Upper Respiratory Tract Infections and Other Infections of the Oral Cavity and Neck
1. Explain the anatomy and structures of the upper respiratory tract, including the three parts of the pharynx.
2. Identify the principal causative organism of pharyngitis; name other organisms capable of causing pharyngitis.
3. Define the following conditions: laryngitis, epiglottis, and parotitis. List the etiologic organisms associated with these conditions.
4. Explain the pathogenic mechanisms (virulence factors) associated with Streptococcus pyogenes pharyngitis.
5. Define Vincent’s angina and peritonsillar abscesses. What organism do they share as the causative agent of disease?
6. Describe the disease process caused by pharyngeal infection with Corynebacterium diphtheriae; name the hallmark symptom of this infection and list the complications associated with infection.
7. Differentiate between stomatitis and thrush, and explain the testing process for each disease.
8. Outline the steps used in the culture of specimens for the isolation of Streptococcus pyogenes.
9. Explain the signs and symptoms and pathogenic mechanisms associated with disease caused by Bordetella pertussis. What special requirements are needed to detect this organism in culture?
10. List three types of periodontal infections that require culture to identify the causative agent of infection; name the bacteria associated with these infections.
11. Explain the unique characteristics of C and G streptococcus, and explain how they contribute to their pathogenesis.
General Considerations
Anatomy
The respiratory tract is generally divided into two regions, the upper and the lower.
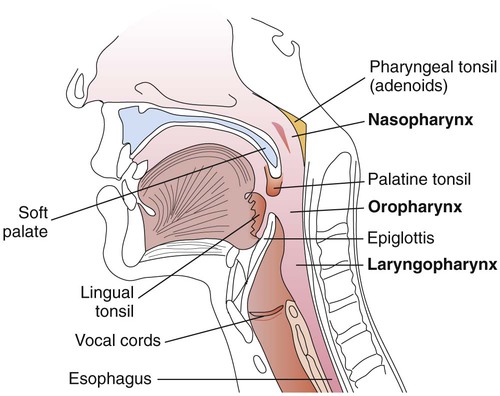
The upper respiratory tract includes all the structures down to the larynx: the sinuses, throat, nasal cavity, epiglottis, and larynx; the throat is also called the pharynx. These anatomic structures are shown in Figure 70-1.
The pharynx is a tubelike structure that extends from the base of the skull to the esophagus (see Figure 70-1). Made of muscle, this structure is divided into three parts:
• Nasopharynx (portion of the pharynx above the soft palate)
• Oropharynx (portion of the pharynx between the soft palate and epiglottis)
• Laryngopharynx (portion of the pharynx below the epiglottis that opens into the larynx)
Pathogenesis
An overview of the pathogenesis of respiratory tract infections is presented in Chapter 69. It is important to keep in mind that upper respiratory tract infections may spread and become more serious because the mucosa (mucous membrane) of the upper tract is continuous with the mucosal lining of the sinuses, eustachian tube, middle ear, and lower respiratory tract.
Diseases of the Upper Respiratory Tract, Oral Cavity, and Neck
Upper Respiratory Tract
Pharyngitis, Tonsillitis, and Peritonsillar Abscesses
Pharyngitis and Tonsillitis.
Epidemiology/Etiologic Agents.
Although different bacteria can cause pharyngitis or tonsillitis, the primary cause of bacterial pharyngitis is Streptococcus pyogenes (or group A beta-hemolytic streptococci). Viral pharyngitis or other causes of pharyngitis/tonsillitis must be differentiated from that caused by S. pyogenes, because pharyngitis resulting from S. pyogenes is treatable with penicillin and a variety of other anti-microbials, whereas viral infections are not. In addition, treatment is of particular importance because infection with S. pyogenes can lead to complications such as acute rheumatic fever and glomerulonephritis. These complications are referred to as poststreptococcal sequelae (diseases that follow a streptococcal infection) and are primarily immunologically mediated; these sequelae are discussed in greater detail in Chapter 15. S. pyogenes may also cause pyogenic infections (suppurations) of the tonsils, sinuses, and middle ear, or cellulitis as secondary pyogenic sequelae after an episode of pharyngitis. Accordingly, streptococcal pharyngitis is usually treated to prevent both the suppurative and nonsuppurative sequelae, as well as to decrease morbidity.
