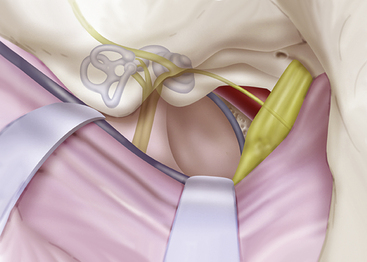
Chapter 38 Tumors Involving the Cavernous Sinus
Until 1965, when Parkinson’s landmark article describing the direct surgical approach to carotid-cavernous fistulas was published, little reference was made in the neurosurgical literature to direct operative attack on lesions of the cavernous sinus.1 This lack of information was largely a result of the inability in the premicrosurgical era to address effectively the extreme risks of significant hemorrhage and damage to the cranial nerves in the region. This anatomic locale has long been considered a true “no man’s land” for direct surgical approaches. The modern era of microneurosurgery has realized expanded capabilities in microsurgical technique and has fostered the work of several neurosurgeons who have made great strides in effectively approaching this region with reduced morbidity.2–20 In particular, the work of Dolenc should be recognized for the development of his combined epidural and subdural approach, which has become the standard method used to directly access lesions in this region.6
More recently, within the last decade, endonasal endoscopic approaches to the cavernous sinus region have been developed as an alternative to an open craniotomy.21–24 These approaches are not commonly utilized, but the current trend in anterior and middle cranial base surgery is to incorporate these strategies as a minimally invasive alternative. These techniques are mentioned as they are becoming an important element of the armamentarium in dealing with these lesions. However, they are complex enough to warrant a separate and entire chapter themselves. Here we will focus on the transcranial techniques in detail.
Indications
The indications for direct operative attack on neoplastic lesions arising in or involving the cavernous sinus have been a matter of debate. New forms of therapy, such as stereotactic radiosurgery, are providing alternatives in our armamentarium for treating these difficult tumors.25–27 A more restrictive set of indications for operative intervention has evolved within the past decade. We briefly consider the presently acceptable indications for a direct operation on these lesions.
The presence of a mass in the cavernous sinus, of course, does not itself constitute an indication for a direct operation. Many variables must be taken into account, including the age and medical condition of the patient, imaging characteristics, adjacent structures involved, time course of the process, and functional severity of symptoms. Many patients, because of poor medical condition or refusal to undergo surgery, may not be candidates for intracavernous microsurgery under any circumstances. The primary indications for surgery in most cases at present depend upon whether radiousurgery can be done safely as the primary treatment or not. Patients that have direct involvement, for example, of the optic apparatus by extension of tumor, cannot have radiosurgical treatment without significant risk of radiation delivery over acceptable minimums to these structures. In such case, an expert debulking of the lesion with creation of sufficient space between residual tumor and the optic apparatus is indicated. Patients with symptoms such as severe retro-orbital pain are also considered candidates for a debulking procedure, in order to relieve them of symptoms. Reports have demonstrated that stereotactic radiosurgery presents a viable alternative in patients with most intracavernous meningiomas.27 At present, most patients who harbor lesions of the cavernous sinus are considered for radiosurgical treatment as primary treatment, with the possibility of surgery as an adjunct to their overall treatment. In some cases, patients with benign, well-circumscribed tumors in the cavernous sinus are candidates for a primary surgical approach for resection of the lesion. Most of these patients have lesions that are consistent with benign tumors of the region (e.g., neurinomas, cavernous hemangiomas, pituitary adenomas, dermoids, chordomas, and chondrosarcomas). These tumors tend to be well-encapsulated masses that are dissectible from the surrounding structures.
Patients with apparent meningiomas of the cavernous sinus, although their tumors are benign, are considered for surgery only in select circumstances. Patients who are able to undergo surgery who have debilitating symptoms, such as rapid visual loss or painful ophthalmoplegia, are offered an operation with the goal of decreasing the mass of the tumor and providing space for the tumor to expand via decompression at the skull base. The goal of such an operation is decompression of the involved structures, with a total resection attempted only when circumstances are very favorable. Patients with asymptomatic, small meningiomas are followed up with serial scans until they show enlargement of the mass or neurologic symptoms. A select few patients with tumors that are located at the lateral wall of the cavernous sinus and involve the temporal dura propria without cavernous invasion may be offered surgery. This is reasonable in such situations as the tumor can be completely removed with a minimum of risk of permanent neurologic deficits. These decisions rely on the judgment of the surgeon experienced with tumors in this area.
Difficult decisions are made in cases in which cavernous sinus involvement occurs by extensions of malignant processes from the paranasal sinuses and pharynx. Procedures treating such disorders are palliative because of the characteristically aggressive nature of these tumors, such as squamous cell carcinoma. En bloc resection of the cavernous sinus and adjacent areas may represent merely a heroic effort on the patient’s behalf, with little realistic chance of long-term survival. Localized malignancies are an entirely different prospect in most cases. Local invasion by chordomas or chondrosarcomas can be effectively resected almost totally in many cases, with long-term recurrence-free survival, even though these tumors are incurable.13
Surgical Anatomy
Work focusing on the microsurgical anatomy of the cavernous sinus and its adjacent structures has made a critical contribution to our understanding and capabilities in dealing with neoplasms involving the cavernous sinus.7,28–32 The individual surgeon’s facility with the anatomic details of this complex region cannot be overemphasized as a basis for successful surgical therapy. The anatomy as presented in conventional texts, although an important initial basis, provides insufficient knowledge for the neurosurgeon operating in this region. An intimate comprehension of the multiple entry corridors and their specific anatomic substrates and boundaries is critical to the safe implementation of these procedures. Adequate preparation, including judicious use of the cadaver dissection laboratory, enhances the chances for a successful approach to these lesions. This has become especially important with the introduction of advanced endonasal endoscopic techniques. These techniques introduce an entirely separate skill set that requires development in the laboratory to gain comfort and facility with the endoscope and the special instruments.
