Ultrasound-guided arterial catheterization
Overview
Arterial catheterization is a frequent and essential procedure that is used in the intensive care unit (ICU) for accurate hemodynamic monitoring and repeated sampling of blood for analysis.1 Blind or palpated catheter insertion can be difficult because pulsations and landmarks are often obscured by edema, obesity, hypotension, hypovolemia, and small-caliber vasculature. Repeated attempts at catheterization are often less successful because of arterial spasm. In addition, the external landmarks used for catheter placement are not necessarily predictive of the underlying anatomy. This is especially true of the femoral vasculature, for which it has been reported that a portion of the common femoral artery overlaps the common femoral vein up to 65% of the time.2–5
Recent studies and meta-analyses have shown the utility of ultrasound for guiding arterial catheterization, with increased success and decreased complication rates similar to those found with the use of ultrasound for central venous catheter placement.6–10 A meta-analysis by Shiloh et al demonstrated a 71% improvement in first-attempt success and a number needed to treat of six when using ultrasound guidance for radial artery catheterization.9 Ultrasound-guided arterial catheterization has been shown to be beneficial in pediatric and adult populations in the ICU, surgical, and interventional settings. B-mode (two-dimensional) ultrasound is most often used for arterial catheterization and has been shown to be superior to Doppler ultrasound techniques. Real-time visual guidance is superior to marking a spot with ultrasound and then trying to locate a vessel without guidance.11–15
Procedure and instrumentation
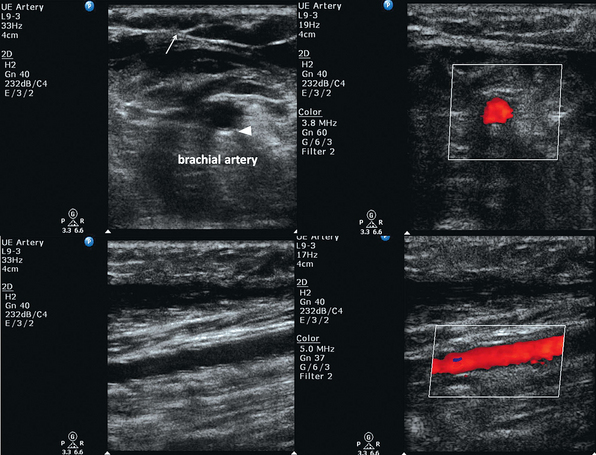
B-mode (two-dimensional) ultrasound is used for arterial catheterization. Arteries appear hypoechoic on B-mode in contrast to the adjacent soft tissue (isoechoic). A key step in ultrasound-guided arterial catheterization is identification of the target artery and the adjacent vein. Pulsatility distinguishes arteries from veins. Partial arterial compression will accentuate pulsations that may be difficult to visualize in smaller-caliber vessels or hypotensive patients.16 As opposed to veins, arteries should not be fully compressible with mild pressure from the transducer. As a rule, the artery should begin to indent when the vein is completely collapsed by pressure from the transducer. In addition, color power Doppler and pulsed wave Doppler can be used to distinguish arterial and venous pulsation.
