Immunization Update III
Perhaps the biggest news in the immunization world over the past 2 years was the 2009 (H1N1) novel influenza A virus outbreak. The virus was initially identified to be the cause of influenza-like illness in two children in the United States during March and April 2009 and the cause of respiratory illness outbreaks in Mexico [1,2]. The virus spread rapidly and within weeks cases were identified across the United States and in several countries around the world. On June 11, 2009 the Director General of the World Health Organization (WHO) Dr Margaret Chan declared a global pandemic. As of August 10, 2010, this has been updated to postpandemic status. This review focuses on discussing the 2009 pandemic briefly along with the global response in terms of vaccine development, recommendations for vaccination for the 2010–2011 influenza season, followed by other recent updates in adolescent and pediatric vaccines.
Influenza vaccines
2009 (H1N1) novel influenza A
As part of the family Orthomyxoviridae, influenza viruses are single-stranded RNA viruses with 3 types: A, B, and C. Influenza A viruses circulate in more than 18 mammalian species (including pigs and humans, although the main reservoir is aquatic wildfowl, including migratory birds) and are responsible for all known major epidemics and pandemics [3]. The influenza A virus genome contains 8 separate gene segments that have the ability to “reassort” or “mix” with other influenza A virus gene segments and thus “shift” antigenic characteristics [3,5]. These viruses are classified according to the subtypes of surface proteins, hemagglutinin (HA) and neuraminidase (NA) that they possess. HA is a surface glycoprotein essential for viral binding and entry into host cells. HA contains the primary epitopes for protective neutralizing antibodies, whereas NA has a somewhat lesser role in protective immunity and is primarily required for viral release [6]. Thus antibody against HA confers protection against infection and illness whereas antibody against NA can reduce the severity of illness. Annual changes in the HA epitopes lend themselves to a “drift” in protective immunity to circulating strains, hence the need for yearly review of the antigenic content of circulating influenza viruses. Pandemics occur when influenza viruses with HA for which there is little or no protective immunity emerge in the human population and transmit virus efficiently from person to person.
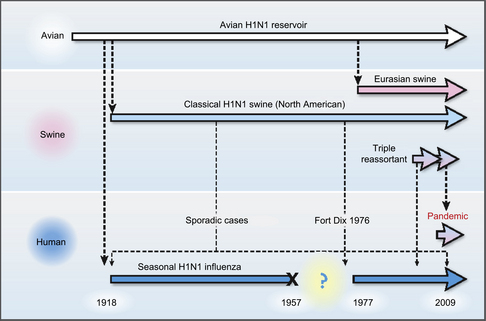
Since the global influenza A(H1N1) virus pandemic of 1918 (Spanish flu), influenza virus gene reassortment has been well documented and observed to occur frequently between human virus subtypes, between human and avian, and among avian influenza viruses [7,8]. Such reassortments led to the global pandemics of 1957 (H2N2) (Asian flu) and 1968 (H3N2) (Hong Kong flu) [8,9]. To have an idea of the magnitude of these epidemics, more Americans died as a result of the 1918 (Spanish flu) pandemic than were killed during World Wars I and II, the Korean, Vietnam, Gulf, Afghanistan, and Iraq wars combined [10].
Nearly all human influenza A infections worldwide since 1918 have been caused by descendants of the 1918 pandemic virus [7]. While influenza A(H1N1) viruses continue to circulate among humans, seasonal epidemics of influenza A virus from 1968 to 2009 were dominated by A(H3N2) virus variants generated by antigenic drift. However, in early April 2009 a new influenza A(H1N1) virus (originally referred to as swine-origin influenza A virus) emerged among humans in California and in Mexico, quickly spreading worldwide through human-to-human transmission, and generating the first influenza pandemic of the twenty-first century. Molecular studies of the new influenza A(H1N1) pandemic virus genome showed that it was antigenically unrelated to human seasonal influenza viruses but genetically derived from several viruses which had been circulating in pigs for a long time [8]. Initial transmission to humans is believed to have taken place at least several months before recognition of the first outbreak, and phylogenetic data suggest that the reassortment of swine lineages may have occurred years before emergence in humans. The 2009 H1N1 is a quadruple-reassortant virus containing gene segments from North American as well as Asian and European swine influenza viruses, some gene segments from North American avian influenza viruses, and one gene segment from a human influenza virus (Fig 1) [3–8]. During the current epidemic, however, there was no evidence that swine were playing any role in the epidemiology or in the worldwide spread of the virus in human populations [9].
Novel influenza A(H1N1), henceforth referred to as 2009 H1N1, for the most part presented as a mild, self-limiting upper respiratory tract illness with (or at times without) fever, cough, sore throat, body aches, fatigue, chills, rhinorrhea, conjunctivitis, headache, and shortness of breath [9,11]. Up to 50% of patients had gastrointestinal symptoms at presentation including diarrhea and vomiting. The spectrum of clinical presentation varied from asymptomatic cases to primary viral pneumonia resulting in respiratory failure, acute respiratory distress, multiorgan failure, and death [12]. While fewer than 1% of estimated cases of 2009 H1N1 in the United States resulted in hospitalization, a quarter of those hospitalized required admission to the intensive care unit (ICU), and more than half required mechanical ventilation. The presence of underlying conditions was common among hospitalized patients; up to 60% of children, and 83% of adults admitted with 2009 H1N1 infection had at least one underlying chronic medical condition (CMC) (asthma, diabetes, cardiovascular disease, neurologic and developmental disorders, the latter 2 being more common in children). Eleven percent of hospitalized adults with 2009 H1N1 were pregnant [13].
