Traumatic Shock and Tissue Hypoperfusion
Nonsurgical Management
In 1934, Blalock suggested four categories of shock: hypovolemic, vasogenic, neurogenic, and cardiogenic.1,2 In more recent clinical practice, additional categories of shock have been proposed.3 Hypovolemic shock, the most common, results from reduction in circulating blood volume. Volume loss may be loss of whole blood, plasma, or extracellular fluid or a combination of all three. Vasogenic shock occurs as a result of changes in the resistance of vessels so that a normal blood volume fails to occupy the available space. Neurogenic shock (spinal shock) is a form of vasogenic shock in which spinal anesthesia or spinal cord injury leads to vasodilation. Septic shock is another form of vasogenic shock in which there is increased capacitance. A decrease in peripheral arterial resistance, a decrease in venous capacitance, and a peripheral arteriovenous maldistribution occur. Cardiogenic shock results from failure of the heart as a pump. Obstructive shock results from mechanical obstruction to cardiac function, as seen with tamponade, tension pneumothorax, or massive pulmonary embolism.4 Traumatic shock includes several components of the conditions mentioned previously.5 Hypovolemia caused by blood loss is compounded by neurogenic, cardiogenic, or obstructive shock plus the vasogenic component of maladaptive mediator cascades initiated by tissue injury. Traumatic shock involves hemorrhage in combination with soft tissue trauma and fractures. As a result, study of pure hemorrhagic shock may have limited relevance to the pathophysiologic condition of traumatic shock. Most studies have shown significant differences in the biologic condition of traumatic shock compared with that of pure hemorrhagic shock based on the activation of mediator cascades.2
Conflicting observations in literature are due at least in part to the assumption that hemorrhagic shock and traumatic shock are identical insults.2,3 Pulmonary complications after simple hemorrhage are uncommon in clinical practice, but pulmonary dysfunction is a common comorbid condition after major trauma with attendant soft tissue or long bone injury.2,6 Activation of mediator systems is far more intense with traumatic shock than with pure hemorrhage.7 Conflicting data regarding changes in cytokine levels after a traumatic insult are likely due to the fact that systemic cytokine levels do not reflect local production of these mediators. Measurement of tissue levels of mediator production may be necessary to determine accurately whether there is upregulation of various mediator systems after trauma or hemorrhage.
Soft tissue injury alone upregulates mediator systems.2,8 A small animal study with closed femur fractures showed Kupffer cell activation 30 minutes after injury.9 Another study assessed the effects of skeletal muscle injury in combination with hemorrhage in a porcine model of hemorrhagic shock. To reach a given physiologic end point (reduction in cardiac index and oxygen delivery), hemorrhage of 40% of the blood volume was required in a pure hemorrhagic shock model. If skeletal muscle injury was added, hemorrhage of only 29% of blood volume was necessary to reach the same end point.2,10 The ability to maintain cardiac function after hemorrhage was impaired in this study by superimposition of a soft tissue injury, emphasizing the difference between hemorrhagic shock and traumatic shock. A synergy in activation of neuroendocrine and inflammatory mediator systems is likely when traumatic injury and hemorrhagic shock are present. More recent work describing coagulation changes occurring with injury emphasizes the danger of combined injury and hypoperfusion of soft tissue in failure of appropriate coagulation response.11
Classic Neuroendocrine Response
The essential homeostatic response to acute blood loss is preservation of cerebral and cardiac perfusion with maintenance of normal blood pressure as sensed by carotid body and aortic arch receptors. Peripheral vasoconstriction and curtailment of fluid excretion are seen. Cardiac contractility and peripheral vascular tone also are altered. Pain, hypoxemia, acidosis, infection, changes in temperature, and availability of substrates such as glucose affect this response. A decrease in blood volume alone without hypotension may activate the hypothalamic-pituitary axis. The magnitude of neuroendocrine response depends not only on the volume of blood loss, but also the rate at which blood loss occurs. This response may be modified by patient age, prescribed medications, preexisting illness, and the use of ethanol or other drugs. With spinal cord transection, operative intervention below the level of injury does not produce typical activation of the hypothalamic-pituitary axis. Similarly, consciousness is unnecessary for activation of this response because it may occur under anesthesia.2,12–16
The initial effect seen with hemorrhage is sympathetic vasoconstriction. Capacitance of the circulatory system is reduced, and aortic arch or carotid sinus baroreceptors respond to changes in blood pressure by modulation of sympathetic tone.2,17 Atrial receptors respond to changes in vascular wall stretch and pressure. Afferent vagal fibers carry signals leading to loss of tonic inhibition of heart rate and immediate activation of thoracolumbar sympathetic outflow with norepinephrine release from postganglionic sympathetic fibers. As blood loss increases, so does the role played by arterial baroreceptors. Another part of this hormonal response is corticotropin-releasing factor secreted by the hypothalamus, vasopressin release, and growth hormone-releasing factor release.12
The clinician sees cool extremities in response to these changes associated with hypovolemia. Venous capacitance also decreases, resulting in accelerated venous return to the heart. Selective arterial vasoconstriction maintains blood flow to the heart and brain until compensation fails. Intense triggering of sympathetic signals is activated when arterial blood pressure decreases to less than 50 mm Hg and is maximally stimulated when systolic blood pressure is less than 15 mm Hg.2 Although metabolic vasoregulation in the heart and brain helps avoid local vasoconstriction, blood flow to other tissues decreases dramatically. Renal blood flow may be reduced to 5% to 10% of normal with acute hypovolemia. Flow to the splanchnic circulation, skin, and skeletal muscle also decreases. These vasoconstrictor responses are mediated by epinephrine and norepinephrine from the adrenal medulla and local sympathetic activity at the vasculature. With increases in acidosis and hydrogen ion concentration, coronary vasodilation occurs as opposed to constriction of arteries in skeletal muscle and the splanchnic circulation.3,18,19
Multiple endocrine responses are seen with trauma and associated hypovolemia. Plasma levels of glucagon, growth hormone, cortisol, and corticotropin (adrenocorticotropic hormone) increase.2,3,5 The renin-angiotensin-aldosterone axis is stimulated with release of vasoconstrictive angiotensin II. Vasopressin release also occurs after hemorrhage, resulting in water absorption in the distal tubule of the kidney. Vasopressin induces splanchnic vasoconstriction. Research suggests that with prolonged hemorrhage, vasopressin depletion may occur, and supplements of this hormone by clinicians may be warranted. Growth hormone and glucagon promote gluconeogenesis, lipolysis, and glycogenolysis. Catecholamines that inhibit insulin release and hyperglycemia and increase blood osmolarity are thought to shift fluid from cells and the interstitium into the intravascular space. More recent data associate hyperglycemia in the setting of injury with adverse outcome, however. The cellular mechanism for this response remains unclear. Loss of fluid or salt through the kidneys also is limited by these hormonal effects, which serve to conserve the circulating blood volume.18,20–22
Compensated acute hypovolemia occurs when the aforementioned mechanisms are sufficient to avoid widespread cellular injury and organ decompensation.2 If volume loss continues, or resuscitation is inadequate, a cycle of decline occurs with regional perfusion defects leading to tissue and microcirculatory changes. Progression from compensated to decompensated and irreversible shock is often defined in retrospect. Frequently, a patient with acute irreversible hemorrhage has been hypotensive for an extended period and cannot be resuscitated despite fluid administration and use of vasoactive drugs.23 Presumed mechanisms in this situation include microcirculatory failure with loss of vasomotor response and integrity of the vascular bed. Patients with subacute but ultimately irreversible shock can be resuscitated initially, but progressive organ injury and end-organ dysfunction follow.
Inflammation in Shock after Injury
In addition to blood loss, extensive research suggests that trauma may be considered an inflammatory disease.24–27 It has been shown that a variety of mediators and indicators of inflammatory response are elevated in severely injured patients. For many of these factors, it could be shown that they were significantly elevated in patients eventually dying compared with survivors, and that prediction of outcome is possible with a significant degree of accuracy. Peak inflammatory activity as measured by plasma values has been noted within hours of injury. Although it cannot at present be decided which of these parameters may play a direct pathophysiologic role in development and promotion of inflammatory response and consecutive organ dysfunction, and which is an indicator of this reaction, inflammatory mediators may reflect pathophysiologically relevant disturbances set off by tissue injury and blood loss with consecutive ischemia and reperfusion incidents.28
Shock after trauma differs from pure hypovolemic shock in that effects of release of mediators by tissue injury are superimposed on hypovolemia. It also is clear that not all damage after shock is the result of tissue hypoxia, and that much of cellular damage follows reperfusion and subsequent inflammation. Loci of this inflammatory response are the wound, with activation of macrophages and production of proinflammatory mediators, and the microcirculation, with activation of blood elements and the endothelium.28,29
Cellular Energetics
With blood loss, classic circulatory variables, such as systolic blood pressure, remain normal or supranormal until 30% of blood loss occurs.2,30 With progressive cellular hypoxia, mitochondria still may be able to metabolize oxygen.2 Nonetheless, with significant hypovolemia, total oxygen available to tissue is severely reduced, causing anaerobic metabolism, which is energy inefficient because one molecule of glucose is no longer able to contribute to resynthesis of 32 mol of adenosine triphosphate but only to 2 mol. Glucose must reach cells through the circulation, which is critically reduced. In addition, the end product is no longer carbon dioxide, which can be eliminated by ventilation, but lactic acid and hydrogen ions, leading to metabolic acidosis. Acidosis drives cellular swelling with loss of extracellular fluid volume into the cells. Lactate finally is metabolized by the liver, which also is hypoxic. Transcapillary refill and lymph flow direct interstitial fluid to increase the circulating blood volume, but ultimately capillaries are damaged by hypoxia and the action of activated neutrophils, which increases interstitial edema. Finally, autoregulation of microcirculation is destroyed, leading to fluid sequestration and sludging in the microvasculature. These factors are responsible for increased diffusion distance for oxygen from capillaries to the mitochondria, which further impairs oxygen extraction. Tissue hypoxia also is the most potent stimulus for proinflammatory activation of macrophages and release of vasoactive or arachidonic acid metabolites, such as prostaglandins and thromboxane. Hypovolemia, shock, and any other cause of brain hypoxia also are detrimental to recovery, particularly in patients with head injury because these conditions induce secondary brain damage.
