Chapter 111 Tumors at the Foramen Magnum
Regional Challenges
Tumors in the region of the foramen magnum have long challenged surgeons who confront not only the difficulties of diagnosis but also the technical obstacles of a hazardous tumor resection. Historically, determined efforts often ended in respiratory failure and death. In the 1954 series of Love et al., for example, 34 of 74 patients died postoperatively, most often as a result of respiratory failure.1 However, with the advent of MRI and microsurgical technique, and knowledge of the regional anatomy, surgical outcome has greatly improved as reflected by the results of larger, more recent series.2–6
A wide array of tumors, both malignant and benign, arise in the region of the foramen magnum. Collectively they comprise about 5% of all spinal tumors and 1% of intracranial tumors.7 It is useful to divide all foramen magnum tumors into intra-axial, intradural extramedullary, and extradural masses. Each location is associated with a specific group of tumors and special topographic relationships that present unique surgical considerations.
More than 90% of foramen magnum tumors are intradural extramedullary tumors, and most commonly occur ventrolaterally in relation to the spinal cord. The majority of these tumors are meningiomas and neurofibromas, the former being considerably more common than the latter.2,7 Intra-axial tumors (e.g., brainstem gliomas) and extradural tumors (e.g., chordomas) comprise fewer than 10% of foramen magnum tumors.3
The results of surgical management of these tumors have greatly improved over the years, because, with the advent of advanced microsurgical techniques, intraoperative monitoring, and a detailed knowledge of foramen magnum anatomy, these lesions are amenable to safe surgical resection. Given the variable pathologic anatomy in the region of the foramen magnum and the availability of a variety of surgical options with varying advantages and disadvantages, the surgeon must carefully choose an appropriate surgical approach. The rational basis for this selection is outlined in this chapter, with emphasis placed on the problems presented by intradural extramedullary lesions. There has been increasing interest in the radiosurgical treatment of foramen magnum meningiomas.8 Radiosurgery has been employed as a primary tool in patients with comorbidity or advanced age. It has also been used as adjuvant treatment in patients who have incomplete resection or aggressive tumors. In patients with inoperable tumors, conventional chemotherapy and inhibitors of epidermal growth factor receptor (EGFR), farnesyl transferase, cyclooxygenase 2 (COX-2), the protein kinase MEK-1, and the intracellar signaling pathway PI3k/Akt are being investigated.9
History
A foramen magnum tumor was first described by Hallopeau in 187410 in a case report of a 50-year-old woman who presented with spastic upper extremity weakness that progressed to quadriparesis with brainstem signs. The patient eventually died of respiratory failure. Autopsy revealed a foramen magnum tumor, “the size of a small chestnut,” that caused compression of the lateral funiculi of the spinal cord bilaterally.
Although early attempts at surgical removal of foramen magnum tumors were met with disastrous consequences,11,12 Elsberg and Strauss successfully removed a foramen magnum meningioma from a woman, aged 36 years, who presented with Brown-Séquard syndrome.13 Despite several intraoperative episodes of respiratory failure, the patient enjoyed full neurologic recovery postoperatively. The report of this case in 1929 was accompanied by the first systematic evaluation of foramen magnum tumors.
In their classic 1938 treatise, Cushing and Eisenhardt divided foramen magnum tumors into craniospinal and spinocranial tumors on the basis of their predominant anatomic location and associated clinical symptomatology.14 Since that account, several series of patients with foramen magnum tumors have been reported in the literature, with progressive improvement in outcome.1,3–6,15–19
Historically, most lesions have been approached dorsally. However, for ventrally and ventrolaterally located lesions, two other surgical approaches have been employed to minimize retraction of the neural structures. The transoral approach was originally described by Kanavel in 1919 in a report on the transoral removal of a bullet that was lodged between the atlas and the base of the skull.20 Although the technique has been described most commonly as an approach to extradural lesions,21–24 intradural lesions have also been treated this way.25–28 Like the transoral approach, the far lateral approach was originally described to manage other lesions, and in this instance, vertebral and vertebrobasilar artery lesions were managed rather than foramen magnum tumors.29 This approach has since been modified and adopted to deal with tumors in the region of the foramen magnum.30,31
Pathology and Epidemiology
Major features of the largest series of foramen magnum tumors are presented in Table 111-1. Conceptually, tumors in the region of the foramen magnum are best categorized as intra-axial, intradural extramedullary, and extradural tumors, similar to the classification used for spine tumors. Intra-axial tumors are predominantly brainstem gliomas but also include gangliogliomas, anaplastic astrocytomas, ependymomas, and cavernous hemangiomas. Caudal extension of medulloblastomas and hemangioblastomas into the foramen magnum occurs in children and adults, respectively.32 Intradural extramedullary tumors consist mainly of meningiomas and nerve sheath tumors and a much smaller number of epidermoid tumors and paragangliogliomas.32,33
Extradural neoplasms are primarily osteocartilaginous tumors, of which chordoma is, by far, the most common. Chondromas and chondrosarcomas may also arise in this region.32 Occasionally, meningiomas extend extradurally. This type of meningioma is associated with more aggressive pathologic features and clinical course.
This distribution of tumors is reflected in the series by Bruneau and George.3 They reviewed 230 cases of extramedullary intradural and extradural tumors of the foramen magnum (intra-axial tumors were excluded). The intradural tumors that comprised almost 80% of the cases reviewed included meningiomas (60%) and neurofibromas (30%). Fifty percent of extradural tumors were chordomas. The most frequently occurring tumors (in order of decreasing frequency) were meningioma (106 cases), neurofibroma (49 cases), and chordoma (28 cases).
The topography of foramen magnum meningioma is of special interest to surgeons. Three characteristics define the lesion.2,3 First is the compartment of origin. Tumors are divided into intradural (representing the majority of lesions), intra-extradural, and extradural (rare). The intradural lesions are further divided into three groups based on their point of attachment. Ventral tumors are attached to the ventral portion of the foramen magnum (dura, spinal root, or spinal cord) on both sides of the midline; lateral tumors originate between the midline and the dentate ligament; and dorsal tumors have a point of origin dorsal to the dentate ligament.3 Using these strict criteria, George et al. found that among the 106 meningiomas in their series, 56% occurred laterally, 31% ventrally, and 13% dorsally.3,33 Other authors have reported a similar distribution.4–6,16,18 Third, the relationship to the vertebral artery is defined. Tumors arising caudal to the vertebral artery displace the lower cranial nerves upward, tumors arising above the vertebral artery displace the nerves caudally, and tumors spanning the craniocaudal extent of the vertebral artery displace the nerves unpredictably.2,3
The relative rarity of foramen magnum tumors belies their clinical importance. Compared with other CNS neoplasms, foramen magnum tumors occur infrequently: they account for only 5% of all spinal neoplasms and 1% of all intracranial neoplasms. Considering meningiomas alone, those occurring in the region of the foramen magnum account for only 1.2% to 3.2% of meningiomas.6,16,18
Among the large series, the age range of patients with foramen magnum tumors was 2 to 81 years, but the majority of these tumors occur around the fifth decade.1,4–6,16–19,34 The average time between onset of symptoms and diagnosis was 2.5 years. The mean age was 47 years, with a female-to-male ratio of 1.5:1.3 These figures are consistent among authors.4–618 Female predominance of meningiomas in general is also a consistent finding.
