Urogenital System
The urogenital system arises from the intermediate mesoderm of the early embryo (see Fig. 6.7). Several major themes underlie the development of urinary and genital structures from this common precursor. The first is the interconnectedness of urinary and genital development, in which early components of one system are taken over by another during its later development. A second is the recapitulation during human ontogeny of kidney types (the equivalent of organ isoforms) that are terminal forms of the kidney in lower vertebrates. A third theme comprises the dependence of differentiation and the maintenance of many structures in the urogenital system on epithelial-mesenchymal interactions. Finally, the sexual differentiation of many structures passes from an indifferent stage, in which male and female differences are not readily apparent, to a male or female pathway, depending on the presence of specific promoting or inhibiting factors acting on the structure. Although phenotypic sex is genetically determined, genetic sex can be overridden by environmental factors, thus leading to a discordance between the two. Clinical Correlations 16.1 and 16.2, later in this chapter, discuss abnormalities of the urinary and genital systems, respectively.
Urinary System
Early Forms of the Kidney
The common representation of mammalian kidney development includes three successive phases beginning with the appearance of the pronephros, the developmental homologue of the type of kidney found in only the lowest vertebrates. In human embryos, the first evidence of a urinary system consists of the appearance of a few segmentally arranged sets of epithelial cords that differentiate from the anterior intermediate mesoderm at about 22 days’ gestation. These structures are more appropriately called nephrotomes. The nephrotomes connect laterally with a pair of primary nephric (pronephric) ducts, which grow toward the cloaca (Fig. 16.1). The earliest stages in the development of the urinary system depend on the action of retinoic acid, which sets the expression limits of Hox 4-11 genes that determine the craniocaudal limits of the early urinary system. The molecular response by the intermediate mesoderm is the expression of the transcription factors Pax-2 and Pax-8, which then induce Lim-1 (Lhx-1) in the intermediate mesoderm. Lim-1 is required for the aggregation of the mesenchymal cells of the intermediate mesoderm into the primary nephric ducts.
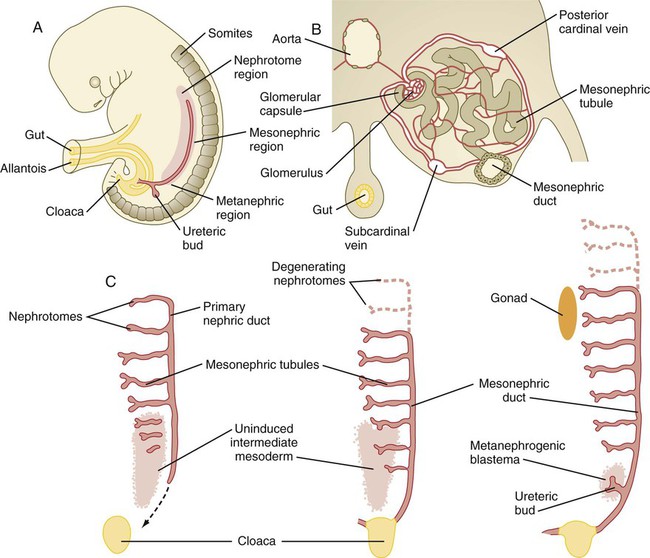
A, Subdivision of the intermediate mesoderm into areas that will form nephrotomes, mesonephros, and metanephros. B, Cross section through mesonephros showing a well-developed mesonephric tubule and its associated vasculature. C, Caudal progression of formation of the mesonephros and degeneration of the most cranial segments of the primitive kidney.
As the primary nephric ducts extend caudally, they stimulate the intermediate mesoderm to form additional segmental sets of tubules. The conversion of the mesenchymal cells of the intermediate mesoderm into epithelial tubules depends on the expression of Pax-2, and in the absence of this molecule, further development of kidney tubules does not occur. These tubules are structurally equivalent to the mesonephric tubules of fishes and amphibians. A typical mesonephric unit consists of a vascular glomerulus, which is partially surrounded by an epithelial glomerular capsule. The glomerular capsule is continuous with a contorted mesonephric tubule, which is surrounded by a mesh of capillaries (see Fig. 16.1B). Each mesonephric tubule empties separately into the continuation of the primary nephric duct, which becomes known as the mesonephric (wolffian) duct.
The formation of pairs of mesonephric tubules occurs along a craniocaudal gradient. The first 4 to 6 pairs of mesonephric tubules (and the pronephric tubules) arise as outgrowths from the primary nephric ducts. Farther caudally, mesonephric tubules, up to a total of 36 to 40, take shape separately in the intermediate mesoderm slightly behind the caudal extension of the mesonephric ducts. By the end of the fourth week of gestation, the mesonephric ducts attach to the cloaca, and a continuous lumen is present throughout each. There is a difference in the developmental controls between the most cranial 4 to 6 pairs of mesonephric tubules and the remaining caudal tubules. Knockouts for the WT-1 (Wilms’ tumor suppressor) gene result in the absence of posterior mesonephric tubules, whereas the cranial tubules that bud off the pronephric duct form normally. As is the case in the formation of the metanephros (see later), WT-1 regulates the transformation from mesenchyme to epithelium during the early formation of renal (mesonephric) tubules. Very near its attachment site to the cloaca, the mesonephric duct develops an epithelial outgrowth called the ureteric bud (see Fig. 16.1A).
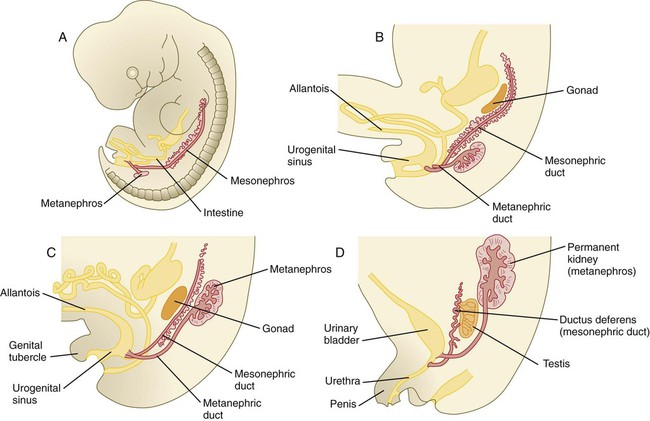
The mesonephros is most prominent while the definitive metanephros is beginning to take shape. Although it rapidly regresses as a urinary unit after the metanephric kidneys become functional, the mesonephric ducts and some of the mesonephric tubules persist in the male and become incorporated as integral components of the genital duct system (Fig. 16.2).
Metanephros
Development of the metanephros begins early in week 5 of gestation, when the ureteric bud (metanephric diverticulum) grows into the posterior portion of the intermediate mesoderm. Mesenchymal cells of the intermediate mesoderm condense around the metanephric diverticulum to form the metanephrogenic blastema (see Fig. 16.1C). Outgrowth of the ureteric bud from the mesonephric duct is a response to the secretion of glial cell line–derived neurotrophic factor (GDNF) by the undifferentiated mesenchyme of the metanephrogenic blastema (Fig. 16.3A). This inductive signal is bound by c-Ret, a member of the tyrosine kinase receptor superfamily, and the coreceptor Gfra-1, which are located in the plasma membranes of the epithelial cells of the early ureteric bud. The formation of GDNF in the metanephric mesenchyme is regulated by WT-1. The posterior location of the ureteric bud results from a combination of repression of GDNF expression in the more anterior regions by the actions of Slit-2/Robo-2 in the mesenchyme and Sprouty, which reduces the sensitivity of the anterior mesonephric duct to the action of GDNF. Bone morphogenetic protein (BMP) signaling in the surrounding mesoderm is also inhibitory to outgrowth of the ureteric bud, but within the metanephrogenic blastema its action is counteracted by the BMP-inhibitory actions of gremlin, which is produced within the blastema itself.
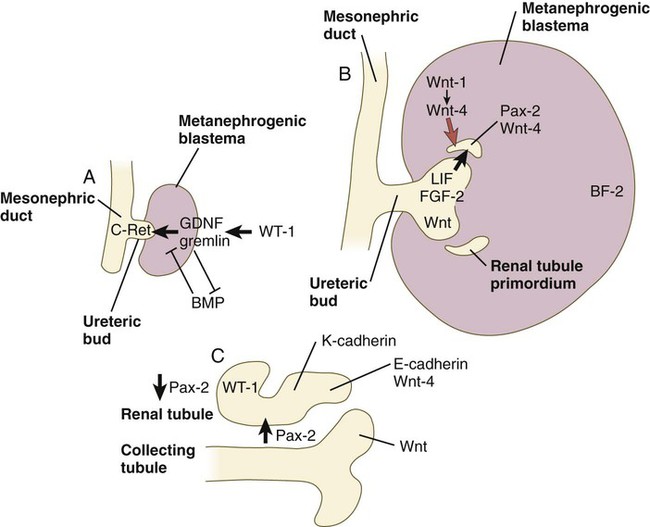
A, Early induction of metanephros. B, Branching of ureteric bud. C, Early tubule formation. FGF, fibroblast growth factor; GDNF, glial cell line–derived neurotrophic factor; LIF, leukemia inhibitory factor.
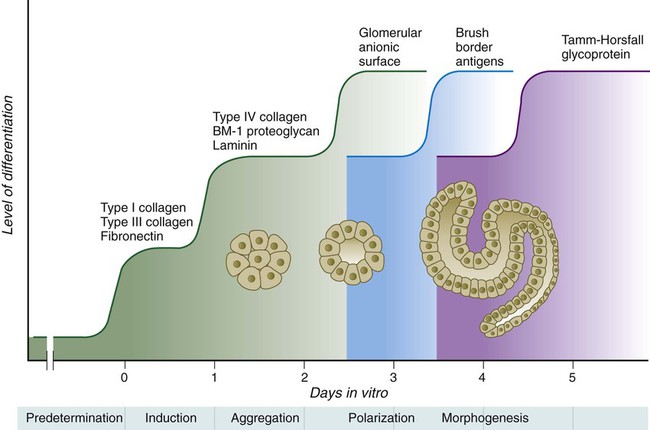
The formation of individual functional tubules (nephrons) in the developing metanephros involves three mesodermal cell lineages: epithelial cells derived from the ureteric bud, mesenchymal cells of the metanephrogenic blastema, and ingrowing vascular endothelial cells. The earliest stage is the condensation of mesenchymal blastemal cells around the terminal bud of the ureteric bud (later to become the metanephric duct). The preinduced mesenchyme contains several interstitial proteins, such as types I and III collagen and fibronectin. As the mesenchymal cells condense after local induction by the branching tips of the ureteric bud, these proteins are lost and are replaced with epithelial-type proteins (type IV collagen, syndecan-1, laminin, and heparin sulfate proteoglycan), which are ultimately localized to the basement membranes (Fig. 16.4).
