CHAPTER 391 Treatment of Other Intracranial Dural Arteriovenous Fistulas
Intracranial dural arteriovenous fistulas (DAVFs) are acquired arteriovenous shunts confined to the dura and are supplied by branches of the external carotid artery, tentorial branches of the internal carotid artery, meningeal branches of the vertebral artery, and rarely, pial branches of the cerebral arteries (Fig. 391-1).1 Venous drainage is anterograde via the dural venous sinuses or retrograde into other dural or leptomeningeal venous channels. These fistulas are frequently idiopathic but can be associated with venous thrombosis,2,3 trauma, tumor, previous neurological surgery,1,4 meningitis,5 or sinus infection.1
Classification
The terms dural arteriovenous malformation and dural arteriovenous fistula are often used interchangeably. DAVFs are classified according to their venous drainage, which determines both the natural history and treatment recommendations. Houser and colleagues first established the association of clinical symptoms with patterns of venous drainage in a series of 28 patients with DAVFs.6 Although several classification systems exist for DAVFs,7–9 we refer primarily to the Borden10 and Cognard1 classifications, which have been described in detail elsewhere and are summarized in Tables 391-1 and 391-2. According to the Borden classification, type I fistulas have anterograde drainage into a dural venous sinus or meningeal vein. These fistulas are benign, often asymptomatic or characterized by a cranial bruit, and have a high rate of spontaneous remission. Borden type II fistulas have both anterograde venous sinus drainage and retrograde drainage into subarachnoid veins. The venous sinus may be patent but largely defunctionalized because of high-flow venopathy causing reversal of flow into arterialized leptomeningeal veins.
TABLE 391-1 Borden Classification of Dural Arteriovenous Fistulas
| Type I | Anterograde drainage into the dural sinus/meningeal vein |
| Type II | Anterograde drainage into the dural sinus plus retrograde drainage into the subarachnoid veins |
| Type III |
TABLE 391-2 Cognard Classification of Dural Arteriovenous Fistulas
| Type I | Anterograde drainage into the main sinus |
| Type IIa | Drainage into the main sinus with reflux into another sinus or sinuses |
| Type IIb | Drainage into the main sinus with reflux into cortical veins |
| Type IIa + IIb | Drainage into the main sinus with reflux into another sinus or sinuses plus cortical veins |
| Type III | Direct cortical venous drainage without venous ectasia |
| Type IV | Direct cortical venous drainage with venous ectasia (>5 mm and 3× larger than the diameter of the draining vein) |
| Type V | Drainage into spinal perimedullary veins |
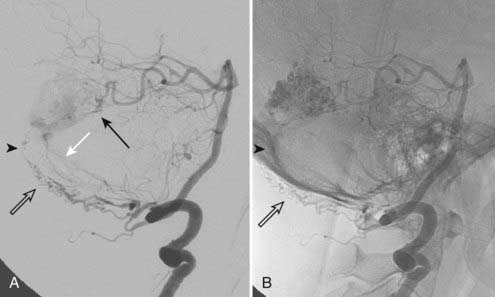
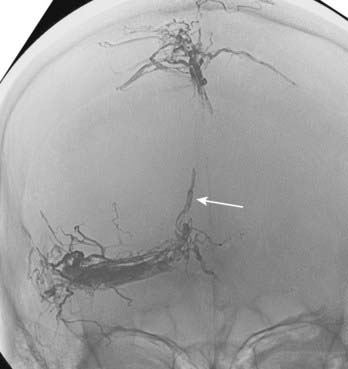
Borden type III DAVFs have exclusive retrograde drainage into arterialized subarachnoid veins at or on the wall of dural venous sinuses. Such drainage is frequently but not always due to occlusion of the arterialized dural sinus. When the sinus is patent, the point of the fistula is located either between the meningeal artery and leptomeningeal vein or between the meningeal artery and a segment of arterialized dural venous sinus that is thrombosed at either end or somehow isolated from the rest of the sinus, thereby causing reversal of drainage into leptomeningeal veins (Fig. 391-2).
Natural History
Borden type I DAVFs have a benign clinical course and a high rate of spontaneous remission. Up to 10% of Borden type II and III DAVFs with cortical venous reflux may spontaneously close as well.11 The risk for intracranial hemorrhage and stroke is dictated by the presence of cortical venous reflux, galenic drainage,11,12 and venous congestion. Lasjaunias and associates proposed that the source of intracranial hemorrhage in these cases is not the fistula itself but rather ectatic, thin-walled arterialized veins.13 Persistence of cortical venous reflux in intracranial DAVFs carries an annual risk for hemorrhagic or nonhemorrhagic neurological deficits of 15% along with an annual mortality rate of 10.4%.11 Cognard and coworkers noted intracranial hemorrhage in 40% of patients with type III and in 65% with type IV DAVFs.1
Little is known about the rebleeding rate for DAVFs, but in small case series it is as high as 43% within the first few days of hospitalization10 and up to 35% in the initial 2 weeks after the first hemorrhage.14 Davies and coauthors reported a 20% annual morbidity and mortality rate after an initial aggressive neurological manifestation.15
Treatment
Observation
Borden type I DAVFs can be observed, especially since they may spontaneously thrombose, which has been noted with cavernous sinus DAVFs.16 Factors associated with spontaneous regression of DAVFs are unknown, but sinus thrombosis may play a role.17 Occasionally, treatment is offered if a patient cannot tolerate symptoms such as a constant bruit. If conservative treatment is chosen, patients are advised to avoid antiplatelet or anticoagulant agents, if possible, to prevent interference with spontaneous thrombosis of the DAVF. High-flow venopathy in sinuses not involved in the fistulous drainage, however, may predispose to undesirable venous sinus thrombosis; such patients are advised to take at least 81 mg of aspirin daily.
