CHAPTER 62 Treatment of the Infected Total Elbow Arthroplasty
INTRODUCTION
Despite multiple improvements in total elbow arthroplasty design, infection was a relatively common and potentially catastrophic complication, with reported rates as high as 11% in earlier studies.* The rate had decreased considerably, and was 3% in our practice overall.39 Others have reported similar findings, with a slightly higher infection rate of approximately 8% in the revision setting,32 and 5% in patients with rheumatoid arthritis.1,11,30,35 Although not demonstrated with certainty, the rate seems to have increased since the introduction of the disease-remitting agents (DMARDs) to treat rheumatoid arthritis (see Chapter 54).
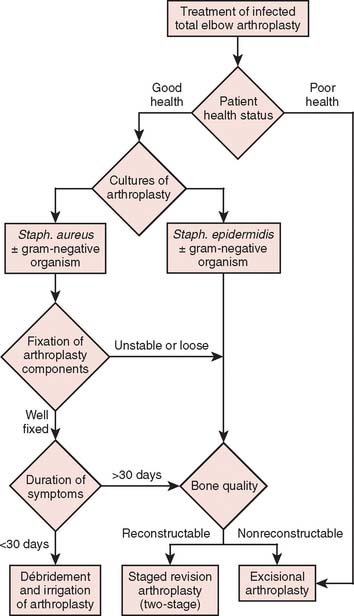
This difficult complication can be treated with either open débridement with placement of antibiotic beads and staged bushing exchange, removal followed by staged reimplantation of components, or resection arthroplasty, depending on the onset and chronicity of symptoms from the time of index arthroplasty. With little information on which to base treatment decisions, poorly functioning and sometimes painful resection arthroplasty has been the definitive procedure of choice, particularly in elderly patients who have relatively low demands on the elbow.14,27,38 Treatment options that have allowed concurrent eradication of the infection with either prosthetic retention or reimplantation have been explored with relatively good results in the past decade, especially at the knee and hip.15,16,21,22,24 Thus, this treatment strategy has been employed in the treatment of the infected total elbow arthroplasty under certain circumstances.6,39,40 Patients who have clinical evidence of infection but have negative cultures represent a treatment dilemma. Most are treated as if they are infected, with resection of the prosthesis, and staged reimplantation after cultures are followed to completion, with a course of parenteral antibiotics. However, this strategy increases the morbidity from a repeat general anesthetic as well as another surgical insult to the elbow. If the implants are well fixed, removal of the prosthesis may pose technically challenging.28,38 The objective of this chapter is to review the evaluation and treatment of the infected total elbow arthroplasty. In particular, patient presentation including health profile, duration of symptoms, fixation of components, and bacteriology are discussed in relation to indications for various treatment strategies for the infected elbow arthroplasty (Fig. 62-1).
ETIOLOGY AND INCIDENCE
Since the initial reports on infections of total elbow arthroplasties, the clinical presentation of these prosthetic infections has changed. Increased awareness of this complication has led to a high index of suspicion and hence earlier recognition.14 However, even at 3%, the rate of infection for elbow arthroplasties remains well above that for the lower extremity arthroplasties, in part because of the high prevalence of severe rheumatoid arthritis or post-traumatic arthritis.11,14,27,38 Risk factors for total elbow infections include rheumatoid arthritis,1,11,35 previous surgical procedures,27 previous local infections38,40 and use of tumor necrosis factor (TNF) antagonists.17 The elbow is a subcutaneous joint with a relatively thin soft tissue envelope, which increases its susceptibility to wound healing complications. In addition, patients with rheumatoid arthritis are often immunocompromised and are taking DMARDs, which may increase the incidence of periprosthetic infection.17 Those with post-traumatic arthritis frequently have undergone multiple operations that compromise the vascularity of the soft tissues and thus increase the risk of wound healing complications.14,38 Delayed wound healing, wound drainage longer than 10 days postoperatively, and reoperation are prognostic factors associated with increased infection rates.38
The incidence of infections following total elbow arthroplasties appears to have declined with improvements in surgical techniques but is now possibly increasing because of the use of the DMARDs. Our experience has shown a decrease in infection rate from an initial report of 8% to a more recent incidence of about 3%.25,27,39 In one long-term series of total elbow arthroplasty, there was no reported infections after the institution of routine use of antibiotic impregnated cement.37 It appears that the use of antibiotic-impregnated cement to implant the components, along with meticulous postoperative hematoma control, has been helpful in lowering the incidence of infections. By protocol at the Mayo Clinic, postoperative elbows are immobilized and elevated in full extension for a period of 24 to 36 hours to allow for edema control and wound epithelialization, before allowing range-of-motion exercises.