Although bacteria other than group A streptococci may cause pharyngitis, this occurs less often. Large colony isolates of groups C and G streptococci (classified as Streptococcus dysgalactiae subsp. equisimilis) are pyogenic streptococci with similar virulence traits as S. pyogenes; symptoms of pharyngitis caused by these agents are also similar to S. pyogenes. In contrast to S. pyogenes, these agents are rarely associated with poststreptococcal sequelae, namely glomerulonephritis and possibly rheumatic fever. Recent studies have demonstrated that these streptococci can exchange genetic information with S. pyogenes and thus potentially obtain virulence factors usually associated with S. pyogenes such as M proteins, streptolysin O, and superantigen genes. Arcanobacterium haemolyticum is also a cause of pharyngitis among adolescents. Examples of agents that can cause pharyngitis or tonsillitis are listed in Table 70-1.
TABLE 70-1
Examples of Bacteria That Can Cause Acute Pharyngitis and/or Tonsillitis
| Organism | Disease | Relative Frequency |
| Streptococcus pyogenes | Pharyngitis/tonsillitis/rheumatic fever/scarlet fever | 15% to 35% |
| Group C and G beta-hemolytic streptococci | Pharyngitis/tonsillitis | <3% to 11% |
| Arcanobacterium (Corynebacterium) haemolyticum | Pharyngitis/tonsillitis/rash | <1% to 10% |
| Neisseria gonorrhoeae | Pharyngitis/disseminated disease | Rare* |
| Corynebacterium ulcerans | Pharyngitis | Rare |
| Mycoplasma pneumoniae | Pneumonia/bronchitis/pharyngitis | Rare |
| Yersinia enterocolitica | Pharyngitis/enterocolitis | Rare |
| Human immunodeficiency virus-1 | Pharyngitis/acute retroviral disease | Rare |
Rhinitis
Rhinitis (common cold) is an inflammation of the nasal mucous membrane or lining. Depending on the host response and the etiologic agent, rhinitis is characterized by variable fever, increased mucous secretions, inflammatory edema of the nasal mucosa, sneezing, and watery eyes. With rare exceptions, rhinitis is typically associated with viral infections (20%-25%); some of these agents are listed in Box 70-1. Rhinitis is common because of the large number of different causative viruses, and reinfections may occur. Bacterial agents associated with rhinitis (10%-15%) include Chlamydia pneumoniae, Mycoplasma pneumoniae, and Group A streptococci.
Miscellaneous Infections Caused by Other Agents.
Corynebacterium diphtheriae.
Pharyngitis caused by Corynebacterium diphtheriae is less common than streptococcal pharyngitis. After an incubation period of 2 to 4 days, diphtheria usually presents as pharyngitis or tonsillitis. Patients are often febrile and complain of sore throat and malaise (body discomfort). The hallmark for diphtheria is the presence of an exudate or membrane that is usually on the tonsils or pharyngeal wall. The gray-white membrane is a result of the action of diphtheria toxin on the epithelium at the site of infection. Complications occur frequently with diphtheria and are usually seen during the last stage of the disease (paroxysmal stage). The most feared complications are those involving the central nervous system such as seizures, coma, or blindness. Information as to how this organism causes disease is discussed in Chapter 69. Additional specifics regarding this organism are provided in Chapter 17.
Bordetella pertussis.
Following an incubation period of 7 to 13 days, the patient with symptomatic infection develops upper respiratory symptoms, including a dry cough, fever, runny nose, and sneezing. After about 2 weeks, this may progress to spells of paroxysmal coughing. As these episodes worsen, the characteristic whoop, caused by attempted inspiration through an epiglottis undergoing spasm, begins. Vomiting may occur, and usually a lymphocytosis is present. This phase of the illness may last as long as 6 weeks. Bacterial culture for B. pertussis is effective using nasopharyngeal specimens during the first 2 weeks when symptoms are evident. Amplification and polymerase chain reaction may demonstrate positive results within 0-4 weeks of the onset of symptoms. However, positive results should be interpreted with caution and in correlation with patient signs and symptoms. More information regarding B. pertussis is provided in Chapter 37.
Oral Cavity
Stomatitis
Periodontal Infections
Etiologic Agents.
The bacteriology is similar in all of these infections and involves primarily anaerobic bacteria and streptococci except for perimandibular space infections, which may also involve staphylococci and Eikenella corrodens in about 15% of patients. The streptococci are microaerobic or facultative and are usually alpha-hemolytic (particularly the Streptococcus anginosus group—see Chapter 15); they are usually found in 20% to 30% of dental infections.
