Overview of the arterial system
Procedural and diagnostic vascular ultrasound applications are essential in the intensive care unit (ICU). This chapter presents a synopsis of arterial disorders, whereas evaluation of venous thrombosis and ultrasound-guided vascular procedures are presented in Chapters 9 to 18.
The arterial system
The left-sided aorta couples the left ventricle to the arterial system. Transthoracic ultrasound detects the ascending aorta (left parasternal view) and the aortic arch (suprasternal view). In the ICU, however, these acoustic windows may be impossible to obtain. Transesophageal echocardiography (TEE) is invaluable in visualizing the thoracic aorta in the ICU but typically cannot image the entirety of the thoracic aorta. Usually, the brachiocephalic trunk, left common carotid artery (CCA), and left subclavian artery (SCA) originate from the arch, in that order. The right CCA begins at the bifurcation of the brachiocephalic trunk, whereas the right vertebral artery (VA) originates from the right SCA. One third of patients have a common origin of the left CCA and brachiocephalic trunk, or the former originates from the proximal segment of the latter. The left VA may originate from the arch, and the right SCA may exhibit an aberrant origin. A right-sided aorta is rare and frequently associated with other types of congenital cardiovascular disease.1,2
The CCA is detected sonographically in the lateral aspect of the neck by using high-frequency transducers (7.5 to 10 MHz) in both the longitudinal and transverse planes. The CCAs run upward to about the upper border of the thyroid cartilage before branching. The normal Doppler spectral waveform (DSW) of the CCA has a narrow peak systolic and forward diastolic flow (Figure 8 E-1). The CCA bifurcates into the internal (ICA) and external (ECA) carotid arteries. The ICA does not branch in the neck and has a low-resistance DSW with forward diastolic flow. The ECA branches in the neck and has a high-resistance DSW with reversed diastolic flow. The VA runs toward the sixth cervical vertebra and enters the base of the skull accompanied by its vein. The SCA becomes the axillary artery after crossing the first rib, and the latter continues as the brachial artery (BA) after the posterior axillary fold. The BA ends about 1 cm distal to the elbow by dividing into the radial artery (RA) and ulnar artery (UA). The RA becomes the deep and the UA the superficial palmar arch, respectively, which join in the hand. The DSW of upper extremity arteries is triphasic.
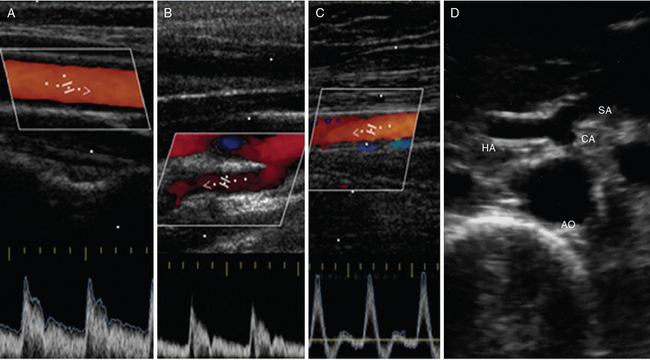
Figure 8 E-1 Normal Doppler spectral waveform of the common carotid artery (A), internal carotid artery (B), and superficial femoral artery (C). D, Abdominal aorta (AO) giving off the celiac artery (CA), which branches into the splenic artery (SA) and hepatic artery (HA).
