Use of transcranial doppler ultrasonography in the pediatric intensive care unit
(CONSULTANT LEVEL EXAMINATION)
Overview
As mentioned in preceding chapters, transcranial Doppler (TCD) ultrasonography is a noninvasive technique that uses low-frequency transducers for real-time evaluation of cerebral arteries in adults and in children with a closed anterior fontanelle.1–3 TCD has been increasingly used for cerebral circulation monitoring in the pediatric intensive care unit (PICU), and it has become an essential tool for managing sickle cell disease in children.2,3 Two types of TCD devices are available: blind TCD and transcranial color Doppler imaging (TCDI) ultrasonography, which links pulsed-wave Doppler to color mapping for a more accurate identification of arteries. In this chapter, we present TCDI applications in the PICU.
Technique
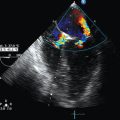
TCDI transducers are sector or phased-array low-frequency (1.8-4.0 MHz) dedicated probes with small footprints that facilitate insonation of the intracranial arteries via the temporal window. The transducer is placed just anterior to the ear’s tragus and above the zygoma, and the axial grayscale view of the base of the brain depicts the hypoechoic “butterfly-shaped” cerebral peduncles and the echogenic “star-shaped” suprasellar cistern (reference landmarks).3 The circle of Willis, which is depicted on color mode, projects anteriorly (Figure 5-1). The middle cerebral artery (MCA) is coded red, with blood flow circulating toward the transducer. Color-scale settings should be optimized according to the blood velocities. After switching to the spectral Doppler mode, the 4- to 5-mm–wide Doppler sample gate is placed on the initial part of the MCA, and the recording is optimized by slightly tilting and sliding the transducer to obtain the cleanest and highest-velocity spectrum, which indicates that the axis of the vessel and the Doppler beam are closely aligned. Two to three spectra are recorded, and the most powerful signal at the highest mean blood velocity is used for measurement. The temporal window also allows for recording the internal carotid artery and the anterior and posterior cerebral arteries. However, because the MCA is the main cerebral artery that supplies blood to 60% to 80% of the hemisphere, its acute blood flow changes may reflect hemodynamic alterations in the entire hemisphere. Hence scanning other arterial segments is not as important as scanning the MCA, except in specific clinical scenarios. The tracing is assumed to be obtained at an optimal angle of 0 degrees. Terminal segments of the vertebral arteries and basilar artery can be insonated via the suboccipital window. The transorbital window can be used when assessing the carotid siphon if no temporal window is available. Although no side effects have been described with TCDI, it should be set at the lowest power output possible, according to the ALARA (As Low As Reasonably Achievable) principle (see Chapter 1).
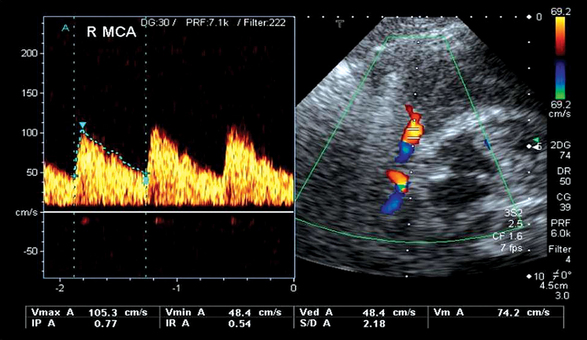
Figure 5-1 Transcranial color Doppler imaging (TCDI) (temporal window) performed in a healthy child. Middle cerebral artery (MCA) is coded red, and the spectrum is above the baseline because blood flow is moving toward the transducer (pulsatility index [PI] = 0.77, resistive index [RI] = 0.54, mean velocity = 74 cm/sec, and end-diastolic velocity = 48 cm/sec).
Doppler measurements
Common Doppler-derived variables are end-diastolic velocity (VD); time-averaged mean maximal velocity (TAMX), obtained after outlining manually or electronically the envelope of the waveform over a cardiac cycle, also called mean velocity (VM); and peak systolic velocity (VS) (see Figure 5-1). Spectral Doppler waveforms are described by the pulsatility index (PI) and resistive index (RI). PI is calculated according to the formula PI = (VS − VD)/VM (normal values = 0.82 ± 0.11). RI is calculated according to the formula RI = VS − VD/VS (normal values = 0.55 ± 0.66, after the neonatal period). When territorial vascular resistance decreases (e.g., vasodilation), thereafter increments of blood flow are observed during diastole, and thus PI and RI are decreased. In contrast, intracranial hypertension results in increments of PI and RI values as resistance to cerebral blood flow is increased. PI and RI are unitless and independent of insonation angles. The Lindegaard ratio helps to distinguish between vasospasm and hyperemia by comparing the VM of the MCA with the VM of the extracranial portion of the ipsilateral internal carotid artery. The normal value is less than 3.0 (median, 1.7). With an elevated VM in the MCA, a ratio of less than 3 is considered hyperemia, 3 to 6 is mild vasospasm, and greater than 6 is severe vasospasm. TCDI measurements are subjected to many limitations: inability to obtain an optimized signal or obtaining a weak signal, aliasing, absent temporal window, operator experience, clinical conditions, and effects of therapy other factors (age, anemia, dehydration, hypotension, hypoventilation or hyperventilation, pharmacologic agents).