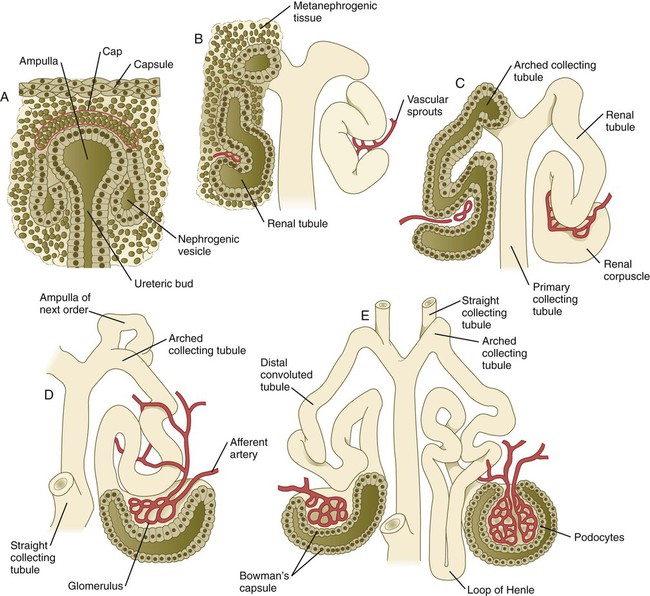
As the terminal bud of the metanephric duct branches, each tip is surrounded by a cap of condensed mesenchyme. Soon, this cap becomes subdivided into a persisting mesenchymal cap and, at its end, a region where the mesenchyme is transforming into an epithelial nephrogenic vesicle (Fig. 16.5A). A single condensation of mesenchymal cells undergoes a defined series of stages to form a renal tubule. After a growth phase, mitotic activity within the rounded blastemal mesenchyme decreases, and the primordium of the tubule assumes a comma shape. Within the comma, a group of cells farthest from the end of the metanephric duct becomes polarized and forms a central lumen and a basal lamina on the outer surface. This marks the transformation of the induced mesenchymal cells into an epithelium—the specialized podocytes, which ultimately surround the vascular endothelium of the glomerulus.
A consequence of this epithelial transformation is the formation of a slit just beneath the transforming podocyte precursors in the tubular primordium (Fig. 16.5B). Precursors of vascular endothelial cells grow into this slit, which ultimately forms the glomerulus. Induced metanephric mesenchyme stimulates the ingrowth of endothelial cells, possibly by the release of a factor similar to FGF. Uninduced mesenchyme does not possess this capability. The endothelial cells are connected with branches from the dorsal aorta, and they form a complex looping structure that ultimately becomes the renal glomerulus. Cells of the glomerular endothelium and the adjoining podocyte epithelium form a thick basement membrane between them. This basement membrane later serves as an important component of the renal filtration apparatus.
As the glomerular apparatus of the nephron takes shape, another slit forms in the comma-shaped tubular primordium, thus transforming it into an S-shaped structure (Fig. 16.5C). Cells in the rest of the tubule primordium also undergo an epithelial transformation to form the remainder of the renal tubule. This transformation involves the acquisition of polarity by the differentiating epithelial cells. It is correlated with the deposition of laminin in the extracellular matrix along the basal surface of the cells and the concentration of the integral membrane glycoprotein uvomorulin (E-cadherin), which seals the lateral borders of the cells (Fig. 16.6). As the differentiating tubule assumes an S shape, differing patterns of gene expression are seen along its length. Near the future glomerular end, levels of Pax-2 expression decrease as WT-1 becomes strongly expressed (see Fig. 16.3). Lim-1 expression and the downstream Delta/Notch system are now known to play a prominent role in generating the proximal convoluted tubule. At the other end of the tubule (future distal convoluted tubule), Wnt-4 and E-cadherin remain prominent, whereas in the middle (future proximal convoluted tubule), K-cadherin is a prominent cellular marker. Many of the uninduced mesenchymal cells between tubules undergo apoptosis.
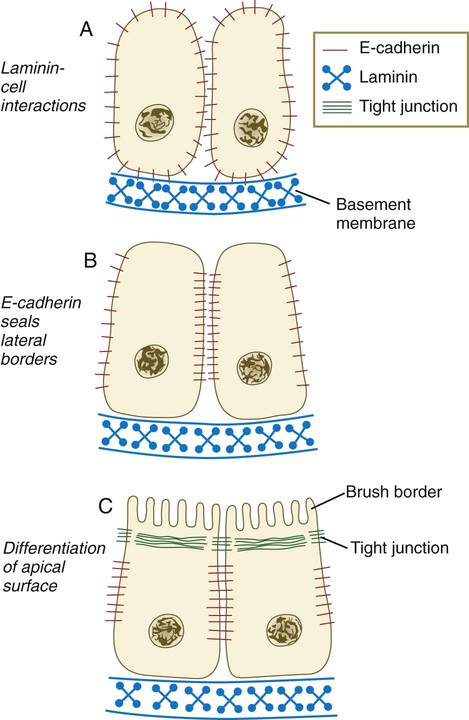
A, Development of polarity is triggered by interactions between laminin and the cell surface, but E-cadherin is still distributed in a nonpolar manner. B, E-cadherin redistribution occurs, and E-cadherin interactions seal the lateral borders of the cells. C, Apical border of epithelial cells differentiates, as seen by formation of a brush border. (Based on Ekblom P: FASEB J 3:2141-2150, 1989.)
Differentiation of the renal tubule progresses from the glomerulus to the proximal and then distal convoluted tubule. During differentiation of the nephron, a portion of the tubule develops into an elongated hairpin loop that extends into the medulla of the kidney as the loop of Henle. As they differentiate, the tubular epithelial cells develop molecular features characteristic of the mature kidney (e.g., brush border antigens or the Tamm-Horsfall glycoprotein [see Fig. 16.4]).
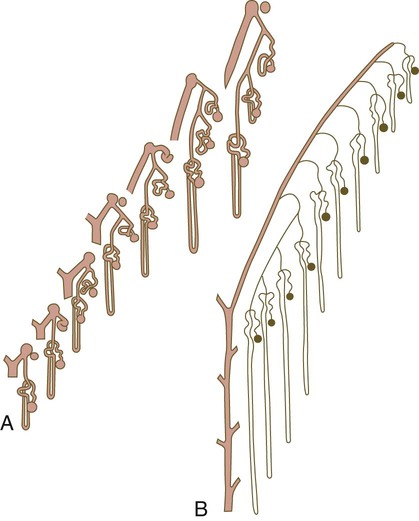
Growth of the kidney involves the formation of approximately 15 successive generations of nephrons in its peripheral zone, with the outermost nephrons less mature than the nephrons farther inward. Development of the internal architecture of the kidney is complex, involving the formation of highly ordered arcades of nephrons (Fig. 16.7). Details are beyond the scope of this text.
Later Changes in Kidney Development
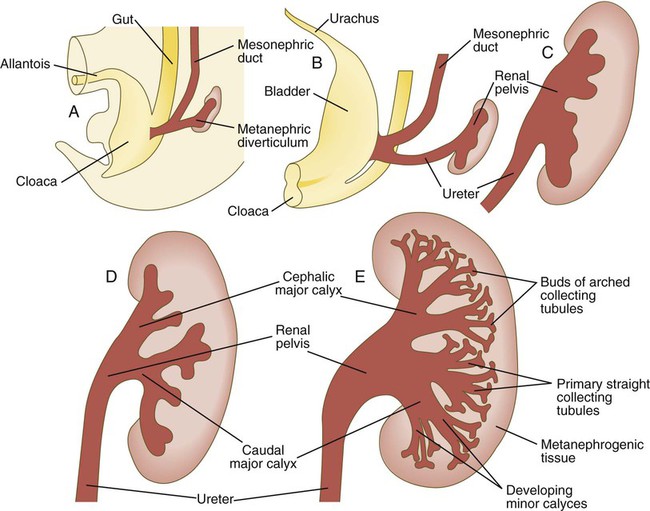
While the many sets of nephrons are differentiating, the kidney becomes progressively larger. The branched system of ducts also becomes much larger and more complex, and it forms the pelvis and system of calyces of the kidney (Fig. 16.8). These structures collect the urine and funnel it into the ureters. During much of the fetal period, the kidneys are divided into grossly visible lobes. By birth, the lobation is already much less evident, and it disappears during the neonatal period.
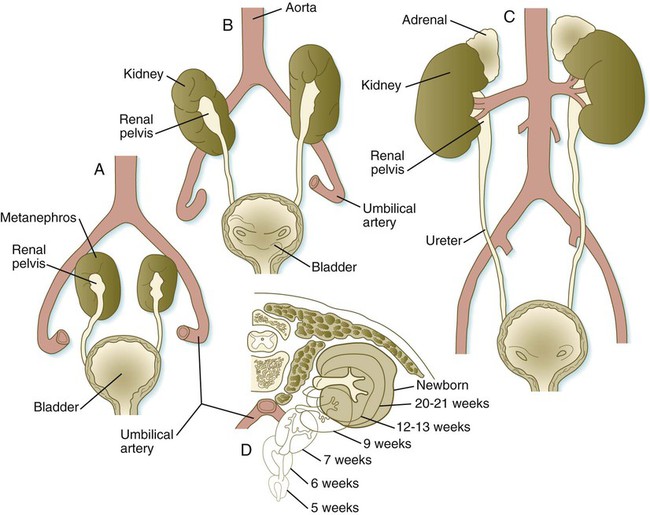
When they first take shape, the metanephric kidneys are located deep in the pelvic region. During the late embryonic and early fetal period, they undergo a pronounced shift in position that moves them into the abdominal region. This shift results partly from actual migration and partly from a marked expansion of the caudal region of the embryo. Two concurrent components to the migration occur. One is a caudocranial shift from the level of L4 to L1 or even the T12 vertebra (Fig. 16.9). The other is a lateral displacement. These changes bring the kidneys into contact with the adrenal glands, which form a cap of glandular tissue on the cranial pole of each kidney. During their migration, the kidneys also undergo a 90-degree rotation, with the pelvis ultimately facing the midline. As they are migrating out of the pelvic cavity, the kidneys slide over the large umbilical arteries, which branch from the caudal end of the aorta. All these changes occur behind the peritoneum because the kidneys are retroperitoneal organs. During the early phases of migration of the metanephric kidneys, the mesonephric kidneys regress. The mesonephric ducts are retained, however, as they become closely associated with the developing gonads.