Besides neoplasms, other entities can present as foramen magnum lesions and should be considered in the differential diagnosis. Calcium pyrophosphate deposition in the transverse ligament can form a tumor-like mass that compresses the cervicomedullary junction.35 This condition is common in the elderly and rarely becomes symptomatic. It can be diagnosed by CT, which demonstrates calcification around the odontoid. Tuberculosis can also affect the cervicomedullary junction in isolation.36 Although uncommon, this condition should be considered in individuals with systemic tuberculosis, patients from geographic areas where it is endemic, and patients with HIV. A recent series of 29 cases of craniocervical tuberculosis describes frequent destruction of the condyles, clivus and dens, and ventral arch of the atlas. The majority of patients harbored space-occupying soft tissue masses in the epidural and paravertebral spaces, and they were large enough to cause myelopathy in 12 out of 29 cases. Cervicomedullary compression can also occur in craniometaphyseal dysplasia,37 a sclerosing bone disorder characterized by bony encroachment of neural foramina.
Clinical Presentation
Many authors have noted that no signs or symptoms are pathognomonic for foramen magnum tumors.16,38 As early as 1937, Symonds and Meadows observed that “the clinical picture which results from compression of the spinal cord at, or near, the level of the foramen magnum is not always easy of recognition.”19 Indeed, the clinical presentation of foramen magnum tumors usually varies and includes such ubiquitous symptoms as neck pain and limb dysesthesias, which are also associated with several more common diseases. The rarity of foramen magnum tumors may therefore cause them to be overlooked by clinicians.39 Even a series as recent as that of Bruneau and George, which reviews 230 cases of foramen magnum tumors from 1985 to 1995, reports a misdiagnosis rate as high as 13.5%.3
The most common presenting symptoms of foramen magnum tumors, in order of decreasing frequency, are suboccipital or neck pain, dysesthesias of the extremities more frequent in the upper than in the lower extremities, gait disturbance, and weakness more frequent in the upper extremities than in the lower. Other common early symptoms include clumsiness of the hands, bladder disturbance, dysphagia, nausea and vomiting, headache, “drop attacks,” and dizziness.4,5,16,18 Usually a patient presents with a constellation of symptoms. Rarely, the presentation is characterized by brainstem symptoms primarily (e.g., nausea) or pure nerve dysfunction (e.g., hemifacial spasm,40 dysphagia,41 occipital neuralgia42).
Suboccipital or upper cervical pain, probably caused by irritation of the dura and C2 nerve root, is the most common presenting complaint and may precede other symptoms by months or years. C2 distribution sensory loss frequently accompanies the pain, and together, these symptoms should suggest the diagnosis of foramen magnum tumor. In the series by Stein et al.,18 Meyer et al.,5 and Guidetti and Spallone,4 suboccipital or upper cervical neck pain was the initial complaint in 65% to 80% of patients. By the time of admission, 100% of Guidetti and Spallone’s patients complained of neck pain.
Limb dysesthesias are frequently present and may occur in the form of a burning4,6 or cold13,43 sensation; proprioceptive loss is also common.44 Weakness typically accompanies the sensory changes and tends to involve the upper extremities more than the lower, and the ipsilateral more than the contralateral side.16 An unusual feature of the weakness is its occasional association with wasting of the intrinsic hand muscles.45,46 Taylor and Byrnes have cogently argued, on the basis of their own experimental evidence, that foramen magnum lesions produce hand wasting by causing venous obstruction in the upper cervical cord, which leads to venous infarction in the lower cervical gray matter.47 If the motor symptoms dominate the presentation, the combination of hyperreflexia and hand wasting can resemble the presentation of amyotrophic lateral sclerosis.
The initial neurologic examination of the patient with a foramen magnum tumor most commonly reveals weakness, sensory loss, hyperreflexia, Babinski sign, and spastic gait. Typically, the weakness first affects the ipsilateral arm and then evolves over time into a progressive spastic quadriparesis. Sensory loss may involve the modalities of pain and temperature, proprioception, or both. The burning and cold dysesthesias have been mentioned. Other less common, but still frequent, signs include nystagmus (classically, downbeat), accessory nerve palsy, and atrophy of the intrinsic muscles of the hand. Infrequent signs include atrophy of the arms and legs, papilledema, Horner syndrome, and cranial neuropathies involving cranial nerves V, VII to X, and XII.44
The nonspecific signs and symptoms produced by foramen magnum tumors must be distinguished from those occurring in several more common conditions. Although modern neuroimaging has lessened this problem, the neurosurgical literature is replete with examples of tumors in the region of the foramen magnum that were misdiagnosed on initial presentation. Even a recent series of foramen magnum tumors found a 13.5% incidence of misdiagnosis.3 The failure to establish the correct diagnosis most commonly occurs because a foramen magnum tumor has not been included in the differential diagnosis.48 The clinical entities most commonly confused with foramen magnum tumors include cervical spondylosis, multiple sclerosis, syringomyelia, intramedullary tumors, carpal tunnel syndrome, normal pressure hydrocephalus, Chiari malformation, and amyotrophic lateral sclerosis.
Surgical Anatomy
A thorough knowledge of foramen magnum anatomy is critical to safe surgical exposure in this region. For a more detailed review of the microsurgical anatomy, the reader is referred to the elegant anatomic studies of Oliveira et al.,49 Rhoton et al.,50 and Wen et al.51
Osseous Structures
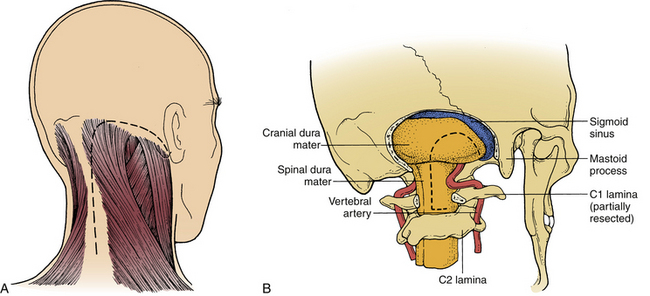
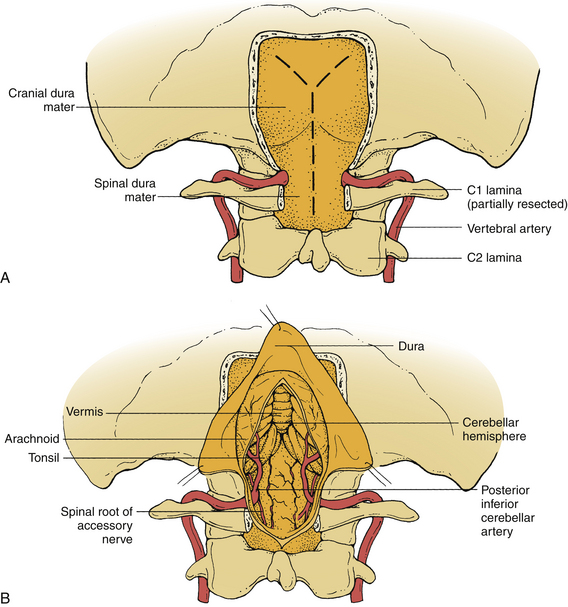
The foramen magnum is formed by the occipital bone, which consists of three parts: basilar, lateral, and squamosal. The basilar part is formed by a fusion between the occipital bone and the clivus. The lateral parts consist of the occipital condyles, which articulate with the atlas. Behind and above the foramen is the occipital squama, whose internal surface is marked by a prominent midline ridge—the internal occipital crest, which serves as the attachment for the falx cerebelli. The ventral margin of the foramen magnum is termed the basion and the opposite margin, the opisthion. The shape of the foramen magnum varies. It is generally oval in shape, and the wider portion is located dorsally.49 It measures on average 35 mm in length and 29 mm in width. The foramen magnum transmits the medulla oblongata; the meninges; the ascending portion of the spinal accessory nerve; and the vertebral, anterior, and posterior spinal arteries.