Diagnosis of Upper Respiratory Tract Infections
Collection and Transport of Specimens
Nasopharyngeal swabs are better suited for recovery of Bordetella pertussis, Neisseria spp., along with several viruses including respiratory syncytial virus, parainfluenza virus, and the other viruses causing rhinitis. Optimum conditions for the collection and transport of specimens for viral detection or culture are described in Chapter 65. Although swabs made of calcium alginate are commonly used to collect nasopharyngeal specimens (excluding those specimens for chlamydia or viral culture), nasopharyngeal secretions collected by either aspiration or washing will improve recovery for Bordetella pertussis because a larger amount of material is obtained.
Direct Visual Examination or Detection
Fungal elements, including yeast cells and pseudohyphae, may be visualized with a 10% potassium hydroxide (KOH) preparation, calcofluor white fluorescent stain, or periodic acid-Schiff (PAS) stain. Direct examination of material obtained from the nasopharynx of suspected cases of whooping cough using a fluorescent antibody stain (see Chapter 37) has been shown to yield some early positive results for detection of B. pertussis. However, direct fluorescent antibody (DFA) staining of nasopharyngeal secretions often lack sensitivity and specificity depending on the antibody used. Numerous studies have demonstrated that PCR-based assays for B. pertussis in nasopharyngeal secretions are superior to both DFA and culture. Various methods, including fluorescent antibody stain reagents, enzyme immunoassays, and nucleic acid amplification methods are also commercially available to detect numerous viral agents (see Chapter 65).
Improvement in the development of rapid methods for detection of group A streptococcal antigen or nucleic acid has obviated the need for culture of pharyngeal specimens. At least 40 commercial products are available to identify group A streptococcal antigens using membrane enzyme immunoassays or liposomal and optical immunoassay techniques. Although the specific procedures vary with the products, several generalizations can be made. Throat swabs are incubated in an acid reagent or enzyme to extract the group A specific carbohydrate antigen. Dacron swabs seem to be most efficient at releasing antigen, although other types of swabs may yield acceptable results. In laboratory comparisons between a rapid antigen method and conventional culture methods for detecting the presence of group A streptococci in throat swabs, the commercial kits have shown relatively acceptable (62% to more than 90%) sensitivity and specificity. Specimens with a negative direct antigen test for group A streptococci should be cultured (requires collection of specimen with two swabs) or confirmed using a nucleic acid method. Group A streptococci can be directly detected from pharyngeal specimens by nucleic acid testing using different molecular assay formats. The commercially available assay (Probe Group A Strep Direct Test (GAS Direct), Hologic-GenProbe, Inc., San Diego, California) that employs a nonisotopic, chemiluminescent, single-stranded DNA probe complementary to the rRNA target of the group A Streptococcus. The assay detects organisms directly from swab specimens by lysing the bacterial cells before amplification. Dacron swabs are acceptable for use with this assay. Sensitivities of the Gen-Probe Group A Strep Direct Test range from 91.7% to 99.3% when compared with culture. A rapid-cycle real-time PCR method, the Light Cycler Strep-A (Roche Applied Science, Indianapolis, Indiana), also detects S. pyogenes directly from throat swabs. Using this technology, 32 samples (including controls) can be tested per run in about 1.5 hours. Isothermol DNA amplification is also available for the detection of Group A Streptococcus from throat swabs (Illumigene Group A Streptococcus, Meridian Bioscience, Inc., Cincinnati, Ohio) and demonstrates sensitivity equal to the Group A Strep Direct test. See Chapter 8 for more information on isothermal DNA amplification.
Culture
Streptococcus pyogenes (Beta-Hemolytic Group A Streptococci)
Because the primary cause of bacterial pharyngitis in North America is Streptococcus pyogenes, most laboratories routinely screen throat cultures for this organism. Group A streptococci are usually beta-hemolytic, with less than 1% being nonhemolytic. Three variables must be taken into consideration regarding successful culture of group A streptococci from pharyngeal specimens: medium, atmosphere, and duration of incubation. Kellogg recommended four combinations of media and atmosphere of incubation for throat specimens; these are listed in Table 70-2. Regardless of the medium and atmosphere of incubation employed, culture plates should be incubated for at least 48 hours before reporting as negative for group A streptococci. In addition, the incubation of sheep blood agar in 5% to 10% CO2 was strongly discouraged.