Basic cerebrovascular hemodynamics
Flow velocities in arterial circuits depend on a vessel’s cross-sectional area and its blood flow. VM variations reflect variations in cerebral blood flow if neither the diameter of the insonated artery nor the angle of insonation change during the examination. PI and RI may be used for indirect evaluation of intracranial pressure (ICP). Invasive ICP measurements are strongly correlated with PI values (within the range of ICP values of 5-40 mm Hg).4 If ICP is increased, diastolic velocity is decreased, and the Doppler waveform has a high resistance profile (increased PI and RI). Because proximal segments of intracranial arteries have limited vasodilation capacities, low velocity reflects low blood flow. With increased cerebral blood flow (hyperemia), Doppler velocities are increased bilaterally, and the waveform has a low resistance profile with decreased RI and PI (Lindegaard ratio < 3). Cerebral blood flow depends on cerebral perfusion and cerebrovascular resistance. The ability of the cerebrovascular system to constrict or dilate in response to perfusion pressure changes is termed autoregulation. Various disorders, including traumatic brain injury (TBI), can impair cerebral autoregulation, rendering the brain susceptible to inadequate (ischemia) or excessive (hyperemia) cerebral blood flow. Alterations in Doppler flow velocities in response to changes in arterial blood pressure and arterial carbon dioxide pressure (Paco2) may be used as markers of cerebrovascular autoregulation. Doppler measurements are influenced by various factors, such as arterial blood pressure, hematocrit, fever, and Paco2. Doppler flow velocities increase with low hematocrit as increased cardiac output and decreased blood viscosity result in reduced intracranial resistance, thus allowing sustained normal brain oxygenation despite anemia. CO2 is a powerful modulator of cerebral blood flow. Hyperventilation induces vasoconstriction of distal intracranial arterioles and significantly increases PI and RI values, whereas hypoventilation induces vasodilation and significantly decreases PI and RI values. Cerebral vasoreactivity to Paco2 can be diminished or abolished by drugs, ischemia, TBI, and some forms of metabolic encephalopathy. Sleeping can slightly increase velocities with hypercapnia. Crying can decrease velocities with hypocapnia. Fever increases blood flow by about 10%. In the PICU, the influence of anesthetic medications on TCDI results should be considered. Halothane increases by 30% the MCA Doppler velocities during general anesthesia, whereas thiopenthal has a mild inverse effect. Lesions producing large diastolic runoff (e.g., large patent ductus arteriosus or aortic valve insufficiency) decrease diastolic blood flow to the brain and consequently the diastolic component of the TCDI waveform. TCDI velocities vary with age. Velocities increase rapidly after birth and then more slowly until the age of 6 to 8 years; then they decrease slowly to about 70% of the maximal velocities by the age of 18 years. Velocities are lower in the vertebrobasilar compared with the carotid system.5
Transcranial color doppler imaging ultrasonography in the pediatric intensive care unit
Monitoring of cerebral hemodynamics after traumatic brain injury
TBI has a detrimental effect on child morbidity and mortality. It influences cerebrovascular hemodynamics by causing cerebral hyperemia, ischemia, and vasospasm. TCDI and other monitoring tools (e.g., computed tomography scans, invasive ICP measures) may aid in guiding TBI management in the PICU. Hyperemia accompanies diffuse cerebral edema, leading to increased ICP and poor outcome in pediatric patients with TBI. Hyperemia can occur a few hours after TBI, lasts 2 to 4 days, and is followed by patterns of high intracranial resistance consistent with elevated ICP.6 Of note, hyperemia was also observed in the first hours after an ischemic event in neonates and children. It can provoke intracerebral hemorrhage and is believed to occur because of autoregulation loss. TCDI detects increased blood flow velocities that are above the mean velocity plus 2 standard deviations in both MCAs; PI and RI are decreased, and the Lindegaard ratio is less than 3. Cerebral ischemia is another important pathophysiologic element of an evolving TBI and is usually observed after the acute phase. TCDI can detect ischemia with associated increased ICP in pediatric patients (Figure 5-2). In a prospective pediatric study of 36 children with moderate or severe TBI, diastolic velocity less than 25 cm/sec and PI greater than 1.3, revealed by TCDI performed on arrival in the emergency department after the first resuscitation phase (correction of low blood pressure, anemia, hypoxia, hypoventilation), were associated with poor prognosis.7 TCDI is also used to monitor therapeutic effects. Vasopressors used to increase mean arterial pressure may also decrease PI and increase VD and VM, whereas mannitol increases TCDI velocities by reducing edema and increasing cerebral blood flow.8 The level of “useful” mean arterial pressure may be estimated by TCDI.