Color Doppler can be used to distinguish the flow of blood in relation to the transducer. Blood cells moving toward the transducer produce a positive Doppler shift, and by convention the latter is represented as red color flow on color mode. A negative Doppler shift, produced by blood cells moving away from the probe, is represented by blue color flow accordingly. As blood velocity increases, so does the Doppler frequency. Lighter shades of red or blue indicate higher velocity, whereas deeper shades indicate lower velocity. Absent flow or low-flow states fail to produce a Doppler shift and are represented as black. For color flow to be present there must be flow in the direction of the ultrasound beam. The angle of insonation (the angle between the direction of flow and the ultrasound beam) is the key factor when using color Doppler (see Chapters 1 and 8). Doppler frequency increases as the ultrasound beam becomes more aligned with the direction of flow (parallel to the vessel). If the flow is perpendicular to the beam, no relative motion will be detected. It is dependent on the operator to appropriately angle the transducer in a fashion that will create flow toward the transducer as arterial or red-shifted. In addition to being red-shifted, arterial flow will be visualized as pulsatile and of higher velocity than venous flow. Venous flow will be blue-shifted and demonstrate continuous flow when compared with arterial flow. Similarly, pulsed wave Doppler can be used to measure changing blood velocity at a single point. A flow waveform of frequency shift (velocity) over time is created. Arterial flow demonstrates pulsatility, representative of the cardiac cycle, with a systolic peak and diastolic nadir. Venous flow is represented by continuous, low-velocity flow. It is important to not rely on Doppler techniques alone, because pulsatile venous Doppler flow can be demonstrated in patients with elevated right atrial and central venous pressure.17,18
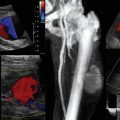
Before the procedure a preliminary scan of prospective sites should be performed to identify the most appropriate vessel for catheterization. Frequently, there is asymmetry in the size of the arteries between the two sides of the body. Common locations for arterial catheterization include the radial, femoral (Figure 16-1), brachial, axillary, and dorsalis pedis arteries. After determining the target artery, the site is prepared in sterile fashion with full barrier precautions. An assistant applies sterile conducting gel to the uncovered probe and holds it vertically. The sterile operator inserts a hand into the sheath, holds the probe, and inverts the sheath over the probe and cable. Additional sterile ultrasound gel is then applied to the sterile probe.
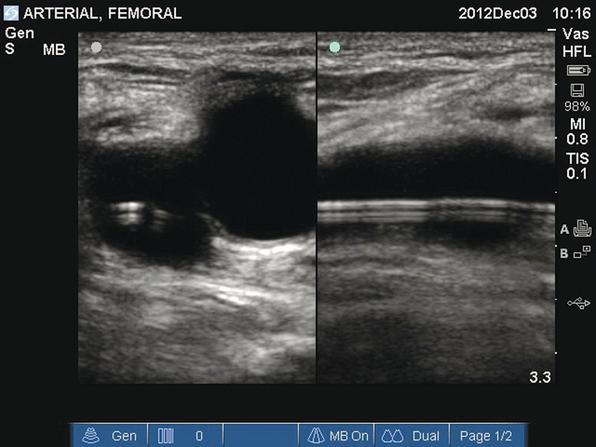
Figure 16-1 Transverse and longitudinal views of the femoral artery. A femoral arterial catheter can be visualized within the lumen of the vessel.
If using a one-person technique, the operator controls the needle with the dominant hand and the transducer with the nondominant hand. When using a two-person technique, an assistant in full sterile barrier precautions controls the transducer. When compared with the two-person technique, the one-person technique has been reported to be learned easily and has improved first-pass and overall success for central venous catheterization.11,15
The artery is localized and centered on the screen in the transverse plane. Transverse and longitudinal views can both be used for catheterization. The transverse view allows easier visualization and catheterization of smaller (radial, dorsalis pedis) and tortuous arteries. The longitudinal view allows the direct visualization of the needle at all times, which may reduce perforation of the posterior vessel wall, but it also limits visualization of the surrounding structures because of a smaller plane of view. The longitudinal view is obtained by rotating the probe 90 degrees after localizing the artery in the transverse view while keeping the artery within the region of interest at all times.19
Site-specific tips
Radial artery
In the transverse view the radial artery and accompanying veins are well visualized. The veins that are often accidentally catheterized when using the palpation technique can be compressed with light pressure from the transducer. When catheterizing the radial artery, an ultrasound-guided Allen test using Doppler signal can be performed to ensure good flow to the hand.16 Ultrasound evidence demonstrates that the dimensions of the radial artery are unaltered when the wrist is extended up to 45 degrees. Extension beyond 60 degrees results in a decrease in radial artery diameter, thereby rendering catheterization more difficult.20
Femoral artery
Though often used to delineate the inguinal ligament, the inguinal crease, a common puncture site for femoral artery catheterization, is an unreliable landmark for the underlying vascular anatomy. Use of the inguinal crease alone as a landmark coupled with an oblique puncture angle above the inguinal ligament may possibly lead to intraabdominal or peritoneal puncture.4 Ultrasound guidance can be used to exclude intraperitoneal structures and avoid catastrophic complications.
