CHAPTER 23 Treatment of Genetic Disease
Many genetic disorders are characterized by progressive disability or chronic ill health for which there is, at present, no effective treatment. Consequently, one of the most exciting aspects of the developments in biotechnology is the prospect of new treatments mediated through gene transfer, RNA modification, or stem cell therapy. It is important, however, to keep a perspective on the limitations of these approaches for the immediate future and to consider, in the first instance, conventional approaches to the treatment of genetic disease.
Conventional Approaches to Treatment of Genetic Disease
Most genetic disorders cannot be cured or even ameliorated using conventional methods of treatment. Sometimes this is because the underlying gene and gene product have not been identified so that there is little, if any, understanding of the basic metabolic or molecular defect. If, however, this is understood then dietary restriction, as in phenylketonuria (p. 167), or hormone replacement, as in congenital adrenal hyperplasia (p. 174), can be used very successfully in the treatment of the disorder. In a few disorders, such as homocystinuria (p. 172) and some of the organic acidurias (p. 183), supplementation with a vitamin or co-enzyme can increase the activity of the defective enzyme with beneficial effect (Table 23.1).
Table 23.1 Examples of Various Methods for Treating Genetic Disease
| Treatment | Disorder |
|---|---|
| Enzyme Induction by Drugs | |
| Phenobarbitone | Congenital non-hemolytic jaundice |
| Replacement of Deficient Enzyme/Protein | |
| Blood transfusion | SCID resulting from adenosine deaminase deficiency |
| Bone marrow transplantation | Mucopolysaccharidoses |
| Enzyme/Protein Preparations | |
| Trypsin | Trypsinogen deficiency |
| α1-Antitrypsin | α1-Antitrypsin deficiency |
| Cryoprecipitate/factor VIII | Hemophilia A |
| β-Glucosidase | Gaucher disease |
| α-Galactosidase | Fabry disease |
| Replacement of Deficient Vitamin or Coenzyme | |
| B6 | Homocystinuria |
| B12 | Methylmalonic acidemia |
| Biotin | Propionic acidemia |
| D | Vitamin D–resistant rickets |
| Replacement of Deficient Product | |
| Cortisone | Congenital adrenal hyperplasia |
| Thyroxine | Congenital hypothyroidism |
| Substrate Restriction in Diet | |
| Amino acids | |
| Phenylalanine | Phenylketonuria |
| Leucine, isoleucine, valine | Maple syrup urine disease |
| Carbohydrate | |
| Galactose | Galactosemia |
| Lipid | |
| Cholesterol | Familial hypercholesterolemia |
| Protein | Urea cycle disorders |
| Drug Therapy | |
| Aminocaproic acid | Angioneurotic edema |
| Dantrolene | Malignant hyperthermia |
| Cholestyramine | Familial hypercholesterolemia |
| Pancreatic enzymes | Cystic fibrosis |
| Penicillamine | Wilson disease, cystinuria |
| Drug/Dietary Avoidance | |
| Sulfonamides | G6PD deficiency |
| Barbiturates | Porphyria |
| Replacement of Diseased Tissue | |
| Kidney transplantation | Adult-onset polycystic kidney disease, Fabry disease |
| Bone marrow transplantation | X-linked SCID, Wiskott-Aldrich syndrome |
| Removal of Diseased Tissue | |
| Colectomy | Familial adenomatous polyposis |
| Splenectomy | Hereditary spherocytosis |
SCID, Severe combined immunodeficiency.
Protein/Enzyme Replacement
If a genetic disorder is found to be the result of a deficiency of or an abnormality in a specific enzyme or protein, treatment could, in theory, involve replacement of the deficient or defective enzyme or protein. An obviously successful example of this is the use of factor VIII concentrate in the treatment of hemophilia A (p. 309).