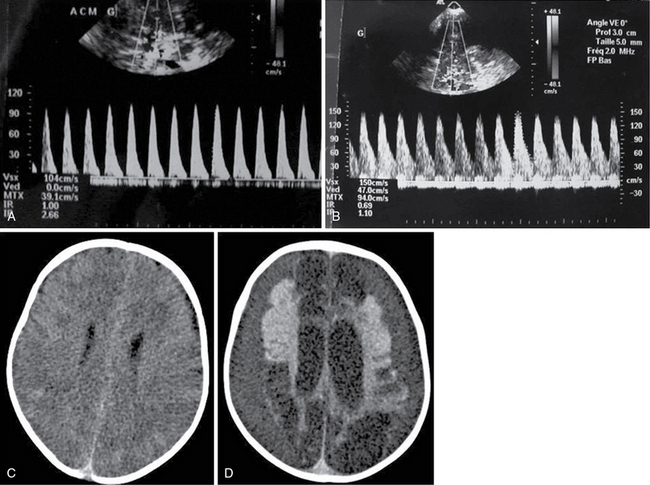
Figure 5-2 Two-month-old boy with shaken-baby syndrome and poor outcome. Transcranial color Doppler imaging (TCDI) was performed upon admission (A) and revealed high-resistance-profile blood flow (pulsatility index [PI] = 2.66) in the middle cerebral artery (MCA). The waveform was normal at day 12 (B) (PI = 1.1). Brain computed tomography (CT) scan revealed diffuse brain edema upon admission (C) and encephalomalacia with bilateral ex vacuo subdural hematomas 1 month later (D).
Recent studies underlined the role of impaired autoregulation as a poor prognostic marker in brain-injured pediatric patients. Impaired autoregulation has been reported in about 40% of children at some point after TBI.9 However, this varied according to the postinjury time, and therefore single measurements may not reflect true incidence.10 Intracranial vasospasm is a usual complication of subarachnoid hemorrhage after rupture of cerebral aneurysm or TBI in adults, but it appears to be uncommon in children with TBI.11–13
Diabetic ketoacidosis in children
Death caused by diabetic ketoacidosis is most often related to the development of acute brain edema. The latter occurs in approximately 1% of cases of ketoacidosis, usually during the first 24 hours of treatment.14 Its pathogenesis is complex and poorly understood. Hyperemia caused by intracranial arteriolar vasodilation secondary to acidosis may occur first (Figure 5-3), leading to intracranial hypertension and finally inadequate cerebral perfusion and ischemia.15,16 Intracerebral hemorrhage may also occur and is believed to be caused by autoregulation loss (see Figure 5-3). The role of vasopressin, insulin, the sodium ion/hydrogen ion (Na+/H+) pump, hypoxia-ischemia, cytokines, as well as of insulin and fluid therapy is still debatable.
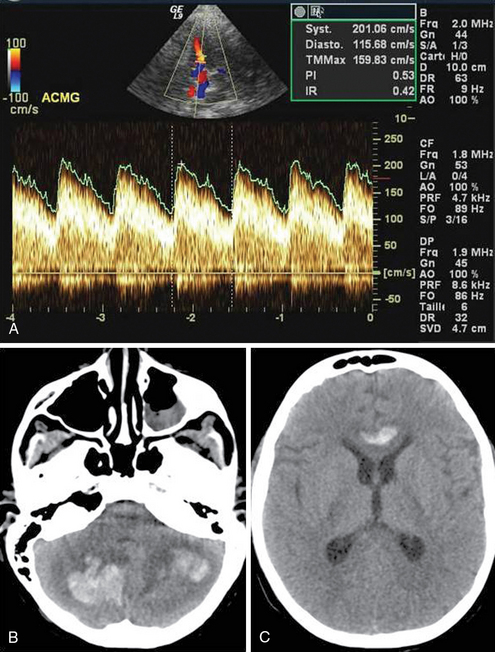
Figure 5-3 An 11-year-old boy in diabetic ketoacidotic coma. A, Upon admission, intracranial flow velocity was abnormally high, and the pulsatility index (PI) was low (hyperemia, mean velocity = 159 cm/sec, PI = 0.53 in the middle cerebral artery [MCA]). B and C, After 7 days, brain magnetic resonance imaging (MRI) depicted intracerebral hematomas.