Compression Therapy
Compression therapy is seldom used currently, except in patients with Borden type I fistulas as a possible first step before neuroendovascular therapy. Compression of the ipsilateral carotid or occipital artery (if the latter vessel is a known feeder to the fistula) is performed for 30 minutes at a time, three times a day. If compression of the carotid artery is chosen, patients are instructed to use the contralateral arm. In case hemispheric ischemia ipsilateral to the compressed artery and paresis of the contralateral upper extremity develop as a result of overzealous compression, the arm will fall away and the compression will necessarily be self-limited. In 25% of simple DAVFs of the transverse/sigmoid sinuses supplied by the occipital artery, compression of the occipital artery results in complete thrombosis of the DAVF within several weeks.18 In patients with subjective improvement or disappearance of pulsatile tinnitus, a follow-up cerebral angiogram can be performed to evaluate whether thrombosis of the DAVF has occurred since the last evaluation.
Transvenous Therapy
Because neuroendovascular techniques have evolved considerably over the past decade, open surgery for cure of DAVFs has become a secondary treatment. Transvenous coil embolization plus occlusion of the recipient venous pouch is the mainstay of endovascular therapy and offers the best chance of cure.19 It is particularly useful for Borden type II DAVFs, which tend to be high flow and may have multiple associated fistulas. The risk of occluding the sinus/arterialized pouch and causing venous infarction is low because the sinus is usually defunctionalized. The use of balloon test occlusion has been recommended for assessing the impact of embolizing a sinus in communication with normal cortical veins1,20,21; however, this is not our practice because all transvenous/transarterial embolizations for DAVFs at our institution are performed with the patient under general anesthesia.
As Cognard and colleagues pointed out, it is essential to differentiate between type IIb and type III DAVF (according to their classification) when considering the option of transvenous coil embolization. Transvenous treatment is appropriate for a Cognard type IIb fistula, in which there is drainage into the sinus and then reflux into a cortical vein, but it is not appropriate for a Cognard type III fistula, in which there is drainage into a cortical vein and then the sinus. In the latter case, transvenous occlusion of the sinus blocks eventual venous egress, thus further elevating pressure in the arterialized subarachnoid vein.1
If patent, venous access is via the transfemoral ipsilateral internal jugular vein and sigmoid sinus route. A triaxial system consisting of a 6 French shuttle sheath advanced to the jugular bulb, a 4 or 5 French vertebral catheter, and a microcatheter provides maximum support. Differentiation of the recipient arterialized venous pouch from the sinus responsible for normal cortical venous drainage is crucial because in the latter drainage may occur via the sinus wall instead of the sinus.3 The arterialized sinus or fistulous pouch is then fully packed with thrombogenic fibered or platinum coils (Fig. 391-3). Inadequate embolization of a sinus with leptomeningeal venous drainage or embolization of the parent sinus without occlusion of the parallel venous channel receiving arterial inflow22 may aggravate the venous hypertension by impeding venous egress and diverting arterialized blood to the subarachnoid veins.10,19
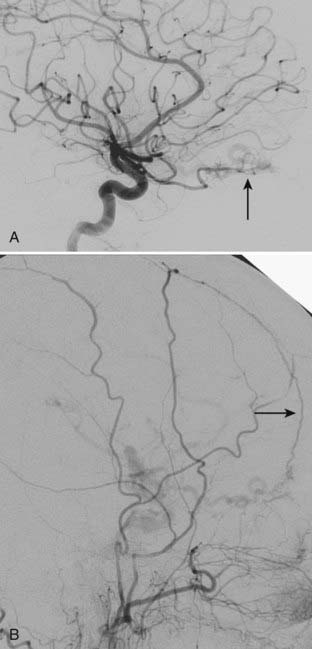
Transvenous embolization for a Borden type III fistula is successful only if the catheter can be carefully manipulated across the thrombosed or trapped segment of the sinus over a guidewire into the arterialized recipient pouch. There is a small risk of perforation and particularly subdural extravasation with this approach (Fig. 391-4).21
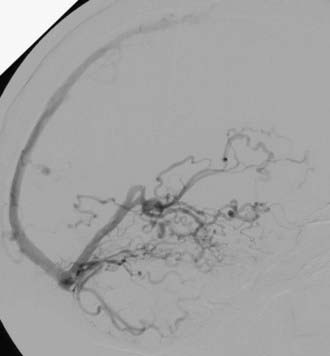
Sinus thrombosis is not an absolute contraindication to passage of the microcatheter,23,24 especially with the current availability of high-performance, small-caliber, hydrophilic guidewires and catheters. When entry to the diseased sinus is not feasible because of venous thrombosis, venopathy, or tortuosity, access can sometimes be secured from the contralateral transverse sinus across the torcular Herophili (Fig. 391-5).25 Recanalization of thrombosed transverse-sigmoid sinuses with balloon angioplasty plus closure of the intramural septations in the wall of the proximal sinus forming the fistula by overlapping stents has been reported. This technique may prove to be useful for eradicating DAVFs of the lateral sinus.26–28 Care should be taken to observe for alterations in venous drainage patterns after balloon angioplasty.29 Primary stent placement in a stenotic sinus to normalize venous outflow after treatment of the DAVF may also relieve venous congestive symptoms,2,30 although long-term venous stent patency rates are not yet known.