Immune Mediator Cascades
Although a variety of initiating events may occur, the subsequent inflammatory response is qualitatively similar.2 Local activation of the complement cascade produces anaphylatoxins, which are strong attractants and stimulants of neutrophils. Local endothelium expresses endothelial leukocyte adhesion molecules, which attract the neutrophil population. Activated neutrophils also express adhesion molecules, leading to aggregation, margination in the vascular endothelium, and migration through vessel walls at the area of injury. This inflammatory response produces a respiratory burst with formation of oxygen radicals and synthesis of proteolytic enzymes (elastase). Local release of bradykinin, histamine, and prostaglandin induces local vasodilation and increased capillary permeability from macromolecules, resulting in a protein-rich exudate. Local phagocytes release messenger molecules, such as granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor, which activate the bone marrow to produce more inflammatory cells. Neutrophils injure otherwise healthy tissues.2,31–34
In a slower response, the monocyte population is attracted to the site of injury, where it differentiates to macrophages and contributes to the inflammatory process by phagocytosing and killing bacteria or disposing of necrotic tissue or both. Macrophages are activated further by triggers such as hypoxia or C5a, macrophage-activating factor, and interleukin (IL)-1-like activity from neutrophils. On stimulation, macrophages release a variety of classes of secretory products, which may be proinflammatory (proteolytic enzymes, oxygen radicals, IL-1, IL-6, tumor necrosis factor) or anti-inflammatory (IL-10, prostaglandin E2). Macrophage mediators such as prostaglandin E2, tumor necrosis factor, IL-1, IL-2, and IL-6 provide systemic signals adapting metabolic and defense mechanisms. Macrophages take several days after activation to develop full inflammatory capacity. They also may release nitric oxide and cytotoxic radicals. In the setting of injury, this local inflammatory process spills over to cause an exaggerated systemic response with inflammatory damage to otherwise healthy cells and organs distant to the site of injury. Secondary infection may occur in the compromised host, leading to generalized inflammation and multiorgan dysfunction (Box 27.1).2,35,36
Neuroimmune Response to Trauma
More recent work examines the link between the autonomic nervous system and modulation of immune response during traumatic injury. Anatomic interactions with immune-competent cells have been identified, and functional consequences of this interaction in the host are now being examined. Integrated hemodynamic, metabolic, behavioral, and immune responses allowing host adaptation are the stress response.37–41
Catecholamines are neurotransmitters that affect immune response humorally through circulating adrenal-derived epinephrine and locally through neuronal release of norepinephrine. There is anatomic evidence of central nervous system (CNS)–lymphoid organ connection through autonomic and sensory fibers and immune tissues, including bone marrow, thymus, spleen, and lymph nodes.37 This sympathetic innervation of lymphoid organs is found across species and has been confirmed by immunohistochemistry. In bone marrow, myelinated and nonmyelinated fibers are distributed with vascular plexuses where they influence hematopoiesis and cell migration. In the lungs, noradrenergic nerve fibers supply tracheobronchial smooth muscle and glands. In addition, nerve fibers have been shown throughout the different compartments of the bronchus-associated lymphoid tissue forming close contact with mast cells, cells of the macrophage/monocyte lineage, or other lymph node cells. In the thymus, noradrenergic nerve fibers have been localized in the subcapsular, cortical, and corticomedullary regions associated with blood vessels and intralobular septa branching into cortical parenchyma where they reach to thymocytes.37,42
The functional effects of catecholamines on cells of the immune system have been confirmed in human volunteers. In addition, relevance of this control mechanism and the implications for dysregulation have been shown by rapid systemic release of IL-10 and the high incidence of infection in patients with sympathetic storm from accidental or iatrogenic brain trauma.37 Although detrimental effects of sustained and exaggerated sympathetic nervous system activation on cardiovascular and metabolic homeostasis have long been recognized, attention is now directed to the likelihood of immune dysregulation as well.
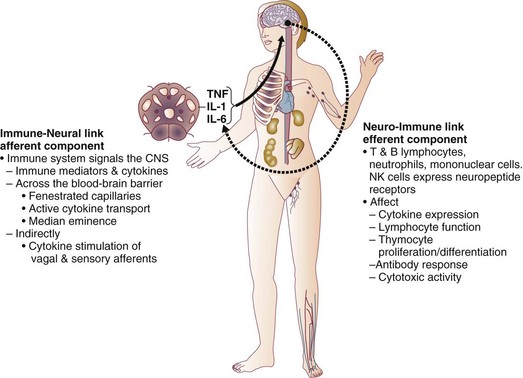
The neuroimmune axis is a bidirectional network composed of descending pathways linking the CNS to peripheral immune tissues and a parallel afferent arm linking the immune system with the CNS. The integrity of this loop allows for communication between the CNS and peripheral immune system integrating neuronal and immune signals in the periphery and in the CNS. Cells from the immune system express functional receptors and signal transduction pathway components for several neuroendocrine mediators allowing functional cellular responses to agonist stimulation. Similarly, cells in the CNS are capable of synthesizing, secreting, and responding to inflammatory and immune molecules. There is considerable evidence that the peripheral immune system can signal the brain to elicit a sickness response during infection, inflammation, and injury. Peripheral immune molecules such as cytokines influence CNS action through mechanisms including entry into the brain through a saturable transport mechanism or through areas that lack the blood-brain barrier. Afferent neurons of the vagus nerve also are activated (Fig. 27.1).43–45
Severe trauma is characterized by the classic activation of the sympathetic nervous system and the recently recognized contribution of the inflammatory and neuroimmune response to injury.37 The sympathetic nervous system has significant anatomic and functional interaction with cells of the immune system and plays an important role in control of the magnitude of early inflammatory response to injury by ensuring expression of adequate cytokine balance.37 Sympathetic neural pathways exert direct effects on cells of the immune system, affecting cytokine expression, lymphocyte function, and cytotoxic activity. In return, the inflammatory mediators released communicate with the CNS through stimulation of sensory and vagal afferents or by crossing the blood-brain barrier through active transport mechanisms and pathways allowing access to hypothalamic-pituitary structures. Immune-derived mediators, such as cytokines and chemokines, can modulate neurotransmission affecting activation of descending autonomic and neuroendocrine pathways.37
Acute Coagulopathy after Trauma
Historical Perspective
Hemorrhagic shock accounts for a significant number of deaths in patients arriving at hospital with acute injury. Patients with uncontrolled hemorrhage continue to die despite adoption of new surgical techniques with improved transport and emergency care.46,47 Coagulopathy, occurring even before resuscitation, contributes significantly to the morbidity associated with bleeding.48,49 Recognition of the morbidity associated with bleeding and coagulation abnormality dates to the Vietnam conflict. At that time, standard tests including prothrombin time (PT) and partial thromboplastin time (PTT) correlated poorly with effectiveness of acute resuscitation efforts. Similar work in the late 1970s was performed in civilian patients receiving massive transfusion. Again, PT, PTT, and bleeding time were only helpful if markedly prolonged.50,51
Studies in the 1970s and 1980s provided additional detail regarding the limitation of simple laboratory parameters and factor levels.51,52 In a study of multiple patients requiring massive transfusion, platelet counts fell in proportion to the size of transfusion although factors V and VIII correlated poorly with the volume of blood transfused. Where coagulopathy appeared, patients seemed to respond to platelet administration. In subsequent studies, patients receiving a large number of blood products were followed for microvascular bleeding. Moderate deficiencies in clotting factors were common, but they were not associated with microvascular bleeding. Microvascular bleeding was associated with severe coagulation abnormalities such as clotting factor levels less than 20% of control values. In statistical analysis, clotting factor activities less than 20% of control levels were predicted by significant prolongation of PT and PTT. These earlier investigators also suggested that empiric blood replacement formulas available at the time were not likely to prevent microvascular bleeding because consumption of platelets or clotting factors did not consistently appear and simple dilution caused by resuscitation fluids frequently did not correspond to microvascular bleeding.52
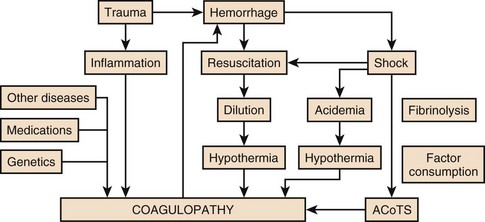
The attention of the American trauma community was drawn to coagulopathy after trauma with the description of the “bloody vicious cycle” by the Denver health team over 20 years ago.48 These investigators noted the contribution of hypothermia, acidosis, and hemodilution associated with inadequate resuscitation and excessive use of crystalloids. Subsequent work extended these observations describing early coagulopathy that could be independent of clotting factor deficiency.53 In a more recent trial, early coagulopathy was noted in the setting of severe injury, which was present in the field, prior to emergency department arrival and initiation of fluid resuscitation. Coagulopathic patients were at increased risk for organ failure and death.
In a study questioning historical transfusion practice emphasizing administration of packed red blood cells (PRBCs) in the setting of massive trauma, Hirshberg and coworkers, using clinical data, developed a computer model designed to capture interactions between bleeding, hemodynamics, hemodilution, and blood component replacement during severe hemorrhage. Resuscitation options were offered in this model and their effectiveness evaluated.54 After setting thresholds for acceptable loss of clotting factors, platelets, and fibrinogen, the authors modeled behavior of coagulation during rapid exsanguation without clotting factor or platelet replacement. The PT reached a critical level first followed by fibrinogen and platelets. If patients were resuscitated with small amounts of crystalloid, leaving overall blood volume reduced, the effective life of components in the coagulation cascade was increased. More aggressive fresh frozen plasma (FFP) replacement in the patient with significant bleeding was supported by this model. The optimal ratio for administration of FFP to PRBCs in this analysis was 2 : 3. Delayed administration of FFP led to critical clotting factor deficiency regardless of subsequent administration of FFP. Fibrinogen depletion was easier to correct. After administration of 5 units of PRBCs, the hemostatic threshold for fibrinogen was not exceeded if a FFP-to-PRBC ratio of 4 : 5 was employed. Analysis of platelet dilution demonstrated that even if platelet replacement was delayed until 10 units of PRBCs were infused, critical platelet dilution was prevented with a subsequent platelet-to-PRBC ratio of 8 : 10.54
Recent Studies
Brohi and coworkers from the United Kingdom helped to reinvigorate discussion of coagulopathy after injury by adding new coagulation laboratory techniques to previous clinical observations.55 After reviewing over 1000 cases, patients with acute coagulopathy after injury had higher mortality rates throughout the spectrum of Injury Severity Scores (ISS). Contrary to historical teaching that coagulopathy was a function of hemodilution with massive crystalloid resuscitation, these authors noted that the incidence of coagulopathy increased with severity of injury but not necessarily in relationship to the volume of intravenous fluid administered to patients. Brohi and others helped to reemphasize the observation that acute coagulopathy could occur before significant fluid administration, which was attributable to the injury itself and proportional to the volume of injured tissue. Development of coagulopathy was an independent predictor of poor outcome. Mediators associated with tissue trauma including humoral and cellular immune system activation with coagulation, fibrinolysis, complement, and kallikrein cascades have been associated with changes in hemostatic mechanisms similar to those identified in the setting of sepsis.55–57
Factors contributing to coagulopathy in the setting of injury have been further reviewed.58 Hypothermia relates to development of coagulopathy by reduction in platelet aggregation and decreased function of coagulation factors in nondiluted blood. Patients with temperature reduction below 34° C had elevated PT and PTT. Coagulation, like most biologic enzyme systems, works best at normal temperature. Similarly, acidosis occurring in the setting of trauma as a result of bleeding and hypotension also contributes to clotting failure. Animal work shows that a pH less than 7.20 is associated with hemostatic impairment. Platelet dysfunction and coagulation enzyme system changes are noted when blood from healthy volunteers is subjected to an acidic environment.59,60
Hess and coworkers, as part of an international medical collaboration, developed a literature review to increase awareness of coagulopathy independent of crystalloid administration following trauma.57 The key initiating factor is volume of tissue injury. Patients with severe tissue injury but no physiologic derangement, however, rarely present with coagulopathy and have a lower mortality rate.61,62 Tissue damage initiates coagulation as endothelial injury at the site of trauma leads to exposure of subendothelial collagen and activation of the coagulation cascade.