Formation of the Urinary Bladder
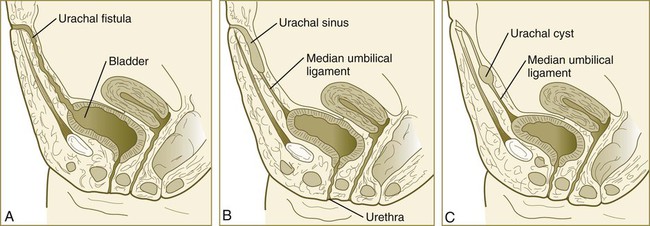
The division of the cloaca into the rectum and urogenital sinus region was introduced in Chapter 15 (see Fig. 15.13). The urogenital sinus is continuous with the allantois, which has an expanded base continuous with the urogenital sinus and an attenuated tubular process that extends into the body stalk on the other end. Along with part of the urogenital sinus, the dilated base of the allantois continues to expand to form the urinary bladder, and its attenuated distal end solidifies into the cordlike urachus, which ultimately forms the median umbilical ligament that leads from the bladder to the umbilical region (see Fig. 16.19).
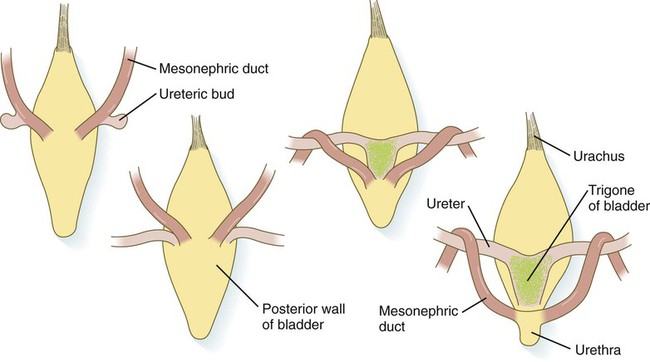
As the bladder grows, its expanding wall, which is derived from tailbud mesenchyme, incorporates the mesonephric ducts and the ureteric buds (Fig. 16.10). The result is that these structures open separately into the posterior wall of the bladder. Through a poorly defined mechanism possibly involving mechanical tension exerted by the migrating kidneys, the ends of the ureters open into the bladder laterally and cephalically to the mesonephric ducts. The region bounded by these structures is called the trigone of the bladder, but much of the substance of the trigone itself is composed of musculature from the bladder. Only small strips of smooth muscle along the edges of the trigone may arise from ureteral smooth muscle. At the entrance of the mesonephric ducts, the bladder becomes sharply attenuated. This region, originally part of the urogenital sinus, forms the urethra, which serves as the outlet of the bladder (see p. 401).
Clinical Correlation 16.1 presents congenital anomalies of the urinary system.
Genital System
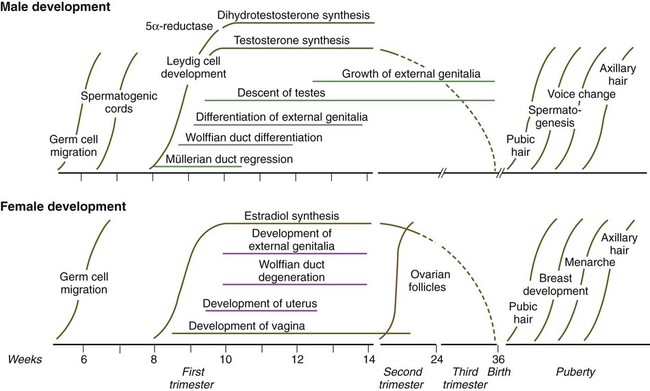
Development of the genital system is one phase in the overall sexual differentiation of an individual (Fig. 16.21). Sexual determination begins at fertilization, when a Y chromosome or an additional X chromosome is joined to the X chromosome already in the egg. This phase represents the genetic determination of gender. Although the genetic gender of the embryo is fixed at fertilization, the gross phenotypic gender of the embryo is not manifested until the seventh week of development. Before that time, the principal morphological indicator of the embryo’s gender is the presence or absence of the sex chromatin (Barr body) in the female. The Barr body is the result of inactivation of one of the X chromosomes. During this morphologically indifferent stage of sexual development, the gametes migrate into the gonadal primordia from the yolk sac.
The phenotypic differentiation of gender is traditionally considered to begin with the gonads* and progresses with gonadal influences on the sexual duct systems. Similar influences on the differentiation of the external genitalia and finally on the development of the secondary sexual characteristics (e.g., body configuration, breasts, hair patterns) complete the events that constitute the overall process of sexual differentiation. Sexual differentiation of the brain, which has an influence on behavior, also occurs.
Genetic Determination of Gender
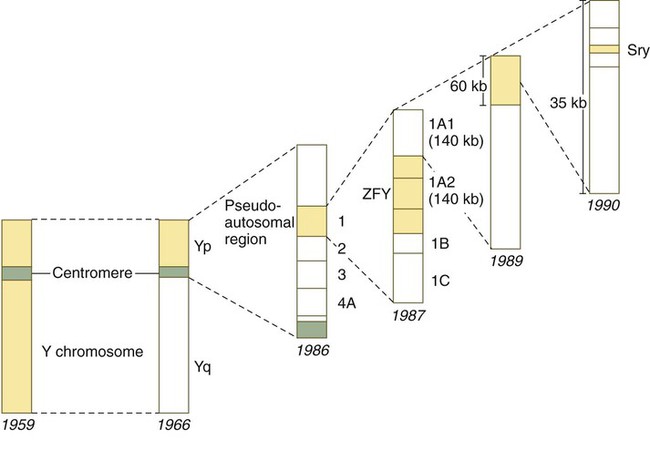
The most recent candidate for the testis-determining gene is one called Sry, a member of the Sox family of transcription factors and probably an evolutionary derivative of Sox-3; it is also located within a 35-kb region on the short arm of the Y chromosome (Fig. 16.22). The Sry gene encodes a 223-amino acid nonhistone protein belonging to a family of proteins that contain a highly conserved 79-amino acid DNA-binding region called a high mobility group box. After the gene was cloned, it was detected in many cases of gender reversal, including XX males with no ZFY genes. The SRY gene on the human Y chromosome is located near the homologous region, thus making it susceptible to translocation to the X chromosome.
Specification of Germ Cells, Migration into the Gonads, and Entry into Meiosis
The early appearance of primordial germ cells (PGCs) in the lining of the yolk sac and their migration into the gonads in human embryos is briefly described in Chapter 1. Both descriptive and experimental studies in the mouse have shown that PGCs originate in the epiblast-derived extraembryonic mesoderm at the posterior end of the primitive streak. In the mouse, as few as six precursor cells become specified to become PGCs in response to BMP-2, BMP-4, and BMP-8b, which are secreted by nearby extraembryonic ectoderm. These cells maintain pluripotency by expressing Sox, Nanog, and Oct-4, much as these genes maintain the undifferentiated condition of blastomeres of cleaving embryos (see p. 42). They are protected by the transcriptional repressor Blimp-1 from entering the default transcriptional program that directs cells of the epiblast to become somatic cells.
Once specified, the PGCs begin a phase of active migration (see Fig. 1.1), which takes them first from the base of the allantois to the future hindgut and, in a second phase, from the hindgut up the dorsal mesentery into the genital ridges (future gonads). During the migratory phase, PGCs are protected from undergoing apoptosis by the actions of Nanos-3, an evolutionarily conserved protein involved in germ cell maintenance. The initial stages of migration of PGCs at some distance from the gonads are accomplished by active ameboid movement of the cells in response to a permissive extracellular matrix substrate. Tissue displacements through differential growth of the posterior region of the embryo may also contribute. During their migration, many PGCs are linked to one another through long cytoplasmic processes. How these interconnections control either migration or settling down in the gonads remains to be determined. As they migrate through the dorsal mesentery, the PGCs proliferate in response to mitogenic factors such as leukemia inhibitory factor and Steel factor (Kit-ligand).
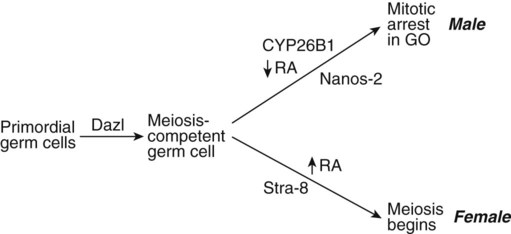
After entry into the genital ridges, PGCs in females enter meiosis, whereas those in males undergo mitotic arrest. Initially, male and female PGCs are equivalent, and under the influence of Dazl (Deleted in azoospermia-like), they both progress to a meiosis-competent stage (Fig. 16.23). At this point, differing environments in male and female gonads exert a profound effect on the PGCs. In the female, retinoic acid, produced in the tubules of the adjoining mesonephros, is found in the gonad. Working through Stra-8, which is required for premeiotic DNA replication, retinoic acid stimulates the PGCs to enter the meiotic cycle. In the male gonad, the action of the cytochrome P450 enzyme Cyp26b1 catabolizes the mesonephrically derived retinoic acid into inactive metabolites. This action, along with the antimeiotic activity of Nanos-2 within the germ cells, prevents entrance of the PGCs into meiosis. Instead, they become arrested at the G0 phase of the mitotic cycle, where they remain until after birth. If Cyp26b1 is inactivated, male PGCs, like their female counterparts, are also propelled into the meiotic cycle. The female gonad suppresses the formation of both these meiosis-inhibiting factors (Cyp26b1 and Nanos-2).
Some PGCs follow inappropriate migratory pathways that lead the cells to settle into extragonadal sites. These cells normally start to develop as oogonia, regardless of genotype; they then degenerate. Rarely, however, the PGCs persist in ectopic sites, such as the mediastinum or the sacrococcygeal region, and ultimately may give rise to teratomas (see Chapter 1).
Establishment of Gonadal Gender
Origin of the Gonads and Adrenal Cortex
One of the earliest genes required for development of the gonads is WT-1, which is expressed throughout the intermediate mesoderm and, as discussed earlier (see p. 376), is important in early kidney formation (see Fig. 16.3). Steroidogenic factor-1 (SF-1) is expressed in the early indifferent gonad and in the developing adrenal cortex. SF-1 apparently is involved in the somatic cells of the early gonad, rather than the PGCs. In keeping with its association with endocrine organs, SF-1 is also expressed in cells of the pituitary and hypothalamus. The other major gene involved in the earliest phase of gonadal development is Lim1. As noted earlier in this text (see Fig. 5.9), the absence of Lim1 expression results in the lack of formation of the anterior head; in addition, neither kidneys nor gonads form.
Differentiation of the Testes
When the genital ridges first appear, those of males and females are morphologically indistinguishable (indifferent stage). The general principle underlying gonadal differentiation is that under the influence of the Sry gene (testis-determining factor) on the Y chromosome, the indifferent gonad differentiates into a testis (Fig. 16.24). In the absence of expression of products of this gene, the gonad later differentiates into an ovary.
