Chapter 151 Treatment Evolution in Management of Cervical Disc Disease
Cervical disc disease may be more neurologically compromising due to anatomic particularities than the more frequently occurring lumbar disc disease. However, cervical degenerative disc disease is the most common cause of acquired disability in patients over the age of 50.1 Despite this, there is a lack of firm evidence regarding the surgical options and prognostic factors associated with its management.2
Historical Development
In 1543, the anatomist and surgeon Andreas Vesalius (1514-1564) was the first to describe the intervertebral disc. However, its role in the development and as cause for various clinical signs and symptoms was not recognized until about 80 years ago. In 1928, Stookey3 reported on several clinical syndromes evoked by herniated cervical discs but interpreted the prolapse as “chondroma or neoplasm of the notochord.” Primarily, investigations by Schmorl4 in Europe and Keyes and Compere5 in the United States contributed to a detailed understanding of the pathophysiology of the intervertebral disc. Shortly after these investigations, Mixter and Barr6 clearly associated a lumbar disc prolapse with the signs and symptoms of nerve root compression; the alterations of the cervical intervertebral discs were identified as reason for neck pain, radiculopathy, and myelopathy.
Historically, the routine surgical approach for symptoms produced by cervical disc disease was posterior. During the last half of the last century, it became clear that the main problem of this approach was the technical challenge of displaying and removing compressive structures that lie anterior to the spinal cord and nerve roots. This need for easier access to reach anterior compressing structures led to the development of an anterior surgical approach to the cervical spine. Bailey and Badgley7 performed the first anterior stabilization of the cervical spine on a lytic process in 1952 and published their technique in 1960. In 1955, Robinson and Smith8 describe their method to stabilize a pathologically changed cervical spine segment using a horseshoe-shaped iliac bone graft. Without knowledge of the results of the other investigators, Cloward9 published his technique of anterior discectomy for removal of compressing structures in 1958.
Since that time, a multitude of variations and modifications have been done in the treatment of degenerative cervical disc disease. Different approaches are competing, as well as numerous implants, varying mainly in material and design. However, the crucial question of “to fuse or not to fuse” has not yet been answered. It is still a controversial issue if a favorable outcome can be achieved by decompression followed by solid fusion or if microsurgical decompression or decompression with arthroplasty can provide long-term preservation of motility.
Laminectomy and Laminoplasty
Initially, the routine surgical approach for symptoms produced by cervical disc disease was posterior. Mixter and Barr6 published their report, which described the use of laminectomy to treat 19 cases of intervertebral disc herniation, including four ruptured cervical discs, in 1934. Since then, posterior approaches and techniques evolved from laminectomy into small keyhole foraminotomy and various laminoplasty methods.
The main indication for laminectomy in degenerative cervical disc disease is single- or multilevel stenosis of the spinal canal caused by various types of pathology, including spondylosis and ossification of the posterior longitudinal ligament, usually presenting with myelopathy or radiculomyelopathy. Laminectomy is preferably employed for patients over 65 years of age who demonstrate variable ventral pathology but with multilevel spondylosis and stenosis in the presence of a well-preserved cervical lordotic curvature. In addition, a medial facetectomy and foraminotomy can be performed at the necessary levels to address a specific disc herniation and spur.10
Open posterior approaches to the cervical spine with laminectomy avoid the approach-related complications associated with anterior approaches but require extensive subperiosteal stripping of the paraspinal musculature, which often results in significant postoperative pain. Moreover, accelerated degeneration of adjacent motion segments after anterior fusion, or so-called adjacent segment disease (ASD), is avoided.11 Nevertheless, apart from anatomic limitations in reaching centrally or paracentrally located soft discs from this approach, long-segment decompression in patients who experience a preoperative loss of lordosis increases the risk for postoperative sagittal plane deformity.12
Complications after laminectomy include a 0% to 10% incidence of spinal cord injury and up to a 12.8% frequency of root injury.10 Nerve root deficits, typically involving C5, are variously attributed to direct mechanical manipulation or to “tethering” of the root after rapid cord decompression and dorsal migration after laminectomy.13
Laminectomy is successful in 77% to 85% of patients, while 10% to 15% may acutely deteriorate and another 23% exhibit delayed deterioration over 10 or more years.14 Rodrigues15 analyzed 51 patients who underwent laminectomy and disc removal for the treatment of paramedian and posterolateral soft cervical disc herniation and noticed 96% total relief of pain, 76% motor improvement, and 63% sensory improvement.
The clinical long-term outcome after cervical laminectomy,10 as well as the biomechanical alterations induced by multilevel cervical laminectomy,16 have been analyzed frequently. Performing extensive multilevel laminectomies typically does not immediately destabilize an otherwise intact spine17 but rather results in denervation and atrophy of the posterior cervical muscles. In addition, surgery denervates and disturbs the facet joints and the loss of the posterior tension band increases the force on the anterior vertebral body, which in turn worsens the sagittal deformity and results in a kyphotic angulation.
Several strategies have been used to prevent postoperative sagittal deformity after multilevel laminectomies. One option involves posterior segmental instrumentation and fusion performed at the time of the initial surgery. This adds considerable time and morbidity to the operation,18 results in a substantial decrease in mobility, and subjects adjacent levels to larger stress and accelerated degeneration. Another option includes laminoplasty or osteoplastic laminotomy. Because of concerns over complications of nonfusion-related laminectomy (spinal deformity, instability, compression by “laminectomy membrane,” and late neurologic deterioration), laminoplasty was developed in Japan in the 1970s as an alternative to laminectomy.19 A variety of laminoplasty techniques have been described; in all, the laminae are preserved, but the size of the spinal canal is expanded because the freed or partially freed laminae are positioned more posteriorly (Fig. 151-1).
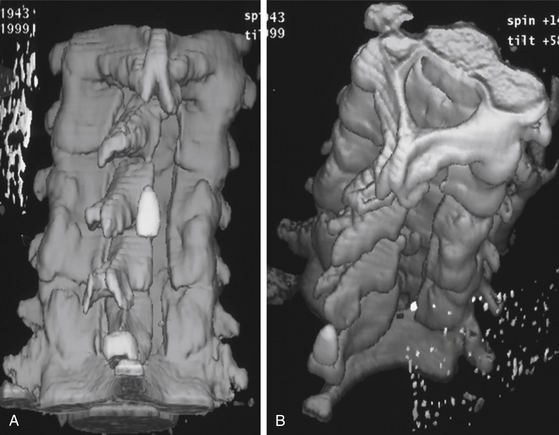
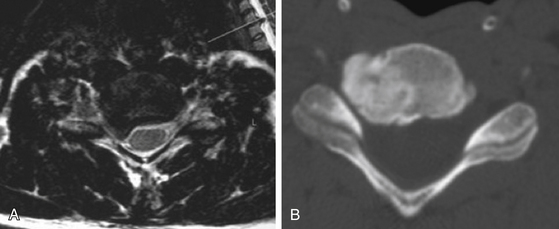
FIGURE 151-1 Postoperative three-dimensional computed tomography (CT) scan after four-level cervical laminoplasty.
