Chapter 25 Osteoporosis
INTRODUCTION
Osteoporosis has reached epidemic proportions, affecting both genders and all races.1,2 In the United States, elderly white women are the at highest risk, and African-American or Hispanic American men may be at lowest risk.3 A 50-year-old white woman has a 40% lifetime risk of fracture and a 14% risk for hip fracture.
The cost of treating fractures is rising.4 Direct costs alone for the treatment of osteoporotic fractures in the United States total greater than $10 billion annually.1,5,6 In a study of over 200,000 postmenopausal women in 34 states in the United States who underwent bone mineral density (BMD) testing using peripheral measurement techniques, almost 40% had low BMD and 7% had osteoporosis.6
Great advances have been made in the past few years in both the diagnosis and treatment of osteoporosis. By the turn of the millennium in the United States, measurement of BMD had become widely available, making the diagnosis of osteoporosis easier. In addition, several biochemical markers of bone turnover allow more precise monitoring of patients on therapy. Newer scoring methods for assessing fracture risk have been developed.7 Lifestyle changes, fall prevention programs, nutritional supplements, and hip pads decrease the risk of fracture.1,8–10 Several medications are currently available that effectively decrease the risk of future fracture.
Unfortunately, despite these advances, studies show that many women age 40 and older have not had the opportunity to discuss management of this disease with their physicians,11 and many patients who suffer from an osteoporotic fracture are not evaluated or treated for their disease.12 Although management of fractures is appropriate, the primary aim for the treating physician should be fracture prevention.
PATHOPHYSIOLOGY
Definition
Osteoporosis is a disease of the skeletal system characterized by decreased bone strength leading to increased susceptibility to fracture.1 Microarchitectural changes result in less bone, which is of poorer quality, thus predisposing the skeleton to fractures that may occur with little or no trauma.1 The first released Surgeon General’s report contains a comprehensive report on osteoporosis.2
Basic Bone Physiology
Early in life, growth and accumulation of skeletal tissue occur. Peak bone mass is reached in adulthood, but the age at which this is achieved varies depending on the skeletal site and measurement techniques used. Currently available evidence suggests this occurs at the hip as soon as the third decade, several years later for the whole body, and maybe even longer in other bones.1,13
Peak bone mass is a major determinant of future osteoporosis risk and optimal achievement is usually multifactorial. Genetic influences account for the majority of variability in BMD and is the most critical determinant of peak bone mass in adulthood.13–16 Daughters of women with osteoporosis, and mothers with hip fractures attain lower peak bone mass than age-matched controls.14,15 Peak bone mass differs among racial groups, with African-American women having the highest BMD and others differing between measured sites and studies.3,17,18 Asian women may have lower BMD than white women but, interestingly, also have lower rates of hip fracture. Differences are likely related to genetic variation between races, local environmental factors, and primarily body weight and bone size.18,19
Basic Mechanisms of Bone Disorders
Uncoupling of bone turnover leading to either increased or decreased formation may result in either net bone gain, as occurs in Paget’s disease of bone, or net bone loss as occurs in postmenopausal women, in primary hyperparathyroidism, and in rheumatoid arthritis.20–23 Most disorders of the skeleton result from excessive osteoclastic activity.20 As a consequence of estrogen deficiency after menopause, there is early and rapid loss of cancellous bone, concomitant with and followed by a more gradual loss of cortical bone. Exogenous estrogen can decrease or prevent this altered skeletal remodeling.24
This bone resorption occurs by two main biochemical pathways: the cathepsin K and the metalloproteinase-dependent pathways. Postmenopausal bone loss is thought to occur mainly via the former.25
Medical Problems Associated with Osteoporosis
Certain medical disorders and medications may significantly impact the attainment of peak bone mass. Malabsorption syndromes, cystic fibrosis, anorexia nervosa, inflammatory arthritis, and other diseases are associated with lower bone mass compared to age-matched controls. Although weight can have a significant impact on the measurement of BMD,18 severely underweight young women have lower BMD and a higher risk of fracture.
Certain medications such as glucocorticoids and phenytoin, vitamin D deficiency, and low calcium diets can also impair skeletal acquisition. Both vitamin D deficiency and low calcium intake are prevalent in our society and increasingly recognized worldwide. Periods of more rapid growth may require higher calcium intake.13,20 Recent evidence suggests that genetic alteration of vitamin D-binding proteins may increase the risk of premenopausal fractures.21
Social Behavior and Bone Mass
Although weight-bearing exercise generally increases BMD, the effects may be short-lived and may return to baseline levels after cessation of exercise regimens.28 Although moderate doses of alcohol have no deleterious effects on BMD, and, in fact, may even be of benefit,29,30 excessive alcohol consumption is associated with lower bone mass and an increased risk of osteoporosis.31 Although not all studies agree, smoking is generally considered to have a deleterious effect on the skeleton and is associated with an increased risk of fracture.24
In young women, eating disorders such as anorexia nervosa and excessive athletic training are associated with loss of BMD or BMD.1,13,26 Young women with anorexia nervosa may have irreversible skeletal changes and have a significantly higher risk of fracture.26 The female athlete triad of intense exercise regimens, with resultant loss of critical body fat and subsequent hypothalamic amenorrhea and low bone mass, are important elements to consider in young active women. This triad is frequently associated with eating disorders that may be further detrimental to skeletal health.
Female athletes weighing less than 50kg have a higher incidence of amenorrhea than those weighing more than 60kg and amenorrheic female athletes have significantly lower BMD than controls and a higher number of fractures. These women may present with stress fractures as the first sign of their disease; thus, every woman with a stress fracture should have a careful dietary, exercise, and menstrual history taken. Importantly, these women may not achieve their peak bone mass if undetected. Bone loss in these young women is likely multifactorial.13,26
Hormonal Influence on Bone Mass
Estrogen
At the cellular level there are two main cell types responsible for the maintenance of skeletal homeostasis: osteoclasts and osteoblasts, influenced by many regulatory genes; several hormones, particularly estrogen, testosterone, parathyroid hormone, and vitamin D; and a variety of cytokines.20,24,32 Estrogen increases osteoclast apoptosis, but its role in osteoblastic function is not fully understood.24
Estrogen decreases osteoclast activity and bone resorption by modulating the production of two essential cytokines that regulate osteoclast number and activity, and possibly osteoblast function as well.20 Estrogen inhibits production of tumor necrosis factor α (TNF-α)33 but increases production of osteoprotegerin (OPG).24 TNF-α promotes bone resorption by decreasing osteoblast activity and directly inducing the differentiation of osteoclast progenitors into mature osteoclasts. TNF-α also appears to induce osteoblastic cells to stimulate osteoclastic bone resorption. Thus, estrogen decreases bone resorption by inhibiting TNF-α production.
In postmenopausal women estrogen therapy inhibits TNF-α release in a dose-dependent manner from peripheral blood mononuclear cells34 and markedly reduces TNF-α mRNA expression in bone biopsy specimens.35,36
In murine models, blockade of TNF-α is not detrimental to skeletal maturation.37 Mice deficient in the p55 TNF receptor are protected against ovariectomy-induced bone loss.38 Surgically induced estrogen deficiency in mice causes significant bone loss, which is mediated via the p55 TNF receptor and can be prevented by blockade of TNF-α.23,37
Mice with excess OPG exhibit increased osteoclastic activity and an osteopetrotic phenotype,39 whereas mice deficient in OPG suffer severe osteoporosis.40 Conversely, OPG protects TNF transgenic mice from generalized bone loss.41 1,25-OH active vitamin D potentiates the action of OPG.
Androgens
Androgens also play a critical role in skeletal development, maturation, and preservation. Differences in estrogen and testosterone production and in tissue sensitivity to these hormones probably account for most of the skeletal differences seen between men and women. At the present time it appears that both are essential for bone health in women and men. Sources of testosterone in women arise mainly from conversion in peripheral tissues but also from central production in small amounts in the ovaries and adrenal glands, whereas in men 95% is derived from testicular secretion, and production of the various isoforms is highly tissue specific. More detailed explanations of the production and removal of these hormones can be found elsewhere in the book.
