TSH-Producing Adenomas
Pituitary thyrotropin-producing adenomas (TSH-omas) are rare tumors that cause hyperthyroidism by chronically stimulating an intrinsically normal thyroid gland.1–4 The first case of hyperthyroidism related to TSH-oma (central hyperthyroidism) was reported in 1960 when serum TSH levels were measured with a bioassay.5 In 1970, Hamilton and coworkers6 documented the first case of TSH-oma that was indisputably proved by radioimmunoassay techniques. Since then, about 350 patients have been reported in the literature. Although early reports describe these tumors as invasive macroadenomas that cause high morbidity and, in general, are difficult to be removed surgically, some cases now are cured more easily owing to earlier diagnosis. In fact, with the advent of ultrasensitive immunometric assays for TSH measurement, which are performed routinely in association with direct measurement of circulating free thyroid hormones (free thyroxine [FT4] and free triiodothyronine [FT3]), it is expected that patients with TSH-oma at the stage of microadenoma will be recognized with increasing frequency, thus permitting an improved clinical outcome.
Classically, TSH-omas, together with resistance to thyroid hormone,7–9 were defined as syndromes of “inappropriate secretion of TSH,” based on the common hormonal profile characterized by high levels of FT4 and FT3 in the presence of measurable TSH concentrations—a finding that contrasted with that observed in primary hyperthyroidism in which TSH is always undetectable. Nonetheless, the term central hyperthyroidism seems to be more pertinent for these disorders. However, clinically and biochemically, euthyroid patients with pituitary adenomas that secrete TSH molecules, possibly with reduced bioactivity, have been described but not clearly documented.10,11 Moreover, pituitary hyperplasia and, in rare instances, true adenoma12–14 related to long-standing primary hypothyroidism are well-known clinical conditions.4 In most of these so-called feedback tumors, resolution of the pituitary lesion and normalization of TSH levels occur after levothyroxine (LT4) replacement therapy, thus bringing into question the actual functional autonomy of such tumors.
Epidemiology
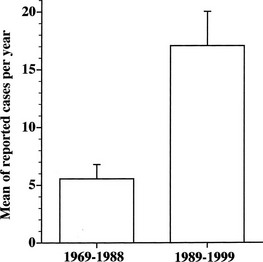
TSH-producing adenoma is a rare disorder, accounting for about 0.5% to 2% of all pituitary adenomas in both clinical and surgical or pathologic series.1,15–17 The prevalence in the general population is 1 to 2 cases per million. Indeed, the number of reported cases of TSH-omas has tripled in the decade from 1989 to 1999 (Fig. 10-1). This increased number of recorded cases results from the introduction of ultrasensitive immunometric assays for TSH as a first-line test for the evaluation of thyroid function. On the basis of the finding of measurable serum TSH levels in the presence of elevated thyroid hormone concentrations, many patients who previously were thought to have Graves’ disease can be diagnosed correctly as having a TSH-secreting pituitary adenoma or, alternatively, resistance to thyroid hormone. Moreover, increased awareness by the endocrinologist and general practitioner regarding the existence of central hyperthyroidism has contributed greatly to the disclosure of a higher number of patients with such a rare disorder.
Pathology and Etiopathogenesis
The thyrotroph is the cell type of origin in TSH-omas. These tumors are nearly always benign; at present, transformation of a TSH-oma into a carcinoma with multiple metastases has been reported in only two patients.18,19 Most of them (72%) secrete TSH alone, although this often is accompanied by unbalanced hypersecretion of the α subunit. About one fourth of TSH-omas are mixed adenomas, characterized by concomitant hypersecretion of other anterior pituitary hormones, mainly growth hormone (GH), prolactin (PRL), or both, which are known to share with TSH the common transcription factor Pit-1. Indeed, hypersecretion of TSH and GH is the most frequent association (16%), followed by hypersecretion of TSH and PRL (10.4%) and occasionally TSH and gonadotropins (1.4%) (Fig. 10-2). No association with adrenocorticotropic hormone (ACTH) hypersecretion has been documented to date. Two ectopic TSH-producing adenomas have been documented in the pharyngeal hypophysis.20,21
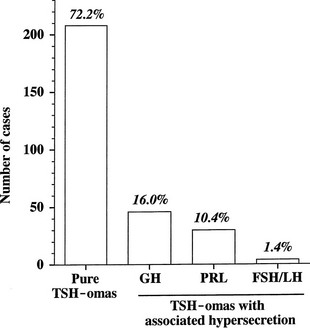
FIGURE 10-2 Classification of pituitary thyrotropin (TSH)-producing adenomas based on hormone secretion into circulation.
At morphologic and histopathologic analysis, most TSH-omas are macroadenomas (87%), frequently with fibrous consistency, even in the absence of prior surgery or radiotherapy, and high local invasiveness.22 However, previous thyroid ablation by surgery or radioiodine has deleterious effects on the size and invasiveness of the tumor (Fig. 10-3).4 In fact, invasive macroadenomas were found in 49% of patients who had undergone thyroid ablation versus 27% in those who were untreated, whereas the figure was reversed in patients with microadenomas (diameter <1 cm) or intrasellar macroadenomas. Therefore, previous thyroid ablation may induce an aggressive transformation of the tumor, as is observed in Nelson’s syndrome after adrenalectomy for Cushing’s disease.
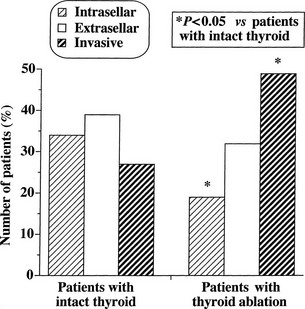
FIGURE 10-3 Effects of previous thyroid ablation on the size of pituitary thyrotropin (TSH)-producing adenomas. Intrasellar refers to both microadenomas and intrasellar macroadenomas, extrasellar to macroadenomas with suprasellar extension, and invasive to invasive macroadenomas. Data were calculated from 253 reported patients (163 with intact thyroid and 90 with thyroid ablation). Statistical analysis was carried out by Fisher’s exact test.
Light microscopy shows that adenoma cells are chromophobic, although they occasionally stain with basic or acid dyes. Ultrastructurally, adenomatous cells frequently appear monomorphous, even if they hypersecrete TSH, α subunit, and other pituitary tropins.23–26 Cells with abnormal morphologic features or mitoses,27 which may be misinterpreted as pituitary malignancy or metastases from distant carcinomas, are present in poorly differentiated adenomas that are characterized by the presence of fusiform cells with sparse and small secretory granules (80 to 200 nm). Indeed, there are no clear criteria of malignancy for TSH-omas except for the presence of metastases. It is worth noting that the first carcinoma reported in the literature exhibited a progressive malignant transformation accompanied by a decline in TSH and α subunit secretion.18
Immunostaining studies show the presence of TSH-β, either free or combined with the α subunit. In very few cases, a negative TSH-β immunostaining has been reported, possibly due to the extremely fast secretion rate of newly synthesized TSH molecules.4,34 With the use of double immunostaining, the existence of mixed TSH-α subunit adenomas composed of one cell type secreting α subunit alone and another cosecreting α subunit and TSH has been documented.28 In addition to α subunit, TSH frequently colocalizes with other pituitary hormones in the same tumoral cell29 or even in the same secretory granule.24,28,30,31 Nonetheless, positive immunohistochemistry panels for one or more pituitary hormones do not necessarily correlate with hypersecretion in vivo.32 Indeed, positive immunostaining for ACTH and gonadotropins without evidence of in vivo hypersecretion has been reported.33–37
TSH-omas have been shown to be monoclonal in origin,38 and several studies have screened a substantial number of adenomas for proto-oncogene activation32,33,39–41 or loss of antioncogenes,40,42 yielding negative results.4 A highly variable expression of thyrotropin-releasing hormone (TRH) and dopamine receptors was documented in several adenomas,43–45 whereas functional somatostatin receptors were detected constantly in TSH-omas,46–48 thus providing the rationale for their medical treatment with somatostatin analogues. Indeed, loss of heterozygosity at the locus of the somatostatin receptor type 5 gene appears to be associated with resistance to somatostatin analogues and a more aggressive phenotype.49
Recently, somatic mutations50 and aberrant alternative splicing51 of thyroid hormone receptor β have been reported, along with dysregulation of iodothyronine deiodinase enzyme expression and function.52,53 These findings at least in part may explain the defects in negative regulation of TSH secretion by thyroid hormones in some tumors.