In their critical review on cervical laminoplasty, Ratliff and Cooper19 found no difference in neurologic outcome based on different laminoplasty techniques or when laminoplasty is compared to laminectomy. Furthermore, there was no benefit to laminoplasty over laminectomy in adult patients in terms of spinal alignment, incidence of kyphotic deformity, and the development of the postlaminectomy membrane. Cervical range of motion (ROM) decreased substantially after laminoplasty, with progressive loss in long-term follow-up studies and final ROM similar to that seen in patients who had undergone laminectomy and fusion. This gradual laminoplasty-induced decrease in cervical ROM may allow for gradual compensation by adjacent segments, thus limiting adjacent-segment disease.
Some studies compare the long-term outcome after laminoplasty and anterior spinal fusion for cervical radiculomyelopathy due to soft disc herniation20 and for multilevel myelopathy.21 Because the two procedures provided the same neurologic improvement, the risks of bone graft complication with the anterior approach must be weighed against the risks of chronic neck pain associated with laminoplasty for determining the best technique. Furthermore, the posterior technique in this context may be reserved for multilevel soft disc herniation in older and multimorbid individuals.
The feasibility of minimal invasive access multilevel laminectomy and laminoplasty techniques were first shown in cadaver models.22–24 Both techniques showed a 43% expansion of the cross-sectional areas of the spinal canal. In a clinical study on 10 patients with degenerative compressive myelopathy treated with endoscopic partial laminectomy, Yabuki and Kikuchi25 showed that the technique was safely practicable and that all patients experienced symptomatic improvement. Furthermore, due to less muscle trauma and preservation of much of the normal osteoligamentous anatomy of the cervical spine, the postoperative wound pain and the risk for postlaminectomy kyphosis was reduced. Perez-Cruet22 reported on four patients with minimally invasive cervical laminoplasty and satisfactory postoperative neurologic improvement. Technically feasible, the author noted technical difficulties associated with elevation of the lamina and insertion of bone grafts due to the limited approach.
Posterior Foraminotomy
The posterior foraminotomy was pioneered by Frykholm26 and Spurling and Scoville27 and later modified by Scoville et al.28 Accessible pathologies are laterally located, soft disc herniation and foraminal stenosis (hard disc), usually presenting with radicular symptoms (Fig. 151-2). Posterior foraminotomy may be performed unilaterally at one or more levels, bilaterally at one or more levels, or in combination with a laminectomy or laminoplasty.10 The detailed technique has been described elsewhere.10,29 A major benefit of posterior foraminotomy versus posterior and anterior techniques with fusion is the preservation of stability and mobility. The avoidance of approach- and graft-related complications, impending in anterior techniques, is another important advantage. Limitations are the removal of compressive structures that lie anterior to the spinal cord and nerve roots.
Numerous publications focus on the indications, advantages, and long-term outcome of patients with lateral cervical soft disc herniation or osteophytes (foraminal stenosis), treated with posterior (keyhole) foraminotomy.30–33 Successful relief of radiculopathy symptoms are seen in 90% to 98%, with complication rates between 0% and 4%, and recurrence rates of 0% to 6%.
Limiting the degree of medial facetectomy to less than 50% prevents postoperative instability34 (Fig. 151-3). Because fusion is not applied, concerns about associated morbidity and the later development of adjacent-level disease theoretically are relatively minor. Nevertheless, Clarke et al. found in 303 patients with cervical radiculopathy, who were treated with posterior foraminotomy between 1972 and 1992, development of symptomatic adjacent-level disease in 4.9% after a mean follow-up of 7.1 years.35
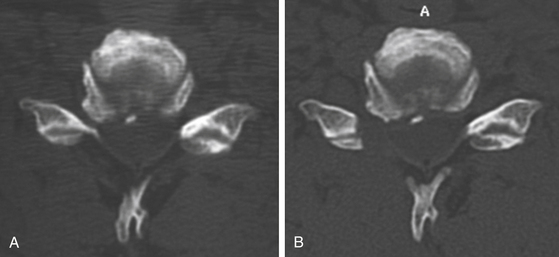
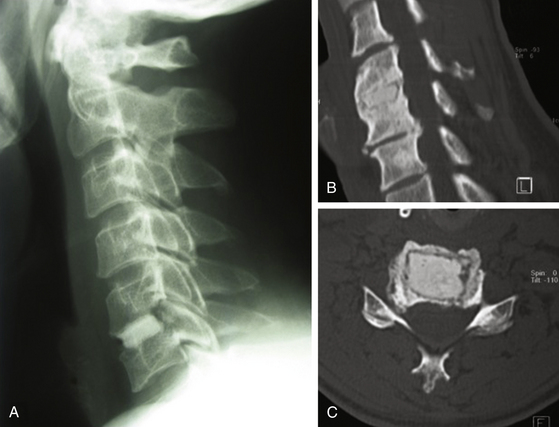
FIGURE 151-3 (A) Pre- and (B) postoperative CT scan after posterior foraminotomy, depicting the amount of bone removal.
Posterior cervical microendoscopic foraminotomy (MEF) and discectomy are the endoscopic alternative of microsurgical “open” posterior foraminotomy. The alternative was developed to address cervical nerve root compression by direct visualization of pathologic findings while minimizing tissue destruction and exposure. Muscle and ligamentous attachments to the spine are preserved, thus decreasing postoperative pain and spasm and helping maintain long-term stability.36 Initial cadaveric studies using this technique demonstrated that the average vertical and transverse diameters of the laminotomy defect were identical for the MEF or open technique.37 The average amount of facet removed and the length of neural decompression were greater with MEF than with the open approach. Initial clinical results have been quite favorable for the MEF procedure.38 Operative times, blood loss, length of hospitalization, and need for postoperative pain medications have all generally been reduced when MEF is used, compared with the open procedure, yielding equivalent clinical results.11,39,40 However, MEF is technically demanding, has a steep learning curve, and may require training within a cadaver laboratory.36
Anterior Discectomy, Arthrodesis, and Arthroplasty
Anterior cervical discectomy (ACD) for spinal cord or nerve root compression was first described independently by Cloward,9 and by Smith and Robinson41 more than 50 years ago. Evolved from posterior techniques due to limited anatomic access, the common feature of this technique is the substantial or complete removal of the disc. Recently, anterior cervical foraminotomy was introduced,42,43 which reduces the extent of disc removal but still has the anterior approach. The anterior approach to the cervical spine generally implies several approach-related complications (recurrent laryngeal nerve palsy, dysphagia, and esophagus and hypopharynx perforation)44–46 that do not exist in posterior approaches. In his systematic review using evidence-based medicine to identify the best techniques for anterior cervical nerve root decompression, Matz47 concluded that ACD, ACDF, and arthroplasty are effective techniques for addressing surgical cervical radiculopathy. However, the controversial question in recent decades has been, “Does anything have to be inserted in the empty disc space after anterior decompression,48,49 and if so, what is the ideal implant/cage material and design for that?”50,51 More questions have arisen, focusing on required additional anterior instrumentation and whether it might not be better to replace arthrodesis by arthroplasty.