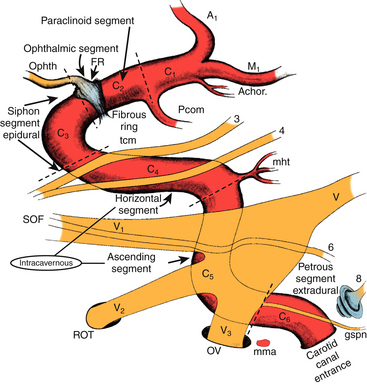
The anatomy of the intracavernous carotid artery deserves special attention. The artery enters the cavernous sinus, piercing the true cavernous membrane, at the foramen lacerum. It is surrounded here by a thickening of this connective tissue, which forms a fibrous ring around the artery. The artery then bends anterosuperiorly toward the superior orbital fissure. Just distal to this bend, the meningohypophyseal trunk typically arises on the superomedial side. This trunk has three branches: (1) the tentorial (Bernasconi-Cassinari), (2) the dorsal meningeal, and (3) the inferior hypophyseal arteries, all of which display some variability. The carotid artery usually gives rise to the artery of the inferior cavernous sinus on its lateral side as it courses anteriorly. This vessel traverses the sinus, usually crossing over cranial nerve VI, and anastomoses with several branches of the internal maxillary artery. These anastomoses include: (1) the recurrent meningeal artery at the superior orbital fissure, (2) the artery of the foramen rotundum, (3) the accessory meningeal artery at the foramen ovale, and (4) the middle meningeal artery at the foramen spinosum. The tentorial artery is absent from the meningohypophyseal trunk in some cases, and in these situations, a marginal tentorial artery is typically found arising from the artery of the inferior cavernous sinus.29 In a few patients (~10%), branches off the medial side of this segment (known as McConnell’s capsular arteries) supply the capsule of the pituitary gland.29
The artery makes another bend in the anterior portion of the cavernous sinus superomedially. This segment of the artery exits the cavernous sinus and pierces the enveloping membrane. The membrane in this region is called the carotico-oculomotor membrane, because it spans the gap between the oculomotor nerve in the medial wall of the cavernous sinus and the carotid artery.29,33 This loop is then completed in the extracavernous, extradural space under the anterior clinoid process. This loop has been designated the siphon segment, or clinoidal segment, and continues posteriorly a short distance before piercing the dura. Here, it is surrounded by a fibrous ring of dura, and the ophthalmic artery typically originates just inside this fibrous dural ring.33–35
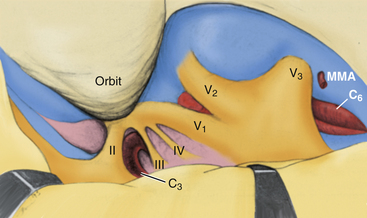
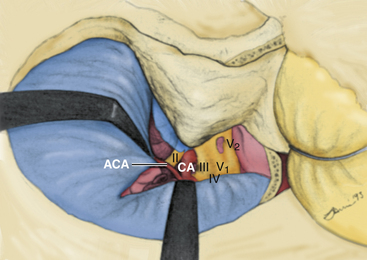
The ICA has been assigned nomenclature that divides it into several segments by different authors. We have been using the system described by Fischer in 1938, which numbers the segments beginning from the carotid bifurcation.36 We make a small modification to the original system with regard to numbering the petrous carotid segment (Fig. 38-1). The C1 segment begins at the carotid bifurcation and extends to the origin of the posterior communicating artery. C2 is the ophthalmic segment described by Day, stretching from the posterior communicating artery to the fibrous dural ring.33 The extradural, extracavernous clinoid segment is given the designation of C3. C4 is the true intracavernous segment of the artery and is delimited by the carotico-oculomotor membrane anteriorly and the origin of the meningohypophyseal trunk posteriorly. From the meningohypophyseal trunk, the artery is designated as C5 until it has passed under the trigeminal nerve. The intrapetrous portion begins at the point at which V3 crosses over the artery and extends to its entrance into the carotid canal in the infratemporal fossa. This segment is designated as C6.
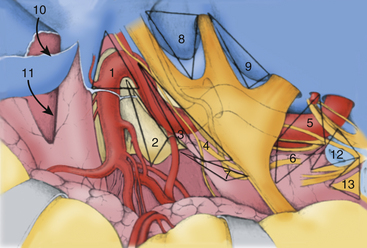
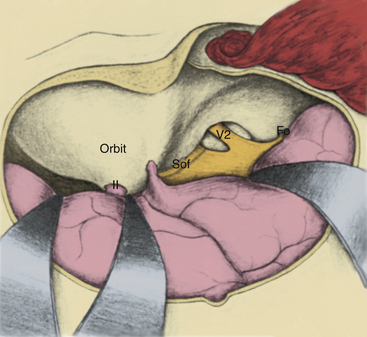
Crucial to the surgeon’s understanding of the relevant surgical anatomy of the cavernous sinus is a thorough working knowledge of the multiple triangular entry corridors into the region. The various entry points have been described by various authors and were brought together into a unified geometric construct of the region in 1986 by Fukushima.7 This scheme is illustrated in Fig. 38-2. Surgical facility with cavernous sinus lesions requires intimate knowledge of the entry spaces into the cavernous sinus in order to minimize morbidity. The anatomy as encountered from the endonasal endoscopic approaches is an important additional consideration. Understanding of the cavernous sinus from this additional perspective is an absolute prerequisite to such procedures.
Anterior Triangle
The anterior triangle describes an epidural space that contains the C3 portion of the ICA. It is exposed by removal of the anterior clinoid process, either intradurally or extradurally. The boundaries of the triangle are the extradural optic nerve, the fibrous dural ring, and the medial wall of the superior orbital fissure.4–637 The C3 carotid segment enters this space by piercing the carotico-oculomotor membrane. It is important to bear in mind the proximity of the oculomotor nerve, which runs in the medial wall of the superior orbital fissure, thus in apposition to the lateral boundary of this space.
Medial Triangle
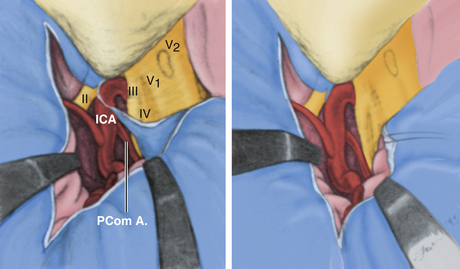
The medial triangle is delimited by the intradural carotid artery, the posterior clinoid process, the porus oculomotorius, and the siphon angle of the carotid artery.9 This space is the primary corridor of access to the C4 portion of the carotid and thus is used for the direct approach to most intracavernous aneurysms. This space is also critical in terms of exposure for most intracavernous tumors.
Superior Triangle
The medial and lateral boundaries of the superior triangle are cranial nerves III and IV, respectively.7 The posterior margin is the edge of the dura along the petrous ridge. This triangle is the entry corridor used to locate the meningohypophyseal trunk.
Lateral Triangle
Described by Parkinson in 1965, the lateral triangle is a very narrow space that is delimited by the trochlear nerve medially and by the ophthalmic division of the trigeminal nerve laterally.1 Again, the dura of the petrous ridge forms the posterior margin. This triangle can be opened to expose cranial nerve VI as it crosses the C5 segment of the carotid artery.
Posterolateral Triangle
The posterolateral triangle, first described by Glasscock in 1968, describes the location of the horizontal intrapetrous carotid artery.38 Exposure of the artery in this space is a critical maneuver in gaining proximal control of the carotid artery. The foramen ovale, foramen spinosum, posterior border of the mandibular division of the trigeminal nerve, and cochlear apex define this space. Removal of the bone of this triangle exposes approximately 10 mm of the C6 segment of the carotid artery.39,40
Posteromedial Triangle
The posteromedial triangle describes the anterior petrous projection of a volume of bone that can be removed to make a window in the petrous apex to the posterior fossa. First described by Kawase and colleagues, this space is delimited by the cochlea, the porus trigeminus, and the posterior border of V3 at the posterior apex of the posterolateral triangle.41,42 If this triangle is used to make a window in the petrous bone, the anterior brain stem and root of the trigeminal nerve can be reached without encountering neural or vascular structures in the bone.
