Urologic Procedures
Testicular Torsion
Although acute scrotal pain accounts for less than 1% of overall emergency department (ED) visits, it may provoke great anxiety in the patient or caretaker given its highly sensitive nature.1 One of the most challenging aspects of scrotal complaints is that a wide variety of clinical conditions may have similar signs and symptoms: a male patient complaining of an acute, painful, swollen, and tender hemiscrotum. The most common urologic emergencies manifested as acute scrotal pain include testicular torsion, severe infectious epididymitis, and Fournier’s necrotizing fasciitis. Other painful conditions include torsion of the appendix testis, inguinal hernia, trauma, testicular cancer, referred pain (such as ureterolithiasis and diverticulitis), Henoch-Schönlein purpura, and orchitis (mumps, brucellosis, coxsackievirus disease, etc.). Hydrocele, varicocele, spermatocele, and testicular cancer tend to be characterized by painless, isolated swelling. Although the differential diagnosis is extensive, testicular torsion is the principal threat to fertility that needs to be ruled out. Definitive management of testicular torsion involves surgical exploration and orchiopexy. Manual detorsion, with or without spermatic cord anesthesia, can be attempted while simultaneously preparing for operative intervention.
Background
An acute scrotum is defined as an acute painful swelling of the scrotum or its contents, accompanied by local signs or general symptoms.2 Acute epididymitis is commonly the cause of acute scrotal pain in adolescents and adults. Torsion of testicular (or epididymal) appendages is another frequent cause of acute scrotal pain in prepubertal boys.
Anatomy and Physiology
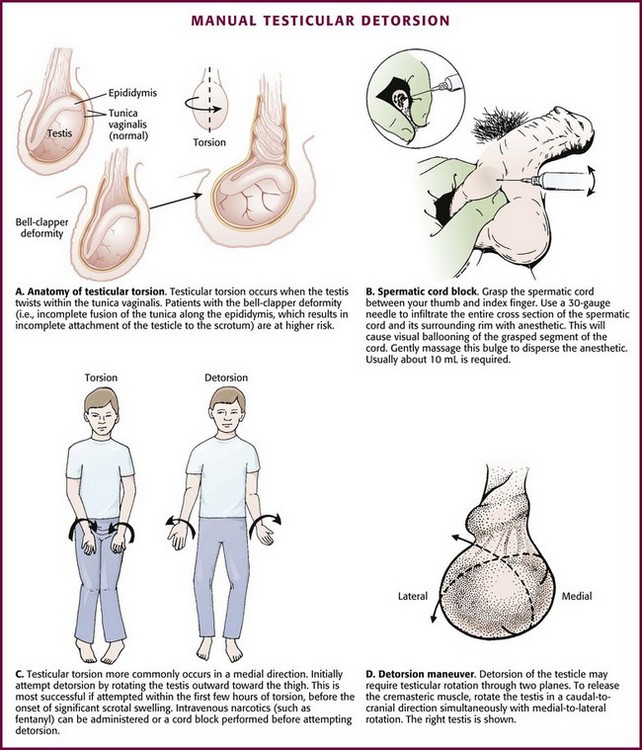
A congenital anomaly of fixation of the testis, termed the bell-clapper deformity, is associated with the development of testicular torsion.3 It occurs when the intrascrotal portion of the spermatic cord lacks firm posterior adhesion to the scrotal wall and remains surrounded by the tunica vaginalis (Fig. 55-1A). As a result of the abnormal attachment, the testis may be suspended horizontally.4 These anatomic features predispose the affected testis to rotation.
Pathophysiology
Testicular salvage rates decrease with time. A metaanalysis of 1140 patients in 22 series demonstrated a greater than 90% salvage rate when surgery was performed within 6 hours of the onset of pain. 5 Testicular atrophy may lead to decreased fertility. Furthermore, testicular loss may result in dysfunction of the contralateral testis through immune-mediated or other mechanisms.5
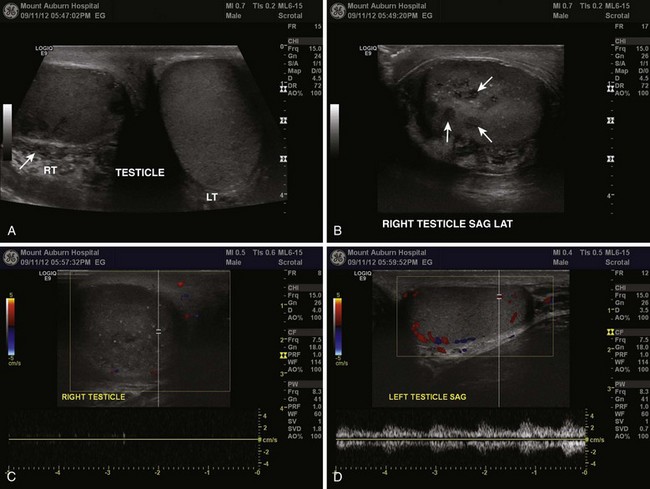
When used in the appropriate clinical setting, ultrasound remains the most useful diagnostic modality in the evaluation of genitourinary (GU) complaints (Fig. 55-2). A patient with compelling findings of testicular torsion on the history and physical examination does not require any preoperative diagnostic tests. Color flow Doppler ultrasonography (CDUS) may be very helpful in all other cases. The classic sonographic finding of testicular torsion is diminished intratesticular blood flow. In addition, examination of the spermatic cord with high-resolution gray-scale ultrasonography may reveal “coiling” or “kinking” of the cord at the site of torsion.6–8
Sonography is used not only to exclude testicular torsion but also to search for alternative causes of acute scrotal pain.9 With epididymitis, perfusion may be normal (or increased) because of the effects of inflammatory mediators on local vascular beds, although this is a nonspecific finding.10,11 An infarcted appendage may be visualized on ultrasound as well.12 Emergency physicians with appropriate training may be able to accurately assess intratesticular blood flow with bedside sonography in patients with acute scrotal pain.13
CDUS has long been regarded as the diagnostic modality of choice in assessing for testicular torsion. However, false-negative ultrasound results have been reported.14–20 Many of these studies are case reports or case series limited by small numbers and retrospective design. Two larger series reported documented intratesticular blood flow with CDUS in 6 of 23 (26%) and 50 of 208 cases (24%), respectively, of confirmed testicular torsion.6,21 Unfortunately, Doppler ultrasound may reveal seemingly adequate intratesticular blood flow in those with partial torsion, which can mislead the practitioner.22
Radionuclide scintigraphy and CDUS have similar sensitivity, as well as false-negative rates, for the diagnosis of testicular torsion.23 However, given the widespread availability of and expertise in ultrasound technology, combined with the risks associated with exposure to radioactive isotopes, radionuclide procedures have fallen out of favor. The use of magnetic resonance imaging has been explored, but limitations include speed of imaging and availability.24,25
Indications
Testicular torsion can be relieved by manual detorsion. A study of 162 cases of testicular torsion revealed that the anticipated lateral-to-medial rotation occurred in 67% of cases, with medial-to-lateral rotation seen in the remaining 33%.26 This challenges the standard dogma of medial-to-lateral rotation, or “opening the book,” as the standard method of detorsion. As noted, there may be a cranial-caudal component to the torsion as well. The end point of manual detorsion is relief of pain or return of intratesticular blood flow as seen on ultrasound imaging.27 Although manual detorsion may allow reperfusion of the testis, a lesser degree of residual torsion may remain. Given that infarction can occur with as little as 180 degrees of torsion, immediate surgical exploration is still advocated after what is thought to successful manual detorsion.26 The bottom line is that specialty consultation and plans for possible immediate surgical exploration need to occur regardless of the outcome of the detorsion procedure.
Procedure
Manual Detorsion and Spermatic Cord Anesthesia
Spermatic Cord Anesthesia: Local anesthesia of the spermatic cord can be induced with 1% plain lidocaine injected at the external or superficial inguinal ring (Fig. 55-1B).28 First prepare the skin with an antiseptic solution. Grasp the cord between the thumb and index finger, and inject 10 mL of 1% plain lidocaine (in an adult) directly into the cord. If the cord is swollen, which is frequently the case with testicular torsion, or if the testicle is lying very high in the hemiscrotum because of spermatic cord torsion (thus precluding grasping), palpate the cord at the pubic tubercle as it passes over the pubis and inject the lidocaine at this landmark. Lee and colleagues29 were able to perform manual detorsion with local spermatic cord anesthesia in 70% of their cases of torsion in adults. Kresling and associates30 had success in 15 of 16 patients and noted a fair amount of associated cremasteric muscle spasm, which must also be relieved.
Manual Detorsion: The goal of manual detorsion is to reestablish or increase blood flow to a previously ischemic testis. This should be done while the operating suite is being prepared. It should never delay operative intervention.
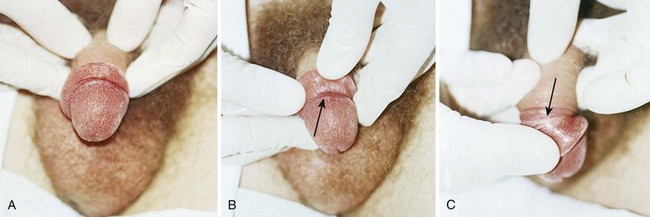
Begin manual detorsion with the clinician standing comfortably at the side of the bed or stretcher, preferably on the patient’s right side if the clinician is right handed or on the left side if left handed. Begin detorsion just as one would open a book (i.e., an initial 180-degree detorsion of the patient’s right testis is done in a counterclockwise fashion) (see Fig. 55-1C and D). The patient’s left testis is detorsed 180 degrees in a clockwise fashion. Ask the patient whether there was any relief of pain after the maneuver. If one rotation relieves some but not all of the pain, continue with another rotation of the testis. The degree of torsion may range from 180 to 1080 degrees, with medians of 360 to 540 degrees.26 Many patients will require two or three rotations to fully alleviate their pain. If the initial detorsion is mechanically difficult (which it will be if detorsion is done in the wrong direction) or makes the pain worse, detorse the testis in the opposite direction and observe the result. Approximately one third of testicular torsions occur in the lateral, or unexpected, direction.26 The objective success or failure of testicular manipulation can be further substantiated by an increase in Doppler signal and symptomatic relief of pain.
Priapism
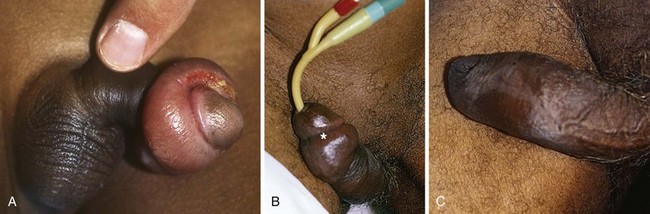
Priapism is defined as a prolonged erection of the penis, generally for more than 4 hours, in the absence of sexual desire or stimulation (Fig. 55-3). This medical condition was named after Priapus, an ancient Greek god of fertility and horticulture who was endowed with oversized genitalia.31
Ischemic priapism can be thought of as a compartment syndrome of the penis.32 The corpora cavernosa become engorged with stagnant, oxygen-depleted venous blood because of either intraluminal obstruction of venous blood flow or an inability of the penile muscle tissue to adequately contract and augment venous outflow.33
Background
More than a third of patients with severe priapism may suffer permanent erectile dysfunction despite treatment, with obvious functional and emotional sequelae.33
Anatomy and Physiology
Priapism is characterized clinically by a soft glans penis and spongy urethra in the presence of two erect penile bodies (corpora cavernosa) (Fig. 55-4). Two important concepts are worthy of mention. First, there is communication of blood flow between the corpora cavernosa; therefore, in most cases the operator needs to access only one of the corpora. Second, the introduction of vasoactive or other agents into the corpora is akin to an intravenous injection and may precipitate systemic effects, particularly when partial detumescence is achieved.
Pathophysiology
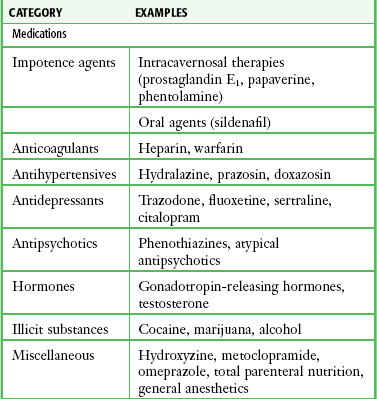
In the past, priapism was most often encountered as a complication of a number of medical conditions, such as hematologic, neoplastic, or drug-related conditions. Today, many cases are iatrogenic and result from the current practice of using vasoactive substances (e.g., papaverine and phentolamine). Another cause is the newer erectile dysfunction medications used to induce penile erections in impotent men. Sickle cell disease continues to be a leading cause of priapism. Sickle cell patients may experience such a high rate of recurrence that home self-injection of vasoactive drugs into the penis has been advocated. Cocaine use is one cause that is likely to be underreported.34 A drug screen may unravel some discrepancies between the clinical findings and history. As an end result, vasoactive drugs promote engorgement of the corpora cavernosa and reduction in venous outflow, which may result in low-flow or ischemic priapism.35–37 Several phosphodiesterase inhibitors and prostaglandin E1 are the drug treatments of impotence approved by the U.S. Food and Drug Administration. These medications act by increasing penile blood flow by enhancing smooth muscle relaxation. The incidence of priapism with these medications is quite low, particularly with the phosphodiesterase inhibitors. Penile rigidity secondary to a nondeflating penile prosthesis (pseudopriapism) or malignant replacement of the corpora in patients with bladder or prostate cancer should not be confused with true priapism.
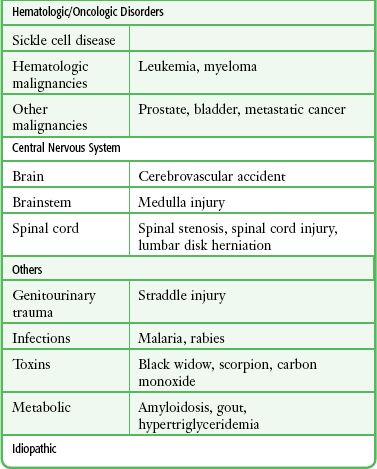
Selected causes of ischemic priapism are listed in Table 55-1.