Transvenous embolization with other liquid nonadhesive agents (Eudragit E mixture) has also been described.31 In DAVFs with a dilated venous channel, creating a “nest” of coils before transvenous injection of a liquid embolic agent may prevent unwanted distal embolization.32 Proper injection technique should be used with liquid embolic agents to avoid retrograde embolization into the leptomeningeal veins, which can produce venous infarction.19
If residual opacification of the tightly packed venous pouch is noted, thrombosis can be enhanced with a 1-g/hr intravenous infusion of ε-aminocaproic acid for 24 to 48 hours, followed by a control angiogram.33 Angiographic resolution of venous hypertension correlates with the disappearance of pulsatile tinnitus and venous congestive symptoms, even if minor residual DAVF is present. However, the risk for rehemorrhage is not eliminated unless the DAVF is angiographically cured.1
Transarterial Therapy
Transarterial therapy is indicated when transvenous routes are inaccessible, the fistula directly communicates with subarachnoid veins, the magnitude of the arteriovenous shunt precludes adequate angiographic evaluation of the sinus or iatrogenic venous infarction is a concern, or the goal is palliation, not cure. Although generally viewed as incomplete treatment,34 transarterial embolization to reduce flow through the fistula has been reported to produce complete thrombosis of the DAVF 60% of the time.10
Although polyvinyl alcohol (PVA) particles, silk sutures, n-butyl-2-cyanoacrylate (NBCA) diluted with iodized oil (e.g., Ethiodol), poly-L-lactic acid (PLLA) microspheres,35 and a variety of other embolic agents have been used with varying success, drawbacks of these approaches include a short duration until biodegradation (PVA and possibly PLLA microspheres) and inadequate distal arteriolar penetration. Secondary recruitment of collateral shunts to the nidus after incomplete embolization is well known.31,32,36 New dural and pial collateral feeders are often very small tortuous arteries that cannot be selectively cannulated, and thus repeated transarterial embolization of these vessels is generally ineffective or impossible. Follow-up angiography after particulate embolization and angiographic “cure” is essential to document the absence of fistula recurrence.37
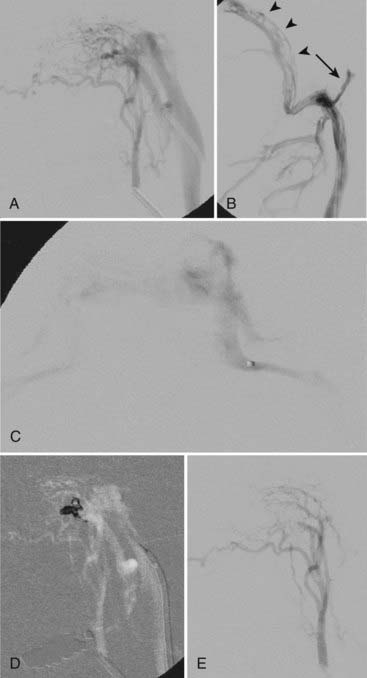
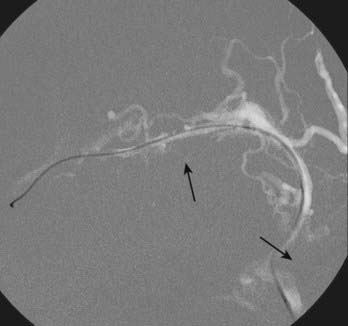
When considering Onyx embolization of a DAVF with multiple feeders from the middle meningeal, occipital, and superficial temporal arteries, the greatest chance of success will generally be with middle meningeal artery embolization because the other vessels usually supply the fistula via transosseous collaterals, which are often tortuous/smaller in caliber and therefore suboptimal for relying on distal permeation of the nidus/draining vein. An example of a small transosseous collateral feeder from the extradural vertebral artery is presented in Figure 391-1. The procedure is terminated when the nidus or foot of the draining vein, or both, are casted with Onyx or enough Onyx refluxes around the microcatheter tip to cause difficulty removing the catheter.