Hyperfibrinolysis is seen as a direct consequence of the combination of tissue injury and shock. Endothelial injury accelerates fibrinolysis because of direct release of tissue plasminogen activator.57,63 Tissue plasminogen activator expression by endothelium is increased in the presence of thrombin. Fibrinolysis is accelerated because of the combined effects of endothelial tissue plasminogen activator release with ischemia and inhibition of plasminogen activator inhibitor in shock. Although hyperfibrinolysis may focus clot propagation on sites of actual vascular injury, with widespread insults, this localization may be lost.
A number of important cofactors must be present to stimulate coagulopathy in the setting of injury.57 Shock is a dose-dependent cause of tissue hypoperfusion. Elevated base deficit has been associated with coagulopathy in as many as 25% of patients in one large study. Progression of shock appears to result in hyperfibrinolysis. One mediator implicated in coagulopathy after injury is activated protein C. Immediate postinjury coagulopathy is likely a combination of effects caused by large volume tissue trauma and hypoperfusion (Fig. 27.2).57
As will be discussed later, equivalent ratios of FFP, PRBCs, and platelets are now considered for management of significant hemorrhage with coagulopathy after injury. Hypothermia and acidemia must be controlled to reduce their impact on enzyme systems.64 Similar to sepsis, cross-talk has been noted between coagulation and inflammation systems with injury. Activation of coagulation proteases may induce inappropriate inflammation with activation of cascades such as complement and platelet degranulation.65,66 Trauma patients are initially coagulopathic with increased bleeding. This condition may progress to a hypercoagulable state, putting them at risk for thrombotic events. This late thrombotic state bears similarities with coagulopathy of severe sepsis and depletion of protein C. Injured and septic patients share a propensity toward multiple organ failure and prothrombotic states.67,68
Fluid Therapy
Warmed isotonic electrolyte solutions are recommended for initial resuscitation of traumatic shock by the Committee on Trauma of the American College of Surgeons. This type of fluid provides transient intravascular expansion and stabilizes the intravascular volume by replacing accompanying fluid losses into the interstitial and intracellular spaces. Lactated Ringer’s solution is the initial fluid of choice. Normal saline is the second choice. Normal saline has the potential to cause hyperchloremic acidosis. This complication is more likely if renal function is compromised (Table 27.1).69
Table 27.1
Estimated Fluid and Blood Losses Based on Initial Clinical Presentation*
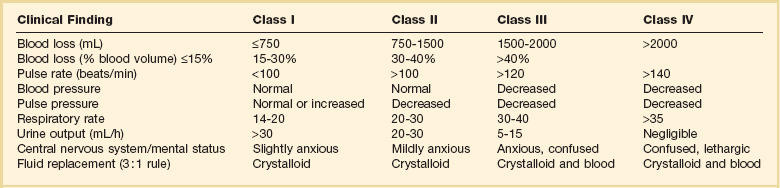
*This is the standard approach to resuscitation of shock after injury as described in the Advanced Trauma Life Support course promulgated by the Committee on Trauma of the American College of Surgeons. The crystalloid of choice used in resuscitation is lactated Ringer’s solution. Clinical parameters are used to estimate the degree of blood loss, and fluid resuscitation begins with 1-2 L of lactated Ringer’s solution given through large-bore peripheral intravenous lines. When the response to resuscitation is limited or transient, O-negative or type-specific blood is added to resuscitation while the cause of shock is sought and additional treatment is given.
From American College of Surgeons Committee on Trauma: Advanced Trauma Life Support for Doctors, 7th ed. Chicago, American College of Surgeons, 2004, pp 69-85.
An initial warm fluid bolus is given rapidly—usually 1 to 2 L for an adult and 20 mL/kg for a child.45 Patient response is observed during this initial fluid resuscitation, and subsequent therapeutic decisions are based on this response. The required amount of fluid and blood is difficult to predict on initial evaluation of the patient. A rough guideline promulgated by the American College of Surgeons for the total amount of crystalloid volume acutely required is 3 mL of crystalloid fluid to replace each 1 mL of blood loss, allowing for restitution of plasma volume lost into interstitial and intracellular spaces. It is most important, however, to assess patient response to fluid resuscitation and evidence of adequate end-organ perfusion as measured by urine output and level of consciousness, rather than provide fluid based on a specific formula. If the amount of fluid required to restore or maintain adequate end-organ function exceeds the previously mentioned estimates, careful reassessment of the situation and exploration for unrecognized injuries, bleeding, or other causes of shock are necessary (Table 27.2).
Table 27.2
Responses to Initial Fluid Resuscitation*
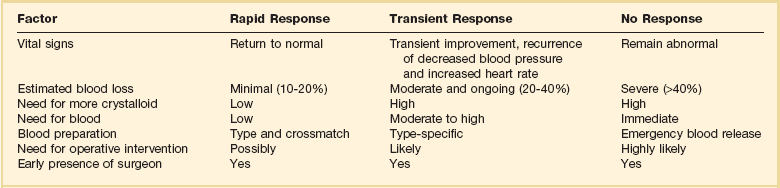
*The Advanced Trauma Life Support course advocates ongoing evaluation of patient response to initial fluid administration. Patients with no response frequently require emergent blood transfusion and transfer to the operating room. Patients with transient response also frequently require operative intervention. Most patients, particularly in centers seeing blunt injury, respond rapidly to an initial 1-2 L of crystalloid and are cleared to proceed to more detailed imaging to determine internal injuries after normalization of clinical parameters.
From American College of Surgeons Committee on Trauma: Advanced Trauma Life Support for Doctors, 7th ed. Chicago, American College of Surgeons, 2004, pp 69-85.
In clinical practice, large volume resuscitation with lactated Ringer’s solution has become common in trauma care.70 However, recent military and laboratory work features a growing concern about tissue edema from large volume resuscitation. In recent decades, a persisting picture of acute lung injury due to increased filtration across pulmonary microcapillaries with pulmonary inflammation emerged. This process would later be called the acute respiratory distress syndrome.71 Other observations included increased interstitial fluid of gut and heart tissues, abdominal compartment syndrome, extremity compartment syndrome in uninjured extremities, and pericardial effusion.72,73
Hemorrhage is a multifactorial disease; circulatory and inflammatory effects of hemorrhagic shock occur simultaneously. Unfortunately, laboratory studies have repeatedly shown that the choice of resuscitation fluid may worsen hemorrhage-induced cellular dysfunction, immune modulation, and inflammation. Fluids affect neutrophil activity by changing life span, activation, and gene expression. Resuscitation fluids also enhance inflammatory cascade through upregulation of cellular receptors and proinflammatory mediators. The choice of fluid also affects cellular gene expression, apoptotic cell death, and extracellular matrix integrity.70,74–76
Isotonic Crystalloids
Of isotonic crystalloids, lactated Ringer’s solution has been most extensively studied to determine its role in hemorrhage-induced immune dysfunction, inflammation, and management of ischemia and reperfusion injury. Lactated Ringer’s solution has been shown to upgrade vascular endothelial adhesion molecules and to increase expression of CD11b and CD18 binding sites on neutrophils. Neutrophil oxidative burst is also stimulated by lactated Ringer’s solution. In other organs, Ringer’s lactate has been found to increase apoptosis in the bowel, the liver, and the lung with multiple cell types affected including macrophages, endothelial cells, epithelial cells, and smooth muscle cells.70,77
Despite laboratory findings about the dangers of lactated Ringer’s solution, it remains the fluid of choice in many centers and the recommended fluid of the Advanced Trauma Life Support (ATLS) protocol. Efforts have been made to examine why lactated Ringer’s solution is cytotoxic and identify ways to improve it. Traditionally, lactated Ringer’s solution came in racemic form; laboratory work implicates the D-isomer of lactate as its primary toxic component.78 The D-isomer was found to increase neutrophil oxidative burst, enhance apoptosis, and drive inflammation. The L-isomer of lactate may confer immune protection through attenuation of neutrophil activation, alteration of leukocyte gene expression, and reduction in apoptosis.79,80
Colloids
Hyperoncotic colloid solutions have also been studied in resuscitation roles for traumatic hemorrhage. The natural colloid albumin does not induce neutrophil oxidative burst and may confer a protective immunologic effect by decreasing neutrophil expression of adhesion molecules.81 At present, albumin sees little application in resuscitation at the scene of injury but has been investigated in critical care practice.
An artificial colloid, 6% hetastarch, has been found to have a number of deleterious resuscitation effects in animal models including increased neutrophil oxidative burst and pulmonary apoptosis. Beneficial effects include decreased neutrophil migration. At present, natural and artificial colloids have failed to show clinical benefits in comparison with crystalloid solutions.82,83 Laboratory concerns and lack of a positive clinical outcomes mandate argue against the use of colloids in early resuscitation of hemorrhagic shock.