Indications
Attempt to identify reversible causes of low-flow priapism and initiate specific corrective therapy as soon as possible. Low-flow priapism in children and young adults may be due to sickle cell disease, and such cases may respond to noninvasive standard antisickling measures. However, the role of transfusion therapy in patients with priapism caused by sickle cell anemia is uncertain.38
Treatment of ischemic priapism is frequently initiated in the ED. The classic teaching is that the initial treatment—oral or subcutaneous terbutaline—is the same regardless of the cause, but its utility is debated.39–41 It is thought that terbutaline, a β2-adrenergic agonist, increases venous outflow from the engorged corpora by relaxing venous sinusoidal smooth muscle. Terbutaline is of unproven benefit and is often ineffective; however, given its limited propensity for adverse effects, it is reasonable to initiate a trial in selected circumstances.42
Contraindications
A subtype of ischemic priapism is known as stuttering priapism. This entity is typically observed in patients with sickle cell disease. Patients experience recurrent episodes of priapism that often last less than 3 hours and frequently do not require emergency treatment unless the symptoms become markedly prolonged.43 High-flow (non-ischemic) priapism is treated surgically (nonurgent). Although perhaps intuitive treatment, transfusion therapy for acute priapism of sickle cell disease is controversial. Exchange transfusion has been associated with acute neurological events (headache, seizures, obtundation) that are thought to be due to a rapid elevation of hemoglobin (greater than 12 g/dL) and a release of procoagulant and vasoactive factors from the corpus carvernosa (ASPEN syndrome). Partial exchange transfusion (target hemoglobin less than 10 g/dL) has not been associated with these condition.
Procedure
A suggested algorithm for the initial treatment of acute nonischemic priapism in the emergency setting is presented in Box 55-1.
Minimally Invasive Technique—Simple Injection
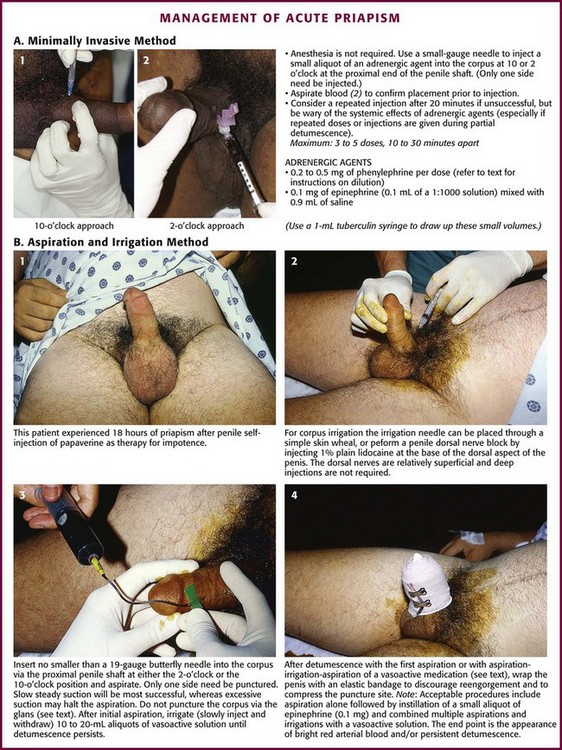
Relief of priapism by simple injection of vasoactive solutions into the corpus cavernosum is a procedure that has been reported.44 Intercavernous injection therapy for the management of priapism is simple to perform, less traumatic, and less invasive than aspiration and irrigation. This minimally invasive procedure may be attempted as an initial approach. The same procedure may be used as a self-injection technique for home treatment of recurrent priapism. With this technique, a 25- to 27-gauge needle (tuberculin or insulin syringe) is used to inject vasoactive substances into the corpus at the proximal end of the penis (2 to 4 cm distal to the origin of the shaft), with the goal of pharmacologically reversing the priapism (Fig. 55-5A). The principle is sympathomimetic-initiated contraction of the cavernous smooth muscle to permit venous outflow. Frequently, this small needle injection can be accomplished without anesthesia and is usually barely perceived by the patient. The most recommended technique is to inject 0.2 to 0.5 mg of phenylephrine into the corpus every 10 to 15 minutes to a maximum of four to five doses, although more frequent dosing has also been advocated. Use the lower dose in patients with cardiovascular pathology. Phenylephrine is available as a 1% solution (10 mg/mL), which must be diluted for this procedure. If 1 mL (10 mg) of the 1% solution of phenylephrine is added to 9 mL of saline, the final phenylephrine concentration is 1 mg/mL. Then withdraw 0.2 mL (0.2 mg) or 0.5 mL (0.5 mg) of this diluted solution with a tuberculin or insulin syringe and add saline to increase the final volume for injection to 1 mL. Phenylephrine is preferred by the American Urologic Society because of minimal cardiovascular side effects. An alternative, readily available injection solution is a mixture of epinephrine and saline. Draw up 0.1 mg of epinephrine (0.1 mL of 1 : 1000) in a tuberculin or insulin syringe and dilute it with 0.9 mL of saline (total volume of 1 mL). Systemic effects, such as hypertension, headache, tremors, and cardiac arrhythmias, are potential side effects of any sympathomimetic injection, so caution is advised, especially if multiple injections are used in a semi-erectile state.
Puncture the corpus with the needle at the 10- or 2-o’clock position at the base of the penis (with 12 o’clock being the dorsal vein of the penis). Aspirate blood to confirm the correct position, and then inject the solution. If not successful in 20 to 30 minutes, repeat the injection up to a total of three injections. In one small study, successful detumescence was achieved in eight of nine patients by simple intracorporal injection of phenylephrine via this regimen, and three or fewer injections were required.45 Only one side needs to be injected. Two or three injections might be necessary. Wait at least 20 minutes after each injection before trying additional interventions. Note that this is essentially an intravenous injection and systemic effects may occur, especially if partial detumescence has been achieved. Accordingly, proceed with caution in patients with cardiovascular disease. Success has also been noted by injecting the corpus cavernosum with 1 mL of the local anesthetic lidocaine (2%) with epinephrine (1 : 100,000) into each side or 2 mL into one side.44
Table 55-2 lists adrenergic agents used for intracavernosal injections.
Table 55-2
Adrenergic Agents Used for Intracavernous Injections
| AGENT | DOSE | VOLUME |
| Phenylephrine* | 0.2-0.5 mg | Dilute with saline; final volume, 1 mL* |
| Epinephrine (1 : 1000) | 0.1 mg (0.1 mL) | Dilute with 0.9 mL saline; final volume, 1 mL |
| Lidocaine (2%) with epinephrine (1 : 100,000) (local anesthetic solution) | 40 mg lidocaine 0.02 mg epinephrine (2 mL) |
1 mL injected into each side of the corpus cavernosum; final volume, 2 mL† |
*Single side injected with the entire amount. See text for preparation of the phenylephrine injection solution.
†Total amount divided into two doses. Inject each side with half the total volume (1 mL) or inject the total volume (2 mL) into one side.
From Roberts JR, Price C, Mazzeo T. Intracavernous epinephrine: a minimally invasive treatment for priapism in the emergency department. J Emerg Med. 2009;36:3:285-289.
Aspiration/Irrigation Technique
If injection is unsuccessful or if the erection has persisted for more than 4 to 6 hours, aspiration of blood from the corpus cavernosum, with or without irrigation, is performed. This is usually combined with an intracavernosal injection of a sympathomimetic drug. Box 55-2 lists the equipment needed for aspiration and irrigation of the corpus cavernosum. This procedure entails drainage of blood from the erect penis, irrigation with saline if necessary (i.e., inadequate return of blood with lack of detumescence), and finally, instillation of a vasoactive medication. As an alternative to instillation of a sympathomimetic drug, irrigation with aliquots of a dilute vasoactive solution (1 mg of epinephrine added to 1 L of saline) may be effective (aspirate-infuse-aspirate cycle as needed).
Place the patient in the supine position. Use parenteral analgesia and sedation. Local anesthesia is recommended and may be achieved with a generous local wheal of lidocaine injected at the puncture site but is best obtained with a penile nerve block (Fig. 55-5B, step 2). Perform the block by injecting 1% plain lidocaine at the base of the dorsal and ventral surface of the penis for a dorsal penile nerve block, or place a circumferential penile block. The nerves are relatively superficial and deep injections are not required. Prepare and drape the penis in sterile fashion. Grasp the shaft of the penis with the nondominant thumb and index finger. Palpate the engorged corpus cavernosum laterally (2- and 10-o’clock positions), and insert a 19-gauge butterfly needle into the corpus cavernosum (see Fig. 55-5B, step 3).46 A dialysis access butterfly needle may also be used (Fig. 55-6). Small butterfly needles and standard intravenous catheters are not ideally suited for the procedure but may be used. If palpation fails to identify the corpus, blindly insert the needle at either 10 or 2 o’clock to gain access to this large vascular structure. Because there is communication of blood flow between both sides, only one of the corpora needs to be aspirated or irrigated. Either side may be punctured. The site of needle placement is typically anywhere from the base to the proximal end of the shaft, approximately 2 to 4 cm distal to its origin. Do not use the glans as a puncture site.
If detumescence is achieved and maintained after initial aspiration, no further treatment may be necessary. Even if this is successful, some advise instilling an aliquot of a vasoactive substance. Phenylephrine is recommended as the agent of choice because it may minimize the risk for cardiovascular side effects that are caused by the more common sympathomimetic agents.41 Inject 0.2 to 0.5 mg of phenylephrine (diluted in 1 mL of normal saline). The concentration recommended for instillation is similar to that suggested for the minimally invasive technique detailed earlier. Use lower concentrations in smaller volumes for patients with cardiovascular risk factors or for children. If irrigation has been performed with a dilute vasoactive substance as delineated below, additional instillation of medication is not suggested.
Aftercare
Observe the patient in the ED for recurrence. Although the ideal observation period is unknown, 2 hours has been suggested.44 Figure 55-5B, step 4, demonstrates the entire penis loosely wrapped with an elastic (Ace) bandage to prevent hematoma formation at the injection site or sites. Provide strict return precautions for recurrence of priapism, and ensure that urgent urology follow-up is possible. A short course of an oral α-adrenergic agent, such as pseudoephedrine for 3 days, is often recommended.44 However, this intervention is of questionable value and unproven benefit.
Complications
Although hematoma and infection can occur after properly performed aspiration, these complications are infrequent. Injected or instilled vasoactive agents can be absorbed systemically, with potential toxic effects.47 Therefore, the intracavernosal use of vasoactive agents is relatively contraindicated in patients with conditions that are sensitive to these agents (e.g., severe hypertension, dysrhythmias, monoamine oxidase inhibitor use). Monitor blood pressure and cardiac rhythm throughout the procedure if the patient is at risk. Failure to aspirate blood is a potential complication, usually because of a misplaced needle, application of excessive suction, or clotted blood. Because impotence is a well-recognized complication of priapism regardless of the cause or promptness of therapeutic intervention, advise the patient regarding this potential complication.
Conclusion
Because prolonged priapism increases the risk for subsequent erectile dysfunction, an aggressive management strategy is advised. After 4 hours of persistent priapism there is heightened release of inflammatory cytokines in the acidotic and hypoxic corpora cavernosa. Inflammation may result in changes in smooth muscle, including cell death and fibrosis, which may cause permanent erectile dysfunction.48 Recurrence is not uncommon, and some patients require multiple procedures on a recurring basis. Surgical shunting procedures might be required if these other measures are not met with success.
Paraphimosis
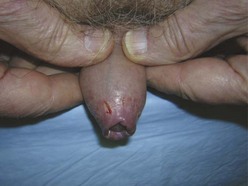
Paraphimosis may occur at any age but is often seen in the extremes of life (Fig. 55-7). The condition can be quite subtle and may be either unrecognized or misdiagnosed as an allergic reaction, penile trauma, infection, or edema resulting from systemic volume overload (i.e., congestive heart failure, nephrotic syndrome) (Fig. 55-8).
Background
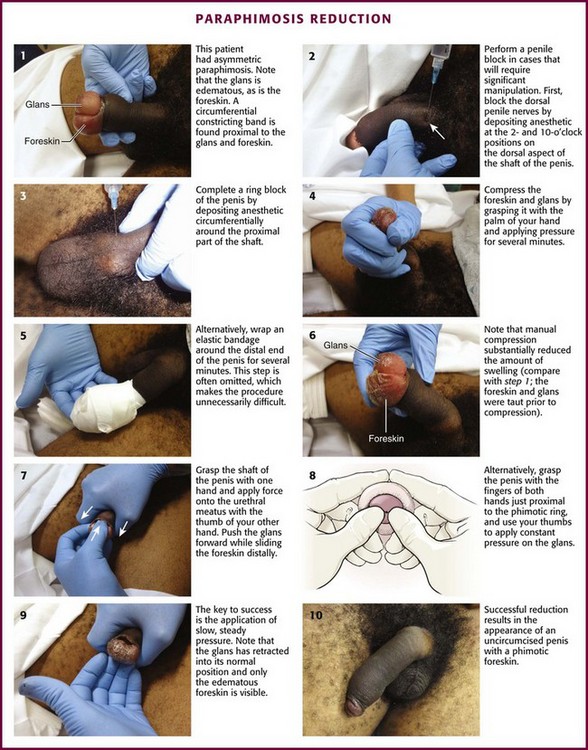
Paraphimosis is a urologic emergency that must be treated promptly to prevent necrosis of the glans. It can frequently be managed in the ED without the need for emergency specialty consultation. Many methods for successful reduction of paraphimosis have been reported; however, the most commonly used initial maneuver involves manual compression of the distal end of the glans penis to decrease edema, followed by reduction of the glans penis back through the proximal constricting band of foreskin.49
Anatomy and Physiology
The penis consists of the paired corpora cavernosa, or erectile bodies, which lie dorsal to the corpus spongiosum (see Fig. 55-4). The corpus spongiosum surrounds the penile urethra. The corpora cavernosa and the corpus spongiosum are wrapped in a thin connective tissue layer called the tunica albuginea. The glans is the distal head of the penis. The distal foreskin, or prepuce, in uncircumcised males lies over the glans and can be retracted proximally to expose the glans. The coronal sulcus distinguishes the glans penis from the penile shaft (see Figs. 55-7B and 55-8B).
