CHAPTER 58 Urinary tract infection
Introduction
Urinary tract infections (UTIs) are a common cause of morbidity in women, affecting 50% of adult women at least once in their lives. In women aged 20–65 years, at least 20% will suffer an infection each year. Each year, approximately 5% of women will present to their general practitioners with dysuria and frequency (Hamilton-Miller 1994), and approximately half of these will have a UTI. It has been estimated that, on average, a case of uncomplicated acute cystitis results in 6.1 days of symptoms, 2.4 days of restriction of activities and 0.4 days in bed (Foxman and Frerichs 1985a). In women, the incidence of UTI increases with age and with the onset of sexual activity, and is highest amongst the elderly. In children under 1 year of age, the prevalence is higher in boys than girls, with a male:female ratio of 3 : 1–5 : 1. UTI in the neonate should be considered to be secondary to an underlying anatomical abnormality until proven otherwise (Kunin 1987).
The prevalence of UTI increases significantly with increasing age, and is higher in women than in men. In the elderly, the prevalence may be as high as 50%, especially if the woman is institutionalized (Boscia and Kaye 1987). This high prevalence in the elderly is thought to be secondary to cerebrovascular accidents, reduced mental and functional capacity, the use of bladder catheters and diabetes.
Approximately 25–30% of affected women will develop recurrent UTI that is not related to an underlying anatomical or functional abnormality of the urinary tract (Hooton 2001, Finer and Landau 2004).
Terminology and Definitions
UTI is defined as inflammation of the urinary tract due to microbial invasion. There is considerable overlap in the clinical presentations of the various syndromes, including cystitis, pyelonephritis and urethritis. Complicated UTI is UTI associated with functional or anatomical abnormalities that increase the risk of serious complications or treatment failure, such as conditions which cause obstruction or relative stasis of urinary flow (Box 58.1).
Urine samples collected directly from the bladder, ureter or renal pelvis should be sterile. Urine passed through the urethra always contains some bacteria derived from the terminal urethra. Significant bacteriuria is defined by the culture of increased numbers of bacterial colony-forming units (CFUs). The absolute number needed to define significant bacteriuria depends on the sample type. The threshold of more than 105 CFU/ml has been a standard for the definition of significant bacteriuria using carefully collected midstream urine (MSU) since the 1950s (Kass 1956). A significant proportion of patients with UTI will have less than 105 CFU/ml (Johnson and Stamm 1987, 1989). Therefore, current recommendations (Warren et al 1999) suggest more than 103 CFU/ml for a diagnosis of cystitis, and more than104 CFU/ml for a diagnosis of pyelonephritis. An important consideration with these diagnostic criteria is that they rely on careful collection of the MSU. This requires that care is taken in the instruction and support that patients are given to collect these samples. Bacteriuria is common in association with any long-term catheter and is not in itself an indication for treatment of UTI.
Asymptomatic bacteriuria is defined as the presence of more than 105 CFU/ml in two MSU samples in the absence of symptoms (Zhanel et al 1990). Cystitis is an inflammation of the bladder which may be due to infection or a variety of other causes. Urethritis is inflammation of the urethra and may be a consequence of a wide range of causes including UTI, sexually transmitted diseases such as chlamydia, vaginitis, trauma and allergy. Bacterial pyelonephritis is infection of the renal pelvices which may be acute or chronic.
Microbiology of Urinary Tract Infection
The majority of UTIs are caused by facultative bacteria and, occasionally, by fungi and viruses. Escherichia coli accounts for up to 70% of community-acquired infections (Grüneberg 1994), with the remainder predominantly caused by Staphylococcus saprophyticus and a variety of Gram-negative rods within the Enterobacteriaceae. In hospital-acquired infections, approximately 50% are caused by E. coli, 15% by Enterococcus spp. and the remainder by members of the Enterobacteriaceae, Pseudomonas spp., Staphylococcus spp. and yeasts (Bryan and Reynolds 1984). Hospital-acquired UTIs are frequently associated with iatrogenic risk factors such as instrumentation, and also with patient comorbidities. Antibiotic resistance is also much more likely to complicate hospital-acquired UTI.
Pathogenesis
Bacterial virulence factors
The ability of bacteria to adhere to uroepithelial cells is a prerequisite for infection to occur, and reduces the chance of the bacteria being cleared from the urinary tract during voiding. There are various adherence factors, called ‘adhesins’; E. coli possess surface organelles called ‘pili’ that act as adhesins. These adhesins attach to complementary structures on the uroepithelial cell wall, and act not only to promote infection but also to help promote growth and toxin production (Zafriri et al 1987). There are many different types of adhesins, such as type 4 pili, outer membrane proteins, curli, filamentous haemagglutinins and adhesive pili. Other virulence factors that may facilitate infection are specific to each pathogen. These include the surface antigens on E. coli and haemolysins that are produced to help degrade cells, and aerobactins that enhance iron uptake which encourages E. coli growth.