Recent reviews suggest important differences in safety among colloids. Examination of data comparing colloids with crystalloids must take into account materials employed. When albumin was used as a reference, the incidence ratio for anaphylactoid reactions was 4.51 after administration of hydroxyethyl starch, 2.32 after dextran, and 12.4 after gelatin. Artificial colloid administration was consistently associated with coagulopathy and clinical bleeding, most frequently in cardiac surgery patients receiving starches. Albumin had the lowest rate of total adverse events and serious adverse events.84 Although albumin is isolated from human plasma, no evidence of viral disease transmission has been consistently identified. Life-threatening anaphylactoid reactions were infrequent for all colloids. Hydroxyethyl starch, as compared with albumin, more than quadrupled the incidence of anaphylactic reactions, whereas dextran more than doubled them. The incidence of these reactions in recipients of gelatin was greater by an order of magnitude than after albumin infusion. Because artificial colloids are derived from nonhuman source materials, they may be recognized as foreign and are more likely to provoke this immune-mediated response. The foreign nature of artificial colloids also may hinder metabolic clearance and promote tissue deposition. On the basis of extensive evidence, albumin is the safest colloid for consideration in resuscitation of traumatic shock.84 Although factors such as desirability of anticoagulant activity may favor other artificial colloids, this is not true in the setting of injury.85–87
Multicenter data comparing albumin and saline for fluid resuscitation were obtained in Australia and published in 2004.88 Nearly 7000 patients were randomly assigned to administration of 4% albumin or normal saline for intravascular fluid resuscitation procedures. Mortality rate and the incidence of single and multiple organ dysfunction were comparable in the two groups. Subset analysis suggests, however, poorer outcomes in the setting of injury. In the subgroup of 140 patients included with principal diagnoses of trauma, a treatment effect seemed to favor administration of saline. In this trial, the increased relative risk of death among patients with trauma compared with patients without trauma resulted from an excess number of deaths among patients who had trauma with brain injury. The difference in mortality rates between albumin and saline groups among patients with trauma involving brain injury must be viewed cautiously because the number of involved subjects is small. In the Australian trial, patients with traumatic brain injury constituted only 7% of the study population, and the excess number of deaths in the albumin group was 21. Other parameters that could be helpful in evaluation of the impact of albumin in the setting of brain injury, such as functional neurologic status, were not provided. In contrast with the experience in trauma, the Australian trial suggests some evidence of treatment benefit favoring administration of albumin in patients with severe sepsis. Given contemporary resuscitation technology, factors influencing the choice of resuscitation for critically ill patients include specific clinician concerns, treatment tolerance, safety, and cost.
Hypertonic Saline
Increased transmembrane sodium gradient caused by hypertonic saline generates intravascular volume expansion similar to hyperoncotic colloids and superior to conventional isotonic crystalloids such as lactated Ringer’s solution and normal saline. Animal models suggest that hypertonic saline solutions dilate precapillary arterioles and shunt oxygen to vital organs.89,90 Hypertonic saline solutions also have fewer proinflammatory properties than other clinical crystalloids and colloids. Hypertonic saline does not induce expression of inflammatory cytokine receptor genes in multiple studies and blunts hemorrhage-induced increase in plasma levels of proinflammatory cytokines, IL-10, and granulocyte-macrophage colony-stimulating factor. Hypertonic saline also does not increase apoptotic cell death in liver, lung, or bowel.70
What about the impact of hypertonic saline and associated hypernatremia on head injury?91 Studies in experimental animals and humans suggest that hypertonic saline may be highly effective in treating head injury, either alone or associated with hemorrhagic hypotension. Tissue swelling in a closed cranium threatens to cause major pressure-induced brain damage or death, and concomitant hemorrhage hypotension reduces cerebral oxygen delivery, resulting in a secondary ischemic insult. Historical data suggest a twofold higher incidence of adverse outcomes in patients with brain injury combined with hypotension. Early data suggest that patients treated with hypertonic saline with dextran are more likely to survive to discharge than individuals treated with standard resuscitation care.92,93
Hypertonic-Hyperoncotic Fluids
Mixture of hypertonic saline with dextran has been the most extensively tested hypertonic-hyperoncotic fluid.70 Use of combinations of hypertonic saline and dextran suggests that this material is effective in expanding plasma volume, restoring hemodynamics, and improving microcirculatory perfusion. In the laboratory, hypertonic saline and dextran solutions blunt hemorrhage-induced inflammatory response by neutrophils and, in clinical trials, decreased adhesion molecule expression.94 As with hypertonic saline solutions, there has been concern that hypertonic saline mixed with dextran could accelerate hemorrhage, increase mortality rate, and cause hypernatremia and hyperchloremia.95 Despite multiple clinical trials comparing hypertonic saline and dextran solutions to more traditional resuscitation products, no improvement in mortality rate or change in the pattern of organ failure is seen.96
Mechanisms by which hypertonic/hyperoncotic resuscitation may be effective in models of head injury and hemorrhage show reduction in water content in noninjured portions of the brain with reduction in intracranial pressure and cerebral edema. In a large animal model, when hypertonic saline was compared with a synthetic colloid, colloid alone had no effect on brain water content.97,98
Crystalloids Versus Colloids
Plasma and blood were the fluid replacements of choice in traumatic shock until the early 1960s, when a variety of investigators showed the need to replace the extracellular fluid deficit with crystalloid solutions. These observations were followed by a variety of clinical studies comparing colloid, typically albumin, solutions with crystalloids, typically lactated Ringer’s solution. Consistent with early studies, colloids, when given on an equal volume basis, more effectively increase cardiac output and oxygen transport. Another finding of this early work was the need to give crystalloids in far greater quantities than colloids to achieve consistent hemodynamic objectives.99,100
Later studies from the Vietnam era compared resuscitation of patients who were given whole blood and crystalloids with patients given whole blood plus 5% albumin. Fluid infusion volumes were far higher in the patients given crystalloid solutions. There was no evidence of pulmonary edema, and patients treated with crystalloids seemed to fare better than patients treated with resuscitation containing albumin. Albumin seemed to have less effect on restoration of renal function with suggestion of detrimental effects in pulmonary response, myocardial contractility, and coagulation. Large animal models suggested that pulmonary compromise could relate to increased capillary permeability to albumin. Increased losses of albumin to the heart, kidneys, liver, and brain also were reported.99,101 More extensive studies in injured patients supported reservations regarding the use of albumin. Evaluation of patients randomly selected to receive 150 g of albumin per day intraoperatively and postoperatively noted poorer outcomes than in patients receiving lactated Ringer’s solution. Both groups received whole blood and FFP. Patients treated with albumin required greater ventilator support and had poorer oxygenation.99,102,103 In another carefully conducted trial of patients with multiple trauma, no differences in cardiopulmonary function between patients resuscitated with lactated Ringer’s solution and patients given 5% albumin and lactated Ringer’s solution were identified.104 Normal cardiac index was used as a therapeutic end point. To maintain adequate cardiac output, patients who received crystalloids required far more resuscitation volume than patients treated with albumin. These authors concluded that cardiac output was an appropriate end point for resuscitation, and that no advantage was accrued based on the type of fluid employed. A clear cost advantage of crystalloids was identified.99
Guyton and Lindsey105 examined the effect of colloid oncotic pressure on pulmonary edema. They observed that reducing the serum protein level lowered the threshold of left atrial pressure at which pulmonary edema could occur. Zarins and colleagues106 subsequently showed that a low colloid oncotic pressure alone did not cause an elevation in extravascular lung water. Because of the remarkable efficiency of pulmonary lymphatics, arterial blood gases, shunt fraction, and lung compliance were unchanged despite a 14% increase in body weight caused by infusion of lactated Ringer’s solution to keep high pulmonary artery occlusion pressures. No pulmonary edema was created despite the presence of ascites and marked peripheral edema. Demling and coworkers107 confirmed these findings with a chronic lung lymph fistula in sheep. Holcroft and coworkers108 produced pulmonary edema in baboons during resuscitation from hemorrhage by continuously administering large volumes of lactated Ringer’s solution sufficient to elevate pulmonary artery occlusion pressures 15 mm Hg above baseline levels. With cessation of infusion, filling pressure rapidly returned to normal.
Blood Component Therapy
Despite work from multiple groups suggesting that simple replacement of PRBCs was not a sufficient answer for the most severely injured patient, particularly in the setting of coagulopathy, the concept of combination blood component replacement remained outside the mainstream of trauma care for over 20 years.48,52,109 It took armed conflicts and experience in a multinational group of trauma centers to bring awareness of the need for multiple blood component therapy in massive bleeding to the level of general trauma practice.
The 1970s and 1980s saw several groups propose resuscitation of significant hemorrhage with combinations of blood components. Kashuk and Moore proposed multicomponent blood therapy in patients with significant vascular injury.48 In a study of patients with major abdominal vascular injury, Kashuk and coworkers noted frequent deviation from a standard ratio of 4 : 1 or 5 : 1 for units of PRBCs to units of FFP. The ratio was 8 : 1 in nonsurvivors and 9 : 1 where overt coagulopathy was noted. Fifty-one percent of patients in this series were coagulopathic after vascular control was obtained. Using multivariate analysis, Ciavarella and coworkers from the Puget Sound Blood Center and Harborview Medical Center proposed aggressive supplementation of platelets in the setting of massive transfusion. These investigators noted that platelet counts below 50 × 109/L correlated highly with microvascular bleeding in trauma and surgery patients. Fibrinogen repletion was also emphasized. Guides to resuscitation included fibrinogen level, PT, and PTT. Supplemental FFP or cryoprecipitate was recommended for low fibrinogen levels.52 Lucas and Ledgerwood, summarizing extensive preclinical and clinical studies, suggested administration of FFP after 6 units of PRBCs had been infused. Additional FFP was recommended for every five additional PRBC transfusions. Monitoring included platelet count, PT, and PTT after each 5 units of PRBCs are administered. Platelet transfusion is generally unnecessary unless the platelet count falls below 50,000.109
Rhee and coworkers, using the massive database of the Los Angeles County Level I Trauma Center, examined transfusion practices in 25,000 patients.110 Approximately 16% of these patients received a blood transfusion. Massive transfusion (≥10 units of PRBCs per day) occurred in 11.4% of transfused patients. After excluding head-injured patients, these authors studied approximately 400 individuals. A trend toward increasing FFP use was noted during the 6 years of data that were reviewed (January 2000 to December 2005). Logistic regression identified the ratio of FFP to PRBC use as an independent predictor of survival. With a higher ratio of FFP : PRBC, a greater probability of survival was noted. The optimal ratio in this analysis was an FFP : PRBC ratio of 1 : 3 or less. Rhee and coworkers provide a large retrospective data set demonstrating that earlier more aggressive plasma replacement can be associated with improved outcomes after bleeding requiring massive transfusion. Ratios derived in this massive retrospective data review support the observations of Hirshberg and coworkers.54 Like the data presented by Kashuk and coworkers in another widely cited report, this retrospective data set suggests improved clinical outcome with increased administration of FFP.111
Another view of damage control hematology comes from Vanderbilt University Medical Center in Nashville, Tennessee. This group implemented a trauma exsanguination protocol involving acute administration of 10 units PRBC with 4 units FFP and 2 units platelets. In an 18-month period, 90 patients received this resuscitation and were compared to a historical set of control subjects. The group of patients receiving the trauma exsanguination protocol as described by these investigators had lower mortality rates, higher blood product use in initial operative procedures, and more frequent use of products in the initial 24 hours, though overall blood product consumption during hospitalization was decreased.112
The strongest multicenter civilian data report examining the impact of plasma and platelet administration along with red blood cells on outcome in massive transfusion comes from Holcomb and coworkers.113 These investigators report over 450 patients obtained from 16 adult and pediatric centers. Overall survival in this group is 59%. Patients were gravely ill as reflected by an admission base deficit of −11.7, pH 7.2, Glasgow Coma Scale score of 9, and a mean ISS of 32. Examination of multicenter data reflects an improvement in outcome as the ratio of FFP to PRBCs administered approaches 1. FFP, however, is not the sole solution to improved coagulation response in acute injury. These workers also examined the relationship of aggressive plasma and platelet administration in these patients. Optimal outcome in this massive transfusion group was obtained with aggressive platelet as well as plasma administration. Worst outcomes were seen when aggressive administration of plasma and platelets did not take place. When either FFP or platelets were given in higher proportion in relationship to PRBCs intermediate results were obtained. Not surprisingly, the cause of death that was favorably affected was truncal hemorrhage.