For most of the inborn errors of metabolism in which an enzyme deficiency has been identified, recombinant DNA techniques may be used to biosynthesize the missing or defective gene product; however, injection of the enzyme or protein may not be successful if the metabolic processes involved are carried out within cells and the protein or enzyme is not normally transported into the cell. Modifications in β-glucocerebrosidase as used in the treatment of Gaucher disease enable it to enter the lysosomes, resulting in an effective form of treatment (p. 178). Another example is the modification of adenosine deaminase (ADA) by an inert polymer, polyethylene glycol (PEG), to generate a replacement enzyme that is less immunogenic and has an extended half-life.
Drug Treatment
In some genetic disorders, drug therapy is possible; for example, statins can help to lower cholesterol levels in familial hypercholesterolemia (p. 175). Statins function indirectly through the low-density lipoprotein (LDL) receptor by inhibiting endogenous cholesterol biosynthesis at the rate-limiting step that is mediated by hydroxymethyl glutaryl co-enzyme A (HMG-CoA) reductase. This leads to upregulation of the LDL receptor and increased LDL clearance from plasma.
In others, avoidance of certain drugs or foods can prevent the manifestation of the disorder, for example sulfonamides in glucose-6-phosphate dehydrogenase (G6PD) deficiency (p. 187). Drug therapy might also be directed at a subset of patients according to their molecular defect. An example is a trial in which gentamicin was administered via nasal drops to patients with cystic fibrosis. Aminoglycoside antibiotics such as gentamicin or PTC124 cause read-through of premature stop codons in vitro and only patients with nonsense mutations (p. 25) showed evidence of expression of full-length cystic fibrosis transmembrane conductance regulator (CFTR) protein in the nasal epithelium. However, although gentamicin and PTC124 were effective in the mdx mouse model of Duchenne muscular dystrophy (DMD), a clinical trial with 174 patients failed to show convincing functional improvement after daily treatment for 48 weeks.
Therapeutic Applications of Recombinant DNA Technology
Biosynthesis of Gene Products
Insulin used in the treatment of diabetes mellitus was previously obtained from pig pancreases. This had to be purified for use very carefully, and even then it occasionally produced sensitivity reactions in patients. However, with recombinant DNA technology, microorganisms can be used to synthesize insulin from the human insulin gene. This is inserted, along with appropriate sequences to ensure efficient transcription and translation, into a recombinant DNA vector such as a plasmid and cloned in a microorganism such as Escherichia coli. In this way, large quantities of insulin can be made. An artificial gene that is not identical to the natural gene needs to be constructed for this purpose. However, synthetically produced genes cannot contain the non-coding intervening sequences, or introns (p. 17), found in the majority of structural genes in eukaryotic organisms, as microorganisms such as E. coli do not possess a means for splicing of the messenger RNA (mRNA) after transcription.
Recombinant DNA technology is being employed in the production of a number of other biosynthetic products (Table 23.2). The biosynthesis of medically important peptides in this way is usually more expensive than obtaining the product from conventional sources because of the research and development involved. For example, the cost of treating one patient with Gaucher disease can exceed £100,000 per year. However, biosynthetically derived products have the dual advantages of providing a pure product that is unlikely to induce a sensitivity reaction and one that is free of the risk of chemical or biological contamination. In the past, the use of growth hormone from human cadaver pituitaries has been associated with the transmission of Creutzfeldt-Jakob disease, and human immunodeficiency virus (HIV) has been a contaminant in cryoprecipitate containing factor VIII used in the treatment of hemophilia A (p. 309).
Table 23.2 Proteins Produced Biosynthetically Using Recombinant DNA Technology
| Protein | Disease |
|---|---|
| Insulin | Diabetes mellitus |
| Growth hormone | Short stature resulting from growth hormone deficiency |
| Factor VIII | Hemophilia A |
| Factor IX | Hemophilia B |
| Erythropoietin | Anemia |
| α-Galactosidase A | Fabry disease (X-linked lysosomal storage disorder) |
| β-Interferon | Multiple sclerosis |
Gene Therapy
Gene therapy has been defined by the UK Gene Therapy Advisory Committee (GTAC) as ‘the deliberate introduction of genetic material into human somatic cells for therapeutic, prophylactic, or diagnostic purposes’. It includes techniques for delivering synthetic or recombinant nucleic acids into humans; genetically modified biological vectors (such as viruses or plasmids), genetically modified stem cells, oncolytic viruses, nucleic acids associated with delivery vehicles, naked nucleic acids, antisense techniques (e.g., gene silencing, gene correction or gene modification), genetic vaccines, DNA or RNA technologies such as RNA interference, and xenotransplantation of animal cells (but not solid organs).