Anterolateral Triangle
The anterolateral triangle is defined by the area between the first and second divisions of the trigeminal nerve as they exit the middle cranial fossa. The anterior border of the triangle is an imaginary line drawn from the lateral edge of the superior orbital fissure to the medial lip of the foramen rotundum. This space is the entry point for exposure of the superior orbital vein and cranial nerve VI and is used to gain access to carotid-cavernous fistulas.43
Lateralmost Triangle (Lateral Loop)
Analogous to the anterolateral triangle, this space is bounded by V2, V3, and an imaginary line from the foramen rotundum to the foramen ovale. Lateral extensions of cavernous sinus tumors are also reached through this route. In some patients, the sphenoidal emissary foramen and vein are found here, communicating the cavernous sinus with the pterygoid venous plexus.29
Premeatal Triangle
The premeatal triangle is used to help define the location of the cochlea from the middle fossa angle of view. The boundaries are the medial lip of the internal acoustic meatus, the intrapetrous carotid genu, and the geniculate ganglion. The cochlea is located in the basal portion of this triangle. This triangle is important in cases in which the petrous apex is removed through the extradural middle fossa approach.39,40
Postmeatal Triangle
The postmeatal triangle delimits the volume of bone located between the internal auditory canal (IAC) and the superior semicircular canal and is used to maximize bone removal of the petrous apex through an extradural middle fossa approach.39 The boundaries are the geniculate ganglion, the lateral lip of the internal acoustic meatus, and the posterior end of the arcuate eminence.
Anesthetic and Monitoring Techniques
The ability of modern neuroanesthesia to facilitate operative procedures by providing increased relaxation of neural tissue and pharmacologic protection against ischemia has realized great improvements over the past forty years. Several maneuvers are used in transcranial cases to help maximize exposure while minimizing retraction of the brain. Administration of osmotic diuretic agents is routine at the beginning of each surgery. We infuse 20% mannitol solution (0.5 mg/kg) along with furosemide (20–40 mg) at the time of skin incision. Further relaxation is attained by maintenance of end-tidal carbon dioxide in the range of 25 to 30 mm Hg. In some cases, these maneuvers alone may not be adequate to provide adequate relaxation, necessitating the use of cerebrospinal fluid (CSF) drainage. This procedure is performed either through a ventricular catheter or a lumbar drain. We rarely use lumbar drainage of CSF in our transcranial cases, mainly because of personal preference. Patients with significant elevation of intracranial pressure are not well served by insertion of a lumbar drain at the beginning of the operation. If necessary, the safest and least complicated method is insertion of a catheter into the frontal horn of the lateral ventricle; this provides ample and accurate drainage of CSF throughout the operation. Administration of osmotic diuretic agents is not on the whole necessary in endonasal endoscopic trans-sphenoidal approaches to the cavernous sinus. In some cases, the pressure gradient between the intracranial compartment and the sphenoid sinus works to the advantage of the surgeon in terms of delivery of tumor into the field for removal. In general, lumbar drains are inserted in these cases when diversion of cerebrospinal fluid is necessary for help in closure of dural defects.
Neurophysiologic monitoring is routinely used in all cases. The specific configuration is tailored to each case, taking into consideration the operative approach and the neural and vascular structures likely to be compromised. Somatosensory-evoked potentials and electroencephalographic data are always recorded when there is a potential for temporary occlusion of the carotid artery. When the operative approach involves exposure of any part of the facial nerve, facial nerve monitoring is employed.44 Visual and brain-stem auditory-evoked potentials have not found much application in our cases of tumors involving primarily the cavernous sinus. But certainly in cases of posterior fossa skull base tumors with cavernous sinus extension, facial nerve monitoring is mandatory.
Surgical Approaches
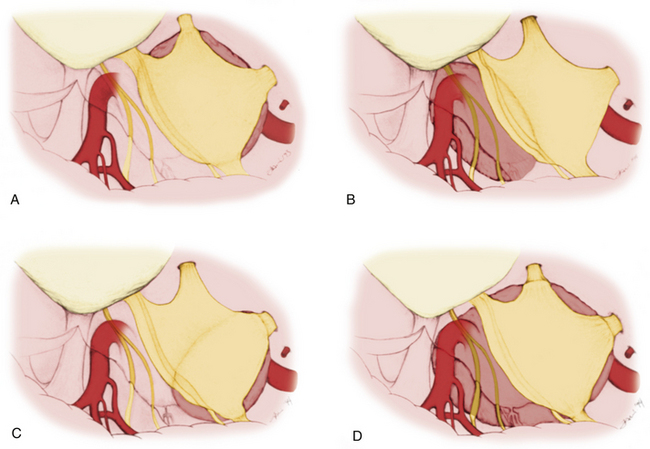
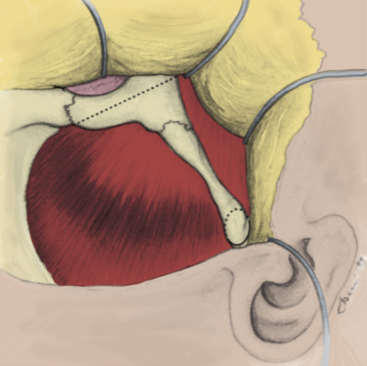
Mainly the specific entry corridors to the cavernous sinus expected to be used to resect the lesion dictate surgical strategy. The cavernous sinus can be divided into four separate quadrants. Lesions involving the anteromedial region are approached via the anteromedial and anterolateral triangles. Because these two triangles are exposed extradurally, in selected cases (e.g., neurinoma of V2), opening the dura might not be necessary in resecting such a lesion. This concept similarly applies to lesions located in the anterolateral quadrant, approached via the lateral loop and posterolateral triangles (Fig. 38-3A). This location of tumor also invites consideration of an endonasal endoscopic extended trans-sphenoidal approach. More posterior lesions, involving the posteromedial and posterolateral regions of the cavernous sinus, usually require exposure through the medial, superior, and lateral triangles (see Fig. 38-3B). These triangles, although possible to open through an extradural route, are typically entered intradurally. Posteromedial lesions without extension lateral to the cavernous carotid artery may also be considered for an endonasal endoscopic approach. Masses confined mainly to the posterolateral quadrant of the region are best approached in our opinion laterally through the middle fossa (see Fig. 38-3C). Lesions involving more than one of these four areas, for example, a mass with extensive posterior cavernous involvement with extension into the posterior fossa, may require a combined approach for adequate exposure (see Fig. 38-3D). This type of lesion requires a combined strategy via an anterolateral and middle fossa transpetrosal approach. Many lesions require more than one of the standard approaches for satisfactory exposure, and the experience and judgment of the surgeon are necessary to adequately plan the procedure. We outline the standard approaches to intracavernous neoplasms used in our practice and discuss the general indications for their use.
Frontotemporal Epidural and Subdural Approach to the Cavernous Sinus
Dolenc is credited with the initial development and use of the combined epidural and subdural frontotemporal approach (anteromedial transcavernous approach), originally used to directly approach intracavernous aneurysms.4,6 This technique has become the standard by which lesions within the cavernous sinus are approached. This strategy effectively exposes lesions confined to the cavernous sinus and those with extension to the supratentorial compartment. Lesions with extension into the petroclival area and the posterior fossa are not well exposed by this approach. The method is, however, easily combined with a more lateral approach (e.g., middle fossa transpetrosal) to gain access to such posterior extensions of tumor. Dolenc’s combined epidural and subdural strategy has been modified in several ways.37,47–49 These modifications largely center around the bone flap used and the extent of extradural bone removal at the skull base. The following discussion presents these modifications as alternatives to the basic approach; the modifications are selected on the basis of the exposure expected to be necessary.
