CHAPTER 79 Pallidal Interventions for Parkinson’s Disease
In the context of movement disorders such as Parkinson’s disease (PD), pallidal surgery refers to lesioning or chronic electrical stimulation (deep brain stimulation [DBS]) of the posterior GPi. Lesioning of the GPi, or pallidotomy, was first performed in patients with PD in the 1940s and 1950s, before any theoretical understanding of its function. The first modern study of pallidotomy for PD (performed in the era of computed tomography and magnetic resonance imaging) was that of Laitinen and colleagues1 in 1992. In the 1990s, chronic DBS emerged as a reversible, adjustable alternative to stereotactic lesioning and was used at all targets where lesioning was effective. GPi-DBS was first reported by Siegfried and Lippitz2 in 1994. Several controlled studies have shown that either GPi-DBS or STN-DBS can modify all cardinal motor signs of PD.3 DBS is safer than pallidotomy for bilateral intervention. Currently, for patients undergoing DBS for PD, the STN is the more commonly selected target, based on evidence that STN-DBS allows a greater reduction in antiparkinsonian medications and a lower stimulation voltage than GPi-DBS. However, randomized studies comparing GPi and STN are showing comparable efficacy,4 and there may be subgroups of PD patients for whom GPi-DBS is preferred. At this time, GPi-DBS for PD is an “on-label” indication for the Medtronic Activa DBS system (Minneapolis, MN).
Mechanism of Action
In the parkinsonian state, neuronal discharge in the GPi and STN is abnormal in several ways: spontaneous firing rates are elevated, neurons that normally discharge independently develop abnormal synchrony, bursting discharge is increased, neurons show oscillations in both the theta to alpha (3 to 8 Hz) and low beta (8 to 20 Hz) ranges, and cells that normally respond to movement of a single joint show responses to multiple joints (loss of joint selectivity).5 Pallidotomy, when it covers the majority of the motor territory of the GPi, eliminates these abnormalities. Although it clearly does not restore normal function (presumably, it reduces GPi output to zero), it partially corrects the cortical metabolic abnormalities found in PD.6 Apparently, motor-controlling cortical areas in adults can function more normally without basal ganglia output than under the influence of the highly abnormal basal ganglia output characteristic of PD.
With regard to GPi-DBS, the mechanism of action is less clear. Originally conceived of as a “reversible lesion,” it now appears that DBS at the frequencies used in PD surgery (100 to 200 Hz) drives the efferent axons of the stimulated structure. The effect on neuronal cell bodies is not certain. GPi-DBS decreases the firing rates in one of its major efferent targets, the ventrolateral thalamus, consistent with activation of the GABAergic pallidothalamic projection.7 Thus, unlike pallidotomy, GPi-DBS does not suppress the abnormally increased GPi firing rates, although it probably does reduce bursting and oscillatory activity in the most detrimental (alpha to beta) frequency ranges.
Indications
Indications for pallidal surgery in PD are as follows:
Contraindications to pallidal surgery in PD are as follows:
In addition to the indications listed, good candidates for pallidal lesioning include patients with asymmetric symptoms (because pallidotomy can be safely performed on only one side) and patients who will have difficulty complying with the follow-up required for the management of deep brain stimulators. For patients requiring bilateral surgery and who can comply with follow-up appointments, bilateral DBS is generally preferred. The exact indications for GPi-DBS versus STN-DBS have not been well defined.4,8 There is no evidence that either pallidotomy or GPi-DBS is neuroprotective against ongoing brain degeneration in PD; thus, they should not be performed early in the disease when symptoms are well managed medically.
Results
Outcomes for surgical therapy in PD are described in terms of standard rating scales of parkinsonian motor signs and symptoms, the most prevalent of which is the Unified Parkinson’s Disease Rating Scale (UPDRS). In a randomized trial of pallidotomy with a medical control arm and blinded evaluations, unilateral pallidotomy produced a 32% decrease (improvement) in the total UPDRS score at 1 year, which was significant compared with a 5% increase in the control group.9 A European study produced similar results at 6-month follow-up.10
Unilateral pallidal stimulation produced similar results to unilateral pallidotomy in a randomized study.11 Unilateral GPi-DBS has also been compared with unilateral STN-DBS, and both procedures produced similar improvements in contralateral bradykinesia12 and manual dexterity.13 The only published randomized comparison of bilateral GPi-DBS and bilateral STN-DBS showed a 38% improvement in motor UPDRS in the GPi group not taking medication, compared with 48% in the STN group. However, cognitive and behavioral complications were observed only in the STN group.4 A large study (300 patients) is in progress comparing bilateral pallidal DBS (and bilateral STN-DBS) with a medical control arm.