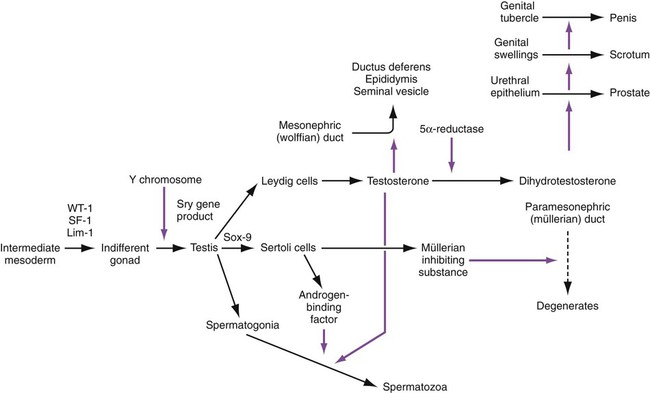
In males, transcripts of the Sry gene are detected only in the genital ridge just at the onset of differentiation of the testis. Neither expression of the Sry gene nor later differentiation of the testis depends on the presence of germ cells. The sex-determining genes act on the somatic portion of the testis and not on the germ cells. In males, Sry stimulates the expression of Sox-9, which initiates the pathway of differentiation of undifferentiated stromal cells into Sertoli cells. Sox-9 stimulates FGF-9 activity, which reinforces Sox-9 activity (see Fig. 16.27).
The morphology of early gonadal differentiation has been controversial, with several proposed scenarios of cell lineage and interactions. According to more recent morphological evidence, the genital ridges first appear midway in the fifth week through the proliferation of coelomic epithelial cells along the medial border of the mesonephros (Fig. 16.25). Later in the fifth week, the PGCs enter the early genital ridge, and the coelomic epithelium sends short epithelial pillars toward the interior of the gonad. Early in the sixth week, under the influence of the transcription factor Sox-9, a set of primitive sex cords takes shape in the genital ridge, and the PGCs migrate into the primitive sex cords. The primitive sex cords are partitioned from one another by endothelial cells that grow into the genital ridge, probably from the mesonephros. The cords are then surrounded by a thin layer of myoid cells of local origin. The myoid cells, which in the adult contract and help to move developing sperm cells along the seminiferous tubules, have no equivalent in the ovary. It is still unclear how many types of cells migrate into the gonad from the mesonephros, and if so, what is the nature of the stimulus for their migration. Ovarian tissue does not attract these cells.
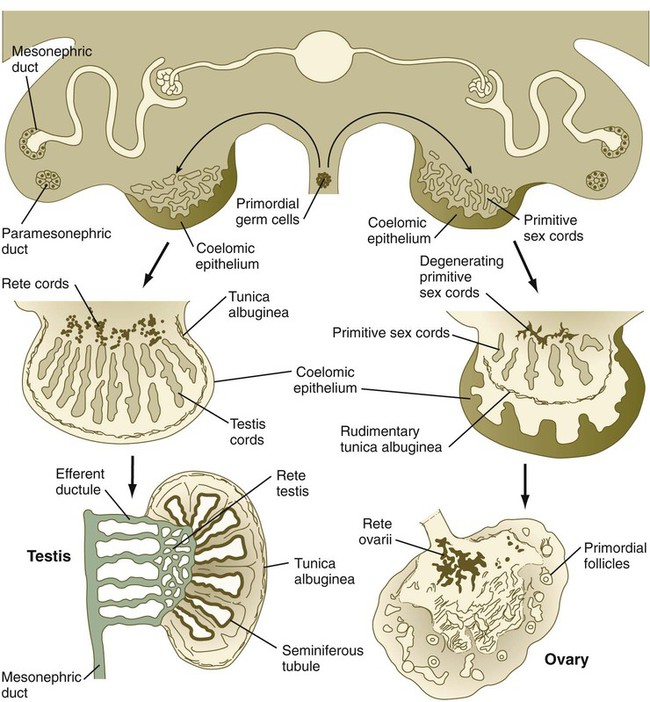
Late in the sixth week of gestation, the testis shows evidence of differentiation. The primitive sex cords enlarge and are better defined, and their cells are thought to represent the precursors of the Sertoli cells (Box 16.1). As the sex cords differentiate, they are separated from the surface epithelium (germinal epithelium) by a dense layer of connective tissue called the tunica albuginea. The deepest portions of the testicular sex cords are in contact with the fifth to twelfth sets of mesonephric tubules. The outer portions of the testicular sex cords form the seminiferous tubules, and the inner portions become meshlike and ultimately form the rete testis. The rete testis ultimately joins the efferent ductules, which are derived from mesonephric tubules.
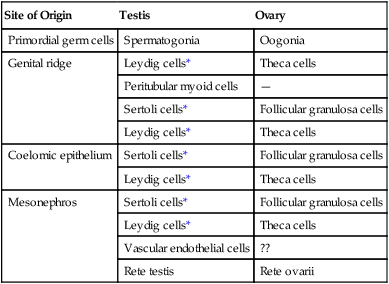
For the first 2 months of development, Leydig cells are not identifiable in the embryonic testis. In addition to local steroidogenic cells arising from the genital ridge, Leydig cell precursors may migrate into the testis from the mesonephros (Table 16.1). These become recognizable during the eighth week and soon begin to synthesize androgenic hormones (testosterone and androstenedione). This endocrine activity is important because differentiation of the male sexual duct system and the external genitalia depends on the sex hormones secreted by the fetal testis. Fetal Leydig cells secrete their hormonal products at just the period when differentiation of the hormonally sensitive genital ducts occurs (9 to 14 weeks). After weeks 17 and 18, the fetal Leydig cells gradually involute and do not reappear until puberty, when they stimulate spermatogenesis. The fetal Leydig cells can be viewed as a cellular isoform that is later replaced by the definitive adult form of the cells. By 8 weeks, the embryonic Sertoli cells produce müllerian inhibiting substance (see p. 394), which also plays an important role in shaping the sexual duct system by causing involution of the precursors of the female genital ducts.
Table 16.1
Origins of Cellular Components of Embryonic Gonads
| Site of Origin | Testis | Ovary |
| Primordial germ cells | Spermatogonia | Oogonia |
| Genital ridge | Leydig cells* | Theca cells |
| Peritubular myoid cells | — | |
| Sertoli cells* | Follicular granulosa cells | |
| Leydig cells* | Theca cells | |
| Coelomic epithelium | Sertoli cells* | Follicular granulosa cells |
| Leydig cells* | Theca cells | |
| Mesonephros | Sertoli cells* | Follicular granulosa cells |
| Leydig cells* | Theca cells | |
| Vascular endothelial cells | ?? | |
| Rete testis | Rete ovarii |
Differentiation of the Ovaries
Despite considerable research, the factors leading to the differentiation of ovaries remain incompletely understood. According to one hypothesis, Wnt-4 and Rspo-1 signals repress FGF-9 expression, thus leading to a reduction of Sox-9 (see Fig. 16.27). This reduction inhibits testis development and leads to formation of an ovary. In contrast to the testes, the presence of viable germ cells is essential for ovarian differentiation. If PGCs fail to reach the genital ridges, or if they are abnormal (e.g., XO) and degenerate, the gonad regresses, and streak ovaries (vestigial ovaries) result.
After the PGCs have entered the future ovary, they remain concentrated in the outer cortical region or near the corticomedullary border. Similar to the testis, the ovary contains primitive sex cords in the medullary region, but these are not as well developed as the sex cords in the testis. The origin of the cells that form the ovarian follicles has not been established. Three sites of origin have been proposed for the follicular epithelial cells: (1) the coelomic epithelium (secondary sex cords), (2) the primitive sex cords of mesonephric origin, and (3) the stroma of the genital ridge itself. Shortly after they arrive in the gonad, clumps of PGCs in the medullary region become partially surrounded by follicular cells and take the form of cell nests. The PGCs, now properly called oogonia, briefly proliferate by mitosis until, under the influence of retinoic acid, they enter prophase of the first meiotic division. The retinoic acid, which is produced in the mesonephros (see earlier) is probably associated with the mesonephrically derived rete ovarii, which is located in the medulla of the ovary. By week 22, oogonia in the cortical region also enter meiosis. By the fetal period, the oogonia, now called oocytes, separate from the cell nests and become individually associated with follicular cells to form primordial follicles (see Fig. 1.5). The oocytes continue in meiosis until they reach the diplotene stage of prophase of the first meiotic division. Meiosis is then arrested, and the oocytes remain in this stage until the block is removed. In adults, this occurs in individual oocytes just days before ovulation. In premenopausal women, 50 years may have elapsed since these oocytes entered the meiotic block in embryonic life.
The developing ovary does not maintain a relationship with the mesonephros. Normally, the mesonephric tubules in the female embryo degenerate, leaving only a few remnants (Table 16.2).