Incision and Flap Elevation
We use three different methods of initial scalp incision and elevation, the choice of which depends mainly on the amount of inferior-to-superior exposure desired. Also, three different methods of craniotomy are used, again depending on the degree of inferior-to-superior exposure necessary.
Two-Layer Technique
The two-layer technique is used when increased inferior-to-superior trajectory is necessary, because this technique results in reflection of the temporalis muscle inferiorly and laterally. This method rotates the muscle away from the orbital rim and frontozygomatic recess, thus preventing the muscle mass from creating an obstruction when the microscope is radically rotated to obtain a more rostral view. The skin incision is typically started slightly more inferiorly, exposing the entire zygomatic root. Beginning medially, the galeal layer is elevated from the pericranium, and the areolar bands, which span the two layers, are sharply divided. Again, the supraorbital and supratrochlear nerves must be preserved with the galeal layer. As the superior temporal line is reached, the areolar connective tissue that is continuous with the pericranial layer is elevated with the galea, which exposes bare temporalis fascia. The critical step in this maneuver is handling the temporal fat pad. This fat pad consists of superficial and deep components. The superficial fat pad is surrounded by the loose areolar connective tissue overlying the temporalis fascia and contains the frontalis branches of the facial nerve. The fat pad is elevated with the areolar tissue and the galeal layer. The galeal layer is elevated to expose the supraorbital rim, lateral orbital rim, and entire zygomatic process, which is covered by fascia. The deep fat pad is situated over the inferior portion of the temporalis muscle as it passes under the zygomatic arch and is covered by fascia. This pad of fat is left in place and retracted with the muscle (Fig. 38-4).
Craniotomy and Extradural Bone Removal
Transzygomatic Craniotomy
This craniotomy method is infrequently employed. It is probably best suited for lesions with a large middle fossa extension where expanded access to the middle fossa is necessary. After the galeal layer is reflected by use of the two-layer technique, the periosteum of the zygomatic process and the lateral orbital rim is incised and elevated. We make this incision in such a way that leaves a cuff of tissue for later reapproximation. The temporalis muscle is freed from the temporal squama and of its attachment to the inner surface of the zygoma. The zygoma is now cut with a sagittal or reciprocating saw (Fig. 38-5). The anterior cut is made parallel to the lateral orbital rim, beginning at the frontozygomatic suture, leaving as little bone overhanging the frontozygomatic recess as possible. The posterior cut is made roughly parallel to the surface of the temporal squama through the root of the temporal zygomatic process, and care is taken to avoid invasion of the temporomandibular joint. This technique results in what we call a “T-bone” cut and maximizes inferior temporalis muscle retraction, resulting in an increased ability to gain an inferior-to-superior view. A frontotemporal craniotomy is now made as described earlier. The remaining temporal squama is removed to obtain a flat viewing angle along the middle cranial fossa floor.
Orbitozygomatic Craniotomy
Two burr holes are then made, one in the keyhole area and the second about 5 cm posteriorly, inferior to the superior temporal line. Next, the thick bore that connects the anterior temporal base to the orbital wall is drilled away. Then, a cut is made with the craniotome beginning at the posterior burr hole, proceeding inferiorly toward the middle fossa floor, and then curving upward over the temporal line to meet the pterional burr hole (Fig. 38-6). A sagittal or reciprocating saw is now used to cut the zygoma root parallel to the squamosal surface, as described earlier (Fig. 38-7). The next cut is made roughly parallel to, and several millimeters above, the zygomaticomaxillary suture, cutting into the lateral wall of the orbit. The medial supraorbital rim is cut and continued posteriorly several millimeters into the orbital roof. The orbital wall is next incised, either with the sagittal saw or a small osteotome, from medial to lateral, thus freeing the supraorbital and lateral orbital rims (Fig. 38-8). The final cut made is through the articulation of the zygomatic and sphenoid bones from posterior. These bone incisions free the flap as a single unit. Sometimes the flap needs to be freed from some remaining attachment of the sphenoid wing; this is easily accomplished via fracturing of that remaining attachment.
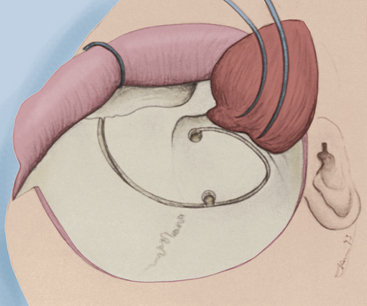
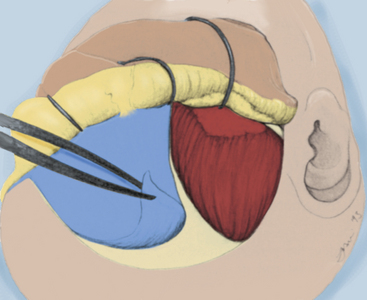
FIGURE 38-6 The initial craniotome osteotomy is illustrated, extending through the medial orbital rim.
Extradural Bone Removal
Extradural removal of bone at the cranial base provides several advantages. Primarily, reduction of cranial base bone volume reduces the degree of necessary retraction of neural structures. Second, removal of bone surrounding neural structures as they pass through bone canals results in mobility of these structures without impingement against bone surfaces, which may result in pressure-induced ischemia. Third, transposition of neural and vascular structures from their bone canals results in wider corridors of access. In this chapter, we describe the technique for maximal removal of the anterolateral base; however, the extent of removal of the cranial base is individualized for each case. Risk is associated with every degree of bone removal at the skull base, and for this reason, determination of the exposure for each particular case is an important step in surgical planning.
Next, the full anterolateral cranial base is skeletonized, and the neural structures become capable of being mobilized, after being freed from the constraints of their respective bone foramina (Fig. 38-9). Hemostasis is attained with the use of bone wax and monopolar cautery. Monopolar cautery should be used only in areas that do not have underlying sensitive structures. A typical example is in the middle fossa in the region of the tegmen tympani. Heat transfer through bone here can damage cranial nerve VII or the hearing apparatus.
When extradural exposure of the intrapetrous carotid artery is desired, it is appropriately exposed in the posterolateral triangle (Fig. 38-9). The dura must be elevated from the middle cranial fossa to expose the greater superficial petrosal nerve running in the major petrosal groove. The middle meningeal artery must be coagulated and divided as it exits the foramen spinosum. This vessel is usually surrounded by a plexus of veins, which must be effectively coagulated. With the greater superficial petrosal nerve exposed, the landmarks delineating the position of the carotid artery are apparent because the artery lies under the nerve and is running parallel, toward V3. Drilling is begun posterior to V3, just medial to the foramen spinosum. The greater superficial petrosal nerve is typically divided near V3 and reflected posteriorly for greater exposure of the ICA. Bone over the artery is removed from the tensor tympani muscle lateral to bone that lies under V3. The greatest danger in this procedure is violation of the cochlea, which lies 1 to 2 mm from the carotid genu. Excessive bone removal posterior to the carotid genu carries significant risk for cochlear violation.
