Chapter 142 Urticaria (Hives) and Angioedema
Etiology and Pathogenesis
Acute urticaria and angioedema are often caused by an allergic immunoglobulin (Ig) E–mediated reaction (Table 142-1). This form of urticaria is a self-limited process that occurs when an allergen activates mast cells in the skin. Systemically absorbed allergens that can induce generalized urticaria include foods, drugs (particularly antibiotics), and stinging insect venoms. If an allergen (latex, animal dander) penetrates the skin locally, hives can develop at the site of exposure. Acute urticaria can also result from non–IgE-mediated stimulation of mast cells, caused by radiocontrast agents, viral agents including hepatitis B and Epstein-Barr virus, opiates, and nonsteroidal anti-inflammatory agents. The diagnosis of chronic urticaria is established when lesions recur at least twice a week for > 6 wk and are not physical urticaria or recurrent acute urticaria with repeated exposures to a specific agent (Table 142-2). Often, chronic urticaria is accompanied by angioedema. Rarely, angioedema occurs without urticaria.
| Foods | Egg, milk, wheat, peanuts, tree nuts, soy, shellfish, fish, strawberries (direct mast cell degranulation) |
| Medications | Suspect all medications, even over-the-counter or homeopathic |
| Insect stings | Hymenoptera (honeybee, yellow jacket, hornets, wasp, fire ants), biting insects (papular urticaria) |
| Infections | Bacterial (streptococcal pharyngitis, Mycoplasma, sinusitis); viral (hepatitis, mononucleosis [Epstein-Barr virus], coxsackievirus A and B); parasitic (Ascaris, Ancylostoma, Echinococcus, Fasciola, Filaria, Schistosoma, Strongyloides, Toxocara, Trichinella); fungal (dermatophytes, Candida) |
| Contact allergy | Latex, pollen, animal saliva, nettle plants, caterpillars |
| Transfusion reactions | Blood, blood products, or IV immunoglobulin administration |
From Lasley MV, Kennedy MS, Altman LC: Urticaria and angioedema. In Altman LC, Becker JW, Williams PV, editors: Allergy in primary care, Philadelphia, 2000, WB Saunders, p 232.
Table 142-2 ETIOLOGY OF CHRONIC URTICARIA
| Idiopathic | 75-90% of chronic urticaria cases are idiopathic, and 35-40% have immunoglobulin (Ig) G, anti-IgE, and anti-FcεRI (high-affinity IgE receptor α chain) autoantibodies |
| Physical | Dermatographism |
| Cholinergic urticaria | |
| Cold urticaria | |
| Delayed pressure urticaria | |
| Solar urticaria | |
| Vibratory urticaria | |
| Aquagenic urticaria | |
| Rheumatologic | Systemic lupus erythematosus |
| Juvenile rheumatoid arthritis | |
| Endocrine | Hyperthyroidism |
| Hypothyroidism | |
| Neoplastic | Lymphoma |
| Mastocytosis | |
| Leukemia | |
| Angioedema | Hereditary angioedema (autosomal dominant inherited deficiency of C1-esterase inhibitor) |
| Acquired angioedema | |
| Angiotensin-converting enzyme inhibitors |
From Lasley MV, Kennedy MS, Altman LC: Urticaria and angioedema. In Altman LC, Becker JW, Williams PV, editors: Allergy in primary care, Philadelphia, 2000, WB Saunders, p 234.
Physical Urticaria
Physically induced urticaria and angioedema share the common property of being induced by environmental factors, such as a change in temperature or direct stimulation of the skin with pressure, stroking, vibration, or light (Table 142-3).
| DIAGNOSIS | DIAGNOSTIC TESTING |
|---|---|
| Food and drug reactions | Elimination of offending agent, skin testing, and challenge with suspected foods |
| Autoimmune urticaria | Autologous serum skin test; anti-thyroid antibodies |
| Thyroiditis | Thyroid-stimulating hormone; anti-thyroid antibodies |
| Infections | Appropriate cultures or serology |
| Collagen vascular diseases and cutaneous vasculitis | Skin biopsy, CH50, C1q, C4, C3, factor B, immunofluorescence of tissues, antinuclear antibodies, cryoglobulins |
| Malignancy with angioedema | CH50, C1q, C4, C1-INH determinations |
| Cold urticaria | Ice cube test |
| Solar urticaria | Exposure to defined wavelengths of light, red blood cell protoporphyrin, fecal protoporphyrin, and coproporphyrin |
| Dermatographism | Stroking with narrow object (e.g., tongue blade, fingernail) |
| Pressure urticaria | Application of pressure for defined time and intensity |
| Vibratory urticaria | Vibration for 4 min |
| Aquagenic urticaria | Challenge with tap water at various temperatures |
| Urticaria pigmentosa | Skin biopsy, test for dermographism |
| Hereditary angioedema | C4, C2, CH50, C1-INH testing by protein and function |
| Familial cold urticaria | Challenge by cold exposure, measurement of temperature, white blood cell count, erythrocyte sedimentation rate, and skin biopsy |
| C3b inactivator deficiency | C3, factor B, C3b inactivator determinations |
| Chronic idiopathic urticaria | Skin biopsy, immunofluorescence (negative result), autologous skin test |
Diagnosis
Urticaria is transient, pruritic, erythematous, raised wheals, with flat tops and edema that may become tense and painful. The lesions may coalesce and form polycyclic, serpiginous, or annular lesions (Figs. 142-1 and 142-2). Individual lesions usually last 20 min to 3 hr, and rarely more than 24 hr. The lesions often disappear only to reappear at another site. Angioedema involves the deeper subcutaneous tissues in locations such as the eyelids, lips, tongue, genitals, and dorsum of the hands or feet.