Complications
The most serious intraoperative or early postoperative complication of DBS or pallidotomy is intracerebral hemorrhage. In the series reported by Binder and colleagues,14 the risk of symptomatic hemorrhage from DBS surgery on a per patient basis was 2.1%, which is consistent with other large series. Early postoperative complications include mental status changes and hardware infection. In the report by Sillay and coworkers,15 the incidence of perioperative (within 6 months) device infection requiring a return to the operating room for partial or complete hardware removal was 4.5% per patient. Long-term hardware-related complications include skin erosion, lead fracture, pulse generator migration, and lead twisting. The incidence of lead fracture and other hardware complications increases with longer postoperative follow-up time and may be as high as 8.4% per electrode-year.16
The risk of hemorrhagic stroke is probably similar for pallidotomy and pallidal DBS. Lesioning surgery does not entail any hardware-related complications and has a much lower rate of infection owing to the absence of an indwelling device. Lesioning, however, carries certain risks that are not present with DBS surgery. It is possible to create inadvertent thermal lesions in critical structures surrounding the intended target, such as the corticobulbar tract (CBT), cortisospinal tract (CST), or optic tract (OT). Bilateral lesioning of the GPi carries a high risk of permanent speech or cognitive dysfunction, even when lesions are correctly placed.17,18 Delayed ischemic subcortical infarction appears to be more common following pallidotomy than after GPi-DBS.19,20 This may relate to thermal damage to neighboring perforating vessels at the time of radiofrequency lesioning.
Surgical Technique
The optimal methods for movement disorder surgery are far from standardized and continue to evolve.
Stereotactic Localization
Figure 79-1 shows the intended active contact location for GPi-DBS within the posterior motor territory of the nucleus. Leads are placed so that the active contact is 3 to 4 mm from the border between the GPi and the internal capsule; this allows the stimulation-induced electrical effect to spread throughout most of the motor territory of the GPi without spreading to the internal capsule. For pallidotomy, the lesion includes the point shown but should extend to the border of the internal capsule posteromedially, the border of the GPe dorsolaterally, and the base of the pallidum inferiorly. Including the GPe in the lesion reduces the efficacy of pallidotomy.21
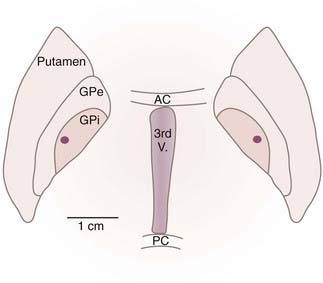
FIGURE 79-1 Typical active contact location for deep brain stimulation of the globus pallidus interna (GPi; dot) with respect to axial plane anatomy drawn from the Schaltenbrand and Wahren26 atlas of the human brain. The axial plane selected passes through the intercommissural line. AC, anterior commissure; GPe, globus pallidus externa; PC, posterior commissure; 3rd V, third ventricle.
(Modified from Starr PA. Placement of deep brain stimulators into the subthalamic nucleus or globus pallidus internus: technical approach. Stereotact Funct Neurosurg. 2002;79:118-145.)
Target Selection
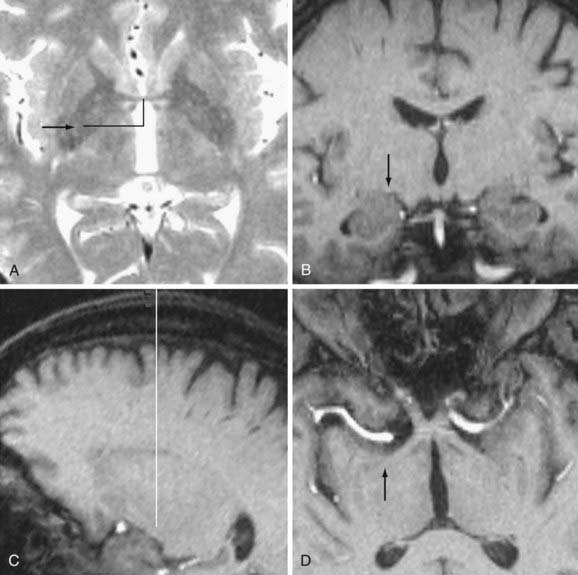
Stereotactic coordinates for the initial anatomic target are first determined indirectly on reformatted 3D-GRE images. Typical coordinates are as follows: anteroposterior (AP) = +2 mm and vertical = −5 mm with respect to the midcommissural point, and lateral = 17.5 mm from the third ventricular wall. The lateral and vertical coordinates are then adjusted with respect to the individual nuclear anatomy on T2-FSE images or IR-FSE images, as shown in Figure 79-2. The target for the DBS lead tip or the inferiormost extent of a pallidotomy lesion is at the base of the GPi immediately superior to the lateral border of the OT. The active DBS contact, or the center of a pallidotomy lesion, is generally on the axial plane passing through the commissures. The active contact is 1 to 2 mm medial to the external accessory lamina, which forms the boundary between the GPi and GPe (see Fig. 79-2), and 3 to 4 mm from the internal capsule. Individual variation in the lateral position of the GPi is significant.