Table 16.2
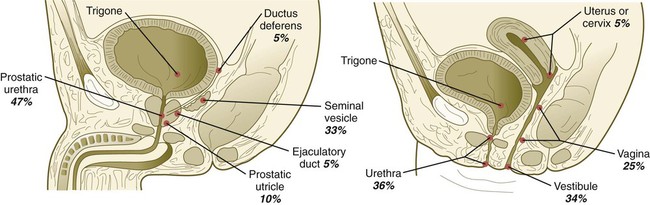
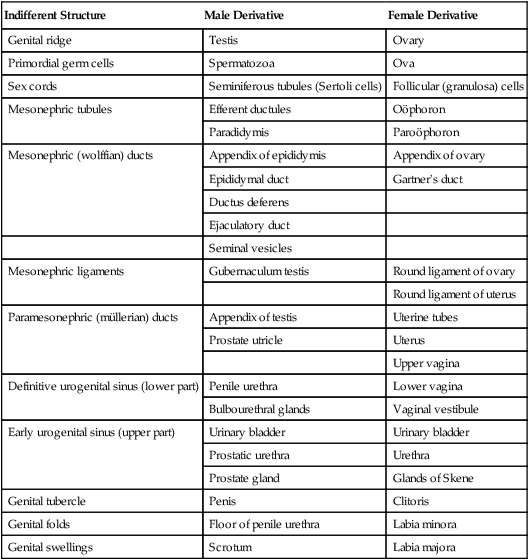
Homologies in the Male and Female Urogenital Systems
| Indifferent Structure | Male Derivative | Female Derivative |
| Genital ridge | Testis | Ovary |
| Primordial germ cells | Spermatozoa | Ova |
| Sex cords | Seminiferous tubules (Sertoli cells) | Follicular (granulosa) cells |
| Mesonephric tubules | Efferent ductules | Oöphoron |
| Paradidymis | Paroöphoron | |
| Mesonephric (wolffian) ducts | Appendix of epididymis | Appendix of ovary |
| Epididymal duct | Gartner’s duct | |
| Ductus deferens | ||
| Ejaculatory duct | ||
| Seminal vesicles | ||
| Mesonephric ligaments | Gubernaculum testis | Round ligament of ovary |
| Round ligament of uterus | ||
| Paramesonephric (müllerian) ducts | Appendix of testis | Uterine tubes |
| Prostate utricle | Uterus | |
| Upper vagina | ||
| Definitive urogenital sinus (lower part) | Penile urethra | Lower vagina |
| Bulbourethral glands | Vaginal vestibule | |
| Early urogenital sinus (upper part) | Urinary bladder | Urinary bladder |
| Prostatic urethra | Urethra | |
| Prostate gland | Glands of Skene | |
| Genital tubercle | Penis | Clitoris |
| Genital folds | Floor of penile urethra | Labia minora |
| Genital swellings | Scrotum | Labia majora |
Sexual Duct System
Indifferent Sexual Duct System
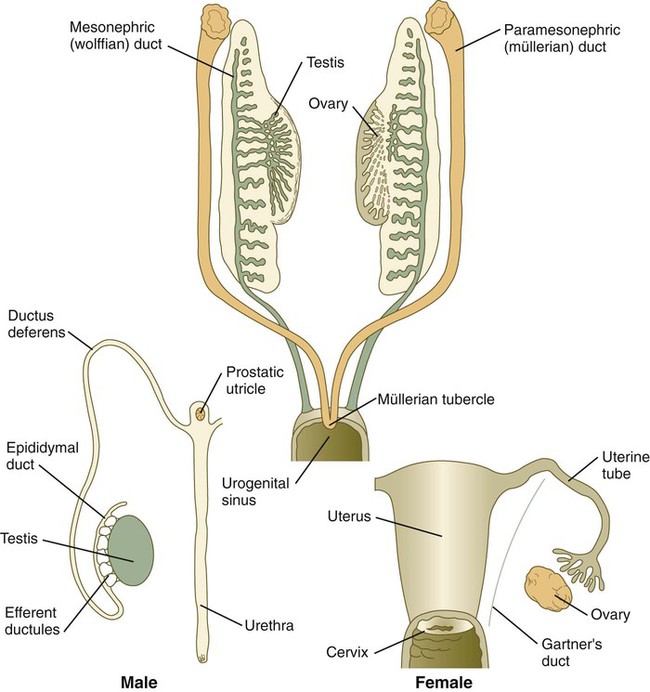
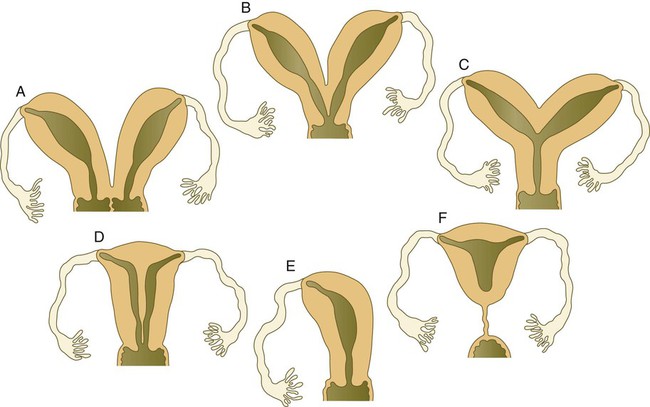
The indifferent sexual duct system consists of the mesonephric (wolffian) ducts and the paramesonephric (müllerian) ducts (Fig. 16.26). The paramesonephric ducts appear between 44 and 48 days of gestation as longitudinal invaginations of the coelomic mesothelium along the mesonephric ridge lateral to the mesonephric ducts. Arising from thickened placodelike structures, the invaginations, which take on the form of epitheliumlike cords, extend toward the mesonephric ducts under the influence of Wnt-4 produced by the mesonephros. When associated with the mesonephric ducts, the tips of the paramesonephric ducts form a proliferative center and depend on a Wnt-9b signal from the mesonephric ducts for their continued caudal advancement toward the urogenital sinus. If the mesonephric ducts are interrupted, the caudally elongating paramesonephric ducts do not extend past the cut ends. The paramesonephric ducts do not develop a true lumen until they have contacted the urogenital sinus. The cranial end of each paramesonephric duct opens into the coelomic cavity as a funnel-shaped structure. The fate of the indifferent genital ducts depends on the gender of the gonad.
Sexual Duct System of Males
Development of the sexual duct system in the male depends on secretions from the testis. Under the influence of müllerian inhibiting substance (sometimes called antimüllerian hormone), a glycoprotein of the transforming growth factor-β family secreted by the Sertoli cells of the testes at 8 weeks’ gestation, the paramesonephric ducts degenerate, leaving only remnants at their cranial and caudal ends (Figs. 16.27 and 16.28; see Table 16.2). Müllerian inhibiting substance apparently does not directly affect the epithelium of the paramesonephric ducts, but rather affects the surrounding mesenchyme. These mesenchymal cells express a gene that encodes a serine-threonine kinase membrane-bound receptor, which binds the müllerian inhibiting substance. Then the surrounding mesenchymal cells instruct the epithelial cells of the müllerian duct to regress through apoptosis and transformation of the epithelial cells into mesenchyme.
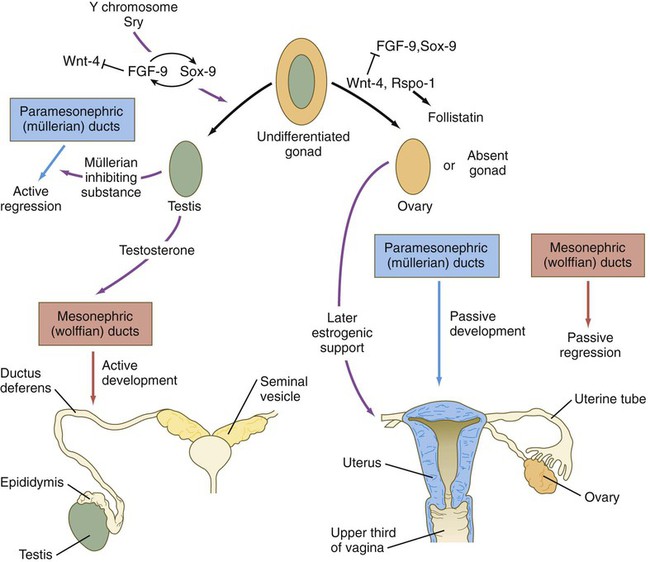
FGF, fibroblast growth factor. (After Hutson JM and others: In Burger H, deKrester D, eds: The testis, ed 2, New York, 1989, Raven Press, pp 143-179.)
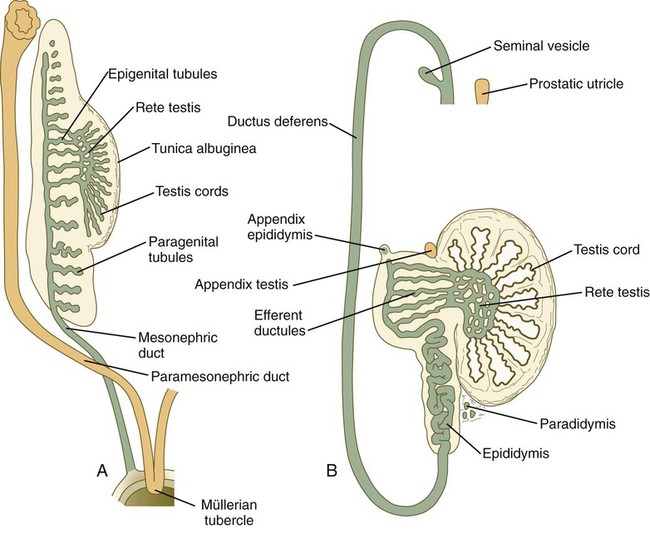
A, At the end of the second month. B, In the late fetus. (Adapted from Sadler T: Langman’s medical embryology, ed 6, Baltimore, 1990, Williams & Wilkins.)
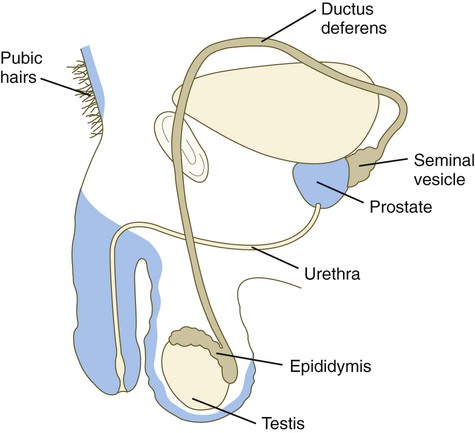
Associated with development of the male genital duct system (both the ductus deferens and the urethra) is the formation of the male accessory sex glands: the seminal vesicles, the prostate, and the bulbourethral glands (Fig. 16.29). These glands arise as epithelial outgrowths from their associated duct systems (seminal vesicles from the ductus deferens and the others from the urogenital sinus, the precursor of the urethra), and their formation involves epithelial-mesenchymal interactions similar to those of other glands. In addition, these glands depend on androgenic stimulation for their development. Specifically, the mesenchymal cells develop androgen receptors and seem to be the primary targets of the circulating androgenic hormones. (At this stage, the epithelial cells do not contain androgen receptors.) After stimulation by the androgens, the mesenchymal cells act on the associated epithelium through the local paracrine effects of growth factors and cause this epithelium to differentiate with gland-specific characteristics.
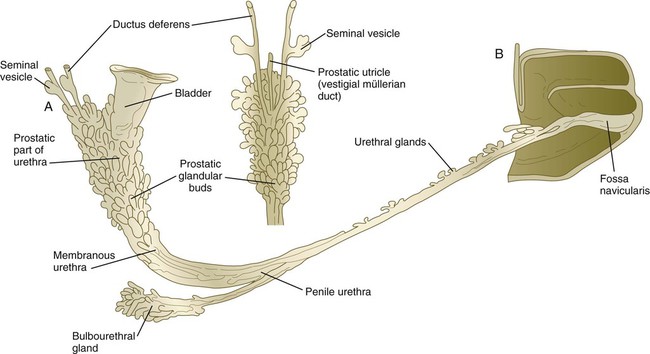
A, Lateral view. B, Dorsal view of prostatic region. (After Didusch. From Johnson FP: J Urol 4:447-502, 1920.)
In the embryo, the tissues around the urogenital sinus synthesize an enzyme (5α-reductase) that converts testosterone to dihydrotestosterone. Through the action of appropriate receptors of either form of testosterone, crucial tissues of the male reproductive tract are maintained and grow (Fig. 16.30).