The United States experienced its first wave of 2009 H1N1 pandemic activity in the spring of 2009, followed by a second wave of 2009 H1N1 activity in the fall. Activity peaked during the second week in October and then declined quickly to below baseline levels in January. The early increase in flu activity in October is in contrast to nonpandemic influenza seasons. From April 2009 to April 2010, a total of 61 million cases were reported, estimated range 43 to 89 million, with an estimated 195,000 to 403,000 hospitalizations and estimated deaths 8870 to 18,300 [14].
In the United States reports from around the country for pediatric cases seemed to follow a common theme. In California 345 children younger than 18 years with laboratory-confirmed 2009 H1N1 were reported over a 3-month period. The median age was 6 years; hospitalization rates were highest in infants younger than 6 months (13.9 per 100,000) versus overall rates (3.5 per 100,000 per 110 days). Two-thirds (230; 67%) had comorbidities. More than half (163 of 278; 59%) had pneumonia, 94 (27%) required intensive care, and 9 (3%) died; in 3 fatal cases (33%), children had secondary bacterial infections. More than two-thirds (221 of 319; 69%) received antiviral treatment, 44% (88 of 202) within 48 hours of symptom onset [15]. Similar numbers have been reported from other parts of the country [16].
In the authors’ own hospital in Northeast Florida, of the 119 hospitalized children, 25 (21%) were admitted to the pediatric ICU and 94 (79%) were admitted to the general medical ward [17]. Mean age was 6.4 years with 52 (44%) patients younger than 5 years. More than 70% of patients had at least one CMC, with the 3 most common being pulmonary, immunosuppression, and neurodevelopmental. The incidence of microbiologically proven coinfections and mortality rate were 6.7% and 0.8%, respectively. Comparison showed statistically that patients in the pediatric ICU had a significantly higher rate of CMCs, complications, and longer length of stay than patients admitted to the general medical ward. Minority populations also seemed to be affected disproportionately. This result may be partly related to the fact that they seemed to suffer more from underlying conditions, putting them at higher risk [18].
Review of reports published from countries in both the northern and southern hemispheres corroborated reports from across the United States that 2009 H1N1 occurred more frequently in young and middle-aged adolescents followed by older children and adolescents [19,20]. This course differs from that of seasonal influenza, which tends to affect people at the two extremes of life, namely the very young and the elderly. It is hypothesized that elderly individuals may have substantial levels of protective antibody against 2009 H1N1 due to past encounters with influenza virus with antigenic epitopes similar to the pandemic strain. Surveys of serum collected before 2009 showed that 4% of United States residents born after 1980 had preexisting neutralizing antibody titers of ≥40 against 2009 H1N1 whereas 34% of those born before 1950 and 57% of those born before 1940 had neutralizing antibody titers of ≥80 [21]. Fatal outcomes were seen at any age, however, and were more frequent in those with an underlying CMC.
Groups at risk for particularly severe infection included pregnant women as well as those with obesity. In the United States more than one-third of pregnant women with confirmed 2009 H1N1 infection had to be hospitalized because of acute respiratory distress syndrome. Pregnancy is known to increase the risk for severe influenza infection because of the physiologic changes of decreased pulmonary tidal volume and increased cardiac output. Suppressed T-helper cell-mediated immune responses also impair maternal responses to infection with several viruses. Seasonal influenza related hospitalization in healthy pregnant women occurs at a rate of 1 to 2 per 1000, a risk that is 18-fold greater than that for healthy nonpregnant women [22]. Obese individuals may also have been at increased risk related to changes in physiology as a result of altered body composition. It should also be noted that while the vast majority of cases reported in the literature were those of hospitalized patients, many cases of 2009 H1N1 influenza probably remained unrecognized in the community with a mild unrecognized respiratory illness; therefore, the true extent of the pandemic may actually be underreported.
The propensity to primarily affect children and young adults as well as its rapid spread made the 2009 H1N1 virus unique from other reassortant viruses. One theory is that the H1N1 virus binds α2,3-sialic acid receptors found on the surface of cells located deep in the lungs that seasonal influenza virus cannot bind, suggesting why people with the pandemic influenza seemed to experience more severe pulmonary symptoms [23].
Influenza vaccines are licensed based on immunogenicity and not efficacy. Seasonal influenza vaccines contain 3 strains: A(H1N1), A(H3N2), and influenza B. Antibodies specific to HA are believed to be the best correlate of protection against influenza virus protection, and are the primary end point used to determine vaccine immunogenicity. The accepted correlate of protection is a hemagglutination inhibition (HI) titer of 1:40 or more (this test measures the ability of serum to compete with the binding of influenza virus to red blood cells) [24]. Influenza vaccines are made using the same reassortment process that leads to pandemic strains [25]. A virus seed strain adapted for growth in eggs using reassortment techniques is made and then serially passaged in eggs. This process gives way to the development of a high growth variant. This process clearly is extremely time consuming, depends on the egg supply, and is fraught with other technical challenges [26]. Cell culture–based vaccines may eventually be the way to overcome these issues.