Intradural Transcavernous Dissection
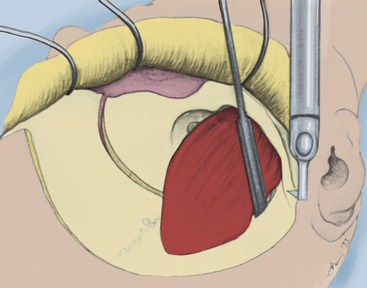
Neoplastic lesions that escape the bounds of the cavernous sinus typically require intradural exposure of adjacent regions. In these cases, the cavernous sinus may be opened through the medial, superior, and lateral triangles via an intradural dissection. This intradural approach to the cavernous sinus begins with opening of the dura using a T-shaped incision. The incision starts at the anterior frontal corner of the exposure and curves downward, close to the posterior bone margin, toward the anterior temporal corner. A cut is then made along the dura that covers the sylvian fissure and proceeds toward the optic nerve dura, completing the T. The dural flaps are retracted forward and tacked down with fine suture.
Anterolateral Temporopolar Transcavernous Approach
This approach provides access to the cavernous sinus from a more lateral trajectory than the standard frontotemporal method.47 The technique makes use of extradural retraction of the frontal and temporal lobes, both to protect the cortical surface and to preserve the venous drainage of the temporal tip. The extensive extradural dissection provides a very wide corridor of access to the cavernous sinus region, as well as wide access to the infrachiasmatic and upper clival areas.
Beginning at the superior orbital fissure apex, the meningo-orbital fibrous band is coagulated and divided. The temporal dura is retracted posteriorly. Elevation of the dural margin begins at the superior orbital fissure and extends laterally to the foramen ovale. At the junction of the periorbital fascia and dura, the cleavage plane is sharply developed, and the connective tissue fibrils bridging the dura and the outer cavernous membrane are divided, as the dura is retracted posteriorly. In this way, the dura is reflected from the outer cavernous membrane toward the petrous ridge (see Fig. 38-10). If this maneuver is performed properly, little bleeding occurs from the cavernous sinus. Cavernous sinus bleeding from small tears in the outer cavernous membrane is stopped by packing small pieces of Surgicel into the openings. The anteromedial limit in dural elevation is the tentorial edge, which is handled after the dura is opened.
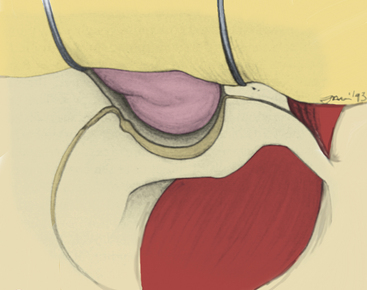
The dura is now ready to be opened. An L-shaped incision is made beginning along the dura covering the sylvian fissure, approximately 5 cm from its attachment to the carotid artery. The incision is extended through optic nerve sheath dura and is then carried medially across the tuberculum sellae for 2 to 3 cm (Fig. 38-11). The retractors are replaced to provide posterior retraction on both the frontal and temporal lobes, and the fibrous dural ring surrounding the carotid artery is sharply freed from the vessel. The lateral portion of this fibrous ring is met by the tentorial edge, formed by a fold in the dura. The two layers composing this fold are then split. This maneuver elevates the temporal dura from the outer cavernous membrane over the medial triangle and effectively frees the medial margin of temporal dura, resulting in full lateral and posterior retraction. Some arachnoidal dissection around the porus oculomotorius is typically a prerequisite to this move. At this juncture, the structures of the lateral wall of the cavernous sinus should be plainly visible through the thin veil of the outer cavernous membrane, from the sella to the trigeminal third division. The medial, superior, and lateral triangles are well delineated (Fig. 38-12).
The sylvian fissure is usually split to decrease the required retractor pressure, even though the degree of frontal and temporal lobe retraction is somewhat lessened with this approach. No more than the anterior 1 to 2 cm of the fissure need be split in most cases. During the dissection, it will be clear that the temporal tip bridging veins need not be sacrificed with this approach because the temporal dura is retracted with the temporal lobe, obviating sacrifice of these vessels. The arachnoid is opened over the optic nerve and chiasm, as well as the carotid artery to expose the A1 and M1 segments (Fig. 38-13). The medial and anterolateral portions of the cavernous sinus are exposed at this point of the dissection, and tumor resection may proceed.
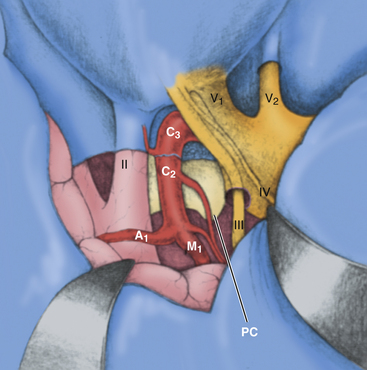
FIGURE 38-13 The final exposure of the anteromedial and posteromedial cavernous sinus via the temporopolar approach.
Lateral Approach to the Posterior Cavernous Sinus Region (Anterior Transpetrosal Approach)
Although the posterior cavernous sinus region may be reached strictly through an anterior trajectory, the exposure is narrow, and cranial nerve V is an obstacle to adequate vision. This narrow corridor provides limited access to the posteroinferior triangle and region surrounding the porus trigeminus. For this reason, a more lateral and posterior approach is indicated, either subtemporal or transpetrosal, that provides a wider operative corridor and access inferolateral to the trigeminal complex. The extradural middle fossa anterior transpetrosal approach provides such exposure through a subtemporal route and is easily combined with the frontotemporal approach.39–42,50,51 When bone removal through this approach is maximized, near-total resection of the petrous apex results.39
Avoidance of complications from this technique depends mainly on an intimate knowledge of the internal anatomy of the petrous bone and the relationships between internal structures and surface landmarks. The major potential complications of the procedure are hearing loss resulting from cochlear or bone labyrinth violation and compromise of facial nerve integrity and function. In an attempt to simplify the technique, we have devised a geometric construct of key middle fossa landmarks that delineates the volume of bone to be resected. This construct helps to locate, and thus avoid, internal structures of the petrous pyramid.39
The approach is performed with the head in the 90-degree lateral position, through a 4-cm by 4-cm temporal craniotomy centered two thirds anterior and one third posterior over the external auditory meatus. When performed in combination with a frontotemporal approach, a more generous temporal extension of the craniotomy must be made that reaches posterior to the root of the zygomatic process. Middle fossa dural elevation begins posteriorly and laterally, over the petrous ridge, and continues anteromedially to the foramen ovale. Dura is elevated in this manner to avoid traction on the greater superficial petrosal nerve (GSPN), which may result in facial nerve compromise. The GSPN lies in the major petrosal groove of the middle fossa floor and is covered by a thin layer of periosteum. The middle meningeal artery and surrounding venous plexus are coagulated and divided near the artery’s exit from the foramen spinosum. The dura is then separated from the trigeminal complex at the foramen ovale, continuing posteriorly toward the porus trigeminus. Tapered retractors are used to retract the dura medially and posteriorly to expose the entire middle fossa floor (Fig. 38-14).