Anterior Discectomy without Graft
In 1958, Cloward9 first introduced the concept of ACD without fusion and direct surgical decompression of dorsal osteophytes. Anterior removal of the cervical disc is an effective and reliable treatment for nerve root or cord compression caused by disc herniation or spondylosis. Although physicians have traditionally included fusion as a part of this procedure, recent experience has suggested that this may not be necessary. The majority of published data focusing on ACD date from the 1970s to the 1990s.48,52–55 Sonntag and Klara56 express opposing views on the need for fusion after discectomy: Sonntag believes that the majority of patients are well served with discectomy alone, avoiding the complications of graft harvest and potential nonunion. Klara feels that the interposed graft restores foraminal height and maintains cervical lordosis, both of which are important to a good outcome.
Of 64 patients with radiculopathy and/or myelopathy treated with ACD between 1994 and 1998, Donaldson and Nelson57 found a 91% success rate after a mean time of follow-up of 8.5 months. In a multicenter, prospective, randomized, controlled study, Oktenoglu et al.58 operated on 11 patients, applying simple anterior microdiscectomy technique, and on 9 patients via ACD and fusion with a semirigid plate technique. Satisfactory results were achieved in both groups. However, although fusion with a semirigid plate preserved disc height and neural foramen height at the operated level in the immediate postoperative period, it did not stop subsidence permanently.
Haden et al.59 conducted a prospective, observational cohort study to investigate the relationships among loss of disc height, cervical spine alignment, and clinical outcome in 140 patients undergoing ACD without interbody graft or cage. At a minimum of 12 months, there was no relationship between loss of disc height and outcome. Loss of the overall cervical lordosis was present in 71 patients, and segmental kyphosis was found in 69 patients. Analysis of clinical outcome showed no significant differences between patients with preserved and abnormal cervical alignment. Neither loss of disc height nor disturbance of cervical alignment compromised clinical outcome in the first year following ACD.
Anterior Discectomy with Fusion
Data from a nationwide survey on the contemporary practice of spinal fusion in the United States60 indicate that cervical spinal fusion experienced a rapid increase in utilization between 1993 and 2003. These changes are more pronounced for patients over 40 years of age and for degenerative disc disease. Similar preferences were seen among Canadian surgeons61 concerning the treatment of single-level cervical degenerative disc disease causing radiculopathy and/or myelopathy. Fusion was employed 93% of the time following ACD, with autologous bone as fusion material in 76% of cases. Additional anterior plating was added in 42% of ACDF cases. Posterior techniques were not mentioned. In a comparable survey among 22 orthopedic surgeons and 8 neurosurgeons within the United States about variation in decision making,62 the greatest agreement occurred for single-level disc herniation, with all surgeons choosing an anterior discectomy and 28 of the 29 respondents recommending fusion.
A number of neurosurgeons7,63 recommended fusion of the involved segment without decompression of the spinal canal from dorsal osteophytes more than 50 years ago. After immobilization of the degenerated segment, resorption of dorsal osteophytes was documented.63 Autograft from the iliac crest has mostly been used for interbody fusion.64,65 However, harvesting of autologous bone is associated with complications such as prolonged pain, cosmetic deformity, wound infection, hematomas, and peripheral nerve irritation or injury.46,66,67 Therefore, alternative interbody implants were sought.
Cloward9 first successfully used allograft for cervical interbody fusion, and allograft became one of the most frequently used interbody implants in the United States.49 Anterior cervical interbody fusion for degenerative disc disease has a higher tendency to fuse with autograft than allograft and shows better clinical outcomes according to one study,68 but other reviews fail to demonstrate significant differences in clinical or radiologic outcomes.69 However, the morbidity associated with autograft harvest was eliminated by the application of allograft.
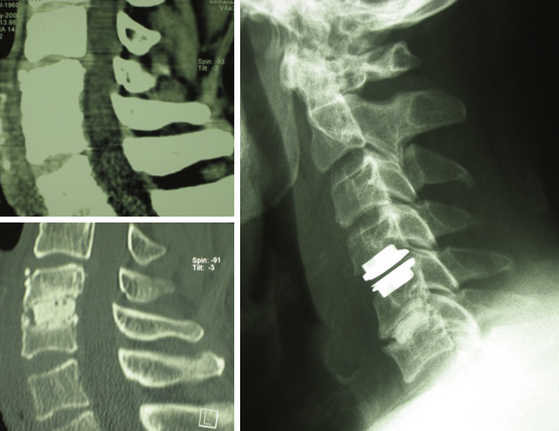
Polymethyl methacrylate (PMMA) was introduced in the 1960s as an interbody spacer for degenerative disc disease of the cervical spine70 (Fig. 151-4). This material is easy to handle, is inexpensive, and has been used until recently, despite some skepticism, mainly in the Western European community.71–74 Various other materials applied include ceramics (hydroxyapatite and tricalciumphosphates,75,76 carbon fiber cages,77,78 titanium cages79,80 (Fig. 151-5), polyetheretherketone (PEEK) cages81 (Fig. 151-6A), Bagby and Kuslich cages,82 and bioresorbable cages.83 These cages show substantial differences not only in material used but also in design84 and thereby in their biomechanical properties (subsidence, primary stability, and dislocation). Preferably, in ceramic materials, supplements of osteoinductive substances, like bone morphogenetic protein,85 have been added to promote intervertebral bone matrix formation.
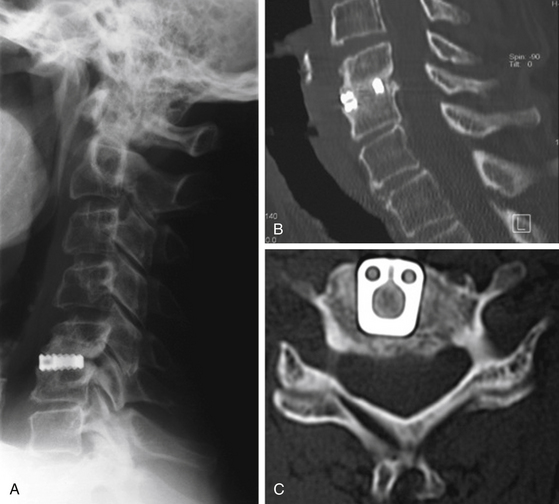
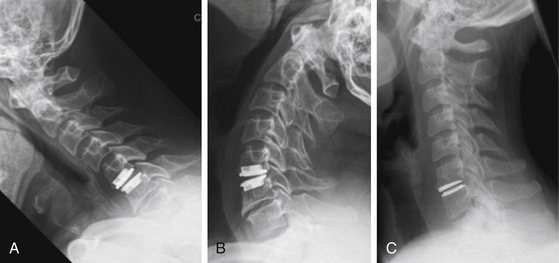
FIGURE 151-5 (A) Lateral radiograph, (B) sagittal, and (C) axial CT scan 7 years after titanium cage interposition and fusion.
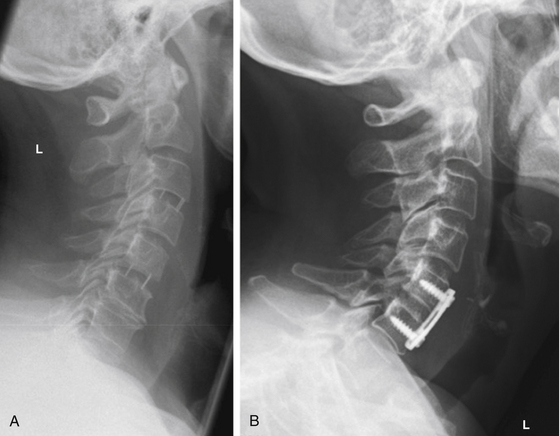
FIGURE 151-6 Lateral radiographs (A) after PEEK cage interbody fusion C3-C4 and C5-C6 and (B) after ACDF and plating C5-C6.