Preliminary reports from a study done in Australia indicated that a single 15-μg dose of an inactivated split influenza A 2009 H1N1 vaccine induced a HI assay titer of 1:40 or more in 95% of 18- to 64-year-old healthy, nonpregnant subjects [27]. The robust immune response observed in the 18- to 49-year-old volunteer cohort was surprising. The findings suggested that there may be some cross-protection from previous exposure to antigenically drifted strains of H1N1 subtype. In addition, 2009 H1N1 shares 3 gene sequences with circulating seasonal H1N1, suggesting that there was more similarity between the 2009 H1N1 virus and the prevailing seasonal virus strains than had been recognized. Similarly, another randomized, observer-blind, age-stratified, parallel group study in 370 healthy infants aged 6 months to children under 9 years old in Australia, using 2 doses of either 15 μg HA or 30 μg HA monovalent, unadjuvanted 2009 H1N1 vaccine showed that a single 15-μg dose was immunogenic in children in this age group. No significant adverse events were reported [28].
In a United States phase 2 trial on 410 children and 724 adults who received a single dose (15 μg HA) of inactivated 2009 H1N1 vaccine, protective serologic titers of greater than 1:40 were detected at 21 days after vaccination in 45% to 50% of 6- to 35-month-old infants, 69% to 75% of 3- to 9-year-old children, 95% to 100% of 18- to 64-year-old adults, and 93% to 95% of adults older than 64 years. No vaccine-related severe adverse events were reported, but about 50% of every age and vaccine group reported injection-site and systemic reactions with no noticeable differences in the vaccine as compared with the placebo group [29]. Similarly, a multicenter, double-blind, randomized trial on 12,691 individuals 3 years of age and older in China receiving a single dose (7.5 μg HA) of a split virion 2009 H1N1 vaccine showed that protective serologic titers were detected on day 21 in 76.7% of 3- to 12-year-old children, 96.8% of 12- to 18-year-old adolescents, 89.5% of 18- to 60-year-old adults, and 80.3% of adults older than 60 years. In children, the administration of a second dose of the 7.5-μg formulation increased the seroprotection rate to 97.7%. Adverse reactions were mild to moderate [30].
Most recently another phase 2, multicenter, randomized, placebo-controlled, observer-blind, clinical trial using 2009 H1N1 vaccine in 1313 young adults (18–64 years) and older (≥65 years) conducted in 11 sites across the United States demonstrated that a single 7.5-μg dose of a monovalent, unadjuvanted 2009 H1N1 vaccine induced protective HI antibody levels in adults of all ages, including elderly adults [31]. Also, at baseline, 28.8% of young adults (18–64 years), 43.9% of younger elderly adults (65–74 years), and 62.9% of very elderly adults (≥75 years) had HI titers to 2009 H1N1 vaccine. Both older age (≥75 years) and receipt of the seasonal influenza vaccine in the previous season contributed independently to the antibody titer at baseline. Other studies in adults and children also suggest that receipt of seasonal influenza vaccine did have some cross-protection against 2009 H1N1 [32–35]. The Centers for Disease Control and Prevention (CDC) recommends immunization against both seasonal and 2009 H1N1 infection [36].
Given the safety and immunogenicity of the 2009 H1N1 vaccine in multiple trials around the world as well as the documented increased risk of complications from influenza and influenza-related illness, vaccination during pregnancy as well as in individuals with CMCs was considered high priority during the pandemic [37,38]. Vaccination of pregnant women was also seen as an effective strategy to prevent infection in infants too young to be vaccinated [39,40]. Health care workers were another group targeted for priority vaccination during the first round of immunizations. In fact, mandatory 2009 H1N1 vaccination was ordered in New York State for health care workers who had direct contact with patients or who had the potential to expose patients to the seasonal and 2009 H1N1 influenza. Ultimately this order was rescinded because of a shortage of both seasonal and 2009 H1N1 vaccine and opposition from some professional organizations. This mandatory vaccination policy raised several ethical questions and since then has come under much scrutiny [41]. Recently the American Academy of Pediatrics (AAP), the Society for Healthcare Epidemiology of America (SHEA), and the Infectious Diseases Society of America (IDSA) endorsed mandatory vaccination of health care workers to reduce the risk of infection among patients and employees [42,43]. The only exceptions would be people with medical contraindications for receipt of the vaccine. It was noted that the benefits of immunization would include prevention of disease from health care workers to patients, decrease health care workers’ risk of infection, create herd immunity, prevent excessive absenteeism during outbreaks, and set an example among the general public regarding the importance of immunization. Other priority groups included individuals with an underlying cardiovascular or respiratory medical condition including asthma, autoimmune disorders, and diabetes, as well as young children.
Studies of intranasal, live-attenuated, monovalent 2009 H1N1 influenza vaccine in children and adults have also demonstrated similar safety and immunogenicity when compared with similar seasonal influenza vaccine [44].
The safety of the 2009 H1N1 vaccines continues to be thoroughly monitored. Current data show that the vaccines are well tolerated and behave just as the corresponding seasonal influenza vaccines in terms of safety and lack of severe adverse events. It is of importance that the 2009 H1N1 vaccines are manufactured in exactly the same way as the seasonal influenza vaccines and therefore are not really “new” vaccines. A small number of Guillain-Barré syndrome (GBS) cases have been reported to be temporally associated after H1N1 vaccine administration in large-scale vaccination campaigns, but they all reverted quickly [45,46]. Clinical trials are still in progress in certain at-risk subpopulations.
Vaccination against 2009 H1N1 influenza was first implemented in China, followed by a large number of countries [47]. In the United States, the Food and Drug Administration (FDA) licensed the first 2009 H1N1 vaccines on September 15, 2009 [48]. None of these vaccines contained any adjuvants. The WHO was responsible for coordinating the effort to ensure adequate supply and access to 2009 H1N1 vaccine in underresourced countries.