PATIENT PROFILE
The most important consideration in treating a patient with an infected total elbow arthroplasty is the overall status of the host. The health status comprises both the patient’s medical condition and his or her functional needs and expectations. Many patients with rheumatoid arthritis are medically debilitated owing to immuno-suppressive medications, anemia of chronic disease, previous surgery, and, sometimes, poor nutrition. Our protocol for these patients includes cessation of DMARDs, which are TNF antagonists (etanercept, infliximab, or adalimumab) at least 2 weeks before surgery and until 2 weeks postoperatively, because these medications have been shown in some studies to increase the incidence of periprosthetic infection.17
The clinical presentation of an infected total elbow arthroplasty may be subtle and only recognized by maintaining a high index of suspicion.38 Patients with an infected olecranon bursa should be assumed to have a deep infection unless proven otherwise. Systemic signs of sepsis (fever and tachycardia) may be absent,27 with the patient complaining of increased pain or pain at rest. Acute inflammation is usually detectable by local signs such as the presence of warmth, erythema, and tenderness. In some patients, there may be drainage from the wound or soft tissues.6,14,27,38 These points become important in determining the onset and thus chronicity of an infection.
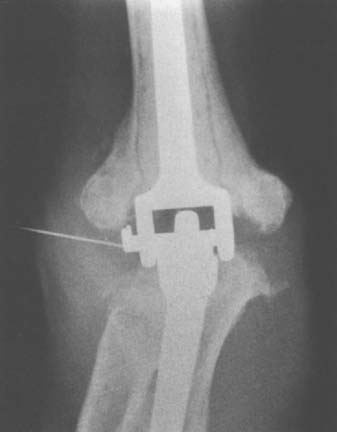
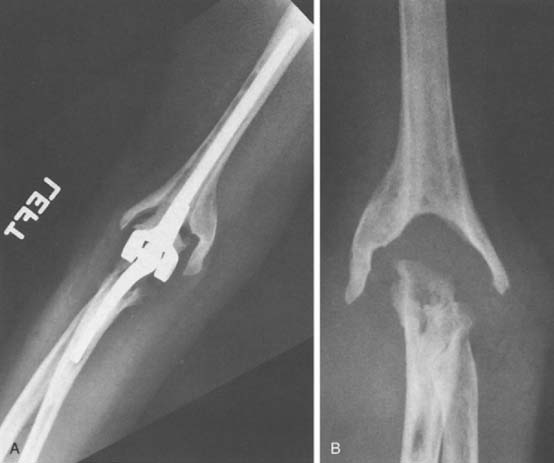
Preoperative evaluation is critical to establish range of motion, stability of the elbow, neurologic status, and function of the biceps and triceps muscles. Laboratory data may be of limited value, with most patients having a normal leukocyte count but an elevated neutrophil count on differential analysis.27 The erythrocyte sedimentation rate (ESR) is often elevated but not specific, because many have systemic inflammatory disease. C-reactive protein (CRP) is now routinely collected to aid in the sensitivity of detecting occult infection.6 The definite step is to aspirate the joint (Fig. 62-2). Patients are considered infected when there are positive cultures or strong clinical suspicion (based on presence of a draining sinus, high white blood cell count, erythrocyte sedimentation rate, CRP, operative observations, and so on) in the context of supportive microscopic pathology.
DURATION OF SYMPTOMS
Traditionally, infections have been classified according to length of time from surgery. An infection is considered acute if it developed within 3 months of the index operation, subacute if presentation was between 3 months and 1 year, and late if it was recognized 1 year after surgery.27,38 The time interval from the index procedure to the development of infection has traditionally been thought to correlate with the ability to eradicate the infection, but we were unable to demonstrate this in our most recent assessment.6 The duration of symptoms, as in the experience with total knee arthroplasty, has demonstrated a correlation with successful treatment by irrigation and débridement.21,31 Therefore, delineating the onset of symptoms has correlated better with the onset of infection and has direct implications on the treatment strategy.