Regulatory Requirements
There has been much publicity about the potential uses and abuses of gene therapy. Regulatory bodies have been established in several countries to oversee the technical, therapeutic, and safety aspects of gene therapy programs (p. 368). There is universal agreement that germline gene therapy, in which genetic changes could be distributed to both somatic and germ cells, and thereby be transmitted to future generations, is morally and ethically unacceptable. Therefore all programs are focusing only on somatic cell gene therapy, in which the alteration in genetic information is targeted to specific cells, tissues or organs in which the disorder is manifest.
More than 1500 clinical trials of gene therapy have been approved for children and adults for a variety of genetic and non-genetic disorders. For the most part, these appear to be proceeding without event, although the unexpected death of a patient in one trial in 1999 and the development of leukemia in 3 of 20 children who received gene therapy for X-linked severe combined immunodeficiency (XL-SCID) (p. 201) has highlighted the risks of gene therapy.
Animal Models
One of the basic prerequisites for assessing the suitability of gene therapy trials in humans is the existence of an animal model. Although there are naturally occurring animal models for some inherited human diseases, for most there is no animal counterpart. The techniques used to generate animal models for human disease are outside the scope of this book, but much effort has focused on the production of animal models that faithfully recreate disease phenotypes. Animal models for cystic fibrosis, DMD, Huntington disease, and Friedreich ataxia have been generated and provide just a few examples that may be used to evaluate gene therapy before trials in humans.
In Utero Fetal Gene Therapy
The report of successful adenovirus vector-mediated in utero gene therapy in a cystic fibrosis mouse model in 1997 means that fetal gene therapy in utero may be possible in humans. At present it is considered unacceptable because of the possibility of inadvertent germ-cell modification. The use of stem cells genetically modified ex vivo should reduce this risk. However, in utero stem-cell transplantation without genetic modification offers the best prospects for the successful treatment of serious neurodegenerative disorders with a very early onset, such as Krabbe disease or Hurler syndrome (p. 177).
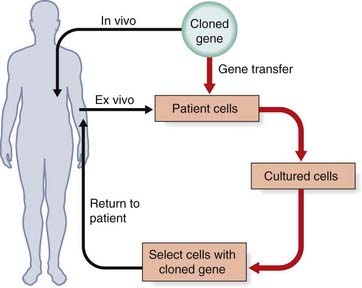
Gene Transfer
Gene transfer can be carried out either ex vivo by treatment of cells or tissue from an affected individual in culture, with reintroduction into the affected individual, or in vivo if cells cannot be cultured or be replaced in the affected individual (Figure 23.1). The ex vivo approach is limited to disorders in which the relevant cell population can be removed from the affected individual, modified genetically, and then replaced. The in vivo approach is the most direct strategy for gene transfer and can theoretically be used to treat many hereditary disorders.
Target Organs
Bone Marrow
In the treatment of disorders affecting the bone marrow, problems arise from the small numbers of stem cells, which need to be immortalized. Ex vivo gene therapy has been reported in two young boys with X-linked adrenoleukodystrophy for whom suitable bone marrow donors were not available. Stem cells were removed from their bone marrow, genetically corrected with a lentiviral vector encoding the normal ABCD1 gene, and then re-infused after the patients received myeloablative treatment. A year later, the progressive cerebral demyelination had stopped, a clinical outcome comparable to that achieved by allogeneic bone marrow transplant.
There are two main methods for delivering gene transfer, viral and non-viral.