TABLE 70-2
| Media | Atmosphere of Incubation |
| Sheep blood agar | Anaerobic |
| Sheep blood agar with coverslip over the primary area of inoculation | Aerobic |
| Sheep blood agar with trimethoprim-sulfamethoxazole | 5%-10% CO2 or anaerobic |
Drawbacks to culture include an extended incubation time of 24 to 48 hours for visible colony formation with additional manipulations of the beta-hemolytic organisms for definitive identification (see Chapter 15). If sufficient numbers of pure colonies are not available for identification, a subculture requiring additional incubation is necessary. By placing a 0.04-unit differential bacitracin filter paper disk, available commercially directly on the area of initial inoculation, presumptive identification of S. pyogenes can be made after overnight incubation (all of group A and a very small percentage of group B streptococci are susceptible). However, use of the bacitracin disk in the primary area of inoculation reduces the sensitivity and specificity of culture and identification of S. pyogenes. Sometimes growth of too few beta-hemolytic colonies or overgrowth of other organisms makes interpretation difficult. Therefore, using the bacitracin disk as the only method of identification of S. pyogenes is not recommended. New selective agars, such as streptococcal selective agar, have been developed that suppress the growth of almost all normal flora and beta-hemolytic streptococci except for groups A and B and Arcanobacterium haemolyticum. Direct antigen or nucleic detection tests or the PYR test (see Chapter 15) can also be carried out on isolated beta-hemolytic colonies.
Corynebacterium diphtheriae
If diphtheria is suspected, the physician must communicate this information to the clinical laboratory. Because streptococcal pharyngitis is included in the differential diagnosis of diphtheria and because dual infections do occur, cultures for Corynebacterium diphtheriae should be plated onto sheep blood agar or streptococcal selective agar, as well as onto special media for recovery of this agent. These special media include a Loeffler’s agar slant and a cystine-tellurite agar plate. Chapter 17 discusses the identification of the organism. Recovery of this organism is improved when culturing specimens from the throat and nasopharynx of potentially infected patients. In addition to culture, rapid toxigenicity assays, including immunoassays and polymerase chain reaction, may be used to assist in the diagnosis. Caution should be used when interpreting molecular assays, because positive results have been associated with related species of Corynebacteria.
Bordetella pertussis
Freshly prepared Bordet-Gengou agar was the first medium developed for isolation of Bordetella pertussis. However, because it was inconvenient to use, other media were subsequently developed (see Chapter 37). Today, Regan-Lowe or charcoal horse blood agar is recommended for use in diagnostic laboratories. Because the organisms are extremely delicate, specimens should be plated directly onto media, if possible. The yield of positive isolations from clinical cases of pertussis seems to vary from 20% to 98% depending on the stage of disease, previous treatment of the patient, age of the patient, and laboratory techniques. Due to the fastidious growth requirements, additional methods, including 16SrRNA sequencing and matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) have proven effective. See Chapter 7 for a description of MALDI-TOF methodology.
Neisseria
Specimens received in the laboratory for isolation of Neisseria meningitidis (for detection of carriers) or N. gonorrhoeae should be plated to a selective medium, either modified Thayer-Martin or Martin-Lewis agar. After 24 to 48 hours of incubation in 5% to 10% carbon dioxide, typical colonies of Neisseria spp. may be visible (see Chapter 40).
Epiglottitis
Clinical specimens from cases of epiglottitis (swabs obtained by a physician) should be plated to sheep blood agar, chocolate agar (for recovery of Haemophilus spp.), and a streptococcal selective medium. Staphylococcus aureus, Streptococcus pneumoniae, and beta-hemolytic streptococci are all potential etiologic agents of this disease. Refer to Table 5-1 for an overview of the methods used to collect, transport, and process different specimens from the upper respiratory tract.
Diagnosis of Infections in the Oral Cavity and Neck
Collection and Transport
Culture
Infections such as peritonsillar abscesses, oral and dental infections, and neck space infections usually involve anaerobic bacteria. The anaerobes involved typically originate in the oral cavity and are often more delicate than anaerobes isolated from other clinical material. Very careful methods are required in order to provide optimal specimens for anaerobic cultivation, as well as collection and transport for the recovery and identification of the etiologic agents. See Chapter 41 for more information related to anaerobic organisms.