The precise molecular pathways in bone resulting from androgenic stimulation are less well-described than for estrogens. Many of the effects of androgens on skeletal tissue may be mediated by increased production of transforming growth factor (TGF)-β and decreased production of interleukin-6.24,42 Studies in female rats have shown that blockade of the androgen receptor may result in significant bone loss, and in men androgen deprivation results in bone loss.43,44 The remainder of this discussion applies mainly to female bone health.
Pregnancy and the Postpartum Period
Significant bone loss occurs during pregnancy, as assessed by BMD testing.45,46 Although earlier reports have suggested that heparin use in pregnancy may result in significant bone loss during pregnancy, a recent randomized trial showed treatment with low molecular weight heparin throughout the course of pregnancy did not result in significantly more bone loss in this study of women with recurrent miscarriages compared to individuals who used only aspirin throughout the course of their pregnancy.49 Breastfeeding in the postpartum period can further exacerbate bone loss, but a recent study shows that the effect may not be sustained, with the majority of women returning to baseline within a year after the birth of their child.46
Age and Postmenopausal Status
With aging, there is an alteration in the rates of bone formation and destruction where the process switches from one of gradual net gain to one of loss. This loss along with microarchitectural deterioration results in significant compromise of bone strength.47 More dramatic changes occur most noticeably during and after menopause. (In contrast, bone loss in men tends to be more constant over time.) Postmenopausal bone loss is biphasic in women (who are not on antiremodeling therapy), with an initial rapid bone loss in the first few years of menopause followed by a more gradual decline in later years.48
Women with higher levels of bone markers lose more bone and have a higher rate of fracture than women with normal or low levels, even after adjustment for BMD and hormonal status. However, postmenopausal women with high bone turnover markers and low estradiol levels have an even higher rate of fracture.48–50
Estrogen therapy preserves BMD and suppresses bone turnover markers, but discontinuation leads to a rapid increase in bone turnover markers and significant bone loss similar to that seen in recently postmenopausal women but greater than age-matched controls.51 Women undergoing surgical menopause may be at higher risk for short-term bone loss compared to women experiencing natural menopause.48
Hyperparathyroidism
Secondary hyperparathyroidism, impaired vitamin D metabolism, and vitamin D and calcium deficiency are also critical elements in age-related bone loss, and their importance and prevalence has been increasingly recognized. Calcium absorption varies among individuals and is influenced by many factors, primarily vitamin D.13,52 Decreased ability to absorb calcium is associated with increased risk of fracture in elderly women.53 Primary osteoporosis is associated with decreased calcium absorption, lower vitamin D levels, and compensatory increases in parathyroid hormone levels.24,54
Vitamin D Deficiency
Vitamin D increases absorption of calcium and phosphorus from the gut, increases their reabsorption from the kidney, and decreases skeletal mobilization of calcium. Deficiency of vitamin D impairs absorption of calcium, which is counteracted by increased parathyroid hormone levels and osteoclastic activity to resorb skeletal tissue to maintain serum calcium level.13,52,54
Vitamin D deficiency is prevalent in elderly women with hip fractures,55 due to decreased skin conversion, decreased sun exposure, and low dietary intake.52
CLASSIFICATION AND GENERAL PRINCIPLES OF INVESTIGATION
Secondary osteoporosis should be considered whenever there is any clinical suspicion of a disorder known to affect bone or calcium metabolism. A careful history, including a list of medications both past and present, is essential given the extensive list of diseases and treatments associated with or known to cause low bone mass or bone loss (Table 25-1).
Table 25-1 Common Associations or Secondary Causes of Low Bone Mass and Secondary Causes of Osteoporosis56–59,61,74
| Category | Specific Examples |
|---|---|
| Medications |
Previous studies have shown that up to 70% of patients with osteoporosis may have other diagnoses contributing to low bone mass.56,57 Secondary causes of low bone mass may be more prevalent in perimenopausal women and in men, although studies are limited on the prevalence of such disorders in postmenopausal women with low bone mass.1,58 An evaluation of possible causes of low bone mass should be undertaken in anyone for whom this is suspected. It is generally recommended that at least basic chemistries are obtained, including complete blood count, thyrotropin level, 24-hour urine for calcium excretion, intact parathyroid hormone level, and a 25-hydroxyvitamin D3 level in all patients.56,59,60
Glucocorticoid-induced Osteoporosis
Glucocorticoid-induced osteoporosis is a distinct form of secondary osteoporosis. Although an extensive list of medications can both directly and indirectly affect the skeleton, glucocorticoid-induced osteoporosis is the most common drug-induced metabolic bone disease.61,62 Glucocorticoids are often prescribed in both young and older individuals for treatment of medical conditions, such as rheumatoid arthritis, asthma, or other chronic obstructive pulmonary disease. Vertebral fractures will develop in 30% to 50% of patients taking chronic glucocorticoids, and patients have an increased risk of fracture at any site compared to nonusers. The risk of fracture is related to the daily dose, duration of therapy, and older age.63
Acute corticosteroid excess leads to decreased calcium absorption from the gut, a marked early increase in bone resorption, and decrease in bone formation with resultant increased excretion of urinary calcium. There can be very significant bone loss during this initial phase. Chronic use leads to other changes, including hypogonadism, resulting in a generalized suppression of skeletal remodeling.62 The American College of Rheumatology has published guidelines for the management of patients with glucocorticoid-induced osteoporosis.63
CLINICAL EVALUATION
History
Osteoporosis is generally a painless and symptom-free disease until fractures occur. Fractures may be very painful or painless, with up to two thirds of vertebral fractures being clinically silent.64 Loss of height is the most common finding in women with osteoporosis. History taking should include questions about height loss, back pain, dietary calcium intake, eating disorder history, age of menarche and menopause, and cycle history during menstrual years. Other risk factors for low bone mass should also be evaluated, including major illnesses, medications (particularly glucocorticoid use), a history of prolonged amenorrhea, and risk factors for fracture such as poor vision, recent falls, and findings of risk on a fall risk assessment.
Diagnostic Imaging
Any history of a fragility fracture should immediately alert the physician to consider a diagnosis of osteoporosis. Although microarchitectural changes seen on bone biopsy specimens definitively diagnose osteoporosis, this is not a practical approach for most women. The need for noninvasive methods to measure bone strength has led to the development of dual energy absorptiometry (DXA) techniques that measure BMD of the skeleton. BMD accounts for the majority of bone strength. Studies show that measurements obtained by this technique have a strong linear correlation with fracture risk.6,65,66 Additional factors influencing bone strength include bone size and quality. Osteoporosis may be diagnosed with findings of low BMD on DXA in postmenopausal women. Lastly, newer methods for assessing fracture risk and bone strength continue to be developed, with exciting techniques such as virtual bone biopsy using microcomputed tomography or magnetic resonance imaging appearing very promising.
Dual Energy Absorptiometry
The advent of DXA has revolutionized our ability to quickly and accurately measure bone mass noninvasively. Results are expressed in grams per centimeter squared and as T- and Z-scores, which are expressions of BMD in standard deviations compared to a normally distributed reference population. The T-score compares the results to young, healthy white women at peak bone mass, whereas the Z-score compares subjects to age-matched, and usually sex-matched and racially matched, controls (depending on the DXA machine used). A strong linear relationship exists between BMD and fracture risk, such that for each standard deviation below 1 there is an approximately doubling of fracture risk.1,6,65,66
A low BMD is the best individual predictor of future fracture risk in postmenopausal women without prior fracture.6 Women with low BMD and previous fractures have a much higher risk of future fracture.7,64 The addition of age66 and other risk factors such as estrogen deficiency greatly increases the utility of BMD testing to predict fracture risk.7,65 Although World Health Organization criteria can be applied to BMD measurements to diagnose osteoporosis,67 they reflect a consensus of expert opinion, and significant problems exist using such a method as the sole means of diagnosing osteoporosis68 (Table 25-2).