Clinical Features
Patients with TSH-oma present with the signs and symptoms of either hyperthyroidism or the mass effect of an expanding intracranial tumor (Table 10-1). TSH-omas may occur at any age (range, 11 to 84 years), although most patients are in the third to sixth decade of life. Unlike the female predominance seen with other common thyroid disorders, TSH-omas occur with equal frequency in males and females. Goiter and clinical thyrotoxicosis are the most common presenting symptoms. Most patients presented with a long history of thyroid dysfunction, often mistakenly diagnosed as Graves’ disease, and one third had inappropriate thyroidectomy, radioiodine thyroid ablation, or both. Thus, patients with TSH-omas may present to the specialist with hyperthyroidism that has been refractory to previous therapeutic attempts. In general, clinical features of hyperthyroidism are milder than expected on the basis of circulating thyroid hormone levels. Moreover, individual patients with untreated TSH-oma were reported to be clinically euthyroid.10,54–56 This emphasizes the importance of systematic measurement of TSH and FT4 in all patients with pituitary tumor, to disclose those with central hyperthyroidism or central hypothyroidism. In some acromegalic patients, signs or symptoms of hyperthyroidism are missed, as they are overshadowed by those of acromegaly.24,57 Severe thyrotoxic features, such as atrial fibrillation, cardiac failure, and episodes of periodic paralysis,58–60 are observed in about one fourth of cases.
Table 10-1
Clinical Characteristics of Patients With Pituitary Thyrotropin (TSH-oma) (Data from reports published until December 2007)
| Patients With TSH-oma % (n/total)* | |
| Age, years | 40.9 ± 14.5 (312)† |
| Sex, female | 55 (180/325) |
| Previous thyroid ablation | 33 (95/290) |
| Severe thyrotoxicosis | 29 (60/204) |
| Goiter | 93 (219/235) |
| Thyroid nodule(s) | 72 (46/64) |
| Macroadenomas | 87 (227/261) |
| Visual field defect | 40 (61/154) |
| Headache | 20 (23/117) |
| Menstrual disorders‡ | 33 (27/81) |
*n/total refers to the number of patients for whom the information was available.
‡Data include women with or without associated prolactin (PRL) hypersecretion.
The presence of a goiter is the rule (93%), even in patients who have undergone previous partial thyroidectomy. Because the thyroid is intrinsically normal in this disorder, it may regrow even after near total resection as a consequence of TSH hyperstimulation. Occurrence of multinodular goiter has been reported in several patients,61 and differentiated thyroid carcinoma has been reported in other patients.62–65 Progression toward functional autonomy seems to be infrequent.66,67 In contrast to Graves’ disease, the occurrence of circulating antithyroid autoantibodies is similar to that found in the general population. Unilateral exophthalmos due to orbital invasion by pituitary tumor was reported in three patients with TSH-omas, whereas Graves’-associated bilateral ophthalmopathy was reported in five patients.4
Most patients bearing a TSH-producing macroadenoma seek medical attention with signs and symptoms of an expanding intracranial tumor. Indeed, as a consequence of tumor suprasellar extension or invasiveness, signs and symptoms of tumor mass prevail over those of thyroid hyperfunction in many patients. Visual field defects are present in 40% of patients and headache in one fifth. Moreover, partial hypopituitarism is common, and loss of gonadal function is present in about one third of patients.22,68 Galactorrhea was recorded in almost all patients with mixed TSH- and PRL-secreting tumors.69,70
Finally, TSH-omas may occur in families with multiple endocrine neoplasia type I71–73 and in McCune-Albright syndrome.74
Biochemical Findings
TSH and Thyroid Hormone Levels
High concentrations of thyroid hormones in the presence of detectable TSH levels typically are present in patients with hyperthyroidism due to a TSH-oma or with resistance to thyroid hormone. In the case of replacement therapy for prior thyroidectomy or thyroid ablation, it is crucial to assess patients in steady state, as TSH levels need 4 to 6 weeks to adjust to a change in LT4 dose. Thus, the diagnosis of TSH-producing adenoma may be difficult to establish in any patient who has had a dramatic change in thyroid hormone replacement therapy resulting from physician instruction or poor compliance. Conversely, the finding of elevated TSH levels in patients who have undergone thyroid ablation and have been overtreated with LT4 should be regarded as a possible sign of previously undiagnosed TSH-oma.75
Various abnormalities in the pituitary-thyroid axis, as well as laboratory artifacts, may cause a biochemical profile similar to that of central hyperthyroidism. These different conditions are more common than are TSH-omas and resistance to thyroid hormone and should be excluded before an extensive clinical assessment of the possible presence of central hyperthyroidism is conducted. Familial or drug- or estrogen-induced increases in circulating thyroxine-binding globulin (TBG) or variants of albumin or transthyretin have led to increases in the levels of total serum thyroid hormone, particularly T4, thus producing a biochemical profile that may be confused with that of TSH-omas. Therefore, measurement of free thyroid hormones is mandatory in these conditions and should be performed by means of direct “two-step” methods (i.e., techniques by which contact between serum proteins and tracer can be avoided at the time of assay).76,77 Indeed, normal levels of total T4 were recorded in several patients with TSH-oma, and only the measurement of FT4 allowed the right diagnosis of central hyperthyroidism. Furthermore, inhibition of T4 to T3 conversion induced by iodine-containing drugs or nonthyroidal illness may cause hyperthyroxinemia and nonsuppressed TSH that are, however, associated with normal or low-normal T3. In clinically ambiguous situations, the differential diagnosis rests on recognition of the underlying disorder, as well as on documentation of normalization of thyroid function test results at a later stage or after recovery of drug withdrawal.
Several laboratory artifacts may cause falsely high serum levels of TSH or thyroid hormones (Table 10-2). The more common factors that interfere with TSH measurement are heterophilic antibodies directed against mouse gamma globulins78 or anti-TSH antibodies. However, preventing formation of the “sandwich” anti-TSH antibodies usually leads to an underestimation of the actual levels of TSH and rarely to an overestimation. The presence of anti-T4 or anti-T3 autoantibodies or both may cause FT4, FT3, or both to be overestimated, particularly when “one-step” analog methods are employed.77 Finally, because patients with a TSH-oma may have T3 toxicosis, as in other forms of hyperthyroidism, there is a need to measure T3, in particular, free T3, when T4 levels are normal.