Large amounts of overlap may occur between the common femoral artery and vein. Life-threatening complications of arterial catheterization, such as hematomas, arterial pseudoaneurysms, and arteriovenous fistulas, can be avoided, detected, and promptly treated when using ultrasound as detailed elsewhere (Chapter 8).
Hematomas
Because of mixing of liquid and coagulated blood, acute hematomas may be visualized on ultrasound as solid or mixed echogenic structures. With time, liquefactive necrosis of the hematoma results in a cystic structure, until ultimately a hypoechoic structure (with a possible hematocrit sign) is produced.21
Pseudoaneurysms
When compared with palpation-guided puncture, use of ultrasound-guided puncture for endovascular interventions was found to decrease rates of pseudoaneurysm formation.22 Ultrasound is the method of choice for detection of pseudoaneurysms (Chapter 8). Findings include a communicating collection adjacent to the femoral artery. Turbulent internal blood flow and flow through the communicating channel can be detected with Doppler ultrasound. Ultrasound-guided percutaneous thrombin injection and ultrasound-guided compression are initial therapies for femoral pseudoaneurysms.23 Potential embolic complications can be reduced by avoiding needle puncture through visualized atheromas.
Arteriovenous fistulas
Arteriovenous fistulas are defined by a direct connection between an artery and vein (see Chapter 8). Color and pulsed wave Doppler are diagnostic and demonstrate flow in the tract between the vascular structures. The jet of arterial flow into the vein causes turbulent flow and, in severe cases, an arterial waveform within the vein.21
Axillary artery
Nerve injury can be prevented by visualization and identification of the branches of the brachial plexus when catheterizing the axillary artery. Ultrasound guidance can be used to catheterize the artery via the traditional approach through the axillary fossa or via a transpectoral approach through the chest wall. Because of its cleaner and drier location, the transpectoral approach may potentially reduce the incidence of catheter infection.24
Dorsalis pedis
Even though the complication rates of dorsalis pedis and radial artery catheterization are similar, the success rate is much lower when placing dorsalis pedis catheters via palpation techniques, probably because of the relatively small caliber and tortuous course of the dorsalis pedis artery.25 Ultrasound guidance would probably improve the success rate of catheterization.
Pearls and highlights
• Blind or palpated catheter insertion can be difficult because pulsation and landmarks are often obscured by edema, obesity, hypotension, hypovolemia, and small-caliber vasculature.
• Recent studies and meta-analyses have shown the utility of ultrasound for guiding arterial catheterization, with increased success and decreased complication rates similar to those found with the use of ultrasound for central venous catheter placement.
• Ultrasound-guided arterial catheterization increases first-pass success rates by 71% over landmark techniques.
References
1. Seneff, M. Arterial line placement and care in intensive care medicine. In: Irwin RS, Rippe JM, eds. Intensive care medicine. ed 5. Philadelphia: Lippincott, Williams & Wilkins; 2003:36–45.
2. Baum, PA, Matsumoto, AH, Teitelbaum, GP, et al, Anatomic relationship between the common femoral artery and vein: CT evaluation and clinical significance. Radiology. 1989;173(3):775–777.
3. Denys, BG, Uretsky, BF, Anatomical variations of internal jugular vein location: impact on central venous access. Crit Care Med. 1991;19(12):1516–1519.
4. Lechner, G, Jantsch, H, Waneck, R, et al, The relationship between the common femoral artery, the inguinal crease, and the inguinal ligament: a guide to accurate angiographic puncture. Cardiovasc Intervent Radiol. 1988;11(3):165–169.
5. Warkentine, FH, Clyde Pierce, M, Lorenz, D, et al, The anatomic relationship of femoral vein to femoral artery in euvolemic pediatric patients by ultrasonography: implications for pediatric femoral central venous access. Acad Emerg Med. 2008;15(5):426–430.