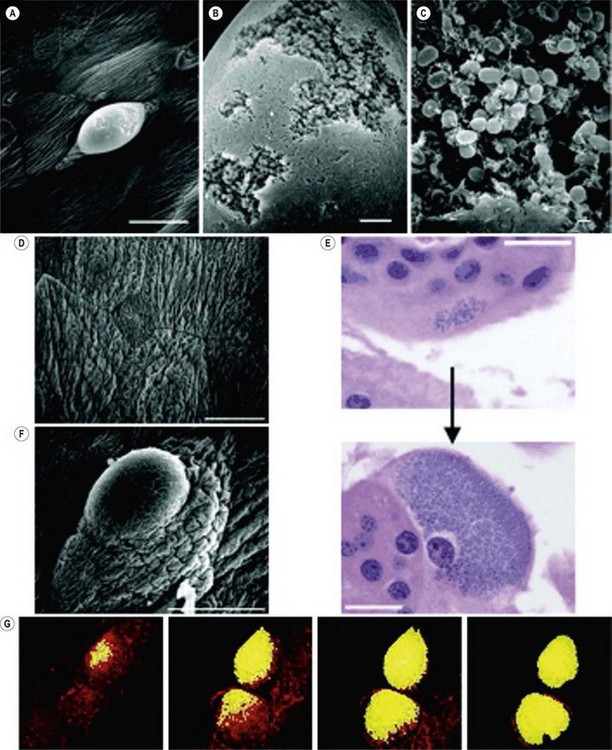
Much of our understanding of UTI comes from the study of uropathogenic E. coli (UPEC). The type of pili of the different strains of UPEC may determine the site of disease in the urinary tract as they have specific cell affinity (Gunther et al 2002). The virulence of UPEC has been attributed mainly to the presence of type 1 fimbriae, a mannose-binding adhesion protein called ‘FimH’ (Abraham et al 1988). Another pathogenic mechanism is the development of intracellular UPEC pods which act as a reservoir for infection (Anderson et al 2003). These pods contain bacteria that are encased in a polysaccharide matrix and protected by a uroplakin coating which help to evade host defence mechanisms and antimicrobials. This initiates the invasion into cells to develop intracellular bacterial communities. It is this reservoir that can serve as a source of bacteria that may reinitiate infection (Figure 58.1)
Host factors
Regular voiding flushes the urinary tract of pathogens and the acidity of urine inhibits bacterial growth. A healthy vaginal flora is thought to be essential in reducing infection. This flora is predominantly lactobacilli and this maintains an acidic pH in the vagina. Lactobacilli and uromucoid in the urine are thought to interfere with bacterial adherence and colonization. It is also thought that the composition of the flora is important as it provides a continuous microbial stimulus to the host immune system, such that it is primed to respond to pathogens. In women with recurrent UTI, the flora has reduced lactobacillus composition (Kirjavainen et al 2009). The glycosaminoglycan layer of the bladder also serves as a protective layer preventing bacterial adherence.
Factors that increase the risk of infection include:
In addition to all these factors, there is a well-developed and effective innate and adaptive host response to bacterial invasion. The mucosal lining of the urinary tract has a number of immune surveillance molecules that function to recognize invading pathogens. The best characterized of these is the Toll-like receptor (TLR) family (Samuelsson et al 2004, Zhang et al 2004, Anderson-Nissen et al 2007). These receptors function to initiate appropriate host immune defences when triggered by a pathogen. There are 11 TLRs; TLR4 is the best characterized and is present on the epithelial cells of the bladder and kidney, and promotes cytokine and chemokine responses to Gram-negative pathogens. This TLR4 response can still occur even once intracellular bacterial invasion has occurred. The importance of host-mediated immunity can be seen in women with recurrent UTI. A study comparing vaginal, urine and blood samples from 22 women wirh recurrent UTI and 17 controls showed an aberrant immune response. Women with recurrent UTI had defective T-cell activation and a lower concentration of tissue-repair-associated vascular endothelial growth factor (Kirjavainen et al 2009).
Natural History
In children up to 1 year of age, 1.1% of girls and 1.2% of boys may suffer a symptomatic UTI. In school-age children, a UTI has been reported in 8% of girls and 2% of boys (Hansson et al 1997). The sequelae of UTI in neonates and young children include pyelonephritis and renal scarring, especially if infection occurred before 5 years of age. Vesicoureteric reflux is a significant aetiological factor in the occurrence of UTI in the young.