Table 25-2 World Health Organization Diagnosis of Osteoporosis in White Women Using Dual Energy Absorptiometry67,68
| Bone Mineral Density T-Score | World Health Organization Classification |
|---|---|
| ≥ −1.0 | Normal |
| −1.1 to −2.4 | Osteopenia |
| ≤ −2.5 | Osteoporosis |
| ≤ −2.5 and the presence of a fragility fracture | Severe (established) osteoporosis |
Although these criteria may be used to identify individuals at increased risk for fracture, they are not a panacea for either diagnosis or treatment of this disease. First, most fractures occur in individuals who do not have osteoporosis using these criteria. Second, these criteria were established for use with central DXA technology; their application to other modalities used to measure BMD remains unclear. Third, they were intended for use primarily in postmenopausal white women; their applicability to other populations such as premenopausal women is less clear. Some of these shortcomings were evident in a recent very large study of postmenopausal women in the United States, in which Asian and Hispanic women had significantly lower BMD at one measured site than white women but a similar risk of fracture.6 Finally, other fracture risk factors may be significant, such as age or prior fractures, and the addition of such factors to fracture risk assessment greatly increases the prediction of future fracture risk.7,65,66,68 (Table 25-3).
Table 25-3 Risk Factors for Fracture from Highest to Lowest Risk
| Prior fragility fracture |
| Low bone density (particularly T-score ≤ − 1.7)4 |
| Maternal history of osteoporotic fracture |
| Low body mass index |
| Smoking history |
As a result, attempts have been made to clarify how to best use DXA technology to diagnose osteoporosis in different populations and using different technologies,69 and also how to predict increased fracture risk.7,65 In conclusion, although a useful measure for predicting increased fracture risk, BMD measurement is only one assessment tool for such predictions, and an overall clinical assessment of an individual’s fracture risk should be used when assessing whether a woman is likely to sustain an osteoporotic fracture. A T-score of ≤ –1.7 appears to be an important cutpoint for fracture risk.4
Because osteoporosis is an asymptomatic disease (until a fracture occurs), screening with DXA should be considered in certain individuals, such as postmenopausal women with additional risk factors for osteoporosis. Presently the International Society for Clinical Densitometry recommends BMD testing for the following groups of people:69
Although T-scores can be used to diagnose osteoporosis with central DXA technology, there are clarifications and important exceptions to this outlined in the recent International Society for Clinical Densitometry consensus statement:69
Although peripheral DXA is not as accurate as central DXA and has significantly less precision, peripheral studies are valuable screening tests given their ease of use, and they do predict fracture risk.6,65,66 Individuals with low BMD using peripheral devices should be referred for central DXA of the hip and spine, which remains the gold standard for measuring BMD. Monitoring of therapy should be performed by central DXA only. Lastly, discordance has been noted between measurements of BMD at different sites, where there may be significant differences between hips or the hip and spine readings.
Fracture risk prediction is most accurate for the actual site assessed by BMD, and the lowest International Society for Clinical Densitometry recommended site should be used for diagnosis.70 Currently, the International Society for Clinical Densitometry does not recommend using Ward’s area or individual vertebra for this assessment.67
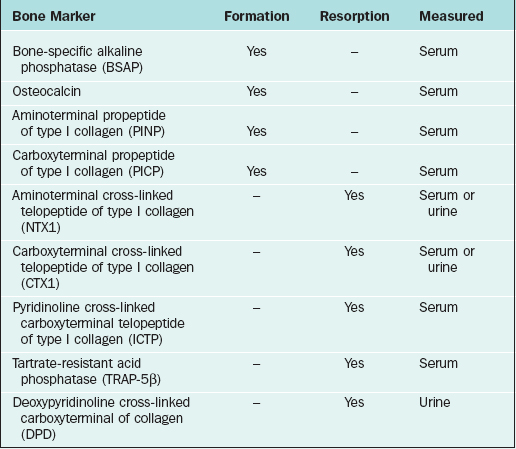
Biochemical Markers of Bone Turnover
Among the more widely available resorption tests are serum and urine aminoterminal cross-linked telopeptide of collagen and carboxyterminal cross-linked telopeptide of collagen, which should be performed after an overnight fast. Formation tests include serum bone-specific alkaline phosphatase and osteocalcin (Table 25-4). Newer assays are cheaper, easier to run, and provide more reliable measurements with markedly better performance characteristics than their predecessors.
Bone turnover markers tend to respond significantly faster to treatment for osteoporosis than BMD, making their measurement particularly useful in monitoring therapy. With certain medications, such as the oral bisphosphonates, significant changes can occur within several weeks of starting therapy.71–73
Many diseases and medications increase a woman’s risk for developing osteoporosis (see Table 25-1). Routine laboratory studies are necessary to look for metabolic abnormalities or diagnoses and to ensure normal renal and calcium metabolism before prescribing therapy (Table 25-5).
Table 25-5 Recommended Baseline Testing for Occult Causes of Low Bone Mass or Secondary Causes of Bone Loss
| Complete chemistry (including calcium, phosphorus, creatinine, and bicarbonate levels and liver function tests) |
| Complete blood count |
| 24-hour urine collection for calcium excretion |
| Intact parathyroid hormone |
| 25-hydroxyvitamin D level |
| Thyrotropin |
Vitamin D levels in the lower limit of normal warrant replacement until the level is normalized.43,74 More profound vitamin D deficiency and osteomalacia is uncommon, but an elevated alkaline phosphatase level or increased parathyroid hormone level should prompt further evaluation of vitamin D metabolism, especially in the presence of normal liver function tests. Parathyroid hormone levels may be mildly elevated in the face of vitamin D deficiency and should return to normal with correction.54
Other Diagnostic Modalities
Bone Biopsy
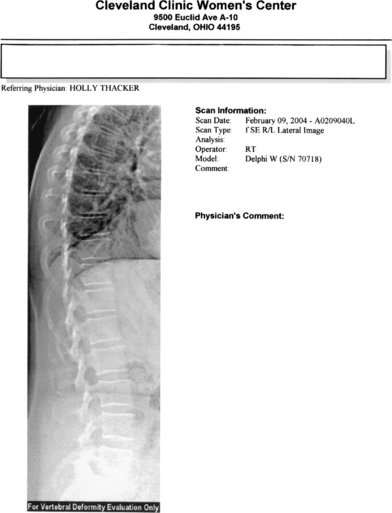
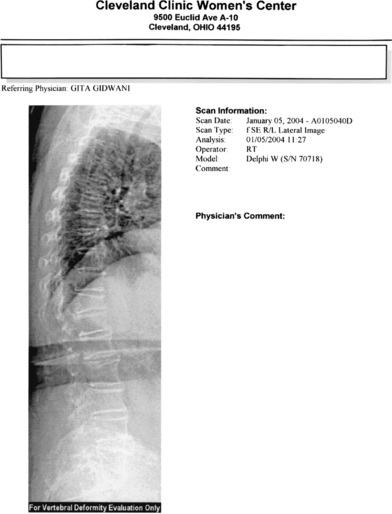
Bone biopsy is usually unnecessary, with a few noteworthy exceptions where there may be doubt about the diagnosis, such as in renal osteodystrophy. Most of the newer DXA technologies now come with the point-of-service imaging of the thoracic and lumbar spine. The procedure, such as lateral vertebral assessment (Figs. 25-1 and 25-2), can be used to image the spine for fractures quickly and easily at significantly lower cost and radiation dose than conventional imaging techniques.75 This is particularly useful for decision-making, with spine fractures often being asymptomatic, and the presence of a fracture being the single best predictor of future fractures.
Prevalent spine fractures are associated with a fivefold increase in the risk of fracture over the next 12 months.64 In the presence of fragility fractures where the T-score does not necessarily meet the World Health Organization BMD criteria for osteoporosis, treatment for osteoporosis is warranted. Plain x-rays and review of old x-ray films may also be useful in certain instances.