The efficacy and outcome after ACD, ACDF, and posterior foraminotomy for the treatment of cervical radiculopathy have been compared in several prospective and retrospective studies.73,86 Wirth et al.86 found all three procedures equally successful for treatment of radiculopathy caused by a herniated cervical disc. In addition, they saw a trend for higher recurrence at the same level with posterior foraminotomy and recurrence at other levels with ACDF. Another retrospective study on 292 patients with monoradiculopathy, treated either with posterior foraminotomy or ACDF, showed similar results after 72.1 months.73 Although the success rate was higher in the ACDF group (93.6%) than in the foraminotomy group (85.1%, P < 0.05), complications related to surgery occurred more frequently in the ACDF group (6.5%) than in the foraminotomy group (1.8%, P < 0.05).
Anterior Discectomy with Fusion and Plating
After anterior instrumentation with a plate and screws was introduced for the treatment of traumatic cervical instability in the 1960s,49 the combined use of auto- or allograft and other cages and plating for fusion after decompression of cervical degenerative disc disease became an attractive option that has gained increasing popularity among spine surgeons and neurosurgeons (Fig. 151-6B). The use of internal fixation with plates attempts to increase the fusion rate and preserve or restore segmental lordosis in the affected cervical spine segments. In addition, the period and extent of immobilization after ACDF is reduced, the risk of graft extrusion is minimized, and donor site morbidity can be eliminated by the use of allografts. With all these advantages, Yue et al.87 found no radiologic and clinical compromise in their 5- to 11-year follow-up study. Similar results are found when other cage materials are used, in addition to plating.76,88,89 However, osteoinductive recombinant human bone morphogenetic protein-2 has been added safely and effectively in such constructions to enhance and accelerate fusion.90
Recently, bioabsorbable anterior cervical plating91,92 achieved fusion rates similar to those previously reported in the literature with metallic implants and was superior to noninstrumented fusion. The bioabsorbable polymer plate and screws appear to be well tolerated by the patients. In a prospective, randomized study on 42 patients with cervical radiculopathy, the 2-year outcome after ACD, ACDF, or ACDF with plating was analyzed.93 The technique of reconstruction played no role in clinical results, while ACD alone resulted in segmental kyphosis compared to ACDF and ACDF with plating. The authors concluded that patient selection and surgical decompression remain the keys to achieving desirable clinical outcomes after cervical discectomy for radiculopathy.
Anterior Foraminotomy and Endoscopic Foraminotomy
Similar to posterior techniques, getting less invasive and establishing the use of the endoscope, an alternative has been adopted increasingly for anterior procedures in recent years. Anterior cervical foraminotomy was developed under the concept of “functional spine surgery,”42 which directly eliminates compressive pathologic factors while preserving functional anatomic features, thus preventing the acceleration of adjacent-level degeration.43 In current series,42,43,94 good and excellent results were seen in 91% to 98% of cases, with few complications and high preservation of mobility of the operated segment. Furthermore, the minimized approach was associated with a less painful postoperative course,94 allowing the patients an almost immediate return to unrestricted full activity.
However, Hacker and Miller95 identified 30% of their 23 patients who had failed anterior cervical foraminotomy, requiring at least one additional procedure because of persistence and recurrence of symptoms. With this reoperation rate, which is considerably higher than that of the most series of anterior cervical surgery for radiculopathy, they do not recommend this technique as stand-alone procedure.
Eight years earlier, Brigham and Tsahakis96 presented their experience in 43 patients with radiculopathy due to spondylosis or lateral herniated soft discs treated with anterior foraminotomy and fusion with autogenous bone graft. In this series, 91% of the patients had excellent or good results; however, the fusion rate was 93%, implying loss of motion and risk of increased stress on adjacent segments.
Only one publication dealing with endoscopic anterior cervical foraminotomy has been available until now.97 After the practicability of the technique was determined on cadavers, a clinical study of 16 patients was conducted. After a mean follow-up of 13.8 months, the overall subjective patient satisfaction rate was 87.6%, and no major surgery-related complications were encountered. The authors concluded, as in an increasing number of publications focusing on less invasive techniques to decompress the nerve root, that one advantage of the endoscopic technique is the preservation of the intervertebral disc and the motion segment.
Cervical Arthroplasty
After anterior cervical fusion, significant increases in intradiscal pressure and segmental motion at levels adjacent to the fusion have been reported in cadaveric98,99 and clinical studies.100–102 By definition, adjacent segment degeneration is radiographic changes of degeneration at levels adjacent to a spinal fusion, and ASD means development of new symptoms correlating with adjacent segment degeneration103 (Fig. 151-7).
Hilibrand et al. reported in their study in 1999 a relatively constant incidence of ASD of 2.9% per year,100 which means that within 10 years more than one fourth of all patients after an anterior cervical arthrodesis may be affected. Ishihara et al. saw an incidence of 19% of their 112 patients followed for 2 years after ACDF.101 However, in 2004, Hilibrand and Robbins103 challenged this theory and postulated that there appears to be an incidence of adjacent segment degeneration and disease after arthrodesis that may be related to natural degeneration. Recently, a cadaveric study104 and a clinical study105 demonstrated this theory.
Against this background, the use of artificial cervical discs was introduced.106 The underlying rationale of cervical arthroplasty (CA) is to decompress the neural structures and to maintain physiologic segmental cervical motion after ACD (Fig. 151-8). Through this, adjacent-level motion is decreased, and the incidence of ASD should be eliminated or reduced.49 There is no need for graft; the anatomic disc space height is maintained, as is the normal segmental lordosis; and the need for bracing is precluded.
Recently, many clinical studies107–110 have focused on experience with, and outcome and complications of, one individual with various CA devices. The results are consistently promising, and new devices are increasingly surging onto the market. But with every new technology, issues have to be learned and new problems arise. Issues that might be new for neurosurgeons relate to nomenclature,111 materials, and alloys—and therewith topics like wear, toxicity,111 biocompatibility,112,113 and device-specific side effects.114 Sekhon et al. demonstrated impressively that postoperative visualization of neural structures and adjacent levels after CA is variable among currently available devices.115
Heterotopic ossification or fusion around cervical artificial discs, another challenge for CA, has been reported initially in case descriptions.116,117 In a multicenter, prospective clinical trial, 17.8% of the studied patients experienced some degree of heterotopic ossification after 12 months,118 and in a recent study two thirds of the patients had such ossification with the same follow-up period.119
The current indications and contraindications change with time and depend on a multitude of factors. Mainly, patients younger than 60 years suffering from radiculopathic symptoms due to a median or paramedian soft disc herniation are good candidates. The inclusion and exclusion criteria of the recent randomized, U.S. Food and Drug Administration–approved, investigational device trial120 were more limited. However, Sekhon presented good results without complications121 in 11 patients with cervical myelopathy, who were treated with anterior decompression and artificial cervical disc replacement.