A total of 26 vaccine manufacturers from America, Europe, Russia, Australia, and Asia have now developed or are presently developing pandemic A(H1N1) vaccines, whether inactivated whole-virus vaccines, split inactivated vaccines, subunit vaccines, live-attenuated vaccines, or other formulations. The silver lining to this pandemic was the emergence of several new vaccine manufacturers in China, India, Thailand, and South America [49].
Despite the widespread publicity as well as education campaigns by the CDC and state and local public health officials, public attitudes toward 2009 H1N1 immunization at best remained lukewarm both in the United States and globally. Despite all the available safety data from multiple studies the public, including individuals at high risk, remained skeptical about vaccine safety [50–54]. This situation continues to highlight the need for ongoing education and public health campaigns. Immunization remains the key to the control of future disease outbreaks. Concerns over the development of resistance to antiviral medications currently used for the treatment of influenza are beyond the scope of this discussion, yet remain another important reason why prevention is so critical. One recent study suggested that any practice visits during October through January as well as evening/weekend influenza vaccine clinics seemed to improve influenza vaccine uptake in young children. This study looked at simultaneous practice and child characteristics as well as practice strategies that are associated with influenza vaccination in geographically different states [55].
Other issues that have implications for future public health policy regarding influenza immunization include school-based immunization as well as immunization within households. There are mixed data regarding transmission within households. It does not appear that 2009 H1N1 transmission was any different from seasonal influenza [56,58]. Similarly, transmission within schools also depended on several factors including rapid identification of cases, implementation of prophylaxis, and school closure in certain instances [59]. It is imperative that we consider the implications of secondary cases due to household and school contacts when formulating public health policy during future pandemics and when formulating future strategies for immunization and containment [60,61].
Seasonal influenza
In the United States, 2009 H1N1 remained the dominant circulating virus during 2009. Few seasonal influenza viral infections were reported, the most being due to influenza A/H3N2 or influenza B. Seasonal influenza A/H1N1 almost seemed to disappear. Similar trends were seen worldwide [62].
Immunization recommendations for the current influenza season include immunization of all high-risk groups previously identified for seasonal influenza immunization (Box 1) [63,64]. An algorithm for immunization in children who may or may not have received the 2009–2010 seasonal as well as 2009 H1N1 vaccine is also outlined in the policy statement by the AAP. Recommendations that are new this year include expansion of routine immunization to include adults 19 to 49 years regardless of any underlying medical condition. Vaccines available for the 2010–2011 influenza season are listed in Table 1.
Box 1 AAP influenza vaccine recommendations for the 2010–2011 season
Table 1 Currently available influenza vaccines, 2010–2011 season
| Vaccine and Manufacturer | Age Labeling and Thimerosol Content (μg mercury per 0.5 mL dose) |
|---|---|
| Afluria (inactivated) CSL Biotherapeutics Ltd |
≥6 mo Not for use in children 6 mo–8 y (due to increased risk of fever and seizures) May be given in children 5–8 y if no other influenza vaccine available 25 μg mercury for multidose vial, 0 μg for prefilled syringes |
| Fluarix (inactivated) GSK Biologicals |
≥3 y No mercury |
| Flulavala (inactivated) ID Biomedical Corp. of Quebec |
≥18 y 25 μg mercury |
| Flumist (live attenuated) MedImmune LLC |
2–49 y No mercury |
| Agriflua (inactivated) Novartis Vaccines and Diagnostics Inc |
≥18 y 0 μg mercury (prefilled syringe) |
| Fluvirin (inactivated) Novartis Vaccines and Diagnostics Inc |
≥4 y 25 μg mercury for multidose vial and <1 μg for prefilled syringe |
| Fluzone (inactivated) Sanofi Pasteur, Inc |
6 mo and older New high dose licensed for ≥65 y 25 μg for 5 mL multidose vial, 0 μg for prefilled syringe |
While influenza vaccines generally are not licensed for use in infants younger than 6 months, an age when they are as vulnerable to complications related to influenza as the elderly, a double-blind, randomized, placebo-controlled trial conducted in 1375 healthy United States infants 6 to 12 weeks of age, receiving 2 doses of trivalent inactivated influenza vaccine 1 month apart in combination with other age-appropriate vaccines, demonstrated that at least 90% of vaccine recipients had antibody titers of 1:40 or more for at least one vaccine strain and 49.6% for 2 strains, versus 16.4% and 0.9% in placebo recipients. The investigators concluded that the vaccine was safe and immunogenic for use in infants aged 6 to 12 weeks [65].
So far in 2010, between June 13 and September 25, the United States has experienced low levels of influenza activity, and the southern hemisphere has experienced seasonal patterns of influenza activity and a mix of 2009 H1N1, influenza A/H3N2, and influenza B viruses cocirculated in tropical regions [66].
Adolescent immunizations
Estimated vaccination coverage among adolescents 13 to 17 years of age continued to improve during 2009 although it remains well below the Healthy People 2010 objectives [67]. Immunization coverage did vary among states, with 4 states achieving greater than 60% coverage for the 3 routine adolescent vaccines (meningococcal conjugate MenACWY, tetanus-diphtheria-acellular pertussis Tdap, and human papilloma virus HPV). During the 2009–2010 school years, on entry into middle school 27 states required Tdap, 7 required MenACWY, and 2 required HPV with opt-out provisions.