Entry Point and Trajectory Determination
A typical trajectory is 60 degrees from the AC-PC line in the sagittal projection, and 0 degrees lateral from the vertical in the coronal projection (e.g., a parasagittal approach). This standard trajectory is visualized on volumetric MRI using “navigation” views. If the trajectory will pass through the ventricle, I adjust the arc angle to a more lateral trajectory that avoids the ventricle. If the trajectory will pass through any sulci, I adjust the arc or ring angles (usually no more than 5 degrees) to avoid these and decrease the risk of damaging a cortical vessel. Figure 79-2 shows satisfactory trajectories to the GPi in navigation views.
Microelectrode Recording and Microstimulation
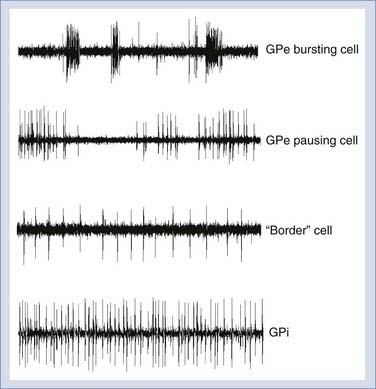
Action potential discharge patterns encountered in the region of the GPi are shown in Figure 79-3. The microelectrode usually encounters the striatum (caudate or putamen) and then the GPe before the GPi. The majority of striatal neurons have very low (0 to 10 Hz) spontaneous discharge rates. In PD, GPe neurons have spontaneous discharge rates of 30 to 60 Hz and typically discharge in “bursting” or “pausing” patterns; GPi neurons are faster, with spontaneous discharge rates of 60 to 100 Hz. Cells with regular discharge rates of 20 to 40 Hz, known as “border” cells, are typically found in the white matter laminae surrounding the GPe and GPi. The OT is identified by light-evoked fiber activity below the inferior margin of the GPi.
Cells in the GPi that are responsive to joint movements usually respond to the movement of one or several joints in a restricted region on the contralateral side of the body. The motor territory of the GPi is somatotopically organized, with leg representations more dorsal and more medial than arm representations.22–24 Thus, noting the distribution of neuronal receptive fields helps determine the mediolateral coordinate of an electrode track. In patients with tremor, cells with discharges grouped at the tremor frequency are often recorded. Microstimulation can be used to localize both the CBT-CST and the OT. The CBT-CST in the internal capsule is identified by evoking muscle contractions (usually of the tongue, face, or hand) at low current thresholds (e.g., 10 µA at 300 Hz, 200 µsec pulse width). The OT is identified when the patient reports visual phenomena (focal scintillating scotomata) at low current thresholds.
Internal Globus Pallidus Lesioning Technique
For pallidotomy, the localization techniques are identical to those for GPi-DBS. After MER localization, lesioning is planned based on the MER map to correspond to the total mapped motor area. MER mapping for lesioning is more extensive than for DBS because localization with absolute certainty is imperative. Lesioning is performed with a 3-mm cylindrical radiofrequency electrode (Radionics, Burlington, MA) at multiple depths and along multiple (two to three) trajectories of the lesioning probe, at 70°C to 85°C for 1 minute each.22
Postoperative Management
Magnetic Resonance Imaging
Medtronic has issued specific guidelines for the use of MRI in the presence of an implanted Activa DBS system. I routinely obtain 1.5-tesla MRI postoperatively and have had no adverse events in more than 1000 examinations.25 Postoperative imaging of the DBS lead is important for documentation of its exact location (Fig. 79-4).
Alberts JL, Okun MS, Vitek JL. The persistent effects of unilateral pallidal and subthalamic deep brain stimulation on force control in advanced Parkinson’s patients. Parkinsonism Relat Disord. 2008;14:481-488.
Anderson ME, Postupna N, Ruffo M. Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J Neurophysiol. 2003;89:1150-1160.
Anderson VC, Burchiel KJ, Hogarth P, et al. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol. 2005;62:554-560.