The occipital condyles are located lateral to the ventral half of the foramen magnum. The occipital condyles are oval, and their inferior surface is convex. They are oriented in a dorsolateral-to-ventromedial direction. They articulate with the superior facet of the atlas, which overlies its lateral mass. The anatomy of the occipital condyles, as it pertains to the transcondylar approach, has been reviewed.52
The hypoglossal canal is located within the occipital bone, ventral to the junction between the ventral and middle third of the occipital condyles.51 The hypoglossal nerve is the only structure that travels through the hypoglossal canal. The jugular foramen is located lateral to the ventral half of the occipital condyles, at the junction of the petrous part of the temporal bone and the occipital bone. It is irregular in shape and has a smaller anterior division and a larger posterior division. The anterior division transmits the inferior petrosal sinus and the glossopharyngeal nerve. The posterior division transmits the vagus and spinal accessory nerves, the internal jugular vein, and the meningeal branches of the ascending pharyngeal and occipital arteries.49
Neural Structures
Cranial Nerves
Any of the lower four cranial nerves may be affected by lesions arising in the foramen magnum. The hypoglossal nerve is formed by a series of rootlets that arise in the ventrolateral sulcus between the pyramid and the olive, along a line that is continuous with the ventral spinal roots. The hypoglossal rootlets course ventrolaterally through the subarachnoid space on their way to the hypoglossal canal, passing dorsally in relation to the vertebral artery. If the course of the vertebral artery is short and straight, there may be no contact between the artery and the hypoglossal nerve. A tortuous vertebral artery, however, may displace the nerve dorsally and medially against the medulla, stretching and damaging its fibers.53 Infrequently, the artery passes through the rootlets of the nerve.49
The glossopharyngeal, vagus, and cranial portions of the accessory nerves all arise in series along the dorsolateral sulcus, between the olive and the tuber cinereum. They exit the skull together through the jugular foramen. The cranial part of the accessory nerve is joined by a spinal part that arises as a series of rootlets between the ventral and dorsal rootlets and ascends through the foramen magnum between the dentate ligament and the dorsal roots. The hypoglossal nerve and, less commonly, the glossopharyngeal, vagus, and accessory nerves may be displaced dorsomedially by a thickened and atheromatous vertebral artery.53
Spinal Nerve Roots
The C1 nerve root often lacks a dorsal rootlet. The accessory nerve frequently contributes a root to the C1 nerve root when the C1 dorsal root, as is commonly the case, is absent. Before exiting the dura mater, the C1 ventral root and the dorsal root, if present, attach to the dorsal caudal surface of the intradural segment of the vertebral artery. The ventral and dorsal roots then exit the dura around the vertebral artery and unite within or just beyond the dural exit.
Vascular Structures
In most individuals, the left vertebral artery is dominant. After ascending through the C1 foramina, the vertebral arteries continue medially with the C1 nerve root along a groove on the rostral surface of the dorsal arch of the atlas, behind the lateral mass. Frequently, this groove forms a complete bony canal that surrounds the vertebral artery.49 Between C6 and C2, the vertebral arteries are thus protected dorsally by the lateral masses. However, as the arteries course dorsal to the C1 lateral mass and enter the region of the foramen magnum, they lose their dorsal bony protection.
Before entering the dura mater, the vertebral artery gives rise to the posterior meningeal and posterior spinal arteries, branches to the deep cervical musculature, and, infrequently, the posterior inferior cerebellar artery. Lang found only a 4% incidence of an extradural origin of the posterior inferior cerebellar artery.54,55
After giving off these branches, the vertebral arteries enter the dura mater just caudal to the lateral edge of the foramen magnum behind the occipital condyles, accompanied by the first cervical nerve and the posterior spinal artery.49
The initial intradural segment of the vertebral artery passes rostral to the dorsal and ventral roots of the first cervical nerve and ventral to the posterior spinal artery, the dentate ligament, and the spinal portion of the accessory nerve. In its ascent along the lower lateral and upper ventral aspect of the medulla, the vertebral artery remains ventral to the lower cranial nerves.7 Variations in this relationship do exist, however, and the vertebral artery may lie dorsal to some cranial nerve rootlets.7 Connections between the hypoglossal nerve, glossopharyngeal nerve, spinal accessory nerve, and C2 cervical root provide the anatomic substrate for various “neck and tongue” syndromes.56
As the vertebral arteries ascend ventromedially along the lateral and then the ventral surface of the medulla, they run adjacent to the occipital condyles, the hypoglossal canals, and the jugular tubercles and then come to rest on the clivus. At or near the pontomedullary junction, the arteries join together to form the basilar artery. The precise point at which the arteries join varies with the size and tortuosity of the vessels.49
The posterior inferior cerebellar artery usually originates from the intradural portion of the vertebral artery just above the foramen magnum, although it rarely arises extradurally at or below the foramen.49 As mentioned, 4% of the posterior inferior cerebellar arteries examined by Lang arose extradurally.54,55 In its course along the ventrolateral and then the dorsolateral medulla, this artery may pass rostrally, caudally, or between the hypoglossal rootlets or above, below, or between the rootlets of the glossopharyngeal, vagus, and accessory nerves.56 Like the vertebral artery, the relationship between the posterior inferior cerebellar artery and the lower cranial nerves is an intimate one and frequently leads to deformation and stretching of the nerves.
After passing through or around the rootlets of the nerves, the posterior inferior cerebellar artery comes to lie dorsal to the glossopharyngeal, vagus, and accessory nerves and then takes a variable course to reach the dorsal medulla, where it bifurcates into a medial and a lateral trunk.56 The medial trunk supplies the vermis and the adjacent cerebellar hemisphere; the lateral trunk supplies the tonsil and hemispheres.
The anterior spinal artery is usually formed by the union of the paired anterior ventral spinal arteries. These arise from the vertebral artery, supply the paramedial ventral medulla, converge, and run caudally along the ventral median fissure of the spinal cord. In a common variant, the anterior spinal artery may arise from a single vertebral artery, supplemented by supply from vertebral radicular branches at C2 or C3.54 The posterior spinal arteries have widely variable origins. They may arise from the posterior inferior cerebellar artery or from the intradural or extradural segment of the vertebral artery.54
The arteries that supply the dura mater in the region of the foramen magnum include the paired anterior and posterior meningeal branches of the vertebral artery, the ascending pharyngeal artery, the meningohypophyseal trunk of the intracavernous internal carotid artery, and the occipital artery. A variable number of radiculomuscular branches also arise from the extradural vertebral artery.54 The size and flow of any of these vessels may markedly increase when supplying a dura-based tumor of the foramen magnum.
