evidence for therapeutic efficacy of selective estrogen receptor modulators for uterine leiomyoma. J Soc Gynecol Invest. 2000;7:249-256.
59 Porter KB, Tsibris JC, Porter GW, et al. Effects of raloxifene in a guinea pig model for leiomyomas. Am J Obstet Gynecol. 1998;179:1283-1287.
60 Palomba S, Sammartino A, Di Carlo C, et al. Effects of raloxifene treatment on uterine leiomyomas in postmenopausal women. Fertil Steril. 2001;76:38-43.
61 Palomba S, Orio F, Morelli M, et al. Raloxifene administration of women treated with gonadotropin-releasing hormone agonist for uterine leiomyomas: Effects on bone metabolism. J Clin Endocrinol Metab. 2002;87:4476-4481.
62 Palomba S, Orio F, Morelli M, et al. Raloxifene administration in premenopausal women with uterine leiomyomas: A pilot study. J Clin Endocrinol Metab. 2002;87:3603-3608.
63 Palomba S, Rosso T, Orio F, et al. Effectiveness of combined GnRH analogue plus raloxifene administration in the treatment of uterine leiomyomas: A prospective, randomized, single-blind, placebo-controlled trial. Hum Reprod. 2002;17:3213-3219.
64 Chwalisz K, De Manno D, Garg R, et al: Therapeutic potential for the selective progesterone receptor modulator asoprisnil in the treatment of leiomyomata. Semin Reprod Med 22:113–9.
65 Elger W, Bartley J, Schneider B, et al. Endocrine-pharmacological characterization of progesterone antagonists and progesterone receptor modulators (PRMs) with respect to PR-agonistic and antagonistic activity. Steroids. 2000;65:713-723.
66 Chwalisz K, Elger W, McCrary K, et al. Reversible suppression of menstruation in normal women irrespective of the effect on ovulation with the novel selective progesterone receptor modulator (SPRM) J867. J Soc Gynecol Invest. 9(Suppl), 2002.
67 Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: A randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15:2663-2668.
68 Wilcox LS, Koonin LM, Pokras R, et al. Hysterectomy in the United States, 1988–1990. Obstet Gynecol. 1994;83:549-555.
69 Ribeiro SC, Ribeiro RM, Santos NC, Pinotti JA. A randomized study of total abdominal, vaginal and laparoscopic hysterectomy. Int J Gynecol Obstet. 2003;83:37-43.
70 Reich H. Laparoscopic hysterectomy. Surgical Laparoscopy and Endoscopy. 1992;2:85-88.
71 ACOG: Surgical Alternatives to Hysterectomy in the Management of Leiomyomas. Compendium of Selected Publications. ACOG Practice Bulletin no.16, May 2000, pp 776–784.
72 Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: A prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654-658.
73 Bulletti C, Polli V, Negrini V, et al. Adhesion formation after laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 1996;3:533-536.
74 Takeuchi H, Kinoshita K. Evaluation of adhesion formation after laparoscopic myomectomy by systematic second-look microlaparoscopy. J Am Assoc Gynecol Laparosc. 2002;9:442-446.
75 Dubuisson JB, Fauconnier A, Chapron C, Kreiker G, Norgaard C. Second look after laparoscopic myomectomy. Hum Reprod. 1998;13:2102-2106.
76 Doridot V, Dubuisson J-B, Chapron C, et al. Recurrence of leiomyomata after laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2001;8:495-500.
77 Nezhat FR, Roemisch M, Nezhat CH, et al. Recurrence rate after laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 1998;5:237-240.
78 Rossetti A, Sizzi O, Soranna L, et al. Long-term results of laparoscopic myomectomy: Recurrence rate in comparison with abdominal myomectomy. Hum Reprod. 2001;16:770-774.
79 Ravina JH, Herbreteau D, Ciraru-Vigneron N, et al. Arterial embolisation to treat uterine myomata. Lancet. 1995;346:671-672.
80 Worthington-Kirsch RL, Popky GL, Hutchins FLJr. Uterine arterial embolization for the management of leiomyomas: Quality-of-life assessment and clinical response. Radiology. 1998;208:625-629.
81 Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for uterine leiomyoma. Obstet Gynecol. 2001;98:29-34.
82 Burn PR, McCall JM, Chinn RJ, et al. Uterine fibroleiomyoma: MR imaging appearances before and after embolization of uterine arteries. Radiology. 2000;214:729-734.
83 deSouza NM, Williams AD. Uterine arterial embolization for leiomyomas: Perfusion and volume changes at MR imaging and relation to clinical outcome. Radiology. 2002;222:367-374.
84 Jha RC, Ascher SM, Imaoka I, Spies JB. Symptomatic fibroleiomyomata: MR imaging of the uterus before and after uterine arterial embolization. Radiology. 2000;217:228-235.
85 Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: Clinical results in 400 women with imaging follow up. BJOG. 2002;109:1262-1272.
86 Watson GM, Walker WJ. Uterine artery embolisation for the treatment of symptomatic fibroids in 114 women: Reduction in size of the fibroids and women’s views of the success of the treatment. BJOG. 2002;109:129-135.
87 Spies JB, Ascher SM, Roth AR, et al. Complications after uterine artery embolization for leiomyomas. Obstet Gynecol. 2002;100:873-880.
88 Goodwin SC, Wong GC. Uterine artery embolization for uterine fibroids: A radiologist’s perspective. Clin Obstet Gynecol. 2001;44:412-424.
89 Chrisman HB, Saker MB, Ryu RK, et al. The impact of uterine fibroid embolization on resumption of menses and ovarian function. J Vasc Intervent Radiol. 2000;11:699-703.
90 Lanocita R, Frigerio L, Patelli G, et al: A fatal complication of percutaneous transcatheter embolization for the treatment of fibroids. Second International Symposium on Embolization of Uterine Myomata/Society for Minimally Invasive Therapy, 11th International Conference, Boston, 1999.
91 de Blok S, de Uries C, Prinssen H, et al. Fatal sepsis after uterine artery embolization with microspheres. J Vasc Intervent Radiol. 2003;14:779-783.
92 Wingo PA, Hunter C, Rubin GL, et al. The mortality risk associated with hysterectomy. Am J Obstet Gynecol. 2000;152:803-808.
93 Hurst BS, S.D., Matthews ML, Marshburn PB. Uterine artery embolization for symptomatic uterine myomas. Fertil Steril. 2000;74:855-869.
94 Stewart EA, Gedroyc W, Tempany C, et al. Focused ultrasound treatment of uterine fibroid tumors: Safety and feasibility of a noninvasive themoablative technique. Am J Obstet Gynecol. 2003;189:48-54.
95 Zupi E, Piredda A, Marconi D, et al. Directed laparoscopic cryomyolysis: A possible alternative to myomectomy and/or hysterectomy for symptomatic leiomyomas. Am J Obstet Gynecol. 2004;190:639-643.
96 Cowan BD. Myomectomy and MRI-directed cryotherapy. Semin Reprod Med. 2004;22:143-148.
97 Zriek TG, Rutherford TJ, Palter SF, et al. Cryomyolysis, a new procedure for the conservative treatment of uterine fibroids. J Am Assoc Gynecol Laparosc. 1998;5:33-38.
98 Park KH, Kim JY, Shin JS, et al. Treatment outcomes of uterine artery embolization and laparoscopic uterine artery ligation for uterine myoma. Yonsei Med J. 2003;44:694-702.