Despite the ease of diagnosis, most published studies show that persons who fracture or are at high risk of fracture are often not evaluated or treated for their disease. A recent study shows that an alarmingly small percentage of women with hip fractures undergo formal BMD testing or treatment for their disease.12 In another study of more than 62,000 women older than age 50 in England, 3.2% of whom were taking glucocorticoids, less than half of those on steroids were on fracture prevention therapy, and this number declined to less than one third in the oldest age group with the highest number of fractures.76
REDUCTION OF FRACTURE RISK AND TREATMENT OF OSTEOPOROSIS
Once a woman is diagnosed with osteoporosis and has undergone an evaluation of their disease status, attention is then focused on instituting appropriate therapy. Treatment for established osteoporosis involves a comprehensive assessment of the woman’s modifiable and nonmodifiable risk factors and associated conditions to provide the most appropriate therapeutic options. Appropriate pharmacologic intervention should then be instituted.
Nonpharmacologic Intervention for Fracture Reduction
A multifactorial approach to fall prevention is effective.8 Regular weight-bearing exercise has been shown to increase or preserve BMD in young, middle-aged, and elderly individuals in numerous studies9,77,78 and also increases muscle tone, helping prevent falls. Hip protectors, if worn by the patient, have been shown to prevent hip fractures in patients who are at significant risk of falling.10 Smokers have lower hip BMD than nonsmokers,79 and smoking cessation may have favorable effects on bone turnover markers.80 Alcohol in moderation may have a positive effect on BMD, but excessive alcohol use should be discouraged given its association with lower bone mass and increased risk of fracture.
Calcium and Vitamin D
Both calcium and vitamin D stabilize BMD, at least temporarily,81–83 have favorable effects on biochemical markers of bone turnover,84,85 and reduce the risk of fracture.83,86 Chapuy and colleagues, in a large randomized, placebo-controlled trial of more than 3000 women with osteoporosis, have demonstrated that a combination of calcium and vitamin D significantly reduced the risk of vertebral, nonvertebral, and hip fractures.86 This may, in part, be explained by the observation that additional supplementation with vitamin D has been shown to significantly reduce fall risk in elderly women compared to calcium supplementation alone.87 Stabilization of bone mass appears to be mediated through the reversal of secondary hyperparathyroidism seen in chronic vitamin D deficiency states.83
Pharmacologic Therapy
Therapies such as systemic estrogen therapy, selective estrogen receptor modulators (SERMs), and bisphosphonates have been shown to preserve BMD, decrease markers of bone turnover, and reduce the risk of fracture in postmenopausal women. The American College of Obstetrics and Gynecology recommends treatment of osteoporosis in all postmenopausal women with fragility fractures and postmenopausal women with central DXA T-scores <−2, or T-scores <−1.5 who have additional risk factors, and recommends FDA-approved therapies proven to be safe and effective.88
Risk Factors for Pharmacologic Therapy
In selecting pharmacologic treatment, the clinician should should also screen the woman for other risk factors that may affect choice of therapy. Avoiding use of estrogen or estrogen-like therapies in those women with a personal history or strong family history of thromboembolic phenomena may be prudent.89,90 Breast cancer risk assessment by history and Gail model predictors may lead the clinician to the choice of a SERM such asraloxifene (if there are no menopausal symptoms). Similarly, raloxifene should be avoided in persons with a history or significant risk factors for venous thromboembolism.91
Bisphosphonates should not be used in persons whose creatinine clearance is less than 35mL/minute, and alendronate should be avoided in persons with a history or suggestive symptoms of peptic ulcer disease, gastroesophageal reflux, or esophageal stricture.92 Teriparatide should not be used in persons with hyperparathyroidism, hypercalcemia, those with malignancy or a significant history of radiation treatment (such as radiation for breast cancer), or persons with Paget’s disease of bone.91
Estrogen Therapy
Estrogen therapy has been considered the mainstay of osteoporosis therapy for postmenopausal women, especially in terms of prevention. Much of the rationale for this emanated from work showing that estrogen deficiency leads to accelerated bone loss and that elderly women with the lowest estrogen levels have the highest risk of fracture.93,94 Hormone therapy (HT) increases BMD,95 slows bone loss,94–96 decreases skeletal turnover,94,97 and prevents both vertebral, nonvertebral, and hip fractures.94,96,98–100 Discontinuation of estrogen therapy leads to further loss of bone.51,95–105
Media attention to the results from the Women’s Health Initiative (WHI) study have raised concern as to the overall benefit-to-risk ratio of HT in a large population of late postmenopausal women who were not risk stratified as to a general preventive therapy. Although there was an increased risk of stroke, deep venous thrombosis, gallbladder diseases, and breast cancer diagnosis in those older women taking combined estrogen/progestin therapy, the WHI also showed that HT reduced the risk of hip fracture by 50% as well as the risk of colon cancer.90 The estrogen-only arm of the WHI showed that there was a similar increased risk of nonfatal stroke but no increased risk of breast cancer or cardiovascular disease, and fracture reduction was seen at all sites.89 Although data from the recent WHI have shown that treating all postmenopausal women with estrogen for osteoporosis prevention is not prudent, it remains unclear whether lower doses or alternative bone preparations are safer.
In premenopausal women with the athletic triad and eating disorders, hormonal contraceptive therapy may be of benefit.26,102 Treatment that includes nutritional supplementation, counseling, calcium and vitamin D supplementation, and hormonal contraceptives can be beneficial.13,26,102–104 The benefits of estrogen alone on BMD are unclear in women with eating disorders, with some studies showing little or no benefit.26,104 A more detailed review of the risks and benefits of estrogen therapy in premenopausal and postmenopausal women is outside the scope of this chapter.
However, the International Menopause Society Position Statement 2004 asserts that HT remains a principal tool for preventing postmenopausal bone wasting, fracture, and loss of connective tissue, as well as effects on quality of life for symptomatic women.
Selective Estrogen Receptor Modulators (SERMs)
SERMs are pharmacologic compounds that have estrogen-like effects in certain tissues. This is an area of intense investigation by the pharmaceutical industry as they attempt to provide “designer” therapies that can be targeted to individuals with different disease risk factors. Raloxifene was approved by the U.S. FDA in 1997 for the prevention and treatment of osteoporosis at an oral dose of 60mg daily.100 The results of the MORE trial, a large, multicenter, randomized, placebo-controlled trial, showed that treatment with raloxifene preserved BMD,105 reduced bone turnover,85,100 and reduced the risk of vertebral fracture.105 Of concern, the study failed to show a significant reduction in nonvertebral fractures.
Although the compound may be well tolerated by many postmenopausal women and appears to reduce the risk of estrogen receptor breast cancer in women with osteoporosis, it can increase the chances of hot flashes and leg cramps, and has a similar increase in the risk of venous thromboembolism as estrogen therapy.100
Bisphosphonates
Bisphosphonates have been used to treat osteoporosis for more than a decade. Originating from pyrophosphates used to remove scale from calcified industrial pipes and plaque from teeth, these antiresorptive drugs are the most widely prescribed class of compounds for the treatment of osteoporosis in the United States.106 Recent large randomized trials have clearly shown that both alendronate (Fosamax) and risedronate (Actonel) maintain or improve BMD, reduce markers of bone turnover,107,108 and significantly reduce the risk of vertebral, nonvertebral, and hip fracture.81,82,84,109–112 Both alendronate and risedronate have been shown, in large well-designed trials, to preserve BMD and to significantly reduce the risk of fractures in glucocorticoid-induced osteoporosis, with risedronate having FDA approval for the prevention of glucocorticoid-induced osteoporosis.112,113
Alendronate appears to have a significant post-treatment suppression of bone turnover.114 One can envision that this may be a significant advantage if it can be shown that a significant antifracture effect persists and that this could be achieved with drug holidays with potentially significant savings to patients. Taking such a compound after menopause for several years may thus be all that is needed to prevent fractures for a significant amount of time in the future. Alternatively, if there are significant benefits to treatment with anabolic agents over bisphosphonates, if prolonged suppression of skeletal turnover may blunt potential benefits from therapy, or if significant long-term side effects emerge, persistent drug effects may be very problematic. So far 10 years of treatment appears to be safe, but clearly further studies are needed to provide definitive answers to these questions and more.