FIXATION OF COMPONENTS
Component fixation in the context of infection is based on the appearance of the bone cement interface on serial radiographs.6,30 The quality of implant fixation has been enhanced in recent years by the improvement in cement techniques. High-quality and comparison radiographs are necessary for the detection of the progression of radiolucent lines, interval changes in implant position, cortical erosions, and osteolysis, suggesting loosening of the prosthesis. Component retention is, of course, a contraindication with loose or poorly fixed components.
BACTERIOLOGY
The microorganisms of implant infections, as opposed to soft tissue infections, are often difficult to eradicate and continue to be a significant problem. This has been demonstrated in total elbow infections in which the type of organism has had a profound impact on the treatment methods.6,39 Organisms vary in virulence, adherence, and the elaboration of extracellular components. Many factors influence the adherence of bacteria to the prosthesis, including alterations in host immune competence and the ability of bacteria to produce an extracellular matrix.7,13 Unlike total hip and total knee replacements, the infection rate of elbow replacements with gram-negative microorganisms has been low.6,39 Studies of infected orthopedic implants have shown that up to 76% of the infectious microorganisms produce a significant biofilm extracellular matrix to improve adherence to the implant.7,13 Of these, coagulase-negative staphylococci, particularly Staphylococcus epidermidis, have been the most common and the most problematic biofilm producers.13,36
More virulent microorganisms such as coagulase-positive staphylococci (i.e., Staphylococcus aureus) have the capacity to invade and infect healthy tissues but have a lesser ability to form a significant biofilm. Coagulase-negative staphylococcal organisms, particularly S. epidermidis, have been recognized as the primary pathogen of orthopedic device infections owing to their unusual capacity to attach to and to colonize orthopedic implants.2,36 Although a relatively nonvirulent pathogen that normally exists on the skin, it can form a tenacious bacterial biofilm (“slime”), or polysaccharide glycocalyx (protein plus carbohydrate), that envelopes the bacteria. This biofilm promotes colonization and adherence, and protects the bacteria from desiccation and host defense mechanisms.7 It also protects from antibiotic penetration and can even permit adherence to antibiotic-impregnated cement. This accounts for the persistence of S. epidermidis and its resistance to treatment.13 Not surprisingly, the presence of S. epidermidis has thus been associated with high incidence of treatment failure in the setting of infected total elbow arthroplasty,6,39 especially with efforts at reimplantation.6,39 Based on our recent studies,6 in the context of resection arthroplasty for infection followed by reimplantation of total elbow arthroplasty, the most common infecting organism from cultures taken at the time of resection was S. epidermidis (44.8%), followed by methicillin-sensitive S. aureus (24%), and Klebsiella pneumoniae (7%). Negative cultures were found in 10.3%, in which case infection was diagnosed clinically on the basis of wound dehiscence, or a draining wound with an exposed prosthesis. Other organisms accounted for the remaining 14%: group A streptococcus in one elbow, aerobic diptheroids in one elbow, Propionobacter acnes in one elbow, and methicillin-resistant S. aureus in one elbow (Table 62-1).
TABLE 62-1 Distribution of Infectious Organisms in Patients Receiving a Reimplantation Following Management of the Septic Condition
| Organism | Percent |
|---|---|
| Staphylococcus epidermidis | 45 |
| Methicillin-resistant Staphylococcus aureus | 24 |
| Klebsiella pneumoniae | 7 |
| “Other” | 14 |
| Negative culture | 10 |
TREATMENT OPTIONS
Once the diagnosis of an infected total elbow arthroplasty is suspected or confirmed, treatment is focused on surgical intervention. In choosing a particular treatment plan, strong consideration must be given to (1) duration of symptoms, (2) the component fixation, (3) the bacteriology, and (4) the patient’s health status (see Fig. 62-1). Debilitated patients who are unable to withstand the rigors of multiple surgical procedures are best served with a resection arthroplasty.
IRRIGATION AND DÉBRIDEMENT WITH RETENTION OF THE COMPONENTS
The initial experience with irrigation and débridement with retention of the components resulted in poor outcomes, with this treatment failing in most patients. Wolfe and coworkers37 reported eight failures in 11 patients with this technique, with the other three elbows deemed successes despite intermittent wound drainage. The Mayo Clinic’s initial experience was similar, with only one of nine patients treated successfully. We are aware of one case of an S. aureus–infected total elbow arthroplasty, successfully treated by arthroscopic irrigation and synovectomy in a patient with acute onset of symptoms at 2 months postoperatively.23
With increased awareness and earlier detection of infection, patients are now often seen acutely with well-fixed components without apparent bone involvement. In Wolfe’s series,38 three of eight patients sustained fractures of the humerus or ulna with component removal. In aseptic revisions, Morrey and Bryan28 noted fractures in 11 of 33 subjects. These results exemplify the difficulty in removal of the components without compromising the bone structure and have renewed interest in component retention.