In this manner, Onyx can be a useful adjunct or a substitute for transvenous coil embolization if transarterial penetration of the arterialized venous pouch is possible (Fig. 391-6) or if transvenous deployment of Onyx is better suited to the lesion than coils. In high-flow DAVFs with feeding pedicles larger than 3 mm in diameter, transvenous coil placement would reduce flow through the fistula before transarterial injection of Onyx and lessen the risk for unintended vessel embolization. Onyx can be combined with other approaches, such as a covered stent, for complex DAVFs.38
The approach to pediatric patients with congenital DAVFs and congestive heart failure involves diminution of flow to permit survival and growth, usually by transarterial or mixed transarterial/transvenous techniques. If residual DAVF is present, cure is attempted at a later date (3 to 6 months of age).32
Surgery
When transvenous or transarterial embolization with Onyx fails, surgery is considered. Initial surgical attempts to resect DAVFs involve massive blood loss.36 However, excision of fistulas, including adjacent arteries and veins, is occasionally performed.39,40 If a dural sinus must be preserved, skeletonization or surgical isolation of the sinus can be carried out to minimize sacrifice of any meningeal arteries that may be supplying potentially important structures.41 In Borden type III DAVFs, coagulation, excision, or ligation of the draining vein is performed at the point of the fistula. The vein draining a DAVF may be safely interrupted42 or ligated43 before removing the rest of the fistula. This is in distinction to a pial arteriovenous malformation, for which primary resection of the draining vein could lead to premature edema/hemorrhage because of a rapid increase in intranidal pressure.10
Injection of glue after surgical exposure of the feeding arteries and draining veins has also been reported.41 Newer techniques involving a small craniotomy performed under local anesthesia for direct sinus coil packing of small DAVFs of the transverse and sigmoid sinuses have been described.17,44,45 The efficacy of packing can be monitored by sequential pressure and blood gas measurements of the diseased sinus. Sinus pressure is 29% to 58% of mean systemic blood pressure, and blood gas is purely arterial at the beginning of the procedure and returns to normal venous values after occlusion of the fistula.17
Radiosurgery
Stereotactic radiosurgery is an infrequent secondary or tertiary option to obviate a craniotomy46 and has been used to eradicate small DAVFs, even those with leptomeningeal venous drainage.47,48 It is sometimes performed after endovascular palliation of arteriovenous shunting.49 Disadvantages of this approach include a waiting period of at least 2 years (and possibly longer to determine the side effects of radiosurgery), and intermittent cerebral angiography is necessary to determine the benefit of treatment. Future studies are needed to address dose planning to achieve selective occlusion of fistulas, although 14 Gy seems to be the minimum dose required for DAVF occlusion. If using radiation monotherapy, marginal doses of greater than 20 Gy are recommended.48
Clinical and Angiographic Features and Treatment of Dural Arteriovenous Fistulas According to Location
Transverse-Sigmoid Sinus Dural Arteriovenous Fistulas
In an analysis of 377 DAVFs by Awad and colleagues, transverse and sigmoid sinus lesions accounted for 62.9% of the DAVFs.12 Nearly half of these patients had a cranial bruit because of proximity of the draining sinus to the middle ear.16 Aggressive neurological symptoms, as defined by symptoms or signs of intracranial hypertension, intracranial hemorrhage, stroke, or ascending myelopathy, were noted in 27% of patients in the series of Cognard and coworkers.1 Treatment is recommended for all symptomatic transverse-sigmoid sinus DAVFs because they occlude spontaneously only 5% of the time and usually do so after hemorrhagic events.16 Transvenous embolization is the favored approach, if technically feasible.
Superior Sagittal Sinus Dural Arteriovenous Fistulas
AVFs involving the SSS account for 7.4% to 8% of intracranial DAVFs.5,12,16 Unlike DAVFs in other locations, which have a strong female preponderance, SSS DAVFs have an equal gender distribution.37 They have a high incidence of associated retrograde drainage into the parasagittal cortical veins. Treatment is often challenging because of multiple and bilateral arterial feeders, most commonly the middle meningeal arteries.37 Variant SSS DAVF is a separate entity that occurs after severe head injury50 and involves direct shunts to cortical veins without participation of the SSS. Consequently, venous congestive encephalopathy, cerebral edema, seizures, hemorrhage, and dementia are frequent sequelae. Headache occurs in 50% of patients with SSS DAVFs.16 Transarterial embolization50 and surgical coagulation of the draining vein are the preferred treatment options for variant SSS fistulas or those located in the anterior third of the SSS. A combination of surgical and endovascular techniques,51 including direct-puncture transvenous embolization during surgery5 or transvenous embolization alone,52 has been described.