Alendronate may have greater potential for upper gastrointestinal adverse events than risedronate115 (which may be better tolerated in people who are otherwise intolerant of other bisphosphonates).116 Both medications are available in several doses and dosing schedules: alendronate tablets are available in 5mg and 10mg daily and 35mg and 70mg weekly dosing preparations, and an oral liquid preparation. Risedronate is available in a 5-mg daily tablet and a once-weekly 35-mg tablet. Bisphosphonates should be taken on an empty stomach 30 to 40 minutes before ingestion of food, liquids other than water, or other medications to maximize their poor oral absorption. Patients should be instructed to take the medication with 6 to 8 ounces of plain tap water and to remain in an upright position for at least 60 minutes after taking the medication to decrease the chances of pill esophagitis.92,117
Several other bisphosphonates are available in the United States. Etidronate was the first commercially available bisphosphonate and has been shown to preserve BMD and reduce the risk of fracture118 but is still not approved by the U.S. FDA for treatment of osteoporosis.119 The usual dose is two 200-mg tablets to be taken for 14 days every 3 months. Given its narrow therapeutic index, the cumbersome nature of having to remember this regimen, and the advent of newer, more potent, second- and third-generation bisphosphonates, etidronate use has largely been surpassed. In 2003, ibandronate received FDA approval for the treatment of osteoporosis at an oral dose of 2.5mg daily. It produces favorable BMD and biochemical marker changes and reduces the risk of vertebral fractures but thus far has not been shown to reduce the risk of nonvertebral fractures.120 Intravenous zoledronic acid, at varying doses and dosage intervals, has been shown to markedly reduce bone turnover markers and produces similar increases in BMD as other bisphosphonates in postmenopausal women.121 Although zoledronic acid is presently only approved for treatment of bone metastases, some preliminary work shows that it might be a useful alternative in patients who cannot otherwise tolerate oral bisphosphonates122; however, reports of maxillary osteonecrosis associated with regular use are a source of concern. Similarly intravenous pamidronate infusions have been shown to have similar efficacy in reducing markers of bone turnover and maintaining or improving BMD in patients with osteoporosis as other bisphosphonates.123,124
Calcitonin
Calcitonin is FDA approved for the treatment of postmenopausal osteoporosis in women. Older preparations of calcitonin derived from salmon and injected subcutaneously often resulted in local hypersensitivity reactions and sometimes antibody formation to the protein, making this a less favorable approach. In 1995, the recombinant form of calcitonin received approval as a nasal spray. Results of a large randomized trial, the PROOF study, have since been published with disappointing results. The attrition rate during the trial was large, and only the 200 IU dose produced a statistically significant reduction in vertebral fractures. There was no significant decrease in nonvertebral fractures.125
Parathyroid Hormone
Parathyroid hormone preparations are an exciting class of compounds and the main class of anabolic agents currently available for treatment of osteoporosis. Only the 1-34 truncated version of teriparatide (Forteo) is FDA approved. In 2002, it was approved for the treatment of postmenopausal osteoporosis (and in men with primary or hypogonadal osteoporosis).91 A large clinical trial showed that individuals who had an average of 18 months of therapy with either 20 or 40μg of teriparatide by daily subcutaneous injection had significant increases in their BMD, significant increases in levels of biochemical markers of bone turnover, and significant reductions in the incidence of both vertebral and nonvertebral fractures.126
Parathyroid hormone has also been shown to prevent bone loss associated with GnRH antagonist use.101 Side effects include injection site reactions and mild transient hypercalcemia. Patients need to be cautioned about the significant increase in the risk of osteosarcoma in rats treated with high doses of teriparatide for long periods, although it is not clear how this risk pertains to humans at this time.91 Reports of osteosarcoma in subjects with chronic hyperparathyroidism are exceedingly rare, with only four cases reported in the literature.127
Combination Therapy
Combination therapy is an intriguing option for osteoporosis. Although combination therapies have shown promise with greater changes in BMD and bone turnover markers in some trials, no large published clinical trial to date has shown any combination to have a significant fracture reduction over monotherapy.54,128
A therapeutic protocol that decreases bone loss and increases bone formation would be ideal and may be possible with combinations of testosterone and estrogen.129 Addition of androgens to estrogen therapy in women may have more favorable skeletal responses than estrogen alone.129,130 Precisely which combination would be most beneficial in treating persons with hypogonadal bone loss remains unclear at this time, and larger, long-term studies need to be done.
Estrogen combined with alendronate and estrogen combined with risedronate have additive reductions in bone turnover markers, with improved BMD changes compared to either agent alone.107 What has become an intriguing question is in what combinations and what sequence these medications should be prescribed and the need for evaluation, although in general combination therapy is discouraged based on expense. Some studies show that there may be a delay or blunting of the anabolic effects of parathyroid hormone in individuals who were pretreated with alendronate.93,101 The clinical significance of this remains unclear at this time. However, it may be prudent to use teriparatide first, based on this preliminary evidence, followed by an oral bisphosphonate.
Several novel therapies for osteoporosis are presently being evaluated. Although high-dose statins have been shown to have significant anabolic skeletal effects in laboratory animals,131 they have not been shown to improve BMD or decrease fracture risk in humans at usual oral doses.132 A recent randomized, placebo-controlled trial with simvastatin in postmenopausal women failed to show significant effects on BMD or markers of bone turnover.133 A recent large trial showed that daily oral strontium increases BMD, decreases bone resorption, and increases bone formation, making it the first “dual-action” compound, and reduced by half the risk of vertebral fracture in postmenopausal women.134 Recent trials on human subjects with low bone mass show significant promise with a recombinant injectable form of OPG [results reported at American Society of Bone and Mineral Research—ASBMR, Minneapolis 2003]. Although fluoride was once considered a promising anabolic agent that significantly increased BMD,135 more recent studies show that despite significant changes in bone mass, there was no fracture benefit and possibly even an increase in fractures, in subjects treated with fluoride and calcium compared to placebo.136
Treatment of Glucocorticoid-induced Osteoporosis
For patients who are already receiving long-term therapy, they recommend similar measures and institution of a bisphosphonate if the patient has a low T-score (< −1.0). Again caution is advised with the use of bisphosphonates in premenopausal women based on pregnancy and fetal concerns. Because many patients receiving chronic glucocorticoids may become hypogonadal, evaluation and hormonal replacement if deficient is also recommended. Calcitonin may be used in individuals intolerant of bisphosphonate therapy.63
Patient Monitoring
Despite numerous trials showing the efficacy of these treatments, studies show that greater than 50% of patients who are prescribed osteoporosis therapy do not fill their first prescription and of those who do, many discontinue them within the first year.137 Regular follow-up coupled with testing of biochemical markers of bone turnover may be useful interventions to improve adherence.138 Changes in bone turnover markers seen with osteoporosis therapy occur early with antiremodeling therapy,108 but may be slower for SERMs,85 and may explain a greater part of the fracture risk reduction with therapy than changes in BMD.85,108,139 However, given the significant variability in assays, they cannot be recommended as the sole means of monitoring patients today.