Prior experience with infected total knee arthroplasty has demonstrated a high correlation between the duration of symptoms of infection (21 days or less) and outcome with component retention.22,31 Using this principle of symptom duration of less than 30 days, a recent study reports a 50% long-term success rate (at a mean 71-month follow-up).39 Furthermore, bacteriology played a significant role, with this treatment protocol failing all four patients infected by S. epidermidis whereas six of eight were successfully eradicated of the S. aureus infection.39 Therefore, traditionally, treatment with débridement and component retention is considered dependent on both the duration of symptoms and the bacteriologic findings. Of note, our recent assessment of the success of reimplantation did not demonstrate a prognostic difference in those with early or delayed diagnosis and treatment.6
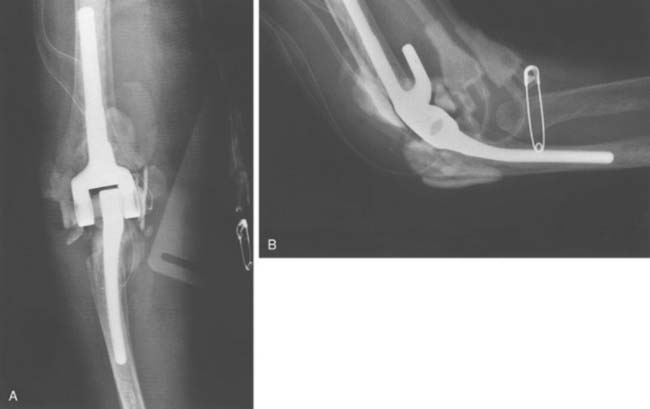
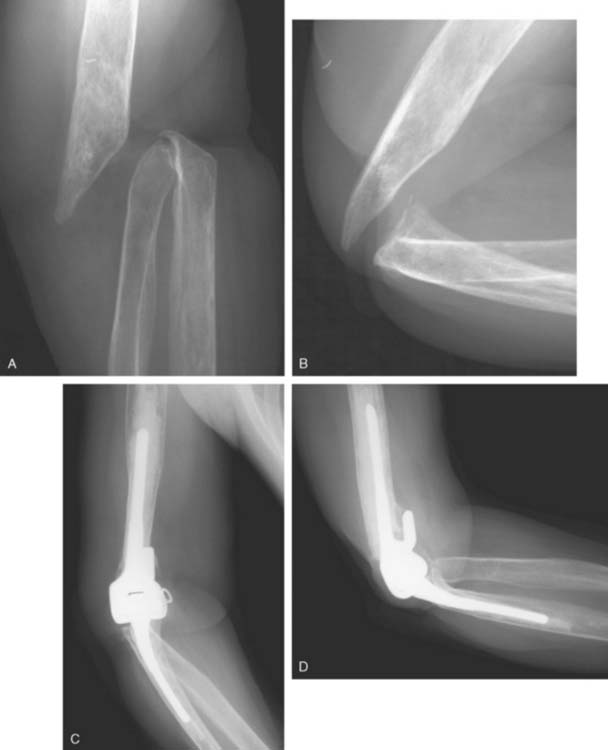
The technique for irrigation and débridement with component retention in our practice is through a posterior approach using the previous incision.6,39 The stability of the components is confirmed, followed by complete disarticulation of the components, including removal of the bushings (Fig. 62-3). This is an essential component of the procedure. The joint is débrided of all necrotic debris and copiously irrigated with pulsatile saline lavage. Antibiotic-impregnated polymethyl methacrylate (PMMA) beads, with a concentration of 2 g tobramycin per half-package of cement, are placed in the wound before wound closure. Patients often return to the operating room every fourth day for repeat irrigation and débridement with antibiotic PMMA bead exchange until the wound bed appears clean. The patient concurrently receives bacteria-sensitive intravenous antibiotics for a minimum period of 6 weeks (based on serum minimal inhibitory and bactericidal concentrations).6,39 The use of chronic oral suppressive antibiotics after completion of parenteral antibiotics is controversial and surgeon dependent.