Tentorial Dural Arteriovenous Fistulas
Tentorial DAVFs represent 12% to 14% of all DAVFs.16 The arterial feeders of tentorial DAVFs are derived from the meningohypophysial trunk, occipital artery, ascending pharyngeal artery, and anterior and posterior meningeal branches of the vertebral artery.53 The initial neurological symptoms are tinnitus (70% to 88%), headache (8% to 24%), and cranial nerve palsies (14% to 17%).16 Ninety-two percent of these lesions give rise to aggressive neurological symptoms,1 with a 60% to 74% incidence of intracranial hemorrhage. Venous drainage is always leptomeningeal16 and is usually cortical, although spinal perimedullary venous drainage has been demonstrated.54 Treatment paradigms are similar to those for DAVFs in other locations.
Anterior Fossa Dural Arteriovenous Fistulas
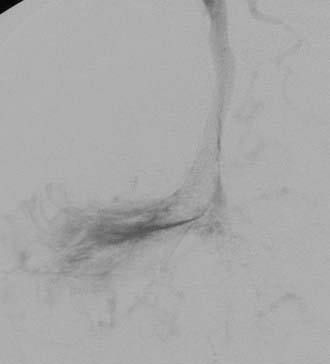
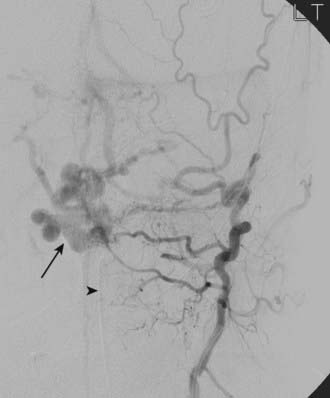
DAVFs of the anterior cranial fossa are located at the cribriform plate at the level of the foramen cecum.55 These DAVFs are invariably supplied by small, usually bilateral ethmoidal branches of the ophthalmic arteries and sometimes by branches of the internal maxillary and middle meningeal arteries (Fig. 391-7). Unlike intracranial DAVFs in other locations, the overwhelming majority of anterior fossa DAVFs occur in males. In contrast to posterior fossa DAVFs, frontobasal fistulas are nearly always symptomatic because of a high incidence of associated leptomeningeal venous drainage and venous hypertension.1 These fistulas are frequently manifested as subarachnoid hemorrhage.53 Surgical coagulation of the fistula or draining veins (or both) is an excellent option for DAVFs in this location. Endovascular cure is more challenging because the small caliber of feeding vessels limits transarterial penetration of embolic agents. Transvenous access may be cumbersome as well as a result of leptomeningeal drainage into tortuous,16 deep, aneurysmally dilated56 cerebral veins (nearly 50% into the basal vein of Rosenthal in one series).55
Other Dural Arteriovenous Fistulas
DAVFs of the torcular Herophili and foramen magnum (Fig. 391-8) have an extremely high incidence of aggressive neurological symptoms (100% for torcular DAVFs in the series of Cognard and associates1). Hypoglossal canal57 and marginal sinus DAVFs have rarely been reported (see Fig. 391-3). There is often significant arterial input from ascending pharyngeal artery branches, especially the neuromeningeal trunk. Because sacrificing this trunk will result in eloquent deficits, a transvenous approach is usually preferred. Foramen magnum DAVFs also derive their vascular supply from the occipital arteries, tentorial branches of the internal carotid artery, and posterior meningeal branches of the vertebral arteries.57 Retrograde drainage into the spinal perimedullary veins is not uncommon and causes progressive myelopathy in 50% of patients because of venous hypertension of the spinal cord.2,30
Outcome
Angiographic cure with transvenous coil or combined transvenous coil/liquid agent embolization is thought to be permanent, as is surgical interruption of the leptomeningeal draining vein/fistula. However, even documented angiographic resolution of the DAVF after transarterial embolization must be regarded as palliative or temporary unless sufficient penetration of the nidus/recipient venous pouch has occurred (see Fig. 391-6). Annual follow-up cerebral angiograms, at least in the short term, are routine for patients who have undergone transarterial embolization, even if the symptoms have resolved.
Complications of the different neuroendovascular techniques are discussed earlier. Vasoparalysis from long-standing venous ischemia probably contributes to postoperative hyperperfusion, as has been documented by single-photon emission computed tomography.58 Preoperative embolization of the DAVF may avoid this complication by reducing the rapid hemodynamic changes. Direct sinus packing is highly effective and associated with low morbidity.17
Awad IA, Little JR, Akarawi WP, et al. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg. 1990;72:839-850.
Barnwell SL, Halbach VV, Higashida RT, et al. Complex dural arteriovenous fistulas. Results of combined endovascular and neurosurgical treatment in 16 patients. J Neurosurg. 1989;71:352-358.
Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166-179.
Caragine LP, Halbach VV, Dowd CF, et al. Parallel venous channel as the recipient pouch in transverse/sigmoid sinus dural fistulae. Neurosurgery. 2003;53:1261-1266.
Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671-680.
