Chapter 34 Treatment of Seizure Disorders
| Abbreviations | |
|---|---|
| EEG | Electroencephalogram |
| GABA | γ-Aminobutyric acid |
| NMDA | N-methyl-D-aspartate |
Therapeutic Overview
| Therapeutic Overview |
|---|
| Partial (Focal) Seizures |
| Simple seizures |
| No loss of consciousness, may or may nor be preceded by an aura, includes sensory, motor, autonomic, or psychic features |
| Complex seizures |
| Impaired consciousness, dreamy dysaffective state with or without automatisms |
| Secondarily generalized tonic-clonic seizures |
| Evolves from simple or complex partial seizure, impaired consciousness with rigid extension of trunk and limbs (tonic phase) and rhythmic contractions of arms and legs (clonic phase) |
| Generalized Seizures |
| Tonic-clonic (grand mal) seizures |
| As above for partial with secondarily generalized tonic-clonic seizures |
| Absence seizures |
| Abrupt loss of consciousness with staring and cessation of ongoing activity with or without eye blinks |
| Other types of seizures |
| Myoclonic—sporadic, isolated jerking movements |
| Clonic—repetitive jerking movements |
| Tonic—muscle stiffness and rigidity |
| Atonic (atypical)—loss of muscle tone |
of familial epilepsy. The causes of isolated seizures and epilepsy (recurrent seizures) are summarized in Box 34-1.
BOX 34–1 Causes of Seizures
Mechanisms of Action
Antiepileptic drugs have been classified and selected for many years based on seizure type (Box 34-2). Although the intricate cellular alterations in the neuronal events mediating the generation of seizures is not totally understood, studies have provided evidence of likely alterations involved in both partial seizures and absence seizures to enable a mechanistic-based approach for treatment.
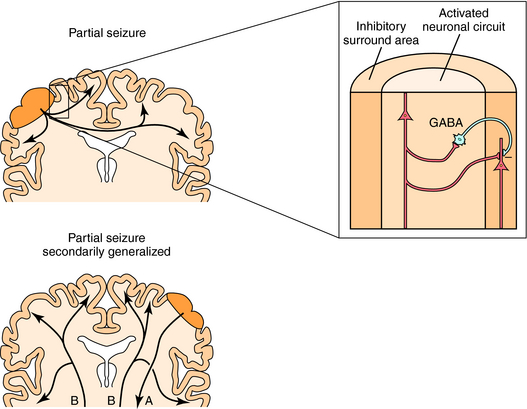
Partial seizures are thought to develop as a consequence of the loss of surround inhibition, a process that normally prevents the activation of neurons adjacent to a focus (Fig. 34-1). This loss of surround inhibition may result from impaired γ-aminobutyric acid (GABA) transmission, alterations in dendritic structure, changes in voltage-gated ion channel activity or density, or alterations in intracellular ion concentrations. If the seizure generalizes secondarily to involve both hemispheres, tonic-clonic effects are manifest. The tonic phase of muscle contraction is thought to reflect prolonged neuronal depolarization as a consequence of the loss of GABA-mediated inhibition and dominance of excitatory glutamate transmission. As the seizure evolves, neurons repolarize and afterhyperpolarizations are apparent, which reflect the reappearance of GABA-mediated inhibition and diminished glutamate excitation, producing the clonic phase. Thus drugs that increase surround inhibition and prevent the spread of synchronous activity are used for the treatment of partial seizures.
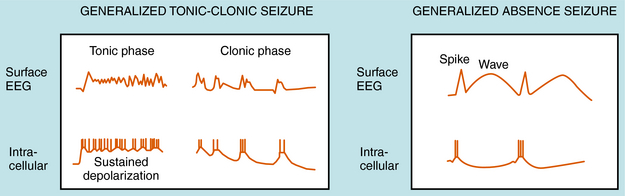
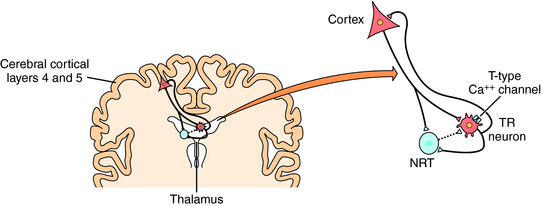
Our understanding of the onset of generalized tonic-clonic seizures is limited. However, there are some clues concerning the cellular mechanisms underlying absence seizures, which are characterized by the sudden appearance of spike-wave discharges synchronized throughout the brain. The EEGs recorded during an absence seizure compared with a generalized tonic-clonic seizure are shown in Figure 34-2. Studies support a major role of thalamocortical circuits in the pathogenesis of absence seizures with abnormal oscillations between cortical and thalamic neurons. The circuit involves excitatory glutamatergic cortical pyramidal and thalamic relay neurons and inhibitory GABAergic thalamic reticular neurons (Fig. 34-3). Thalamic relay neurons exhibit spike-wave discharges that generate normal cortical rhythms and participate in the generation of sleep spindles. The normal bursting pattern of these neurons results from the activation (depolarization) of low voltage-gated T-type (transient inward current) Ca++ channels, followed by hyperpolarization mediated by GABA released from thalamic reticular neurons. Thus drugs that block these T-type Ca++ currents are effective for the treatment of absence seizures.