The overall success rate of the Mayo series reported by Yamaguchi and coworkers39 was 50% but increased to 70% when those patients infected with S. epidermidis were eliminated. Outcomes produced good functional results but carried a 43% incidence of complication, including a 21% incidence of wound breakdown or triceps avulsion and 21% with peripheral nerve injury.
STAGED EXCHANGE ARTHROPLASTY
With the success of staged exchange arthroplasty for lower extremity infections,16,34 this technique has been successfully used for infected total elbow arthroplasties. We reported an 80% success rate at the Mayo Clinic with staged revision, with the only failures occurring in patients infected with S. epidermidis.39 The procedure’s success is dependent on the complete eradication of the pathogenic microorganism, necessitating the complete removal of all prosthetic components including PMMA.6,39,40
After reimplantation of total elbow arthroplasty following resection arthroplasty for infection reported by Cheung et al,6 there were 52% good to excellent, 10% fair, and 38% poor results at mean 7.4 years follow-up. Overall, there was a 28% rate of failure to eradicate the infection. The success rate of eradication of infection did not correlate with the chronicity of the infection. A success rate of 80% for eradication of infection was achieved if the duration of symptoms was less than 3 months at the time of resection arthroplasty. By comparison, the success rate decreased to 66% when the onset of symptoms was greater than 3 months from the primary arthroplasty. As in previous studies,39,40 elbows infected with S. epidermidis had a higher likelihood of treatment failure following reimplantation.
The most common indications for staged exchange arthroplasty in our practice are (1) radiographic or intraoperative evidence of loose components with sufficient bone stock for reconstruction, (2) duration of symptoms longer than 30 days, and (3) a medically fit patient (Fig. 62-4). A relative contraindication, dependent on the surgeon and the patient, is an infection caused by S. epidermidis.
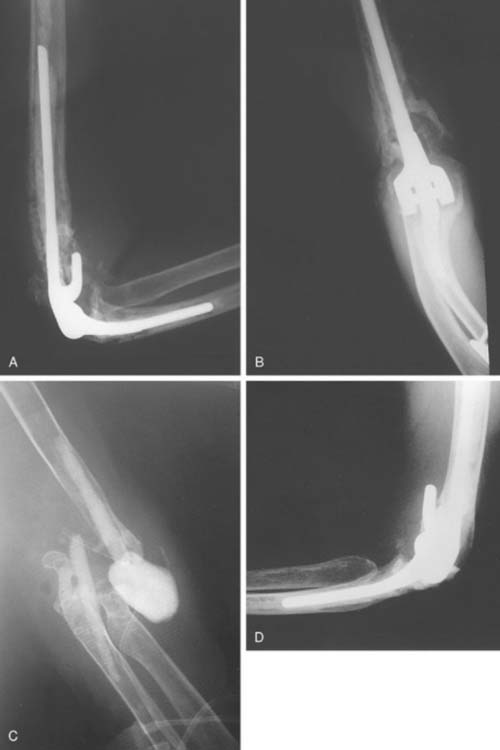
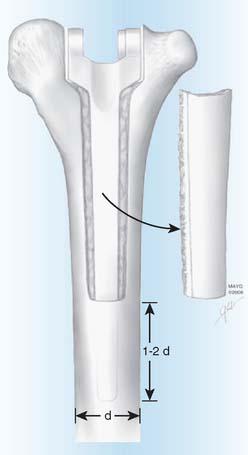
The surgical technique uses the previous posterior incision to expose the joint, with consideration of flaps for soft tissue coverage.28,39 While preserving bone stock (see Fig. 62-4), the components and any remaining cement are removed in a meticulous fashion during resection arthroplasty with the use of small curettes, a motorized burr, a router, or an ultrasonic cement removal device. Recently, this has been facilitated at the humerus by removing the posterior cortex of the distal humerus as a cortical window. Care is taken to avoid fracture of the condyle and to preserve the intact humerus so the tip of the reimplanted component is two diameters proximal to the osteotomized cortical window (Fig. 62-5). Every effort is made to preserve the integrity of the condyles. It is important to preserve the integrity of the condyles because they provide structural stability in case the patient is left with a resection arthroplasty, in which the ulna should articulate within the space between the medial and lateral condyles. The condyles also serve as the origins of the common flexor and extensor muscles of the fore-arm, and loss of their integrity would theoretically further compromise function in cases of resection arthroplasty.