Tetanus, diphtheria, and acellular pertussis containing vaccine: Tdap
Even though immunization rates continue to increase, it is important that providers not become complacent but continue to educate their patients about the risks of vaccine-preventable diseases. The 2010 outbreak of pertussis in California, as well as the increased numbers of cases being reported in Michigan, Pennsylvania, New York, and South Carolina, continue to serve as reminders that vaccine-preventable diseases have been far from eradicated [68]. Adolescents and young adults who have not received the booster doses of Tdap continue to serve as carriers within schools, households, and the community [69,70]. A school-based immunization strategy has been used to control an outbreak in a school previously, and seems to be a logical approach when large numbers of individuals need immunization in an outbreak situation [71].
From January 1 through December 2011, 9,477 confirmed, probable and suspect cases of pertussis have been reported to the California Department of Health for a state rate of 24.2 cases/100,000 [72]; this is the highest number of cases reported since 1945. At the time of writing of this article 10 deaths have been reported, 9 of them in Hispanic infants. Nine fatalities were in infants younger than 2 months at the time of disease onset and who had not received any pertussis containing vaccine. Highest rates of diseases were seen in infants younger than 6 months, predominantly of Hispanic ethnicity. This outbreak highlights the importance of immunization for not only young children but also young adults. Household contacts including mothers of newborns have been documented as the primary source of disease for young infants [73]. The case for maternal immunization is important. Not only does it protect young infants from acquiring disease, but transplacental acquisition of maternal antibody is also the mainstay of protection for young infants until they are old enough to be immunized themselves [74]. Current recommendations in the United States call for immunization of mothers not previously immunized to be given Tdap vaccination as soon as possible in the postpartum period and before discharge from the hospital [75].
Following the California outbreak, the ACIP voted to recommend the “off-label” use of Tdap in two patient groups to try to capture those who might be at risk of passing disease to young infants. The first group is children 7 to 9 years who may not have completed the primary childhood series, and the second group is adults aged 65 and older who have contact with young infants. A dose of Tdap may also be given to people in this age group who have not previously received the vaccine [76]. California also issued expanded pertussis immunization recommendations to include immunization of the two groups as recommended by the ACIP in addition to immunization of pregnant women during or after pregnancy, as well as immunization of individuals who had contact with pregnant women or infants.
Meningococcal vaccines
The first tetravalent conjugate meningococcal vaccine (Menactra, MCV4; Sanofi Pasteur, Swiftwater, PA, USA) was approved for use in adolescents 11 through 55 years of age in 2005 [77]. Since then a second conjugate meningococcal vaccine (Menveo, MenACWY-CRM; Novartis Vaccines and Diagnostics, Sovicille, Italy) was licensed by the FDA on February 10, 2010 [78]. The two vaccines contain serogroups A/C/Y/W 135. The vaccines differ in the carrier to which the meningococcal polysaccharide capsule is conjugated: MenACWY-diphtheria CRM 197 and MCV4-diphtheria toxoids. MCV4 is approved for use for ages 2 through 55 years whereas MenACWY-CRM is approved for ages 11 through 55 years. Neither vaccine provides protection against serogroup B disease.
A recently published study looked to determine the immunogenicity and safety of MenACWY-CRM compared with MCV4 in 2- to 10-year-olds. A total of 2907 children were divided in to two groups (2–5 years and 6–10 years) and were randomized to receive a single dose of MenACWY-CRM or MCV4. Seroresponse to MenACWY-CRM showed statistical superiority for groups W and Y and was noninferior for group C in both groups. For group A, noninferiority criteria were not met. When the two age groups were combined, MenACWY-CRM was noninferior to MCV4 for all 4 serogroups and statistically superior for groups C/W/Y. There was no difference in safety parameters for both vaccines across both age groups [79]. Licensure for MenACWY-CRM for the 2- to 10-year-old age group is pending FDA licensure. Of the two licensed vaccines Menveo (MenACWY-CRM) is the only one that is Halal certified, and should be given to all Muslims needing meningococcal vaccine especially before embarking on the annual Haj pilgrimage to Makkah.
Prelicensure studies did not show an increase in cases of GBS with either vaccine. Initial postmarketing studies suggested a temporal association and a potential increased risk for GBS with the use of MCV4; however, ongoing studies have not been able to confirm a causal relationship, therefore either meningococcal vaccine may be used when meningococcal immunization is indicated. A history of GBS is a precaution to administration of MCV4 [80].
During the October 2010 meeting, the ACIP recommended changes for meningococcal vaccination as well. For children in high-risk groups it was already recommended that they receive MCV4 at age 2 years; now they would receive a booster 3 to 5 years following the initial immunization. The committee also voted to recommend a booster dose for adolescents at age 16 if they received the first dose at the 11- to 12-year visit. For those who received the immunization at ages 13 to 15, the booster dose would be 5 years after the initial immunization [81]. The basis for these recommendations is increasing evidence that levels of protective antibody decline with time since vaccination; invasive disease develops rapidly, therefore immune memory alone cannot be relied on for protection, and one cannot rely on herd immunity for protection of adolescents [82].
The quadrivalent meningococcal polysaccharide vaccine (MPSV4) remains available for use in children older than 2 years and older individuals at high risk for meningococcal disease. Periodic case reports have also linked MPSV4 to the development of Henoch-Schönlein purpura (HSP). A recent review of the Vaccine Safety Datalink cohort did not show any association of HSP with the vaccine in the 16- to 20-year age cohort [83]. No cases were seen in the 42 days following MPSV4 after 42,027 doses.