6. Levin, PD, Sheinin, O, Gozal, Y. Use of ultrasound guidance in the insertion of radial artery catheters. Crit Care Med. 2003; 31(2):481–484.
7. Schwemmer, U, Arzet, HA, Trautner, H, et al. Ultrasound-guided arterial cannulation in infants improves success rate. Eur J Anaesthesiol. 2006; 23(6):476–480.
8. Shiloh, AL, Eisen, LA, Ultrasound-guided arterial catheterization: a narrative review. Intensive Care Med. 2010;36(2):214–221.
9. Shiloh, AL, Savel, RH, Paulin, LM, et al, Ultrasound-guided catheterization of the radial artery: a systematic review and meta-analysis of randomized controlled trials. Chest. 2011;139(3):524–529.
10. Shiver, S, Blaivas, M, Lyon, M. A prospective comparison of ultrasound-guided and blindly placed radial arterial catheters. Acad Emerg Med. 2006; 13(12):1275–1279.
11. Feller-Kopman, D, Ultrasound-guided internal jugular access: a proposed standardized approach and implications for training and practice. Chest. 2007;132(1):302–309.
12. Schummer, W, Schummer, C, Tuppatsch, H, et al, Ultrasound-guided central venous cannulation: is there a difference between Doppler and B-mode ultrasound. J Clin Anesth. 2006;18(3):167–172.
13. Hosokawa, K, Shime, N, Kato, Y, et al. A randomized trial of ultrasound image–based skin surface marking versus real-time ultrasound-guided internal jugular vein catheterization in infants. Anesthesiology. 2007; 107(5):720–724.
14. Mansfield, PF, Hohn, DC, Fornage, BD, et al. Complications and failures of subclavian-vein catheterization. N Engl J Med. 1994; 331(26):1735–1738.
15. Milling, TJ, Jr., Rose, J, Briggs, WM, et al, Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) Trial. Crit Care Med. 2005;33(8):1764–1769.
16. Jarvis, MA, Jarvis, CL, Jones, PR, et al, Reliability of Allen’s test in selection of patients for radial artery harvest. Ann Thorac Surg. 2000;70(4):1362–1365.
17. Abu-Yousef, MM, Kakish, ME, Mufid, M, Pulsatile venous Doppler flow in lower limbs: highly indicative of elevated right atrium pressure. AJR Am J Roentgenol. 1996;167(4):977–980.
18. Cozcolluela, MR, Sarria, L, Sanz, L, et al. Correlation of central venous pressure with Doppler waveform of the common femoral veins. J Ultrasound Med. 2000; 19(8):587–592.
19. Sandhu, NS, Ultrasound imaging in anesthesia: an overview of vascular access and peripheral nerve blocks. Semin Anest. 2007; 26:197–209.
20. Mizukoshi, K, Shibasaki, M, Amaya, F, et al. Ultrasound evidence of the optimal wrist position for radial artery cannulation. Can J Anaesth. 2009; 56(6):427–431.
21. Davison, BD, Polak, JF, Arterial injuries: a sonographic approach. Radiol Clin North Am. 2004;42(2):383–396.
22. Gabriel, M, Pawlaczyk, K, Waliszewski, K, et al. Location of femoral artery puncture site and the risk of postcatheterization pseudoaneurysm formation. Int J Cardiol. 2007; 120(2):167–171.
23. Ahmad, F, Turner, SA, Torrie, P, et al. Iatrogenic femoral artery pseudoaneurysms—a review of current methods of diagnosis and treatment. Clin Radiol. 2008; 63(12):1310–1316.
24. Sandhu, NS, The use of ultrasound for axillary artery catheterization through pectoral muscles: a new anterior approach. Anesth Analg. 2004;99(2):562–565.
25. Martin, C, Saux, P, Papazian, L, et al. Long-term arterial cannulation in ICU patients using the radial artery or dorsalis pedis artery. Chest. 2001; 119(3):901–906.