Meningitis and meningoencephalitis in children
Meningitis is still associated with high mortality mainly because of cerebral herniation and compression of the brainstem as a result of increased ICP. Monitoring cerebrovascular hemodynamic changes may be important for early detection of cerebral perfusion compromise and to prevent further cerebral insult (Figure 5-4). In the early phase, when increased ICP may be present, the RI is useful, and RI values greater than 0.65 for children and greater than 0.80 for neonates and young infants suggest significant perfusion compromise, especially when mean flow velocity is also lower than normal.17 PI above 1.30 and VD less than 25 cm/sec are also used as thresholds for increased ICP in this setting. TCDI can also detect arterial stenosis or vasospasm as a complication of meningitis, encephalitis, brain abscess, and sepsis. Infection likely leads to cerebral ischemia via several mechanisms, such as systemic inflammatory response, hypercoagulable state, and/or direct invasion of the endothelium.
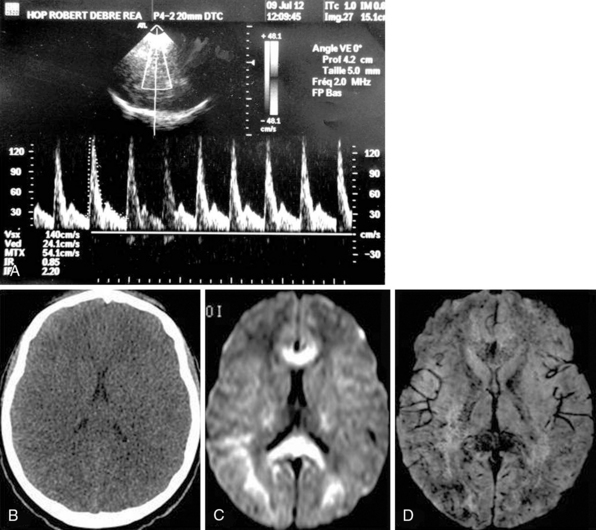
Figure 5-4 A 14-year-old girl into febrile coma after a trip to Africa (cerebral malaria). A, Both middle cerebral arteries (MCAs) exhibited high-resistance-profile spectra (resistive index [RI] = 0.85, pulsatility index [PI] = 2.20), whereas brain computed tomography (CT) scan revealed diffuse brain swelling (B). Magnetic resonance imaging (MRI) with diffusion-weighted imaging revealed extensive cytotoxic edema (C) and with susceptibility-weighted imaging demonstrated diffuse petechial hemorrhage throughout the brain (D).
Sickle cell disease
Screening children aged 2 to 16 years who have sickle cell anemia to assess for and prevent ischemic stroke is the best-established, evidence-based use of TCDI (type A level of evidence and class I recommendation). Sickle cell disease is associated with progressive occlusion of intracranial arteries (most frequently the intracranial internal carotid artery and MCA), leading to stroke caused by total occlusion of these arteries or related to hemodynamic failure in the downstream territory. According to TCDI criteria for sickle cell disease, a VM less than 170 cm/sec is considered normal, and a VM of 170 to 199 cm/sec is considered “conditional.” VM greater than or equal to 200 cm/sec is considered abnormal and indicates the need for transfusion therapy.2,3 A VM greater than or equal to 200 cm/sec is associated with a risk of stroke of 40% within the next 3 years.18 Transfusion with reduced hemoglobin S, to less than 30% of total hemoglobin, will lower this risk by 90% compared with standard care alone.19 TCDI is repeated every 12 months after a normal scan and every 3 months with a “conditional” scan. The efficacy of this protocol has been shown in several series, as in the longitudinal French newborn cohort, with the risk of stroke by age 18 significantly decreased from 11% to 1.9%.20
Pearls and highlights
• Impaired autoregulation has been reported in about 40% of children at some point after TBI. In its early stages, hyperemia characterized by increased Doppler MCA flow velocities (decreased PI and RI values, Lindegaard ratio < 3) is observed. As TBI evolves, cerebral ischemia commonly occurs. PI values greater than 1.3 and end-diastolic velocities less than 25 cm/sec are suggestive of intracranial hypertension and indicate the need for intensive treatment.