Non-Viral Methods
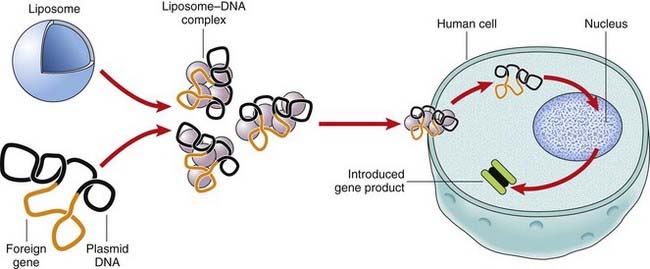
Liposomes
Liposomes are lipid bilayers surrounding an aqueous vesicle that can facilitate the introduction of foreign DNA into a target cell (Figure 23.2). A disadvantage of liposomes is that they are not very efficient in gene transfer and the expression of the foreign gene is transient, so that the treatment has to be repeated. An advantage of liposome-mediated gene transfer is that a much larger DNA sequence can be introduced into the target cells or tissues than with viral vector systems.
RNA Modification
Antisense Oligonucleotides
The identification of exon-splicing enhancer (ESE) sequences has increased our understanding of the process of exon splicing. If an ESE is mutated, the exon is more likely to be spliced out. Some proteins with in-frame whole-exon deletions retain some residual activity; for example, dystrophin mutations in Becker muscular dystrophy (p. 307). Blocking an ESE using an antisense oligonucleotide in patients with DMD aims to restore the reading frame and ameliorate the phenotype to that of Becker muscular dystrophy. However, a specific antisense oligonucleotide is required to target each different exon. The potential for antisense therapy is perhaps greater in spinal muscular atrophy (p. 306) where the non-expressed SMN2 gene could be converted to generate functional SMN1 protein in virtually all patients.
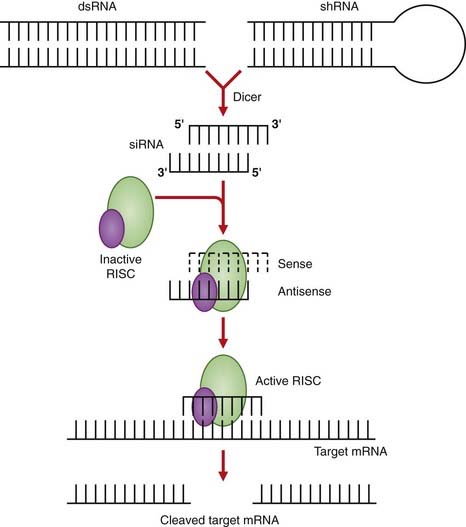
RNA Interference
This technique also has broad therapeutic application, as any gene may be a potential target for silencing by RNA interference. In contrast to antisense oligonucleotide therapy where the target mRNA is bound, as a result of RNA interference the target mRNA is cleaved and it is estimated to be up to 1000-fold more active. RNA interference works through the targeted degradation of mRNAs containing homologous sequences to synthetic double-stranded RNA molecules known as small interfering RNAs (siRNAs) (Figure 23.3). The siRNAs may be delivered in drug form using strategies developed to stabilize antisense oligonucleotides, or from plasmids or viral vectors. The first of these drugs is bevasiranib, an siRNA therapy designed to silence the genes that produce vascular endothelial growth factor, believed to be responsible for the vision loss in patients with the “wet” form of age-related macular degeneration.
Targeted Gene Correction
A promising new approach is to repair genes in situ through the cellular DNA repair machinery (p. 27). Proof of principle has been demonstrated in an animal model of Pompe disease. The point mutation was targeted by chimeric double-stranded DNA-RNA oligonucleotides containing the correct nucleotide sequence. Repair was demonstrated at the DNA level and normal enzyme activity was restored.
Diseases Suitable for Treatment Using Gene Therapy
Disorders that are possible candidates for gene therapy include both genetic and non-genetic diseases (Table 23.3).