Follow-up BMD testing should be performed at regular intervals every 2 years to make sure that there is no further bone loss. It is important to understand the concept of least significant change when monitoring therapy with BMD testing. This is a measure, calibrated regularly for each individual DXA machine, a change greater than which is clinically significant. Although this usually is approximately 3% in the best centers, it is extremely important to note that this may vary considerably between skeletal sites, machines, and technicians and can only be accurately assessed by performing the necessary calibration for each individual BMD machine and center.140 Knowing the least significant change is important when interpreting serial bone density scans. Annual rates of bone loss in untreated postmenopausal women vary between 0.6% to 1.7% but may be as high as 4%.86
Parathyroid hormone preparations have shown the greatest annual gains in BMD (2% to 5% at the hip and 5% to 10% at the spine) followed by estrogen or bisphosphonates (1% to 4% at the hip, 2% to 5% at the lumbar spine) followed by raloxifene (0 to 1% at the hip and 0.5% to 2% at the spine).51,81,82,84,95,101,105,107,109,112,126 Stabilization or gain of bone mass is considered a therapeutic success. The magnitude of BMD gain does not explain all the fracture risk reductions. If there is significant loss of bone mass on therapy, adherence should be reviewed, and this may also be an indication to consider other secondary causes of low bone mass.69
Investigational Treatment Approaches
Phytoestrogens
Phytoestrogens are naturally occurring compounds that have estrogen-like activity. Many are derivatives of soy protein products, may be even more potent than currently available estrogen preparations or SERMs, and are currently under scrutiny and intense investigation. Two recently published randomized trials using isoflavone treatment in postmenopausal women revealed conflicting results for significant BMD changes after 1 year of therapy.141,142 Longer treatment periods may be needed to see significant differences in BMD with certain treatments.
Dehydroepiandrosterone
Dehydroepiandrosterone (DHEA), currently available over the counter in the United States, may have potential, showing preservation of BMD in treated patients with systemic lupus erythematosus.143 The addition of DHEA to other treatments is under investigation.144
Growth Hormone
Growth hormone preparations also appear promising in some studies.145 Lastly, several small trials have shown favorable results of long-term treatment with the SERM tibolone as an estrogen alternative.146,147 However, until large rigorous scientific trials show that specific compounds are safe and effectively reduce vertebral and nonvertebral fracture risk, patients should not be advised to consider their use for the treatment or prevention of osteoporosis.
1 NIH Consensus Development Conference. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
2 U.S. Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Issued 14. Available at: http://www.hhs.gov/surgeongeneral/library/bonehealth/, October 2004. Accessed 4 January 2005.
3 Looker AC, Orwoll ES, Johnston CCJr, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12:1761-1768.
4 Siris ES, Chen YT, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108-1112.
5 World Health Organization. Aging and Osteoporosis. Issued 7. Available at: http://www.who.int/archives/whday/en/documents1999/osteo.html, April 1999. Accessed 21 September 2004.
6 Siris ES, Miller PD, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: Results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815-2822.
7 Miller PD, Barlas S, Brenneman SK, et al. An approach to identifying osteopenic women at increased short-term risk of fracture. Arch Intern Med. 2004;164:1113-1120.
8 Tinetti ME, Baker DI, McAvay G, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. NEJM. 1994;331:821-827.
9 Katz WA, Sherman C. Osteoporosis: The role of exercise in optimal management. Phys Sportsmed. 1998;26:33-43.
10 Kannus P, Parkkari J, Niemi S, et al. Prevention of hip fracture in elderly people with use of a hip protector. NEJM. 2000;343:1506-1513.
11 Gallagher TC, Geling O, Comite F. Missed opportunities for prevention of osteoporotic fracture. Arch Intern Med. 2002;162:450-456.
12 Harrington JT, Broy SB, Derosa AM, et al. Hip fracture patients are not treated for osteoporosis: A call to action. Arthritis Rheum. 2002;47:651-654.
13 Heaney RP, Abrams S, Dawson-Hughes B, et al. Peak bone mass. Osteoporos Int. 2000;11:985-1009.
14 Barthe N, Basse-Cathalinat B, Meunier PJ, et al. Measurement of bone mineral density in mother-daughter pairs for evaluating the family influence on bone mass acquisition: A GRIO survey. Osteoporos Int. 1998;8:379-384.
15 Seeman E, Tsalamandris C, Formica C, et al. Reduced femoral neck bone density in the daughters of women with hip fractures: The role of low peak bone density in the pathogenesis of osteoporosis. J Bone Miner Res. 1994;9:739-743.
16 Livshits G, Deng HW, Nguyen TV, et al. Genetics of bone mineral density: Evidence for a major pleiotropic effect from an intercontinental study. J Bone Miner Res. 2004;19:914-923.
17 Wu XP, Liao EY, Huang G, et al. A comparison study of the reference curves of bone mineral density at different skeletal sites in native Chinese, Japanese, and American Caucasian women. Calcif Tissue Int. 2003;73:122-132.
18 Finkelstein JS, Lee ML, Sowers M, et al. Ethnic variation in bone density in premenopausal and early perimenopausal women: Effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87:3057-3067.
19 Meyer HE, Berntsen GK, Sogaard AJ, et al. Higher bone mineral density in rural compared with urban dwellers: The NOREPOS study. Am J Epidemiol. 2004;160:1039-1046.
20 Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337-342.
21 Whitson H, DeMarco D, Reilly D, et al. Uncoupling of bone turnover following hip replacement. Calcif Tissue Int. 2002;71:14-19.
22 Garnero P, Jouvenne P, Buchs N, et al. Uncoupling of bone metabolism in rheumatoid arthritis patients with or without joint destruction: Assessment with serum type I collagen breakdown products. Bone. 1999;24:381-385.
23 Martin TJ, Rodnan GA. Osteoporosis. In: Markus B, editor. Coupling of Bone Resorption and Formation during Bone Remodeling. 2nd ed. San Diego: Academic Press; 2001:361-372.
24 Riggs BL, Khosla S, Melton LJ3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279-302.
25 Halleen JM. Tartrate-resistant acid phosphatase 5B is a specific and sensitive marker of bone resorption. Anticancer Res. 2003;23:1027-1029.
26 Gordon CM. Normal bone accretion and effects of nutritional disorders in childhood. J Womens Health (Larchmt). 2003;12:137-143.
27 Lauridsen AL, Vestergaard P, Hermann AP, et al. Female premenopausal fracture risk is associated with gc phenotype. J Bone Miner Res. 2004;19:875-881.
28 Gustavsson A, Olsson T, Nordstrom P. Rapid loss of bone mineral density of the femoral neck after cessation of ice hockey training: A 6-year longitudinal study in males. J Bone Miner Res. 2003;18:1964-1969.
29 Williams FM, Cherkas LF, Spector TD, MacGregor AJ. The effect of moderate alcohol consumption on bone mineral density: A study of female twins. Ann Rheum Dis. 2004;69:309-310.
30 Bainbridge KE, Sowers M, Lin X, Harlow SD. Risk factors for low bone mineral density and the 6-year rate of bone loss among premenopausal and perimenopausal women. Osteoporos Int. 2004;15:439-446.
31 Laitinen KD, Valimaki M. Alcohol and bone. Calcif Tissue Int. 1991;49(Suppl):S70-S73.
32 Lewiecki EM, Watts NB, McClung MR, et al. Official positions of the International Society for Clinical Densitometry. J Clin Endocrinol Metab. 2004;89:3651-3655.
33 Kimble RB, Bain S, Pacifici R. The functional block of TNF but not of IL-6 prevents bone loss in ovariectomized mice. J Bone Miner Res. 1997;12:935-941.
34 Ralston SH, Russell RG, Gowen M. Estrogen inhibits release of tumor necrosis factor from peripheral blood mononuclear cells in postmenopausal women. J Bone Miner Res. 1990;5:983-988.
35 Ralston SH. Analysis of gene expression in human bone biopsies by polymerase chain reaction: Evidence for enhanced cytokine expression in postmenopausal osteoporosis. J Bone Miner Res. 1994;9:883-890.
36 Udagawa N, Takahashi N, Yasuda H, et al. Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology. 2000;141:3478-3484.
37 Ammann P, Rizzoli R, Bonjour JP, et al. Transgenic mice expressing soluble tumor necrosis factor-receptor are protected against bone loss caused by estrogen deficiency. J Clin Invest. 1997;99:1699-1703.