Alteration of Anatomy by the Tumor
The most important consideration in the selection of a surgical approach is the topographic relationship between the tumor and the neurovascular structures. These structures include the rostral spinal nerve roots; the glossopharyngeal, vagus, accessory, and hypoglossal nerves; and the vertebral artery and its branches. Obviously, the direction of displacement of the neurovascular structures varies with the location and size of the tumor (Fig. 111-1). Although there are variations, the following guidelines generally apply:
• Dorsal midline tumors displace neurovascular structures ventrally.
• Ventral midline tumors displace neurovascular structures dorsally.
• Ventrolateral tumors displace the spinal cord and brainstem dorsomedially, the cranial and spinal nerves dorsally, and the vertebral artery and its branches ventrally.
Selection of a Surgical Approach
In selecting an approach, the surgeon has to weigh the likelihood of maximally resecting the tumor with the morbidity of the approach, and his or her skill and experience with a given approach. Although many surgical approaches to the region of the foramen magnum have been described, the following three are the most common: (1) the dorsal approach, (2) the far lateral approach, and (3) the transoral approach. The advantages and disadvantages of each of these approaches are outlined in Table 111-2.7 The approach is generally open, although the endoscopic approach has also been employed with ventral approaches.
TABLE 111-2 Advantages and Disadvantages of Surgical Approaches to the Foramen Magnum
| Approach | Advantages | Disadvantages |
|---|---|---|
| Posterior | Most familiar Least demanding Minimal risk of infection Permits stabilization and fusion for craniocervical instability |
Poor visualization of cord-tumor interface Exposure limited by neurovascular structures Limited proximal control of vertebral artery |
| Far lateral | Minimal risk of infection Direct visualization of cord-tumor interface Exposes anterior aspect of thecal sac Obviates need for cord retraction Excellent proximal control of vertebral artery Rarely produces craniocervical instability Permits stabilization and fusion for craniocervical instability |
More complex exposure Associated with postoperative hydrocephalus Time consuming |
| Transoral | Obviates need for cord retraction No neurovascular obstacles in midline Exposes midline of anterior thecal sac |
Deep operative field Limited lateral exposure Cord-tumor interface not seen until end of operation High risk for cerebrospinal fluid leak and meningitis Destabilizes craniocervical junction Stabilization or fusion of craniocervical junction requires separate incision No proximal control of vertebral artery |
In laterally located meningiomas, with an origin ventral to the dentate ligament, a far lateral approach offers advantages over a dorsal midline approach. Although it involves more dissection and greater bone removal, the far lateral approach allows the dissection of the spinal cord/tumor junction to be carried out under direct vision. It also provides excellent control of the vertebral artery.7,57–59 It may also be used to approach ventrally located tumors because it exposes the entire ventral aspect of the thecal sac. Compared with the transoral approach, the instrumentation used in the far lateral approach is also simpler and the risk of infection is minimized. Also, in contrast to the transoral approach, destabilization of the craniocervical junction can be addressed with a fusion and instrumentation procedure. The latter is performed easily concomitantly, if required, based on the amount of removal of the occipital condyle.
The transoral approach is best suited for midline ventral tumors, especially extradural ones. Its advantages include lack of neurovascular obstacles and avoidance of any retraction of the neuraxis. The drawbacks of the transoral approach, however, are many: laterally placed or broad-based tumors are inadequately exposed; vascular control is lacking; the spinal cord/tumor junction is not observed until the end of the operation; and iatrogenic spinal destabilization, which invariably occurs, requires a separate stabilizing procedure. Furthermore, because the operative field is necessarily contaminated and because a watertight dural closure is difficult to achieve, there is a high incidence of postoperative cerebrospinal fluid (CSF) fistula and meningitis. From a technical standpoint, intradural ventral surgery is rendered difficult by the long reach through a narrow surgical corridor. Consideration has been given recently to performing these operations endoscopically or even with robot-assisted surgery, using methodologies applied previously in cardiac and prostate surgery.60
Preoperative Considerations
Electrophysiologic monitoring, including somatosensory evoked potentials, motor-evoked potentials, and brainstem auditory evoked potentials, is employed. Due to the rostral nature of the lesion, D-wave motor monitoring is not practical. The vagus nerve can be monitored using an electromyographic endotracheal tube.61 Electromyography can also be performed on the accessory and hypoglossal nerves. Although employed uncommonly in these operations, perioperative placement of a ventriculostomy should be considered when hydrocephalus is present or seems likely to occur. Finally, the surgical approach should be selected on the basis of the location of the tumor and its relationship to the neural and osseous structures.
Dorsal Approach
Dorsally located tumors are directly accessible with this approach and usually present little risk of vascular injury or cranial neuropathy. These lesions are often amenable to en bloc resection. By contrast, ventrolateral lesions require care to avoid injury to the vertebral or posterior inferior cerebellar arteries, which may be attached to or encased by the tumor. Furthermore, ventrolateral lesions tend to displace the spinal cord dorsally, stretching one or more rootlets of the cranial nerves or rostral spinal roots over the tumor. In tumors arising ventral to the dentate ligament, tumor resection is carried out between the involved rootlets in a piecemeal fashion, with care taken to avoid injury to the radicular vessels that run along the rostral cervical nerve roots. Sectioning the dentate ligaments may improve exposure. Often, the lesion must be removed in multiple small fragments, working between rootlets of the spinal accessory nerve, to avoid excessive rootlet retraction.
The tumor capsule is best opened sharply. It is coagulated and incised. The ultrasonic aspirator can be useful for debulking the intracapsular portion of the tumor. The tumor capsule itself is dissected free from surrounding neurovascular structures and is excised along with involved dura mater. A frozen section is typically obtained intraoperatively, since other entities such as sarcoid can resemble a meningioma and may not merit a radical resection.62
The need for the radical resection of meningioma-involved dura mater to prevent tumor recurrence is controversial.63,64 Curettage of the inner surface of tumor-involved dura with a Penfield dissector or a small curette, followed by bipolar coagulation, may be sufficient to prevent tumor recurrence when involved dura mater cannot be completely excised.
Far Lateral Approach
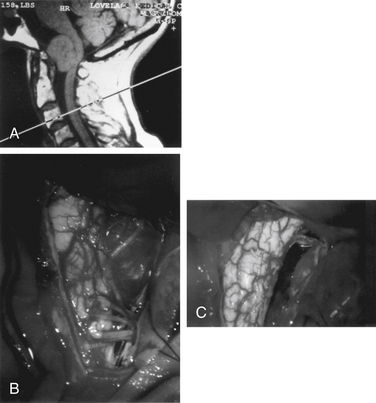
The far lateral approach is best suited for tumors located ventrally or ventrolaterally.7,57–59,61 This provides exposure of the ventral brainstem and makes excessive retraction of neural elements unnecessary. At least 15 variations exist in the far lateral approach, as described in the literature. One should therefore tailor the amount and the order of the dissection and bone removal to the pathology. It is important to weigh the added morbidity of extensive dissection against its associated gain in visualization and surgical freedom of movement.65 Immediately after anesthesia is induced, and before surgery, a lumbar drain is inserted. The patient is then positioned in a modified “park bench” position. The lower arm is allowed to drop from the end of the table, where it is cradled in a sling. The head is flexed, rotated downward, and tilted away from the ipsilateral shoulder for maximal opening of the space between the atlas and the foramen magnum. The upper shoulder is pulled down toward the feet with tape to create a larger space for movement of the microscope. The entire body is secured with tape to allow full rotation of the table. Alternatively, this operation has been performed in the sitting position. This may provide the advantage of improved visualization due to a reduction of venous distention and bleeding, but is associated with air embolism. This is especially true when working around the vertebral artery and hypoglossal nerve, which are surrounded by a rich venous plexus.