Table 10-2
Circulating Factors That May Interfere With the Measurement of Pituitary Thyrotropin (TSH) or Total and Free Thyroid Hormones Giving Overestimation of the Actual Serum Levels of These Hormones and Thus Simulating the Presence of a TSH-Producing Adenoma
Heterophilic antibodies directed against mouse γ-globulins, leading to interference with monoclonal antibodies used in the immunometric assay*
Anti-TSH autoantibodies or antibodies cross-reacting with TSH†
Anti-iodothyronine autoantibodies (anti-T4 and/or anti-T3)‡
Abnormal forms of albumin or transthyretin (e.g., familial dysalbuminemic hyperthyroxinemia)‡
*This interference is commonly prevented by the addition of a few microliters of mouse serum to the assay buffer.
†Overestimation of TSH is very rare in the presence of such antibodies. This interference cannot be prevented, but it can be documented by performing dilution and recovery tests in the immunoassay.
‡To prevent misdiagnosis, measure free T4 and free T3 by direct “two-step” methods.76,77
In TSH-omas, extremely variable levels of serum TSH and thyroid hormones have been reported (Table 10-3). It is interesting to note that in patients who were treated previously with thyroid ablation, TSH levels were dramatically higher than in untreated patients, although free thyroid hormone levels were still in the hyperthyroid range and the reduction of total thyroid hormone levels was minimal. The conserved sensitivity of tumoral thyrotroph cells to even small reductions in circulating free thyroid hormone levels is confirmed by the rapidly increased rate of TSH secretion during antithyroid drug administration.57
Table 10-3
Biochemical Data of Patients With Pituitary Thyrotropin (TSH-oma) (Data from reports published until December 2007)
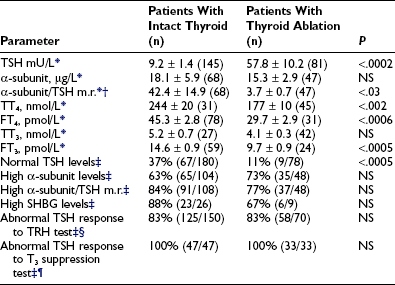
*Mean ± standard error (SE) (n).
†To calculate the α subunit/TSH molar ratio, divide α subunit (µg/L) by TSH (mU/L) and multiply by 10, provided that TSH IRP 80/558 is used in the immunometric assay.
¶Lack of complete TSH inhibition after 8 to 10 days of LT3 administration (80 to 100 µg/day).
Although patients with TSH-oma have TSH-dependent hyperstimulation of the thyroid gland, any significant correlation between immunoreactive TSH and free thyroid hormone levels is lacking, even though only untreated patients are taken into account. Moreover, in one third of these patients, high levels of free thyroid hormones are associated with immunoreactive TSH levels within the normal range. Variations in the biologic activity of secreted TSH molecules most likely account for these findings.79 The first demonstration that circulating TSH in patients with TSH-oma may possess an enhanced bioactivity was made in one patient with a mixed GH-TSH–secreting pituitary adenoma in whom a ratio between biologic and immunologic activities of TSH significantly higher than that of controls was documented.24 Other studies indicate that the circulating TSH biologic/immunologic activity ratio may be normal, reduced, or increased in patients with TSH-oma,22,79,80 probably because of altered glycosylation of circulating TSH molecules. In fact, both intrapituitary and circulating TSH exist as multiple isoforms characterized by heterogeneity of oligosaccharide chains, which has a great impact on hormone biologic properties, such as biologic activity and metabolic clearance rate. Tumoral transformation may be accompanied by variable alterations in posttranslational processing within thyrotrophs, leading to the secretion of TSH molecules with peculiar glycosylation and biologic properties.79,81,82
Glycoprotein Hormone α Subunit
TSH-omas commonly secrete excessive quantities of the free α subunit, resulting in high levels of circulating free α subunit in two thirds of patients (see Table 10-3). This is another expression of the altered synthetic process within tumoral thyrotropes and represents a helpful diagnostic clue to the presence of a TSH-oma. Secretion of the α subunit in these tumors is in excess not only of the TSH-β subunit, but also of the intact TSH molecule. This generally results in a molar ratio of α subunit to TSH that is higher than 1. Although previous studies have suggested that a ratio greater than 1.0 is indicative of the presence of TSH-producing adenoma,83 similar values have been observed in normal controls, particularly in postmenopausal women, indicating the need for appropriate control groups matched for TSH and gonadotropin levels.4,61,84 It is interesting to note that microadenomas that frequently have α subunit levels within the normal range may show a high α subunit/TSH molar ratio. Furthermore, it has been suggested that extremely high levels of free α subunit might portend future malignant behavior, and that a spontaneous and marked decrease in both TSH and α subunit might indicate that the tumor is becoming less differentiated and might correlate with invasive and metastatic behavior.18
Parameters Evaluating Peripheral Thyroid Hormone Action
Measurements of several parameters of peripheral thyroid hormone action both in vivo (basal metabolic rate, cardiac systolic time intervals, Achilles’ reflex time) and in vitro (sex hormone–binding globulin [SHBG], cholesterol, angiotensin-converting enzyme, osteocalcin, blood red cell sodium content, carboxyterminal cross-linked telopeptide of type I collagen [ICTP], and so on)85 may help in quantifying the degree of peripheral hyperthyroidism, particularly in patients with mild clinical signs and symptoms34,57,61,86–89 (see Table 10-3). In particular, evaluations of SHBG and ICTP may help to differentiate hyperthyroid patients with TSH-oma, in whom these parameters are elevated, from those with resistance to thyroid hormone, in whom they are in the range of those of euthyroid subjects.
Dynamic Testing
Several stimulatory and inhibitory tests have been employed to evaluate TSH secretory dynamics in patients with TSH-oma. None of these tests is of clear-cut diagnostic value, and the combination of some of them may enhance their accuracy in disclosing the pituitary adenoma. Among the stimulatory tests, TRH-induced TSH secretion is absent or blunted in 83% of patients (see Table 10-3). Although the α subunit response to the preceding stimulatory agents usually has paralleled that of TSH, discrepancy between α subunit and TSH response to TRH has been recorded in some cases. Such a discrepancy may be due to the presence of mixed adenomas, composed of distinct cell types that possess different receptor expression.24,28 Most TSH-omas are unable to increase TSH secretion after administration of dopamine antagonists such as domperidone or sulpiride. Long-term treatment with antithyroid drugs induces an increase in serum TSH levels in most patients because of both the high sensitivity of adenomatous cells to the reduction of circulating levels of FT4 and FT357 and recovered TSH secretion by normal thyrotrophs surrounding the adenoma in response to the activated feedback mechanism.4,90 In keeping with this are the observations of significantly higher TSH levels in patients who have undergone thyroid ablation, as well as the more active proliferation of tumoral cells in treated patients.