Agents used for the treatment of epilepsy depress aberrant neuronal firing by primarily altering ion channel activity, enhancing GABA-mediated inhibitory neurotransmission, or dampening glutamate-mediated excitatory neurotransmission. It is important to note that although some drugs have a single mechanism of action, several of these agents have more than one mechanism. Anticonvulsant drugs classified according to mechanisms of action are listed in Box 34-3.
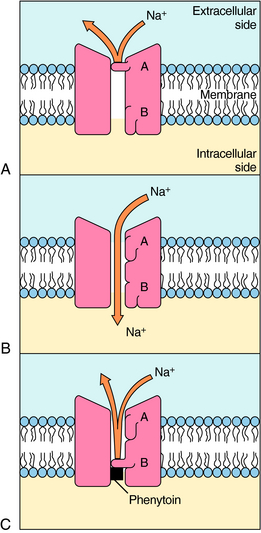
The voltage-gated Na+ channel blockers are widely used antiseizure drugs with demonstrated effectiveness for partial and secondarily generalized seizures. These drugs include phenytoin, carbamazepine, oxcarbazepine, lamotrigine, topiramate, valproic acid, and zonisamide. These agents reduce the repetitive firing of neurons by producing a use-dependent blockade of Na+ channels (Fig. 34-4). By prolonging the inactivated state of the Na+ channel and thus the relative refractory period, these drugs do not alter the first action potential but rather reduce the likelihood of repetitive action potentials. Neurons retain their ability to generate action potentials at the lower frequencies common during normal brain function. Because these drugs block repetitive firing, they are better at controlling partial and tonic-clonic seizures than absence seizures.
As indicated, T-type Ca++ currents provide for slow rhythmic firing of thalamic neurons and are thought to be involved in generating cortical discharge characteristic of absence seizures. Ethosuximide, valproic acid, and zonisamide have all been shown to inhibit these low threshold currents, the former two at clinically relevant concentrations. It is likely that the effectiveness of ethosuximide and valproic acid for absence seizures reflects their action on these Ca++ currents, whereas the efficacy of valproic acid for partial and tonic-clonic seizures may reflect its inhibitory effects at both Na+ and Ca++ channels.
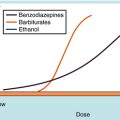
As mentioned, excessive neuronal firing may occur as a consequence of either decreased inhibition or increased excitation of neurons. GABA, the major inhibitory neurotransmitter in the brain, activates ligand-gated Cl− channels (see Chapter 31), thereby hyperpolarizing neurons and rendering them less likely to activate. GABAA receptors contain separate and distinct binding sites for both the benzodiazepines and barbiturates (see Chapter 31). Benzodiazepines enhance the actions of GABA by increasing the frequency of Cl− channel openings, whereas barbiturates prolong the duration of Cl− channel openings upon activation of the receptor by GABA. The benzodiazepine clonazepam is used both as monotherapy and as adjunctive therapy for akinetic and myoclonic seizures and in absence seizures in patients who fail to respond to ethosuximide. Clorazepate is used only as adjunctive therapy, and diazepam and lorazepam are used for the treatment of status epilepticus. Among the barbiturates, phenobarbital and mephobarbital are used for generalized tonic-clonic and partial seizures. Although all barbiturates suppress seizures, they are not useful clinically because they have strong sedative effects.
Pharmacokinetics
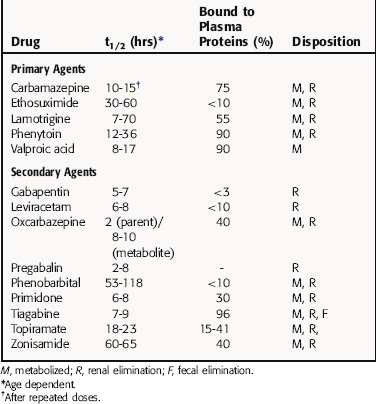
Many antiepileptic drugs are highly bound to plasma proteins, which is clinically important because the usual determinations of blood concentrations indicate total drug (bound plus free) in serum, even though it is only free drug that is active. The half-life of antiepileptic agents varies with the age of the patient and exposure to other drugs. The pharmacokinetic parameters of antiepileptic agents are summarized in Table 34-1. The pharmacokinetics of the benzodiazepines are presented in Chapter 31.
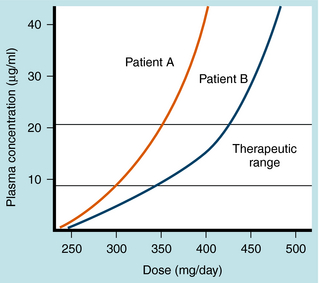
Phenytoin metabolism is characterized by saturation, or zero-order kinetics (see Chapter 2). At low doses there is a linear relationship between the dose and the serum concentration of the drug. At higher doses, however, there is a much greater rise in serum concentration for a given increase in dose (nonlinear), because when serum concentrations rise above a certain value, the liver enzymes that catalyze phenytoin metabolism become saturated. The dose at which this transition occurs varies from patient to patient but is usually between 400 and 600 mg/day (Fig. 34-5). Because of this kinetic pattern, doses of phenytoin must be individualized.