Sexual Duct System of Females
If ovaries are present, or if the gonads are absent or dysgenic, the sexual duct system differentiates into a female phenotype. In the absence of testosterone secreted by the testes, the mesonephric ducts regress, leaving only rudimentary structures (see Table 16.2). In contrast, the absence of müllerian inhibitory substance allows the paramesonephric (müllerian) ducts to continue to develop into the major structures of the female genital tract (Fig. 16.31).
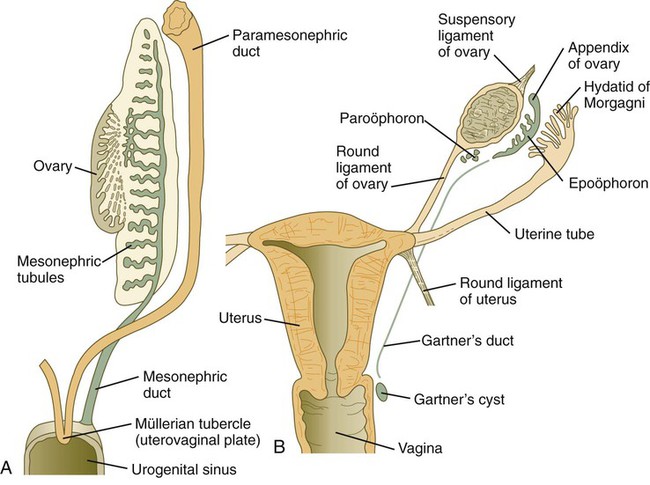
A, At the end of the second month. B, Mature condition. (Adapted from Sadler T: Langman’s medical embryology, ed 6, Baltimore, 1990, Williams & Wilkins.)
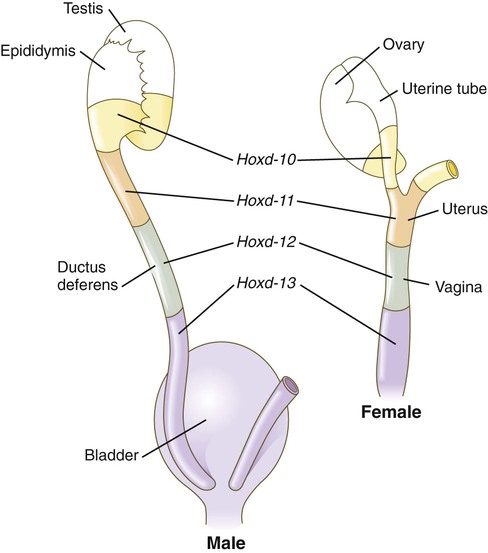
Early formation of the paramesonephric ducts depends on Wnt signaling. In the absence of Wnt-4, the paramesonephric ducts fail to form. Wnt-7a, which is also involved in setting up the dorsoventral axis of the developing limb, is expressed in the epithelium throughout the entire paramesonephric ducts and is required for their normal development. Later in development, Wnt-7a expression becomes restricted to the uterine epithelium. In some manner, Wnt-7a seems to be involved in maintaining the expression of a sequence of Hox genes (Hoxd-10 through Hoxd-13 and the Hoxa paralogues) that are spread along the female reproductive tract (Fig. 16.32): Hoxa-9 is expressed in the uterine tubes; Hoxa-10, in the uterus; Hoxa-11, in the uterus and cervix; and Hoxa-12, in the upper vagina.
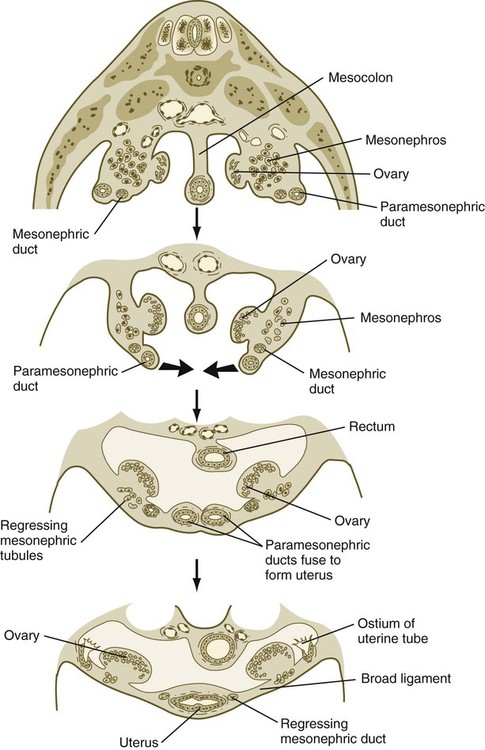
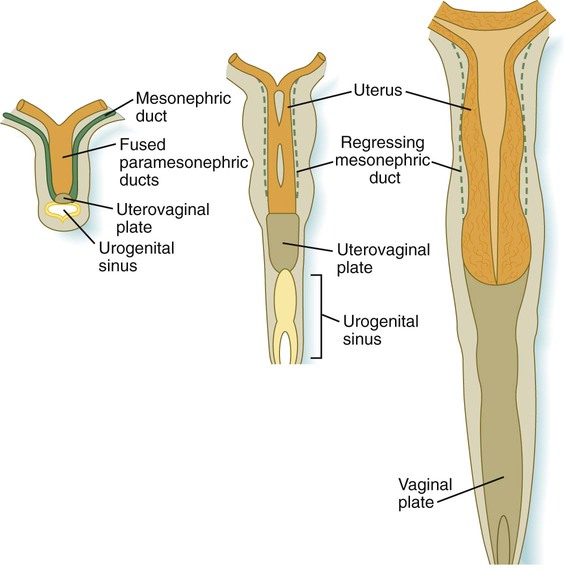
The cranial portions of the paramesonephric ducts become the uterine tubes, with the cranial openings into the coelomic cavity persisting as the fimbriated ends. Toward their caudal ends, the paramesonephric ducts begin to approach the midline and cross the mesonephric ducts ventrally. This crossing and ultimate meeting in the midline are caused by the medial swinging of the entire urogenital ridge (Fig. 16.33). The region of midline fusion of the paramesonephric ducts ultimately becomes the uterus, and the ridge tissue that is carried along with the paramesonephric ducts forms the broad ligament of the uterus.
The formation of the vagina remains poorly understood, and several explanations for its origin have been posited. According to one commonly held hypothesis, the fused paramesonephric ducts form the upper part of the vagina, and epithelial tissue from the müllerian tubercle (uterovaginal plate) hollows out to form the lower part (Fig. 16.34). More recently, several investigators have suggested that the most caudal portions of the mesonephric ducts participate in the formation of the vagina either by directly contributing cells to its wall or by inductively acting on the paramesonephric tissue, which appears to diverge toward the mesonephric ducts at the very tip of the fused portion. Full development of the female reproductive tract depends on estrogenic hormones secreted by the fetal ovaries.
Descent of the Gonads
Descent of the Testes
The testes do not remain in their original site of development; they migrate from their intra-abdominal location into the scrotum (Fig. 16.35). Similar to the kidneys, the testes are retroperitoneal structures, and their descent occurs behind the peritoneal epithelium. Before their descent, the testes are anchored cranially to the cranial suspensory ligament, derived from the diaphragmatic ligament of the mesonephros, and caudally to the inguinal (caudal) ligament of the mesonephros, which in later development is called the gubernaculum.
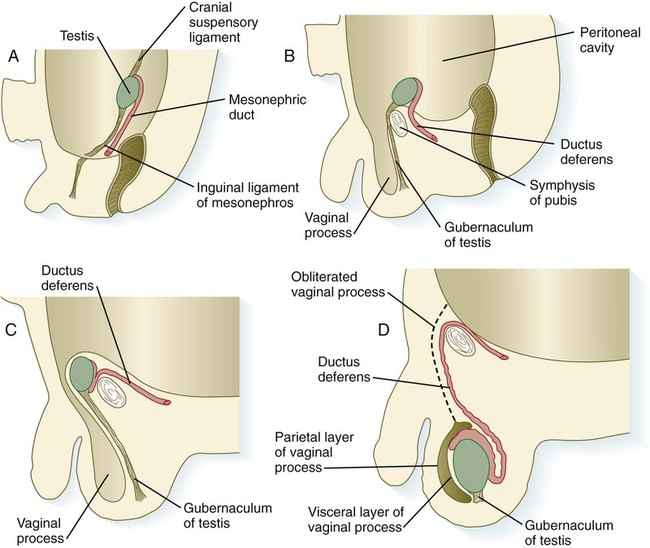
A, In the second month. B, In the third month. C, In the seventh month. D, At term.
Testicular descent begins during the seventh month and may not be completed until birth. As it descends into the scrotum, the testis slides behind an extension of the peritoneal cavity known as the vaginal process (see Fig. 16.35C). Although this cavity largely closes off with maturation of the testis, it remains as a potential mechanical weak point. With straining, it can open and permit the herniation of intestine into the scrotum.
Descent of the Ovaries
Although not so dramatically as the testes, the ovaries also undergo a distinct caudal shift in position. In conjunction with their growth and the crossing over of the paramesonephric ducts, the ovaries move caudally and laterally. Their position is stabilized by two ligaments, both of which are remnants of structures associated with the mesonephros. Cranially, the diaphragmatic ligament of the mesonephros becomes the suspensory ligament of the ovary. The superior portion of the inguinal ligament (called the caudal gonadal ligament by some authors) develops into the round ligament of the ovary, and the inferior portion of the inguinal ligament becomes the round ligament of the uterus (see Fig. 16.31). The most caudal ends of the round ligaments of the uterus become embedded in the dense fascial connective tissue of the labia majora.