An inverted J-shaped incision is begun at the mastoid prominence, curved to the midline, and extended caudally to the C6 level (Fig. 111-2A). The nuchal fascia is cut transversely, leaving a 1-cm cuff for reattachment. The paraspinal muscles are split and elevated from the spinous processes and lamina. The muscle flap is dissected from the suboccipital bone to the mastoid process, and the entire flap is retracted laterally and caudally with fishhooks that are attached to a table-mounted device, such as a Leyla bar (B. Braun Medical, Inc., Bethlehem, PA). The midline aspect of the wound is retracted contralaterally with fishhooks from a second Leyla bar. The lateral mass of C1 and the vertebral artery from C1 to the dura mater are exposed. Bleeding from the venous plexus surrounding the vertebral artery is controlled with bipolar cauterization and packing with oxidixed cellulose (Surgicel). Care must be taken to avoid injury to the vertebral artery or to one of its extracranial branches. Occasionally, the posterior inferior cerebellar artery arises extracranially and could be inadvertently coagulated and divided.66 It has even been noted to originate between C1 and C2.67 The same is true of a hypoplastic vertebral artery.51 The dorsal arch of C1 is then removed with a high-speed drill (Midas Rex; Midas Rex Institute, Inc., Fort Worth, TX), using a B1 bit and foot plate. The ipsilateral lamina is cut at its far lateral extent, as well as on the contralateral side, slightly across the midline. The C1 arch is saved and replaced at closure.
A suboccipital craniotomy is performed with the same drill. The drilling begins at the foramen magnum and extends contralaterally across the midline and ipsilaterally as far laterally as possible. The ipsilateral rim of the foramen magnum is removed with a bone rongeur to the occipital condyle. The dorsal occipital condyle and the rostral lateral mass and facet of C1 are removed using the drill with the B1 bit. The safest technique is to drill away the inner portion of the occipital condyle, leaving a thin shell of cortical bone to protect the surrounding structures. The shell is carefully removed with small curettes and rongeurs until the dorsolateral third of the condyle has been removed (Fig. 111-2B). Bleeding from the condylar veins can be controlled easily with bone wax. The extradural vertebral artery should be protected with a small dissector while the condyle is being drilled. The hypoglossal canal is located in the ventral medial third of the condyle and is not threatened by removal of the dorsolateral third. The degree of resection of the occipital condyle varies depending on the degree of exposure needed. It is possible to perform a far lateral approach without any condylar resection.68 Indeed, with increasing experience in the resection of these lesions, the tendency is for less resection of the condyle or for frank use of a retrocondylar approach,69 which has the obvious advantage of eliminating the need for a fusion. At the other extreme, occasionally, a total condylectomy is necessary to remove an extradural lesion with condylar involvement.65
The dura mater is opened over the midline of the upper cervical spinal cord. At its caudal extent above C2, the dural incision is curved to the ipsilateral side. Rostrally, the dural opening extends in a curvilinear fashion to the rostral lateral aspect of the craniotomy. This configuration allows the dural flap to be hinged laterally, where it is tented up with #4-0 sutures. Because the occipital condyle is removed as necessary for identification of the anatomy, excellent visualization of the proximal intradural vertebral artery is achieved after the dura is opened. Minimal retraction of the cerebellar hemisphere improves the more distal exposure of the vertebral artery and the tumor (Fig. 111-3).
The stability of the craniocervical junction is an important issue to address following the far lateral approach.65,70 The surgeon must carefully balance the trade-off between improving visualization and causing instability. In a series of 25 patients with a far lateral approach, Bejjani et al. performed an occipitocervical fusion in eight cases.70 The authors delineated three indications for fusing across the occipitocervical region: (1) the presence of a painful head tilt, (2) instability on flexion-extension radiographs, or (3) complete resection of the occipital condyle. As a general rule, patients with less than 70% removal of the condyle do not require fusion. This corresponds to resection up to the hypoglossal canal. Any further resection increases the likelihood of craniocervical instability.
Transoral Approach
The transoral approach has been widely used for the treatment of extradural lesions and bony abnormalities of the craniovertebral junction.21–24,71–76 This approach also provides the most direct access to the intradural foramen magnum lesions located at the ventral midline.25,27,28,77–79 The lateral exposure achieved by this approach, however, is limited by the vertebral arteries, the hypoglossal nerves, and the jugular foramen structures. A further disadvantage of this approach is the inability to directly close the dura mater in a watertight fashion, leading to increased risk of CSF leak and postoperative meningitis.
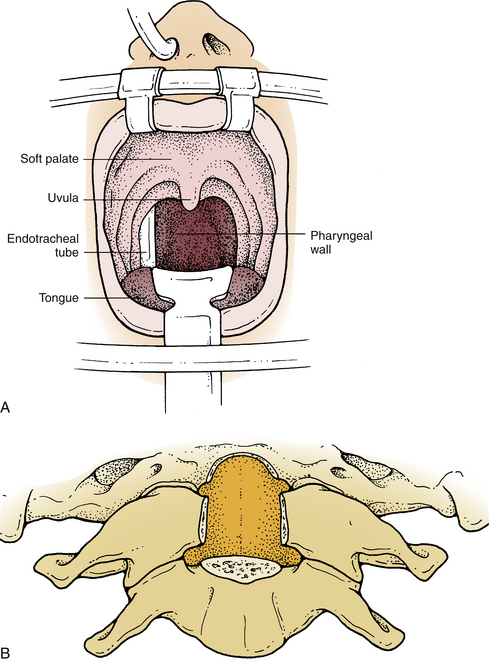
Improved exposure of the dorsal nasopharynx is obtained by suturing the uvula to a rubber catheter passed through the nose and applying gentle upward retraction. This elevates the uvula and soft palate out of the way for C1-2 exposure. If necessary for exposure of the caudal clivus, the soft palate and uvula can be divided to expose the pharyngeal wall. The latter is incised in the midline. The soft palate incision should be brought to one side of the uvula, leaving it intact to the opposite side (Fig. 111-4A). Dissection is continued through the prevertebral musculature and prevertebral fascia, which are retracted laterally. Next, the anterior atlanto-occipital and longitudinal ligaments are detached from the clivus and the upper cervical vertebrae. The drill is then used to remove the lower third of the clivus, the ventral C1 arch, the odontoid peg, and part of the C2 body (Fig. 111-4B). Care is taken to avoid excessively lateral dissection that might injure the vertebral arteries, the hypoglossal nerves, or the jugular foramen structures. Once it is adequately exposed, the dura mater is opened in a cruciate fashion.
After excision of the tumor, the dura mater is closed. This may be accomplished by primary closure, including duroplasty, but this is difficult and is rarely watertight. The dural closure may be further sealed with fibrin glue. Where approved, a sealant (DuraSeal; Confluent Surgical, Waltham, MA) can be applied over the incision.80 A fat graft may be used as an additional seal and to obliterate the retropharyngeal space. Finally, the longus colli is reapproximated with single interrupted polyglactin 910 (Vicryl) sutures, and the pharyngeal and soft palate mucosa are closed with running chromic gut suture.