In young women, UTI can result in asymptomatic bacteriuria, cystitis and pyelonephritis. These do not usually affect renal function, except in the elderly where bacteriuria has been associated with a decrease in glomerular filtration rate. In pregnancy, UTIs have been associated with an increased risk of prematurity, perinatal mortality and perinatal complications (Maclean 2000). The incidence of asymptomatic bacteriuria is similar to non-pregnant women (4–7%) (Patterson and Andriole 1997); however, in pregnancy the risk of developing a symptomatic UTI is much higher, and 10–30% of women with asymptomatic bacteriuria develop pyelonephritis.
Management
Investigations
Investigations should be aimed to help select appropriate treatment and to exclude any underlying cause which may predispose to recurrence (Arnold 2000).
Urinalysis
Commercial stick tests are available for detection of various urinary components. The tests which are most useful are those for the detection of white blood cell leukocyte esterase and nitrites (formed from the conversion of urinary nitrate by bacteria). A clear freshly voided urine with negative nitrite and leukocyte tests indicates that UTI is unlikely (a high negative predictive value). The stick tests are not reliable to exclude asymptomatic bacteriuria when the patient has recently received antibiotics, in the immunocompromised, if there are delays in testing the urine, or if the number of bacteria is less than 105 CFU/ml (Stevens 1989). Patients with suspected UTI should be treated empirically and promptly, even with negative stick test results. It is imperative that urine culture is performed in pregnancy, in the immunocompromised, in those with complicated infections, and where previous empirical therapy has failed.
Imaging studies
Plain abdominal radiograph
Plain abdominal radiography can be used to supplement an ultrasound to detect stones or foreign bodies. If stones are present, 90% will be visualized as they contain calcium or cystine and so are radio-opaque. Calcification in lymph nodes or renal tumours may also be seen. In combination with ultrasound, it has been shown to be superior to an intravenous urogram and incurs less radiation exposure (Lewis-Jones et al 1989, Spencer et al 1990).
Nuclear medicine scanning
The DMSA (dimercaptosuccinate) nucleotide is retained in the renal tubules and therefore this scan delineates renal anatomy and function. In patients with acute pyelonephritis, the affected area or scarring may be seen, along with any deterioration in proximal function. It should be considered in women with severe or unresolving pyelonephritis. In children under 5 years of age, it is more sensitive than ultrasound and intravenous urography in detecting renal scars (Mansour et al 1987). If obstruction is suspected, a DTPA (diethylenetriamine penta-acetic acid) or MAG3 (mercaptoacetyl triglycerine) scan is useful, as obstruction will result in delayed washout of the isotope from the renal pelvis. It will also allow calculation of the glomerular filtration rate and assessment of the contribution of each kidney to total renal function. MAG3 scans are also useful in delineating areas of reduced uptake and renal scars.
Computed tomography
In the majority of cases, ultrasound and intravenous urography are sufficient to reach a diagnosis; however, CT scanning is a useful adjunctive test where ultrasound and intravenous urography fail to make a diagnosis. In many units, CT imaging is now readily available and used for its increased sensitivity. It also allows a more global assessment of the pelvis. CT allows detailed anatomical demonstration of the kidney, intrarenal collecting system, ureter and bladder (Silverman et al 2009). It can thus be used for the investigation of haematuria.
CT can be used without contrast; however, the use of contrast does allow better anatomical detail and may give functional information. Contrast is usually injected into the collecting system 2–3 min after injection, and imaging can be performed at different stages of contrast enhancement to delineate areas of low or abnormal attenuation, such as seen with damaged parenchyma (Kawashima et al 1997).
In acute pyelonephritis, CT can be used to delineate focal or diffuse changes in the renal architecture and define the extent of hydronephrosis (Talner et al 1994). Spiral CT scan images, which are very much faster, can also detect ureteric stones or abscesses.
Magnetic resonance imaging
MRI has the advantage over CT that it does not use ionizing radiation or iodinated contrast. However, it is generally more expensive, less accessible and may be less well tolerated by patients and sensitive to motion artefact. It is also less sensitive for detecting renal calculi (Silverman et al 2009).
Treatment
Antimicrobial therapy
Many antibiotics administered systemically reach much higher concentrations in urine than in serum. These include β-lactams, aminoglycosides, fluoroquinolones and trimethoprim, so large doses of these agents are rarely required. Amoxycillin resistance is now so common in laboratory isolates in the UK that it is best avoided in the empirical treatment of UTI, and in some areas, trimethoprim resistance is reaching similar levels. The true level of antibiotic resistance amongst agents of community infection is not known. The levels of resistance in laboratory isolates from patients in the community may be an overestimate because of biases in the way in which laboratories are used. For example, samples may only be sent to the laboratory when patients return to a doctor after failure of empirical treatment. Estimates of the levels of antibiotic resistance for hospital infections may be more accurate because of the relative ease of use of the laboratory. Alternatives to amoxicillin or trimethoprim for oral use include nalidixic acid, penicillin/enzyme inhibitor combinations (such as amoxicillin with clavulanate), nitrofurantoin, oral cephalosporins and quinolones. The British National Formulary gives good advice on antibiotic selection and treatment durations for specific clinical scenarios. The duration of therapy has come under some debate with a move to shorter regimes to increase compliance, as these will have less effect on the faecal and vaginal flora and reduce the risk of resistant strains. Three-day regimes are as effective as 5- and 7-day regimes for those with uncomplicated UTI (Norrby 1990). Ideally, protocols should be developed with local microbiologists and/or infectious disease specialists that take account of local resistance information. Additional information on the use of antibiotics is provided in the section dealing with specific clinical presentations.