External Genitalia
Indifferent Stage
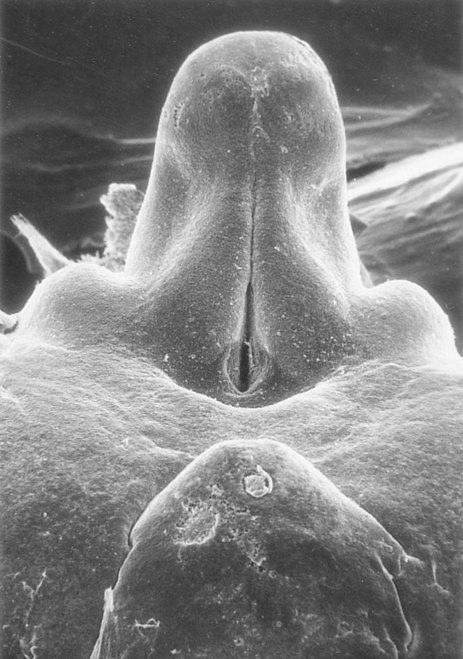
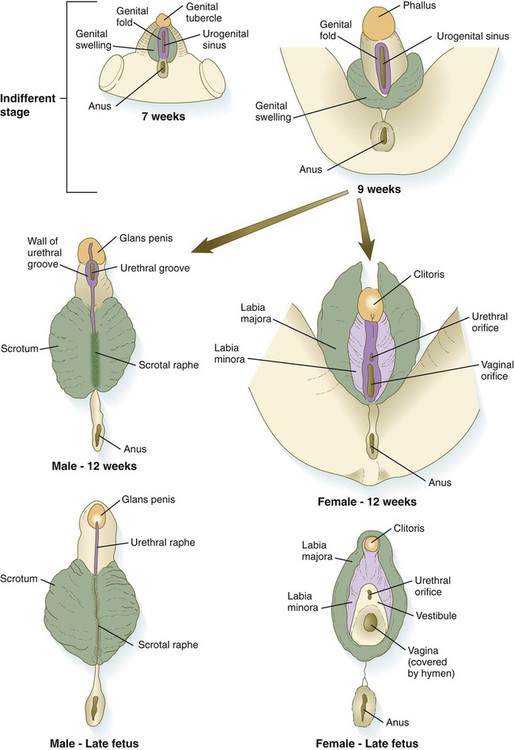
The external genitalia are derived from a complex of mesodermal tissue located around the cloaca. A very early midline elevation called the genital eminence is situated just cephalic to the proctodeal depression. This structure soon develops into a prominent genital tubercle (Fig. 16.36), which is flanked by a pair of genital folds extending toward the proctodeum. Lateral to these are paired genital swellings (Fig. 16.37). When the original cloacal membrane breaks down during the eighth week, the urogenital sinus opens directly to the outside between the genital folds. An endodermal urethral plate lines much of the open urogenital sinus. These structures, which are virtually identical in male and female embryos during the indifferent stage, form the basis for development of the external genitalia.
There are some parallels between development of the genital tubercle and that of the limbs, but significant differences also exist. Both use many of the same molecular players, starting with an underlying basis of Hox gene expression. Being at the terminal part of the urogenital system, the genital tubercle expresses the 5′ elements along the Hox gene clusters, specifically Hoxa-13 and Hoxd-13. The signal initiating the development of the genital tubercle is still not known. Shh, which is expressed in the endodermal urethral epithelial plate, is the principal molecule that acts on the mesenchyme and outer ectoderm to cause outgrowth of the genital tubercle. Many members of the FGF and Wnt families of signaling molecules are active in the genital tubercle, but their exact functions remain incompletely understood. Small pieces of genital tubercle exhibit polarizing activity when they are grafted into anterior regions of the limb bud (see Chapter 10). This activity is probably caused by the presence of shh in the endoderm of the tubercle.
External Genitalia of Males
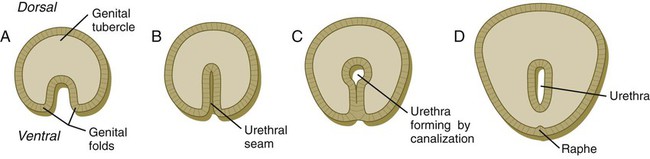
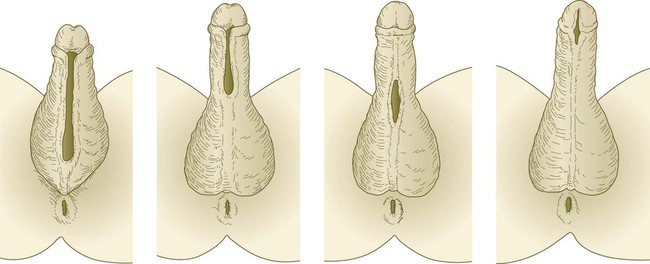
Under the influence of dihydrotestosterone (see Fig. 16.30), the genital tubercle in the male undergoes a second phase of elongation to form the penis, and the genital swellings enlarge to form scrotal pouches (see Fig. 16.37). As this growth is occurring, the urethra takes shape. The male urethra forms in a proximodistal direction by the ventral folding and midline fusion of the genital folds. This results in the formation of a midline epithelial seam on the ventral face of the elongating genital tubercle (Fig. 16.38B). The midline seam undergoes proximodistal remodeling by secondary canalization and detachment from the ventral surface epithelium to form the urethra proper (see Fig. 16.38C). Various signaling systems, including BMP-7, Eph-ephrin, and FGF, are involved in ventral closure of the urethra. The entire length of the urethra is formed from the endodermal lining of the urogenital sinus, and the histological characteristic of the distal epithelial lining (a stratified squamous epithelium) can be accounted for by the specific inductive effect of the glandular mesenchyme acting on the urethral epithelium. After the urethra has formed and has become detached from the ventral epithelial seam, the line of fusion of the urethral folds is marked by the persistence of a ventral raphe, which is continuous with the midline raphe that passes between the scrotal swellings.
Outgrowth of the male phallus is highly testosterone dependent. In the absence of testosterone or the absence of functional testosterone receptors in the testicular feminization syndrome (see Clinical Correlation 16.2), significant outgrowth of the penis does not occur.
External Genitalia of Females
In females, the pattern of external genitalia is similar to the pattern of the indifferent stage (see Fig. 16.37). The genital tubercle becomes the clitoris, the genital folds become the labia minora, and the genital swellings develop into the labia majora. The urogenital sinus remains open as the vestibule, into which the urethra and the vagina open. The female urethra, developing from the more cranial part of the urogenital sinus, is equivalent to the prostatic urethra of the male, which has a similar origin. The lack of outgrowth of the clitoris was traditionally considered to result solely from the absence of a dihydrotestosterone influence, but more recent research has also implicated an inhibitory influence of estrogen receptors. In mice, if estrogen receptors are inactivated, the clitoris undergoes elongation, and partial masculinization of the external genitalia occurs. This may be caused by the masculinizing influence of basal levels of androgens, which, in normal development, is repressed by estrogens.
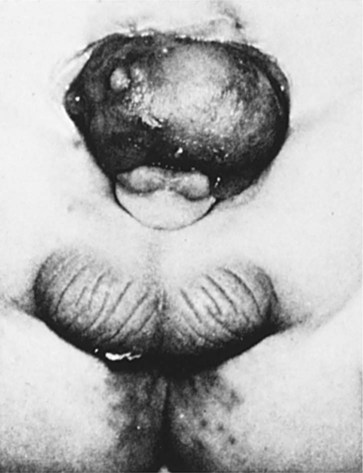
Clinical Correlation 16.2 presents malformations of the genital system.
Summary
 The urogenital system arises from the intermediate mesoderm. The urinary system arises before gonadal development begins.
The urogenital system arises from the intermediate mesoderm. The urinary system arises before gonadal development begins.
 Kidney development begins with the formation of pairs of nephrotomes that connect with a pair of primary nephric ducts. Farther caudally to the nephrotomes, pairs of mesonephric tubules form in a craniocaudal sequence and connect to the primary nephric ducts, which become known as mesonephric ducts. In the caudal part of each mesonephric duct, a ureteric bud grows out and induces the surrounding mesoderm to form the metanephros.
Kidney development begins with the formation of pairs of nephrotomes that connect with a pair of primary nephric ducts. Farther caudally to the nephrotomes, pairs of mesonephric tubules form in a craniocaudal sequence and connect to the primary nephric ducts, which become known as mesonephric ducts. In the caudal part of each mesonephric duct, a ureteric bud grows out and induces the surrounding mesoderm to form the metanephros.
 Outgrowth of the ureteric bud is stimulated by GDNF, produced by the metanephrogenic mesenchyme. This inductive signal is bound by c-Ret on the ureteric bud. FGF-2, BMP-7, and leukemia inhibitory factor, secreted by the ureteric bud, stimulate the formation of renal tubules in the metanephrogenic mesenchyme.
Outgrowth of the ureteric bud is stimulated by GDNF, produced by the metanephrogenic mesenchyme. This inductive signal is bound by c-Ret on the ureteric bud. FGF-2, BMP-7, and leukemia inhibitory factor, secreted by the ureteric bud, stimulate the formation of renal tubules in the metanephrogenic mesenchyme.
 Within the developing metanephros, nephrons (functional units of the kidney) form from three sources: the metanephrogenic blastema, the metanephrogenic diverticulum, and ingrowing vascular endothelial cells. Nephrons continue to form throughout fetal life. The induction of nephrons involves reciprocal inductions between terminal branches of the collecting duct system (ureteric bud) and the metanephrogenic mesoderm. Many molecular interactions mediate these inductions.
Within the developing metanephros, nephrons (functional units of the kidney) form from three sources: the metanephrogenic blastema, the metanephrogenic diverticulum, and ingrowing vascular endothelial cells. Nephrons continue to form throughout fetal life. The induction of nephrons involves reciprocal inductions between terminal branches of the collecting duct system (ureteric bud) and the metanephrogenic mesoderm. Many molecular interactions mediate these inductions.
 The kidneys arise in the pelvic basin. During the late embryonic and early fetal period, the kidneys shift into the abdominal region, where they become associated with the adrenal glands. The urinary bladder arises from the base of the allantois.
The kidneys arise in the pelvic basin. During the late embryonic and early fetal period, the kidneys shift into the abdominal region, where they become associated with the adrenal glands. The urinary bladder arises from the base of the allantois.