Bony resection begins with the exposure of the IAC. By use of a high-speed diamond drill, the IAC is found 3 to 4 mm deep to the middle fossa floor along the bisection axis between lines projected along the GSPN and the arcuate eminence. The entire length of the IAC is exposed. Next, the GSPN is uncovered lateral to the facial hiatus, until the geniculate ganglion is exposed. A thin shell of bone is left over the ganglion for protection. The GSPN is preserved if possible. In some cases, however, it must be sectioned and reflected lateral for exposure needs. The carotid is exposed in the posterolateral triangle from V3 to the tensor tympani muscle. Then, the bone between the IAC and carotid can be safely removed to expose posterior fossa dura (Fig. 38-15). Great care must be taken to avoid the cochlea during this portion of bone dissection.
The cochlea is located in the base of the premeatal triangle, which is defined by the carotid genu, the geniculate ganglion, and the medial lip of the internal auditory meatus.39 Resection of apical petrous bone is continued inferiorly to the level of the inferior petrosal sinus. The apical bone inferior to the trigeminal ganglion can be removed by coring out of the petrous apex. The wedge of bone remaining that lies lateral to the IAC can be removed, to result in an almost 270-degree exposure of the IAC. This lateral wedge of bone is defined by the postmeatal triangle, which is bounded by the geniculate ganglion, the arcuate eminence, and the lateral lip of the internal auditory meatus. It is often helpful to blue-line the superior semicircular canal during removal of this bone to avoid entering the vestibule.
With the bone dissection complete, the posterior and inferolateral cavernous sinus can be widely reached. The limits of this exposure are the foramen of Dorello medially and the inferior petrosal sinus below. The trigeminal complex can be completely freed of dura and then elevated or retracted medially. This exposure provides visualization of the C5 and C6 portions of the carotid artery. A prerequisite to this maneuver for increasing the exposure of the posterior cavernous sinus is extradural liberation of the V2 and V3 divisions in their respective bone canals. Elevation of the trigeminal complex in this way allows full exposure of the posterior cavernous sinus and complete petrous apex resection extradurally under direct vision.40
The dura is opened at the porus trigeminus above the superior petrosal sinus. This incision is carried laterally as far as the arcuate eminence and exposes the superior surface of the tentorium. A parallel incision is then made inferior to the superior petrosal sinus. The superior petrosal sinus is ligated and divided at its medial aspect. The retractors can then be placed on the undersurface of the tentorium, and the temporal lobe is retracted more superiorly under its protection. The trigeminal root is also liberated from its dural attachment at the porus trigeminus. Mobilizing the trigeminal complex medially and superiorly provides a corridor to the posterior cavernous sinus and the entrance of cranial nerve VI into Dorello’s canal. To expose the intracavernous carotid artery, it is necessary to open the outer cavernous membrane between the trigeminal ganglion and the posterolateral fibrous ring surrounding the carotid’s entrance to the cavernous sinus at the foramen lacerum. In this way, the intracavernous carotid can be exposed to the crossing point of cranial nerve VI. This strategy provides full access to the posterolateral quadrant of the cavernous sinus, and tumor resection may proceed. The main venous connections of the posterolateral cavernous sinus are to the pterygoid venous plexus via the sphenoidal emissary, the inferior petrosal sinus, and the basilar venous plexus. Effective hemostasis is obtained via packing oxidized cellulose in the direction of these venous connections.
Techniques of Hemostasis
As the foramina of the cranial nerves are approached, the technical strategy changes. Heat and current from the monopolar cautery can spread for several millimeters through bone and can damage the nerves. Bone bleeding around neural foramina is controlled more with bone wax than with cautery or the diamond drill. Bleeding from the cavernous sinus also occurs via tiny rents in the cavernous membrane when the dura is separated from the margins of the foramina. This bleeding can be controlled by packing tiny pieces of Surgicel into the open cavernous membrane and covering for 1 or 2 minutes with a small Cottonoid. Coagulating the Surgicel for 1 to 2 seconds with the bipolar after it is packed in the hole is sometimes helpful. Bleeding can occur from a tear in the cavernous membrane during the final stages of removal of the anterior clinoid process. Although possible to remove without bleeding, more frequently, the carotico-oculomotor membrane develops a small tear that can bleed profusely. A small piece of Surgicel suffices when packed into the opening and covered with a Cottonoid for 1 to 2 minutes. An important principle in hemostatic technique in the cavernous sinus is patience. Much is gained by packing an area, moving to another area to work, then coming back later to the original bleeding site.
Closure Techniques
Skull Base Carotid Bypass Procedures
Invasive tumors often require sacrifice of the ICA if they are to be completely removed. Balloon test occlusion is performed before surgery to determine tolerance to occlusion of the involved ICA.41 Patients who tolerate occlusion without incident may be treated without bypass, thus avoiding the potential major complications of thromboembolic sequelae and anticoagulation associated with these procedures. In cases in which occlusion is not tolerated, the carotid flow can be preserved by performing a bypass procedure.8,28,30,53,54 This procedure was first performed in 1986 by Fukushima to treat a giant intracavernous aneurysm.7,8,55 Over time, the procedure has evolved to include three variations. The most common form is a bypass between the C3 and C6 segments of the carotid. The second important variation is an anastomosis from the high cervical portion of the ICA to the C3 segment.8,56
The C3-to-C6 bypass procedure requires exposure of those two segments in sufficient length to perform the anastomosis procedure. The C3 portion is exposed in the anterior triangle through removal of the anterior clinoid process and detachment of the fibrous dural ring. The C6 segment is exposed in the posterolateral triangle.57 It is usually helpful to free the dura from the posterior trigeminal complex, as discussed earlier, to gain several additional millimeters of exposure. Clips can then be placed to trap the intracavernous carotid segments. A saphenous vein graft is harvested from the upper thigh and prepared for anastomosis. Any loose adventitia is stripped from the vein, and tributaries are ligated with fine suture. The graft is flushed with heparinized saline solution, and proper orientation is maintained by marking either end of the graft. The distal anastomosis is then performed, either end to end or end to side, with 8-0, 9-0, or 10-0 suture. Suture selection depends on the wall thickness of both the graft and the carotid artery. Control of carotid flow is maintained either by a temporary clip near the genu or by exposure in the neck. The proximal anastomosis is performed between the origins of the ophthalmic artery and the posterior communicating artery. In some cases, the anastomosis may be performed in an end-to-end fashion if the tumor resection leaves a carotid stump including the ophthalmic origin or the ophthalmic artery is taken with tumor resection in the orbit. The anastomosis is performed under electroencephalographic burst suppression induced by barbiturates. Patients are given low-dose heparin therapy for several days postoperatively, and they are then given aspirin or warfarin (Coumadin) for approximately 3 months.
Al-Mefty O., Smith R.R. Surgery of tumors invading the cavernous sinus. Surg Neurol. 1988;30:370-381.
Ceylan S., Koc K., Anik I. Endoscopic endonasal transsphenoidal approach for pituitary adenomas invading the cavernous sinus. J Neurosurg. 2010;112:99-107.
Day J.D. Surgical approaches to suprasellar and parasellar tumors. Neurosurg Clin North Am. 2003;54(2):391-395.
Day J.D. Cranial base surgical techniques for large sphenocavernous meningiomas: technical note. Neurosurgery. 2000;46(3):754-759.