While there are several candidate meningococcal serogroup B vaccines in development, none has been licensed for routine clinical use as yet. Given that this is a significant cause of meningitis and septicemia in many industrialized countries, this remains a challenge. Issues faced in the development of a suitable vaccine are mainly that the polysaccharide capsule of serogroup B, being a homopolymer of α2-8 linked sialic acid, contains epitopes that are cross-reactive with the polysialylated form of the neural cell adhesion molecule. Because this is expressed by several host tissues, it is a poor immunogen even when conjugated to a carrier protein, and any anticapsular antibodies elicited have the potential to cross-react with host antigens and contribute to autoimmune disease. Efforts have therefore focused on using noncapsular antigens; however, the difficulty has been to find surface-exposed noncapsular antigens that are safe, antigenically conserved, and can elicit serum bactericidal antibodies. Various technologies using outer membrane vesicles (OMV) from the meningococcus as well as recombinant proteins alone or in combination with OMV are being used for different candidate vaccines in development [84]. An investigational recombinant vaccine that contains 3 central neisserial proteins with and without OMV from the New Zealand epidemic strain is currently in phase 2 and phase 3 clinical trials in young infants [85,86].
Human papilloma virus vaccines
The second HPV vaccine (HPV2) (Cervarix; GlaxoSmithKline Biologicals, Rixensart, Belgium) was licensed by the FDA on October 16, 2009 [87]. This bivalent vaccine contains types 16 and 18, and is indicated for the prevention of cervical cancer, cervical intraepithelial neoplasia (CIN) grade 2 or worse, adenocarcinoma in situ, and CIN grade 1 caused by HPV types 16 and 18 in females aged 10 through 25 years. Prior to this a quadrivalent HPV vaccine (HPV4 contains types 6/11/16/18) (Gardasil; Merck & Co. Inc, West Point, PA, USA) was licensed in June 2006. Like HPV4, HPV2 is composed of noninfectious, recombinant, virus-like particles (VLP) [88]. The VLP are composed of the viral L1 protein, which is the major capsid protein of the virus and has been shown to elicit strong systemic immune responses, eliciting antibodies that are highly effective in preventing native viral particles from entering epithelial cells and causing infection. Both vaccines can be given as early as 9 years of age. Catch-up vaccination is recommended for females 13 through 26 years old who have not been previously vaccinated, and the series can be completed if the female turns 26 before the series is complete [89].
Given the fact that HPV infections are most often sexually transmitted, that there are approximately 500,000 cases per year of genital warts in the United States in sexually active men and women, 90% being caused by types 6 and 11, that there are 600,000 cases of cancer caused by HPV annually in the United States, the majority being cervical, vaginal, and vulvar cancers in women (70% of cervical cancers are caused by types 16 and 18), and that HPV-associated cancers in men are caused primarily by type 16, it made sense to consider HPV vaccination for men as well. On October 16, 2010 the FDA licensed HPV4 for use in men aged 9 through 26 years old for prevention of HPV 6/11/16/18-related outcomes (genital warts and cancer). Following licensure, the ACIP issued a permissive recommendation that the quadrivalent vaccine may be given to males 9 through 26 years of age to reduce their likelihood of acquiring genital cancers [90]. In a permissive recommendation the ACIP states that administering the vaccine may be beneficial but that it is not an affirmative recommendation. The vaccine is available through the Vaccines for Children (VFC) Program, and VFC providers are required to administer the vaccine if requested by a VFC-eligible patient. Mathematical modeling suggests that adding male HPV vaccination to a female-only HPV vaccination program is not the most cost-effective immunization strategy for reducing HPV-related disease when immunization coverage for females is high (>80%). It may be more beneficial when female immunization rates are less than 80% [91].
Several barriers to HPV immunization leading to suboptimal immunization rates have been identified, Some of these are common to all adolescent vaccines; that is, the fact that adolescents do not visit the doctor as frequently as young children has decreased parental as well as public awareness about adolescent vaccines as opposed to routine childhood vaccines, as well as less acceptance of minor vaccine-related side effects such as injection-site soreness. There have also been specific parental concerns about HPV vaccine. Some parents have been reluctant to have their children vaccinated, fearing it will create a false sense of security about engaging in sexual activity. There has also been some stigma associated with HPV vaccine for similar reasons. Both provider and parental as well as patient education will go a long way toward dispelling some of these fears, therefore ongoing education remains crucial [92,94]. Acceptance and knowledge about HPV vaccine among male patients is also varied [95].
Routine HPV immunization for 12-year-old girls was introduced in the United Kingdom in 2008 and has had uptake rates between 80% and 90% [96]. In the United States the vaccine was recommended for use by the ACIP in 2006, shortly after licensure; however, in 2009 estimated vaccine coverage in 13- to 17-year-old girls for one dose of the vaccine was 44% and only 27% for 3 doses [67]. These rates are an improvement over the 2008 numbers, but still far below what is being reported in the United Kingdom. A recently published survey of physicians nationwide showed that while nearly all physicians surveyed started using the quadrivalent vaccine shortly after licensure, physician recommendation for older adolescents (19–26 years) was greater than for younger adolescents (11–12 years). Parents of younger adolescents also seemed more reluctant to vaccinate their girls [97]. Reasons for refusal have been mentioned in the previous section. Financial concerns were also perceived as a barrier to vaccination.