Prevention
For many women with recurrent infection, suggested preventive measures include maintaining a high fluid intake, instructions on perineal hygiene such that the perineum is wiped from front to back after defaecation and micturition (thus reducing the risk of faecal contamination of the urethra), and the avoidance of bubble baths, vaginal deodorants and specific underwear. The benefits of these practices are unclear and they have not been shown to reduce the frequency of infections in case–control studies (Foxman and Frerichs 1985b, Remis et al 1987).
There is, however, a strong association between sexual behaviour and contraceptive use (Foxman and Chi 1990). If sexual intercourse is a precipitating factor, postcoital treatment and voiding are recommended. In women using spermicides and diaphragms for contraception, alternative methods may be recommended.
The beneficial effects of cranberry juice are receiving increasing attention as a simple remedy that reduces the incidence of recurrent infection; however, most studies have been relatively small and inconclusive. There are two postulated mechanisms of action: competitive inhibition of the E. coli fimbrial subunit to the uroepithelial cells, or prevention of the expression of the normal fimbrial subunits (Patel and Daniels 2000). In a randomized double-blind trial to determine the effect of cranberry juice on bacteriuria and pyuria in 153 elderly women, there was reduced frequency of bacteriuria (15% vs 28%) with daily ingestion of 300 ml of cranberry juice (Avorn et al 1994). There was a decrease in the incidence of symptomatic UTI but this did not reach statistical significance; however, antibiotic use decreased by approximately 50% in the group who drank cranberry juice. The Cochrane analysis of cranberries for prevention of UTI found that the studies assessed were flawed as the amount and type of cranberries given differed, and there was only weak evidence to support the use of cranberry juice for prevention (Jepson et al 2004).
In postmenopausal women, there is increased susceptibility to infection secondary to the changes in the vaginal flora and the uroepithelium secondary to oestrogen deficiency. There are few randomized trials of hormone replacement therapy in the prevention of UTI. In a double-blind, placebo-controlled trial of oestriol cream for the treatment of recurrent infection in 93 women, Raz and Stamm (1993) found that those on topical oestriol had a lower incidence of UTI (0.5 vs 5.9 episodes/patient-year). The decrease in UTI was seen together with a decrease in vaginal pH and recolonization with lactobacilli. In a later study by Cardozo et al (1998), daily use of oestriol cream was found to be superior to placebo in the treatment of recurrent UTI.
The recent Cochrane review (Perrotta et al 2008) of oestrogen use in postmenopausal women with recurrent UTI reviewed nine studies which included 3345 women. The data were difficult to summarize as studies were heterogeneous and used different application methods and doses. Pooled data from four studies showed that oral oestrogens did not reduce UTI compared with placebo. There were two small relevant studies comparing vaginal oestrogens which showed a reduction in UTI.
Low-dose prophylactic antibiotics can be considered if the frequency of attacks is two or more per 6 months or three or more over 12 months (Nicolle and Ronald 1987, Stamm and Hooton 1993). The aim of treatment is to eradicate urinary bacteria without affecting the healthy flora of the bowel and vagina, or causing the development of resistant strains. The usual suppressive dose is one-quarter to one-third of the antimicrobial dose required to treat an acute infection. This is usually prescribed at night to maintain a high antimicrobial concentration for as long as possible. The antibiotics of choice are trimethoprim, trimethoprim and sulphamethoxazole, nitrofurantoin, nalidixic acid and cephalexin (Harding et al 1982). In patients with neuropathic bladders, long-term indwelling catheters or ileal conduits who are at increased risk of recurrent infection, it is not advisable to begin long-term prophylaxis as this increases the risk of resistance and antimicrobial side-effects. In these cases, the patient should be advised to seek early treatment when a UTI is suspected.
Future approaches to treatment
There is much interest in the use of lactobacillus-containing probiotics for preventing recurrent infection. Probiotics are ‘live micro-organisms’ and the rationale for their use is that they restore the commensal flora of the genitourinary tract which inhibits colonization with uropathogens. Four randomized trials (Reid et al 1992, 1995, Baerheim et al 1994, Kontiokari et al 2001) have studied lactobacilli for the prevention of recurrent UTI; however, only one reported a reduction in episodes of recurrent UTI compared with the year prior to the study (Reid et al 1995). There are also concerns regarding the stability of probiotics and strain-specific effects.