The descending thoracic aorta is detected sonographically via the cardiac apical window. The abdominal aorta (AA) begins at the aortic hiatus of the diaphragm and ends at the fourth lumbar vertebra (bifurcation). The AA is detected sonographically on longitudinal, transverse, and oblique planes by using convex transducers (2.5 to 5 MHz), and its DSW is biphasic. The AA gives off several main branches: the celiac artery (CA), which supplies the liver, gallbladder, stomach, intestines, and pancreas and in turn branches into the splenic, left gastric, and common hepatic arteries (see Figure 8 E-1); the superior mesenteric artery (SMA), which originates approximately 1 cm inferior to the CA and supplies the intestines and pancreas; the inferior mesenteric artery (IMA), which supplies the colon and rectum; and the renal arteries, which branch off last and supply the kidneys and adrenal glands. The AA bifurcates into the right and left common iliac arteries at the level of the umbilicus, and these vessels travel distally to divide into the internal (IIA) and external (EIA) iliac arteries. The EIA enters the thigh at the inguinal ligament and becomes the common femoral artery (CFA). The CFA bifurcates into the superficial (SFA) and deep (DFA) femoral arteries. The DFA travels laterally and medially to supply the deep thigh muscles. The SFA travels distally through the adductor canal (Hunter canal) into the popliteal fossa and becomes the popliteal artery (POPA). The POPA gives off several branches (e.g., sural) and bifurcates into the anterior tibial artery (ATA) and the tibioperoneal trunk (TPT). The ATA travels anteriorly and laterally between the tibia and fibula and becomes the dorsalis pedis artery (DPA) as it travels across the foot. The TPT bifurcates into the posterior tibial artery (PTA) and peroneal artery. The PTA travels posterior to the medial malleolus and peroneal artery and down the calf. The PTA bifurcates into the medial and lateral plantar arteries in the foot. The latter join with the plantar metatarsal arteries to form the plantar arch. The lower extremity arteries have a triphasic DSW (see Figure 8 E-1).1,2
Disorders
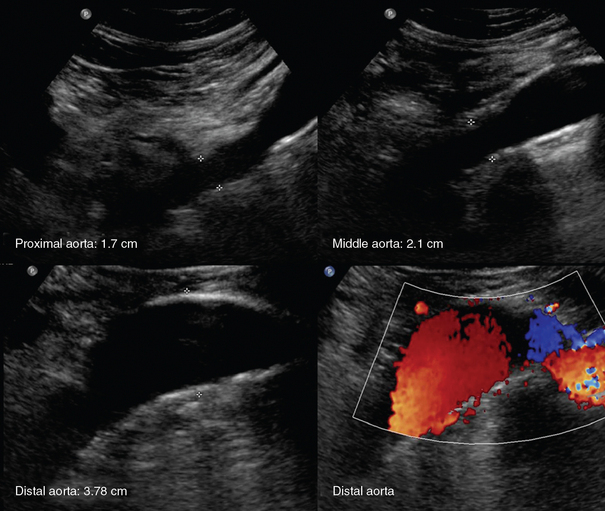
An aortic aneurysm is defined as aortic dilatation (diameter >1.5 times the normal aortic diameter), and rupture of it is associated with high mortality. Aneurysms result from weakening of elastin and collagen within the medial layer of the arterial wall. A thoracic aortic aneurysm (TAA) resembles a mediastinal mass. Use of TEE and computed tomography (CT) is often necessary for accurate diagnosis. TAAs are commonly thrombosed and saccular. An abdominal aortic aneurysm (AAA) is the most common type of aortic aneurysm and is qualitatively defined as having a diameter greater than 3 cm (Figures 8 E-2 and 8 E-3). The AA has no vasa vasorum to facilitate repair; moreover, the ratio of elastic fibers to collagen changes from 3.1:1 in the proximal ascending aorta to 2.8:1 in the midthoracic region to 0.8:1 in the AA. AAAs are true aneurysms (fusiform, saccular) and can remain asymptomatic. They are usually atherosclerotic and infrarenal and have a predicted expansion rate of 0.2 to 0.5 cm/yr. Mild aortic dilatation (>2.0 and <3.0 cm) is described as ectatic, a moderate AAA is 3 to 5 cm in diameter, and a severe AAA is larger than 5 cm and corresponds to increased risk for rupture necessitating surgical repair (>80% mortality). In the ICU, surface ultrasound could be a useful examination to evaluate the aorta, although several limitations such as obesity, edema, abdominal gas, and trauma may hinder proper evaluation. Regardless, the vast majority can be imaged if alternative views such as coronal scanning through the liver are used when needed. Indications for performing ultrasound examination of the AA are surveillance of a known AAA, detection of an abdominal bruit on auscultation, unexplained shock, abdominal pain, pulsatile aorta or mass, history of hypertension or smoking, age older than 50 years with a family history of AAA, aneurysm in another vessel, aortic coarctation, lower extremity arterial disease, and the presence of emboli in the absence of another source. Ultrasound can be used to estimate the anteroposterior and transverse AAA diameter from outer wall to outer wall (Figure 8-1). The DSW through an AAA may reveal systolic dampening and diastolic reversal along with swirling of blood flow. A thrombus (mural or circumferential), usually of mixed echogenicity, or atherosclerotic plaque (hyperechoic areas confined to the aortic wall) may be found. Rupture or leakage is indicated by depiction of free fluid in the abdominal spaces, particularly retroperitoneal hematoma. Contrast-enhanced CT is used to confirm the findings and is the method of choice for diagnosis of leakage.1,3
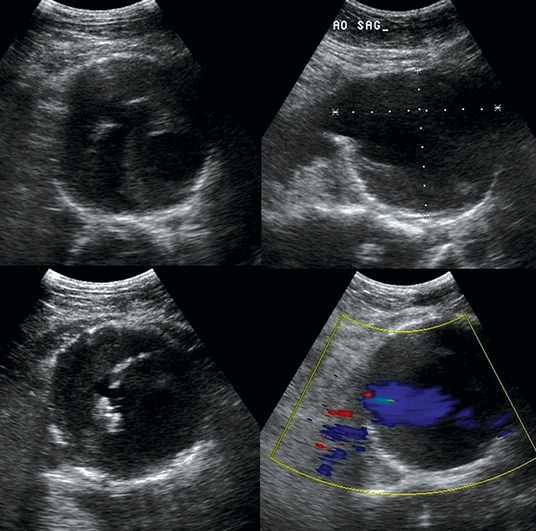
Figure 8-1 Huge infrarenal abdominal aortic aneurysm (anteroposterior diameter, 7.5 cm) with a mural thrombus and calcifications (top: transverse and sagittal views; bottom: transverse views).
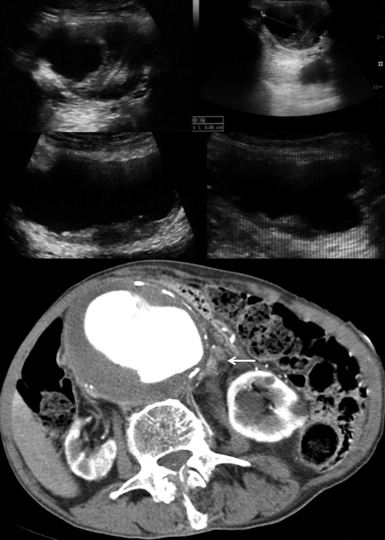
Figure 8 E-3 (Top) Multiple views of an abdominal aortic aneurysm (AAA) with a mural thrombus extending to the common iliac arteries; (bottom) computed tomography scan depicting a ruptured AAA with a mural thrombus (arrow). (Courtesy Dr. G. Anyfantakis.)