A summary statement comes from Holcomb and a combination of military and civilian investigators.56,57 These workers identify a patient group at high risk for coagulopathy and resuscitation failure due to hypothermia, acidosis, hypoperfusion, inflammation, and volume of tissue injury. In the paradigm proposed by these writers, resuscitation begins with prehospital limitation of blood pressure at approximately 90 mm Hg preventing renewed bleeding from recently clotted vessels. Intravascular volume resuscitation is accomplished using thawed plasma in a 1 : 1 or 1 : 2 ratio with PRBCs. Acidosis is managed by use of THAM (tromethamine) and volume loading with blood components as hemostasis is obtained. A massive transfusion protocol for these investigators included delivery of packs of 6 units of plasma, 6 units of PRBC, 6 units of platelets, and 10 units of cryoprecipitate in stored individual coolers. These coolers are supplied until discontinuation by the trauma team. Even in causalities requiring resuscitation with 10 to 40 units of blood products, Holcomb and coworkers found that as little as 5 to 8 L of crystalloid are utilized during the first 24 hours, representing a decrease of at least 50% compared to standard practice. The lack of intraoperative coagulopathic bleeding allows surgeons to focus on surgical hemorrhage. The goal is arrival of the patient in intensive care unit (ICU) in a warm, euvolemic, and nonacidotic state. International normalized ratio (INR) approaches normal and edema is minimized. Subjectively, patients treated in this way are more readily ventilated and easier to extubate than patients with a similar blood loss treated with standard crystalloid resuscitation and smaller amounts of blood products. Holcomb and others suggest that massive transfusion will be required in 6% to 7% of military patients and 1% to 2% of civilian trauma patients.
End Points
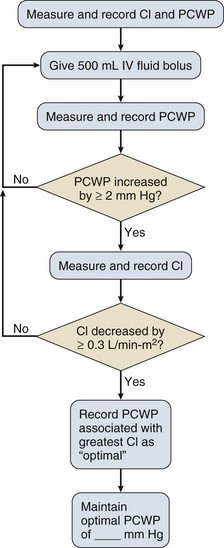
The Problem
Severely injured trauma patients are at high risk of developing multiple organ failure or death. Initial treatment priorities include appropriate fluid administration and rapid hemostasis.114,115 Inadequate tissue oxygenation leads to anaerobic metabolism and tissue acidosis. Depth and duration of shock are associated with cumulative oxygen and metabolic debt. Resuscitation is incomplete until the metabolic debt is paid, and tissue acidosis is eliminated with restoration of aerobic metabolism. Many patients seem to be adequately resuscitated based on normalization of vital signs but have occult hypoperfusion and ongoing tissue acidosis (compensated shock). These individuals are at risk for later organ dysfunction and death.69
As stated in the Advanced Trauma Life Support protocol, the standard of care remains restoration of normal blood pressure, heart rate, and urine output.69 When these parameters remain abnormal (uncompensated shock), the need for additional resuscitation is obvious. After normalization of these parameters, however, many trauma patients still have evidence of inadequate tissue oxygenation or gastric mucosal ischemia. Recognition of this state and its reversal are crucial to reduce the risk of organ dysfunction or death. The optimal marker of adequate resuscitation in injury remains unclear.116
Not all patients can be managed in the same way. More recent literature describing management of neurologic trauma suggests poor outcome with any degree of hypotension during prehospital care, resuscitation, or subsequent in-hospital course. Episodes of hypotension and hypoxia were associated with poor neurologic outcome in a review of more than 700 patients from the Traumatic Coma Data Bank with a Glasgow Coma Scale score less than 9. In this large study, patients without hypotension or hypoxia had a 27% risk of death and a 51% chance of favorable recovery. In the presence of hypotension, with or without hypoxia, the risk of death increased to 65% to 75%. Contrary to the needs of patients with penetrating trauma in whom early aggressive resuscitation may lead to increased bleeding, hypotension should be avoided in head-injured patients. Resuscitation parameters specific to various types of injury have not been reported.117–119
Oxygen Delivery Parameters
Shoemaker and various coworkers provided early stimulus to optimization of hemodynamic management in high-risk surgical patients by examining hemodynamic profiles of survivors of surgical shock states versus patients who died.120 Survivors had significantly higher oxygen delivery and cardiac index values than nonsurvivors. Values correlating with survival included cardiac index greater than 4.5 L/minute/m2, oxygen delivery greater than 600 mL/minute/m2, and oxygen consumption equal to or greater than 170 mL/minute/m2. These initial observations led to a series of articles from this group suggesting reduction in resource consumption and improvement in morbidity and mortality rates with resuscitation to supranormal oxygen delivery parameters. Initial augmentation of oxygen delivery came with volume loading followed by dobutamine and blood transfusions as needed to a hemoglobin level of 14 g/dL.121–124
Attempts by other investigators to replicate these findings met limited success. Moore and coworkers used a resuscitation protocol aimed at maximizing oxygen delivery and found no benefit with resuscitation to achieve supranormal oxygen delivery.116,125 A variety of studies suggested that patients failing to reach resuscitation goals were at increased risk for multiple organ failure. Other workers noted that patients who did not obtain supranormal oxygen delivery values were at high risk of developing organ failure regardless of treatment strategy.126,127 Obtaining hemodynamic and oxygen transport parameters seems to be more predictive of survival than useful as a goal for resuscitation, particularly if fluid administration is adequate.
In addition to conflicting outcomes in oxygen transport trials, technical concerns have been raised.116 These studies cannot be totally blinded. Patients in control groups often obtain similar physiologic end points to those in treatment groups. Other aspects of care were sometimes inconsistent, and entrance criteria varied among investigators. There also is potential mathematical coupling of oxygen delivery and consumption because both are calculated values that share many of the same measured variables.117 Some clinicians argue that the pathologic relationship between oxygen delivery and consumption trials cannot be accepted with confidence, unless oxygen consumption is measured directly. Finally, use of traditional oxygen delivery and consumption as resuscitative end points requires a pulmonary artery catheter and special expertise for operation and insertion. Routine use of pulmonary artery catheterization or central venous catheters has not been a part of acute trauma resuscitation or emergency medical management.69,116,117
Lactate
As an indicator of shock, blood lactate has proved accurate at assessing severity, predicting mortality risk, and assessing response to resuscitation in the hands of various workers.116,117 At the cellular level, the explanation is based on oxygen transport principles. With shock and inadequate oxygen delivery, mitochondrial respiration is impaired. The primary cellular fuel, pyruvate, is shunted from its normal aerobic path (conversion by pyruvate dehydrogenase to acetyl coenzyme A and subsequent entry into the tricarboxylic acid cycle) to the anaerobic pathway (conversion to lactate by lactate dehydrogenase). Anaerobic metabolism makes inefficient use of cellular substrate, and high-energy phosphate stores are rapidly depleted. During cellular ischemia, lactate is released into the bloodstream and ultimately converted to glucose in the liver and kidney via the Cori cycle. Because it directly reflects anaerobic metabolism, lactate is thought to serve as a mirror of global hyperperfusion because increasing lactate levels indicate increasing oxygen debt.117,128
Initial and peak lactate levels and duration of increased lactate concentration correlate with development of multiorgan dysfunction after trauma.116 In a study of trauma patient resuscitation, patients normalizing lactate levels at 24 hours survived, whereas patients who normalized lactate levels between 24 and 48 hours had a 25% mortality rate; patients who did not normalize by 48 hours had an 86% mortality rate.129 Theoretically, severity of metabolic acidosis secondary to tissue hyperperfusion should be reflected in lactate levels, anion gap, and base deficit. This is not a consistent finding among investigators studying trauma resuscitation.130,131 In addition, although lactate levels are rapidly available, conclusive data tying specific lactate levels and targets to improved resuscitation outcomes are unavailable.