The identification of various E. coli adhesins, including the type I and P fimbriae, has stimulated the search for vaccines against the development of UTI. Animal studies have shown that vaccination with antibodies to the FimH adhesin, which mediates binding to the bladder mucosa, reduces bacterial binding, bacteriuria and pyuria (Langermann et al 2000).
As the host immune response may determine susceptibility to infection, modulators of the immune system have been proposed as adjuvants to help boost the normal immunological mechanisms to vaccines (Ishii and Akira 2007, Parker et al 2007). Data from animal studies have shown a greater antigen-specific immune reponse and a reduced bacterial load when immunomodulators have been used (Bishop et al 2007, Huleatt et al 2007).
Specific Clinical Situations
Asymptomatic bacteriuria
There has been much debate on the benefits and cost-effectiveness of screening pregnant women for asymptomatic bacteriuria. The prevalence of asymptomatic bacteriuria has been reported to range from 2% to 13% (Norden and Kass 1968). There is an association with premature birth and low birth weight (Andriole and Patterson 1991), as well as an increased risk of developing pyelonephritis. In a study of 5000 antenatal patients, it was reported that in women with asymptomatic bacteriuria in pregnancy, 36% progressed to acute pyelonephritis if untreated compared with 5% if treated (Little 1965). Current recommendations in the UK (National Institute for Health and Clinical Excellence 2008) support the screening of pregnant women for bacteriuria at the time of booking by urine culture, because treatment with antibiotics reduces the risk of pyelonephritis. Antibiotic choices should be based on the culture result and should take account of the safety profile of the selected agent in pregnancy. Tests for urinary nitrite and leukocyte esterase are unreliable for the diagnosis of asymptomatic bacteriuria (Tincello and Richmond 1998).
Recurrent infection
Up to 20% of women with acute cystitis develop recurrent UTI which is defined as three or more laboratory-confirmed infections per year. These occur due to either reinfection or a relapse of persistent infection. Risk factors such as atrophic vaginitis should be looked for and treated. Maintaining a good urine output is advisable to promote voiding and avoid urinary stasis. Prophylactic use of trimethoprim, nitrofurantoin or norfloxacin therapy may reduce the risk of recurrent attacks but may also select for antibiotic resistance. Cranberry juice may reduce the risk of recurrent UTI without the risk of antibiotic resistance (Kontiokari et al 2001).
Postcoital prophylaxis with a single dose of an antibiotic can be used if infection is related to intercourse. Alternatively, patients can be given antibiotics to use when symptoms occur, or daily prophylaxis with nitrofurantoin, trimethoprim, norfloxacin or cephalexin. A 6-month trial of prophylaxis is usually recommended, with the patient observed to see if infection recurs once the regime is discontinued. However, recurrence seems to occur and a longer period of prophylaxis is now advocated for at least 2 years. Antimicrobials such as trimethoprim have been effective and well tolerated for up to 5 years (Nicolle and Ronald 1987, Nicolle et al 1988).
Acute pyelonephritis
Prompt antimicrobial treatment reduces the risk of serious adverse outcomes, so treatment should be commenced as soon as a diagnosis of urosepsis is considered (clinical signs and symptoms of UTI such as dysuria or loin pain in association with systemic signs of infection such as fever, rigors, hypotension, tachypnoea and tachycardia) and prior to the results of urine culture. Potential complications of pyelonephritis include Gram-negative bacteraemia, endotoxic shock and disseminated intravascular coagulation. In general, acute pyelonephritis resolves without long-term renal damage in the majority of women. However, in the presence of obstruction, such as with stones, infection may result in papillary necrosis, renal or perinephric abscess, or xanthogranulomatous pyelonephritis (Cattell 1998). Treatment consists of aggressive supportive treatment including rehydration and intravascular volume expansion.
Drugs of choice for parenteral therapy include cephalosporins, fluoroquinolones or an aminoglycoside. After the culture results are available, treatment may be changed to the appropriate antibiotic if necessary in consultation with a microbiologist or infectious disease specialist. The duration of treatment is usually 10–14 days (Bailey 1998, Cattell 1998).
Imaging of the renal tract is not usually necessary unless there has been no response to antibiotics or if there is a strong clinical suspicion of renal tract obstruction. Intravenous urography will be normal in 75% of cases of uncomplicated acute pyelonephritis (Fraser et al 1995), as well as renal ultrasound. CT imaging is usually normal unless infection is severe, when changes include renal enlargement, focal swelling and parenchymal attenuation.