Aortic dissection, which is more common in patients with cardiovascular risk factors, is defined as separation of the aortic intima from the media by blood. The Stanford classification divides dissections into those occurring proximal (type A) or distal (type B) to the left SCA. DeBakey classified dissections into type I (involving the entire ascending and descending aorta), type II (involving just the ascending aorta), and type III (involving just the descending aorta), which is further subdivided into IIIa (limited to the descending thoracic aorta) and IIIb (extending below the diaphragm). An intimal flap, which can be depicted sonographically as an echogenic band floating in the anechoic lumen (Figure 8-2), divides the latter into true and false lumens; however, echogenicity may be variable if echogenic thrombotic occlusion or atherosclerotic plaque coexist (Figure 8 E-4). Duplex ultrasound depicts lower flow velocity in the false lumen or absent flow in the case of thrombosis. Changes in the DSW reflect additional hemodynamic abnormalities such as stenosis or occlusion. Invasive evaluation (e.g., degree of lumen compromise, length of dissection) can be performed with intravascular ultrasound (IVUS), as well as with angiography. Thoracic trauma is an indication for cardiac (e.g., hemopericardium) and aortic (e.g., traumatic aortic dissection, aneurysm formation) ultrasound evaluation in the ICU. The role of TEE in detecting aortic dissection, especially DeBakey type II, is important (see Figure 8-2). An abdominal aortic hematoma is usually the result of rupture secondary to trauma or an AAA. Rupture may be confined to the aortic wall or, most commonly, to the retroperitoneum. In cases of intraperitoneal rupture, blood can flow inside the peritoneal cavity and be seen, but it is short-lived because of rapid exsanguination. Rarely, an AAA may rupture into the gastrointestinal tract and cause massive hemorrhage (aortoduodenal fistula). Contrast-enhanced ultrasound may be useful in detecting aortic rupture. Impending rupture is indicated by intramural hematoma (thickened hypoechoic vessel wall). A retroperitoneal hematoma has mixed echogenicity (a hyperechoic zone reflecting clots and a hypoechoic one reflecting serum) and can be detected via a translumbar or abdominal approach.1,3
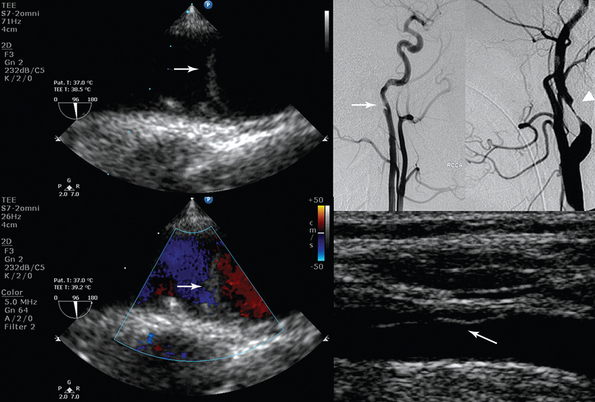
Figure 8-2 (Left) Transesophageal echocardiography showing posttraumatic aortic dissection (type A); (right top) invasive angiography depicting posttraumatic internal carotid artery (ICA) (arrow) and distal ICA (arrowhead) dissection; (right bottom) ultrasound showing common carotid artery dissection. Please note the echogenic flaps within the anechoic lumens (arrows).
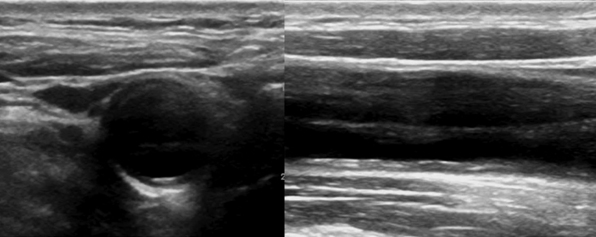
Figure 8 E-4 B-mode showing common carotid artery dissection of variable echogenicity (presence of echogenic flap and thrombus).