Among inhibitory tests, complete inhibition of both basal and TRH-stimulated TSH secretion after a T3 suppression test (Werner test: 80 to 100 µg/day of LT3 for 8 to 10 days) has never been recorded in a patient with a TSH-oma (see Table 10-3), although a slight TSH reduction may occur in a minority of patients. In patients who have undergone previous thyroid ablation, this test is the most sensitive and specific in documenting the possible presence of a TSH-oma. However, high doses of LT3 are contraindicated in elderly patients and in those with coronary heart disease. Dopamine (1 to 4 µg/kg body weight/min intravenously) or dopamine agonists, such as bromocriptine (2.5 mg orally), generally are ineffective in inhibiting TSH secretion, whereas native somatostatin or its analogues reduce TSH levels in most cases and may be predictive of the efficacy of long-term treatment in the majority of patients.61,91,92 We have demonstrated recently that long-term administration of long-acting somatostatin analogues in patients with central hyperthyroidism caused a marked decrease in both FT4 and FT3 circulating levels in all patients but one with TSH-omas, whereas patients with thyroid hormone resistance did not respond at all. Thus, administration of these analogues for at least 2 to 3 months can be useful in distinguishing the two forms of central hyperthyroidism.93
Imaging Studies and Localization
In considering the diagnosis of a TSH-oma, full imaging studies, particularly high-resolution computed tomography (CT) or nuclear magnetic resonance imaging (MRI), are necessary. Various degrees of suprasellar extension or sphenoidal sinus invasion are present in two thirds of cases. It is curious that in two patients, pituitary stones have been described.94
Microadenomas now are reported with increasing frequency, accounting for about 13% of all recorded cases. In contrast to other secreting pituitary tumors,95 no correlation between serum TSH levels and tumor size was found in untreated patients with TSH-oma. Pituitary scintigraphy with radiolabeled Tyr3-substituted octreotide has been shown to successfully image TSH-omas.96 Moreover, in vivo evidence for both somatostatin and dopamine D2 receptors was obtained with the use of single-photon emission tomography with 111In-pentetreotide and 123I-iodobenzamide.92,97 The presence of these receptors correlates with the sensitivity of the tumor to long-term medical treatment. 111In-pentetreotide scintigraphy also may be useful in localizing possible ectopic TSH-producing adenomas. Finally, bilateral petrosal sinus sampling has been used in difficult cases, allowing the identification and lateralization of a microadenoma not seen on radiographic scans.98 However, one should expect a certain number of false lateralizations, as already has been observed for ACTH-secreting pituitary tumors.
Differential Diagnosis
The presence of detectable TSH levels in a hyperthyroid patient rules out primary hyperthyroidism, whereas in patients receiving levothyroxine replacement for primary hypothyroidism, poor compliance is by far the most common cause of apparent inappropriate secretion of TSH, with TSH still too high for the levels of thyroid hormones. This underscores the importance of studying patients in steady state. The first step in the case of hyperthyroxinemia and detectable TSH is to measure free thyroid hormone levels and repeat TSH by ultrasensitive assays. The finding of normal TSH, FT4, and FT3 levels suggests euthyroid hyperthyroxinemia, whereas high FT4 and FT3 concentrations and suppressed TSH definitively indicate the presence of primary hyperthyroidism due to Graves’ disease and other forms of thyrotoxicosis. If FT4 and FT3 concentrations are elevated in the presence of measurable TSH levels, it is important to exclude methodologic interference. When the existence of central hyperthyroidism is eventually confirmed, several diagnostic steps have to be carried out to differentiate a TSH-oma from resistance to thyroid hormone (RTH). This is particularly true for the variant of RTH with predominant pituitary resistance with clear clinical signs and symptoms of hyperthyroidism.7–9 Indeed, alterations of pituitary content on CT or MRI, as well as the possible presence of neurologic signs and symptoms (visual defects, headache), or clinical features of concomitant hypersecretion of other pituitary hormones (acromegaly, galactorrhea, amenorrhea) definitely point to the presence of a TSH-oma. Nevertheless, the differential diagnosis may be difficult when the pituitary adenoma is undetectable by CT or MRI or in the case of confusing (empty sella) or incidental pituitary lesions.99,100 No significant differences in age, sex, previous thyroid ablation, TSH levels, or free thyroid hormone concentrations occur between patients with TSH-oma and those with RTH (Table 10-4). However, in contrast to RTH patients, familial cases of TSH-oma have never been documented. The finding of measurable TSH levels and high concentrations of FT4 and FT3 in one relative definitely points to the diagnosis of RTH. Serum TSH levels within the normal range are found more frequently in RTH, whereas elevated α subunit concentrations or a high α subunit/TSH molar ratio typically is present in patients with TSH-omas. Moreover, absent or impaired TSH responses to TRH administration and to the T3 suppression test favor the presence of a TSH-oma. Circulating SHBG levels are in the hyperthyroid range in patients with TSH-oma, the only patients with low SHBG being those with concomitant hypersecretion of GH, which potently inhibits SHBG secretion. The few patients with RTH and high SHBG levels were those who were treated with estrogens or those who showed profound hypogonadism.88 Other parameters that may be useful in the differential diagnosis are markers of bone turnover, such as ICTP (altered in TSH-omas, normal in RTH) or total cholesterol (rarely high in TSH-omas).9,89 In difficult cases, particularly after thyroidectomy, genetic investigations into thyroid hormone receptor-β1 mutations may be the only diagnostic test. Finally, an apparent association between TSH-oma and resistance to thyroid hormone has been reported in a few patients.86,101 Although genetic studies and familial investigations were not carried out in the Japanese patient, the occurrence of TSH-omas in patients with RTH is theoretically possible and therefore should be considered carefully.
Table 10-4
Differential Diagnosis Between TSH-Producing Adenomas (TSH-omas) and Resistance to Thyroid Hormones (RTH)
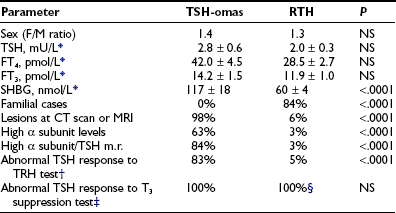
*Only patients with intact thyroid were taken into account. Data were obtained from patients followed at our institution (18 TSH-omas and 68 RTH) and are expressed as mean ± standard error (SE) (n).
‡Werner’s test (80 to 100 µg T3 for 8 to 10 days). Quantitatively normal responses to T3 (i.e., complete inhibition of both basal and TRH-stimulated TSH levels) have never been recorded in either group of patients.
§Although abnormal in quantitative terms, TSH response to T3 suppression test is qualitatively normal in most RTH patients.7–9
Treatment and Outcome
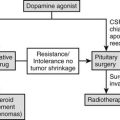
Pituitary Surgery and Radiation Therapy
The primary goal in the treatment of TSH-omas is to remove the pituitary tumor and restore euthyroidism. Therefore, the first therapeutic approach to TSH-producing adenomas should be to surgically remove or debulk the tumor by transsphenoidal or subfrontal adenomectomy, the choice of route depending on the tumor volume and its suprasellar extension.1,16,102,103 This may be particularly difficult because of the marked fibrosis of these tumors and local invasion involving the cavernous sinus, internal carotid artery, or optic chiasm. To restore euthyroidism before surgery, antithyroid drugs or octreotide along with propranolol can be administered. If surgery is contraindicated or declined, pituitary radiotherapy (no less than 45 Gy fractionated at 2 Gy/day or 10 to 25 Gy in a single dose if a stereotactic gamma unit is available) and subsequent somatostatin analogue administration should be considered.
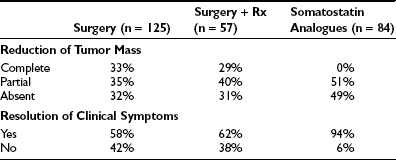
Surgery alone or combined with radiotherapy induces normalization of thyroid hormone levels and apparent complete removal of tumor mass in about one third of patients, whereas normalization of thyroid hormones without complete removal of the adenoma occurs in an another third of patients (Table 10-5). Collectively, about two thirds of TSH-omas are brought under control with surgery, irradiation, or both. In the remaining patients, the large size and the invasiveness of the tumor prevent successful removal of the tumor. Elevation of α subunit or cosecretion of other pituitary hormones does not seem to be an unfavorable prognostic factor. Postsurgical deaths were reported in five cases. Partial or complete hypopituitarism may be the result of surgery. Evaluation of other pituitary functions, particularly ACTH secretion, should be undertaken carefully soon after surgery and should be performed again every year, especially in patients who are treated by radiotherapy. In addition, in the case of surgical cure, postoperative TSH is undetectable and may remain low for many weeks or months, causing central hypothyroidism. The time necessary for the recovery of normal thyrotrophs is variable, and permanent central hypothyroidism occasionally may occur as the result of damage to the normal thyrotroph by the tumor or during surgery. Thus, temporary or permanent LT4 replacement therapy may be necessary. In a few cases, total thyroidectomy was performed after pituitary surgery failed because patients were at risk for thyroid storm.