Catheter-associated infection
Urethral catheterization is a major risk factor for UTI and local trauma, so should not be undertaken lightly. Those who carry out urinary tract catheterization should have received appropriate training in technique and catheter type (Carter et al 1990). A UTI is reported to occur in approximately one-third of patients catheterized in hospital (Hayley et al 1985). The risk of a UTI after an in–out catheter is 1–2% (Turck et al 1962), but the risk is higher in pregnancy, with a high bladder residual and in the immunocompromised. Basic measures such as the use of a closed-drainage system and gravity-dependent drainage of urine decrease the risk of UTI, and a policy of expediting catheter removal as soon as clinically appropriate should be practised.
Long-term urinary catheters become colonized with bacteria, and UTI is a frequent complication. The underlying cause is the development of a pathogenic biofilm on the surface of an indwelling catheter. The rate of bacteriuria is 3–10%/day and approaches 100% in those with long-term catheters (Warren et al 1982, Saint et al 2002). Inhibiting biofilm formation is one mechanism to reduce UTI, and urinary catheters have been modified to promote this. Impregnation of catheters with antimicrobial agents such as silver have been shown to delay or reduce the onset of bacteriuria; however, there is the possibility of future resistance to silver (Brosnahan et al 2004). Treatment may be required when the patient develops systemic signs or symptoms of infection. Antibiotic treatment choice is best based on culture results.
Conclusions
KEY POINTS
Abraham SN, Sun D, Dale JB, Beachey EH. Conservation of the D-mannose adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae. Nature. 1988;336:682-684.
Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105-107.
Andersen-Nissen E, Hawn TR, Smith KD, et al. Cutting edge: TLr5-/- mice are more suspectible to Escherichia coli urinary tract infection. Journal of Immunology. 2007;178:4717-4720.
Andriole VT, Patterson TF. Epidemiology, natural history and management of urinary tract infections in pregnancy. Medical Clinics of North America. 1991;75:359-373.
Arnold E. Investigations. In: Stanton SL, Dwyer P, editors. Urinary Ttract Infections in the Female. London: Martin Dunitz; 2000:35-59.
Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. Journal of the American Medical Association. 1994;271:751-754.
Bailey RR. Vesicoureteric reflux and reflux nephropathy. In: Schrier RW, Gootschalk GW, editors. Diseases of the Kidney. 4th edn. Boston: Little, Brown and Company; 1998:748-783.
Baerheim A, Larsen E, Digranes A. Vaginal application of lactobacilli in the prophylaxis of recurrent lower urinary tract infection in women. Scandinavian Journal of Primary Health Care. 1994;12:239-243.
Bishop BL, Duncan MJ, Somg J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nature Medicine. 2007;13:625-630.
Boscia JA, Kaye D. Asymptomatic bacteriuria in the elderly. Infectious Diseases Clinics of North America. 1987;1:892-903.
Brosnahan J, Jull A, Tracy C 2004 Types of Urethral Catheters for the Management of Short-term Voiding Problems in Hospitalized Adults. Cochrane Library, Issue 2. Update Software, Oxford.
Bryan CS, Reynolds KL. Hospital acquired bacteraemic urinary tract infection: epidemiology and outcome. Journal of Urology. 1984;132:494-498.
Cardozo L. Postmenopausal cystitis. BMJ (Clinical Research Ed.). 1996;313:129.
Cardozo L, Benness C, Abbott D. Low dose oestrogen prophylaxis for recurrent tract infections in elderly women. British Journal of Obstetrics and Gynaecology. 1998;105:403-407.
Carter R, Aitchison M, Mufti GR, Scott R. Catheterisation: your urethra in their hands. BMJ (Clinical Research Ed.). 1990;301:905.
Cattell WR. The patient with urinary tract infections. In: Davison AM, Cameron JS, Grunfeld J, Kerr DNS, Ritz E, Winearls CG, editors. Oxford Textbook of Clinical Nephrology. 2nd edn. New York: Oxford University Press; 1998:1252-1259.
Ditchburn RK, Ditchburn JS. A study of microscopical and chemical tests for the diagnosis of urinary tract infections in general practice. British Journal of General Practice. 1990;40:241-243.
Finer G, Landau D. Pathogenesis of urinary tract infections with normal female anatomy. The Lancet Infectious Diseases. 2004;4:631-635.
Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. Journal of Clinical Epidemiology. 1990;43:329-337.
Foxman B, Frerichs RR. Epidemiology of urinary tract infection. I. Diaphragm use and sexual intercourse. American Journal of Public Health. 1985;75:1308-1313.
Foxman B, Frerichs RR. Epidemiology of urinary tract infections. II. Diet, clothing and urinary habits. American Journal of Public Health. 1985;75:1314-1317.