Pathophysiology
Patients will have a red, painful, and swollen glans penis associated with an edematous, proximally retracted foreskin that forms a circumferential constricting band. The normal anatomy and identification of the foreskin may be obfuscated by edema, and the condition can be asymmetric and rather bizarre appearing (Fig. 55-9, step 1). The penile shaft proximal to the constricting band is typically soft. The entrapped foreskin forms a constricting band on the penile shaft. Compression inhibits venous drainage of the glans, sulcus, and distal part of the foreskin itself and results in a vicious circle of progressive glans and foreskin edema that may further prevent reduction of the foreskin to its natural position. The edema may become so severe that arterial flow is compromised, which can result in necrosis and gangrene of the glans penis.
Indications
Emergency reduction of a paraphimotic foreskin is indicated whenever the condition exists.
Procedure
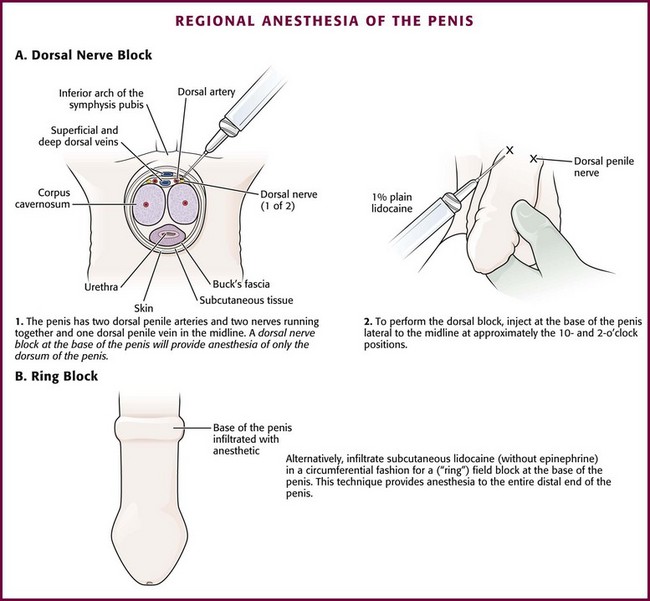
The current standard for reducing paraphimosis is manual reduction. It can be facilitated by applying a nonirritating topical anesthetic lubricant onto the inner surface of the foreskin (not the shaft of the penis) and the glans to reduce friction and decrease the discomfort of the procedure. A dorsal and ventral penile block should be performed in cases that require significant manipulation (see Fig. 55-9, steps 2 and 3). If significant discomfort or patient apprehension is present, systemic analgesia or procedural sedation may be useful adjuncts. For young children, general anesthesia may be necessary.50
Compress the foreskin and glans by snugly grasping it with the palm of the hand and apply pressure for several minutes, or wrap an elastic bandage around the distal end of the penis to reduce as much edema fluid as possible (see Fig. 55-9, steps 4 and 5).51 Compression is often omitted and makes the procedure more difficult. A significant amount of edema can be alleviated with simple compression (see Fig. 55-9, step 6). Place the index and long fingers of both hands in apposition just proximal to the phimotic ring. Align both thumbs on the urethral meatus and apply constant force. Use the thumbs to invert the glans penis proximally, and use the index and long fingers to attempt to reduce the phimotic ring distally over the glans penis into its normal anatomic position (see Fig. 55-9, step 8). Successful reduction results in the appearance of an uncircumcised penis with a phimotic foreskin (see Fig. 55-9, steps 9 and 10). Alternatively, use the thumb to push the glans through the foreskin that is encircled by the entire palm (see Fig. 55-9, step 7). The key to success in both these maneuvers is the application of slow, steady pressure.
Adjunctive Techniques to Assist in Manual Reduction
Use Babcock clamps (noncrushing tissue clamps) to reduce the paraphimotic foreskin (Fig. 55-10A).52 Apply six to eight Babcock clamps spaced evenly around the foreskin and straddling the phimotic ring (one edge just proximal and the other edge just distal to the phimotic ring). Grasp all the clamps and apply simultaneous distal traction to pull the phimotic ring over the glans. After reduction, remove the clamps. It is important to inspect the foreskin afterward for injury.
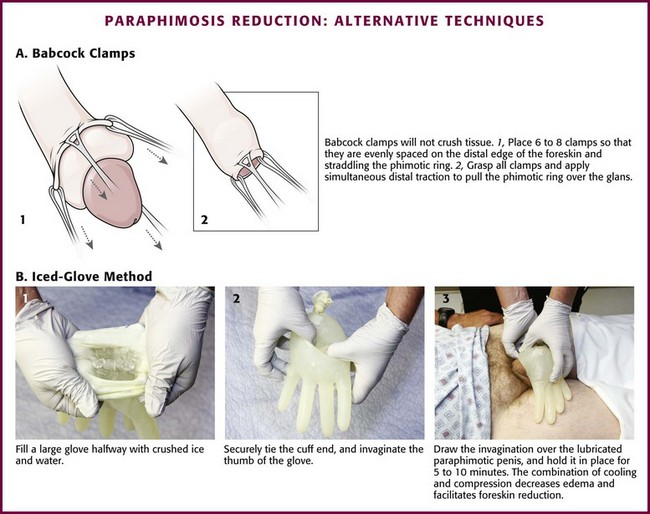
Figure 55-10 Paraphimosis reduction: alternative techniques.
Other techniques focus on reduction of glans or foreskin edema (or both), followed by reduction of the paraphimosis. Diminished glans edema may allow the edematous foreskin to be reduced distally to its natural position. A suggested maneuver involves the application of an ice pack. In the “iced-glove” method, cold compression is used to reduce foreskin swelling and induce vasoconstriction in the glans penis (see. Fig. 55-10B).53 Fill a large glove halfway with crushed ice and water, and tie the cuff end securely. Invaginate the thumb of the glove and then draw it over the lubricated paraphimotic penis. Hold the thumb of the glove securely in place over the glans for 5 to 10 minutes. The combination of cooling and compression usually decreases the edema sufficiently to permit manual reduction of the foreskin. Wrapping the glans (and penis) in a compressive bandage is another option.51
Alternatively, techniques focusing on reduction of foreskin edema have been advocated (Box 55-3). Though not usually pursued in the ED, they will be included for completeness. The Dundee technique involves making multiple micropunctures in the edematous foreskin and then squeezing out the edema fluid.54 Hyaluronidase has been reported to result in rapid reduction of prepuce edema to facilitate manual reduction of the foreskin. This enzyme, when injected into the swollen retracted foreskin, causes hydrolysis of hyaluronic acid, which in turn increases tissue permeability so that the edema in the foreskin is diffused out into the surrounding tissue of the penis.55 There have even been advocates of a noninvasive way to reduce the foreskin edema via the application of granulated sugar to the penis. Sugar forms an osmotic gradient that draws out the fluid with reduction of the edema, but this may take several hours.56 Publications on these alternative procedures are generally observational in design with very small numbers. To date, there have not been any large studies of comparative effectiveness; consequently, it is difficult to recommend any one method as being superior to the others.57
If the aforementioned methods are unsuccessful, it may be necessary to incise the constricting phimotic tissue to permit reduction of the foreskin over the glans (dorsal slit procedure).58,59 In adults, this can generally be carried out under penile block anesthesia, but procedural sedation or general anesthesia may be necessary for young children.
Phimosis
Phimosis is constriction of the foreskin (or prepuce) that limits its retraction proximally over the glans (Fig. 55-11). Uncircumcised infants and young children often have physiologic phimosis secondary to adhesions between the prepuce and glans. This is in contrast to pathologic phimosis, where failure to retract results from distal scarring of the prepuce.
Anatomy and Physiology
Physiologic phimosis consists of a pliant, unscarred preputial orifice on physical examination. The foreskin gradually becomes retractile over time as a result of intermittent erections and keratinization of the inner epithelium. By 3 years of age, 90% of glans can easily be retracted, with nearly all becoming retractile by late adolescence.60,61
Procedure
Box 55-4 lists the equipment needed to perform a dorsal slit of the foreskin. With the patient in the supine position, clean and drape the penis with sterile towels. Clipping of pubic hair is unnecessary. Then infiltrate 1% plain lidocaine without epinephrine into the dorsal midline of the foreskin just beneath the superficial fascia throughout the course of the proposed incision, starting proximally at the level of the coronal sulcus and proceeding distally to the tip of the foreskin (Fig. 55-12, step 1). Consider mixing equal volumes of 1% lidocaine with 0.5% bupivacaine. After 3 to 5 minutes, grasp the foreskin with toothed forceps to test for anesthesia. Be certain that the inner surface of the foreskin is also anesthetized. If this area is not numb, use a dorsal nerve block or “ring block” at the base of the penis (Fig. 55-13).62
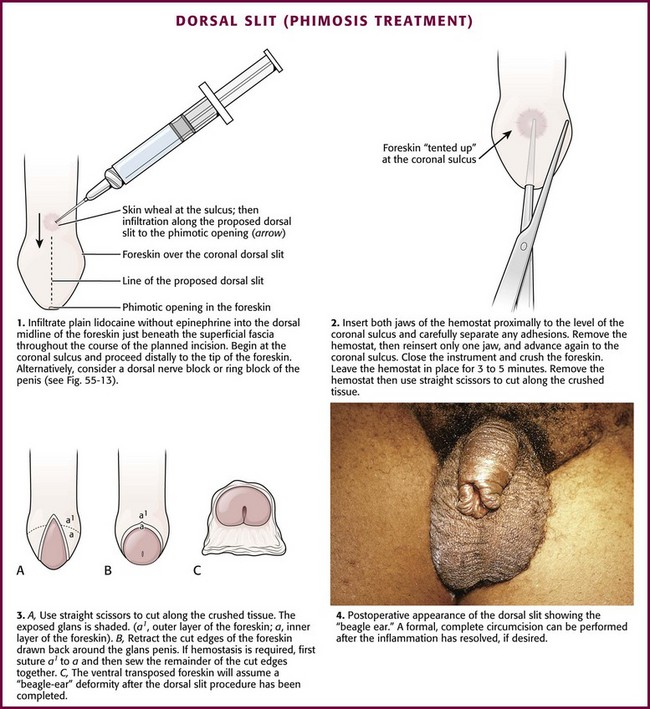
Figure 55-12 Dorsal slit treatment of phimosis.
After achieving both adequate local anesthesia and systemic analgesia (or procedural sedation if needed), take a straight hemostat and carefully advance both jaws of the hemostat proximally to the area of the coronal sulcus between the inner layer of the foreskin and the smooth glans penis and carefully separate any existing preputial adhesions (see Fig. 55-12, step 2). Take care to visualize or palpate the meatus and urethra at all times to avoid inadvertent injury during this maneuver. Once release of adhesions is complete, open the hemostat and place one jaw in the recently developed plane between the glans penis, opened to tent the skin and ensure proper placement, and the superior overlying inner layer of foreskin. Advance the hemostat to the level of the coronal sulcus and then close it, thereby effectively crushing the interposed anesthetized foreskin. Leave the closed hemostat in place for 3 to 5 minutes, and then remove it and cut the resultant serrated crushed foreskin longitudinally with straight scissors throughout the extent of the crushed tissue.
Normally, the incised, anatomically approximated skin edges bleed and ooze. Not infrequently, these skin edges of the foreskin separate into two layers, the outer foreskin and the inner foreskin (see Fig. 55-12, step 3). Two absorbable chromic or Vicryl (4-0 to 5-0 for children and 3-0 to 4-0 for adults) running hemostatic sutures may be placed, each beginning proximally at the apex of the dorsal slit and carried distally, to reapproximate the two leaves of foreskin. Standard antibiotic ointment may be used to lubricate the suture material and facilitate passage of suture through the delicate skin tissue.
If reduction of paraphimosis cannot be achieved with other means, consider surgical interventions. Figure 55-14 describes the dorsal slit procedure, which involves carefully sterilizing the field, anesthetizing the penis on the dorsal aspect, and then making a linear incision.
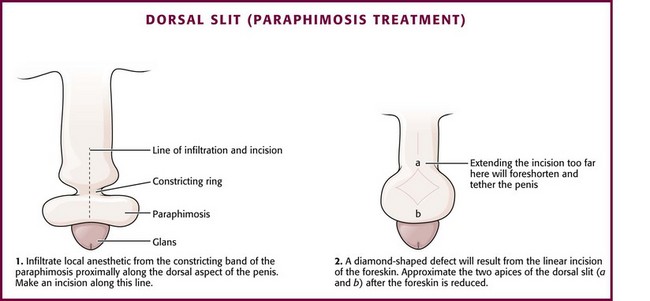
Figure 55-14 Dorsal slit treatment of paraphimosis. This procedure may be necessary if manual reduction of paraphimosis (see Fig. 55-9) is unsuccessful.
Urethral Catheterization
In adult men without anatomic lesions, first-voided midstream specimens can define the presence or absence of culture-proven bacteriuria.63 This certainly represents a user-friendly approach to urine collection in a busy ED setting.
In adult women, properly collected clean-catch midstream specimens have been found to be as bacteriologically reliable as catheterized specimens.64 A few caveats are worth mentioning. Ideally, patients must sit backward on the toilet when collecting the specimen (i.e., patient facing the wall behind the toilet, which theoretically promotes spreading of the labia). Of more concern is the fact that such studies excluded patients with vaginitis, urologic abnormalities, pregnancy, and vaginal bleeding. These conditions represent the clinical circumstances for which urine is commonly examined in young women visiting the ED. In this at-risk population, catheterized urine specimens are optimal. However, this needs to be balanced with the view that catheterization may introduce unnecessary patient discomfort and resource utilization, as well as the risk of introducing bacteria into the bladder.65
Anatomy and Physiology
The female urethral meatus is oval but may appear as an anteroposterior slit with rather prominent margins situated directly superior to the opening of the vagina and approximately 2.5 cm inferior to the glans clitoris (Fig. 55-15). It is the first of three orifices encountered when examining the female genitalia from cephalad to caudad in the lithotomy position. The urethral meatus might be especially difficult to find in very young infants and in older, postmenopausal women. Anticipation of this problem and knowledge of anatomic variations will help mollify any patient discomfort associated with needless catheter tip probing, which is an unsettling experience for both the patient and the person performing the catheterization.