Pediatric vaccines
Pneumococcal conjugate vaccines
Introduction of 7-valent pneumococcal conjugate vaccine (PCV7) to the primary childhood immunization schedule in 2000 resulted in a significant reduction in invasive pneumococcal diseases in children as well as adults. A decrease in pneumococcal infections in unvaccinated children and adults due to indirect effects (herd immunity) was also seen, in addition a decline in diseases due to antibiotic resistant serotypes [98–103]. However, increase in invasive disease due to replacement serotypes was seen, particularly serotype 19A [104]. On February 24, 2010 the FDA licensed the 13-valent pneumococcal conjugate vaccine for use in place of the 7-valent vaccine [105]. This 13-valent vaccine (Prevnar 13; Wyeth Pharmaceuticals Inc, Philadelphia, PA, USA) contains serotypes 1/3/4/5/6A/6B/7F/9V/14/18C/19A/19F/23F. It is licensed for the prevention of invasive serotypes mentioned as well as for the prevention of otitis media caused by serotypes 4/6B/9V/14/18C/19F/23F in children 6 weeks through 5 years of age. Following licensure, the ACIP issued recommendations for use of PCV13 to include routine immunization of all children aged 2 to 59 months, vaccination of children 60 to 71 months with underlying medical conditions placing them at increased risk for invasive pneumococcal disease, and for those children who have previously received one or more doses of PCV7. For children 14 to 59 months who have received 4 or more doses of PCV7, a single supplemental dose is recommended. In addition, a single dose of PCV13 is also recommended for children younger than 71 months with underlying medical conditions, including children who have previously received 23-valent pneumococcal polysaccharide vaccine (PPSV23). PCV13 should be given after an interval of 8 weeks following PCV7 or PPSV23 [106]. Prelicensure studies demonstrated that the safety and immunogenicity of PCV13 were comparable with those of PCV7. The AAP has issued guidelines for the use of PCV13 and recommends replacement of PCV7 with PCV13 [107]. While it is beyond the scope of this review, the ACIP has also issued updated recommendations for the use of PPSV23 in adults [108]. Postlicensure surveillance data in the coming years will hopefully demonstrate changes in the epidemiology of pneumococcal isolates in children as well as adults. The effect on hospitalization due to invasive disease as well as usage of antibiotics in pediatric practices is also expected as we continue to see the anticipated decline in pneumococcal disease.
Rotavirus vaccines
On April 3, 2008, the second rotavirus vaccine was licensed in the United States (Rotarix; GlaxoSmith Kline, Rixensart, Belgium) [109]. Several studies have demonstrated the effectiveness and safety of both rotavirus vaccines since licensure [110–114]. In March 2010, the FDA recommended suspension of Rotarix after it was learnt that the vaccine contained DNA from porcine circovirus 1. Later it was discovered that Rotateq also has small quantities of porcine circovirus 2. However, these viruses cannot be grown in human cell lines and do not infect or cause disease in humans. Later in May 2010 the FDA recommended that use of Rotarix resume and Rotateq continue, because the risk of using these vaccines did not outweigh their benefits [115]. There is no question that these two vaccines have been extensively studied for safety and efficacy, and the AAP considers both vaccines to be equivalent. The FDA did, however, add the use of both rotavirus vaccines as a contraindication to the package insert following reports to the Vaccine Adverse Event Reporting System regarding the development of vaccine-associated diarrhea and prolonged virus shedding in 5 infants with severe combined immunodeficiency. Live-attenuated vaccines are contraindicated in the setting of severe immunodeficiency states involving cellular immunity [116]. Postmarketing surveillance data from Mexico showed a possible increased risk of intussusception of about 1.8-fold in the 30-day period following the first dose of Rotarix, with a clustering of cases in the first week after vaccination. Similar studies from Australia reported an increase in intussusception cases in the first week after vaccination with both rotavirus vaccines [117]. These findings are based on a small number of cases and have all been reported outside the United States. Based on these data, 1 case of intussusception per approximately 100,000 doses of rotavirus vaccine would occur in infants vaccinated according to age-appropriate recommendations. Following these reports, the ACIP recommends that while a small risk of intussusception is possible, given the benefit of rotavirus vaccines immunization with both rotavirus vaccines for prevention of severe rotavirus disease in United States infants and children should continue as recommended. The FDA advised a labeling change for Rotarix to inform health care providers about the potential risk of intussusception. Postmarketing surveillance studies for both rotavirus vaccines are ongoing.
Other childhood combination vaccines on the horizon
Combined haemophilus influenzae type B-neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine
Given the large number of vaccines that are now part of the routine immunization schedule, combination vaccines have become increasingly popular. A new conjugate, combined Haemophilus influenzae type B (Hib)-Neisseria meningitidis Serogroups C and Y (MenCY)-Tetanus Toxoid (TT) vaccine (HibMenCY-TT), is on the horizon, currently awaiting FDA review. So far this vaccine has shown noninferiority compared with monovalent MenC and Hib conjugate vaccines, with a comparable safety profile. Bactericidal antibodies against MenC/Y were induced after 2 doses of HibMenCY-TT [118,119].
In the United States, Tdap-inactivated polio vaccine (IPV)/Hib and Tdap-IPV combination vaccines have been available since 2008. Combination vaccines containing Hib were recommended for use by the CDC in 2008 following the Hib vaccine shortage in 2008 and the death of 5 children due to invasive Hib disease in Minnesota [120]. Combination vaccines available in other countries but not licensed in the United States include Tdap-HBV-IPV/Hib. This vaccine is currently being evaluated in 2-, 4-, and 6-month-old infants in Canada for safety and immunogenicity [121].