Foxman B, Marsh J, Gillespie B, et al. Condom use and first-time urinary tract infection. Epidemiology. 1997;8:637-641.
Fraser IR, Birch D, Fairley KF, et al. A prospective study of corticol scarring in acute febrile pyelonephritis in adults: clinical and bacteriological characteristics. Clinics in Nephrology. 1995;47:13-18.
Gunther IN, Snyder JA, Lockatell V, et al. Assessment of virulence of uropathogenic Escherichia coli type 1 fimbrial mutants in which the invertible element is phase-locked on or off. Infection and Immunity. 2002;70:3344-3354.
Grüneberg RN. Changes in urinary pathogens and their antibiotic sensitivities, 1971–1992. Journal of Antimicrobial Chemotherapy. 1994;33(Suppl A):1-8.
Hamilton-Miller JMT. The urethral syndrome and its management. Journal of Antimicrobial Chemotherapy. 1994;33(Suppl A):63-73.
Hansson S, Martinell J, Stokland E, Jodal U. The natural history of bacteriuria in childhood. Infectious Diseases Clinics of North America. 1997;11:499-512.
Harding GKM, Ronald AR, Nicolle LE, Thomson MJ, Gray GJ. Long term antimicrobial prophylaxis for recurrent urinary infection in females. Review of Infectious Diseases. 1982;4:438-443.
Hayley RW, Culver DH, Waite JW, Morgan WM, Emori TG. The national nosocomial infection rate. A new need for new vital statistics. American Journal of Epidemiology. 1985;121:159-167.
Hopkins WJ, Uehling DT, Wargowski DS. Evaluation of a familial predisposition to recurrent urinary tract infections in women. American Journal of Medical Genetics. 1999;83:422-424.
Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. New England Journal of Medicine. 1996;335:468-474.
Hooton TM. Recurrent urinary tract infection in women. International Journal of Antimicrobial Agents. 2001;17:259-268.
Huleatt JW, Jacobs AR, Tang J, et al. Vaccination with recombinant fusion proteins incorporating Toll-like receptor ligands induces rapid cellular and humoral immunity. Vaccine. 2007;25:763-775.
Ishii KJ, Akira S. Toll or Toll-free adjuvant path towatd the optimal vaccine development. Journal of Clinical Immunology. 2007;27:363-371.
Jepson RG, Mihaljevic L, Craig J 2004 Cranberries for Preventing Urinary Tract Infections. The Cochrane Library, Issue 2. Update Software, Oxford.
Johnson JR, Stamm WE. Diagnosis and treatment of acute urinary tract infections. Infectious Diseases Clinics of North America. 1987;1:773-791.
Johnson JR, Stamm WE. Urinary tract infections in women: diagnosis and treatment. Annals of Internal Medicine. 1989;111:906-917.
Kass EH. Asymptomatic infections of the urinary tract. Transactions of the Association of American Physicians. 1956;69:56-64.
Kawashima A, Sandler C, Goldman S, Raval B, Fishman E. CT of renal inflammatory disease. Radiographics. 1997;17:851-866.
Kirjavainen PK, Paulter S, Baroja ML, et al. Abnormal immunological profile and vaginal microbiota in women prone to urinary tract infections. Clinical Vaccine Immunology. 2009;16:29-36.
Kontiokari T, Sundqvist K, Nuutinen M, Pokka T, Koskela M, Uhari M. Randomised trial of cranberry–lingonberry juice and lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ (Clinical Research Ed.). 2001;322:1571.
Kunin CM. Detection, Prevention and Management of Urinary Tract Infections, 4th edn. Philadelphia: Lea and Febiger; 1987. pp 57–124
Langermann S, Mollby R, Burlein JE, et al. Vaccination against FimH adhesin protects cynomolgus monkeys from colonisation and infection by uropathogenic Escherichia coli. Journal of Infectious Diseases. 2000;181:774-778.
Lewis-Jones HG, Lamb GHR, Hughes PL. Can ultrasound replace the intravenous urogram in the preliminary investigation of urinary tract disease? British Journal of Radiology. 1989;62:977-980.
Little PJ. Prevention of pyelonephritis of pregnancy. The Lancet. 1965;i:567-569.
Lomberg H, Cedergren B, Leffler H, et al. Influence of blood group on the availability of receptors for attachment of uropathogenic Escherichia coli. Infection and Immunology. 1986;51:919-926.
Maclean A. Pregnancy. In: Stanton SL, Dwyer PL, editors. Urinary Tract Infection in the Female. London: Martin Dunitz; 2000:145-160.
Mansour M, Azmy AF, MacKenzie JR. Renal scarring secondary to vesicoureteric reflux. Critical assessment and new grading. British Journal of Urology. 1987;70:32-34.
National Institute for Health and Clinical Excellence. Antenatal Care: Routine Care for the Healthy Pregnant Woman. Clinical Guideline 62. London: NICE; 2008.