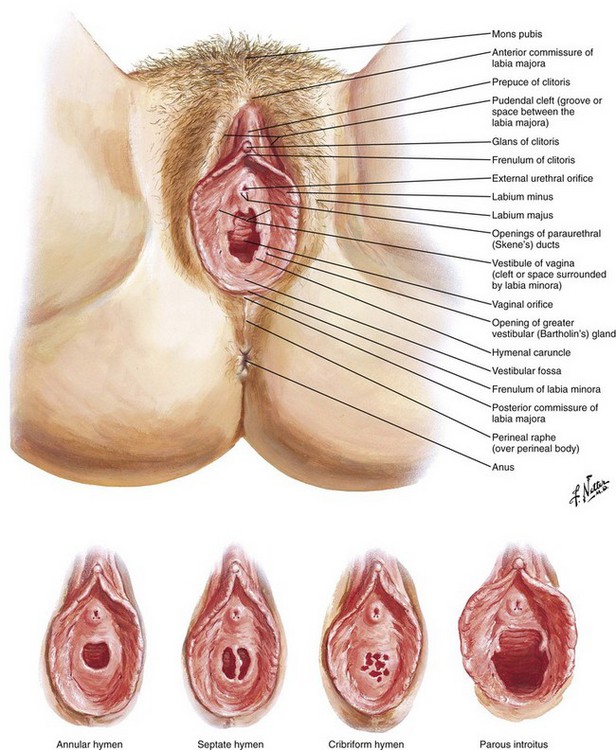
Figure 55-15 External female genitalia. The urethral meatus is directly superior to the vaginal introitus and approximately 2.5 cm inferior to the clitoris. (Netter illustration from www.netterimages.com. © Elsevier, Inc. All rights reserved.)
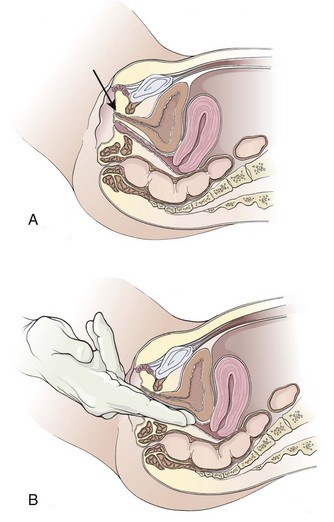
Occasionally, the urethral meatus recedes superiorly into the vagina and is not immediately visible because of either previous surgical procedures or atrophic postmenopausal changes. Anticipation of such cases will allow the examiner to gently advance a nondominant index finger into the vagina in the superior midline. The urethral meatus can usually be palpated and then visualized as a soft mound surrounded by a firmer ring of supporting periurethral tissue. Rarely, the meatus will have receded so far superiorly and intravaginally that it cannot be visualized at all, and catheterization must be carried out by palpation alone. From the meatus (if the patient assumes a supine position), the urethra proceeds straight back to slightly downward as it advances into the bladder just behind the symphysis pubis (Fig. 55-16A).
In women with a urethrocele or cystourethrocele in whom the urethra or the bladder falls into the vagina, the “normal” urethral course might be considerably more posterior. The normal anatomic relationships in these situations may be re-created by spreading the index and long fingers, placing them along the superior vaginal wall, and gently applying upward support (see Fig. 55-16B). This reconstitutes the normal anatomic relationships and permits straight, rapid urethral catheterization. Because the female urethra is so short, only half the total length of the catheter has to be inserted before it is safe to inflate the Foley balloon.
Male Catheterization
Because the urethral meatus is usually evident in most males, it might seem a simple matter to insert a urethral catheter.66 Yet catheterization can be quite difficult. The normal male urethra is approximately 20 cm long from the external urethral meatus to the neck of the bladder (Fig. 55-17). The posterior prostatic urethra is approximately 3.5 cm long, and the contiguous external sphincter or urogenital diaphragm that encompasses the membranous urethra is located 4 cm from the neck of the bladder. In males, any catheter must be fully inserted to the balloon-inflating side arm channel before it is safe to inflate the balloon. At the first egress of urine from the catheter, the balloon is just passing through the membranous urethra. The catheter balloon still has about 3 cm to go before clearing the neck of the bladder. Inflation of the Foley balloon at any point before full insertion of the catheter might result in iatrogenic urethral injury.
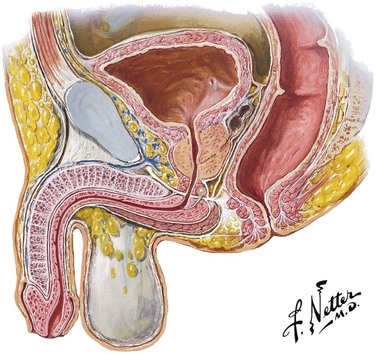
Figure 55-17 Male urethra. The male urethra is approximately 20 cm long from the meatus to the neck of the bladder. The prostatic urethra and membranous urethra can be up to 7 cm combined. Thus, it is essential to advance the urethral catheter to the hilt before balloon inflation to avoid urethral injury. (Netter illustration from www.netterimages.com. © Elsevier, Inc. All rights reserved.)
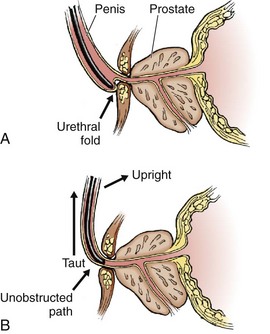
The male urethra is relatively fixed at the level of the urogenital diaphragm and symphysis pubis; traction downward on the penis kinks and promotes urethral folding at the level of the penile suspensory ligament, which creates a level of spurious obstruction (Fig. 55-18A). For this reason, always hold the penis taut and upright during any urethral instrumentation, including catheterization. The catheter then needs to make only a single curve rather than a complex S curve as it traverses into the bladder (see Fig. 55-18B).
Indications
Contraindications
Avoid urethral catheterization when other less invasive procedures are equally effective and informative. A traditional, albeit currently relative contraindication to urethral catheterization (see the following discussion) is a trauma patient with suspected urethral injury who has blood at the urethral meatus; an abnormal-feeling or high-riding prostate on rectal examination; penile, scrotal, or perineal hematoma; or radiographic evidence of urethral or bladder trauma. In the setting of a severely fractured pelvis or diastasis of the pubic symphysis, urethrography should always precede attempts at catheterization. Although some authors67 and more liberal clinical protocols allow gentle attempts at passage of the catheter in the presence of the previously discussed traditional contraindications, these findings dictate a selective approach or the need for retrograde urethrography (RUG), or both, to define the integrity of the urethra before any attempted urethral catheterization.68
Procedure
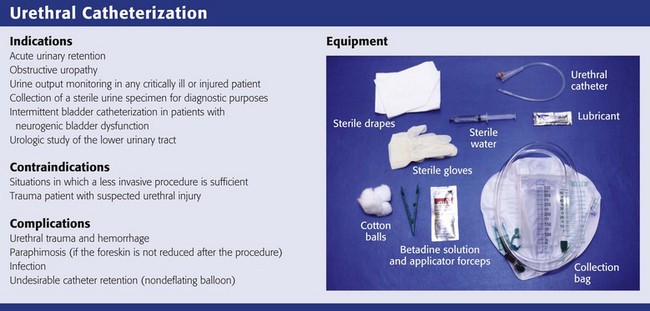
The equipment shown in Review Box 55-1 is included in most standard catheterization trays and must be at hand before attempting urethral catheterization. Check the list of contents before opening the tray because some trays do not include everything that is needed. For most routine adult in-and-out catheterizations, a 14-Fr red rubber catheter or Foley balloon catheter is adequate (Fig. 55-19). In neonates or infants, a 2- or 5-Fr feeding tube taped in place produces the least amount of urethral trauma. In older boys, a 5- to 12-Fr red rubber catheter or Foley balloon catheter may be used. Table 55-3 lists appropriately sized catheters and feeding tubes for urethral catheterization in patients of all ages.69
TABLE 55-3
Pediatric Urethral Catheter Size
| AGE GROUP | PEDIATRIC CATHETER SIZE* |
| Infants | 5- to 8-Fr feeding tube |
| 1-3 yr | 8-Fr feeding tube |
| 4-6 yr | 10-Fr feeding tube |
| 7-12 yr | 10- to 12-Fr red rubber catheter (Robinson) |
| >12 yr | 14-Fr red rubber catheter (Robinson) |
*Sizes are approximate; 6 Fr is the smallest balloon catheter and is quite flimsy, and therefore 8 Fr is recommended; 12 Fr is the smallest coudé catheter.
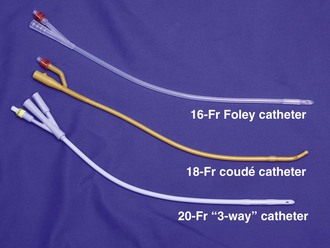
Figure 55-19 Urethral catheters. A wide variety of types and sizes of catheters are available. For most adult catheterizations, a 14- or 16-Fr Foley catheter is sufficient. For male patients with prostatic enlargement, a 14- to 18-Fr coudé catheter may be used (see Fig. 55-20). For patients with gross hematuria, a large (20 Fr and up) “three-way” catheter is ideal because it allows continuous bladder irrigation and its large diameter allows the passage of blood clots.
Consider using a 14- to 18-Fr coudé catheter after unsuccessful passage of a straight Foley balloon catheter or in a male patient with known enlargement of the prostate (Fig. 55-20). If a coudé catheter is not available, attempt catheterization with a larger 18- to 22-Fr Foley balloon catheter. In a male patient with a urethral stricture in whom attempts at catheterization with a straight Foley or coudé catheter have failed, the next step is retrograde cystoscopy, passage of a guidewire, or passage of filiforms or followers under the direction of a urologist. If immediate bladder access is required in an emergency, suprapubic placement of a peel-away sheath and Foley balloon catheter via the Seldinger technique will be needed (as described in the suprapubic cystostomy section of this chapter).70
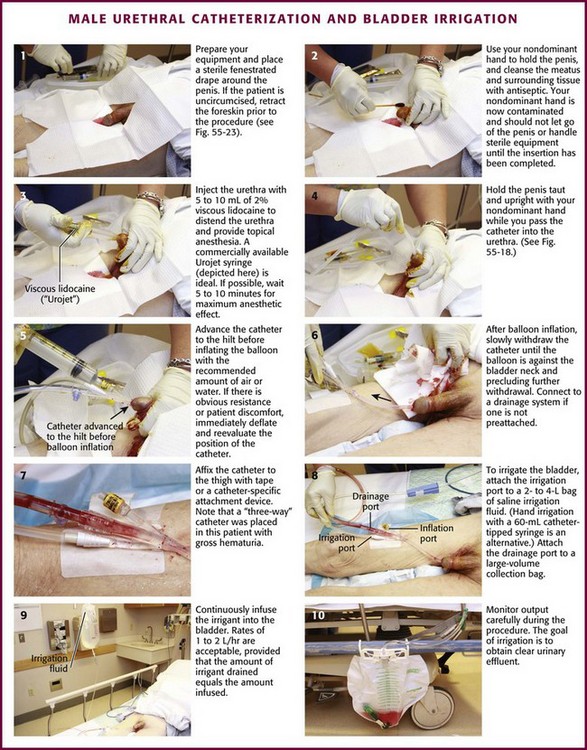
General Procedure (Figs. 55-21 and 55-22)
As stated previously, always use sterile technique during urethral catheterization. Both male and female patients have special urethral meatal considerations. In an uncircumcised or partially circumcised male, absolute total control of the penile foreskin is paramount to ensure success. Before establishing a sterile field, retract the available foreskin to its fullest extent proximal to the glans penis (Fig. 55-23A). Unfold a standard 4 × 4 gauze pad, refold it in its longest dimension, and carefully wrap it around the retracted foreskin at the level of the coronal sulcus (see Fig. 55-23B). This will prevent the tendency for lubricated foreskin to reduce during catheterization and thus provides a continuously dry and sterile field. Secure the folded 4 × 4 gauze around the foreskin between the nondominant long and ring fingers at the beginning the procedure and do not release it until the procedure is completed. This position leaves the nondominant index finger and thumb available for manipulating the catheter. After catheterization, remove the 4 × 4 pad and reduce the penile foreskin to its normal anatomic position to prevent the development of iatrogenic paraphimosis.
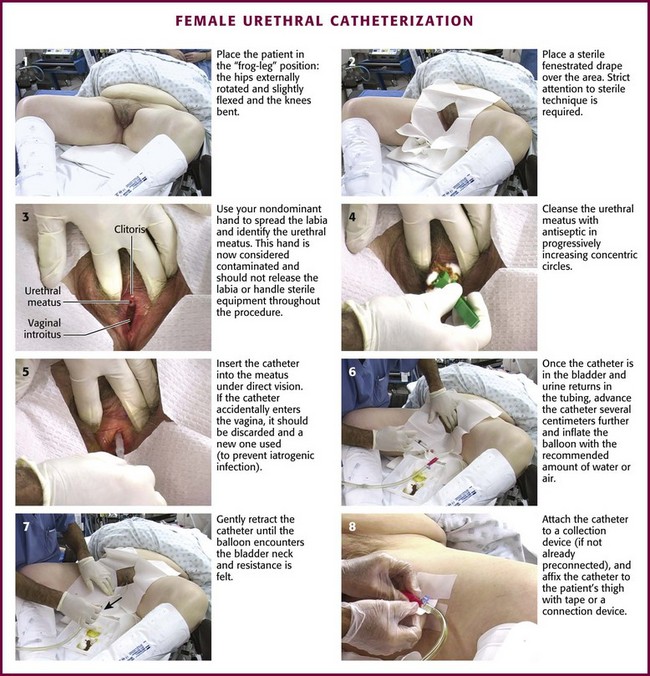
Figure 55-22 Female urethral catheterization.
After passing the catheter “to the hilt” in a male patient, slowly inflate the balloon with 10 mL of air or tap water. Sterile water or saline is not necessary. Most 5-mL balloons will easily accommodate up to 30 to 50 mL of air or water without bursting. If there is obvious resistance or patient discomfort during inflation of the balloon, erroneous urethral positioning may have occurred and mandates reevaluation. In this case, immediately deflate the Foley balloon and reposition or slightly withdraw the catheter. Then pass it to the hilt again before reinflating the balloon. If the second time is unsuccessful, remove the catheter and evaluate the urethra for a potential obstructive problem or false passage. RUG is recommended (see the section “RUG” later in this chapter). It may be difficult or impossible to pass a Foley catheter in a male with a markedly edematous penis (Fig. 55-24). If it cannot be accomplished, a suprapubic catheter may be required.