Antibiotic-impregnated cement (2 g of tobramycin per 20 g of cement) is placed as a spacer between the humerus and ulna, and the incision is closed. The wound is closed and the limb is placed in a cast or hinged orthosis for 4 weeks. Since 1983 at the Mayo Clinic, antibiotic-impregnated cement has been routinely used for fixation of the implant in both primary and revision total elbow arthroplasties. The patient is placed on a 6-week course of intravenous antibiotics, based on organism sensitivity. At our institution, the orthopedic infectious disease service is consulted at the time of initial diagnosis of a prosthetic infection. Repeat irrigation, débridement, and biopsies of the tissues are performed as necessary. Consideration to longer staging intervals and repeat irrigation and débridement may be given to more resistant infections such as S. epidermidis. Arthroplasty reconstruction is performed at some point after 6 weeks using a long-stem semi-constrained arthroplasty with antibiotic-impregnated PMMA.6,40 Before reimplantation of total elbow arthroplasty and after completion of the 6-week course of antibiotics and resection arthroplasty, we routinely check that a complete blood count with differential, erythrocyte sedimentation rate, and CRP are within normal limits. An aspiration of the elbow with cell count, culture and sensitivity is also performed to confirm the absence of active infection. Intraoperatively, we send at least three tissue samples for frozen section pathology to confirm the absence of acute inflammation before cementing a new prosthesis. If there is no evidence of acute inflammation, we proceed with reimplantation. No additional antibiotics are typically given after the immediate perioperative period of the reimplantation. The patients are reminded of our lifetime postoperative activity restrictions, which they receive preoperatively: no lifting greater than 10 pounds as a single event, or repetitive lifting of greater than 2 pounds.
IMMEDIATE EXCHANGE ARTHROPLASTY
There has been very minimal information regarding immediate exchange for infected total elbow arthroplasties. We reported one case in which there was failure to eradicate the infection.40 Recently, Gille and colleagues12 reported successful treatment of S. aureus–infected total elbow arthroplasties in five of six patients treated with single-stage revision arthroplasty and mean follow-up of 6.8 years.
The majority of the lower extremity surgical experience has occurred in Europe, with only anecdotal and early reports in North America. Success rates with immediate exchange arthroplasty for infected total knee replacements vary from 35% to 75%, with suggested improved results with gram-positive non–glycocalyx-producing organisms.16,21,24 The indications for immediate exchange arthroplasty are probably very limited and, at this time, undetermined. The principles of the surgical treatment are similar to those of the staged revision with aggressive débridement with removal of all foreign material, antibiotic-impregnated cement fixation, and concomitant 6 to 12 weeks of intravenous antibiotics.
RESECTION ARTHROPLASTY
Resection arthroplasty has been the standard of treatment for infected elbow arthroplasty and constitutes the largest treatment experience. Functional results are usually limited but can be associated with a high satisfaction rate. Moreover, because many of these patients are debilitated, it is considered the treatment of choice for those medically frail and unfit for extensive or multiple surgical procedures. If successful, it often provides a relatively pain-free satisfactory range of active motion with reasonable stability (Fig. 62-6). Presentation of both condyles is essential for a functional resection arthroplasty.
The technique of elbow resection arthroplasty involves removal of the implant components through the previous incision followed by complete removal of the PMMA. All necrotic and contaminated tissue is excised. If remaining intact, the condyles of the distal humerus are then contoured and deepened to encircle the ulna. The soft tissue coverage is established by primary closure or local rotation flaps. Concurrent treatment with 4 to 6 weeks of appropriate antibiotic therapy is used. The limb is placed in a cast or brace for 3 to 4 weeks to obtain soft tissue stability. We have recently assessed the experience with 40 patients treated by resection arthroplasty for sepsis. At a mean of 6-year surveillance, 78% considered their functional status satisfactory from the perspective of pain relief. On the other hand only 55% considered the salvage to be a functional joint.4 If continued dysfunction and patient dissatisfaction persists, a delayed reimplantation may be considered (Fig. 62-7).