Surgical Results
Meyer et al. reviewed 102 patients with benign extramedullary foramen magnum tumors who underwent operation at the Mayo Clinic between 1924 and 1982.5 They found a 5% mortality rate and an additional 5% rate of tumor-related deaths (from recurrence) within 3 years of surgery. However, the long-term survival rate was 90%, with 75% of the patients returning to productive lives and 12% being only mildly impaired.
Bruneau and George found a 77% rate of complete tumor removal, a 16% rate of subtotal removal, and only a 7% rate of partial removal. Complete tumor removal was least frequently achieved with ventrally located tumors (69%, vs. 81% for lateral tumors and 86% for dorsal tumors). Furthermore, gross total resection was more often attained among intradural tumors (83% vs. 50% for extradural tumors).3 Bruneau and George also observed superior results with the far lateral approach, compared with the standard dorsal approach, for both ventral and lateral tumors: 86% gross total resection with the far lateral approach versus 71% with the straight dorsal approach.3
Samii et al. obtained a complete resection of craniocervical junction meningiomas in 25 out of 40 patients.81 There was a marked disparity in the rate of complete removal between patients with encapsulated lesions and patients with en plaque or aggressive lesions. Incomplete tumor removal was found to be independently associated with encasement of the vertebral artery, an en plaque pattern, infiltrative growth, and an intracranial origin.
Experience with the transoral approach to intradural extramedullary tumors is limited. It is absent in the reports of both Meyer et al.5 and Bruneau and George.3 Among seven ventrally located intradural lesions at the craniovertebral junction, including three meningiomas, two schwannomas, a neurenteric cyst, and a basilar-anterior inferior cerebellar artery junction aneurysm, Crockard et al. achieved a total removal of both schwannomas, a subtotal removal of two of the three meningiomas, a total removal of the neurenteric cyst, and a successful clipping of the aneurysm.22,26,27 Major postoperative complications included spinal instability (two patients), CSF leakage (three patients), and velopharyngeal insufficiency (three patients).
Chono et al. successfully removed a ventrally located foramen magnum meningioma with the transoral approach. The procedure was complicated by a CSF leak that required reoperation; a ventral occipitocervical fusion was performed.78 Bonkowski et al. reported the successful removal of a ventrally located foramen magnum meningioma by the transoral approach that was uncomplicated by spinal instability or CSF leakage.77 The authors’ avoidance of CSF leakage may in part be related to their innovative application of a bone baffle.
The best predictors of poor outcome are the presence of a large ventrally located tumor and poor preoperative neurologic status.1,4,5,15,18,19 Tumor-related deaths most commonly result from respiratory failure.
Complications
The intraoperative and postoperative complications of surgery in the region of the foramen magnum may be divided into categories, as formulated by Sen et al.30 as follows: (1) neurologic, (2) CSF leakage, (3) vascular, (4) infections, and (5) systemic.
Neurologic
Neurologic complications may be caused by intradural or extradural hematoma formation, cerebellar contusion, or injury to the cranial nerves. Postoperative hematoma is best prevented by meticulous hemostasis at the time of operation. Particular attention should be paid to obtaining excellent surgical exposure to prevent undetected bleeding in areas of the wound that are not well visualized. Wide exposure also minimizes the need for excessive retraction. Routine postoperative CT may be used to detect the development of any hematoma or hydrocephalus. Cerebellar contusion is best avoided by applying only gentle retraction at the time of exposure and by providing intermittent periods of relaxation.
The glossopharyngeal, vagus, accessory, and hypoglossal nerves are vulnerable to injury during surgery in the region of the foramen magnum, particularly because of their proximity to and frequent involvement with the lesion.82,83 Gentle and meticulous surgical technique provides the best chance of protecting these structures that may be inadvertently sectioned or stretched. In a series of ventral foramen magnum meningiomas,61 glossopharyngeal and vagus nerve dysfunction was the most common complication postoperatively.
Concomitant lesions of the glossopharyngeal, vagus, and accessory nerves magnify the effects of a hypoglossal nerve lesion. Depending on the severity of injury, patients may require only minimal speech and swallowing therapy, or they may require a comprehensive speech and swallowing rehabilitation program with or without a tracheotomy and gastrostomy. In general, patients with preoperative cranial nerve deficits are at lower risk for postoperative aspiration pneumonia, since they have already adapted to a slowly progressive dysfunction of the lower cranial nerves.81
Velopharyngeal insufficiency is a unique complication of the transoral approach. Three of the seven patients of Crockard’s series developed this problem postoperatively.25 It probably results from removal of the bony support of the dorsal pharynx and should be distinguished from a cranial nerve palsy.79 Because it appears related to bony removal, velopharyngeal insufficiency does not occur after transoral procedures for extradural lesions.74,84 Placement of “bone baffle” has been proposed to prevent this complication.77
Cerebrospinal Fluid Leakage
Once the problem is recognized, the initial management of a CSF leak is with a lumbar spinal fluid drain. If this measure fails to stop the leak, surgical reexploration and dural repair may be necessary. In addition to predisposing to meningitis, a CSF leak can produce a pseudomeningocele. This has been associated with postoperative neurologic deterioration.85–87
Infections
Prophylactic intraoperative antibiotics are used routinely and should be given before the skin is incised (the potential for infection starts with this step). Repeated dosing is required to maintain adequate tissue concentration of the antibiotic. Proper tissue handling helps avert tissue devitalization and desiccation that may later form the nidus for infection. Copious irrigation is performed intermittently throughout the procedure, particularly before closure. Postoperative CSF leakage, which may lead to meningitis, should be prevented or promptly recognized and addressed.
Summary
Although early diagnosis continues to challenge clinicians, advances in the operative and perioperative management of foramen magnum tumors have greatly reduced both the morbidity and the mortality in affected patients. Intradural extramedullary tumors comprise approximately 90% of all foramen magnum tumors and include meningiomas and neurofibromas predominantly.7
Arnautovic K.I., Al-Mefty O., Husain M. Ventral foramen magnum meningiomas. J Neurosurg (Spine 1). 2000;92:71-80.
Bassiouni H., Ntoukas V., Asgari S., et al. Foramen magnum meningiomas: clinical outcome after microsurgical resection via a posterolateral suboccipital retrocondylar approach. Neurosurgery. 2006;59:1177-1187.
Bruneau M., Georges B. Classification system of foramen magnum meningiomas. J Craniovertebr Junction Spine. 2010;1(1):10-17.
de Oliveira F., Rhoton A.L.Jr., Peace D. Microsurgical anatomy of the region of the foramen magnum. Surg Neurol. 1985;24:293-352.
Wen H.T., Rhoton A.L., Katsuta T., et al. Microsurgical anatomy of the transcondylar, supracondylar, and paracondylar extensions of the far-lateral approach. J Neurosurg. 1997;87:555-585.
1. Love J.G., Thelen E.P., Dodge H.W.Jr. Tumors of the foramen magnum. J Int Coll Surgeons. 1954;22:1-17.
2. Bruneau M., George B. Classification system of foramen magnum meningiomas. J Craniovertebr Junction Spine. 2010;1(1):10-17.
3. Bruneau M., George B. Foramen magnum meningiomas: detailed surgical approaches and technical aspects at Labroisiere Hospital and review of the literature. Neurosurg Rev. 2008;31:19-33.