Bladder Irrigation
After prostate surgery (transurethral resection), blood clots may form in the bladder and cause acute urinary retention. Blood loss can be significant. This condition can frequently be easily relieved by using a large three-way irrigation catheter. A Bardex 22- to 26-Fr Foley catheter is preferred. Infuse saline irrigation fluid continuously into the bladder while allowing the egress of fluid and blood clots. Continue the procedure until the bladder remains decompressed or the bleeding stops (see Fig. 55-21, steps 8 to 10). Gravity alone usually provides adequate ingress and egress of fluid, but gentle syringe irrigation with 60-mL aliquots of saline may also be used. Irrigation can be brisk, 1 to 2 L/hr or greater, as long as the volume of drained saline is equal to the volume infused. Use only saline (supplied in 2- or 4-L bags) and infuse it via gravity. Syringe irrigation of a Foley catheter with 60- to 100-mL aliquots is an alternative. The goal is to obtain clear urine.
DUC
Difficult urethral catheterization (DUC) is a common urologic problem. In females DUC is due primarily to vaginal atrophy with retraction of the meatus into the vagina. This problem can typically be overcome by placing the patient in the lithotomy position, using a speculum, or guiding the catheter blindly with the provider’s index and middle fingers. However, DUC in males may present greater challenges. Common causes in males include urethral strictures, contracture of the neck of the bladder, false passage, and prostate disease. A trial of a coudé catheter is generally the first-line approach to DUC. Alternatives used by urologists include flexible cystoscopy, passage of a guidewire, or use of filiforms/followers.71 Consider suprapubic cystostomy an option of last resort given its inherent complications. Various causes and approaches to DUC in the emergency care setting are presented in Table 55-4.
TABLE 55-4
Approach to Difficulties in Male Catheterization
| CONDITION | ISSUE | WORK-AROUND STRATEGY |
| Phimosis | Inability to identify the urethral meatus because of a tight phimotic opening | Dilate the phimotic opening sufficiently to identify the urethral meatus and blindly pass the catheter Dorsal slit of the foreskin to expose the glans and urethral meatus in extreme cases |
| Foreskin edema | Edema may obscure the glans penis and urethral meatus | Attempt to identify the cause of the edema; exclude paraphimosis and foreign body strangulation Manually compress the swollen foreskin by hand or between opposing cold packs If unsuccessful, snugly wrap the distal penis in a compressive dressing for 10 min |
| Meatal stenosis | The urethral meatus may be either congenitally or secondarily narrowed by scarring | Trial of a smaller-caliber catheter If a larger-caliber catheter is necessary, meatal dilation or meatotomy might be necessary under the direction of a urologist |
| Urethral stricture | May develop as a result of trauma, infection (especially STDs), lower urinary tract instrumentation, or long-term indwelling catheter drainage | Trial with a coudé catheter However, manual force should not be used to negotiate or to dilate urethral strictures because it may lead to false passages, bleeding, and future increased scarring Consideration of urethral dilation with the use of filiforms and followers under the direction of a urologist |
| External urethral sphincter spasm | Voluntary or involuntary contraction of the urogenital diaphragm (external sphincter) produces spurious urethral resistance at approximately 16 cm from the meatus | Encourage the patient to lay supine and take slow, deep breaths while consciously trying to relax the perineum and rectum Plantar flexion of the toes and ankles may also aid in relaxation of the pelvic floor Gentle but steady pressure exerted on the syringe or the catheter along with the aforementioned steps may aid in passage The external sphincter is composed of striated muscle and fatigues within a few minutes If continued resistance, assume an anatomic abnormality and consider RUG before further instrumentation |
| High bladder neck | Enlarged intravesical portion of the prostate with a secondary high-riding bladder neck Typically resistance encountered after the catheter has been passed 16-20 cm into the urethra |
Slow instillation or injection of 20-30 mL of sterile lubricating jelly If unsuccessful, consider a trial with a coudé catheter; may be enhanced by digital compression of the prostate during digital rectal examination |
| Pelvic trauma, straddle injury | Partial or complete urethral injury might occur Injudicious catheterization has the potential for conversion of a partial injury (may heal with little or no scarring) to a complete urethral injury (often requires surgical repair and usually results in some degree of postoperative morbidity) Blood at the urethral meatus, a “high-riding” or abnormal-feeling prostate on rectal examination, and penile, scrotal, or perineal ecchymoses might be evidence of potential urethral or bladder injury but have poor sensitivity66 |
Consider precatheterization RUG in suspected cases Maintain a low threshold for urologic consultation Suprapubic catheter placement may be necessary in certain cases |
RUG, retrograde urethrography; STDs, sexually transmitted diseases.
Aftercare
Systemic antimicrobial prophylaxis should not be used routinely in patients with short-term (or long-term) catheterization to reduce catheter-associated bacteriuria or urinary tract infection because of concern about selection of antimicrobial resistance.72
Complications
Infection
The incidence of bacteriuria associated with indwelling catheterization is 3% to 8% per day, and the duration of catheterization is the most important risk factor for the development of catheter-associated bacteriuria.71 However, in hospitalized, elderly, debilitated, or postpartum patients, the rate might be considerably higher. Urinary catheterization is the leading cause of nosocomial infection.71
Long-Term Catheter Use
By 1 month, nearly all patients with an indwelling catheter will be bacteriuric.72 Other less frequent complications of long-term indwelling urethral catheterization include bladder stones, recurring bladder spasm, periurethral abscesses, urethral stricture formation, bladder perforation,73 and urethral erosion.74
Removal of a Nondeflating Catheter
Occasionally, an indwelling catheter balloon fails to deflate, thereby leading to undesired retention of the Foley catheter in the bladder. This problem has challenged and frustrated many clinicians and has produced a number of ingenious solutions.75–77 Unfortunately, pretesting Foley catheter balloons by trial inflation and deflation before insertion does not eliminate the potential for a nondeflating Foley catheter balloon.
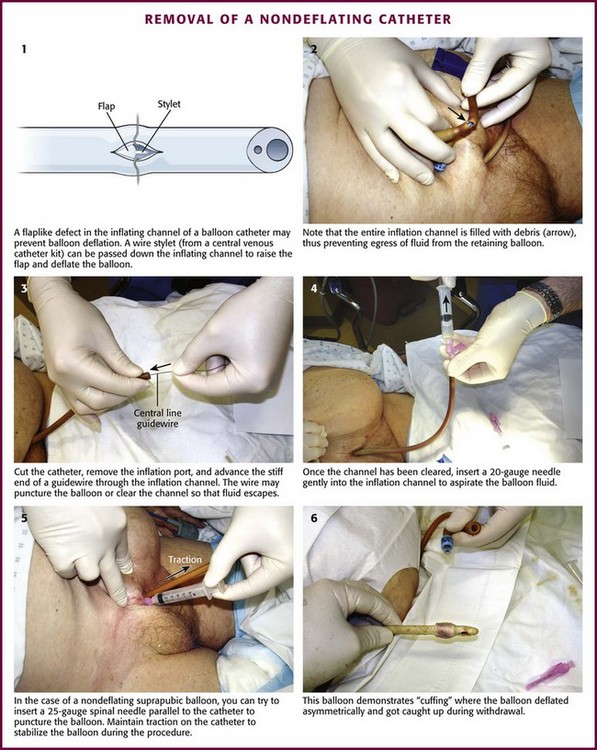
A common cause of a nondeflating catheter balloon is malfunction of the flap-type valve in the balloon lumen of the catheter, which normally allows fluid to fill the balloon of the catheter but prevents passive egress (Fig. 55-25, step 1).75 The ideal solution is one that resolves the problem—deflating the balloon—without creating another problem, such as unnecessary bladder irritation or balloon fragmentation. Of the methods recommended to decompress nondeflating catheter balloons, the only technique that approaches the ideal directly attacks this flap valve deformity.
The inflate-deflate channel normally prevents passive egress of inflating fluid or air. Occasionally, cutting off the inflation port will deflate the balloon, but usually, the problematic area is further along the catheter, closer to the bladder. Once the port has been removed, use a needle/syringe in the inflation channel to suck out the fluid in the balloon (see Fig. 55-25, step 4). Cutting the catheter closer to the bladder may fortuitously cut off the offending area, but be careful to not cut off an excessive length of exposed catheter. More often, the inflation channel may have a valve defect or be filled with debris (see Fig. 55-25, step 2). When presented with this situation, insert a thin, rigid wire into the balloon port lumen in an attempt to deflate the valve flap defect sufficiently and to promote escape of fluid from the balloon (see Fig. 55-25, step 3). Occasionally, the balloon itself may be punctured by this wire. The wire stylets from a central venous or angiographic catheter, guidewires from ureteral catheters, and very small well-lubricated ureteral catheters themselves have all been reported to be successful. When a ureteral catheter guidewire was used in one series, 34 of 39 balloons were deflated without fragmentation.76
Alternative methods for removal of anondeflating catheter are presented in Table 55-5.
TABLE 55-5
Alternative Methods of Removing Nondeflating Catheters
| METHOD | COMMENTS |
| Needle puncture of the balloon (see Fig. 55-25, step 5) | Overinflate the balloon (50-100 mL of saline) Draw the balloon against the bladder neck Use local anesthesia for the suprapubic approach Use a 25- to 27-gauge spinal needle to puncture the balloon Ultrasound guidance may be helpful |
| Hyperinflation of the balloon | High rate of balloon fragmentation: 100 of 100 in one report74 Postprocedure cystoscopic inspection of the bladder essential Consider adding 50-100 mL of saline into the bladder to cushion the effects of subsequent balloon rupture if no urinary retention is present Inject up to 200 mL of air into a 5-mL balloon to induce rupture |
| Injection of an erosive substance into the balloon port (e.g., mineral oil) | This technique has fallen out of favor High rate of balloon fragmentation: 95 of 100 in one report74 Associated with chemical cystitis |
A variation of the nondeflating balloon is “cuffing,” essentially an asymmetric deflation in which the balloon does not deflate flush with the catheter. The irregularity becomes more pronounced as the catheter is withdrawn into the urethra and thus prevents easy withdrawal. It occurs more often when the balloon fluid is aspirated quickly and less often with more gradual withdrawal of the fluid. Inflate the balloon again and try to deflate it slowly if this occurs. Reinflating the balloon to a smooth surface with 0.5 to 1 mL of saline during withdrawal may also obviate this problem. An example of cuffing is shown in Figure 55-25, step 6, and this is more common with suprapubic tubes.
Traumatic Foley Catheter Removal
Occasionally, uncooperative or demented patients will pull out their Foley catheter with the balloon still inflated (Fig. 55-26). One would intuit that urethral injury is possible; however, there are no prospective data on the incidence or type of injury from this maneuver or how to deal with this situation. Usually, blood is apparent at the meatus, and it may be difficult to know whether the patient can urinate spontaneously. If the patient can urinate spontaneously, it would seem reasonable to gently pass another Foley catheter to avoid urethral obstruction by tears or clots and to allow healing of the urethral trauma with a new catheter in place. Most of the time, however, the clinician has minimal data yet is faced with the decision on how to approach this conundrum. Surprisingly, such incidents do not usually result in massive urethral injury. It is assumed that a new catheter will be required, either to continue the original indication for the catheter or to provide a stent while any urethral injury heals. In the absence of available urologic consultation, gently attempt to pass another Foley catheter without routinely performing RUG (described later in this chapter). If the catheter does not pass easily, stop and perform RUG to assess the extent of the pathology. Most of the time, simply replacing the catheter will be the most prudent approach. Prophylactic antibiotics are reasonable under these circumstances. Complete eversion and prolapse of the bladder have been associated rarely with this misadventure.78
Suprapubic Aspiration
Suprapubic aspiration of the bladder, first reported as a method of collecting urine for bacteriologic study in 1956,79 is a relatively simple means of obtaining uncontaminated bladder urine. It is used primarily in young children, although there are limited indications for this procedure in adults as well.
It has been demonstrated that with proper technique, suprapubic aspiration consistently provides an uncontaminated urine specimen on which true bacteriuria can be distinguished from contamination.80,81 However, the perception of increased discomfort and the procedural failure rate have led to a precipitous decline in its use, particularly outside the neonatal intensive care unit.81–83 Nevertheless, suprapubic aspiration continues to be considered the gold standard method of urine collection.84
Despite the lack of experience by most currently practicing clinicians, the procedure may still be necessary in girls with labial adhesions and in boys with phimosis. The introduction of ultrasound technology has led to a significant improvement in success rates and probably in the comfort of the clinician performing the procedure.85–87
Procedure
Place the child supine in a frog-leg position and restrained as necessary (Fig. 55-27, A1). First locate the bladder. A full, palpable, or percussible bladder should be readily apparent, but this can be difficult to discern in all but the thinnest patients. If there is any question about the location or the amount of bladder urine, a quick ultrasound examination is informative. The point of entry in the skin should be 1 to 2 cm above the superior edge of the symphysis pubis. The syringe and needle are passed perpendicular to the abdominal wall toward the bladder, usually at a 10- to 20-degree angle from the true vertical, somewhat cephalad in children (see Fig. 55-27, A2) and somewhat caudad in adults (see Fig. 55-27B). Note that the bladder of a newborn is an abdominal organ and will be missed if the needle is inserted too close to the pubis or angled toward the feet.
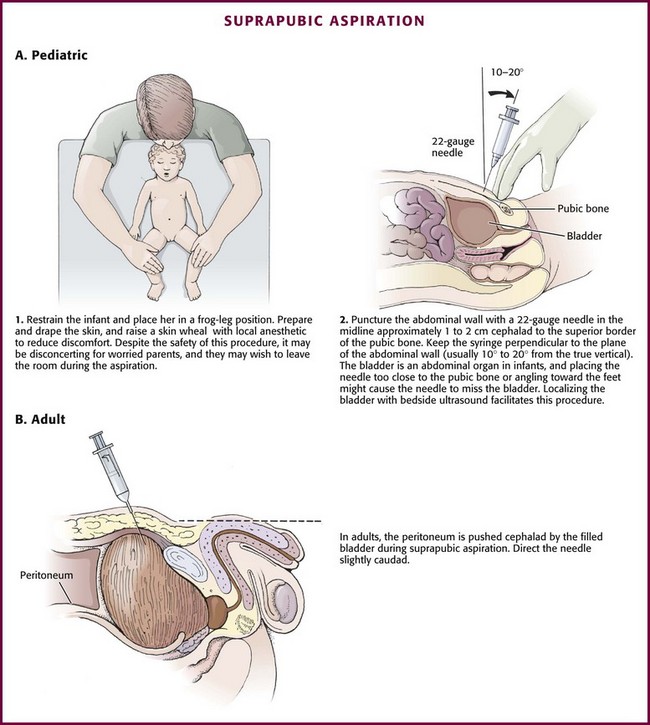
Figure 55-27 Suprapubic aspiration.