Table 23.3 Diseases that Can Potentially Be Treated by Gene Therapy
| Disorder | Defect |
|---|---|
| Immune deficiency | Adenosine deaminase deficiency Purine nucleoside phosphorylase deficiency Chronic granulomatous disease |
| Hypercholesterolemia | Low-density lipoprotein receptor abnormalities |
| Hemophilia | Factor VIII deficiency (A) Factor IX deficiency (B) |
| Gaucher disease | Glucocerebrosidase deficiency |
| Mucopolysaccharidosis VII | β-Glucuronidase deficiency |
| Emphysema | α1-Antitrypsin deficiency |
| Cystic fibrosis | CFTR mutations |
| Phenylketonuria | Phenylalanine hydroxylase deficiency |
| Hyperammonemia | Ornithine transcarbamylase deficiency |
| Citrullinemia | Argininosuccinate synthetase deficiency |
| Muscular dystrophy | Dystrophin mutations |
| Spinal muscular atrophy | SMN1 gene deletion |
| Thalassemia/sickle cell anemia | α- and β-globin mutations |
| Malignant melanoma | |
| Ovarian cancer | |
| Brain tumors | |
| Neuroblastoma | |
| Renal cancer | |
| Lung cancer | |
| AIDS | |
| Cardiovascular diseases | |
| Rheumatoid arthritis |
Genetic Disorders
There are a number of single-gene diseases that have been the focus of gene therapy attempts.
Adenosine deaminase deficiency
One of the first diseases for which gene therapy was attempted in humans is the inherited immunodeficiency disorder caused by adenosine deaminase (ADA) deficiency (p. 179). The most successful conventional treatment for ADA deficiency is bone marrow transplantation but, in the absence of a compatible donor, patients may be treated with PEG-conjugated ADA.
Hemoglobinopathies
Attempts at treating β-thalassemia and sickle-cell disease by gene therapy have not been effective as yet, primarily because the numbers of α- and β-globin chains must be equal (p. 157). Gene therapy must, therefore, be dose-specific, and this is not possible at the present time.
Duchenne muscular dystrophy
An alternative strategy is to use antisense oligonucleotides to force exon skipping and convert out-of-frame deletions that cause DMD to in-frame deletions usually associated with the milder Becker muscular dystrophy phenotype. This approach could be successful for up to 80% of patients with DMD. Proof of concept has been demonstrated in cultured patient cells and the mdx mouse model where dystrophin re-expression was demonstrated. The first clinical trial involved four patients who underwent intramuscular injection of an antisense oligonucleotide to target exon 51. Dystrophin was restored in the vast majority of muscle fibres at levels between 17% and 35%, without any adverse effects. However, intramuscular injection of individual muscles is not feasible as a treatment and various chemistries are under investigation in order to deliver antisense oligonucleotides to muscles throughout the body.
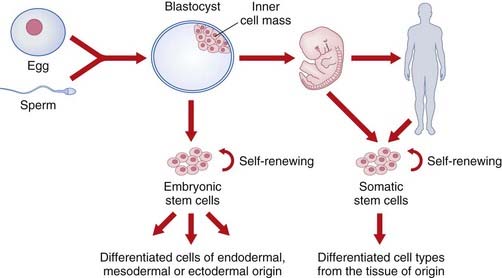
Stem Cell Therapy
Stem cells are unspecialized cells that are defined by their capacity for self-renewal and the ability to differentiate into specialized cells along many lineages. Embryonic stem cells are pluripotent, which means they can give rise to derivatives of all three germ layers (i.e., all cell types that are found in the adult organism). Somatic stem cells can only differentiate into the cell types found in the tissue from which they are derived (Figure 23.4), but can be isolated from any human, whatever their age. Nowadays the term induced pluripotent stem cell (iPS) is used rather than somatic or adult stem cell.
Embryonic Stem Cell Therapy
Teratomas (benign) and teratocarcinomas (malignant) are tumors that are found most commonly in the gonads. Their name is derived from the Greek word ‘teratos’ (monster); it describes their appearance well, as these tumors contain teeth, pieces of bone, muscles, skin, and hair. A key experiment demonstrated that if a single cell is removed from one of these tumors and injected intraperitoneally, it acts as a stem cell by producing all the cell types found in a teratocarcinoma.