• TCDI is useful in monitoring pediatric patients with diabetic ketoacidosis, meningitis, or meningoencephalitis; however, screening children aged 2 to 16 years with sickle cell anemia to assess for and prevent ischemic stroke is its best-established use.
• According to TCDI criteria for sickle cell disease VM values less than 170 cm/sec are considered normal, and values in the range of 170 to 199 cm/sec are considered “conditional.” VM values greater than or equal to 200 cm/sec indicate the need for transfusion because there is a risk of stroke of 40% within the next 3 years.
References
1. American College of Radiology (ACR), Society for Pediatric Radiology (SPR), Society of Radiologists in Ultrasound (SRU): AIUM practice guideline for the performance of a transcranial Doppler ultrasound examination for adults and children. J Ultrasound Med. 2012;31(9):1489–1500.
2. Bulas, D. Transcranial Doppler applications in neonates and children. Ultrasound Clin. 2009; 4:533–551.
3. Verlhac, S. Transcranial Doppler in children. Pediatr Radiol. 2011; 41(Suppl 1):S153–S165.
4. Bellner, J, Romner, B, Reinstrup, P, et al. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP). Surg Neurol. 2004; 62(1):45–51.
5. Bode, H, Wais, U. Age dependence of flow velocities in basal cerebral arteries. Arch Dis Child. 1988; 63(6):606–611.
6. Muttaquin, Z, Uozumi, T, Kuwabara, S, et al. Hyperaemia prior to acute cerebral swelling in severe head injuries:the role of transcranial Doppler monitoring. Acta Neurochir. 1993; 123(1-2):76–81.
7. Trabold, F, Meyer, PG, Blanot, S, et al. The prognostic value of transcranial Doppler studies in children with moderate and severe head injury. Intensive Care Med. 2004; 30(1):108–112.
8. Orliaguet, GA. Cerebral monitoring in children. Paediatr Anaesth. 2004; 14(5):407–411.
9. Udomphorn, Y, Armstead, WM, Vavilala, MS. Cerebral blood flow and autoregulation after pediatric traumatic brain injury. Pediatr Neurol. 2008; 38(4):225–234.
10. Tontisirin, N, Armstead, W, Waitayawinyu, P, et al, Change in cerebral autoregulation as a function of time in children after severe traumatic brain injury: a case series. Childs Nerv Syst. 2007;23(10):1163–1169.
11. Figaji, AA. Practical aspects of bedside cerebral hemodynamics monitoring in pediatric TBI. Childs Nerv Syst. 2010; 26(4):431–439.
12. Mandera, M, Larysz, D, Wojtacha, M. Changes in cerebral hemodynamics assessed by transcranial Doppler ultrasonography in children after head injury. Childs Nerv Syst. 2002; 18(3-4):124–128.
13. Fortier O’Brien, N, Reuter-Rice, KE, Khanna, S, et al. Vasospasm in children with traumatic brain injury. Intensive Care Med. 2010; 36(4):680–687.
14. Foster, JR, Morrison, G, Fraser, DD. Diabetic ketoacidosis-associated stroke in children and youth. Stroke Res Treat. 2011; 2011:219706.
15. Roberts, JS, Vavilala, MS, Schenkman, KA, et al. Cerebral hyperemia and impaired cerebral autoregulation associated with diabetic ketoacidosis in critically ill children. Crit Care Med. 2006; 34(8):2217–2223.
16. Hoffman, WH, Pluta, RM, Fisher, AQ, et al. Transcranial Doppler ultrasound assessment of intracranial hemodynamics in children with diabetic ketoacidosis. J Clin Ultrasound. 1995; 23(9):517–523.
17. Goh, D, Robert A Minns, RA. Cerebral blood flow velocity monitoring in pyogenic meningitis. Arch Dis Child. 1993; 68(1):111–119.
18. Adams, RJ, McKie, VC, Carl, EM, et al. Long-term stroke risk in children with sickle cell disease screened with transcranial Doppler. Ann Neurol. 1997; 42(5):699–704.
19. Adams, RJ, McKie, VC, Hsu, L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998; 339(1):5–11.
20. Bernaudin, F, Verlhac, S, Arnaud, C, et al. Impact of early transcranial Doppler screening and intensive therapy on cerebral vasculopathy outcome in a newborn sickle cell anemia cohort. Blood. 2011; 117(4):1130–1140.