Medical Treatment
In terms of pharmacologic therapy for TSH-omas, antithyroid drugs must not be used because they may cause worsening of goiter size or more rapid growth and invasiveness of the pituitary adenoma, and their use is recommended only as preparation of the patient for neurosurgery. For alleviating the symptoms of hyperthyroidism, β-blockers such as propranolol may be used. Glucocorticoids are effective in reducing TSH secretion, but they induce deleterious side effects in long-term treatment. Dopamine agonists, particularly bromocriptine, have been employed in some TSH-omas with variable results, with the positive effects observed in some patients with mixed TSH- and PRL-secreting adenoma diminishing over time.104,105 Currently, medical treatment for TSH-oma rests on somatostatin analogues (SSa) such as octreotide47,91–93,106–108 or the slow-release formulation of lanreotide.109,110 Both SSa lead to a reduction in TSH and α subunit secretion in almost all cases, with restoration of the euthyroid state in most (see Table 10-5). Moreover, modifications of the TSH glycoisomer distribution pattern during SSa treatment have been documented in one patient,111 suggesting that restoration of euthyroidism in some patients who show no reduction in immunoreactive levels of TSH during SSa therapy may be due to a reduction in the bioactivity of secreted molecules.91,112 During SSa therapy, tumor shrinkage occurs in about half of patients (see Table 10-5), and vision improvement is seen in 75%.
Tachyphylaxis occurred in about one fifth of patients and responded to increasing SSa doses, whereas long-term studies demonstrated true escape from the inhibitory effects in few cases. In only 5% of cases was true resistance to SSa treatment documented. Octreotide treatment was effective in restoring euthyroidism in one pregnant woman with central hyperthyroidism and had no side effects on fetal development and thyroid function.113,114 Patients taking octreotide have to be monitored carefully because untoward side effects, such as cholelithiasis and carbohydrate intolerance, may arise. The dose administered should be tailored for each patient, depending on therapeutic response and tolerance (including gastrointestinal side effects). The marked SSa-induced suppression of TSH secretion and consequent biochemical hypothyroidism seen in some patients may require LT4 substitution. Whether somatostatin analogue treatment may be an alternative to surgery and irradiation in patients with TSH-oma remains to be established. However, the slow-release preparation of somatostatin, lanreotide-SR, and octreotide-LAR may represent useful tools for long-term treatment of such a rare pituitary adenoma.
Criteria of Cure and Follow-Up
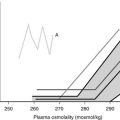
Evidence has accumulated about the criteria of cure and follow-up for patients undergoing operation or irradiation for TSH-omas.4,57,61 In untreated hyperthyroid patients, it is reasonable to assume that cured patients have clinical and biochemical reversal of thyroid hyperfunction. However, normal free thyroid hormone concentrations or indices of peripheral thyroid hormone action in the euthyroid range may be associated with partial removal or destruction of tumoral cells, since transient clinical remission and euthyroidism are observed frequently.57,61 As it occurs for other pituitary tumors, disappearance of neurologic signs and symptoms only partially reflects the radicality of tumor removal, in that it may occur even in the presence of an incomplete debulking of the tumor. Pituitary imaging performed after surgery has low predictivity because of the high frequency of false-negative imaging results. The criteria for normalization of circulating TSH are not applicable to previously thyroidectomized patients or to those with normal basal values of TSH. In our experience, undetectable TSH levels 1 week after surgery are likely to indicate complete adenomectomy, provided that the patient was hyperthyroid and presurgical treatments were stopped at least 10 days before surgery.48 Similarly, although normalization of α subunit or the α subunit/TSH molar ratio in general is a good index for the evaluation of therapy efficacy, both parameters are normal in a remarkable number of patients with TSH-oma. The most sensitive and specific test used to document complete removal of the adenoma remains the T3 suppression test (in the absence of clinical contraindication). In fact, regardless of the restoration of euthyroidism, only patients in whom T3 administration completely inhibits basal and TRH-stimulated TSH secretion appear to be truly cured (Fig. 10-4).
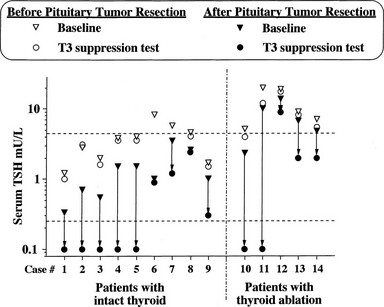
FIGURE 10-4 Results of T3 suppression test carried out before and after pituitary surgery in patients with pituitary thyrotropin (TSH)-producing adenoma. Horizontal dashed lines indicate the normal range of serum TSH. Note the lack of TSH suppression in all patients before neurosurgery. Complete suppression of serum TSH levels (i.e., complete removal of the adenoma) was seen in about half of patients after neurosurgery, independent of previous thyroid ablation.
References
1. Clarke, MJ, Erickson, D, Castro, R, et al. Thyroid-stimulating hormone pituitary adenomas. J Neurosurg. 2008;109:17.
2. Samuels, MH, Ridgway, EC. Glycoprotein-secreting pituitary adenomas. Baillieres Clin Endocrinol. 1995;9:337.
3. Greenman, Y, Melmed, S. Thyrotropin-secreting pituitary tumors. In: Melmed S, ed. The Pituitary. Boston: Blackwell Science; 1995:546.
4. Beck-Peccoz, P, Brucker-Davis, F, Persani, L, et al. Thyrotropin-secreting pituitary tumors. Endocr Rev. 1996;17:610.
5. Jailer, JW, Holub, DA. Remission of Graves’ disease following radiotherapy of a pituitary neoplasm. Am J Med. 1960;28:497.
6. Hamilton, C, Adams, LC, Maloof, F. Hyperthyroidism due to thyrotropin-producing pituitary chromophobe adenoma. N Engl J Med. 1970;283:1077.
7. Refetoff, S, Weiss, RE, Usala, SJ. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993;14:348.
8. Beck-Peccoz, P, Asteria, C, Mannavola, D. Resistance to thyroid hormone. In: Braverman LE, ed. Contemporary Endocrinology: Diseases of the Thyroid. Totowa, NJ: Humana Press; 1997:199.
9. Gurnell M, Beck-Peccoz P, Chatterjee VKK: Resistance to thyroid hormone.
10. Felix, I, Asa, SL, Kovacs, K, et al. Recurrent plurihormonal bimorphous pituitary adenoma producing growth hormone, thyrotropin, and prolactin. Arch Pathol Lab Med. 1994;118:66.
11. Bertholon-Gregoire, M, Trouillas, J, Guigard, MP, et al. Mono and plurihormonal thyrotropic pituitary adenomas: Pathological, hormonal and clinical studies in twelve patients. Eur J Endocrinol. 1999;140:519.
12. Katz, MS, Gregerman, RI, Horvath, E, et al. Thyrotroph cell adenoma of the human pituitary gland associated with primary hypothyroidism: Clinical and morphological features. Acta Endocrinol. 1980;95:41.
13. Ghannam, NN, Hammami, MM, Muttair, Z, et al. Primary hypothyroidism-associated TSH-secreting pituitary adenoma/hyperplasia presenting as a bleeding nasal mass and extremely elevated TSH level. J Endocrinol Invest. 1999;22:419.