Dolenc V.V., Kregar R., Ferluga M., et al. Treatment of tumors invading the cavernous sinus. In: Dolenc V.V., editor. The Cavernous Sinus: Multidisciplinary Approach to Vascular and Tumorous Lesions. Wien: Springer-Verlag; 1987:377-391.
Duma C.M., Lunsford L.D., Kondziolka D., et al. Stereotactic radiosurgery of cavernous sinus meningiomas as an addition or alternative to microsurgery. Neurosurgery. 1993;32:699-705.
Fraser J.F., Mass A.Y., Brown S., et al. Transnasal endoscopic resection of a cavernous sinus hemangioma: technical note and review of the literature. Skull Base. 2008;18:309-315.
Fukushima T., Day J.D., Tung H. Intracavernous carotid artery aneurysms. In: Apuzzo M.L.J., editor. Brain Surgery: complication Avoidance and Management. New York: Churchill Livingstone; 1993:925-944.
Glasscock M.E. Exposure of the intra-petrous portion of the carotid artery. In: Hamberger C.A., Wersall J. Disorders of the Skull Base Region: Proceedings of the Tenth Nobel Symposium, Stockholm, 1968. Stockholm: Almqvist & Wicksell; 1969:135-143.
Hakuba A., Suzuki T., Jin T.B., Komiyama M. Surgical approaches to the cavernous sinus: report of 52 cases. In: Dolenc V.V., editor. The Cavernous Sinus. Wien: Springer-Verlag; 1987:302-327.
Harris F.S., Rhoton A.L. Anatomy of the cavernous sinus: a microsurgical study. J Neurosurg. 1976;44:169-180.
House W.F., Hitselberger W.E., Horn K.L. The middle fossa transpetrous approach to the anterior-superior cerebellopontine angle. Am J Otol. 1986;7:1-4.
Kassam A.B., Gardner P., Snyderman C., et al. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus. 2005;19(1):E6.
Kawase T., Toya S., Shiobara R., Mine T. Transpetrosal approach for aneurysms of the lower basilar artery. J Neurosurg. 1985;63:857-861.
Kitano M., Taneda M., Shimono T., Nakao Y. Extended transsphenoidal approach for surgical management of pituitary adenomas invading the cavernous sinus. J Neurosurg. 2008;108:26-36.
Perneczky A., Knosp E., Czech T. Para- and infraclinoidal aneurysms. Anatomy, surgical technique and report on 22 cases. In: Dolenc V.V., editor. The Cavernous Sinus. Wien: Springer-Verlag; 1987:252-271.
Pichierri A., Santoro A., Raco A., et al. Cavernous sinus meningiomas: retrospective analysis and proposal of a treatment algorithm. Neurosurgery. 2009;64:1090-1101.
Sekhar L.N., Ross D.A., Sen C. Cavernous sinus and sphenocavernous neoplasms. In: Sekhar L.N., Janecka I.P. Surgery of Cranial Base Tumors. New York: Raven Press; 1993:521-604.
Spetzler R.F., FukushimaT Martin N., Zabramski J.M. Petrous carotidto-intradural carotid saphenous vein graft for intracavernous giant aneurysm, tumor, and occlusive cerebrovascular disease. J Neurosurg. 1990;73:496-501.
Umansky F., Elidan J., Valarezo A. Dorello’s canal: a microanatomical study. J Neurosurg. 1991;75:294-298.
Walsh M.T., Couldwell W.T. Management options for cavernous sinus meningiomas. J Neurooncol. 2009;92:307-316.
Zada G., Day J.D., Giannotta S.L. The extradural temporopolar approach: a review of indications and operative technique. Neurosurg Focus. 2008;25(6):E3.
1. Parkinson D. A surgical approach to the cavernous portion of the carotid artery: anatomical studies and case report. J Neurosurg. 1965;23:474-483.
2. Al-Mefty O., Smith R.R. Surgery of tumors invading the cavernous sinus. Surg Neurol. 1988;30:370-381.
3. Cioffi F.A., Bernine F.P., Punzo A., et al. Cavernous sinus meningiomas. Neurochirurgia (Stuttg). 1987;30:40-47.
4. Dolenc V. A combined epi- and subdural direct approach to carotid-ophthalmic artery aneurysms. J Neurosurg. 1985;62:667-672.
5. Dolenc V.V., Kregar R., Ferluga M., et al. Treatment of tumors invading the cavernous sinus. In: Dolenc V.V., editor. The Cavernous Sinus: Multidisciplinary Approach to Vascular and Tumorous Lesions. Wien: Springer-Verlag; 1987:377-391.
6. Dolenc V.V. Cavernous sinus masses. In: Apuzzo M.L.J., editor. Brain Surgery: Complication Avoidance and Management. New York: Churchill Livingstone; 1993:601-614.
7. Fukushima T. Direct operative approach to the vascular lesions in the cavernous sinus: summary of 27 cases. Mt Fuji Workshop Cerebrovasc Dis. 1988;6:169-189.
8. Fukushima T., Day J.D., Tung H. Intracavernous carotid artery aneurysms. In: Apuzzo M.L.J., editor. Brain Surgery: complication Avoidance and Management. New York: Churchill Livingstone; 1993:925-944.
9. Hakuba A., Matsuoka Y., Suzuki T., et al. Direct approaches to vascular lesions in the cavernous sinus via the medial triangle. In: Dolenc V.V., editor. The Cavernous Sinus. Wien: Springer-Verlag; 1987:272-284.
10. Hakuba A., Suzuki T., Jin T.B., Komiyama M. Surgical approaches to the cavernous sinus: report of 52 cases. In: Dolenc V.V., editor. The Cavernous Sinus. Wien: Springer-Verlag; 1987:302-327.
11. Lesoin F., Jomin M., Boucez B., et al. Management of cavernous sinus meningiomas: report of twelve cases and review of the literature. Neurochirurgia (Stuttg). 1985;28:195-198.
12. Lesoin F., Jomin M. Direct microsurgical approach to intracavernous tumors. Surg Neurol. 1987;28:17-22.
13. Sekhar L.N., Ross D.A., Sen C. Cavernous sinus and sphenocavernous neoplasms. In: Sekhar L.N., Janecka I.P. Surgery of Cranial Base Tumors. New York: Raven Press; 1993:521-604.
14. Sephernia A., Samii M., Tatgiba M. Management of intracavernous tumours: an 11-year experience. Acta Neurochir Suppl (Wien). 1991;53:122-126.
15. Zada G., Day J.D., Giannotta S.L. The extradural temporopolar approach: a review of indications and operative technique. Neurosurg Focus. 2008;25(6):E3.
16. Day J.D. Surgical approaches to suprasellar and parasellar tumors. Neurosurg Clin North Am. 2003;54(2):391-395.
17. Day J.D. Cranial base surgical techniques for large sphenocavernous meningiomas: technical note. Neurosurgery. 2000;46(3):754-759.
18. Day J.D., Fukushima T. The surgical management of trigeminal neuromas. Neurosurgery. 1998;42(2):233-240.
19. Pichierri A., Santoro A., Raco A., et al. Cavernous sinus meningiomas: retrospective analysis and proposal of a treatment algorithm. Neurosurgery. 2009;64:1090-1101.