Base Deficit
Inadequate oxygen delivery to tissues leads to anaerobic metabolism. The degree of anaerobiosis is proportional to the depth and severity of hemorrhagic shock, which should be reflected in lactate and base deficit. Arterial pH is not as useful because compensatory mechanisms attempt to normalize this parameter. Serum bicarbonate levels offer better correlation with base deficit (removal or addition of base in the blood).116,132,133
Similar to lactate, base deficit has been carefully studied.116 A greater base deficit has been associated with blood pressure reduction, increased blood loss, and transfusion requirements. A series of studies by Davis and coworkers link base deficit to resuscitation requirements and end-organ dysfunction, such as acute respiratory distress syndrome, renal failure, and coagulopathy. Cytokine and adhesion molecule changes also have been found to parallel changes in base deficit.134–140
Base deficit may vary with patient populations. Concern remains in older patients that base deficit is nonspecific and may reflect metabolic acidosis due to a variety of causes, including renal dysfunction and diabetes.116,117 Similar to temporal changes in lactate, base deficit variation over time may add to the value of this parameter.132 Patients with elevated base deficit also showed impaired oxygen use reflected in lower oxygen consumption. The timing of base deficit measurement also is important. One study suggested that the worst base deficit in the initial 24 hours was predictive of mortality rate along with blood pressure and estimated blood loss.138 Some workers debate whether alcohol intoxication may worsen base deficit for similar levels of injury severity and hemodynamics after trauma. In a large database survey, use of alcohol did not change significant predictive value of admission lactate and base deficit.141,142 Resuscitation with normal saline (hyperchloremic metabolic acidosis) or lactated Ringer’s solution (accumulation of D-lactate) may increase base deficit independent of injury severity. Acidosis associated with hyperchloremia is associated with lower mortality rate than that from other causes, particularly anaerobic metabolism.116,143 Base deficit levels and time to normalization of base deficit are similar to data for lactate in that correlation has been established with the need for resuscitation and risk of organ dysfunction and death after injury. Specific thresholds for outcome have not been determined, however, and there are no multicenter data that conclusively show that using base deficit as an end point for resuscitation improves survival.116
Gastric Mucosal pH
As systemic perfusion decreases, blood flow to vulnerable organs (brain and heart) is maintained at the expense of other organs (skin, muscle, kidneys, and intestines). Detection of subclinical ischemia to these organs may allow identification of patients requiring additional resuscitation despite normalized vital signs.116,144 Gastric tonometry is based on the finding that tissue ischemia leads to an increase in tissue partial pressure of carbon dioxide (PCO2) and subsequent decrease in tissue pH. Because CO2 diffuses readily across tissues and fluids, the PCO2 of gastric secretions rapidly equilibrates with that in gastric mucosa. For elevation in gastric pH values to be accurate, it is important to withhold gastric feedings and suppress gastric acid secretion. To perform gastric tonometry, a semipermeable balloon is attached to a special nasogastric tube and placed in the stomach. The balloon is filled with saline, and CO2 is allowed to diffuse into the balloon for a specific time. PCO2 in the saline is then measured. Continuous CO2 measuring electrodes are sometimes employed. Intramucosal pH is calculated from the Henderson-Hasselbalch equation. The difference between intragastric PCO2 and arterial PCO2, or the intramucosal pH, correlates with the degree of gastric ischemia.145
In studies of a small number of trauma patients, patients with low intramucosal pH (≤7.32) were more likely to develop complications or die.146–148 Patients with normal intramucosal pH fared well. Correlation to other parameters has not been rigorously studied. A larger trial examined the value of intramucosal pH and the gastric mucosal-arterial CO2 gap (difference between intragastric PCO2 and arterial PCO2). Ability to predict multiple organ dysfunction and death was maximized with intramucosal pH less than 7.25 and CO2 gap greater than 18 mm Hg. Similar to studies using blood lactate and base deficit, time course for changes in CO2 gap or intramucosal pH may be important. Ivatury and associates149,150 compared changes in intramucosal pH with oxygen transport values. Although intramucosal pH changes paralleled improvement in oxygen transport, delay in achieving intramucosal pH was more predictive of organ system failure than oxygen transport parameters. The gap between gastric mucosal and arterial PCO2 was similarly predictive. After resuscitation, changes in mucosal pH were an early predictor of complications.
Newer fiber-optic technologies increase the ease of gastric mucosal pH assessment.151 Although this parameter may be predictive of early resuscitation failure, accepted thresholds for failure and outcome data do not support widespread use to guide initial resuscitation after injury (Fig. 27.3).
Near-Infrared Spectroscopy
Measurement of skeletal muscle oxyhemoglobin levels by near-infrared spectroscopy offers a noninvasive measurement for evaluating adequacy of resuscitation from normalization of tissue oxygenation.116,117,145,152 This technology allows simultaneous measurement of tissue partial pressure of oxygen (PO2), PCO2, and pH. In human volunteers, cerebral cortex and calf oxygen saturation as measured by near-infrared spectroscopy decreased in proportion to blood loss. Oxygenation index (oxygenated hemoglobin—deoxygenated hemoglobin) also decreased. Studies in injury suggest correlation of tissue oxygen saturation with systemic oxygen delivery, base deficit, lactate, and gastric mucosal PCO2.153
This technology provides information regarding mitochondrial function. Normally, tissue oxyhemoglobin levels reflecting local oxygenation are tightly coupled to cytochrome function, reflecting mitochondrial oxygen consumption. In preliminary studies, when patients showed change in mitochondrial function, even in the absence of abnormality in systemic oxygen transport, multiple organ failure was more likely.154 Nonetheless, at this time, work in this area is preliminary, and a role for this technology in management of traumatic shock has not been defined.
Clinical Strategies
Clinical observations of shock in injury have been made for hundreds of years, but the optimal treatment continues to be debated.115 Early observations are attributed to Paré, Le Dran, Latta, and Gross.155,156 Crile and Henderson were among the first to attribute the hemodynamic instability of shock to decreased intravascular volume and to propose therapy based on restoration of intravascular volume with administration of intravenous fluid.115,156 During the First World War, physiologists Cannon and Bayliss observed patients in clinical shock.6 These observers noted that patients with crush injuries despite absence of obvious blood loss also developed signs and symptoms of shock.157,158 Cannon later suggested the concept of deliberate hypotension in the treatment of wounds to the torso during war with the intent of minimizing internal bleeding until the time at which operative intervention could control the hemorrhage.159,160 In later studies, other authors reported laboratory models of ongoing arterial hemorrhage and concluded that regardless of the means used to increase blood pressure, either fluid resuscitation or vasopressor, bleeding would increase, with subsequent death.161,162
Current guidelines for the treatment of hypotension secondary to hemorrhage after trauma recommend rapid infusion of crystalloid solutions to restore blood pressure.69,160 This premise is based in part on clinical studies and laboratory data showing that hemorrhagic shock in animals produced with controlled blood loss was reversible when blood loss was replaced with two to three times that volume of a crystalloid solution.163–165 Although controlled hemorrhage is a well-defined laboratory model, resuscitation of a patient with multiple injuries and active or uncontrolled bleeding may represent very different pathophysiology.115
In 1950, Wiggers166 developed a standard hemorrhagic shock model in dogs. He and others showed that severe hypotension over several hours produced a condition in which infusion of withdrawn blood restored arterial pressure only temporarily.115 After intervals ranging from 30 minutes to 3 hours, arterial pressure declined again. Additional infusions of blood were followed by progressively poorer recovery and more rapid development of circulatory failure, ultimately resulting in the demise of the animal. This decompensation point in shock, defining a time at which reinfusion of shed blood could not resuscitate the animal, led to the concept of irreversible shock.167 The approach to resuscitation of cellular, organ, and organism changes after hemorrhagic shock using the Wiggers model has been applied to all types of injury based in part on the elegant experiments of Shires and colleagues163 and a series of other investigators.69,70
Early Limited Resuscitation
Animal Studies
Several large animal studies explored the use of varying degrees of fluid resuscitation in animals receiving injuries leading to uncontrolled hemorrhagic shock. Bickell and coworkers168 created infrarenal aortotomy using a stainless steel wire in 16 anesthetized Yorkshire swine weighing 23 to 40 kg, which had been instrumented with pulmonary artery and carotid artery catheters. When the wire was pulled, a 5-mm aortotomy with subsequent intraperitoneal hemorrhage followed. Animals were alternately assigned to an untreated control group or a treatment group receiving 80 mL/kg of lactated Ringer’s solution as an intravenous bolus. The volume of blood loss and mortality rate were significantly increased in animals treated with lactated Ringer’s solution relative to the untreated control group. All control animals survived, whereas animals treated with lactated Ringer’s solution died in less than 2 hours. Volume of hemorrhage identified in treated animals exceeded 2 L, whereas control animals lost on average less than 800 mL of blood.
Several observations may be made in relation to this widely cited report. First, mortality rate in the control group was low, leading one to question the severity of injury in the animal model. Second, fluid resuscitation administered, although consistent with replacement of two to three times the volume loss in blood with crystalloid, far exceeds standard resuscitation for a human patient of comparable weight. In addition, the rapidity of fluid administration may have served to diminish further any potential positive impact of fluid administration in this model of injury. The effect seen was reproduced, however, with other types of fluid administration in a comparable injury model. Other large animal studies of hypotensive resuscitation used graded resuscitation protocols.169,170
Stern and coworkers169 examined a swine model combining femoral artery hemorrhage via a catheter to a mean arterial pressure of 30 mm Hg with subsequent intra-abdominal aortic laceration producing a 4-mm tear and uncontrolled intraperitoneal hemorrhage. Three groups of animals were resuscitated to mean arterial pressures of 40 mm Hg, 60 mm Hg, and 80 mm Hg. No untreated control group was employed. Resuscitation was begun when the pulse pressure of each animal reached 5 mm Hg. Animals were resuscitated with saline at 6 mL/kg/minute to a maximum of 90 mL/kg, after which resuscitation fluid was changed to shed blood at 2 mL/kg/minute to a maximal volume of 24 mL/kg. Animals were observed for 60 minutes or until death. As noted previously, mortality rate was significantly higher in animals receiving the most aggressive resuscitation compared with less aggressively treated groups. Animals resuscitated most aggressively had higher volumes of intraperitoneal hemorrhage than the two other experimental groups. In addition, oxygen delivery, which was monitored in these animals, was significantly greater in the group resuscitated to a mean arterial pressure of 60 mm Hg than in the two other experimental groups. Similar observations were made in a second report from this same group in a study by Kowalenko and colleagues.170
Clinical and preclinical studies focused on early limitation of crystalloid resuscitation and hemorrhagic shock focus on penetrating torso trauma but do not address initial care of patients with head injury, the leading cause of traumatic death in the United States. Historically, when shock accompanies head injury, the incidence of adverse outcome doubles. Because of the vulnerability of the injured brain to even brief periods of reduced perfusion, guidelines for the management of head injury state that delayed resuscitation cannot be considered applicable in head trauma.118 Nonetheless, in a large animal model using a standard cerebral injury along with uncontrolled hemorrhage secondary to aortotomy, there was no evidence of increased secondary cerebral ischemia with delayed resuscitation. Conventional resuscitation with lactated Ringer’s solution resulted in signs of increased secondary brain injury.171
Clinical Studies
Martin and coworkers172 provided preliminary data in patients on the effect of aggressive versus delayed prehospital resuscitation of uncontrolled hemorrhagic shock after penetrating injury. These workers evaluated the effect of delaying fluid resuscitation until surgical intervention could control the source of hemorrhage on outcome of hypotensive trauma victims. Injury severity was similar in standard resuscitation and delayed resuscitation groups. The rate of survival to hospital discharge was 69% in the delayed resuscitation group and 56% in the standard resuscitation group. The difference between these groups did not reach statistical significance owing to small sample size.