14. Losa, M, Mortini, P, Minelli, R, et al. Coexistence of TSH-secreting pituitary adenoma and autoimmune hypothyroidism. J Endocrinol Invest. 2006;29:555.
15. Kienitz, T, Quinkler, M, Strasburger, C, et al. Long-term management in five cases of TSH-secreting pituitary adenomas: a single center study and review of the literature. Eur J Endocrinol. 2007;157:39.
16. Socin, HV, Chanson, P, Delemer, B, et al. The changing spectrum of TSH-secreting pituitary adenomas: Diagnosis and management in 43 patients. Eur J Endocrinol. 2003;148:433.
17. Mindermann, T, Wilson, CB. Thyrotropin-producing pituitary adenomas. J Neurosurg. 1993;79:521.
18. Mixson, AJ, Friedman, TC, David, AK, et al. Thyrotropin-secreting pituitary carcinoma. J Clin Endocrinol Metab. 1993;76:529.
19. Brown, RL, Muzzafar, T, Wollman, R, et al. A pituitary carcinoma secreting TSH and prolactin: a non-secreting adenoma gone awry. Eur J Endocrinol. 2006;154:639.
20. Cooper, DS, Wenig, BM. Hyperthyroidism caused by an ectopic TSH-secreting pituitary tumor. Thyroid. 1996;6:337.
21. Pasquini, E, Faustini-Fustini, M, Sciarretta, V, et al. Ectopic TSH-secreting pituitary adenoma of the vomerosphenoidal junction. Eur J Endocrinol. 2003;148:253.
22. Gesundheit, N, Petrick, P, Nissim, M, et al. Thyrotropin-secreting pituitary adenomas: Clinical and biochemical heterogeneity. Ann Intern Med. 1989;111:827.
23. Teramoto, A, Sanno, N, Tahara, S, et al. Pathological study of thyrotropin-secreting pituitary adenoma: plurihormonality and medical treatment. Acta Neuropathol. 2004:108–147.
24. Beck-Peccoz, P, Piscitelli, G, Amr, S, et al. Endocrine, biochemical, and morphological studies of a pituitary adenoma secreting growth hormone, thyrotropin (TSH), and α-subunit: Evidence for secretion of TSH with increased bioactivity. J Clin Endocrinol Metab. 1986;62:704.
25. Ozawa, Y, Kameya, T, Kasuga, A, et al. A functional thyrotropin- and growth hormone-secreting pituitary adenoma with ultrastructurally monomorphic feature: A case study. Endocr J. 1998;45:211.
26. Ikeda, H, Ogawa, Y, Yoshimoto, T. Ultrastructural characteristics of TSH-producing adenomas with special reference to its close similarity to BFA-treated pituitary adenoma cells. Pituitary. 1999;1:221.
27. Trouillas, J, Girod, C, Loras, B, et al. The TSH secretion in the human pituitary adenomas. Pathol Res Pract. 1988;183:596.
28. Terzolo, M, Orlandi, F, Bassetti, M, et al. Hyperthyroidism due to a pituitary adenoma composed of two different cell types, one secreting alpha-subunit alone and another cosecreting alpha-subunit and thyrotropin. J Clin Endocrinol Metab. 1991;72:415.
29. Jaquet, P, Hassoun, J, Delori, P, et al. A human pituitary adenoma secreting thyrotropin and prolactin: Immunohistochemical, biochemical and cell culture study. J Clin Endocrinol Metab. 1984;59:817.
30. Kuzuya, N, Inoue, K, Ishibashi, M, et al. Endocrine and immunohistochemical studies on thyrotropin (TSH)-secreting pituitary adenomas: Responses of TSH, alpha-subunit and growth hormone to hypothalamic releasing hormones and their distribution in adenoma cells. J Clin Endocrinol Metab. 1990;71:1103.
31. Malarkey, WB, Kovacs, K, O’Dorisio, T. Response of GH- and TSH-secreting pituitary adenoma to a somatostatin analogue (SMS 201–995): Evidence that GH and TSH coexist in the same cell and secretory granules. Neuroendocrinology. 1989;49:267.
32. Sanno, N, Teramoto, A, Matsuno, A, et al. Clinical and immunohistochemical studies on TSH-secreting pituitary adenoma: Its multihormonality and expression of Pit-1. Modern Pathol. 1994;7:893.
33. Dong, Q, Brucker-Davis, F, Weintraub, BD, et al. Screening of candidate oncogenes in human thyrotroph tumors: Absence of activating mutations of the Gαq, Gα11, Gαs, or thyrotropin-releasing hormone receptor genes. J Clin Endocrinol Metab. 1996;81:1134.
34. Lind, P, Langsteger, W, Koltringer, P, et al. Transient prealbumin-associated hyperthyroxinemia in a TSH-producing pituitary adenoma. Nuklearmedizin. 1990;29:40.
35. Waldhausl, W, Brautsch-Marrain, P, Nowotony, P, et al. Secondary hyperthyroidism due to thyrotropin hypersecretion: Study of pituitary tumor morphology and thyrotropin chemistry and release. J Clin Endocrinol Metab. 1979;49:879.
36. Stanley, JM, Najjar, SS. Hyperthyroidism secondary to a TSH-secreting pituitary adenoma in a 15-year-old-male. Clin Pediatr. 1991;30:109.
37. Patrick, AW, Atkin, SL, MacKenzie, J, et al. Hyperthyroidism secondary to a pituitary adenoma secreting TSH, FSH, alpha-subunit and GH. Clin Endocrinol (Oxf). 1994;40:275.
38. Ma, W, Ikeda, H, Watabe, N, et al. A plurihormonal TSH-producing pituitary tumor of monoclonal origin in a patient with hypothyroidism. Horm Res. 2003;59:257.
39. Pellegrini, I, Barlier, A, Gunz, G, et al. Pit-1 gene expression in the human pituitary and pituitary adenomas. J Clin Endocrinol Metab. 1994;79:189.
40. Boggild, MD, Jenkinson, S, Pistorello, M, et al. Molecular genetics studies of sporadic pituitary tumors. J Clin Endocrinol Metab. 1994;78:387.
41. Bamberger, CM, Fehn, M, Bamberger, AM, et al. Reduced expression levels of the cell-cycle inhibitor p27Kip1 in human pituitary adenomas. Eur J Endocrinol. 1999;140:250.
42. Sumi, T, Stefaneanu, L, Kovacs, K, et al. Immunohistochemical study of p53 protein in human and animal pituitary tumors. Endocr Pathol. 1993;4:95.
43. Chanson, P, Li, JY, LeDafniet, M, et al. Absence of receptors for thyrotropin (TSH)-releasing hormone in human TSH-secreting pituitary adenomas associated with hyperthyroidism. J Clin Endocrinol Metab. 1988;66:447.
44. LeDafniet, M, Brandi, A-M, Kujas, M, et al. Thyrotropin-releasing hormone (TRH) binding sites and thyrotropin response to TRH are regulated by thyroid hormones in human thyrotropic adenomas. Eur J Endocrinol. 1994;130:559.
45. Kim, K, Arai, K, Sanno, N, et al. The expression of thyrotrophin-releasing hormone receptor 1 messenger ribonucleic acid in human pituitary adenomas. Clin Endocrinol (Oxf). 2001;54:309.
46. Takano, K, Ajima, M, Teramoto, A, et al. Mechanism of action of somatostatin on human TSH-secreting adenoma cells. Am J Physiol. 1995;268:E558.