Disorders of the carotid system and peripheral arteries are common. Ultrasound of the CA is performed with high-frequency transducers (5 MHz is reserved for deeper vessels such as the VA or SCA). The sample volume of pulsed wave Doppler is kept as small as possible and placed in the center of the vessel at an insonation angle of 60 degrees. In tortuous vessels, smaller angles can be used, but angles larger than 60 degrees should be avoided because of large errors in estimation of peak systolic velocity (PSV). CA stenosis is observed commonly and is due to atherosclerotic plaque located at the carotid bulb (bifurcation) and proximal ICA. B-mode classifies plaque into (1) anechoic, hypoechoic, or hyperechoic (calcified and when dystrophic may indicate areas of hemorrhage or necrosis); (2) homogeneous, which reflects a uniform echo pattern (e.g., hypoechoic fibrous plaque with soft gray echoes), or heterogeneous, which is more symptomatic and reflects a complex echo pattern (e.g., hypoechoic plaque with hyperechoic echoes); and (3) smooth (more stable) or irregular (more likely to ulcerate or embolize and cause stroke). Plaque can also be monitored with contrast-enhanced ultrasound, three-dimensional techniques, and IVUS. Strict ultrasound criteria exist only for the evaluation of ICA stenosis because these criteria do not apply in the case of ECA or CCA stenosis (Table 8-1). With occlusion of the ICA, PSV is usually undetectable, whereas hemodynamic changes in other vessels secondary to the occlusion (e.g., close to zero flow in end-diastole in the ipsilateral CCA or increased end-diastolic flow in the contralateral CCA) could be present (Figure 8 E-5). Invasive or computed tomographic angiography (CTA) confirms the findings.4–7
TABLE 8-1
Ultrasound Criteria for Stenosis of the Internal Carotid Artery

Plaque was estimated and the diameter of the ICA calculated by B-mode and duplex ultrasound.
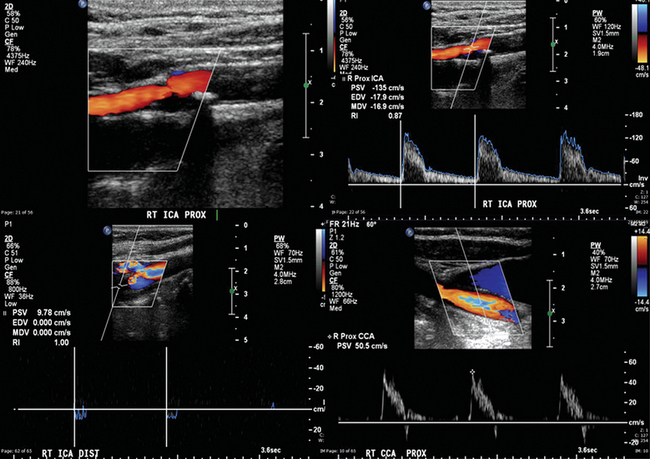
Figure 8 E-5 Right internal carotid artery (ICA) with 50% to 69% stenosis (top) and occlusion (bottom). Flow through the right common carotid artery (CCA) is almost zero in end-diastole because it was redirected through the external carotid artery system (high-resistance arterial conduit). Normally, the Doppler spectral waveform of the CCA reflects the low distal resistance of the ICA and has flow above zero in end-diastole. (Courtesy Dr. G. Anyfantakis.)
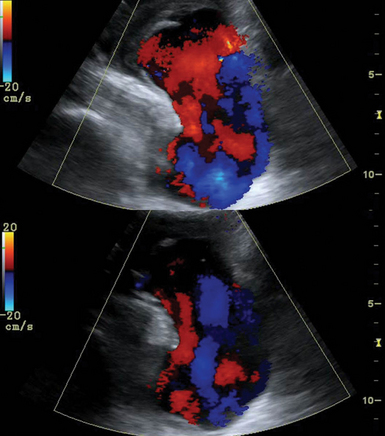
Ultrasound examination of the VAs should assess the direction of flow (antegrade or retrograde), and retrograde flow should raise suspicion for SCA stenosis (occlusion) or subclavian steal syndrome (Figure 8 E-6). SCA stenosis is identified by loss of the reversal component in the DSW, increased PSV, and poststenotic turbulence. Cervical artery dissection (CAD) is usually observed following trauma (see Figure 8-2). Underlying arteriopathies may predispose to CAD as a result of protease hyperactivity (e.g., patients with CAD have an increased incidence of fibromuscular dysplasia of the involved artery). Ultrasound may reveal mural hematoma and thrombosis (thickened, hypoechoic vessel wall). If hemodynamic abnormalities (e.g., stenosis) are present, the sensitivity of duplex examination is increased.