Davies MA, TerBrugge KG, Willinsky R, et al. The natural history and management of intracranial dural arteriovenous fistulae, part 2: aggressive lesions. Interv Neuroradiol. 1997;3:303-311.
Djindjian R, Merland JJ, Theron J. Superselective Arteriography of the External Carotid Artery. Berlin: Springer-Verlag; 1977.
Duffau H, Lopes M, Janosevic V, et al. Early rebleeding from intracranial dural arteriovenous fistulas: report of 20 cases and review of the literature. J Neurosurg. 1999;90:78-84.
Halbach VV, Higashida RT, Hieshima GB, et al. Transvenous embolization of dural fistulas involving the transverse and sigmoid sinuses. AJNR Am J Neuroradiol. 1989;10:385-392.
Halbach VV, Higashida RT, Hieshima GB, et al. Dural fistulas involving the transverse and sigmoid sinuses: results of treatment in 28 patients. Radiology. 1987;163:443-447.
Houser OW, Baker, HL Jr, Rhoton ALJr, et al. Intracranial dural arteriovenous malformations. Radiology. 1972;105:55-64.
Kallmes DF, Marx WF, Jensen ME, et al. Adjuvant use of epsilon-aminocaproic acid (Amicar) in the endovascular treatment of cranial arteriovenous fistulae. Neuroradiology. 2000;42:302-308.
Kincaid PK, Duckwiler GR, Gobin YP, et al. Dural arteriovenous fistula in children: endovascular treatment and outcomes in seven cases. AJNR Am J Neuroradiol. 2001;22:1217-1225.
Kiyosue H, Hori Y, Okahara M, et al. Treatment of intracranial dural arteriovenous fistulas: current strategies based on location and hemodynamics, and alternative techniques of transcatheter embolization. Radiographics. 2004;24:1637-1653.
Kurl S, Saari T, Vanninen R, et al. Dural arteriovenous fistulas of superior sagittal sinus: case report and review of literature. Surg Neurol. 1996;45:250-255.
Lalwani AK, Dowd CF, Halbach VV. Grading venous restrictive disease in patients with dural arteriovenous fistulas of the transverse/sigmoid sinus. J Neurosurg. 1993;79:11-15.
Levrier O, Métellus P, Fuentes S, et al. Use of a self-expanding stent with balloon angioplasty in the treatment of dural arteriovenous fistulas involving the transverse and/or sigmoid sinus: functional and neuroimaging-based outcome in 10 patients. J Neurosurg. 2006;104:254-263.
Roy D, Raymond J. The role of transvenous embolization in the treatment of intracranial dural arteriovenous fistulas. Neurosurgery. 1997;40:1133-1144.
Sundt TMJr, Piepgras DG. The surgical approach to arteriovenous malformations of the lateral and sigmoid dural sinuses. J Neurosurg. 1983;59:32-39.
Urtasun F, Biondi A, Casaco A, et al. Cerebral dural arteriovenous fistulas: percutaneous transvenous embolization. Radiology. 1996;199:209-217.
van Dijk JMC, TerBrugge KG, Willinsky RA, et al. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke. 2002;33:1233-1236.
1 Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671-680.
2 Malek AM, Higashida RT, Balousek PA, et al. Endovascular recanalization with balloon angioplasty and stenting of an occluded occipital sinus for treatment of intracranial venous hypertension: technical case report. Neurosurgery. 1999;44:896-901.
3 Nishio A, Ohata K, Tsuchida K, et al. Dural arteriovenous fistula involving the superior sagittal sinus following sinus thrombosis: case report. Neurol Med Chir (Tokyo). 2002;42:217-220.
4 Sakaki T, Morimoto T, Nakase H, et al. Dural arteriovenous fistula of the posterior fossa developing after surgical occlusion of the sigmoid sinus. J Neurosurg. 1996;84:113-118.
5 Tsutsumi S, Yasumoto Y, Ito M, et al. Atypical dural arteriovenous fistula associated with meningitis: case report. Neurol Med Chir (Tokyo). 2008;48:68-71.
6 Houser OW, Baker, HL Jr, Rhoton ALJr, et al. Intracranial dural arteriovenous malformations. Radiology. 1972;105:55-64.
7 Djindjian R, Merland JJ, Theron J. Superselective Arteriography of the External Carotid Artery. Berlin: Springer-Verlag; 1977.
8 Piton J, Guilleux MH, Guibert-Tranier F, et al. Fistulae of the lateral sinus. J Neuroradiol. 1984;11:143-159.
9 Lalwani AK, Dowd CF, Halbach VV. Grading venous restrictive disease in patients with dural arteriovenous fistulas of the transverse/sigmoid sinus. J Neurosurg. 1993;79:11-15.
10 Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166-179.
11 van Dijk JMC, TerBrugge KG, Willinsky RA, et al. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke. 2002;33:1233-1236.
12 Awad IA, Little JR, Akarawi WP, et al. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg. 1990;72:839-850.
13 Lasjaunias P, Merland JJ, Théron J, et al. Vascularisation méningée de la fosse cérébrale moyenne. J Neuroradiol. 1977;4:361-384.