47. Bertherat, J, Brue, T, Enjalbert, A, et al. Somatostatin receptors on thyrotropin-secreting pituitary adenomas: Comparison with the inhibitory effects of octreotide upon in vivo and in vitro hormonal secretions. J Clin Endocrinol Metab. 1992;75:540.
48. Horiguchi, K, Yamada, M, Umezawa, R, et al. Somatostatin receptor subtypes mRNA in TSH-secreting pituitary adenomas: a case showing a dramatic reduction in tumor size during short octreotide treatment. Endocr J. 2007;54:371.
49. Filopanti, M, Ballarè, E, Lania, AG, et al. Loss of heterozygosity at the SS receptor type 5 locus in human GH- and TSH-secreting pituitary adenomas. J Endocrinol Invest. 2004;27:937.
50. Ando, S, Sarlis, NJ, Oldfield, EH, Yen, PM. Somatic mutation of TRbeta can cause a defect in negative regulation of TSH in a TSH-secreting pituitary tumor. J Clin Endocrinol Metab. 2001;86:5572.
51. Ando, S, Sarlis, NJ, Krishnan, J, et al. Aberrant alternative splicing of thyroid hormone receptor in a TSH-secreting pituitary tumor is a mechanism for hormone resistance. Mol Endocrinol. 2001;15:1529.
52. Tannahill, LA, Visser, TJ, McCabe, CJ, et al. Dysregulation of iodothyronine deiodinase enzyme expression and function in human pituitary tumours. Clin Endocrinol (Oxf). 2002;56:735.
53. Baur, A, Buchfelder, M, Kohrle, J. Expression of 5′-deiodinase enzymes in normal pituitaries and in various human pituitary adenomas. Eur J Endocrinol. 2002;147:263.
54. Yamakita, N, Ikeda, T, Murai, T, et al. Thyrotropin-producing pituitary adenoma discovered as a pituitary incidentaloma. Intern Med. 1995;34:1055.
55. Koide, Y, Kugai, N, Kimura, S, et al. A case of pituitary adenoma with possible simultaneous secretion of thyrotropin and follicle-stimulating hormone. J Clin Endocrinol Metab. 1982;54:397.
56. Scanlon, MF, Howells, S, Peters, JR, et al. Hyperprolactinaemia, amenorrhoea and galactorrhoea due a pituitary thyrotroph adenoma. Clin Endocrinol. 1985;23:35.
57. Losa, M, Giovanelli, M, Persani, L, et al. Criteria of cure and follow-up of central hyperthyroidism due to thyrotropin-secreting pituitary adenomas. J Clin Endocrinol Metab. 1996;81:3084.
58. Kiso, Y, Yoshida, K, Kaise, K, et al. A case of thyrotropin (TSH)-secreting tumor complicated by periodic paralysis. Jpn J Med. 1990;29:399.
59. Alings, AM, Fliers, E, de Herder, WW, et al. A thyrotropin-secreting pituitary adenoma as a cause of thyrotoxic periodic paralysis. J Endocrinol Invest. 1998;21:703.
60. Hsu, FS, Tsai, WS, Chau, T, et al. Thyrotropin-secreting pituitary adenoma presenting as hypokalemic periodic paralysis. Am J Med Sci. 2003;325:48.
61. Brucker-Davis, F, Oldfield, EH, Skarulis, MC, et al. Thyrotropin-secreting pituitary tumors: Diagnostic criteria, thyroid hormone sensitivity, and treatment outcome in 25 patients followed at the National Institutes of Health. J Clin Endocrinol Metab. 1999;84:476.
62. Calle-Pascual, AL, Yuste, E, Martin, P, et al. Association of a thyrotropin-secreting pituitary adenoma and a thyroid follicular carcinoma. J Endocrinol Invest. 1991;14:499.
63. Gasparoni, P, Rubello, D, Persani, L, et al. Unusual association between a thyrotropin-secreting pituitary adenoma and a papillary thyroid carcinoma. Thyroid. 1998;8:181.
64. Kishida, M, Otsuka, F, Kataoka, H, et al. Hyperthyroidism in a patient with TSH-producing pituitary adenoma coexisting with thyroid papillary adenocarcinoma. Endocr J. 2000;47:731.
65. Ohta, S, Nishizawa, S, Oki, Y, et al. Coexistence of thyrotropin-producing pituitary adenoma with papillary adenocarcinoma of the thyroid: A case report and surgical strategy. Pituitary. 2001;4:271.
66. Beckers, A, Abs, R, Mahler, C, et al. Thyrotropin-secreting pituitary adenomas: Report of seven cases. J Clin Endocrinol Metab. 1991;72:477.
67. Abs, R, Stevenaert, A, Beckers, A. Autonomously functioning thyroid nodules in a patient with a thyrotropin-secreting pituitary adenoma: Possible cause-effect relationship. Eur J Endocrinol. 1994;131:355.
68. Sy, ARG, Bernstein, R, Chynn, KI, et al. Reduction in size of a thyrotropin- and gonadotropin-secreting pituitary adenoma treated with octreotide acetate (somatostatin analogue). J Clin Endocrinol Metab. 1992;74:690.
69. Horn, K, Erhardt, F, Fahlbusch, R, et al. Recurrent goiter, hyperthyroidism, galactorrhoea and amenorrhoea due to a thyrotropin and prolactin-producing pituitary tumor. J Clin Endocrinol Metab. 1976;43:137.
70. Adriaanse, R, Brabant, G, Endert, E, et al. Pulsatile thyrotropin and prolactin secretion in a patient with a mixed thyrotropin- and prolactin-secreting pituitary adenoma. Eur J Endocrinol. 1994;130:113.
71. Burgess, JR, Shepherd, JJ, Greenaway, TM. Thyrotropinomas in multiple endocrine neoplasia type 1 (MEN-1). Aust N Z J Med. 1994;24:740.
72. Wynne, AG, Gharib, H, Scheithauer, BW, et al. Hyperthyroidism due to inappropriate secretion of thyrotropin in 10 patients. Am J Med. 1992;92:15.
73. Taylor, TJ, Donlon, SS, Bale, AE, et al. Treatment of a thyrotropinoma with octreotide-LAR in a patient with multiple endocrine neoplasia-1. Thyroid. 2000;10:1001.
74. Gessl, A, Freissmuth, M, Czech, T, et al. Growth hormone-prolactin-thyrotropin-secreting pituitary adenoma in atypical McCune-Albright syndrome with functionally normal Gsα protein. J Clin Endocrinol Metab. 1994;79:1128.
75. Langlois, M-F, Lamarche, JB, Bellabarba, D. Long-standing goiter and hypothyroidism: An unusual presentation of a TSH-secreting adenoma. Thyroid. 1996;6:329.
76. Ekins, R. Measurement of free hormones in blood. Endocr Rev. 1990;11:5.
77. Beck-Peccoz, P, Piscitelli, G, Cattaneo, MG, et al. Evaluation of free thyroxine methods in the presence of iodothyronine binding autoantibodies. J Clin Endocrinol Metab. 1984;58:736.
78. Zweig, MH, Csako, G, Spero, M. Escape from blockade of interfering heterophile antibodies in a two-site immunoradiometric assay for thyrotropin. Clin Chem. 1988;34:2589.
79. Beck-Peccoz, P, Persani, L. Variable biological activity of thyroid-stimulating hormone. Eur J Endocrinol. 1994;131:331.
80. Bevan, JS, Burke, CW, Esiri, MM, et al. Studies of two thyrotropin-secreting pituitary adenomas: Evidence for dopamine receptor deficiency. Clin Endocrinol. 1989;31:59.