20. Walsh M.T., Couldwell W.T. Management options for cavernous sinus meningiomas. J Neurooncol. 2009;92:307-316.
21. Fraser J.F., Mass A.Y., Brown S., et al. Transnasal endoscopic resection of a cavernous sinus hemangioma: technical note and review of the literature. Skull Base. 2008;18:309-315.
22. Ceylan S., Koc K., Anik I. Endoscopic endonasal transsphenoidal approach for pituitary adenomas invading the cavernous sinus. J Neurosurg. 2010;112:99-107.
23. Kitano M., Taneda M., Shimono T., Nakao Y. Extended transsphenoidal approach for surgical management of pituitary adenomas invading the cavernous sinus. J Neurosurg. 2008;108:26-36.
24. Kassam A.B., Gardner P., Snyderman C., et al. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus. 2005;19(1):E6.
25. Barbaro N.M., Gutin P.H., Wilson C.B., et al. Radiation therapy in the treatment of partially resected meningiomas. Neurosurgery. 1987;20:525-528.
26. Carella R.J., Ransohoff J., Newall J. Role of radiation therapy in the management of meningioma. Neurosurgery. 1982;10:332-339.
27. Duma C.M., Lunsford L.D., Kondziolka D., et al. Stereotactic radiosurgery of cavernous sinus meningiomas as an addition or alternative to microsurgery. Neurosurgery. 1993;32:699-705.
28. Al-Mefty O., Khalil N., Elwany M.N., Smith R.R. Shunt for bypass graft of the cavernous carotid artery: an anatomical and technical study. Neurosurgery. 1990;27:721-728.
29. Harris F.S., Rhoton A.L. Anatomy of the cavernous sinus: a microsurgical study. J Neurosurg. 1976;44:169-180.
30. Sekhar L.N., Burgess J., Akin O. Anatomical study of the cavernous sinus emphasizing operative approaches and related vascular and neural reconstruction. Neurosurgery. 1987;21:806-816.
31. Umansky F., Elidan J., Valarezo A. Dorello’s canal: a microanatomical study. J Neurosurg. 1991;75:294-298.
32. Umansky F., Nathan H. The lateral wall of the cavernous sinus with special reference to the nerves related to it. J Neurosurg. 1982;56:228-234.
33. Day A.L. Aneurysms of the ophthalmic segment. J Neurosurg. 1990;72:677-691.
34. Perneczky A., Knosp E., Borkapic P., Czech T. Direct surgical approach to infraclinoidal aneurysms. Acta Neurochir (Wien). 1983;76:36-44.
35. Perneczky A., Knosp E., Czech T. Para- and infraclinoidal aneurysms. Anatomy, surgical technique and report on 22 cases. In: Dolenc V.V., editor. The Cavernous Sinus. Wien: Springer-Verlag; 1987:252-271.
36. Fischer E. Die Lagabweichrugan der vorderen Hirnarterie in Gefassbild. Zentralb Neurochir. 1938;3:300-312.
37. Dolenc V., Skrap M., Sustersic J., et al. A transcavernous-transsellar approach to the basilar tip aneurysms. Br J Neurosurg. 1987;1:251-259.
38. Glasscock M.E. Exposure of the intra-petrous portion of the carotid artery. In: Hamberger C.A., Wersall J. Disorders of the Skull Base Region: Proceedings of the Tenth Nobel Symposium, Stockholm, 1968. Stockholm: Almqvist & Wicksell; 1969:135-143.
39. Day J.D., Fukushima T., Giannotta S.L. Microanatomical study of the extradural middle fossa approach to the petroclival and posterior cavernous sinus region: description of the rhomboid construct. Neurosurgery. 1994;34:1009-1016.
40. Fukushima T., Day J.D., Harahara K. Extradural total petrous apex resection with trigeminal translocation for improved exposure of the posterior cavernous sinus and petroclival region. Skull Base Surg. 1996;6(2):95-103.
41. Kawase T., Shiobara R., Toya S. Anterior transpetrosal-transtentorial approach for sphenopetroclival meningiomas: surgical method and results in 10 patients. Neurosurgery. 1991;28:869-876.
42. Kawase T., Toya S., Shiobara R., Mine T. Transpetrosal approach for aneurysms of the lower basilar artery. J Neurosurg. 1985;63:857-861.
43. Mullan S. Treatment of carotid-cavernous fistulas by cavernous sinus occlusion. J Neurosurg. 1979;50:131-144.
44. Traynelis V.C., Gantz B.J. Intraoperative facial nerve monitoring. In: Loftus C.M., Traynelis V.C. Intraoperative Monitoring Techniques in Neurosurgery. New York: McGraw-Hill; 1994:157-163.
45. Moller A.R. Monitoring techniques in cavernous sinus surgery. In: Loftus C.M., Traynelis V.C. Intraoperative Monitoring Techniques in Neurosurgery. New York: McGraw-Hill; 1994:141-157.
46. Carter L.P. Continuous monitoring of cortical blood flow. In: Loftus C.M., Traynelis V.C. Intraoperative Monitoring Techniques in Neurosurgery. New York: McGraw-Hill; 1994:53-61.
47. Day JD, Giannotta SL, Fukushima T: Extradural temporopolar approach to lesions of the upper basilar artery and infrachiasmatic region. J Neurosurg in press.
48. Fujitsu K., Kuwabara T. Zygomatic approach for lesions in the interpeduncular cistern. J Neurosurg. 1985;62:340-343.
49. Hakuba A., Tanaka K., Suzuki T., Nishimura S. A combined orbitozy-gomatic infratemporal epidural and subdural approach for lesions involving the entire cavernous sinus. J Neurosurg. 1989;71:699-704.
50. Hitselberger W.E., Horn K.L., Hankinson H., et al. The middle fossa transpetrous approach for petroclival meningiomas. Skull Base Surg. 1993;3:130-135.
51. House W.F., Hitselberger W.E., Horn K.L. The middle fossa transpetrous approach to the anterior-superior cerebellopontine angle. Am J Otol. 1986;7:1-4.
52. Horton J.A., Jungreis C.A., Pistoia F. Balloon test occlusion. In: Sekhar L.N., Janecka I.P. Surgery of Cranial Base Tumors. New York: Raven Press; 1993:33-36.
53. Linskey M.E., Sekhar L.N., Sen C. Cerebral revascularization in cranial base surgery. In: Sekhar L.N., Janecka I.P. Surgery of Cranial Base Tumors. New York: Raven Press; 1993:45-68.
54. Sekhar L.N., Sen C.N., Jho H.D. Saphenous vein bypass of the cavernous internal carotid artery. J Neurosurg. 1990;72:35-41.
55. Spetzler R.F., Fukushima T., Martin N., Zabramski J.M. Petrous carotid-to-intradural carotid saphenous vein graft for intracavernous giant aneurysm, tumor, and occlusive cerebrovascular disease. J Neurosurg. 1990;73:496-501.
56. Miyazaki S., Fukushima T., Fujimaki T. Resection of high-cervical paraganglioma with cervical-to-petrous internal carotid artery saphenous vein bypass: report of two cases. J Neurosurg. 1990;73:141-146.
57. Glasscock M.E.III, Smith P.G., Whitaker S.R., et al. Management of aneurysms of the petrous portion of the internal carotid artery by resection and primary anastomosis. Laryngoscope. 1983;93:1445-1453.