About 50% of patients considered for ACDF are now suitable for CA instead. Considering all patients worldwide, this is more attractive for the industry than operation techniques in which no foreign material has to be implanted and motility is preserved (e.g., foraminotomy).
Since the problems of the patients are due to nerve root compression, the short-term results after decompression are comparable to those after any surgical procedure. Currently, only one multicenter, prospective, randomized study has compared the long-term results of cervical disc arthroplasty with ACDF in patients treated for symptomatic single-level cervical degenerative disc disease.120 To summarize, there was statistically overall success in five parameters of CA versus ACDF. The artificial cervical disc device maintained physiologic segmental motion 24 months after implantation and was associated with improved neurologic success, improved clinical outcomes, and a reduced rate of secondary surgeries compared with ACDF.
Future Trends
As alternatives to operative treatment, which is invasive and bears a risk of complications, there are regenerative treatment strategies in spinal surgery,122 representing therapy approaches in the field of gene technology on a molecular–biologic level.123,124 The starting point is the reduction of aggrecan, a proteoglycan in the cervical disc, which is responsible for the water content and therewith for the volume and the elasticity of the cervical disc.123 In animal models, therapeutic genes (the human transforming growth factor beta 1 encoding gene) were transferred successfully in vivo by means of vectors (adeno- or retrovirus) in target cells inside the cervical disc. The proteoglycan synthesis was thereby stimulated, and the degeneration process was stopped. The results are promising124 but far from being a potential approach in humans. Other than the treatment methods described in this review, these future trends could be applied prophylactically, that is, before degenerative alterations become manifest.
Intervertebral disc transplantation125 is another approach with present attempts not only in primates but already in humans. Five years after transplanting fresh–frozen disc allografts in five patients, the neurologic symptoms of all patients improved, and no immunoreaction was encountered.125 Despite signs of mild disc degeneration, the motion and stability of the spinal unit was preserved.126
Baaj A.A., Uribe J.S., Vale F.L., et al. History of cervical disc arthroplasty. Neurosurg Focus. 2009;27:E10.
Clarke M.J., Ecker R.D., Krauss W.E., et al. Same-segment and adjacent-segment disease following posterior cervical foraminotomy. J Neurosurg Spine. 2007;6:5-9.
Cloward R.B. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15:602-617.
Dowd G.C., Wirth F.P. Anterior cervical discectomy: is fusion necessary? J Neurosurg. 1999;90:8-12.
Epstein N.E. A review of laminoforaminotomy for the management of lateral and foraminal cervical disc herniations or spurs. Surg Neurol. 2002;57:226-233.
Feiz-Erfan I., Klopfenstein J.D., Bambakidis N.C., et al. Surgical management of cervical disc disease: from no fusion to fusion and back again. Clin Neurosurg. 2005;52:331-337.
Frykholm R. Cervical root compression resulting from disc degeneration and root sleeve fibrosis. Acta Chir Scand. 1951;160:1-149.
Ishihara H., Kanamori M., Kawaguchi Y., et al. Adjacent segment disease after anterior cervical interbody fusion. Spine J. 2004;4:624-628.
Jho H.D., Kim W.K., Kim M.H. Anterior microforaminotomy for treatment of cervical radiculopathy: part 1—disc-preserving “functional cervical disc surgery.”. Neurosurgery. 2002;51:S46-S53.
Korinth M.C., Kruger A., Oertel M.F., et al. Posterior foraminotomy or anterior discectomy with polymethyl methacrylate interbody stabilization for cervical soft disc disease: results in 292 patients with monoradiculopathy. Spine. 2006;31:1207-1214.
Martz E.O., Goel V.K., Pope M.H., et al. Materials and design of spinal implants—a review. J Biomed Mater Res (Appl Biomater). 1997;38:267-288.
Mummaneni P.V., Burkus J.K., Haid R.W., et al. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine. 2007;6:198-209.
Perez-Cruet M.J. Microendoscopic cervical laminectomy and laminoplasty. In: Fessler R.G., Regan J.J. Endoscopic spine surgery and instrumentation: percutaneous procedures. New York: Thieme, 2005. pp. 74-87
Ratliff J.K., Cooper P.R. Cervical laminoplasty: a critical review. J Neurosurg (Spine). 2003;98:230-238.
Robinson R.A., Smith G.W. Anterolateral disk removal and interbody fusion for cervical disk syndrome. Bull Johns Hopkins Hosp. 1955;96:223-224.
Robinson R.A., Walker A.E., Ferlic D.C., et al. The results of anterior interbody fusion of the cervical spine. J Bone Joint Surg. 1962;44:1569-1587.
Roosen K. Knochenzement als Ersatzmittel zervikaler Bandscheiben. Fortschr Med. 1982;100:2120-2126.
Ruan D., He Q., Ding Y., et al. Intervertebral disc transplantation in the treatment of degenerative spine disease: a preliminary study. Lancet. 2007;369:993-999.
Schmorl G. Über Verlagerung von Bandscheibengewebe und ihre Folgen. Arch Klin Chir. 1928;172:240-276.
Scoville W.B., Whitcomb B.B., McLaurin R.L. The cervical ruptured disc: Report of 115 operative cases. Trans Am Neurol Assoc. 1951;56:222-224.
Sonntag V.K.H., Klara P. Controversy in spine care. Is fusion necessary after anterior cervical discectomy? Spine. 1996;21:1111-1112.
Spurling R.G., Scoville W.B. Lateral rupture of the cervical intervertebral disc. A common cause of shoulder and arm pain. Surg Gynecol Obstet. 1944;798:350-358.
Stookey B. Compression of the spinal cord due to ventral extradural cervical chondromas. Arch Neurol Psychiat. 1928;20:275-291.
van Limbeek J., Jacobs W.C., Anderson P.G., et al. A systematic literature review to identify the best method for a single level anterior cervical interbody fusion. Eur Spine J. 2000;9:129-136.
Zdeblick T.A., Zou D., Warden K.E., et al. Cervical stability after foraminotomy. A biomechanical in vitro analysis. J Bone Joint Surg. 1992;74:22-27.
1. Fehlings M.G., Arvin B. Surgical management of cervical degenerative disease: the evidence related to indications, impact, and outcome. J Neurosurg Spine. 2009;11:97-100.
2. Carette S., Fehlings M.G. Clinical practice. Cervical radiculopathy. N Engl J Med. 2005;353:392-429.
3. Stookey B. Compression of the spinal cord due to ventral extradural cervical chondromas. Arch Neurol Psychiat. 1928;20:275-291.
4. Schmorl G. Über Verlagerung von Bandscheibengewebe und ihre Folgen. Arch Klin Chir. 1928;172:240-276.
5. Keyes D.C., Compere E.L. The normal and pathological physiology of the nucleus pulposus of the intervertebral disc. J Bone Joint Surg. 1932;14A:897-938.
6. Mixter W.J., Barr J.S. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;211:210-215.
7. Bailey R.W., Badgley C.E. Stabilization of the cervical spine by anterior fusion. J Bone Joint Surg. 1960;42:565-594.
8. Robinson R.A., Smith G.W. Anterolateral disk removal and interbody fusion for cervical disk syndrome. Bull Johns Hopkins Hosp. 1955;96:223-224.