Changes in recommendations for vaccines used in special circumstances
Given the increasing number of international adoptions over the last several years in the United States, readers are reminded that immunization schedules vary widely depending on the country of origin of the adoptee. In addition, vaccine-induced immunity may vary depending on several factors, therefore all internationally adopted children should be evaluated carefully once in the United States. As an example, a recently published study looking at hepatitis A virus (HAV) immunity at an international adoption clinic showed that prevalence of immunity to HAV was lowest in children born in the Asia/Pacific Rim (17%) and highest in children born in Africa (72%) [122]. Young children may also remain asymptomatic with acute infection and have prolonged shedding, placing their new families and close contacts at risk for infection. All international adoptees should therefore have a thorough evaluation of their immunization status within a few weeks on arrival in the United States [123].
International vaccine news
Eradication of poliomyelitis still remains elusive, as cases of polio continue to occur in endemic countries (Pakistan, India, Afghanistan, and Nigeria) as well as in countries that have reestablished transmission (Angola, Chad, Republic of Congo) and in 13 other countries mainly in Africa, central Asia, and the former Soviet Republics with importation of the virus. Despite continuous setbacks, global poliomyelitis eradication efforts continue. It is estimated that the economic benefits of polio eradication will be approximately US$40 to 50 billion [124]. The global alliance for vaccines and immunization (GAVI), a private-public partnership supported by country and donor agreements, WHO, UNICEF, the World Bank, the global pharmaceutical industry, the Bill and Melinda gates Foundation, as well as private philanthropists, continues to provide vital support to developing countries in their efforts to support and sustain critically needed immunization programs in their respective countries. For example, in GAVI-supported countries, immunization coverage rates for diphtheria-tetanus-pertussis, hepatitis B, and Hib disease have gone from 60%/20%/0% in 2000 to 80%/ just under 60%/30%, respectively. Funding support for pneumococcal and rotavirus vaccines has also been provided, and efforts are ongoing to extend support to more countries to implement immunization programs for these diseases. Plans are also in place to provide future support for immunization programs for HPV vaccines, HAV, Japanese encephalitis, meningitis A, rubella, and typhoid [125].
As of November 2009, 130 of 193 WHO member states have introduced rubella-containing vaccine into their national immunization schedules. It is interesting that while two-thirds of WHO member states have incorporated rubella vaccine into their programs, these member states only represent less than half of the global birth cohort [126].
References
[9] Acute Respiratory Infections. The A/2009 H1N1 influenza virus pandemic. (update September 2009) Available at: http://www.who.int/vaccine_research/diseases/ari/en/index5.html Accessed November 8, 2010
[14] Updated CDC estimates of 2009 H1N1 influenza cases, hospitalizations and deaths in the United States. Available at http://www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm, April 2009-April 10, 2010. Accessed November 22, 2010
[48] FDA 2009 H1N1 vaccine questions and answers. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/QuestionsaboutVaccines/ucm182335.htm Accessed November 25, 2010
[68] B. Roehr. Whooping cough outbreak hits several US states. BMJ. 2010;341:c4627.
[72] Pertussis Report. California Department of Health. Available at: http://www.cdph.ca.gov/programs/immunize/Documents/PertussisReport2011-03-09.pdf Accessed April 13, 2011
[76] Centers for Disease Control and Prevention. ACIP Presentation slides: October 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/slides-oct10.htm#pertussis Accessed November 25, 2010
[77] FDA Approval Letter-Menactra. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm131181.htm. Accessed November 25, 2010.
[78] FDA Approval Letter-Menveo. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm201343.htm. Accessed November 25, 2010.
[81] Centers for Disease Control and Prevention. ACIP Presentation Slides October 2010. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-oct10/02-5-mening-mcv4.pdf Accessed November 25, 2010
[84] D.M. Granoff. Review of meningococcal group B vaccines. Clin Infect Dis. 2010;50(S2):S54-S65.
[87] FDA Approval Letter-Cervarix. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm186959.htm. Accessed November 27, 2010.
[105] FDA Approval Letter- Prevnar 13. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm201741.htm. Accessed November 28, 2010.
[109] FDA US Food and Drug Administration. Rotavirus Approval. 2008 Biologicals License Applications Approval. Available at: http://www.fda.gov/BiologicsBloodVaccines/DevelopmentApprovalProcess/BiologicalApprovalsbyYear/ucm173571.htm Accessed November 29, 2010
[117] Centers for Disease Control and Prevention. Vaccine preventable diseases: statement regarding Rotarix and Rotateq rotavirus vaccines and intussusception. Available at: http://www.cdc.gov/vaccines/vpd-vac/rotavirus/intussusception-studies-acip.htm Accessed December 8, 2010
[118] T. Nolan, P. Richmond, H. Marshall, et al. Immunogenicity and safety of an investigational combined Haemophilus influenzae type B—Neisseria meningitis serogroups C and Y-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J. 2010. Available at: http://www.ncbi.nlm.nih.gov.lp.hscl.ufl.edu/pubmed/20948453 Accessed November 27, 2010
[121] Immunogenicity and safety of GSK Biologicals’ Infanrix Haxa in infants. Available at: http://clinicaltrials.gov/ct2/show/NCT00753649 Accessed November 28, 2010
[124] Global Polio Eradication Initiative. Infected countries. Available at: http://www.polioeradication.org/Infectedcountries.aspx Accessed November 28, 2010
[125] The GAVI Alliance. Available at: http://www.gavialliance.org/resources/GAVI_Alliance_Nov_2010.pdf. Accessed November 28, 2010.