Much attention has been directed to resuscitation of patients after injury after a report from Bickell and associates173 that appeared in the New England Journal of Medicine. The authors reported a prospective clinical trial of adults with penetrating truncal trauma who were hypotensive in the field as indicated by a systolic blood pressure less than 90 mm Hg. Patients were randomly assigned to placement of intravascular catheters with standard prehospital and trauma center fluid resuscitation using lactated Ringer’s solution or an experimental group in which vascular catheters were placed but intravenous fluids were not administered until patients reached the operating room. Patients were excluded from this trial if they were noted to have a field revised trauma score of zero consistent with cardiopulmonary arrest or had sustained fatal gunshot wounds to the head with neurologic injury that precluded long-term survival.28 In addition, patients with penetrating truncal injury who did not require operation were excluded. After 1069 patients were screened during the 37 months of this study, 598 patients were enrolled—309 in an immediate resuscitation group receiving standard fluids according to Advanced Trauma Life Support protocols and 289 in a delayed resuscitation group, which did not receive intravenous fluids until reaching the operating room.69
The immediate and delayed resuscitation groups were well matched with respect to age, gender, and anatomic injury as measured by the ISS, Revised Trauma Score (physiologic response to injury), and systolic blood pressure.174,175 Field response times for prehospital providers in this trial were short, averaging 30 minutes or less. The trauma center interval (i.e., the interval in the hospital before operation) was surprisingly long—44 minutes in the immediate resuscitation group and 52 minutes on average in the group receiving delayed resuscitation. Prehospital fluid administration averaged less than 900 mL in the immediate resuscitation group versus less than 100 mL in the delayed resuscitation cohort. Fluid administration in the trauma center before operation averaged greater than 1600 mL of fluid in the immediate resuscitation group, whereas the delayed resuscitation patients averaged 283 mL of fluid received. Operative blood loss between the study groups was not different. Among the 289 patients who received delayed fluid resuscitation, 203 (70%) survived and were discharged from the hospital. Of the 309 patients who received immediate fluid resuscitation, 193 (62%) survived (P = 0.04). Patients in the delayed resuscitation group displayed a trend toward reduced postoperative complications, including acute respiratory distress syndrome, sepsis syndrome, acute renal failure, coagulopathy, wound infection, and pneumonia, compared with patients in the immediate resuscitation group (P = 0.08).
A subgroup analysis from this study was reported at a subsequent meeting of the American Association for the Surgery of Trauma. When Wall and coworkers176 examined major subgroups in the patient population reported by Bickell and colleagues, a statistical difference in hospital survival could be shown only in patients who had sustained penetrating cardiac injury.115 Patients with major vascular injury, solid organ injury requiring operation, or noncardiac thoracic injury had comparable survival in the immediate and delayed resuscitation groups.
Although these early clinical studies represent a remarkable accomplishment in design, organization, and data analysis, many questions remain unanswered. None of the studies reported was blinded, and a randomization scheme was not employed. In the trial of Bickell and colleagues, in which the difference in mortality rate rested in a difference in survival of a small number of patients in the experimental groups, 22 patients in the delayed resuscitation group were given intravenous fluids in violation of study design.173 Although these individuals were appropriately included in an intent-to-treat analysis, the impact of selected fluid administration on study outcome is unclear. The authors also have been criticized for excluding patients after randomization because of injuries considered too minor (no operative therapy) or too severe (revised trauma score of zero). Exclusion of these patients may invalidate the statistical approach employed and increase the difficulty of the clinician seeking guidance from this work. Finally, time spent in the trauma center by these hypotensive patients with injuries requiring operation was surprisingly long. Although the resuscitation groups described differed statistically in vital signs and hematologic parameters, it is unclear whether the differences observed had clinical significance.
We await additional data on the military approach to resuscitation, which requires innovation and effectiveness in austere environments.177 Contemporary recommendations include limitation of fluid administration unless systolic blood pressure is less than 80 to 85 mm Hg or is rapidly falling. Another clinical indicator for fluid resuscitation is decreasing mentation without evidence of head injury. Key assessment parameters are mental status and the presence of a radial pulse. In many settings, no fluids are administered in the presence of a strong radial pulse and normal mentation. Pulse deterioration or decreasing level of consciousness are indicators for intervention. When fluids are given, a number of small-volume colloids with high tonicity or colloids in combination with hypertonic saline are being investigated. Even early hospital resuscitation is designed to emphasize the use of blood products and minimize crystalloids and nonblood colloids in the setting of major injury.
Clinical Pathway—Early Resuscitation
In all of the preclinical and clinical work described, the mechanism of injury and survival remains unclear. Among considerations are the impact of fluid resuscitation on early clot formation in the setting of uncontrolled hemorrhage.115,169 Other workers suggest that rapidity in resuscitation of pulse pressure may relate to mechanical disruption of initial thrombus.169 Fluid resuscitation may contribute to dilution of clotting factors in the setting of exaggerated bleeding in uncontrolled hemorrhage.115,160 The data to support these observations are limited. Coagulopathy proportional to volume of injured tissue and severity of shock may be seen even before resuscitation fluids are provided.11
Despite provocative preclinical and clinical data, there is insufficient evidence to propose practice guidelines or make recommendations. “Uncontrolled” hemorrhage itself remains undefined. This problem is best seen as injury with blood loss occurring in the absence of surgical or mechanical hemostasis or the “control” provided by regulated blood removal through a vascular cannula. It is unclear whether a vascular injury after a torso gunshot wound and a shattered spleen after an automobile crash are different in this regard. The bottom-line message from all of the studies is that elevation of the blood pressure to normal or supranormal levels results in resumption of bleeding from the uncontrolled site, and rebleeding leads to recurrent shock and death of the experimental animal. Other work shows that animals subjected to shock could be successfully resuscitated at lower than “normal” mean arterial pressures if the bleeding site was controlled as part of the resuscitation program. Shock victims resuscitated with electrolyte solutions are subject to progressive hemodilution, and this may lead to death. The lessons that clinicians should learn from this body of data are as follows.69,115,145,11
1. Operation to control bleeding is part of resuscitation.
2. Blood pressure levels are convenient but possibly misleading end points for shock resuscitation in that resuscitation to normal or supranormal pressures may be harmful if the effort delays operation to control bleeding or the pressure elevation causes rebleeding. Better end points (e.g., tissue oxygenation or other metabolic parameters) are needed.
3. Blood loss is increased in the setting of significant soft tissue insults combined with shock. Early administration of blood products should stimulate use of a balanced administration strategy with PRBCs, FFP, and platelets given in equal proportions.
4. Resuscitation of traumatic shock, similar to fluid management of a burned patient, requires repeated observation, judgment, and skill and cannot be accomplished by recipe or formula.
Management of Traumatic Shock in the Intensive Care Unit
Before admission to the ICU, resuscitation is directed at maintaining blood pressure and reducing heart rate through volume loading with crystalloid and blood products. Relatively simple clinical end points are employed.69 This approach should be adequate for 95% of injured patients. On admission to the ICU, severely injured patients may receive a central venous catheter or a pulmonary artery catheter to monitor hemodynamics and refine further the direction of resuscitation.178 A series of early reports by Shoemaker and coworkers proposed that supranormal oxygen delivery (600 mL/minute/m2) and resuscitation to a plateau oxygen consumption were appropriate clinical end points. Although observations of improved hemodynamic response in survivors of injury make intuitive sense, driving injured patients to supranormal hemodynamic performance was not associated with improvement in clinical outcome.116,122–125 Reduced goals for oxygen delivery (500 mL/minute/m2) are proposed among end points for support of patients receiving pulmonary artery catheter monitoring.179–181
In a series of studies characterizing resuscitation of injured patients in the ICU, Moore and coworkers used the pulmonary artery catheter to describe response to fluid administration. Criteria identifying patients considered for placement of a pulmonary artery catheter and need for ICU resuscitation include major injury (two or more abdominal organs, two or more long bone fractures, complex pelvic fractures, flail chest, or major vascular injury), blood loss (anticipated need for >6 units PRBC during the first 12 hours after hospitalization), and metabolic stress (arterial base deficit > 6 mEq/L during the first 12 hours after hospital admission). A trauma victim older than 65 years with any two of the previous criteria also warrants consideration for pulmonary artery catheter insertion and ICU resuscitation. Patients with these criteria who also incurred severe brain injury, defined as Glasgow Coma Scale score less than or equal to 8 in the trauma ICU and abnormality on brain computed tomography scan, were not resuscitated by protocol during development of this approach unless assessed by the attending neurosurgeon to be at low risk of secondary brain injury with these procedures.180,181 In my practice, I find that the brain, similar to other organs, benefits from aggressive resuscitation (Table 27.3).
Table 27.3
Summary of Protocol for Resuscitation of Shock Resulting from Major Torso Trauma*
| Intervention | Threshold | Method |
| Transfuse (PRBC) | DO2I <500 mL/min/m2; hemoglobin <10 g/dL (age ≥65 years, <12 g/dL) | 1 g hemoglobin/dL/unit PRBC; bolus transfusion; then hemoglobin analysis (bedside); then calculate DO2I |
| Volume load (LR) | DO2I <500 mL/min/m2; hemoglobin ≥10 g/dL (age ≥65 years, ≥12 g/dL); PCWP <15 mm Hg (age ≥65 years, <12 mm Hg) | 1-L LR bolus infusion (age ≥65 years, 0.5 L); then measure PCWP; then calculate DO2I |
| Starling curve (NS) | DO2I <500 mL/min/m2; hemoglobin ≥10 g/dL (age ≥65 years, ≥12 g/dL); PCWP ≥15 mm Hg (age ≥65 years, ≥12 mm Hg) | 0.5- or 0.25-L NS bolus infusion; then measure PCWP and CI: CI-PCWP optimal if ΔCI ≤−0.3; ΔPCWP ≤+4 with two consecutive boluses; then calculate DO2I |
| Inotrope | DO2I <500 mL/min/m2; hemoglobin 10 g/dL (age ≥65 years, ≥12 g/dL); CI and PCWP optimized | Milrinone, 0.1-µg increments to 0.8 µg/kg/min, or dobutamine, 2.5-µg increments to 20 µg/kg/min; calculate DO2I |
| Vasopressor | DO2I <500 mL/min/m2; MAP <65 mm Hg | Norepinephrine, 0.05-µg increments to 0.2 µg/kg/min; measure MAP; calculate DO2I |
*Details of the resuscitation protocol used by McKinley and coworkers are given. Selected drugs for inotropic and vasopressor support are listed. Patients also are treated to age-appropriate hemoglobin levels and given fluid infusion based on a volume loading protocol until filling pressures and DO2I are optimized.
Modified from McKinley BA, Kozar RA, Cocanour CS, et al: Normal versus supranormal oxygen delivery goals in shock resuscitation: The response is the same. J Trauma 2002;53:825-832.
A sequential approach to shock resuscitation using a pulmonary artery catheter is advocated by Moore with McKinley and coworkers.178,179 This approach includes a series of interventions including administration of PRBC and lactated Ringer’s solution to optimize cardiac index and pulmonary capillary wedge pressure as described in a classic Starling curve. Milrinone, dobutamine, and norepinephrine are used as vasoactive agents as necessary to provide mean arterial pressure greater than 65 mm Hg and oxygen delivery index greater than 500 mL/minute/m2. These patients require large volumes of protocol-directed shock resuscitation (approximately 15 L for oxygen delivery index >500 mL/minute/m2). Significant urine output volumes also should be expected. This large net positive balance suggests unrecognized ongoing blood loss or extreme fluid shifts between intravascular, interstitial, and intracellular compartments, or both, for severely injured patients (Fig. 27.4).