38 Roggia C, Gao Y, Cenci S, et al. Up-regulation of TNF-producing T cells in the bone marrow: A key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA. 2001;98:13960-13965.
39 Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309-319.
40 Bucay N, Sarosi I, Dunstan CR, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260-1268.
41 Schett G, Redlich K, Hayer S, et al. Osteoprotegerin protects against generalized bone loss in tumor necrosis factor-transgenic mice. Arthritis Rheum. 2003;48:2042-2051.
42 Wiren KM. Androgen action in bone: Basic cellular and molecular aspects. In: Orwoll ES, Bliziotes M, editors. Osteoporosis: Pathophysiology and Clinical Management. Totowa, N.J.: Humana Press; 2002:349-374.
43 Goulding A, Gold E. Flutamide-mediated androgen blockade evokes osteopenia in the female rat. J Bone Miner Res. 1993;8:763-769.
44 Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. NEJM. 2001;345:948-955.
45 Carlin AJ, Farquharson RG, Quenby SM, et al. Prospective observational study of bone mineral density during pregnancy: Low molecular weight heparin versus control. Hum Reprod. 2004;19:1211-1214.
46 Pearson D, Kaur M, San P, et al. Recovery of pregnancy mediated bone loss during lactation. Bone. 2004;34:570-578.
47 Dempster DW. Bone microarchitecture and strength. Osteoporos Int. 2003;14(Suppl 5):54-56.
48 Rogers A, Hannon RA, Eastell R. Biochemical markers as predictors of rates of bone loss after menopause. J Bone Miner Res. 2000;15:1398-1404.
49 Garnero P, Sornay-Rendu E, Duboeuf F, Delmas PD. Markers of bone turnover predict postmenopausal forearm bone loss over 4 years: The OFELY study. J Bone Miner Res. 1999;14:1614-1621.
50 Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: The OFELY study. J Bone Miner Res. 2000;15:1526-1536.
51 Sornay-Rendu E, Garnero P, Munoz F, et al. Effect of withdrawal of hormone replacement therapy on bone mass and bone turnover: The OFELY study. Bone. 2003;33:159-166.
52 Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S-1688S.
53 Ensrud KE, Duong T, Cauley JA, et al. Low fractional calcium absorption increases the risk for hip fracture in women with low calcium intake. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 2000;132:345-353.
54 Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805-806.
55 LeBoff MS, Kohlmeier L, Hurwitz S, et al. Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA. 1999;281:1505-1511.
56 Tannenbaum C, Clark J, Schwartzman K, et al. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab. 2002;87:4431-4437.
57 Deutschmann HA, Weger M, Weger W, et al. Search for occult secondary osteoporosis: Impact of identified possible risk factors on bone mineral density. J Intern Med. 2002;252:389-397.
58 Licata A. Osteoporosis in men: Suspect secondary disease first. Cleve Clin J Med. 2003;70:247-254.
59 Delaney MF, Leboff MS. Metabolic bone disease. In: Ruddy S, Harris E, editors. Kelley’s Textbook of Rheumatology. 6th ed. Philadelphia: WB Saunders; 2001:1635-1652.
60 Sikon A, Thacker HL, Carey J, et al. Secondary osteoporosis — are we recognizing it? Menopause. 2004;11:676.
61 Tannirandorn P, Epstein S. Drug-induced bone loss. Osteoporos Int. 2000;11:637-659.
62 Canalis E, Giustina A. Glucocorticoid-induced osteoporosis: Summary of a workshop. J Clin Endocrinol Metab. 2001;86:5681-5685.
63 American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis. Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. Arthritis Rheum. 2001;44:1496-1503.
64 Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320-323.
65 Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. NEJM. 1995;332:767-773.
66 Hui SL, Slemenda CW, Johnston CCJr. Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988;81:1804-1809.
67 World Health Organization. Osteoporosis definition: Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO Technical Report Series 843. Available at: http://www.radiology.ab.ca/rcalinksOD.htm, 1994. Accessed 22 September 2004.
68 Kanis JA, Melton LJ3rd, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137-1141.
69 The Writing Group for the International Society for Clinical Densitometry (ISCD) Position Development Conference. Position statement: Introduction, methods, and participants. J Clin Densitom. 2004;7:13-16.
70 Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72-75.
71 Garnero P, Delmas P. Utility of biochemical markers of bone turnover in osteoporosis. In: Markus B, editor. Osteoporosis. 2nd ed. San Diego: Academic Press; 2001:459-478.
72 Khosla S, Kleerkoper M. American Society of Bone and Mineral Metabolism. In: Farris MJ, editor. Primer on the Metabolic Bone Disease and Disorders of Mineral Metabolism. Washington, DC: American Society for Bone and Mineral Research; 2003:166-172.
73 Qvist P, Christgau S, Pedersen BJ, et al. Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): Effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone. 2002;31:57-61.
74 Malabanan AO, Holick MF. Vitamin D and bone health in postmenopausal women. J Womens Health. 2003;12:151-156.
75 Genant HK, Li J, Wu CY, Shepherd JA. Vertebral fractures in osteoporosis: A new method for clinical assessment. J Clin Densitom. 2000;3:281-290.
76 Chantler IW, Davie MW, Evans SF, Rees JS. Oral corticosteroid prescribing in women over 50, use of fracture prevention therapy, and bone densitometry service. Ann Rheum Dis. 2003;62:350-352.
77 Devine A, Dhaliwal SS, Dick IM, et al. Physical activity and calcium consumption are important determinants of lower limb bone mass in older women. J Bone Miner Res. 2004;19:1634-1639.
78 Augestad LB, Schei B, Forsmo S, et al. The association between physical activity and forearm bone mineral density in healthy premenopausal women. J Womens Health (Larchmt). 2004;13:301-313.
79 Gerdhem P, Obrant KJ. Effects of cigarette-smoking on bone mass as assessed by dual-energy X-ray absorptiometry and ultrasound. Osteoporos Int. 2002;13:932-936.
80 Oncken C, Prestwood K, Cooney JL, et al. Effects of smoking cessation or reduction on hormone profiles and bone turnover in postmenopausal women. Nicotine Tob Res. 2002;4:451-458.
81 McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. NEJM. 2001;344:333-340.
82 Heaney RP, Zizic TM, Fogelman I, et al. Risedronate reduces the risk of first vertebral fracture in osteoporotic women. Osteoporos Int. 2002;13:501-505.
83 Chapuy MC, Pamphile R, Paris E, et al. Combined calcium and vitamin D3 supplementation in elderly women: Confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: The Decalyos II study. Osteoporos Int. 2002;13:257-264.
84 Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: A randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344-1352.
85 Bjarnason NH, Sarkar S, Duong T, et al. Six and twelve month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in postmenopausal osteoporosis. Osteoporos Int. 2001;12:922-930.
86 Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. NEJM. 1992;327:1637-1642.
87 Bischoff HA, Stahelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: A randomized controlled trial. J Bone Miner Res. 2003;18:343-351.
88 American College of Obstetricians and Gynecologists (ACOG). Osteoporosis. ACOG Pract Bull. 2004;50:203-216.
89 Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
90 Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
91 Eli Lilly Pharmaceuticals Forteo Product Information. Available at http://www.rxlist.com/cgi/rxlist.cgi?drug=forteo, 2002. Accessed 22 September 2004.
92 Procter & Gamble Pharmaceuticals. Actonel Drug Information. Available at http://www.rxlist.com/cgi/rxlist.cgi?drug=actonel, 2000. Accessed 22 September 2004.
93 Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. NEJM. 2003;349:1207-1215.
94 Thacker HL. The case for hormone replacement: New studies that should inform the debate. Cleve Clin J Med. 2002;69:670-678.
95 Lindsay R. The role of estrogen in the prevention of osteoporosis. Endocrinol Metab Clin North Am. 1998;27:399-409.
96 Marcus R, Holloway L, Wells B, et al. The relationship of biochemical markers of bone turnover to bone density changes in postmenopausal women: Results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial. J Bone Miner Res. 1999;14:1583-1595.