COMPLICATIONS
Treatment of the infected elbow requires multiple irrigation and débridement, and complications are not uncommon: intraoperative and postoperative fracture due to loss of bone stock, triceps insufficiency, nerve injury, and skin or wound breakdown. Thus, special attention should be given to protect surrounding neurovascular structures intraoperatively during implant and cement extraction. Care should also be taken to preserve the triceps insertion, and maintain meticulous soft tissue handling. Special consideration should be taken using triceps-on approaches and proactive planning for possible soft tissue coverage procedures with preemptive consultation to plastic surgery. Triceps insufficiency may need to be addressed with anconeus muscle rotation at the time of reimplantation.5 Of course, the high risk of complications and the associated morbidity are significant considerations that have to be discussed with the patient before any prosthetic salvage strategy is attempted in the context of infected elbow arthroplasty.
CONCLUSION
Infection remains a significant and severe complication of total elbow arthroplasty with an incidence above that of lower extremity joint replacements. Previously, the only treatment option was resection arthroplasty. Recent reports of this problem suggest that both irrigation and débridement and staged exchange arthroplasty can be successful treatment modalities given the appropriate indications (see Fig. 62-1). Our experience suggests a poorer prognosis in those with a history of S. epidermidis and in settings of increasing chronicity of disease. As with other joints, functional outcome is compromised after reimplantation of the prosthesis, even in those with successful treatment in regard to infection. As such, selected treatment methods may improve both the functional and satisfaction rate of this most devastating complication.
1 Aldridge J.M.3rd, Lightdale N.R., Mallon W.J., Coonrad R.W. Total elbow arthroplasty with the Coonrad/Coonrad-Morrey prosthesis. A 10- to 31-year survival analysis. J. Bone Joint Surg. Br. 2006;88:509.
2 Arciola C.R., Campoccia D., Gamberini S., Donati M.E., Pirini V., Visai L., Speziale P., Montanaro L. Antibiotic resistance in exopolysaccharide-forming Staphylococcus epidermidis isolates from orthopaedic implant infection. Biomaterials. 2005;26:6530.
3 Brumfield R.H.Jr, Kuschner S.H., Gellman H., Redix L., Stevenson D.V. Total elbow arthroplasty. J. Arthroplasty. 1990;5:359.
4 Cass, B., Adams, R. A., and Morrey, B. F.: Long-term outcome of resection arthroplasty of the elbow for infection. 2007 (in press).
5 Celli A., Arash A., Adams R.A., Morrey B.F. Triceps insufficiency following total elbow arthroplasty. J. Bone Joint Surg. Am. 2005;87:1957.
6 Cheung E.V., Adams R.A., Morrey B.F. Reim-plantation of total elbow arthroplasty following resection arthroplasty for infection J. Bone Joint Surg. Am. 2008;90:589.
7 Christensen G.D., Baldassarri L., Simpson W.A. Colonization of Medical Devices. 2nd ed. Washington DC: American Society for Microbiology, 1994;45.
8 Davis R.F., Weiland A.J., Hungerford D.S., Moore J.R., Volenec-Dowling O.T.R. Nonconstrained total elbow arthroplasty. Clin. Orthop. Relat. Res. 1982;171:156.
9 Ewald F.C., Simmons E.D.Jr., Sullivan J.A., Thomas W.H., Scott R.D., Poss R., Thornhill T.S., Sledge C.B. Capitellocondylar total elbow replacement in rheumatoid arthritis: Long-term results. J. Bone Joint Surg. 1993;75A:498.
10 Figgie M.P., Gerwin M., Weiland A.J. Revision total elbow replacement. Hand Clin. 1994;10:507.
11 Gill D.R., Morrey B.F. The Coonrad-Morrey total elbow arthroplasty in patients who have rheumatoid arthritis. A ten to fifteen-year follow-up study. J. Bone Joint Surg. Am. 1998;80:1327-1335.
12 Gille J., Ince A., Gonzalez O., Katzer A., Loehr J. Single-stage revision of peri-prosthetic infection following total elbow replacement. J. Bone Joint Surg. Br. 2006;88:1341.
13 Gristina A.G. Biomaterial-centred infection: Microbial adhesion versus tissue integration. Science. 1987;237:1588.
14 Gutow A.P., Wolfe S.W. Infection following total elbow arthroplasty. Hand Clin. 1994;10:521.
15 Haleem A., Berry D., Hanssen A. Mid-term to long-term follow-up of two stage reimplantation for infected total knee arthroplasty. Clin. Orthop. Rel. Res. 2004;428:35.
