Ultrasound in reconstructive microsurgery
(CONSULTANT-LEVEL EXAMINATION)
Overview
Recent innovations in local tissue rearrangement and free tissue transfer have altered surgical approaches from simple wound care or amputation to optimal functional and aesthetic repair.1 Survival of transferred tissue remains a concern in trauma patients.2,3 Apart from the surgical technique, various factors may influence microsurgical operations, such as defect location and size, functional characteristics and underlying disorders, types of affected tissue, anatomic variants, and the presence of trauma or infection. The surgical focus is underlined by an effort to maximize the functional and aesthetic results, although safety remains a paramount requirement. Careful preoperative design, decision-making, and postoperative management in the intensive care unit (ICU) are as critical as the operation itself. Designing reconstructive microsurgical operations is challenging and cannot rely solely on clinical criteria. Ultrasound has been used to study blood flow in free flaps by means of implantable Doppler probes or by surface scanning with high-frequency transducers.4–6 Ultrasound techniques have increasingly been assimilated in surgical planning of flaps during the last decade.7–9
Ultrasound applications in microsurgery
Ultrasound examination of free flaps is achieved with standard two-dimensional and color Doppler techniques.9 Portable ultrasound devices equipped with high-frequency (20-MHz) linear transducers are used for imaging of the small and medium-sized vessels used in microsurgical reconstructions. Ultrasound provides real-time information about arterial and venous conduits (patency, diameter, flow) in both the donor and recipient sites. Doppler spectral waveforms (DSWs) of perforator arteries and accompanying venous tributaries facilitate vascular mapping. Ultrasound has effectively been integrated into free flap design by several microsurgical teams.4–9 According to a German survey, which included 121 plastic surgery institutions, ultrasound was the predominant technique for preoperative perforator mapping in flap surgery (48% of institutions used ultrasound techniques).10 Hence ultrasound is the most frequent imaging modality used in reconstructive microsurgery, followed by computed tomographic angiography (CTA) and magnetic resonance angiography.4–11
Sonography depicts vessel diameter, course, and branching patterns and facilitates preoperative discrimination between septocutaneous and musculocutaneous perforators.12 DSW analysis identifies vascular flow characteristics and can be used as an adjunctive tool for selecting between perforator vessels of the same caliber and value in cases in which perforators might have been damaged. In addition, exploring the patency of accompanying venous tributaries is important since dynamic evaluation of venous drainage affects the design of certain flaps (venous congestion might lead to flap failure).4,9 Perforators are small in size, but their anatomy and vascular network are highly complex and variable. Ultrasound can accurately visualize perforators with a diameter of at least 0.18 mm at the fascia level (Figure 54-1). It is a reliable method for estimation of caliber if its spatial resolution of 0.18 (axial) and 0.35 mm (lateral) is compared with CTA’s axial (0.68 to 0.78 mm) and z resolution (0.65 mm).4,13 Although sonography demonstrates two-dimensional footprints of the perivascular anatomy as opposed to three-dimensional views with CTA, it provides additional information about the condition and thickness of the subcutaneous fat layer along with anatomic characteristics and the status of underlying muscles and fascia.9 Therefore, sonographic data enable preoperative flap design. Intraoperative ultrasound applications include real-time perforator mapping and evaluation of microsurgical anastomoses and flap perfusion.9 In the ICU the method can be used for flap monitoring given its ability to evaluate changes in blood flow.9
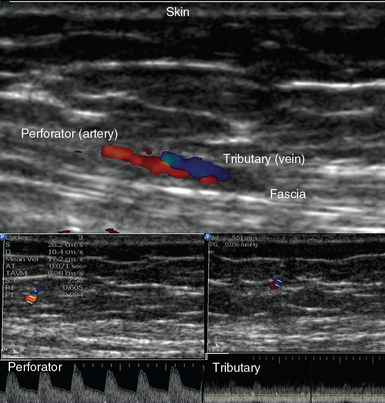
Figure 54-1 (Top) Ultrasound depicting a small perforator with accompanying tributary exiting the abdominal fascia and their Doppler spectral waveforms (bottom).