Complications
Bacteremia does not typically result from this procedure.88 Bowel penetration has occurred in children with distended abdomens from gastrointestinal disturbances.89 The combination of gaseous bowel distention and relative hypovolemia might displace and flatten the relatively empty bladder against the pelvic floor. Even when the large bowel has been penetrated, patients recover uneventfully. Simple penetration of the bowel with a needle is considered an innocuous event and requires no specific treatment.
Suprapubic Cystostomy
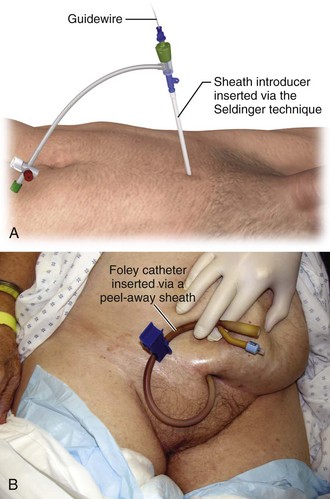
Among the user-friendliest devices for suprapubic bladder access is the Cook peel-away sheath unit.90 It uses the Seldinger (guidewire) technique to gain bladder access and allows suprapubic placement of a Foley balloon catheter for definitive bladder drainage. This device is readily suitable for ED use when compared with other suprapubic bladder access approaches, such as trocar-type devices.
When emergency bladder drainage is required and a Foley catheter cannot be placed transurethrally, any device suitable for central venous access can be inserted suprapubically via the Seldinger technique (Fig. 55-28).
Procedure
The following comments describe placement of the Cook peel-away sheath, as reported by Chiou and colleagues.91 With modifications, the same principles apply to any type of suprapubic catheter placement.
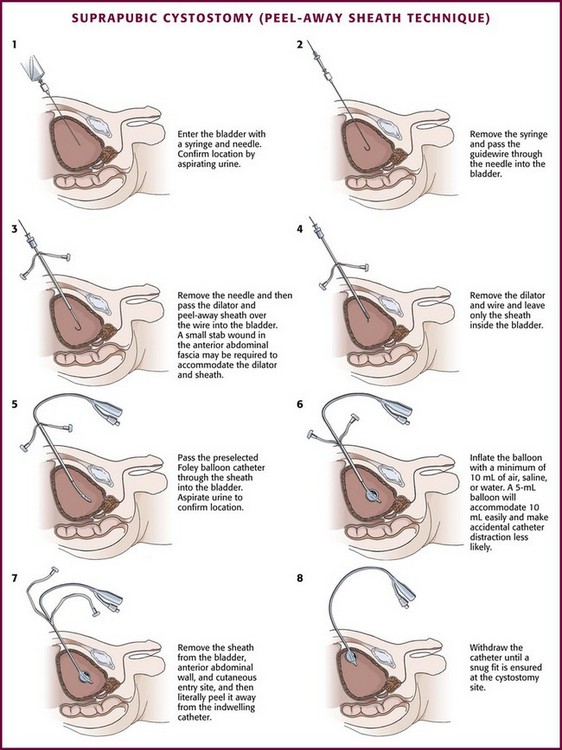
Locate the bladder by advancing the needle in the prescribed direction while aspirating the syringe. Urine is easily aspirated when the bladder is entered (Fig. 55-29, step 1).
Once the bladder has been located, remove the syringe from the needle and advance a guidewire through the needle into the bladder (see Fig. 55-29, step 2). Withdraw the needle while leaving only the guidewire traversing the anterior abdominal wall and positioned inside the bladder. Use a No. 15 scalpel blade to make a stab incision directly posterior to the wire through the skin, subcutaneous tissue, and superficial anterior abdominal wall fascia. Then pass the peel-away sheath and indwelling fascial dilator together over the wire into the bladder (see Fig. 55-29, step 3). Remove the guidewire and fascial dilator and leave only the peel-away sheath inside the bladder (see Fig. 55-29, step 4). Then pass a preselected Foley balloon catheter through the indwelling intravesical sheath into the bladder (see Fig. 55-29, step 5). Aspirate urine to confirm proper placement. Inflate the Foley balloon with a minimum of 10 mL of air, water, or saline (see Fig. 55-29, step 6). Withdraw the peel-away sheath from the bladder and anterior abdominal wall and literally peel it away from the catheter, with only the indwelling suprapubic Foley catheter left in place (see Fig. 55-29, step 7). Withdraw the catheter slowly until the inflated balloon approximates the cystostomy site (see Fig. 55-29, step 8).
Complications
A wide variety of complications have been reported, which serve as reminders that suprapubic cystostomy is not innocuous. Occasionally, despite the best intentions, the suprapubic tube or catheter cannot be positioned or maintained successfully without untoward sequelae (Box 55-5).
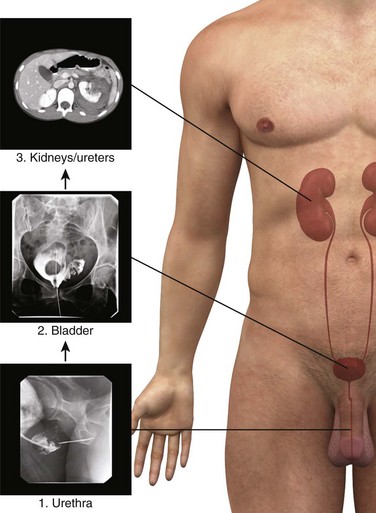
Lower GU Tract Imaging
In patients with urologic trauma, the lower urinary tract should be studied before the upper urinary tract (e.g., study the urethra before the bladder; study the bladder before the kidneys). Consequently, specific diagnostic studies should be conducted in a retrograde fashion (Fig. 55-30).
Anatomy and Physiology
The anatomy of the male urethra is demonstrated Figure 55-17. The male urethra varies from around 17.5 to 20 cm in length in adults and consists of anterior and posterior portions, each of which is subdivided into two parts (pendulous and bulbous for the anterior portion, membranous and prostatic for the posterior portion). The female urethra is approximately 4 cm long and extends from the neck of the bladder at the urethrovesical junction to the vaginal vestibule, where it forms the external meatus between the labia minora (see Figs. 55-15 and 55-16).
Pathophysiology
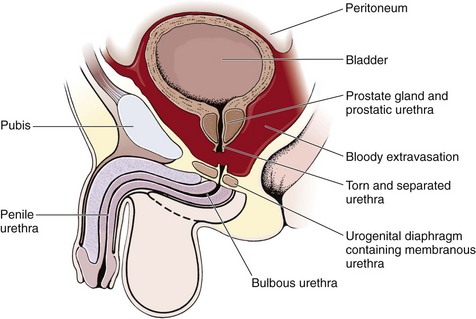
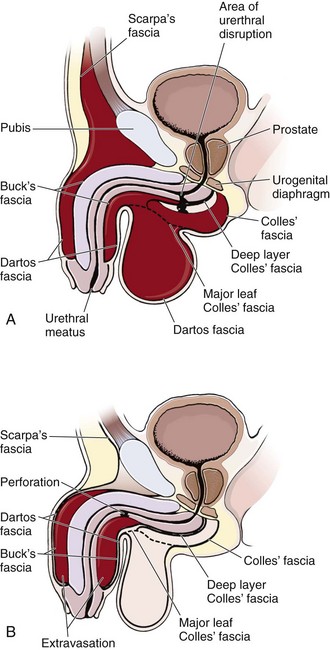
The male posterior urethra, which includes the membranous and prostatic urethra, is injured more frequently than the anterior urethra. The urogenital diaphragm encloses and fixes the membranous urethra; the prostate and prostatic urethra are firmly attached to the posterior surface of the symphysis pubis by the puboprostatic ligaments. Blunt trauma and pelvic fractures, especially in the presence of a full bladder, may result in shearing forces that partially or completely avulse portions of the firmly attached posterior urethra. Usually, the bladder and prostate gland are sheared from the membranous urethra, which results in complete urethral disruption (Fig. 55-31). The female urethra, in contrast, is short and relatively mobile and generally escapes injury with blunt trauma. Occasionally, a significant pelvic fracture will result in a laceration or avulsion of the female urethra at the bladder neck. Direct injuries to the female urethra may also occur secondary to penetrating trauma to the vagina or perineum. These injuries are often disclosed by blood at the introitus or abnormal findings on vaginal examination in a female patient with a pelvic fracture.92
Contusions or lacerations of the male anterior urethra occur when the bulbous urethra is compressed against the inferior surface of the symphysis pubis. This happens most commonly as a result of straddle injuries in males but may be caused by any blunt perineal trauma. Significant trauma to the penile urethra is rare without penetrating injuries or urethral instrumentation. Anterior urethral injuries may result in extravasation of blood or urine into the penis, scrotum, or perineum or along the anterior abdominal wall, depending on whether Buck’s fascia has been violated (Fig. 55-32).93 This is in contrast to posterior urethral injuries, in which blood and urine extravasate into the pelvis.
Indications
RUG is indicated whenever uncertainty exists about the integrity of the urethra. RUG is an anatomic rather than a physiologic examination but is critical in identifying a possible urethral injury before insertion of a urinary catheter to preclude a potentially devastating complication.94
The traditional physical indications to perform RUG in the trauma setting include blood at the urethral meatus, a nonpalpable or high-riding prostate, or urethral trauma. However, the value of these findings to the emergency clinician has been called into question given their low sensitivity.95,96
Procedure
Several different iodinated contrast agents are suitable for retrograde studies (urethrography, cystography). Common agents, dosages, and methods of administration are listed in Table 55-6.
TABLE 55-6
| AGENTS97 | USE | PROCEDURE |
| Diatrizoate (Cystografin) Iohexol (Omnipaque) Iodixanol (Visipaque) |
Use a stock concentration of solution* or Use a one-half stock concentration of solution or Dilute the stock solution with saline in a ratio of 1 part contrast to 10 parts saline (resulting in a <10% solution) |
Urethrography: 10-15 mL of dilute solution injected slowly through the urethral meatus for adults (children: 0.2 mL/kg) Cystography: after a plain film and with the Foley catheter in place, fill the bladder of adults with 400 mL of dilute contrast material introduced under gravity (children: 5 mL/kg) |
*A higher concentration may allow improved image quality; however, extravasation of contrast material into periurethral or perivesical tissues may cause a considerable inflammatory reaction at higher concentrations.
RUG
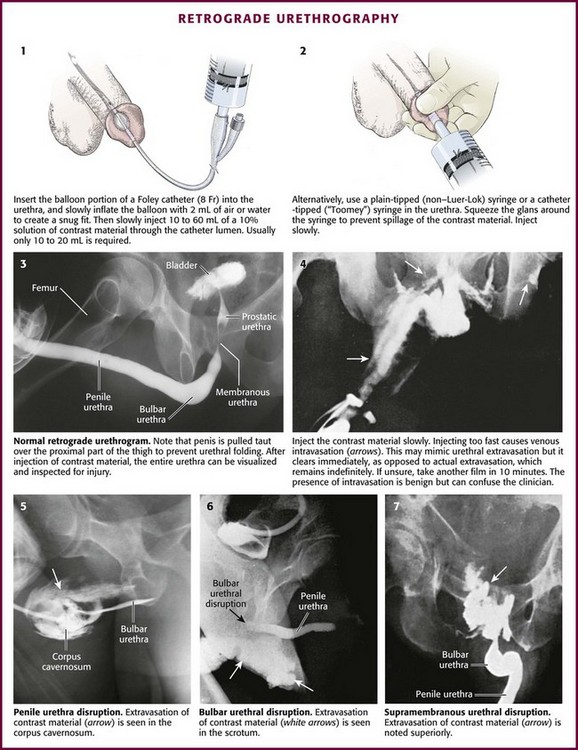
First, take a plain film (kidney, ureter, bladder [KUB]) for reference before injecting any contrast material.98 Retract and secure the penile foreskin with a folded 4 × 4 gauze sponge. Second, hold the penis between the long and ring fingers of the nondominant hand to allow a snug fit of the contrast-filled syringe or catheter inside the urethra. The penis should be stretched laterally over the proximal part of the thigh with moderate traction to prevent folding of the urethra (i.e., the double image of the proximal penile and bulbous urethra superimposed on one another) and allow high-quality RUG (Fig. 55-33, step 3).
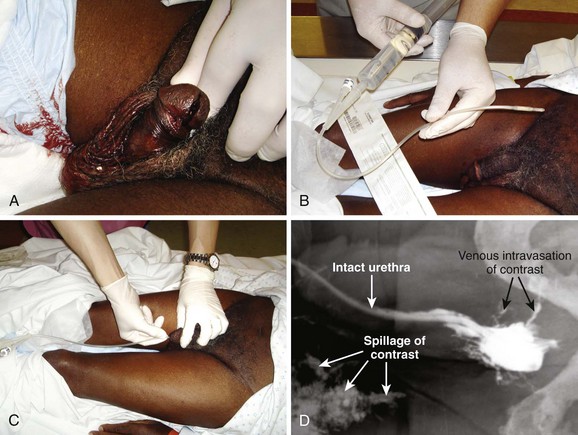
After sterile penile preparation, prepare to inject the dye. A small Foley catheter may be used (see Fig. 55-33, step 1). Seat the balloon portion of the catheter in the fossa navicularis of the penile urethra, and delicately inflate it with 1.0 to 1.5 mL of saline solution to ensure a snug fit.99 Lubrication is not recommended because it may prevent the balloon from remaining in place. Steady manual pressure of the balloon against the external urethral meatus (distal to the fossa navicularis) is an alternative strategy. Another option is to gently advance a catheter-tipped Toomey irrigating syringe or a regular 60-mL syringe with an attached Christmas tree adapter or a non–Luer-Lok syringe inside the urethral meatus until a snug fit is ensured (see Fig. 55-33, step 2). Always gently squeeze the meatus over the injecting device to prevent leakage.