9. Cloward R.B. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15:602-617.
10. Epstein N.E. A review of laminoforaminotomy for the management of lateral and foraminal cervical disc herniations or spurs. Surg Neurol. 2002;57:226-233.
11. Gala V.C., O’Toole J.E., Voyadzis J.M., et al. Posterior minimally invasive approaches for the cervical spine. Orthop Clin North Am. 2007;38:339-349.
12. Albert T.J., Murrell S.E. Surgical management of cervical radiculopathy. J Am Acad Orthop Surg. 1999;7:368-376.
13. Sakaura H., Hosono N., Mukai Y., et al. C5 palsy after decompression surgery for cervical myelopathy: review of the literature. Spine. 2003;28:2447-2451.
14. Kato Y., Iwasaki M., Fuji T., et al. Long-term follow-up results of laminectomy for cervical myelopathy caused by ossification of the posterior longitudinal ligament. J Neurosurg. 1998;89:217-223.
15. Rodrigues M.A., Hanel R.A., Prevedello D.M., et al. Posterior approach for soft cervical disc herniations: a neglected technique? Surg Neurol. 2001;55:17-22.
16. Hansen-Schwartz J., Kruse-Larsen C., Nielsen C.J. Follow-up after cervical laminectomy, with special reference to instability and deformity. Br J Neurosurg. 2003;17:301-305.
17. Deutsch H., Haid R.W., Rodts G.E., et al. Postlaminectomy cervical deformity. Neurosurg Focus. 2003;15:E5.
18. Heller J.G., Edwards C.C.2nd, Murakami H., et al. Laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: an independent matched cohort analysis. Spine. 2001;26:1330-1336.
19. Ratliff J.K., Cooper P.R. Cervical laminoplasty: a critical review. J Neurosurg (Spine). 2003;98:230-238.
20. Sakaura H., Hosono N., Mukai Y., et al. Long-term outcome of laminoplasty for cervical myelopathy due to disc herniation: a comparative study of laminoplasty and anterior spinal fusion. Spine. 2005;30:756-759.
21. Edwards C.C.2nd, Heller J.G., Murakami H. Corpectomy versus laminoplasty for multilevel cervical myelopathy: an independent matched-cohort analysis. Spine. 2002;27:1168-1175.
22. Perez-Cruet M.J. Microendoscopic cervical laminectomy and laminoplasty. In: Fessler R.G., Regan J.J. Endoscopic spine surgery and instrumentation: percutaneous procedures. New York: Thieme, 2005. pp. 74-87
23. Wang M.Y., Green B.A., Coscarella E., et al. Minimally invasive cervical expansile laminoplasty: an initial cadaveric study. Neurosurgery. 2003;52:370-373.
24. Benglis D.M.Jr., Guest J.D., Wang M.Y. Clinical feasibility of minimally invasive cervical laminoforaminotomy. Neurosurg Focus. 2009;25:E3.
25. Yabuki S., Kikuchi S. Endoscopic partial laminectomy for cervical myelopathy. J Neurosurg Spine. 2005;2:170-174.
26. Frykholm R. Cervical root compression resulting from disc degeneration and root sleeve fibrosis. Acta Chir Scand. 1951;160:1-149.
27. Spurling R.G., Scoville W.B. Lateral rupture of the cervical intervertebral disc. A common cause of shoulder and arm pain. Surg Gynecol Obstet. 1944;798:350-358.
28. Scoville W.B., Whitcomb B.B., McLaurin R.L. The cervical ruptured disc: Report of 115 operative cases. Trans Am Neurol Assoc. 1951;56:222-224.
29. Russell S.M., Benjamin V. Posterior surgical approach to the cervical neural foramen for intervertebral disc disease. Neurosurgery. 2004;54:662-66544.
30. Caglar Y.S., Bozkurt M., Kahilogullari G., et al. Keyhole approach for posterior cervical discectomy: experience on 84 patients. Minim Invasive Neurosurg. 2007;50:7-11.
31. Grieve J.P., Kitchen N.D., Moore A.J., et al. Results of posterior cervical foraminotomy for treatment of cervical spondylitic radiculopathy. Br J Neurosurg. 2000;14:40-43.
32. Witzmann A., Hejazi N., Krasznai L. Posterior cervical foraminotomy. A follow-up study of 67 surgically treated patients with compressive radiculopathy. Neurosurg Rev. 2000;23:213-217.
33. Woertgen C., Rothoerl R.D., Henkel J., et al. Long term outcome after cervical foraminotomy. J Clin Neurosci. 2000;7:312-315.
34. Zdeblick T.A., Zou D., Warden K.E., et al. Cervical stability after foraminotomy. A biomechanical in vitro analysis. J Bone Joint Surg. 1992;74:22-27.
35. Clarke M.J., Ecker R.D., Krauss W.E., et al. Same-segment and adjacent-segment disease following posterior cervical foraminotomy. J Neurosurg Spine. 2007;6:5-9.
36. O’Toole J.E., Sheikh H., Eichholz K.M., et al. Endoscopic posterior cervical foraminotomy and discectomy. Neurosurg Clin N Am. 2006;17:411-422.
37. Burke T.G., Caputy A. Microendoscopic posterior cervical foraminotomy: a cadaveric model and clinical application for cervical radiculopathy. J Neurosurg. 2000;93:126-129.
38. Adamson T.E. Microendoscopic posterior cervical laminoforaminotomy for unilateral radiculopathy: results of a new technique in 100 cases. J Neurosurg (Spine). 2001;95:51-57.
39. Fessler R.G., Khoo L.T. Minimally invasive cervical microendoscopic foraminotomy: an initial clinical experience. Neurosurgery. 2002;51:S37-S45.
40. Ruetten S., Komp M., Merk H., et al. A new full-endoscopic technique for cervical posterior foraminotomy in the treatment of lateral disc herniations using 6.9-mm endoscopes: prospective 2-year results of 87 patients. Minim Invasive Neurosurg. 2007;50:219-226.
41. Smith G.W., Robinson R.A. The treatment of certain cervical spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg. 1958;40:607-624.
42. Jho H.D., Kim W.K., Kim M.H. Anterior microforaminotomy for treatment of cervical radiculopathy: part 1—disc-preserving “functional cervical disc surgery.”. Neurosurgery. 2002;51:S46-S53.
43. White B.D., Buxton N., Fitzgerald J.J. Anterior cervical foramenotomy for cervical radiculopathy. Br J Neurosurg. 2007;21:370-374.
44. Bazaz R., Lee M.J., Yoo J.U. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine. 2002;27:2453-2458.
45. Jung A., Schramm J., Lehnerdt K., et al. Recurrent laryngeal nerve palsy during anterior cervical spine surgery: a prospective study. J Neurosurg Spine. 2005;2:123-127.
46. Wang M.C., Chan L., Maiman D.J., et al. Complications and mortality associated with cervical spine surgery for degenerative disease in the United States. Spine. 2007;32:342-347.
47. Matz P.G., Ryken T.C., Groff M.W., et al. Techniques for anterior cervical decompression for radiculopathy. J Neurosurg Spine. 2009;11:183-197.