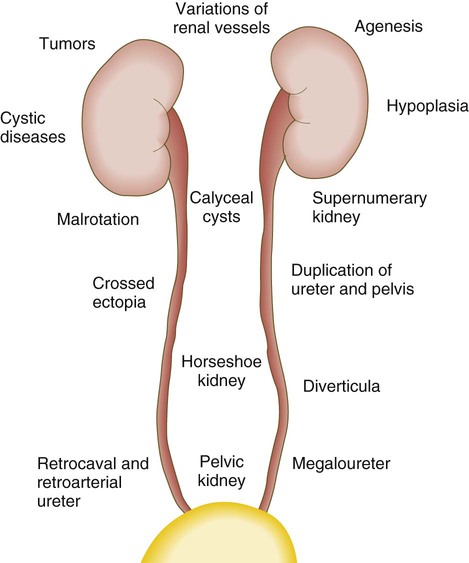
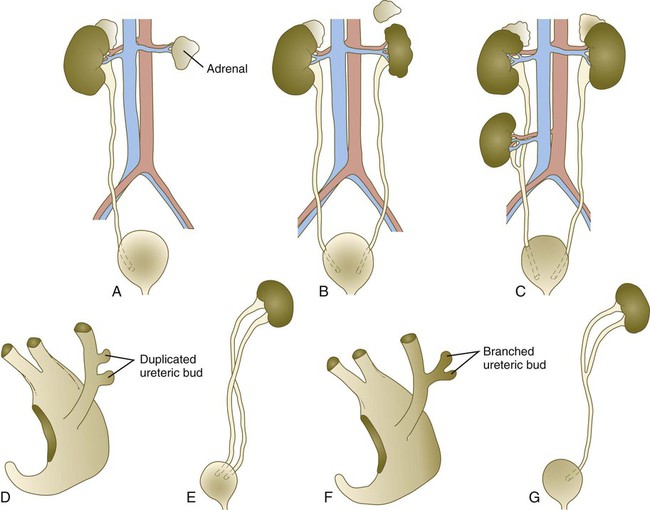
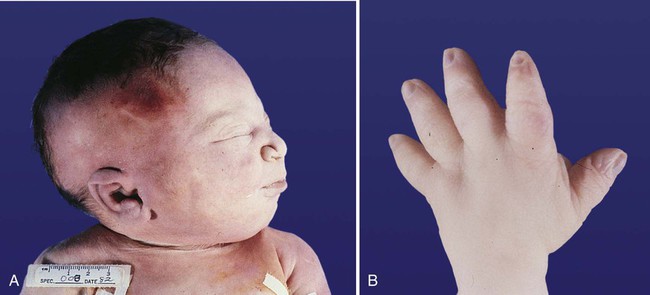
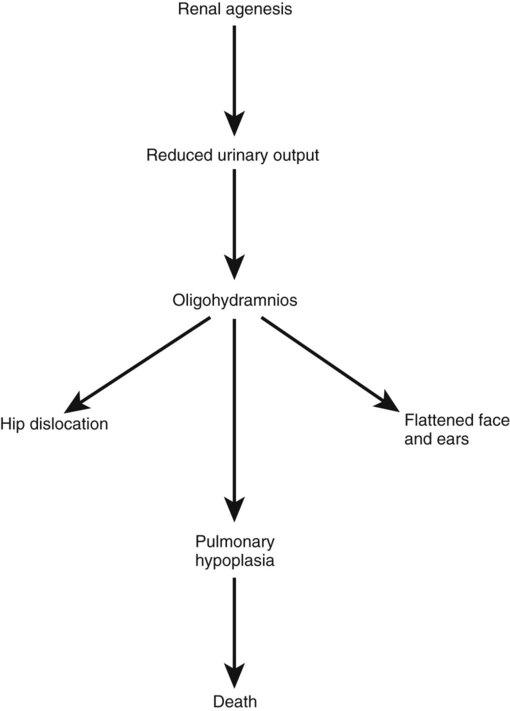
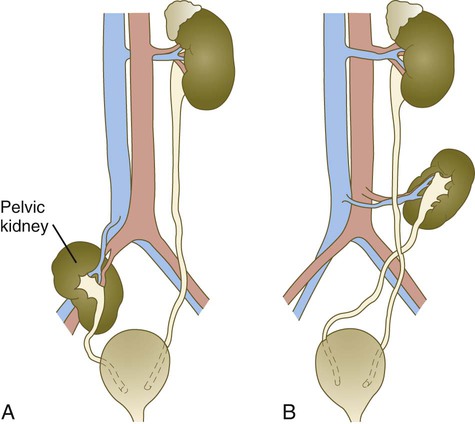
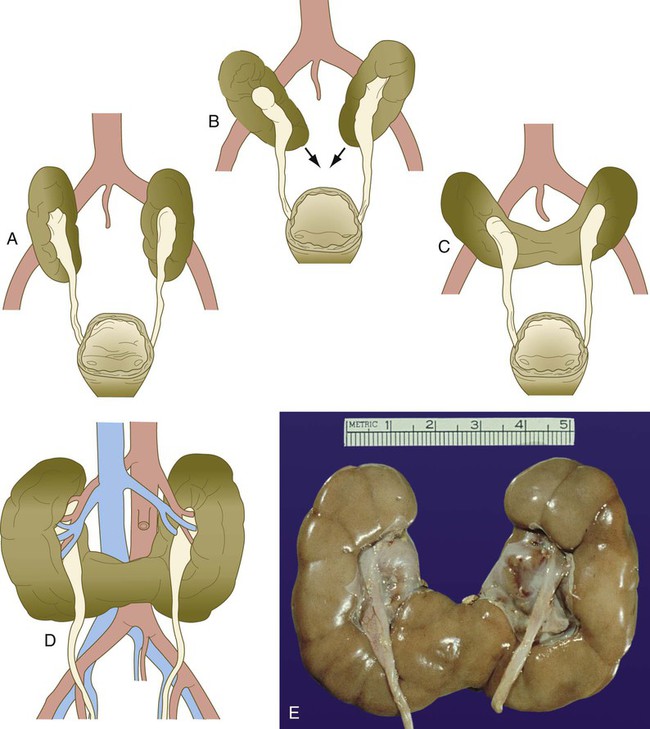
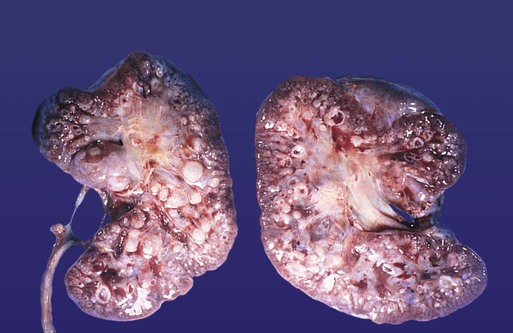
 The urinary system is subject to various malformations. The most severe is renal agenesis, which is probably caused by faulty induction in the early embryo. Abnormal migration can result in pelvic kidneys, other ectopic kidneys, or horseshoe kidney. Polycystic disease of the kidney is associated with cysts in other internal organs. Faulty closure of the allantois results in urachal cysts, sinuses, or fistulas.
The urinary system is subject to various malformations. The most severe is renal agenesis, which is probably caused by faulty induction in the early embryo. Abnormal migration can result in pelvic kidneys, other ectopic kidneys, or horseshoe kidney. Polycystic disease of the kidney is associated with cysts in other internal organs. Faulty closure of the allantois results in urachal cysts, sinuses, or fistulas.
 Sex determination begins at fertilization by the contribution of an X or a Y chromosome to the egg by the sperm. The early embryo is sexually indifferent. Through the action of the Sry gene, the indifferent gonad in the male develops into a testis. In the absence of this gene, the gonad becomes an ovary.
Sex determination begins at fertilization by the contribution of an X or a Y chromosome to the egg by the sperm. The early embryo is sexually indifferent. Through the action of the Sry gene, the indifferent gonad in the male develops into a testis. In the absence of this gene, the gonad becomes an ovary.
 Gonadal differentiation begins after migration of the PGCs into the indifferent gonads. Under the influence of the Sry gene product (testis-determining factor), the testis begins to differentiate. The presence of germ cells is not required for differentiation of testis cords. In the embryonic testis, Leydig cells secrete testosterone, and Sertoli cells produce müllerian inhibitory substance. In the absence of Sry expression, the gonad differentiates into an ovary and contains follicles. Ovarian follicular differentiation does not occur in the absence of germ cells.
Gonadal differentiation begins after migration of the PGCs into the indifferent gonads. Under the influence of the Sry gene product (testis-determining factor), the testis begins to differentiate. The presence of germ cells is not required for differentiation of testis cords. In the embryonic testis, Leydig cells secrete testosterone, and Sertoli cells produce müllerian inhibitory substance. In the absence of Sry expression, the gonad differentiates into an ovary and contains follicles. Ovarian follicular differentiation does not occur in the absence of germ cells.
 The sexual duct system consists of the mesonephric (wolffian) and paramesonephric (müllerian) ducts. The duct system is originally indifferent. In the male, müllerian inhibitory substance causes regression of the paramesonephric duct system, and testosterone causes further development of the mesonephric duct system. In the female, the mesonephric ducts regress in the absence of testosterone, and the paramesonephric ducts persist in the absence of müllerian inhibitory substance.
The sexual duct system consists of the mesonephric (wolffian) and paramesonephric (müllerian) ducts. The duct system is originally indifferent. In the male, müllerian inhibitory substance causes regression of the paramesonephric duct system, and testosterone causes further development of the mesonephric duct system. In the female, the mesonephric ducts regress in the absence of testosterone, and the paramesonephric ducts persist in the absence of müllerian inhibitory substance.
 In males, the mesonephric ducts form the ductus deferens and give rise to the male accessory sex glands. In females, the paramesonephric ducts form the uterine tubes, the uterus, and part of the vagina.
In males, the mesonephric ducts form the ductus deferens and give rise to the male accessory sex glands. In females, the paramesonephric ducts form the uterine tubes, the uterus, and part of the vagina.
 The testes descend from the abdominal cavity into the scrotum later in development. The ovaries also shift to a more caudal position. Faulty descent of the testes results in cryptorchidism and is associated with sterility and testicular tumors.
The testes descend from the abdominal cavity into the scrotum later in development. The ovaries also shift to a more caudal position. Faulty descent of the testes results in cryptorchidism and is associated with sterility and testicular tumors.
 The external genitalia also begin in an indifferent condition. Basic components of the external genitalia are the genital tubercle, genital folds, and genital swellings. Under the influence of dihydrotestosterone, the genital tubercle elongates into a phallus, and the genital folds fuse to form the penile urethra. The genital swellings form the scrotum. In the female, the genital tubercle forms the clitoris, the genital folds form the labia minora, and the genital swellings form the labia majora.
The external genitalia also begin in an indifferent condition. Basic components of the external genitalia are the genital tubercle, genital folds, and genital swellings. Under the influence of dihydrotestosterone, the genital tubercle elongates into a phallus, and the genital folds fuse to form the penile urethra. The genital swellings form the scrotum. In the female, the genital tubercle forms the clitoris, the genital folds form the labia minora, and the genital swellings form the labia majora.
 If an individual possesses only one X chromosome (XO), Turner’s syndrome results. Such individuals have a female phenotype with streak gonads. True hermaphroditism or pseudohermaphroditism can result from various causes. Testicular feminization is found in genetic males lacking testosterone receptors. Such individuals are phenotypic females. Major abnormalities of the sexual ducts are rare, but they can lead to duplications or the absence of the uterus in females.
If an individual possesses only one X chromosome (XO), Turner’s syndrome results. Such individuals have a female phenotype with streak gonads. True hermaphroditism or pseudohermaphroditism can result from various causes. Testicular feminization is found in genetic males lacking testosterone receptors. Such individuals are phenotypic females. Major abnormalities of the sexual ducts are rare, but they can lead to duplications or the absence of the uterus in females.
*More recent research has shown gender differences as early as the preimplantation embryo. The Sry genes (see later section) are already transcribed before implantation. In addition, the XY preimplantation embryo develops more rapidly than the XX embryo. Male and female preimplantation embryos are antigenically distinguishable. This suggests differences in gene expression.