97 Kothari S, Thacker HL. Risk assessment of the menopausal patient. Med Clin North Am. 1999;83:1489-1502.
98 Lufkin EG, Wahner HW, O’Fallon WM, et al. Treatment of postmenopausal osteoporosis with transdermal estrogen. Ann Intern Med. 1992;117:1-9.
99 Nelson HD, Rizzo J, Harris E, et al. Osteoporosis and fractures in postmenopausal women using estrogen. Arch Intern Med. 2002;162:2278-2284.
100 Delmas PD, Ensrud KE, Adachi JD, et al. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: Four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:3609-3617.
101 Finkelstein JS, Hayes A, Hunzelman JL, et al. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. NEJM. 2003;349:1216-1226.
102 Smith A. The female athlete triad: Causes, diagnosis and treatment. Phys Sportsmed. 1996;24:67-86.
103 Erickson SM. Osteoporosis in active women: Prevention, diagnosis and treatment. Phys Sportsmed. 1997;25:61-74.
104 Miller KK. Mechanisms by which nutritional disorders cause reduced bone mass in adults. J Womens Health (Larchmt). 2003;12:145-150.
105 Greendale GA, Espeland M, Slone S, et al. Bone mass response to discontinuation of long-term hormone replacement therapy: Results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med. 2002;162:665-672.
106 Fleisch H. Bisphosphonates in Bone Disease: From the Laboratory to the Patient, 4th ed. San Diego: Academic Press, 2000.
107 Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
108 Eastell R, Barton I, Hannon RA, et al. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003;18:1051-1056.
109 Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83-91.
110 Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535-1541.
111 Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: Results from the Fracture Intervention Trial. JAMA. 1998;280:2077-2082.
112 Wallach S, Cohen S, Reid DM, et al. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int. 2000;67:277-285.
113 Saag KG, Emkey R, Schnitzer TJ, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. NEJM. 1998;339:292-299.
114 Bone HG, Hosking D, Devogelaer JP, et al. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. NEJM. 2004;350:1189-1199.
115 Lanza FL, Hunt RH, Thomson AB, et al. Endoscopic comparison of esophageal and gastroduodenal effects of risedronate and alendronate in postmenopausal women. Gastroenterology. 2000;119:631-638.
116 Delaney MF, Hurwitz S, Shaw J, LeBoff MS. Bone density changes with once weekly risedronate in postmenopausal women. J Clin Densitom. 2003;6:45-50.
117 Merck & Co. Fosamax Product Information. Available at: http://www.drugs.com/fosamax.html. Accessed 22 September 2004.
118 Miller PD, Watts NB, Licata AA, et al. Cyclical etidronate in the treatment of postmenopausal osteoporosis: Efficacy and safety after seven years of treatment. Am J Med. 1997;103:468-476.
119 Procter & Gamble Pharmaceuticals. Didronel: Drug Information. Available at: http://www.rxlist.com/cgi/pharmclips2.cgi?keyword=%20%20Didronel%20%AE%20, 2004. Accessed 21 September 2004.
120 Stakkestad JA, Benevolenskaya LI, Stepan JJ, et al. Intravenous ibandronate injections given every three months: A new treatment option to prevent bone loss in postmenopausal women. Ann Rheum Dis. 2003;62:969-975.
121 Reid IR, Brown JP, Burckhardt P, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. NEJM. 2002;346:653-661.
122 Mikulec KH, Delaney MF, Hurwitz S, LeBoff MS. Safety and efficacy of zoledronic acid: A chart review. J Bone Miner Res. M321(Suppl), 2003.
123 Chan SS, Nery LM, McElduff A, et al. Intravenous pamidronate in the treatment and prevention of osteoporosis. Intern Med J. 2004;34:162-166.
124 Voskaridou E, Terpos E, Spina G, et al. Pamidronate is an effective treatment for osteoporosis in patients with beta-thalassaemia. Br J Haematol. 2003;123:730-737.
125 Chesnut CH3rd, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: The prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med. 2000;109:267-276.
126 Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. NEJM. 2001;344:1434-1441.
127 Jutte PC, Rosso R, de Paolis M, et al. Osteosarcoma associated with hyperparathyroidism. Skeletal Radiol. 2004;33:473-476.
128 Fadanelli ME, Bone HG. Combining bisphosphonates with hormone therapy for postmenopausal osteoporosis. Treat Endocrinol. 2004;3:361-369.
129 Raisz LG, Wiita B, Artis A, et al. Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J Clin Endocrinol Metab. 1996;81:37-43.
130 Watts NB, Notelovitz M, Timmons MC, et al. Comparison of oral estrogens and estrogens plus androgen on bone mineral density, menopausal symptoms, and lipid–lipoprotein profiles in surgical menopause. Obstet Gynecol. 1995;85:529-537.
131 Garrett IR, Mundy GR. The role of statins as potential targets for bone formation. Arthritis Res. 2002;4:237-240.
132 LaCroix AZ, Cauley JA, Pettinger M, et al. Statin use, clinical fracture, and bone density in postmenopausal women: Results from the Women’s Health Initiative Observational Study. Ann Intern Med. 2003;139:97-104.
133 Rejnmark L, Buus NH, Vestergaard P, et al. Effects of simvastatin on bone turnover and BMD: A 1-year randomized controlled trial in postmenopausal osteopenic women. J Bone Miner Res. 2004;19:737-744.
134 Meunier PJ, Roux C, Seeman E, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. NEJM. 2004;350:459-468.
135 Riggs BL, Seeman E, Hodgson SF, et al. Effect of the fluoride/calcium regimen on vertebral fracture occurrence in postmenopausal osteoporosis. Comparison with conventional therapy. NEJM. 1982;306:446-450.
136 Riggs BL, Hodgson SF, O’Fallon WM, et al. Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. NEJM. 1990;322:802-809.
137 McCombs JS, Thiebaud P, McLaughlin-Miley C, Shi J. Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas. 2004;48:271-287.
138 Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: A randomized controlled trial. J Clin Endocrinol Metab. 2004;89:1117-1123.
139 Greenspan SL, Parker RA, Ferguson L, et al. Early changes in biochemical markers of bone turnover predict the long-term response to alendronate therapy in representative elderly women: A randomized clinical trial. J Bone Miner Res. 1998;13:1431-1438.
140 Lenchik L, Kiebzak GM, Blunt BA. What is the role of serial bone mineral density measurements in patient management? J Clin Densitom. 2002;5(Suppl):S29-S38.
141 Kreijkamp-Kaspers S, Kok L, Grobbee DE, et al. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: A randomized controlled trial. JAMA. 2004;292:65-74.
142 Chen YM, Ho SC, Lam SS, et al. Beneficial effect of soy isoflavones on bone mineral content was modified by years since menopause, body weight, and calcium intake: A double-blind, randomized, controlled trial. Menopause. 2004;11:246-254.
143 van Vollenhoven RF. Dehydroepiandrosterone in systemic lupus erythematosus. Rheum Dis Clin North Am. 2000;26:349-362.
144 Delaney MF, Hurwitz S, Chan CK, Leboff MS. Combination therapy with micronized dehydroepiandrosterone and raloxifene or placebo: Changes in markers of bone turnover. J Bone Miner Res. 2003;18:M364.
145 Biermasz NR, Hamdy NA, Pereira AM, et al. Long-term skeletal effects of recombinant human growth hormone (rhGH) alone and rhGH combined with alendronate in GH-deficient adults: A seven-year follow-up study. Clin Endocrinol (Oxf). 2004;60:568-575.
146 Rymer J, Robinson J, Fogelman I. Ten years of treatment with tibolone 2.5mg daily: Effects on bone loss in postmenopausal women. Climacteric. 2002;5:390-398.
147 Prelevic GM, Markou A, Arnold A, et al. The effect of tibolone on bone mineral density in postmenopausal women with osteopenia or osteoporosis–8 years follow-up. Maturitas. 2004;47:229-234.