48. Dowd G.C., Wirth F.P. Anterior cervical discectomy: is fusion necessary? J Neurosurg. 1999;90:8-12.
49. Feiz-Erfan I., Klopfenstein J.D., Bambakidis N.C., et al. Surgical management of cervical disc disease: from no fusion to fusion and back again. Clin Neurosurg. 2005;52:331-337.
50. Martz E.O., Goel V.K., Pope M.H., et al. Materials and design of spinal implants—a review. J Biomed Mater Res (Appl Biomater). 1997;38:267-288.
51. van Limbeek J., Jacobs W.C., Anderson P.G., et al. A systematic literature review to identify the best method for a single level anterior cervical interbody fusion. Eur Spine J. 2000;9:129-136.
52. Abd-Alrahman N., Dokmak A.S., Abou-Madawi A. Anterior cervical discectomy (ACD) versus anterior cervical fusion (ACF), clinical and radiological outcome study. Acta Neurochir (Wien). 1999;141:1089-1092.
53. Savolainen S., Rinne J., Hernesniemi J. A prospective randomized study of anterior single-level cervical disc operations with long-term follow-up: surgical fusion is unnecessary. Neurosurgery. 1998;43:51-55.
54. Thorell W., Cooper J., Hellbusch L., et al. The long-term clinical outcome of patients undergoing anterior cervical discectomy with and without intervertebral bone graft placement. Neurosurgery. 1998;43:268-273.
55. Watters W.C.III, Levinthal R. Anterior cervical discectomy with and without fusion. Results, complications, and long-term follow-up. Spine. 1994;19:2343-2347.
56. Sonntag V.K.H., Klara P. Controversy in spine care. Is fusion necessary after anterior cervical discectomy? Spine. 1996;21:1111-1112.
57. Donaldson J.W., Nelson P.B. Anterior cervical discectomy without interbody fusion. Surg Neurol. 2002;57:219-224.
58. Oktenoglu T., Cosar M., Ozer A.F., et al. Anterior cervical microdiscectomy with or without fusion. J Spinal Disord Tech. 2007;20:361-368.
59. Haden N., Latimer M., Seeley H.M., et al. Loss of inter-vertebral disc height after anterior cervical discectomy. Br J Neurosurg. 2005;19:469-474.
60. Cowan J.A.Jr., Dimick J.B., Wainess R., et al. Changes in the utilization of spinal fusion in the United States. Neurosurgery. 2006;59:15-20.
61. Pickett G.E., Van Soelen J., Duggal N. Controversies in cervical discectomy and fusion: practice patterns among Canadian surgeons. Can J Neurol Sci. 2004;31:478-483.
62. Irwin Z.N., Hilibrand A., Gustavel M., et al. Variation in surgical decision making for degenerative spinal disorders. Part II: Cervical spine. Spine. 2005;30:2214-2219.
63. Robinson R.A., Walker A.E., Ferlic D.C., et al. The results of anterior interbody fusion of the cervical spine. J Bone Joint Surg. 1962;44:1569-1587.
64. Goffin J., Geusens E., Vantomme N., et al. Long-term follow-up after interbody fusion of the cervical spine. J Spinal Disord Tech. 2004;17:79-85.
65. Sonntag V.K., Han P.P., Vishteh A.G. Anterior cervical discectomy. Neurosurgery. 2001;49:909-912.
66. Epstein N.E., Dickerman R.D. Delayed iliac crest autograft fractures following plated single-level anterior cervical corpectomy with fusion. J Spinal Disord Tech. 2002;15:420-424.
67. Silber J.S., Anderson D.G., Daffner S.D., et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28:134-139.
68. An H.S., Simpson J.M., Glover J.M., et al. Comparison between allograft plus demineralised bone matrix versus autograft in anterior cervical fusion. Spine. 1995;20:2211-2221.
69. Floyd T., Ohnmeiss D. A meta-analysis of autograft versus allograft in anterior cervical fusion. Eur Spine J. 2000;9:398-403.
70. Roosen K. Knochenzement als Ersatzmittel zervikaler Bandscheiben. Fortschr Med. 1982;100:2120-2126.
71. Chen J.F., Wu C.T., Lee S.C., et al. Use of a polymethylmethacrylate cervical cage in the treatment of single-level cervical disc disease. J Neurosurg Spine. 2005;3:24-28.
72. Hamburger C., Festenberg F.V., Uhl E. Ventral discectomy with PMMA interbody fusion for cervical disc disease: long-term results in 249 patients. Spine. 2001;26:249-255.
73. Korinth M.C., Kruger A., Oertel M.F., et al. Posterior foraminotomy or anterior discectomy with polymethyl methacrylate interbody stabilization for cervical soft disc disease: results in 292 patients with monoradiculopathy. Spine. 2006;31:1207-1214.
74. Schröder J., Grosse-Dresselhaus F., Schul C., et al. PMMA versus titanium cage after anterior cervical discectomy—a prospective randomized trial. Zentralbl Neurochir. 2007;68:2-7.
75. Agrillo U., Mastronardi L., Puzzilli F. Anterior cervical fusion with carbon fiber cage containing coralline hydroxyapatite: preliminary observations in 45 consecutive cases of soft-disc herniation. J Neurosurg (Spine). 2002;96:273-276.
76. Bruneau M., Nisolle J.F., Gilliard C., et al. Anterior cervical interbody fusion with hydroxyapatite graft and plate system. Neurosurg Focus. 2001;10:E8.
77. Frederic S., Benedict R., Payer M. Implantation of an empty carbon fiber cage or a tricortical iliac crest autograft after cervical discectomy for single-level disc herniation: a prospective comparative study. J Neurosurg Spine. 2006;4:292-299.
78. Vavruch L., Hedlund R., Javid D., et al. A prospective randomized comparison between the Cloward procedure and a carbon fiber cage in the cervical spine. Spine. 2002;27:1694-1701.
79. Moreland D.B., Asch H.L., Clabeaux D.E., et al. Anterior cervical discectomy and fusion with implantable titanium cage: initial impressions, patient outcomes and comparison to fusion with allograft. Spine J. 2004;4:184-191.
80. Türeyen K. Disc height loss after anterior cervical microdiscectomy with titanium intervertebral cage fusion. Acta Neurochirn (Wien). 2003;145:565-570.
81. Mastronardi L., Ducati A., Ferrante L. Anterior cervical fusion with polyetheretherketone (PEEK) cages in the treatment of degenerative disc disease. Preliminary observations in 36 consecutive cases with a minimum 12-month follow-up. Acta Neurochir (Wien). 2006;148:307-312.
82. Matge G. Anterior interbody fusion with the BAK-cage in cervical spondylosis. Acta Neurochir (Wien). 1998;140:1-8.
83. Cahill D.W., Martin G.J., Hajjar M.V., et al. Suitability of bioresorbable cages for anterior cervical fusion. J Neurosurg (Spine). 2003;98:195-201.
84. Kandziora F., Pflugmacher R., Schafer J., et al. Biomechanical comparison of cervical spine interbody fusion cages. Spine. 2001;26:1850-1857.