4. Guidetti B., Spallone A. Benign extramedullary tumors of the foramen magnum. Surg Neurol. 1980;13:9-17.
5. Meyer F.B., Eberld M.J., Reese D.F. Benign tumors of the foramen magnum. J Neurosurg. 1984;61:136-142.
6. Yasuoka S., Okazaki H., Daube J.R., MacCarty C.S. Foramen magnum tumors. J Neurosurg. 1978;49:828-838.
7. Tuite G.F., Crockard H.A. The far lateral approach to the foramen magnum. In: Torrens M., Dickson R.A., editors. Practice of surgery. New York: Churchill Livingstone, 1991.
8. Starke R.M., Nguyen J.H., Reames D.L., et al. Gamma Knife radiosurgery of meningiomas involving the foramen magnum. J Craniovertebr Junction Spine. 2010;1(1):23-28.
9. Johnson M.D., Sade B., Milano M.T., et al. New prospects for management and treatment of inoperable and recurrent skull base meningiomas. J Neurooncol. 2008;86:109-122.
10. Hallopeau H. Note sur deux faits de tumeurs de mesocephale communique a la societe de biologie (le 18 mai 1872). Gazette Med Paris. 1874;3:111-112.
11. Abrahamson I., Grossman M. Tumors of the upper cervical cord. J Nerv Mental Dis. 1923;57:342-363.
12. Frazier C.H., Spiller W.G. An analysis of fourteen consecutive cases of spinal cord tumor. Arch Neurol Psychiatry. 1922;8:455-501.
13. Elsberg C.A., Strauss I. Tumors of the spinal cord which project into the posterior cranial fossa: report of a case in which a growth was removed from the ventral and lateral aspects of the medulla oblongata and upper cervical cord. Arch Neurol Psychiatry. 1929;21:261-273.
14. Cushing H., Eisenhardt L. Meningiomas: their classification, regional behaviour, life history and surgical end results. Springfield, IL: Charles C Thomas; 1938.
15. Cohen L., Macrae D. Tumors in the region of the foramen magnum. J Neurosurg. 1962;19:462-469.
16. Dodge H.W.Jr, Love J.G., Gottlieb C.M. Benign tumors at the foramen magnum. J Neurosurgery. 1956;13:603-617.
17. Smolik E.A., Sachs E. Tumors of the foramen magnum of spinal origin. J Neurosurg. 1954;11:161-172.
18. Stein B.M., Leeds N.E., Taveras J.M., Pool J.L. Meningiomas of the foramen magnum. J Neurosurg. 1963;10:1740-1751.
19. Symonds C.P., Meadows S.P. Compression of the spinal cord in the neighborhood of the foramen magnum. Brain. 1937;60:52-84.
20. Kanavel A.B. Bullet located between the atlas and the base of the skull. Technique for removal through the mouth. Surg Clin. 1919;1:361-366.
21. Ashraf J., Crockard H.A. Transoral fusion for high cervical fractures. J Bone Joint Surg [Br]. 1990;72:76-79.
22. Crockard H.A., Pozo J.L., Ransford A.D., et al. Transoral decompression and posterior fusion for rheumatoid atlanto-axial subluxation. J Bone Joint Surg [Br]. 1986;68:350-356.
23. Di Lorenzo N., Palatinsky E., Bardella L., Maleci A. Benign osteoblastoma of the clivus removed by a transoral approach: case report. Neurosurgery. 1987;20:52-55.
24. Guthkelch A.W., Williams R.G. Anterior approach to recurrent chordomas of the clivus. J Neurosurg. 1967;36:670-672.
25. Crockard H.A., Sen C.N. The transoral approach for the management of intradural lesions at the craniocervical junction: review of 7 cases. Neurosurgery. 1991;28:88-98.
26. Crockard H.A., Bradford R. Transoral transclival removal of a schwannoma anterior to the craniocervical junction. J Neurosurg. 1985;62:293-295.
27. Miller E., Crockard H.A. Transoral transclival removal of anteriorly placed meningiomas at the foramen magnum. Neurosurgery. 1987;20:966-968.
28. Mullan S., Nauton R., Hekmat-Panah J., Vailati G. The use of an anterior approach to ventrally placed tumors in the foramen magnum and vertebral column. J Neurosurg. 1966;24:536-543.
29. Heros R.C. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg. 1986;64:559-562.
30. Sen C., Snyderman C.H., Sekhar L.N. Complications of skull base operations. In: Sekhar L.N., Janecka I.P., editors. Surgery of cranial base tumors. Philadelphia: Lippincott-Raven, 1993.
31. Spetzler R.F., Grahm T.W. The far-lateral approach to the inferior clivus and the upper cervical region: technical note. BNI Quarterly. 1990;6:35-38.
32. Osborn A.G. Diagnostic radiology. St. Louis: CV Mosby; 1994.
33. George B., Lot G. Foramen magnum meningiomas: a review from personal experience of 37 cases and from a cooperative study of 106 cases. Neurosurg Quart. 1995;5:149-167.
34. Love J.G., Adson A.W. Tumors of the foramen magnum. Trans Am Neurol Assoc. 1941;67:78-81.
35. Assaker R., Louis E., Boutry N., et al. Foramen magnum syndrome secondary to calcium pyrophosphate crystal deposition in the transverse ligament of the atlas. Spine. 2001;26:1396-1400.
36. Krishnan A., Patkar D., Patankar T., et al. Craniovertebral junction tuberculosis: a review of 29 cases. J Comput Assist Tomogr. 2001;25:171-176.
37. Day R.A., Park T.S., Ojemann J.G., Kaufman B.A. Foramen magnum decompression for cervicomedullary encroachment in craniometaphyseal dysplasia: case report. Neurosurgery. 1997;41:960-964.
38. Howe J.R., Taren J.A. Foramen magnum tumors. JAMA. 1973;225:1061-1066.
39. Resnikoff S., Cardenas C. Meningioma at the foramen magnum. J Neurosurg. 1964;21:301-302.
40. Harrison G.S., Chovan P., Lee J.H. Hemifacial spasm due to a large distant ipsilateral posterior fossa meningioma. Skull Base Surg. 2000;10(1):43-45.
41. Tso G.J., Tsang M.W., Mobley B.C., Cheng W.W. Foramen magnum meningioma: dysphagia of atypical etiology. J Gen Intern Med. 2007;23(2):206-209.
42. Kim N.H., Yang S.Y., Koo J.B., Jeong S.W. Occipital neuralgia as the only presenting symptom of foramen magnum meningioma. J Clin Neurol. 2009;5:198-200.
43. Beatty R.A. Cold dysesthesia: a symptom of extramedullary tumors of the spinal cord. J Neurosurg. 1970;33:75-78.
44. Scott E.W., Rhoton A.L. Foramen magnum meningiomas. In: Al-Mefty O., editor. Meningiomas. Philadelphia: Lippincott-Raven,; 1991:543-568.
45. Krayenbuhl H. Special clinical features of tumors of the foramen magnum. Schweiz Arch Neurol Neurochir Psychiatry. 1973;112:205-218.
46. Oppenheim H. Weitere beitrage zur diagnose und differential-diagnose des tumor medullae spinalis. Mschr Psychiat Neurol (Berl). 1913;33:451-493.
47. Taylor A.R., Byrnes D.P. Foramen magnum and high cervical cord compression. Brain. 1974;977:473-480.
48. Bull J. Letter: Missed foramen-magnum tumours. Lancet. 1974;1:91.
49. Oliveira F d.e., Rhoton A.L.Jr, Peace D. Microsurgical anatomy of the region of the foramen magnum. Surg Neurol. 1985;24:293-352.