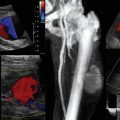
In our practice, ultrasound is used to determine the suitability of vessels as recipients by measuring their diameter and assessing their flow characteristics, as well as the level at which safe anastomosis could be performed (outside the zone of injury).9 The recipient site is evaluated for possible effects of radiation (e.g., actinonecrosis) or scarring from previous surgery. In head and neck reconstructions, the ipsilateral facial and superior thyroid vessels are the recipients of choice.14 Nevertheless, all native common carotid artery branches and jugular vein tributaries are investigated in both the ipsilateral and contralateral regions of the neck. For breast reconstruction, the internal mammary vessels are the recipients of choice. Apart from evaluating the arterial circuits, assessment of the flow, diameter, and patency of the venous tributaries is mandatory (Figure 54-2). Moreover, the width of the second and third intercostal spaces is measured to decide whether a rib-sparing technique will be used for exposure of the vessel. A width of 2 cm is required for easy access and safe, comfortable anastomosis. Ultrasound is effective in detecting preexisting vascular disorders or posttraumatic vascular abnormalities after lower limb reconstruction.4 In cases of pedicled flaps, recipient vessel selection, level of safe anastomosis (e.g., outside the zone of injury), and pedicle length requirements are evaluated regularly. Intraoperatively, sonography is used, under strict sterilization measures, in cases in which recipient vessel or perforator flow appears to be problematic (see Figure 54-2). In the ICU, ultrasound is used to monitor buried flaps or flap perfusion whenever the latter is uncertain based on clinical criteria (see Figure 54-2).
Pearls and highlights
• Ultrasound provides two-dimensional and color Doppler details of the recipient and donor sites in microsurgical operations. It evaluates perforators and the accompanying venous tributaries to provide dynamic vascular mapping, which is crucial in microsurgical planning.
• Sonographic assessment of medium-sized and small vessels is technically demanding and has many limitations.
References
1. Wei, FC, Demirkan, F, Chen, HC, et al, The outcome of failed free flaps in head and neck and extremity reconstruction: what is next in the reconstructive ladder. Plast Reconstr Sur. 2001; 108:1154–1160.
2. Culliford AT, 4th., Spector, J, Blank, A, et al, The fate of lower extremities with failed free flaps: a single institution’s experience over 25 years. Ann Plast Sur. 2007; 59:18–21.
3. Cohn, AB, Lang, PO, Agarwal, JP, et al. Free-flap reconstruction in the doubly irradiated patient population. Plast Reconstr Surg. 2008; 122:125–132.
4. Gravvanis, A, Tsoutsos, D, Karakitsos, D, et al, Blood perfusion of free anterolateral thigh perforator flap: its beneficial effect in the reconstruction of infected wounds in the lower extremity. World J Sur. 2007; 31:11–18.
5. Gravvanis, A, Papalois, A, Delikonstantinou, I, et al. Changes in arterial blood flow of free flaps after the administration of sildenafil in swine. Microsurgery. 2011; 31:465–471.
6. Rosenberg, JJ, Fornage, BD, Chevray, PM, Monitoring buried free flaps: limitations of the implantable Doppler and use of color duplex sonography as a confirmatory test. Plast Reconstr Sur. 2006; 118:109–113.
7. Renshaw, A, Whitwell, KA, Berger, L, Butler, PE. The use of color-Doppler ultrasound in the assessment of vessels for facial transplantation. Ann Plast Surg. 2007; 59:82–86.
8. Blondeel, PN, Beyens, G, Verhaeghe, R. Doppler flowmetry in the planning of perforator flaps. Br J Plast Surg. 1998; 51:202–209.
9. Gravvanis, A, Karakitsos, D, Dimitriou, V, et al, Portable duplex ultrasonography: a diagnostic and decision-making tool in reconstructive microsurgery. Microsurger. 2010; 30:348–353.
10. Knobloch, K, Gohritz, A, Reuss, E, et al, Preoperative perforator imaging in reconstructive plastic surgery: current practice in Germany. Plast Reconstr Sur. 2009; 124:183–184.
11. Gravvanis, A, Dionyssiou, DD, Chandrasekharan, L, et al, Paramuscular and paraneural perforators in DIEAP flaps: radiographic findings and clinical application. Ann Plast Surg. 2009;63:610–615.
12. Dumanian, GA, Free tissue transfer for lower extremity reconstruction: a study of the role of computed angiography in the planning of free tissue transfer in the posttraumatic setting. Plast Reconstr Sur. 2009; 124:530–531.
13. Rozen, WM, Ribuffo, D, Atzeni, M, et al. Current state of the art in perforator flap imaging with computed tomographic angiography. Surg Radiol Anat. 2009; 31:631–639.
14. Gravvanis, A, Tsoutsos, D, Delikonstantinou, I, et al. Impact of portable duplex ultrasonography in head and neck reconstruction. J Craniofac Surg. 2012; 23:140–144.