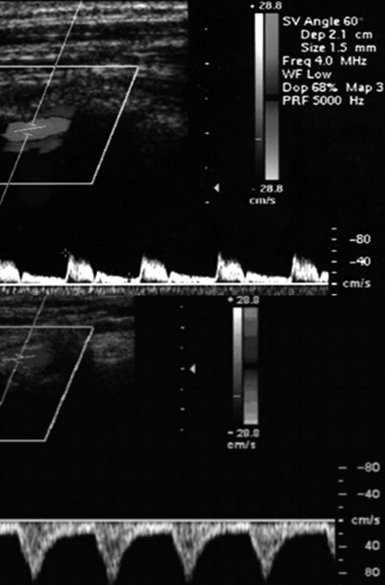
Figure 8 E-6 Normal flow in the right vertebral artery (top) and flow reversal in subclavian steal syndrome (bottom).
Common mechanisms of peripheral arterial disease include atherosclerosis (stenosis or occlusion), thromboembolism (plaque or thrombotic fragments originating from the heart or an aneurysm, hypercoagulable states, insertion of an arterial line), vasospasm (transient constriction, usually of the digital arteries), extrinsic arterial compression (tumors, hematomas, anomalous anatomic configurations), aneurysms, pseudoaneurysms, and congenital or acquired arteriovenous fistula (AVF).1–3 In the lower extremities, arterial obstruction secondary to atherosclerosis is commonly observed in the DFA (60%), at arterial bifurcations, or at the level of the POPA (entrapment syndrome). It may also result from intimal hyperplasia or radiation injury. High-frequency ultrasound is used for evaluation. Stenosis in peripheral arteries is depicted by B-mode as thickening of the vessel wall, narrowing of the lumen, or an echogenic filling defect. Doppler evaluation includes determination of PSV and flow direction, V2/V1 ratio (with V2 representing the PSV of a stenotic segment and V1 being the PSV of the proximal normal segment), and changes from a triphasic DSW to a biphasic (sharp systolic upstroke with loss of diastolic flow reversal), monophasic (blunted systolic upstroke), or severely blunted DSW described as “parvus tardus” (weak and slow systolic upstroke and downstroke). When using Doppler to evaluate arterial stenosis, (1) low velocity can be detected by reducing the velocity scales and applying power Doppler; (2) greater than 50% stenosis corresponds to at least double PSV of the proximal normal segment with accompanying changes in the DSW and poststenotic turbulence; (3) a “staccato” DSW indicates an upcoming occlusion; and (4) with total occlusion, zero intraluminal flow is evident, collaterals are seen in chronic cases, and secondary changes in flow in the arterial and venous circuits of the regional network may be observed (Figures 8 E-7 and 8 E-8).1–3
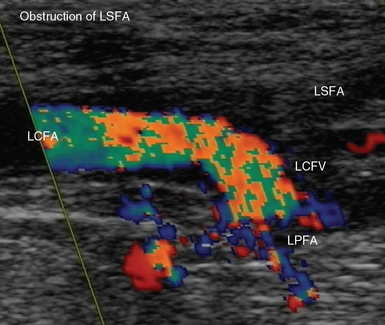
Figure 8 E-7 Acute obstruction of the left superficial femoral artery (LSFA); aliasing is depicted in the left common femoral (LCFA) and profunda (LPFA) arteries, respectively. LCFV, Left common femoral vein.
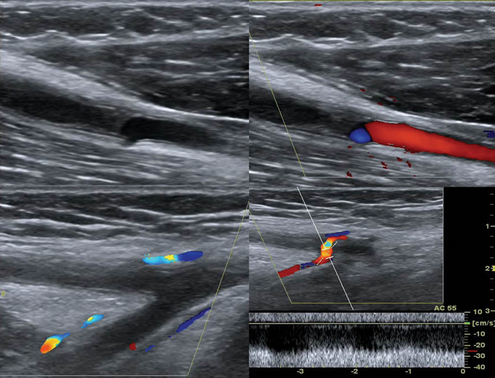
Figure 8 E-8 Chronic thrombosis of the brachial artery causing obstruction (top) and depiction of collaterals in the same case (bottom).