50. Rhoton A.L., Oliveira E d.e. Microsurgical anatomy of the region of the foramen magnum. In: Wilkins R.H., Rengachary S.S., editors. Neurosurgery update. New York: McGraw-Hill; 1990:925-949.
51. Wen H.T., Rhoton A.L., Katsuta T., Oliveira de E. Microsurgical anatomy of the transcondylar, supracondylar, and paracondylar extensions of the far-lateral approach. J Neurosurg. 1997;87:555-585.
52. Dowd G.C., Zeiller S., Awashti D. Far lateral transcondylar approach: dimensional anatomy. Neurosurgery. 1999;45:95-100.
53. Sunderland S. Neurovascular relations and anomalies at the base of the brain. J Neurol Neurosurg Psychiatry. 1948;11:243-257.
54. Lang J. Craniocervical region, blood vessels. Neuroorthopaedics. 1986;2:55-56.
55. Lang J. Anatomy of the posterior cranial fossa. In: Sekhar L.N., Janecka I.P., editors. Surgery of cranial base tumors. Philadelphia: Lippincott-Raven, 1993.
56. Lister R.J., Rhoton A.L., Matsushima T., Peace D.A. Microsurgical anatomy of the posterior inferior cerebellar artery. Neurosurgery. 1982;10:170-295.
56. Orrell R.W., Marsden C.D. The neck-tongue syndrome. J Neurol Neurosurg Psychiatry. 1994;57:348-352.
57. Anson J.A., Spetzler R.F. Endarterectomy of the intradural vertebral artery via the far lateral approach. Neurosurgery. 1993;33:804-811.
58. Kratimenos G.P., Crockard H.A. The far lateral approach for ventrally placed foramen magnum and upper cervical spine tumors. J Neurosurg. 1993;7:12-140.
59. Sen C.N., Sekhar L.N. An extreme lateral approach to intradural lesion of the cervical spine and foramen magnum. Neurosurgery. 1990;27:197-204.
60. Lee J.Y., O’Malley B.W., Newman J.G., et al. Transoral robotic surgery of craniocervical junction and atlantoaxial spine: a cadaveric study. J Neurosurg Spine. 2010;12(1):13-18.
61. Arnautovic K.I., Al-Mefty O., Husain M. Ventral foramen magnum meningiomas. J Neurosurg (Suppl 1). 2000;92:71-80.
62. Sandhu F.A., Schelliger D., Martuza R.L. A vascular sarcoid mass mimicking a convexity meningioma. Neuroradiology. 2000;42(3):195-198.
63. Levy W.J.Jr, Bay J., Dohn D. Spinal cord meningioma. J Neurosurg. 1982;57:804-812.
64. Solero C.L., Fornari M., Giombini S., et al. Spinal meningiomas: a review of 174 operated cases. Neurosurgery. 1989;25:153-160.
65. Spektor S., Anderson G.J., McMenomey S.O., et al. Quantitative description of the far-lateral transcondylar transtubercular approach to the foramen magnum and clivus. J Neurosurg. 2000;92:824-831.
66. Salas E., Ziyal I.M., Bank W.O., et al. Extradural origin of the posteroinferior cerebellar artery: an anatomic study with histological and radiographic correlation. Neurosurgery. 1998;42:1326-1331.
67. Tanaka A., Kimura M., Yoshinaga S., Tomonaga M. Extracranial aneurysm of the posterior inferior cerebellar artery: case report. Neurosurgery. 1993;33:742-745.
68. Nanda A., Vincent D.A., Vannemreddy PSSV, et al. Far-lateral approach to intradural lesion of the foramen magnum without resection of the occipital condyle. J Neurosurg. 2002;96:302-309.
69. Bassiouni H., Ntoukas V., Asgari S., et al. Foramen magnum meningiomas: clinical outcome after microsurgical resection via a posterolateral suboccipital retrocondylar approach. Neurosurgery. 2006;59:1177-1187.
70. Bejjani G.K., Sekhar L., Riedel C.J. Occipitocervical fusion following the extreme lateral transcondylar approach. Surg Neurol. 2000;54:109-116.
71. Alonso W.A., Black P., Connor G.H., Uematsu S. Transoral transpalatal approach for resection of clival chordoma. Laryngoscope. 1971;81:1626-1631.
72. Di Lorenzo N. Transoral approach to extradural lesions of the lower clivus and upper cervical spine. Neurosurgery. 1989;24:37-42.
73. Makhmudov U.B., Tcherekayev V.A., Tanyashin S.V. Transoral approach to tumors of the clivus: report of two cases. J Craniofac Surg. 1992;3:35-38.
74. Menezes A.H., Vangilder J.C. Transoral transpharyngeal approach to the anterior craniocervical junction. J Neurosurg. 1988;69:895-903.
75. Pasztor E. Transoral approach for epidural craniocervical pathological processes. Symon L., editor. Advances and technical standards in neurosurgery, vol 12. New York: Springer-Verlag, 1985. pp 125–170
76. Schmidek H.H., Smith D.A., Sofferman R.A., et al. Transoral unilateral facetectomy in the management of unilateral anterior rotatory atlanto axial fracture dislocation: a case report. Neurosurgery. 1986;18:645-652.
77. Bonkowski J.A., Gibson R.D., Snape L. Foramen magnum meningioma: transoral resection with a bone baffle to prevent CSF leakage. Case report. J Neurosurg. 1990;72:493-496.
78. Chono Y., Abe H., Iwasaki Y., et al. Transoral anterior approach to foramen magnum meningioma. Case report and review. No Shinkei Geka. 1985;13:109-114.
79. Crockard H.A. Transoral surgery: some lessons learned. Br J Neurosurg. 1995;9:283-293.
80. Than K.D., Baird C.J., Olivi A. Polyethylene glycol hydrogel dural sealant may reduce incisional cerebrospinal fluid leak after posterior fossa surgery. Neurosurgery. 2008;63(1 suppl 1):ONS182-ONS186.
81. Samii M., Klekamp J., Carvalho G. Surgical results for meningiomas of the craniocervical junction. Neurosurgery. 1996;39:1086-1095.
82. Marentette L.J., Goding G.S.Jr, Levine S.C. Rehabilitation of the lower cranial nerves. Neurosurg Clin North Am. 1993;4:573-580.
83. Snyderman C.H., Johnson J.T. Rehabilitation of swallowing. In: Sekhar L.N., Janecka I.P., editors. Surgery of cranial base tumors. Philadelphia: Lippincott-Raven; 1993:819-824.
84. Hadley M.N., Martin N.A., Spetzler R.F., Sonntag V.K.H. The transoral approach to the superior cervical spine. J Neurosurg. 1989;71:16-23.
85. Burres K.P., Conley F. Progressive neurological dysfunction secondary to postoperative cervical pseudomeningocele in a C-4 quadriplegic. J Neurosurg. 1978;48:289-291.
86. Helle T.L., Conley F.K. Postoperative pseudomeningocele as a cause of delayed myelopathy. Neurosurgery. 1981;9:314-316.
87. Hosono N., Yonenobu K., Ono K. Postoperative cervical pseudomeningocele with herniation of the spinal cord. Spine (Phila Pa 1976). 1995;20:2147-2150.