Nicolle LE, Ronald AR. Recurrent urinary infection in adult women: diagnosis and treatment. Infectious Diseases Clinics of North America. 1987;1:793-806.
Nicolle LE, Harding GKM, Preiksaitis J, Ronald AR. The association of urinary tract infection with sexual intercourse. Journal of Infectious Diseases. 1982;146:579-584.
Nicolle LE, Harding GKM, Thomson M, Kennedy J, Urias B, Ronald AR. Efficacy of five years of continuous, low-dose trimethoprim–sulfamethoxazole prophylaxis for urinary tract infection. Journal of Infectious Diseases. 1988;157:1239-1241.
Norden CW, Kass EH. Bacteriuria of pregnancy — a critical appraisal. Annual Reviews in Medicine. 1968;19:431-470.
Norrby SR. Short-term treatment of uncomplicated urinary tract infections in women. Review of Infectious Diseases. 1990;12:458-467.
Parker LC, Prince LR, Sabroe I. Translational mini-review series on Toll-like receptors: networks regulated by Toll-like receptors mediate innate and adaptive immunity. Clinical and Experimental Immunology. 2007;147:199-207.
Patel N, Daniels IR. Botanical perspectives on health: of cystitis and cranberries. Journal of the Royal Society of Health. 2000;120:52-53.
Patterson JE, Andriole VT. Bacterial urinary tract infections in diabetes. Infectious Disease Clinics of North America. 1997;11:735-750.
Perrotta C, Aznar M, Mejia R, Albert X, Ng CW 2008 Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database of Systematic Reviews 2: CD005131.
Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopuasal women with recurrent urinary tract infections. New England Journal of Medicine. 1993;329:753-756.
Reid G, Bruce AW, Taylor M. Influence of 3-day antimicrobial therapy and lactobacillus vaginal suppositories on recurrence of urinary tract infections. Clinical Therapeutics. 1992;14:11-16.
Reid G, Bruce AW, Taylor M. Instillation of lactobacillus and stimulation of indigenous organisms to prevent recurrence of urinary tract infections. Microecology and Therapy. 1995;23:32-45.
Remis RS, Gurwith MJ, Gurwith D, Hargett-Bean NT, Layde PM. Risk factors for urinary tract infection. American Journal of Epidemiology. 1987;126:685-694.
Saint S, Lipsky BA, Goold SD. Indwelling urinary catheters: a one-point restraint? Annals of Internal Medicine. 2002;137:125-128.
Samuelsson P, Hang L, Wullt B, Irjala H, Svanborg C. Toll-like receptor 4 expression and cytokine responses in the human urinary tract mucosa 2004. Infection and Immunity. 2004;72:3179-3186.
Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, Rogatko A, Fair WR. Association of Lewis blood-group phenotype with recurrent urinary tract infections in women. New England Journal of Medicine. 1989;320:773-777.
Silverman SG, Leyendecker JR, Amis ES. What is the current role of CT urography and MR urography in the evaluation of the urinary tract? Radiology. 2009;250:309-323.
Spencer J, Lindsell D, Mastorakou I. Ultrasonography compared with intravenous urography in the investigation of urinary tract infection in adults. BMJ (Clinical Research Ed.). 1990;301:221-224.
Stamm WE, Hooton TM. Management of urinary tract infection in adults. New England Journal of Medicine. 1993;329:1328-1334.
Stevens M. Screening urine for bacteriuria. Medical Laboratory Science. 1989;46:194-206.
Talner CB, Davidson AJ, Lebowitz RI, Dalla-Palma L, Goldman SM. Acute pyelonephritis: can we agree on terminology? Radiology. 1994;192:297-305.
Tincello DG, Richmond DH. Evaluation of reagent strips in detecting asymptomatic bacteriuria in early pregnancy: prospective case series. BMJ (Clinical Research Ed.). 1998;316:435-437.
Turck M, Giffe B, Petersdorf RG. The urethral catheter and urinary tract infection. Journal of Urology. 1962;88:834-837.
Warren JW, Tenney JH, Hoopes JM, et al. A prospective study microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. Journal of Infectious Diseases. 1982;146:719-723.
Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clinical Infectious Diseases. 1999;29:745-758.
Zafriri D, Oron Y, Einenstein BI, Ofek I. Growth advantages and enhanced toxicity of Escherichia coli adherent to tissue culture cells due to restricted diffusion of products secreted by cells. Journal of Clinical Investigation. 1987;79:1210-1216.
Zhang D, Zhang G, Hayden MS, et al. A Toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1148-1155.
Zhanel GG, Harding GKM, Guay DRP. Asymptomatic bacteriuria: which patients should be treated. Archives of Internal Medicine. 1990;150:1389-1396.