Relationship of Mechanisms of Action to Clinical Response
As mentioned, antiepileptic medication is selected typically according to seizure type, and the goal of therapy is to prevent seizures and minimize side effects. To this end, relative serum concentration ranges for producing therapeutic responses with minimal side effects have been established for partial and generalized seizures. These “therapeutic” serum concentration ranges for the primary antiepileptic drugs are listed in Table 34-2 and have been determined empirically from general clinical experience in diverse and heterogeneous populations of patients. Thus these values should not be taken as absolute recommendations for individual patients, but they may be used as a guide.
TABLE 34–2 Effective Serum Concentrations of Antiepileptic Drugs Required for Specific Seizure Types
| Drug | Therapeutic Serum Concentration (μg/mL) | Indication |
|---|---|---|
| Carbamazepine | 4-12 | Partial, including secondarily generalized |
| Generalized tonic-clonic (grand mal) | ||
| Ethosuximide | 40-100 | Absence (petit mal) |
| Lamotrigine | 2-20 | Partial, including secondarily generalized |
| Atypical absence, myoclonic, atonic | ||
| Phenytoin | 5-25 | Generalized tonic-clonic (grand mal) |
| 10-20 | Partial, including secondarily generalized | |
| Valproic acid* | 50-150 | Generalized tonic-clonic (grand mal) with absence seizure |
| Absence (petit mal) | ||
| 50-100 | Atypical absence, myoclonic, atonic |
* First choice for absence if primary generalized tonic-clonic seizure is also present.
Ethosuximide is typically used for uncomplicated absence seizures, which respond well to this agent.
Lamotrigine has a broad anticonvulsant profile and is effective as both monotherapy and adjunctive therapy for partial and generalized seizures. It is as effective as carbamazepine and phenytoin for newly diagnosed partial or generalized seizures and is better tolerated than these agents. Lamotrigine has also been shown to be efficacious for Lennox-Gastaut syndrome (see following text).
Pharmacovigilance: Side Effects, Clinical Problems, and Toxicity
Antiepileptic drugs cross the blood-brain barrier and have potential to cause systemic and neurological toxicity. The problems encountered for the primary drugs are listed in the Clinical Problems Box. Side effects of antiepileptic drugs occur in 30% to 50% of patients. However, these are frequently tolerable and require monitoring only. In other cases side effects can be reduced or eliminated by changing the dose or administration schedule. In 5% to 15% of patients, another antiepileptic drug must be prescribed because of toxicity. Serious idiosyncratic effects, such as allergic reactions, are rare but can be life-threatening. They usually occur within several weeks or months of starting a new drug and tend to be dose-independent. Most antiepileptic drugs should be introduced slowly to minimize side effects.
The side effects associated with the use of the benzodiazepines are presented in Chapter 31.
Antiepileptic Drugs during Pregnancy
Because antiepileptic agents are taken for many years or a lifetime, the issue of taking these drugs during pregnancy is important. During pregnancy, 25% of epileptic women experience an increase in seizure frequency, 25% experience a decrease in seizure frequency, and 50% do not experience any change. The possibility of seizures puts both the mother and child at risk. The teratogenic properties of antiepileptic drugs are also a concern. Although fetal exposure to phenytoin, carbamazepine, valproic acid, and phenobarbital has been associated with congenital anomalies, including cardiac, urinary tract, and neural tube defects and cleft palate, most pregnant patients exposed to antiepileptic drugs deliver normal infants. Children of mothers who have epilepsy are at increased risk for malformations even if antiepileptic drugs are not used during pregnancy. Whenever possible, women with epilepsy should be counseled before they become pregnant. If discontinuation of
antiepileptic medication is not an option, monotherapy with the lowest possible dose of the antiepileptic agent should be used.
Drugs for epilepsy. Treat Guidel Med Lett. 2005;3:75-82.
García-Morales et al. 2007 García-Morales I, Rieger JS, Gil-Nagel A, Fernández JL. Antiepileptic drugs: From scientific evidence to clinical practice. Neurologist. 2007;13:S20-S28.
Karceski SC. Seizure medications and their side effects. Neurology. 2007;69:E27-E29.
Loring et al. 2007 Loring DW, Marino S, Meador KJ. Neuropsychological and behavioral effects of antiepilepsy drugs. Neuropsychol Rev. 2007;17:413-425.
Stafstrom CE. Epilepsy: A review of selected clinical syndromes and advances in basic science. J Cereb Blood Flow Metab. 2006;26:983-1004.
Wiebe et al. 2008 Wiebe S, Téllez-Zenteno JF, Shapiro M. An evidence-based approach to the first seizure. Epilepsia. 2008;49(Suppl 1):50-57.