Inject contrast material through the catheter or syringe. If not done carefully, this technique often results in spillage and deposition of contrast agent outside the urethra and onto the patient and the examination table, thereby yielding a spurious result. For this reason, slowly inject anywhere from 10 to 60 mL of contrast material (typically 10 to 20 mL is all that is needed) under constant pressure into the urethra. Not uncommonly, spasm of the external urethral sphincter will be encountered and prevent filling of the posterior urethra. Slow, gentle pressure is usually needed to overcome this resistance.100 Overly forceful injection of contrast material may cause intravasation of contrast material into the venous plexus of the urethra and simulate an injury (see Fig. 55-33, step 4). Finally, during injection of the last 10 mL of contrast material, a film (the urethrogram) is taken (see Fig. 55-33, step 3). Alternatively, the urethrogram can be viewed in real time with use of fluoroscopy.
Extravasation of contrast material from a urethral disruption usually appears as a flamelike density outside the urethral contour (see Fig. 55-33, plates 5 to 7). If any contrast material is seen within the bladder in conjunction with urethral extravasation, a partial rather than complete urethral disruption is more likely. With complete urethral disruption, urethral extravasation will be present without evidence of contrast material within the bladder. The examiner needs to be certain that the lack of bladder contrast is not secondary to voluntary contraction of the external sphincter. Occasionally, as mentioned previously, intravasated contrast material is seen in the periurethral penile venous plexus (see Fig. 55-33, step 4). It is of no clinical significance and should not be mistaken for urethral extravasation. As expected, penile venous intravasation (venous plexus opacification) is noted to clear spontaneously on any postvoid films, unlike urethral extravasation, which remains.
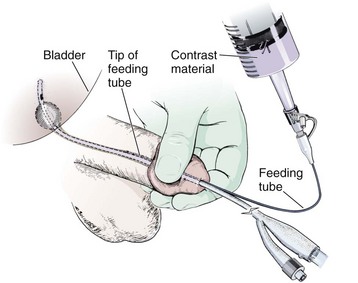
If a Foley catheter has been successfully placed into the bladder and a partial urethral injury is suspected later, such an injury can easily be demonstrated without removing the catheter. Place the lubricated end of a pediatric feeding tube into the penile urethra alongside the existing Foley catheter (Fig. 55-34). Obtain a seal by compressing the glans penis with the nondominant thumb and index finger and gently inject contrast material via a Luer-Lok syringe with the dominant hand. In this way, extravasation can be demonstrated. It should be noted, however, that successful placement of the Foley catheter obviates the need for further treatment of a partial urethral tear in the emergency setting because an indwelling catheter alone is appropriate initial management of this type of injury. The finding of an associated urethral injury must be conveyed to a urologist because it will dictate the duration of definitive Foley catheter drainage.
Retrograde Cystography
Obtain a preliminary KUB film, which will serve as reference for the entire examination. Next, fill the bladder under direct operator supervision by gravity instillation of contrast material. After removing the central piston from a 60-mL catheter-tipped syringe, attach the catheter-tipped end of the syringe to the Foley catheter and hold it above the level of the patient’s bladder. Pour the contrast material into the syringe and allow it to fill the bladder by gravity instillation to one of three end points: (1) 100 mL with evidence of gross extravasation on fluoroscopy or on plain film (if the examiner elects to check at this point); (2) 400 mL in an adult or any child 11 years or older; in children younger than 11 years, bladder capacity and therefore appropriate contrast volumes are estimated from the formula101 (age in years + 2) × 30; or (3) the point of initiating a bladder contraction (see later). Then add an additional 50 mL by hand injection under pressure.
Obtain anteroposterior (AP) and complementary oblique projections as long as no evidence of a pelvic fracture is present (Fig. 55-35, A1). In the presence of a pelvic fracture, obtain all films with the patient in the supine position for the same reasons that were elucidated for RUG. A lateral film may be informative when oblique films are not possible (see Fig. 55-35, A2). Obtain an AP postevacuation film in all cases after bladder drainage to disclose posterior perforation in selected cases, especially those associated with penetrating trauma (see Fig. 55-35, A3). Again, a dilute solution of contrast material (see Table 55-6) may be used rather than full-strength contrast. Some authors recommend a dilute solution of contrast material (≤10%) because extravasation into periurethral or perivesical tissue may cause a considerable inflammatory reaction at higher concentrations. Dilute solutions do not appear to compromise the quality of the study, but this must be a consideration. Retrograde cystography done by any technique other than hand-poured gravity instillation is subject to inadequate bladder filling or disconnection of the connector tubing from the catheter. Both conditions will result in spurious examination results, which may adversely affect important patient management decisions.
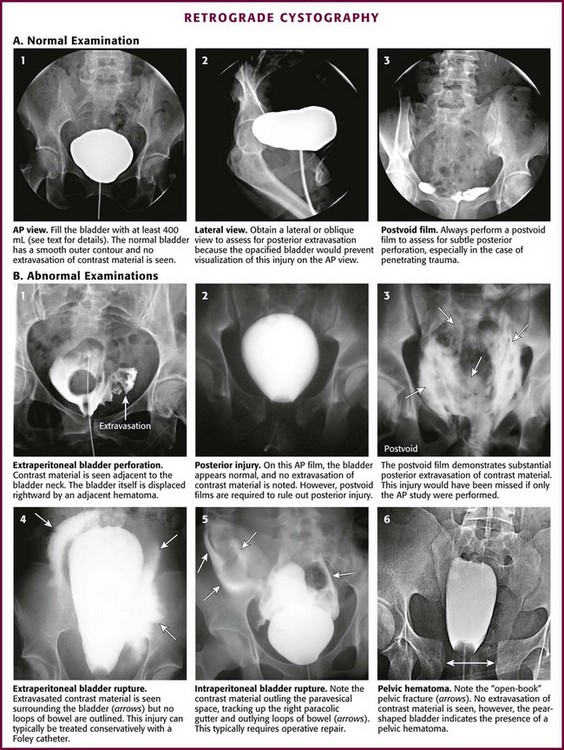
Figure 55-35 Retrograde cystography. AP, anteroposterior.
It must be stressed that in the absence of initial gross extravasation, the bladder must be filled to 400 mL in an adult and to an appropriate capacity in a child and the catheter clamped with a Kelly clamp. Volumes less than 400 mL have been associated with false-negative findings, especially with penetrating bladder injuries.102 At times the patient may have difficulty cooperating with bladder filling because of a head injury or associated pain, and in the case of severe injury, the patient may have involuntary bladder contractions that cause contrast material to back up into the Toomey syringe. If this occurs, refill the bladder to the point of initiating a bladder contraction, clamp the Foley catheter, remove the initial syringe, replace it with a 60-mL contrast-filled syringe, unclamp the catheter, hand-inject the additional 50 mL under pressure, and reclamp the catheter. The goal is to overdistend the bladder. Once the filled-bladder films have been obtained and reviewed, unclamp the Foley catheter and allow the contrast material to drain into a bedside drainage bag. Then obtain an AP postevacuation film to visualize any posterior extravasation that may have been hidden by the distended bladder during the AP filled-bladder film (see Fig. 55-35, B2 and B3). Once again, take care to ensure that contrast material is not spilled onto the patient or the examination table during the procedure. Spilled contrast can lead to spurious examination results.
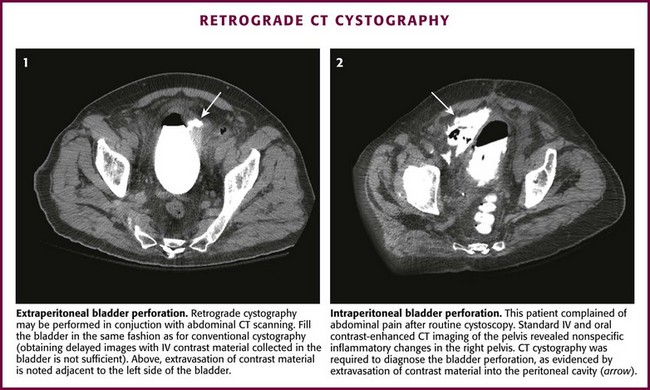
Extravasation from an injured bladder may be intraperitoneal, extraperitoneal, or both. Extraperitoneal extravasation is usually seen as flamelike areas of contrast material confined to the pelvis and projecting laterally to the bladder (see Fig. 55-35, B1 and B4). If contrast material extravasates intraperitoneally, it tends to fill the paracolic gutters and outline the intraperitoneal structures, particularly the bowel, spleen, or liver (see Fig. 55-35, B5). It is important to distinguish extraperitoneal from intraperitoneal injury because the treatment options are totally different: surgical repair for all intraperitoneal injuries and for extraperitoneal injuries that extend into or primarily involve the neck of the bladder, especially in women. Most other extraperitoneal injuries can be managed confidently by Foley catheter drainage alone.
Retrograde cystography may be done in conjunction with contrast-enhanced abdominal CT scanning (Fig. 55-36). The bladder must be filled just as though conventional retrograde cystography were being performed. Clamp the catheter and seek evidence of contrast-enhanced ascites on the CT scan. When this is encountered, bladder injury with extravasation must be searched for with selective images of the pelvis.
Upper GU Tract Imaging
Once the lower GU tract has been exonerated with retrograde urethrography and cystography, the upper tract (kidneys and ureters) should be evaluated. For adult blunt trauma patients with gross hematuria or hypotension (systolic pressure lower than 90 mm Hg), upper tract imaging is indicated.103 Data show that 12.5% of such patients will have a major renal injury as compared with only 0.2% of adults with microscopic hematuria and normal blood pressure.104 In the presence of a major mechanism of injury or other extenuating circumstances, inclusion criteria for upper tract imaging may be broadened. It should be noted that these criteria are not intended for use in pediatric populations, and opinions regarding diagnostic strategies in children remain controversial.
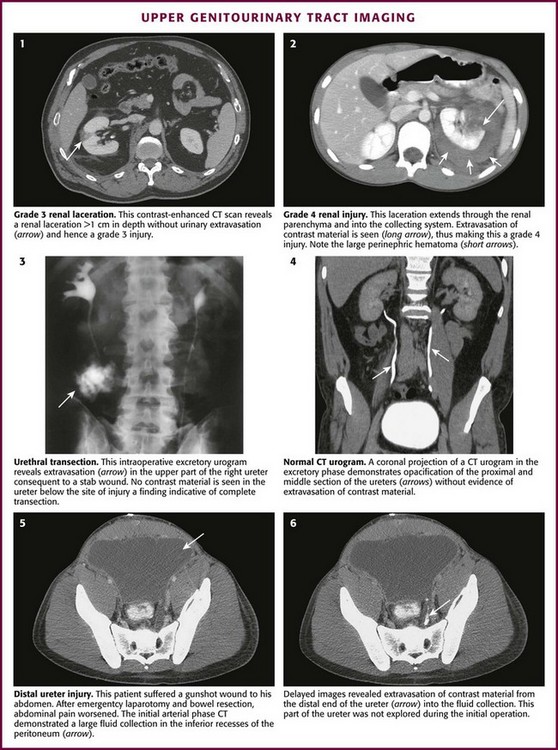
Contrast-enhanced CT is the gold standard for evaluating patients with suspected renal trauma.103 Parenchymal injuries, vascular injuries, and urinary extravasation can all be readily identified on CT (Fig. 55-37, plates 1 and 2). Traumatic renal injuries are graded on a 5-point scale (Box 55-6). For patients with a mandate for emergency laparotomy, upper tract imaging can be performed in the operating room with an excretory urogram (see Fig. 55-37, plate 3).
Ureteral injuries can also be identified on contrast-enhanced CT imaging; however, it is essential to obtain delayed images (i.e., about 10 minutes after the initial intravenous contrast bolus) (see Fig. 55-37, plates 4 to 6). This delay allows the contrast material to become concentrated in the kidneys and excreted through the ureters. Ureteral injury can then be identified by extravasation of contrast material into adjacent tissues.
References
1. Lewis, AG, Bukoswki, TP, Jarvis, PD, et al. Evaluation of the acute scrotum in the emergency department. J Pediatr Surg. 1995;30:277–282.
2. Cavusoglu, YH, Karaman, A, Karaman, I, et al. Acute scrotum—etiology and management. Indian J Pediatr. 2005;72:201–203.
3. Vijayaraghavan, SB. Sonographic differential diagnosis of acute scrotum: real-time whirlpool sign, a key sign of torsion. J Ultrasound Med. 2006;25:563–574.
4. Kamaledeen, S, Surana, R. Intermittent testicular pain: fix the testes. BJU Int. 2003;91:406–408.
5. Visser, AJ, Heyns, CF. Testicular function after torsion of the spermatic cord. BJU Int. 2003;92:200–203.
6. Kalfa, N, Veyrac, C, Lopez, M, et al. Multicenter assessment of ultrasound of the spermatic cord in children with acute scrotum. J Urol. 2007;177:297–301.
7. Arce, JD, Cortes, M, Vargas, JC. Sonographic diagnosis of acute spermatic cord torsion. Rotation of the cord: a key to the diagnosis. Pediatr Radiol. 2002;32:485–491.
8. Kalfa, N, Veyrac, C, Baud, C, et al. Ultrasonography of the spermatic cord in children with testicular torsion: impact on the surgical strategy. J Urol. 2004;172:1692–1695.
9. Sidhu, PS. Clinical and imaging features of testicular torsion: role of ultrasound. Clin Radiol. 1999;54:343–352.
10. Haecker, F-M, Hauri-Hohl, A, von Schweinitz, D. Acute epididymitis in children: a 4-year retrospective study. Eur J Pediatr Surg. 2005;15:180–186.
11. Sakellaris, GS, Charissis, GC. Acute epididymitis in Greek children: a 3-year retrospective study. Eur J Pediatr. 2008;167:765–769.
12. Yang, DM, Lim, JW, Kim, JE, et al. Torsed appendix testis. Gray scale and color Doppler sonographic findings compared with a normal appendix testis. J Ultrasound Med. 2005;24:87–91.
13. Blaivas, M, Sierzenski, P, Lambert, M. Emergency evaluation of patients presenting with acute scrotum using bedside ultrasonography. Acad Emerg Med. 2001;8:90–93.
14. Burks, DD, Markey, BJ, Burkhard, TK, et al. Suspected testicular torsion and ischemia: evaluation with color Doppler sonography. Radiology. 1990;175:815–821.