Gene Therapy Using Embryonic Stem Cells
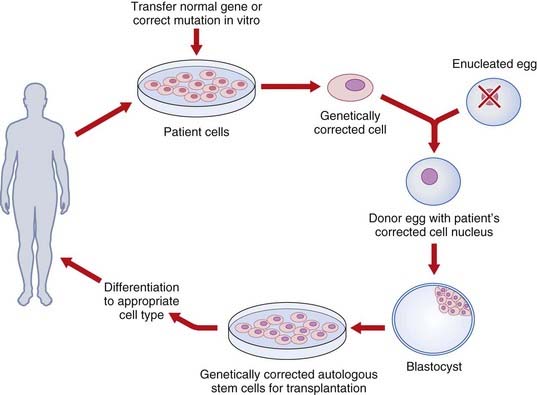
An alternate strategy is to use ESCs as delivery vehicles for genes that mediate phenotype correction through gene-transfer technology. One potential barrier to using human ESCs to treat genetic disorders is immunorejection of the transplanted cells by the host. This obstacle might be overcome by using gene transfer with the relevant normal gene to autologous cells (such as cultured skin fibroblasts), transfer of the corrected nucleus to an enucleated egg from an unrelated donor, development of ‘corrected’ ESCs and, finally, differentiation and transplantation of the corrected relevant cells to the same patient (Figure 23.5).
Induced Pluripotent Stem Cell Therapy
Mesenchymal Stem Cells
Mesenchymal stem cell (MSC) therapy, through its promise of repair and regeneration of cardiac tissue, represents an exciting avenue of treatment for a range of cardiovascular diseases. Cardiovascular disease is the leading cause of death in developed countries. Although cardiomyocytes retain limited plasticity following maturation, the heart is grossly unable to recover from structural damage.
The genetic disorder retinitis pigmentosa (p. 182) results in the loss of photoreceptors, leading to visual symptoms in the teens and blindness by 40 to 50 years of age. Recently, systemic administration of pluripotent bone marrow–derived MSCs in a rat model has demonstrated improved visual function. This is a potentially exciting development for the future treatment of other forms of retinal degeneration and other ocular vascular diseases such as diabetic retinopathy.
A third application of MSC therapy is in bone repair and metabolic bone diseases such as osteogenesis imperfecta (p. 121) and hypophosphatasia, because MSCs can also differentiate to form bone and cartilage.
Limbal Stem Cells
Stem cell therapy has now progressed from preclinical (animal studies) to early clinical trials (in humans) for a variety of disorders. In general, these studies have shown enormous potential in the animal models but more limited success in humans so far. Aside from participation in regulated trials, patients should be advised that stem cell therapy is at an early stage and discouraged from undergoing forms of treatment whose safety and efficacy is not yet proven. An unwanted spin-off from stem cell research has been the development of so called stem cell tourism. Patients have travelled to countries where stem cell–based treatment is not regulated to receive expensive treatments that are scientifically unproven These treatments are at best, ineffective and at worst, dangerous.
Anderson WF. Human gene therapy. Science. 1992;256:808-813.
A consideration of gene therapy by one of its main proponents.
Aartsma-Rus A, van Ommen G-JB. Progress in therapeutic antisense applications for neuromuscular disease. Eur J Hum Genet. 2010;18:146-153.
Belmonte JCI, Ellis J, Hochedlinger K, Yamanaka S. Induced pluripotent stem cells and reprogramming: seeing the science through the hype. Nat Rev Genet. 2009;10:878-883.
Brown BD, Naldini L. Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat Rev Genet. 2009;10:578-585.
Article describing the potential applications of RNA interference.
Graw J, Brackmann HH, Oldenburg J, et al. Haemophilia A: from mutation analysis to new therapies. Nat Rev Genet. 2006;6:488-501.
Review of hemophilia A genetics and gene therapy.
Solter D. From teratocarcinomas to embryonic stem cells and beyond: a history of embryonic stem cell research. Nat Rev Genet. 2006;7:319-327.
Elements