Baron MS, Vitek JL, Bakay RAE, et al. Treatment of advanced Parkinson’s disease by posterior GPi pallidotomy: 1-year results of a pilot study. Ann Neurol. 1996;40:355-366.
Binder D, Rau G, Starr PA. Risk factors for hemorrhage during microelectrode-guided deep brain stimulator implantation for movement disorders. Neurosurgery. 2005;56:722-732.
Chang EF, Turner RS, Ostrem JL, et al. Neuronal responses to passive movement in the globus pallidus internus in primary dystonia. J Neurophysiol. 2007;98:3696-3707.
de Bie RM, de Haan RJ, Nijssen PC, et al. Unilateral pallidotomy in Parkinson’s disease: a randomised, single-blind, multicentre trial. Lancet. 1999;354(9191):1665-1669.
Deep-Brain Stimulation for Parkinson’s Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345:956-963.
Eidelberg D, Moeller JR, Ishikawa T, et al. Regional metabolic correlates of surgical outcome following unilateral pallidotomy for Parkinson’s disease. Ann Neurol. 1996;39:450-459.
Gatev P, Darbin O, Wichmann T. Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov Disord. 2006;21:1566-1577.
Guridi J, Gorospe A, Ramos E, et al. Stereotactic targeting of the globus pallidus internus in Parkinson’s disease: imaging versus electrophysiological mapping. Neurosurgery. 1999;45:278-289.
Higuchi Y, Iacono RP. Surgical complications in patients with Parkinson’s disease after posteroventral pallidotomy. Neurosurgery. 2003;52:558-571.
Laitinen LV, Bergenheim AT, Hariz MI. Leksell’s posteroventral pallidotomy in the treatment of Parkinson’s disease. J Neurosurg. 1992;76:53-61.
Larson PS, Richardson RM, Starr PA, Martin AJ. Magnetic resonance imaging of implanted deep brain stimulators: experience in a large series. Stereotact Funct Neurosurg. 2008;86:92-100.
Lim JY, DeSalles AAF, Bronstein J, et al. Delayed internal capsule infarctions following radiofrequency pallidotomy. J Neurosurg. 1997;87:955-960.
Merello M, Nouzeilles MI, Kuzis G, et al. Unilateral radiofrequency lesion versus electrostimulation of posteroventral pallidum: a prospective randomized comparison. Mov Disord. 1999;14:50-56.
Merello M, Starkstein S, Nouzeilles MI, et al. Bilateral pallidotomy for treatment of Parkinson’s disease induced corticobulbar syndrome and psychic akinesia avoidable by globus pallidus lesion combined with contralateral stimulation. J Neurol Neurosurg Psychiatry. 2001;71:611-614.
Nakamura K, Christine CW, Starr PA, Marks WJJr. Effects of unilateral subthalamic and pallidal deep brain stimulation on fine motor functions in Parkinson’s disease. Mov Disord. 2007;22:619-626.
Oh MY, Abosch A, Kim SH, et al. Long-term hardware-related complications of deep brain stimulation. Neurosurgery. 2002;50:1274-1276.
Okun MS, Foote KD. Subthalamic nucleus vs globus pallidus interna deep brain stimulation, the rematch: will pallidal deep brain stimulation make a triumphant return? Arch Neurol. 2005;62:533-536.
Schaltenbrand G, Wahren W. Introduction to Stereotaxis with an Atlas of the Human Brain. Stuttgart: Georg Thieme; 1977.
Siegfried J, Lippitz B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery. 1994;35:1126-1129.
Sillay KA, Larson PS, Starr PA. Deep brain stimulator hardware-related infections: incidence and management in a large series. Neurosurgery. 2008;62:360-366.
Starr PA. Placement of deep brain stimulators into the subthalamic nucleus or globus pallidus internus: technical approach. Stereotact Funct Neurosurg. 2002;79:118-145.
Vitek JL, Bakay RA, Freeman A, et al. Randomized trial of pallidotomy versus medical therapy for Parkinson’s disease. Ann Neurol. 2003;53:558-569.
Vitek JL, Bakay RA, Hashimoto T, et al. Microelectrode-guided pallidotomy: technical approach and its application in medically intractable Parkinson’s disease. J Neurosurg. 1998;88:1027-1043.
Zhang J, Russo GS, Mewes K, et al. Lesions in monkey globus pallidus externus exacerbate parkinsonian symptoms. Exp Neurol. 2006;199:446-453.
1 Laitinen LV, Bergenheim AT, Hariz MI. Leksell’s posteroventral pallidotomy in the treatment of Parkinson’s disease. J Neurosurg. 1992;76:53-61.