14 Duffau H, Lopes M, Janosevic V, et al. Early rebleeding from intracranial dural arteriovenous fistulas: report of 20 cases and review of the literature. J Neurosurg. 1999;90:78-84.
15 Davies MA, TerBrugge KG, Willinsky R, et al. The natural history and management of intracranial dural arteriovenous fistulae, part 2: aggressive lesions. Interv Neuroradiol. 1997;3:303-311.
16 Kiyosue H, Hori Y, Okahara M, et al. Treatment of intracranial dural arteriovenous fistulas: current strategies based on location and hemodynamics, and alternative techniques of transcatheter embolization. Radiographics. 2004;24:1637-1653.
17 Endo S, Kuwayama N, Takaku A, et al. Direct packing of the isolated sinus in patients with dural arteriovenous fistulas of the transverse-sigmoid sinus. J Neurosurg. 1998;88:449-456.
18 Halbach VV, Higashida RT, Hieshima GB, et al. Dural fistulas involving the transverse and sigmoid sinuses: results of treatment in 28 patients. Radiology. 1987;163:443-447.
19 Halbach VV, Higashida RT, Hieshima GB, et al. Transvenous embolization of dural fistulas involving the transverse and sigmoid sinuses. AJNR Am J Neuroradiol. 1989;10:385-392.
20 Roy D, Raymond J. The role of transvenous embolization in the treatment of intracranial dural arteriovenous fistulas. Neurosurgery. 1997;40:1133-1144.
21 Urtasun F, Biondi A, Casaco A, et al. Cerebral dural arteriovenous fistulas: percutaneous transvenous embolization. Radiology. 1996;199:209-217.
22 Caragine LP, Halbach VV, Dowd CF, et al. Parallel venous channel as the recipient pouch in transverse/sigmoid sinus dural fistulae. Neurosurgery. 2003;53:1261-1266.
23 Gobin YP, Houdart E, Rogopoulos A, et al. Percutaneous transvenous embolization through the thrombosed sinus in transverse sinus dural fistula. AJNR Am J Neuroradiol. 1993;14:1102-1105.
24 Naito I, Iwai T, Shimaguchi H, et al. Percutaneous transvenous embolization through the occluded sinus for transverse-sigmoid dural arteriovenous fistulas with sinus occlusion. Neuroradiology. 2001;43:672-676.
25 Komiyama M, Ishiguro T, Matsusaka Y, et al. Transfemoral, transvenous embolisation of dural arteriovenous fistula involving the isolated transverse-sigmoid sinus from the contralateral side. Acta Neurochir (Wien). 2002;144:1041-1046.
26 Murphy K, Gailloud P, Venbrux A, et al. Endovascular treatment of a grade IV transverse sinus dural arteriovenous fistula by sinus recanalization, angioplasty, and stent placement: technical case report. Neurosurgery. 2000;46:497-501.
27 Liebig T, Henkes H, Brew S, et al. Reconstructive treatment of dural arteriovenous fistulas of the transverse and sigmoid sinus: transvenous angioplasty and stent deployment. Neuroradiology. 2005;47:543-551.
28 Levrier O, Métellus P, Fuentes S, et al. Use of a self-expanding stent with balloon angioplasty in the treatment of dural arteriovenous fistulas involving the transverse and/or sigmoid sinus: functional and neuroimaging-based outcome in 10 patients. J Neurosurg. 2006;104:254-263.
29 Gutierrez A, Do HM, Marks MP. Alteration in the venous drainage of a dural arteriovenous fistula following angioplasty. AJNR Am J Neuroradiol. 2004;25:1086-1088.
30 Troffkin NA, Graham CB3rd, Berkmen T, et al. Combined transvenous and transarterial embolization of a tentorial-incisural dural arteriovenous malformation followed by primary stent placement in the associated stenotic straight sinus. Case report. J Neurosurg. 2003;99:579-583.
31 Ohtakara K, Murao K, Kawaguchi K, et al. Selective transvenous liquid embolization of a type 1 dural arteriovenous fistula at the junction of the transverse and sigmoid sinuses. J Neurosurg. 2000;92:1045-1049.
32 Kincaid PK, Duckwiler GR, Gobin YP, et al. Dural arteriovenous fistula in children: endovascular treatment and outcomes in seven cases. AJNR Am J Neuroradiol. 2001;22:1217-1225.
33 Kallmes DF, Marx WF, Jensen ME, et al. Adjuvant use of epsilon-aminocaproic acid (Amicar) in the endovascular treatment of cranial arteriovenous fistulae. Neuroradiology. 2000;42:302-308.
34 Goto K, Sidipratomo P, Ogata N, et al. Combining endovascular and neurosurgical treatments of high-risk dural arteriovenous fistulas in the lateral sinus and the confluence of the sinuses. J Neurosurg. 1999;90:289-299.