15. Ingram, S, Hollman, AS, Azmy, A. Testicular torsion: missed diagnosis on color Doppler sonography. Pediatr Radiol. 1993;23:483.
16. Steinhardt, GF, Boyarsky, S, Mackey, R. Testicular torsion: pitfalls of color Doppler sonography. J Urol. 1993;150:461–462.
17. Yazbeck, S, Patriquin, HB. Accuracy of Doppler sonography in the evaluation of acute conditions of the scrotum in children. J Pediatr Surg. 1994;29(9):1270–1272.
18. Allen, TD, Elder, J. Shortcomings of color Doppler sonography in diagnosis of testicular torsion. J Urol. 1995;154:1508–1510.
19. Stehr, M, Boehm, R. Critical validation of color Doppler ultrasound in diagnostics of acute scrotum in children. Eur J Pediatr Surg. 2003;13:386–392.
20. Frauscher, F, Klauser, A, Radmayr, C. Ultrasonographic assessment of the scrotum. Lancet. 2001;357:721–722.
21. Baud, C, Veyrac, C, Couture, A, et al. Spiral twist of the spermatic cord: a reliable sign of testicular torsion. Pediatr Radiol. 1998;28:950–954.
22. Frush, DP, Babcock, DS, Lewis, AG, et al. Comparison of color Doppler sonography and radionuclide imaging in different degrees of torsion in rabbit testes. Acad Radiol. 1995;2:945–951.
23. Nussbaum, AR, Bulas, D, Shalaby-Rana, E, et al. Color Doppler sonography and scintigraphy of the testis: a prospective, comparative analysis in children with acute scrotal pain. Pediatr Emerg Care. 2002;18(2):67–71.
24. Terai, A, Yoshimura, K, Ichioka, K, et al. Dynamic contrast-enhanced subtraction magnetic resonance imaging in the diagnostics of testicular torsion. Urology. 2006;67:1278–1282.
25. Wanatabe, Y, Dohke, M, Ohkubo, K, et al. Scrotal disorders: evaluation of testicular enhancement patterns at dynamic contrast enhanced subtraction MR imaging. Radiology. 2000;217:219–227.
26. Sessions, AE, Rabinowitz, R, Hulbert, WC, et al. Testicular torsion: direction, degree, duration and disinformation. J Urol. 2003;169:663–665.
27. Garel, L, Dubois, J, Azzie, G, et al. Preoperative manual detorsion of the spermatic cord with Doppler ultrasound monitoring in patients with intravaginal testicular torsion. Pediatr Radiol. 2000;30:41–44.
28. Smith, DP. Treatment of epididymitis by infiltration at spermatic cord with procaine hydrochloride. J Urol. 1941;46:74.
29. Lee, LM, Wright, JE, McLoughlin, MG. Testicular torsion in the adult. J Urol. 1983;130:93.
30. Kresling, V, Schroeder, D, Panljev, P, et al. Spermatic cord block and manual reduction: primary treatment for spermatic cord torsion. J Urol. 1984;132:921.
31. Papadopoulos, I, Kelami, A. Priapus and priapism from mythology to medicine. Urology. 1988;32:385–386.
32. Huang, YC, Harraz, A, Shindel, A, et al. Evaluation and management of priapism: 2009 update. Nat Rev Urol. 2009;6:262–271.
33. Burnett, B, Bivalacqua, T. Priapism: current principles and practice. Urol Clin North Am. 2007;34:631–642.
34. Green, J, Hakim, L. Cocaine-induced veno-occlusive priapism: importance of urine toxicology screening in the emergency room setting. Clin Urol. 1999;161(4S):180.
35. Volkmer, BG, Nesslauer, T, Kraemer, SC, et al. Prepubertal high flow priapism: incidence, diagnosis and treatment. J Urol. 2001;166:1018.
36. Fernandez, JA, Basha, MA, Wilson, GC. Emergency treatment of papaverine priapism. J Emerg Med. 1987;5:289.
37. Winter, CC, McDowell, G. Experience with 105 patients with priapism: updated review of all aspects. J Urol. 1988;140:980.
38. Merritt, A, Haiman, C, Henderson, S. Myth. Blood transfusion is effective for sickle cell anemia-associated priapism. Can J Emerg Med. 2006;8:119–122.
39. Lowe, JC, Jarow, JP. Placebo-controlled study of oral terbutaline and pseudoephedrine in management of prostaglandin E1–induced prolonged erections. Urology. 1993;42:51–54.
40. Govier, FE, Jonsson, E, Kramer-Levin, D. Oral terbutaline for the treatment of priapism. J Urol. 1994;151:878–879.
41. Erectile Dysfunction Guideline Update Panel, The Management of Priapism, Baltimore, American Urological Association, Inc., 2003. [reaffirmed September 28, 2009]. Available at, http://guidelines.gov/content.aspx?id=3741. [Accessed January 5, 2011].
42. Priyadarshi, S. Oral terbutaline in the management of pharmacologically induced prolonged erection. Int J Impot Res. 2004;16:424–426.
43. Muneer, A, Minhas, S, Arya, M, et al. Stuttering priapism—a review of the therapeutic options. Int J Clin Pract. 2008;62:1265–1270.
44. Roberts, JR, Price, C, Mazzeo, T. Intracavernous epinephrine: a minimally invasive treatment for priapism in the emergency department. J Emerg Med. 2009;36:285–289.
45. Muruve, N, Hosking, DH. Intracorporeal phenylephrine in the treatment of priapism. J Urol. 1996;155:141.
46. O’Brien, WM, O’Connor, KP, Lynch, JH. Priapism: current concepts. Ann Emerg Med. 1989;18:980.
47. Lue, TF, Helstrom, WJ, McAninch, JW, et al. Priapism: a refined approach to diagnosis and treatment. J Urol. 1986;136:104.
48. Bivalacqua, T, Burnett, A. Priapism: new concepts in the pathophysiology and new treatment strategies. Curr Urol Rep.. 2006;7:497–502.
49. Choe, JM. Paraphimosis: current treatment options. Am Fam Physician. 2000;62:2623–2626.
50. Little, B, White, M. Treatment options for paraphimosis. Int J Clin Pract. 2005;59:591–593.
51. Ganti, SU, Sayegh, N, Addonizio, JC. Simple method for the reduction of paraphimosis. Urology. 1985;25:77.
52. Skoglund, RW, Chapman, WH. Reduction of paraphimosis. J Urol. 1970;104:137.
53. Houghton, GR. The “iced-gloved” method of treatment of paraphimosis. Br J Surg. 1973;60:876.
54. Reynard, J, Barua, J. Reduction of paraphimosis the simple way—the Dundee technique. BJU Int. 1999;83:859–860.
55. DeVries, C, Miller, A. Reduction of paraphimosis with hyaluronidase. Urology. 1996;48:464–465.
56. Kerwat, R, Shandall, A, Stephenson, B. Reduction of paraphimosis with granulated sugar. Br J Urol. 1998;82:755.
57. Mackway-Jones, K. Ice, pins, or sugar to reduce paraphimosis. Emerg Med J. 2004;21:77–78.
58. Coutts, AG. Treatment of paraphimosis. Br J Surg. 1991;78:252.
59. Williams, JC, Morrison, PM, Richardson, JR. Paraphimosis in elderly men. Am J Emerg Med. 1995;13:351–353.
60. McGregor, TB, Pike, JG, Leonard, MP. Pathologic and physiologic phimosis: approach to the phimotic foreskin. Can Fam Physician. 2007;53:445–448.
61. Oster, J. Further fate of the foreskin: incidence of preputial adhesions, phimosis, and smegma among Danish schoolboys. Arch Dis Child. 1968;43(228):200–203.
62. Goulding, FJ. Penile block for postoperative pain relief in penile surgery. J Urol. 1981;126:337.
63. Lipsky, BA, Inui, TS, Plorde, JJ, et al. Is the clean catch midstream void procedure necessary for obtaining urine culture specimens from men? Am J Med. 1984;76:257.
64. Walter, FG, Knopp, RK. Urine sampling in ambulatory women: midstream clean-catch versus catheterization. Ann Emerg Med. 1989;18:166.
65. Wilson, ML, Gaido, L. Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis. 2004;38:1150–1158.
66. Blandy, JP. Acute retention of urine. Br J Hosp Med. 1978;19:109.
67. Shlamovitz, GZ, McCullough, L. Blind urethral catheterization in trauma patients suffering from lower urinary tract injuries. J Trauma. 2007;62:330.
68. Spirnak, JP. Pelvic fracture and injury to the lower urinary tract. Surg Clin North Am. 1988;68:1057.
69. The Harriet Lane Handbook, 18 ed. Philadelphia, Elsevier Mosby, 2009.
70. O’Brien, WM. Percutaneous placement of a suprapubic tube with peel-away sheath introducer. J Urol. 1991;145:1015.
71. Villanueva, C, Hemstreet, GP, Difficult Catheterization: Tricks of the Trade. AUA Update Series 2011, Volume 30, Lesson 5, Linthicum, MD, American Urological Association, 2011.
72. Hooton, TM, Bradley, SF, Cardenas, DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–663.
73. Farraye, MJ, Seaberg, D. Indwelling Foley catheter causing extraperitoneal bladder perforation. Am J Emerg Med. 2000;18:497.
74. Steidle, CP, Mulcahy, JJ. Erosion of penile prostheses: a complication of urethral catheterization. J Urol. 1989;142:737.
75. Eichenberg, HA, Amin, M, Clark, J. Non-deflating Foley catheters. Int Urol Nephrol. 1976;8:171.
76. Patterson, R, Little, B, Tolan, J, et al. How to manage a urinary catheter balloon that will not deflate. Int Urol Nephrol. 2006;38:57.
77. Moisey, CA, Williams, LA. Self-retained balloon catheter: a safe method for removal. Br J Urol. 1980;52:67.
78. Acharya, A, Mishra, DR. Complete eversion and prolapse of the bladder following pulling out a Foley catheter concurrent with uterine prolapse. Indian J Urol. 2007;23:474.
79. Beeson, PB, Guze, LB. Observations on the reliability and safety of bladder catheterization for bacteriologic study of the urine. N Engl J Med. 1956;255:474.
80. Pryles, CV, Atkin, MD, Morse, TS, et al. Comparative bacteriologic study of urine obtained from children by percutaneous suprapubic aspiration of the bladder and by catheter. Pediatrics. 1959;24:983–991.
81. Pollack, CV, Pollack, ES, Andrew, ME. Suprapubic bladder aspiration versus urethral catheterization in ill infants: success, efficiency, and complications rates. Ann Emerg Med. 1994;23:225–230.
82. Kozer, E, Rosenbloom, E, Goldman, D, et al. Pain in infants who are younger than 2 months during suprapubic aspiration and transurethral bladder catheterization: a randomized, controlled study. Pediatrics. 2006;118:e51.
83. El-Naggar, W, Yiu, A, Mohamed, A, et al. Comparison of pain during two methods of urine collection in preterm infants. Pediatrics. 2010;125:1224–1229.
84. American Academy of Pediatrics; Committee on Quality Improvement, Subcommittee on Urinary Tract Infection. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics. 1999;103:843–852.
85. Buys, H, Pead, L, Hallett, R, et al. Suprapubic aspiration under ultrasound guidance in children with fever of undiagnosed cause. BMJ. 1994;308:690.
86. Munir, V, Barnett, P, South, M. Does the use of volumetric bladder ultrasound improve the success rate of suprapubic aspiration of urine? Pediatr Emerg Care. 2002;18:346–349.
87. Chu, RW, Wong, YC, Luk, SH, et al. Comparing suprapubic urine aspiration under real-time ultrasound guidance with conventional blind aspiration. Acta Paediatr. 2002;91:512–516.
88. Mustonen, A, Uhari, M. Is there bacteremia after suprapubic aspiration in children with urinary tract infection? J Urol. 1978;119:822.
89. Weuthers, WT, Wenzl, JE. Suprapubic aspiration: perforation of the viscus other than the bladder. Am J Dis Child. 1969;117:590.
90. O’Brien, WM. Percutaneous placement of a suprapubic tube with peel-away sheath introducer. J Urol. 1991;145:1015.
91. Chiou, RK, Morton, JJ, Engelsgjerd, JS, et al. Placement of large suprapubic tube using peel-away introducer. J Urol. 1995;153:1179–1181.
92. Perry, MO, Husmann, DA. Urethral injuries in female subjects following pelvic fractures. J Urol. 1992;147:139.
93. Spirnak, JP. Pelvic fracture and injury to the lower urinary tract. Surg Clin North Am. 1988;68:1057.
94. Sty, JR, Pan, CP. Genitourinary imaging techniques. Pediatr Clin North Am. 2006;53:339–361.
95. Shlamovitz, GZ, McCullough, L. Blind urethral catheterization in trauma patients suffering from lower urinary tract injuries. J Trauma. 2007;62:330.
96. Shlamovitz, GZ, Mower, WR, Bergman, J, et al. Poor test characteristics for the digital rectal examination in trauma patients. Ann Emerg Med. 2007;50:25.
97. American College of Radiology (ACR) Committee on Drugs and Contrast Media. ACR Manual on Contrast Media, Version 7, 2010. Reston, VA: American College of Radiology; 2010.
98. Santucci, RA, Langenburg, SE, Zachareas, MJ. Traumatic hematuria in children can be evaluated as in adults. J Urol. 2004;171:822.
99. Sandler, CM, Corriere, JN, Jr. Urethrography in the diagnosis of acute urethral injuries. Urol Clin North Am. 1989;16:283–289.
100. Kawashima, A, Sandler, CM, Wasserman, NF, et al. Imaging of urethral disease: a pictorial review. Radiographics. 2004;24:S195–S216.
101. Berger, RM, Maizels, M, Moran, GC, et al. Bladder capacity (ounces) equals age (years) plus 2 predicts normal bladder capacity and aids in diagnosis of abnormal voiding patterns. J Urol. 1983;129:347–349.
102. Cass, AS. False-negative retrograde cystography with bladder rupture owing to external trauma. J Trauma. 1984;24:168.
103. Jankowski, JT, Spirnak, P. Current recommendations for imaging in the management of urologic traumas. Urol Clin North Am. 2006;33:365–376.
104. Miller, KS, McAninch, JW. Radiographic assessment of renal trauma: our 15-year experience. J Urol. 1995;154:352–355.