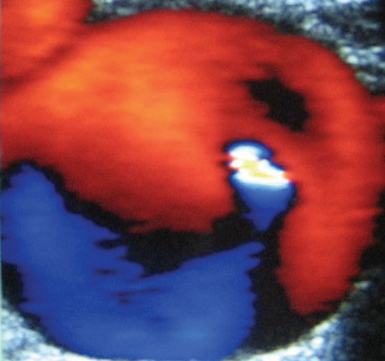
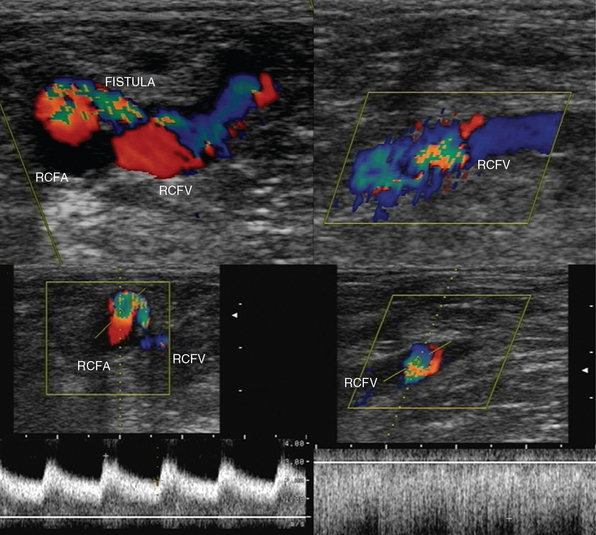
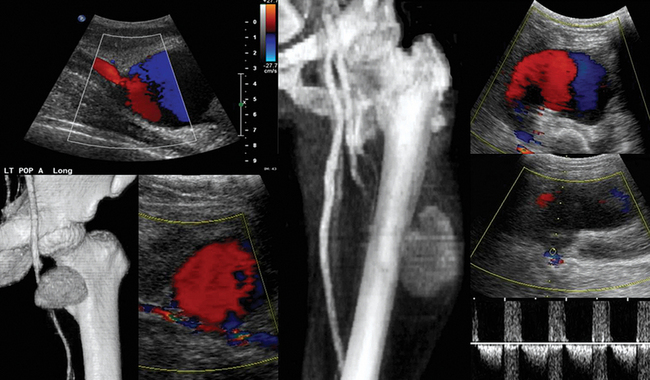
Pseudoaneurysms are pulsating, encapsulated hematomas in communication with the lumen of a ruptured vessel via a patent neck, and their walls may consist of adventitia, hematoma, and fibrous tissue. Color mode depicts turbulent flow (“yin-yang” sign) in the sac of the pseudoaneurysm (Figure 8 E-9), and Doppler mode detects to-and-fro flow patterns within its neck; coexisting thrombosis is common. In the ICU, pseudoaneurysms may form as a result of trauma or invasive procedures and can be repaired by ultrasound-guided compression (60% to 90% success rate). They could be also treated by ultrasound-guided injection of thrombin: the transducer is directed over the pseudoaneurysm, a 22-gauge spinal needle is inserted into its center, and 0.2 to 1.0 mL (1000 U/mL) of thrombin is injected. Peripheral aneurysms are dilated arterial segments twice the size of normal proximal arterial segments. Within the aneurysm, slow whirling of blood flow may be observed (Figure 8 E-10). Common sites of peripheral aneurysms are the POPA and CFA (3% of patients may have a POPA aneurysm). POPA or CFA aneurysms can coexist with an AAA (60% of male patients with a POPA aneurysm and more than 50% of patients with a CFA aneurysm could have an AAA). An important complication of POPA aneurysms is not rupture, but thrombosis. Surgical repair is reserved for peripheral aneurysms with a diameter of 2 cm or larger. An AVF is an abnormal connection of an artery and vein and may be posttraumatic or iatrogenic (Figure 8 E-11). It is characterized by color noise on duplex along with high velocity and a low-resistance DSW as arterial flow bypasses tissues and capillaries and drains straight into the vein (enhanced pulsatile venous flow).1,8 Ultrasound guides procedures such as venous and arterial cannulation, placement of inferior vena cava filters, vascular bypass, and carotid surgery. It can also be used to monitor the patency of vascular grafts and endovascular stents by identifying aneurysms, endoleaks or perigraft leaks, stenosis, thrombosis, and stent malposition.9,10 Finally, experimental studies have targeted plaque inflammation and neovascularization with contrast-enhanced ultrasound, and promising results have been shown.11,12
Pearls and highlights
• Arterial stenosis is commonly due to atherosclerosis. It is diagnosed by increments in Doppler PSV and changes in DSW. Strict ultrasound criteria exist only for classifying ICA stenosis.