35 Yamamoto T, Hayakawa K, Tabata Y, et al. Transcatheter arterial embolization using poly-L-lactic acid microspheres. Radiat Med. 2003;21:150-154.
36 Sundt TMJr, Piepgras DG. The surgical approach to arteriovenous malformations of the lateral and sigmoid dural sinuses. J Neurosurg. 1983;59:32-39.
37 Kurl S, Saari T, Vanninen R, et al. Dural arteriovenous fistulas of superior sagittal sinus: case report and review of literature. Surg Neurol. 1996;45:250-255.
38 Shi ZS, Qi TW, Gonzalez NR, et al. Combined covered stent and Onyx treatment for complex dural arteriovenous fistula involving the clivus and cavernous sinus. Surg Neurol. 2009;72:169-174.
39 Nomura S, Anegawa S, Nakagawa S, et al. Subarachnoid hemorrhage caused by dural arteriovenous fistula of the sphenobasal sinus. Neurol Med Chir (Tokyo). 2002;42:255-258.
40 Lewis AI, Rosenblatt SS, Tew JJ. Surgical management of deep-seated dural arteriovenous malformations. J Neurosurg. 1997;87:198-206.
41 Barnwell SL, Halbach VV, Higashida RT, et al. Complex dural arteriovenous fistulas. Results of combined endovascular and neurosurgical treatment in 16 patients. J Neurosurg. 1989;71:352-358.
42 Thompson BG, Doppman JL, Oldfield EH. Treatment of cranial dural arteriovenous fistulae by interruption of leptomeningeal venous drainage. J Neurosurg. 1994;80:617-623.
43 Collice M, D’Aliberti G, Talamonti G, et al. Surgical interruption of leptomeningeal draining as treatment for intracranial dural arteriovenous fistulas without dural sinus drainage. J Neurosurg. 1994;84:810-817.
44 Kuwayama N, Akai T, Horie Y, et al. Dural arteriovenous fistulae involving the transverse-sigmoid sinus and foramen magnum. Surg Neurol. 1994;41:389-395.
45 Kasai K, Iwasa H, Yamada N, et al. Combined treatment of a dural arteriovenous malformation of the lateral sinus using transarterial and direct lateral sinus embolisation. Neuroradiology. 1996;38:494-496.
46 Ratliff J, Voorhies RM. Arteriovenous fistula with associated aneurysms coexisting with dural arteriovenous malformation of the anterior inferior falx: case report and review of the literature. J Neurosurg. 1999;91:303-307.
47 Chandler HC, Friedman WA. Successful radiosurgical treatment of a dural arteriovenous malformation: case report. Neurosurgery. 1993;33:139-141.
48 Shin M, Kurita H, Tago M, et al. Stereotactic radiosurgery for tentorial dural arteriovenous fistulae draining into the vein of Galen: report of two cases. Neurosurgery. 2000;46:730-733.
49 Friedman JA, Pollock BE, Nichols DA, et al. Results of combined stereotactic radiosurgery and transarterial embolization for dural arteriovenous fistulas of the transverse and sigmoid sinuses. J Neurosurg. 2001;94:886-891.
50 Fukai J, Terada T, Kuwata T, et al. Transarterial intravenous coil embolization of dural arteriovenous fistula involving the superior sagittal sinus. Surg Neurol. 2001;55:353-358.
51 Barnwell SL, Halbach VV, Dowd CF, et al. A variant of arteriovenous fistulas within the wall of dural sinuses. Results of combined surgical and endovascular therapy. J Neurosurg. 1991;74:199-204.
52 Cloft HJ, Kallmes DF, Jensen ME, et al. Percutaneous coil embolization of a type 4 sagittal sinus dural arteriovenous fistula: case report. Neurosurgery. 1997;41:1191-1193.
53 Viñuela F, Fox AJ, Pelz DM, et al. Unusual clinical manifestations of dural arteriovenous malformations. J Neurosurg. 1986;64:554-558.
54 Bret P, Salzmann M, Bascoulergue Y, et al. Dural arteriovenous fistula of the posterior fossa draining into the spinal medullary veins—an unusual cause of myelopathy: case report. Neurosurgery. 1994;35:965-969.
55 Reul J, Thron A, Laborde G, et al. Dural arteriovenous malformations at the base of the anterior cranial fossa: report of nine cases. Neuroradiology. 1993;35:388-393.
56 Defreyne L, Vanlangenhove P, Vandekerckhove T, et al. Transvenous embolization of a dural arteriovenous fistula of the anterior cranial fossa: preliminary results. AJNR Am J Neuroradiol. 2000;21:761-765.
57 Kiyosue H, Tanoue S, Okahara M, et al. Ocular symptoms associated with a dural arteriovenous fistula involving the hypoglossal canal: selective transvenous coil embolization. Case report. J Neurosurg. 2001;94:630-632.
58 Kuroda S, Ushikoshi S, Houkin K, et al. Postoperative hyperperfusion in dural arteriovenous fistula associated with venous ischemia: case report. Surg Neurol. 1998;49:406-411.