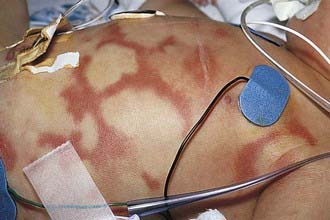
Figure 142-1 Polycyclic lesions of urticaria associated with prostaglandin E2 infusion.
(From Eichenfield LF, Friedan IJ, Esterly NB: Textbook of neonatal dermatology, Philadelphia, 2001, WB Saunders, p 300.)
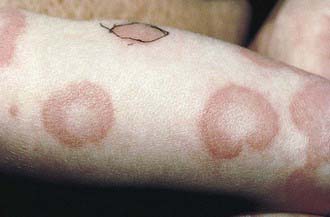
Figure 142-2 Annular urticaria of unknown etiology.
(From Eichenfield LF, Friedan IJ, Esterly NB: Textbook of neonatal dermatology, Philadelphia, 2001, WB Saunders, p 301.)
An exogenous cause of chronic urticaria is rarely identified, reflecting an autoimmune or idiopathic etiology. An ASST is useful in establishing the diagnosis of autoimmune urticaria. In vitro testing for serum-derived activity that activates basophils involves detection of the expression of the surface marker CD63 or CD203c by donor basophils after incubation with patient serum. The clinical applicability and significance of these tests remains debated. The differential diagnosis of chronic urticaria includes cutaneous or systemic mastocytosis, complement-mediated disorders, malignancies, mixed connective tissue diseases, and cutaneous blistering disorders (e.g., bullous pemphigoid) (see Table 142-2). In general, laboratory testing should be limited to a complete blood cell count with differential, ESR determination, urinalysis, thyroid autoantibody testing, and liver function tests. Further studies are warranted if the patient has fever, arthralgias, or elevated ESR (see Table 142-3). Hereditary angioedema, a potentially life-threatening form of angioedema associated with deficient C1 inhibitor activity, is the most important familial form of angioedema (Chapter 128.3), but is not associated with typical urticaria. In patients with eosinophilia, stools should be obtained for ova and parasite testing, because infection with helminthic parasites has been associated with urticaria. A syndrome of episodic angioedema/urticaria and fever with associated eosinophilia has been described in both adults and children. In contrast to other hypereosinophilic syndromes, this entity has a benign course.
Physical urticaria should be considered in any patient with chronic urticaria and a suggestive history (see Table 142-3). Papular urticaria commonly occurs in small children, generally on the extremities. It manifests as grouped or linear, highly pruritic wheals or papules mainly on exposed skin at the sites of insect bites.
Exercise-induced anaphylaxis manifests as varying combinations of pruritus, urticaria, angioedema, wheezing, laryngeal obstruction, or hypotension after exercise (Chapter 143). Cholinergic urticaria is differentiated by positive results of heat challenge tests and the rare occurrence of anaphylactic shock. The combination of ingestion of various food allergens (shrimp, celery, or wheat) and postprandial exercise has been associated with urticaria/angioedema and anaphylaxis. In patients with this combination disorder, food or exercise alone does not produce the reaction.
Treatment
Acute urticaria is a self-limited illness requiring little treatment other than antihistamines and avoidance of any identified provocateur. Hydroxyzine and diphenhydramine are sedating but are effective and commonly used for treatment of urticaria. Loratadine, fexofenadine, and cetirizine are also effective and are preferable because of reduced frequency of drowsiness and longer duration of action (Table 142-4). Epinephrine 1 : 1,000, 0.01 mL/kg (maximum: 0.3 mL) usually provides rapid relief of acute, severe urticaria/angioedema but is seldom required. A short course of oral corticosteroids should be given only for very severe episodes of urticaria and angioedema that are unresponsive to antihistamines.