• Total arterial occlusion is characterized by zero intraluminal flow and secondary hemodynamic changes in the arterial and venous circuits of the regional network.
• AAAs are usually atherosclerotic and infrarenal, and their predicted expansion rate is 0.2 to 0.5 cm/yr. If an AAA is larger than 5 cm, the risk for rupture increases and surgery is necessary (mortality rates exceed 80%).
• POPA and CFA aneurysms are the most common peripheral aneurysms and may coexist with an AAA.
• Pseudoaneurysms are usually posttraumatic and can be repaired by ultrasound-guided compression or thrombin injection.
References
1. Rzucidlo, EM, Zwolak, RM. Arterial duplex scanning. In Rutherford RB, ed.: Vascular Surgery, ed 6, Philadelphia: Elsevier Saunders, 2005.
2. Hallett JW, Brewster DC, Rasmussen TE, eds. Noninvasive vascular testing. In Handbook of patient care in vascular diseases. Philadelphia: Lippincott, Williams & Wilkins, 2001.
3. Zierler RE, ed. Strandess’s duplex scanning disorders in vascular diagnosis, ed 4, Philadelphia: Wolters, Kluwer, Lippincott, Williams & Wilkins, 2010.
4. Gerhard-Herman, M, Gardin, JM, Jaff, M, et al, Guidelines for noninvasive vascular laboratory testing: a report from the American Society of Echocardiography and the Society of Vascular Medicine and Biology. J Am Soc Echocardiog. 2006; 19:955–972.
5. Diethrich, EB, Pauliina-Margolis, M, Reid, DB, et al, Virtual histology intravascular ultrasound assessment of carotid artery disease: the Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL) study. J Endovasc The. 2007; 14:676–686.
6. Fayad, ZA, Fuster, V. Clinical imaging of the high-risk or vulnerable atherosclerotic plaque. Circ Res. 2001; 89:305–316.
7. Fenster, A, Landry, A, Downey, DB, et al. 3D ultrasound imaging of the carotid arteries. Curr Drug Targets Cardiovasc Haematol Disord. 2004; 4:161–175.
8. Van Bockel JH, Hamming, JF. Lower extremity aneurysms. In Rutherford RB, ed.: Vascular Surgery, ed 6, Philadelphia: Elsevier Saunders, 2005.
9. Back, MR, Novotney, M, Roth, SM, et al. Utility of duplex surveillance following iliac artery angioplasty and primary stenting. J Endovasc Ther. 2001; 8:629–637.
10. Lal, BK, Hobson, RW, 2nd., Goldstein, J, et al, Carotid artery stenting: is there a need to revise ultrasound velocity criteria. J Vasc Sur. 2004; 39:58–66.
11. Leong-Poi, H, Christiansen, J, Heppner, P, et al. Assessment of endogenous and therapeutic arteriogenesis by contrast ultrasound molecular imaging of integrin expression. Circulation. 2005; 111:3248–3254.
12. Vicenzini, E, Giannoni, MF, Benedetti-Valentini F, Lenzi, GL. Imaging of carotid plaque angiogenesis. Cerebrovasc Dis. 2009; 27:48–54.