2 Siegfried J, Lippitz B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery. 1994;35:1126-1129.
3 Deep-Brain Stimulation for Parkinson’s Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s diseasee. N Engl J Med. 2001;345:956-963.
4 Anderson VC, Burchiel KJ, Hogarth P, et al. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol. 2005;62:554-560.
5 Gatev P, Darbin O, Wichmann T. Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov Disord. 2006;21:1566-1577.
6 Eidelberg D, Moeller JR, Ishikawa T, et al. Regional metabolic correlates of surgical outcome following unilateral pallidotomy for Parkinson’s disease. Ann Neurol. 1996;39:450-459.
7 Anderson ME, Postupna N, Ruffo M. Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J Neurophysiol. 2003;89:1150-1160.
8 Okun MS, Foote KD. Subthalamic nucleus vs globus pallidus interna deep brain stimulation, the rematch: will pallidal deep brain stimulation make a triumphant return? Arch Neurol. 2005;62:533-536.
9 Vitek JL, Bakay RA, Freeman A, et al. Randomized trial of pallidotomy versus medical therapy for Parkinson’s disease. Ann Neurol. 2003;53:558-569.
10 de Bie RM, de Haan RJ, Nijssen PC, et al. Unilateral pallidotomy in Parkinson’s disease: a randomised, single-blind, multicentre trial. Lancet. 1999;354(9191):1665-1669.
11 Merello M, Nouzeilles MI, Kuzis G, et al. Unilateral radiofrequency lesion versus electrostimulation of posteroventral pallidum: a prospective randomized comparison. Mov Disord. 1999;14:50-56.
12 Nakamura K, Christine CW, Starr PA, Marks WJJr. Effects of unilateral subthalamic and pallidal deep brain stimulation on fine motor functions in Parkinson’s disease. Mov Disord. 2007;22:619-626.
13 Alberts JL, Okun MS, Vitek JL. The persistent effects of unilateral pallidal and subthalamic deep brain stimulation on force control in advanced Parkinson’s patients. Parkinsonism Relat Disord. 2008;14:481-488.
14 Binder D, Rau G, Starr PA. Risk factors for hemorrhage during microelectrode-guided deep brain stimulator implantation for movement disorders. Neurosurgery. 2005;56:722-732.
15 Sillay KA, Larson PS, Starr PA. Deep brain stimulator hardware-related infections: incidence and management in a large series. Neurosurgery. 2008;62:360-366.
16 Oh MY, Abosch A, Kim SH, et al. Long-term hardware-related complications of deep brain stimulation. Neurosurgery. 2002;50:1274-1276.
17 Merello M, Starkstein S, Nouzeilles MI, et al. Bilateral pallidotomy for treatment of Parkinson’s disease induced corticobulbar syndrome and psychic akinesia avoidable by globus pallidus lesion combined with contralateral stimulation. J Neurol Neurosurg Psychiatry. 2001;71:611-614.
18 Higuchi Y, Iacono RP. Surgical complications in patients with Parkinson’s disease after posteroventral pallidotomy. Neurosurgery. 2003;52:558-571.
19 Lim JY, DeSalles AAF, Bronstein J, et al. Delayed internal capsule infarctions following radiofrequency pallidotomy. J Neurosurg. 1997;87:955-960.
20 Baron MS, Vitek JL, Bakay RAE, et al. Treatment of advanced Parkinson’s disease by posterior GPi pallidotomy: 1-year results of a pilot study. Ann Neurol. 1996;40:355-366.
21 Zhang J, Russo GS, Mewes K, et al. Lesions in monkey globus pallidus externus exacerbate parkinsonian symptoms. Exp Neurol. 2006;199:446-453.
22 Vitek JL, Bakay RA, Hashimoto T, et al. Microelectrode-guided pallidotomy: technical approach and its application in medically intractable Parkinson’s disease. J Neurosurg. 1998;88:1027-1043.
23 Guridi J, Gorospe A, Ramos E, et al. Stereotactic targeting of the globus pallidus internus in Parkinson’s disease: imaging versus electrophysiological mapping. Neurosurgery. 1999;45:278-289.
24 Chang EF, Turner RS, Ostrem JL, et al. Neuronal responses to passive movement in the globus pallidus internus in primary dystonia. J Neurophysiol. 2007;98:3696-3707.
25 Larson PS, Richardson RM, Starr PA, Martin AJ. Magnetic resonance imaging of implanted deep brain stimulators: experience in a large series. Stereotact Funct Neurosurg. 2008;86:92-100.
26 Schaltenbrand G, Wahren W. Introduction to Stereotaxis with an Atlas of the Human Brain. Stuttgart: Georg Thieme; 1977.