| CLASS/DRUG | DOSE | FREQUENCY |
|---|---|---|
| ANTIHISTAMINES, TYPE H1 (2ND GENERATION) | ||
| Fexofenadine | 6-11 yr: 30 mg | bid |
| >12 yr: 60 mg | ||
| Adult: 180 mg | Once daily | |
| Loratadine | 2-5 yr: 5 mg | Once daily |
| >6 yr: 10 mg | ||
| Desloratadine | 6-11 mo: 1 mg | Once daily |
| 1-5 yr: 1.25 mg | ||
| 6-11 yr: 2.5 mg | ||
| >12 yr: 5 mg | ||
| Cetirizine | 6-24 mo: 2.5 mg | 6-12 mo: once daily |
| 2-6 yr: 2.5-5 mg | 12-24 mo: 1-2 daily | |
| >6 yr: 5-10 mg | 2-12 yr: once daily | |
| ANTIHISTAMINES, TYPE H2 | ||
| Cimetidine | Infants: 10-20 mg/kg/day | Divided q6-12h |
| Children: 20-40 mg/kg/day | ||
| Ranitidine | 1 mo-16 yr: 5-10 mg/kg/day | Divided q12h |
| Famotidine | 3-12 mo: 1 mg/kg/day | Divided q12h |
| 1-16 yr: 1-2 mg/kg/day | ||
| LEUKOTRIENE PATHWAY MODIFIERS | ||
| Montelukast | 12 mo-5 yr: 4 mg | Once daily |
| 6-14 yr: 5 mg | ||
| >14 yr: 10 mg | ||
| Zafirlukast | 5-11 yr: 10 mg | bid |
| IMMUNOMODULATORY DRUGS | ||
| Cyclosporine | 4-6 mg/kg/day | Once daily* |
| Sulfasalazine | >6 yr: 30 mg/kg/day | Divided q6h† |
| Intravenous immunoglobulin (IVIG) | 400 mg/kg/day | 5 consecutive days |
* Monitor blood pressure and serum creatinine, potassium, and magnesium levels monthly.
† Monitor complete blood count and liver function tests at baseline, every 2 wk for 3 mo, and then every 1-3 mo.
Chronic urticaria only rarely responds favorably to dietary manipulation. Removal of recognized urticarial aggravators such as salicylates and β-blockers should be considered. The mainstay of therapy is the use of nonsedating or low-sedating H1 antihistamines. In those patients not showing response to standard doses, pushing the H1 blockade with higher than the usual recommended doses of these agents is a common next approach. The combined use of H1– and H2 antihistamines is sometimes helpful to control chronic urticaria when H1-type antihistamines alone at higher than standard doses do not work (see Table 142-4). Doxepin is an antagonist of both H1 and H2 receptors and can be helpful, but its usefulness is limited by adverse effects. H2-type antihistamines alone may exacerbate urticaria. Antileukotriene agents in combination with antihistamines are occasionally helpful. If hives persist after maximal H1– and/or H2-receptor blockade has been achieved, a brief course of oral corticosteroids may be considered, but long-term steroid use is best avoided. Treatment with cyclosporine 4-6 mg/kg/day has been effective in some adults with chronic urticaria but its use is limited by hypertension and/or nephrotoxicity. Immunomodulatory agents that have been used but remain to be formally proven effective include hydroxychloroquine, sulfasalazine, colchicine, dapsone, mycophenylate and omalizumab (anti-IgE), intravenous immunoglobulin, and plasmapheresis have been used to treat autoimmune chronic urticaria refractory to other therapies.
Hereditary Angioedema
Hereditary angioedema (HAE) is an inherited disease caused by low levels of the plasma protein C1 inhibitor (C1-INH) (Chapter 128). Patients typically report episodic attacks of angioedema or deep localized swelling, most commonly on a hand or foot, that begin during childhood and become much more severe during adolescence. Cutaneous nonpitting and nonpruritic edema not associated with urticaria is the most common symptom. Patients usually have a prodrome, a tightness or tingling in the area that will swell, lasting most frequently for several hours, followed by the development of angioedema. The swelling usually becomes more severe over about 10 days and then resolves over about the same period. In some patients attacks are preceded by the development of a rash that is erythematous, not raised, and not pruritic. The second major symptom complex noted by patients is attacks of severe abdominal pain caused by edema of the mucosa of any portion of the gastrointestinal tract. The intensity of the pain can approximate that of an acute abdomen, often resulting in unnecessary surgery. Either constipation or diarrhea during these attacks can be noted. The gastrointestinal edema generally follows the same time course to resolution as the cutaneous attacks.
Boguniewicz M. Chronic urticaria in children. Allergy Asthma Proc. 2005;26:13-17.
Dibbern DAJr. Urticaria: Selected highlights and recent advances. Med Clin North Am. 2006;90:187-209.
Frank MM. Hereditary angioedema: the clinical syndrome and its management in the United States. Immunol Allergy Clin North Am. 2006;26:653-668.
Grattan CE, Humphries F. Guidelines for evaluation and management of urticaria in adults and children. Br J Dermatol. 2007;157:1116-1123.
Kaplan AP, Greaves M. Pathogenesis of chronic urticaria. Clin Exp Allergy. 2009;39:777-787.
Morgan M, Khan DA. Therapeutic alternatives for chronic urticaria: an evidence based review, part 2. Ann Allergy Asthma Immunol. 2008;100:517-526.
Poon M, Springs A, Reid C. Do steroids help children with acute urticaria? Arch Dis Child. 2004;89:85-86.
Powell RJ, Du Toit GL, Siddique N, et al. BSACI guidelines for the management of chronic urticaria and angio-oedema. Clin Exp Allergy. 2007;37:631-650.
Sackesen C, Sekerel BE, Orhan F, et al. The etiology of different forms of urticaria in childhood. Pediatr Dermatol. 2004;21:102-108.