81. Magner, JA, Kane, J. Binding of thyrotropin to lentil lectin is unchanged by thyrotropin-releasing hormone administration in three patients with thyrotropin-producing pituitary adenomas. Endocr Res. 1992;8:163.
82. Magner, JA, Klibanski, A, Fein, H, et al. Ricin and lentil lectin affinity chromatography reveals oligosaccharide heterogeneity of thyrotropin secreted by 12 human pituitary tumors. Metabolism. 1992;41:1009.
83. Kourides, IA, Ridgway, EC, Weintraub, BD, et al. Thyrotropin-induced hyperthyroidism: Use of alpha and beta subunit levels to identify patients with primary tumors. J Clin Endocrinol Metab. 1977;45:534.
84. Beck-Peccoz, P, Persani, L, Faglia, G. Glycoprotein hormone α-subunit in pituitary adenomas. Trends Endocrinol Metab. 1992;3:41.
85. Franklyn, J, Shephard, M, Evaluation of thyroid function in health and disease. DeGroot LJ, ed., Thyroid disease, 2008. http://www.thyroidmanager.org.
86. Watanabe, K, Kameya, T, Yamauchi, A, et al. Thyrotropin-producing adenoma associated with pituitary resistance to thyroid hormone. J Clin Endocrinol Metab. 1993;76:1025.
87. Azarnivar, A, Chopra, IJ. Tension pneumoencephalus after transsphenoidal resection of a thyrotropin (TSH)-secreting pituitary adenoma. Endocrinologist. 1995;5:308.
88. Beck-Peccoz, P, Roncoroni, R, Mariotti, S, et al. Sex hormone-binding globulin measurement in patients with inappropriate secretion of thyrotropin (IST): Evidence against selective pituitary thyroid hormone resistance in nonneoplastic IST. J Clin Endocrinol Metab. 1990;71:19.
89. Persani, L, Preziati, D, Matthews, CH, et al. Serum levels of carboxyterminal cross-linked telopeptide of type I collagen (ICTP) in the differential diagnosis of the syndromes of inappropriate secretion of TSH. Clin Endocrinol. 1997;47:207.
90. Rubello, D, Busnardo, B, Girelli, ME, et al. Severe hyperthyroidism due to neoplastic TSH hypersecretion in an old man. J Endocrinol Invest. 1989;12:571.
91. Chanson, P, Weintraub, BD, Harris, AG. Octreotide therapy for thyroid stimulating hormone-secreting pituitary adenomas: A follow-up of 52 patients. Ann Intern Med. 1993;119:236.
92. Losa, M, Magnani, P, Mortini, P, et al. Indium-111 pentetreotide single-photon emission tomography in patients with TSH-secreting pituitary adenomas: Correlation with the effect of a single administration of octreotide on serum TSH levels. Eur J Nucl Med. 1997;24:728.
93. Mannavola, D, Persani, L, Vannucchi, G, et al. Different responses to chronic somatostatin analogues in patients with central hyperthyroidism. Clin Endocrinol (Oxf). 2005;62:176.
94. Webster, J, Peters, JR, John, R, et al. Pituitary stone: Two cases of densely calcified thyrotropin-secreting pituitary adenomas. Clin Endocrinol. 1994;40:137.
95. Nabarro, JDN. Acromegaly. Clin Endocrinol (Oxf). 1987;26:481.
96. Lamberts, SWJ, Krenning, EP, Reubi, J-C. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocr Rev. 1991;12:450.
97. Verhoeff, NPLG, Bemelman, FJ, Wiersinga, WM, et al. Imaging of dopamine D2 and somatostatin receptors in vivo using single-photon emission tomography in a patient with a TSH/PRL-producing pituitary macroadenoma. Eur J Nucl Med. 1993;20:555.
98. Frank, SJ, Gesundheit, N, Doppman, JL, et al. Preoperative lateralization of pituitary microadenomas by petrosal sinus sampling: Utility in two patients with non-ACTH-secreting tumors. Am J Med. 1989;87:679.
99. Mariotti, S, Anelli, S, Bartalena, L, et al. Familial hyperthyroidism due to nonneoplastic inappropriate TSH secretion associated with sellar abnormalities [Abstract]. J Endocrinol Invest. 1987;10(Suppl 1):20.
100. Hall, WA, Luciano, MG, Doppman, JL, et al. Pituitary magnetic resonance imaging in normal human volunteers: Occult adenomas in the general population. Ann Intern Med. 1994;120:817.
101. Safer, JD, Colan, SD, Fraser, LM, et al. A pituitary tumor in a patient with thyroid hormone resistance: A diagnostic dilemma. Thyroid. 2001;11:281.
102. McCutcheon, IE, Weintraub, BD, Oldfield, EH. Surgical treatment of thyrotropin-secreting pituitary adenomas. J Neurosurg. 1990;73:674.
103. Losa, M, Mortini, P, Franzin, A, et al. Surgical management of thyrotropin-secreting pituitary adenomas. Pituitary. 1999;2:127.
104. Mouton, F, Faivre-Defrance, F, Cortet-Rudelli, C, et al. TSH secreting adenoma improved with cabergoline. Ann Endocrinol (Paris). 2008;69:244.
105. Zuniga, S, Mendoza, V, Espinoza, IF, et al. A plurihormonal TSH-secreting pituitary microadenoma: Report of a case with an atypical clinical presentation and transient response to bromocriptine therapy. Endocr Pathol. 1997;8:81.
106. Comi, R, Gesundheit, N, Murray, L, et al. Response of thyrotropin-secreting pituitary adenomas to a long-acting somatostatin analogue. N Engl J Med. 1987;317:12.
107. Gourgiotis, L, Skarulis, MC, Brucker-Davis, F, et al. Effectiveness of long-acting octreotide in suppressing hormonogenesis and tumor growth in thyrotropin-secreting pituitary adenomas: report of two cases. Pituitary. 2001;4:135.
108. Caron, P, Arlot, S, Bauters, C, et al. Efficacy of the long-acting octreotide formulation (octreotide-LAR) in patients with thyrotropin-secreting pituitary adenomas. J Clin Endocrinol Metab. 2001;86:2849.
109. Gancel, A, Vuillermet, P, Legrand, A, et al. Effects of a slow-release formulation of the new somatostatin analogue lanreotide in TSH-secreting pituitary adenomas. Clin Endocrinol. 1994;40:421.
110. Kuhn, JM, Arlot, S, Lefebvre, H, et al. Evaluation of the treatment of thyrotropin-secreting pituitary adenomas with a slow release formulation of the somatostatin analog lanreotide. J Clin Endocrinol Metab. 2000;85:1487.
111. Francis, TB, Smallridge, RC, Kane, J, et al. Octreotide changes serum thyrotropin (TSH) glycoisomer distribution as assessed by lectin chromatography in a TSH macroadenoma patient. J Clin Endocrinol Metab. 1993;77:183.
112. Hill, S, Falko, J, Wilson, C, et al. Thyrotropin-producing pituitary adenomas. J Neurosurg. 1982;57:515.
113. Caron, P, Gerbaud, C, Pradayrol, L, et al. Successful pregnancy in an infertile woman with a thyrotropin-secreting macroadenoma treated with somatostatin analog (octreotide). J Clin Endocrinol Metab. 1996;81:1164.
114. Blackhurst, G, Strachan, MW, Collie, D, et al. The treatment of a thyrotropin-secreting pituitary macroadenoma with octreotide in twin pregnancy. Clin Endocrinol (Oxf). 2002;57:401.