85. Kandziora F., Bail H., Schmidmaier G., et al. Bone morphogenetic protein-2 application by a poly(d,l-lactide)–coated interbody cage: in vivo results of a new carrier for growth factors. J Neurosurg (Spine). 2002;97:40-48.
86. Wirth F.P., Dowd G.C., Sanders H.F., et al. Cervical discectomy. A prospective analysis of three operative techniques. Surg Neurol. 2000;53:340-346.
87. Yue W.M., Brodner W., Highland T.R. Long-term results after anterior cervical discectomy and fusion with allograft and plating: a 5- to 11-year radiologic and clinical follow-up study. Spine. 2005;30:2138-2144.
88. Majd M.E., Vadhva M., Holt R.T. Anterior cervical reconstruction using titanium cages with anterior plating. Spine. 1999;24:1604-1610.
89. Mobbs R.J., Rao P., Chandran N.K. Anterior cervical discectomy and fusion: analysis of surgical outcome with and without plating. J Clin Neurosci. 2007;14:639-642.
90. Baskin D.S., Ryan P., Sonntag V., et al. A prospective randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with Cornerstone-SR™ allograft ring and the Atlantis™ anterior cervical plate. Spine. 2003;28:1219-1225.
91. Aryan H.E., Lu D.C., Acosta F.L.Jr., et al. Bioabsorbable anterior cervical plating: initial multicenter clinical and radiographic experience. Spine. 2007;32:1084-1088.
92. Vaccaro A.R., Carrino J.A., Venger B.H., et al. Use of bioabsorbable anterior cervical plate in the treatment of cervical degenerative and traumatic disc disruption. J Neurosurg (Spine). 2002;97:473-480.
93. Xie J.C., Hurlbert R.J. Discectomy versus discectomy with fusion versus discectomy with fusion and instrumentation: a prospective randomized study. Neurosurgery. 2007;61:107-116.
94. Saringer W., Nöbauer I., Reddy M., et al. Microsurgical anterior cervical foraminotomy (uncoforaminotomy) for unilateral radiculopathy: clinical results of a new technique. Acta Neurochir (Wien). 2002;144:685-694.
95. Hacker R.J., Miller C.G. Failed anterior cervical foraminotomy. J Neurosurg (Spine). 2003;98:126-130.
96. Brigham C.D., Tsahakis P.J. Anterior cervical foraminotomy and fusion. Surgical technique and results. Spine. 1995;20:766-770.
97. Saringer W.F., Reddy B., Nobauer-Huhmann I., et al. Endoscopic anterior cervical foraminotomy for unilateral radiculopathy: anatomical morphometric analysis and preliminary clinical experience. J Neurosurg (Spine). 2003;98:171-180.
98. Eck J.C., Humphreys S.C., Lim T.H., et al. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine. 2002;27:2431-2434.
99. Schwab J.S., Diangelo D.J., Foley K.T. Motion compensation associated with single-level cervical fusion: where does the lost motion go? Spine. 2006;31:2439-2448.
100. Hilibrand A.S., Carlson G.D., Palumbo M.A., et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519-528.
101. Ishihara H., Kanamori M., Kawaguchi Y., et al. Adjacent segment disease after anterior cervical interbody fusion. Spine J. 2004;4:624-628.
102. Robertson J.T., Papadopoulos S.M., Traynelis V.C. Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty: a prospective 2-year study. J Neurosurg Spine. 2005;3:417-423.
103. Hilibrand A.S., Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4:190S-194S.
104. Rao R.D., Wang M., McGrady L.M., et al. Does anterior plating of the cervical spine predispose to adjacent segment changes? Spine. 2005;30:2788-2792.
105. Kolstad F., Nygaard O.P., Leivseth G. Segmental motion adjacent to anterior cervical arthrodesis: a prospective study. Spine. 2007;32:512-517.
106. Baaj A.A., Uribe J.S., Vale F.L., et al. History of cervical disc arthroplasty. Neurosurg Focus. 2009;27:E10.
107. Coric D., Finger F., Boltes P. Prospective randomized controlled study of the Bryan Cervical Disc: early clinical results from a single investigational site. J Neurosurg Spine. 2006;4:31-35.
108. Nabhan A., Ahlhelm F., Shariat K., et al. The ProDisc-C prosthesis: clinical and radiological experience 1 year after surgery. Spine. 2007;32:1935-1941.
109. Shim C.S., Lee S.H., Park H.J., et al. Early clinical and radiologic outcomes of cervical arthroplasty with Bryan Cervical Disc prosthesis. J Spinal Disord Tech. 2006;19:465-470.
110. Yoon D.H., Yi S., Shin H.C., et al. Clinical and radiological results following cervical arthroplasty. Acta Neurochir (Wien). 2006;148:943-950.
111. Mummaneni P.V., Haid R.W. The future in the care of the cervical spine: interbody fusion and arthroplasty. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:155-159.
112. Oskouian R.J., Whitehill R., Samii A., et al. The future of spinal arthroplasty: a biomaterial perspective. Neurosurg Focus. 2004;17:E2.
113. Smith H.E., Wimberley D.W., Vaccaro A.R. Cervical arthroplasty: material properties. Neurosurg Focus. 2004;17:E3.
114. Pickett G.E., Sekhon L.H., Sears W.R., et al. Complications with cervical arthroplasty. J Neurosurg Spine. 2006;4:98-105.
115. Sekhon L.H., Duggal N., Lynch J.J., et al. Magnetic resonance imaging clarity of the Bryan, Prodisc-C, Prestige LP, and PCM cervical arthroplasty devices. Spine. 2007;32:673-680.
116. Bartels R.H., Donk R. Fusion around cervical disc prosthesis: case report. Neurosurgery. 2005;57:E194.
117. Parkinson J.F., Sekhon L.H. Cervical arthroplasty complicated by delayed spontaneous fusion. Case report. J Neurosurg Spine. 2005;2:377-380.
118. Leung C., Casey A.T., Goffin J., et al. Clinical significance of heterotopic ossification in cervical disc replacement: a prospective multicenter clinical trial. Neurosurgery. 2005;57:759-763.
119. Mehren C., Suchomel P., Grochulla F., et al. Heterotopic ossification in total cervical artificial disc replacement. Spine. 2006;31:2802-2806.
120. Mummaneni P.V., Burkus J.K., Haid R.W., et al. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine. 2007;6:198-209.
121. Sekhon L.H. Cervical arthroplasty in the management of spondylotic myelopathy: 18-month results. Neurosurg Focus. 2004;17:E8.
122. Hegewald A.A., Ringe J., Sittinger M., et al. Regenerative treatment strategies in spinal surgery. Front Bioscience. 2008;13:1507-1525.
123. Sobajima S., Kim J.S., Gilbertson L.G., et al. Gene therapy for degenerative disc disease. Gene Ther. 2004;11:390-401.
124. Wallach C.J., Gilbertson L.G., Kang J.D. Gene therapy applications for intervertebral disc degeneration. Spine. 2003;28:93-98.
125. Ruan D., He Q., Ding Y., et al. Intervertebral disc transplantation in the treatment of degenerative spine disease: a preliminary study. Lancet. 2007;369:993-999.
126. Luk K.D., Ruan D.K. Intervertebral disc transplantation: a biological approach to motion preservation. Eur Spine J. 2008;17:S504-S510.