16 Hanssen A. Managing the infected knee: as good as it gets. J. Arthroplasty. 2002;17:98.
17 Howe C.R., Gardner G.C., Kadel N.J. Perioperative medication management for the patient with rheumatoid arthritis. J. Am. Acad. Orthop. Surg. 2006;14:544.
18 Kasten M.D., Skinner H.B. Total elbow arthroplasty: An 18-year experience. Clin. Orthop. Rel. Res. 1993;290:177.
19 Kraay M.J., Figgie M.P., Inglis A.E., Wolfe S.W., Ranawat C.S. Primary semiconstrained total elbow arthroplasty. J. Bone Joint Surg. 1994;76B:636.
21 Marculescu C.E., Berbari E.F., Hanssen A.D., Steckelberg J.M., Osmon D.R. Prosthetic joint infection diagnosed postoperatively by intraoperative culture. Clin. Orthop. Relat. Res. 2005;439:38.
22 Marculescu C.E., Berbari E.F., Hanssen A.D., Steckelberg J.M., Harmsen S.W., Mandrekar J.N., Osmon D.R. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin. Infect. Dis. 2006;42:471.
23 Mastrokalos D.S., Zahos K.A., Korres D., Soucacos P.N. Arthroscopic debridement and irrigation of periprosthetic total elbow infection. Arthroscopy. 2006;22:1140.
24 Mitchell P., Masri B., Garbuz D., Greidanus N., Duncan C. Cementless revision for infection following total hip arthroplasty. Instr. Course Lect. 2003;52:323.
25 Morrey B.F., Adams R.A. Semiconstrained arthroplasty for the treatment of rheumatoid arthritis of the elbow. J. Bone Joint Surg. 1992;74A:479.
26 Morrey B.F., Bryan R.S. Complications of total elbow arthroplasty. Clin. Orthop. Rel. Res. 1982;170:204.
27 Morrey B.F., Bryan R.S. Infection after total elbow arthroplasty. J. Bone Joint Surg. 1983;65A:330.
28 Morrey B.F., Bryan R.S. Revision total elbow arthroplasty. J. Bone Joint Surg. 1987;69A:523.
29 Ruth J.T., Wilde A.H. Capitellocondylar total elbow replacement: A long-term follow-up study. J. Bone Joint Surg. 1992;74A:95.
30 Schneeberger A.G., Meyer D.C., Yian E.H. Coonrad-Morrey total elbow replacement for primary and revision surgery: A 2- to 7.5-year follow-up study. J. Shoulder Elbow Surg. 2007;16(3 Suppl):S47.
31 Schoifet S.D., Morrey B.F. Treatment of infection after total knee arthroplasty by débridement with retention of the components J. Bone Joint Surg. 1990;72A:1383.
32 Sneftrup S.B., Jensen S.L., Johannsen H.V., Søjbjerg J.O. Revision of failed total elbow arthroplasty with use of a linked implant. J. Bone Joint Surg. Br. 2006;88:78.
33 Trancik T., Wilde A.H., Borden L.S. Capitellocondylar total elbow arthroplasty. Clin. Orthop. Rel. Res. 1987;223:175.
34 Tsukayama D., Estrada R., Gustilo R. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J. Bone Joint Surg. Am. 1996;78:512.
35 van der Lugt J.C., Geskus R.B., Rozing P.M. Primary Souter-Strathclyde total elbow prosthesis in rheumatoid arthritis. J. Bone Joint Surg. Am. 2004;86-A:465.
36 Van Pett K., Schurman D.J., Smith R.L. Quantitation and relative distribution of extracellular matrix in Staphylococcus epidermidis biofilm. J. Orthop. Res.. 1990;8:321.
37 Weiland A.J., Weiss A.P.C., Wills R.P., Moore J.R. Capitellocondylar total elbow replacement. A long-term follow-up study. J. Bone Joint Surg. 1989;71A:217.
38 Wolfe S.W., Figgie M.P., Inglis A.E., Bohn W.M., Ranawat C.S. Management of infection about total elbow prostheses. J. Bone Joint Surg. 1990;72A:198.
39 Yamaguchi K., Adams R.A., Morrey B.F. Infection after total elbow arthroplasty. J. Bone Joint Surg. 1998;80A:481.
40 Yamaguchi K., Adams R.A., Morrey B.F. Semiconstrained total elbow arthroplasty in the context of treated previous infection. J. Shoulder Elbow Surg. 1999;8:461.