The protocol-driven approach described has provided a variety of observations.182 First, even elderly patients respond to ICU resuscitation after injury.180 In general, the maximal oxygen delivery response is less than that of younger patients, and elderly patients have a greater requirement for inotropic support.183 Second, a Starling curve generation approach is feasible and reliably improves hemodynamic resuscitation from major trauma. Supranormal resuscitation is neither necessary nor desirable in the management of patients with trauma associated with shock.178 Third, aggressive resuscitation, particularly in the setting of ongoing bleeding, increases the risk of elevated intra-abdominal pressure and abdominal compartment syndrome.184 Preload-driven resuscitation may cause bowel edema with subsequent venous obstruction, declining cardiac output, decreased urinary output, and compromise of systemic oxygenation. Finally, although many end points for interventions for goal-directed resuscitation in critical injury exist, systemic oxygen transport is the current state of the art in the most severely injured patients and is the basis for future development of clinical processes for resuscitation of shock caused by major trauma (Fig. 27.5).182
The utility of the pulmonary artery catheter in the management of patients with severe injury is suggested by a study using data obtained in the National Trauma Data Bank.185 From more than 450,000 records, 53,000 patients were reviewed. These patients were admitted between January 1994 and December 2001. Patients survived more than 48 hours and underwent at least one diagnostic or therapeutic procedure. The patients were 16 to 90 years old and distinguished by ISS and initial base deficit. Approximately 2000 patients who had insertion of a pulmonary artery catheter during hospitalization were compared with 51,000 patients who did not. Logistic regression analysis was used to develop a model that examined mortality rate after injury. Factors included in the model were use of a pulmonary artery catheter, age, emergency department base deficit, ISS, comorbid conditions, mechanism of injury, and specific injury patterns as identified by the Abbreviated Injury Scale. Overall, patients managed with a pulmonary artery catheter were older and had a higher ISS, greater emergency department base deficit, and higher mortality rates (29.7% with pulmonary artery catheter versus 9.8% without pulmonary artery catheter). Patients with spine, abdominal, chest, or head injury and patients with at least one Abbreviated Injury Scale score equal to or greater than 3 were more likely to be managed with a pulmonary artery catheter.
Although these observations come from a large database, retrospective study design and subgroup analysis are not optimal for definitive hypothesis testing. Finally, neither timing of placement for pulmonary artery catheters nor cause of death and specific relationship to placement of the pulmonary artery catheter could be conclusively examined by analysis of the National Trauma Data Bank. Nonetheless, these data suggest that injured patients may derive benefit from pulmonary artery catheter-guided resuscitation to avert complications related to persistent perfusion deficits. Further focused examination of patients with risk factors for poor outcome is warranted.185
Massive Transfusion
Independent of mechanism of injury, hemorrhagic shock consistently is the second leading cause of early death among injured patients, with only CNS injury consistently more lethal.186 Primary CNS injury is devastating and has a high rate of prehospital mortality; prevention is the best strategy.187 Hemorrhagic shock accounts for 30% to 40% of trauma deaths and is more amenable to interventions to reduce mortality and morbidity rates.186 In addition, approximately 25% of CNS injuries are complicated by hemorrhagic shock.188,189 Hemorrhage contributes to death during the prehospital period in 33% to 56% of cases, and exsanguination is the most common cause of death among individuals found dead on arrival of emergency medical services personnel.47 Hemorrhage accounts for the largest proportion of mortality rates occurring within the first hour of trauma center care and greater than 80% of operating room deaths after major trauma.186,190 Although the need for massive transfusion (defined as administration of ≥10 units of PRBC in <24 hours) is probably necessary in only 3% of patients in busy trauma centers, this intervention can be lifesaving, and preliminary data suggest that early aggressive administration of blood products reduces morbidity and mortality rate and decreases overall product use.191
Numerous general observations can be made.191–193 Most patients receiving massive transfusion are treated initially with crystalloid fluids followed by non-cross-matched type O red blood cells. Plasma therapy is typically delayed while waiting for blood typing and plasma to thaw. Platelets frequently are not given until patients have multiple units of PRBCs. Coagulopathy is common and difficult to correct. Plasma and platelets are inadequately used and greater emphasis is needed on plasma and platelet administration.
A typical massive transfusion protocol begins in the emergency department when the senior trauma practitioner orders transfusion of O-negative PRBC and invokes an organization-specific massive transfusion protocol.193,194 This is followed by administration of 4 to 6 additional typed or O-negative units of PRBC and a similar number of units of FFP and platelets. Therapy continues with containers sent from the blood bank, each containing red blood cells, plasma, and platelets. Goals are normalization of the PT and elevation of the platelet count to 50 to 100 × 109/L. The fibrinogen level is checked after 6 to 12 units of PRBC, and cryoprecipitate is given if the fibrinogen level is less than 1 g/L. This triggers administration of 10 units of cryoprecipitate. Once a common component of massive transfusion strategies, recombinant activated factor VII has been deemphasized in both military and civilian practice.195–198
A number of scoring systems have been developed to rapidly predict the patient requiring massive transfusion.199–201 Of these scores, the ABC (assessment of blood consumption) score, which incorporates penetrating mechanism of injury, positive ultrasound examination of the abdomen in the emergency department, arrival systolic blood pressure of 90 mm Hg or less, and arrival heart rate of 120 beats/minute or more, has been reported and validated in large patient sets. Although multiple more sophisticated criteria have been proposed, having an ABC score of two criteria or greater correctly classified the patient requiring massive transfusion in up to 85% of cases. Other investigators point out that hypotension and evidence of coagulopathy are the strongest predictors of massive transfusion. Additional data from military and civilian practice is awaited to bring further clarity to appropriate transfusion triggers.
Tranexamic Acid
Tranexamic acid is a derivative of amino acid lysine that inhibits fibrinolysis by blocking binding sites on plasminogen. This agent has been used in a variety of surgical trials and has been demonstrated to reduce blood transfusion requirement. The massive CRASH-2 study, incorporating over 20,000 patients, evaluates tranexamic acid as a means to address fibrinolysis occurring as a component of coagulopathy after trauma.202,203 CRASH-2 was conducted in over 250 hospitals and 40 countries. Over 20,000 patients with significant bleeding or at risk for significant bleeding were assigned within 8 hours of injury to either tranexamic acid or matching placebo. All-cause mortality rate was significantly reduced with tranexamic acid. Specific risk of death due to bleeding was also significantly reduced. This remarkable outcome was accomplished without a significant increase in thrombotic events. Subsequent analysis of the CRASH-2 data, however, reveals that greatest efficacy came when treatment was initiated within 3 hours after injury. In fact, treatment after 3 hours seemed to increase the risk of death due to bleeding. A recent review of military experience with tranexamic acid, the MATTERs trial, also demonstrates improved outcomes with early administration of this inexpensive drug shortly after injury. This military experience with tranexamic acid suggests improvement in outcome in patients receiving as little as 1 unit of PRBCs. Incremental risk with administratin of tranexamic acid was not demonstrated.204
The role for tranexamic acid in trauma systems where massive transfusion protocols incorporate FFP containing all indigenous antifibrinolytic elements in plasma remains unclear. In developed trauma systems, the best place for tranexamic acid may actually be in the prehospital environment as this material can readily be maintained in helicopter and road transport programs. Prehospital administration of blood products, especially plasma, is uncommon in civilian settings; thus, tranexamic acid offers an early opportunity to manage coagulopathy.205 Opportunities for use of this promising material in the critical care setting are being defined.
Risks of Early Red Blood Cell Transfusion
Blood transfusion in trauma has been identified as an independent predictor of multiple organ failure, systemic inflammatory response syndrome, increased postinjury infection, and increased mortality rate in multiple studies.206 Cumulative risks have been related to the number of units of PRBC transfused, increased storage time of transfused blood, and possibly the presence of leukocytes in donor blood. Many authors have concluded that blood transfusion in an injured patient should be minimized whenever possible.207
Large single-institution data sets examined the impact of blood transfusion in postinjury multiple organ failure.136,208,209 Variables identified as early independent predictors of multiple organ failure included age older than 55 years, ISS equal to or greater than 25, and greater than 6 units of PRBC in the first 12 hours after admission. Base deficit greater than 8 mEq/L in the first 12 hours and lactate greater than 2.5 mol/L also were independent predictors of multiple organ failure. Subsequent prospective work confirmed the importance of blood transfusion as an independent risk factor for postinjury multiple organ failure after controlling for other indices of shock, including base deficit and lactate. Additional studies of blood product use after injury associate blood transfusion with increased mortality rate. Potential confounding shock variables, including base deficit, serum lactate, age, gender, race, Glasgow Coma Scale score, and ISS, were controlled in this analysis.
Factors contributing to complications associated with red blood cell transfusion include storage time, increased endothelial adherence of stored red blood cells, nitric oxide binding by free hemoglobin in stored blood, donor leukocytes, host inflammatory response, and reduced red blood cell deformability.205,210 Nonetheless, transfusion of an injured patient with balanced blood component therapy is the only option for treatment of severe hemorrhagic shock. Although other hemoglobin-based oxygen carriers hold great promise and ultimately may provide better outcomes for injured patients, these materials have not come to be used. In an effort to minimize adverse events, attempts to minimize the use of blood transfusion in injury are appropriate outside major hemorrhage.
Special Problems
Abdominal Compartment Syndrome
A compartment syndrome is a condition in which increased pressure within a confined anatomic space adversely affects function and viability of tissues contained within. Confined anatomic spaces associated with compartment syndromes are fascial spaces of the extremities, the globe as in glaucoma, and the cranial cavity as in epidural or subdural hematoma. Abdominal compartment syndrome is a condition in which sustained pressure within the abdominal wall, pelvis, diaphragm, and retroperitoneum adversely affects the function of the gastrointestinal tract and related extraperitoneal organs. Abdominal compartment syndrome is receiving increasing recognition as a complication of massive resuscitation after trauma, burns, or other surgical procedures (Box 27.3). Operative decompression is frequently required. Pressures around 5 to 7 mm Hg in the peritoneal cavity are normal. Short-duration pressure increases frequently occur with coughing, Valsalva maneuvers, defecation, and weightlifting. Intra-abdominal pressure can be nonpathologically increased in obese individuals. Elevated intra-abdominal pressure is a common finding among critically ill medical and surgical patients.211–213
A more recent consensus conference on abdominal compartment syndrome has created improved definitions in relation to abdominal compartment syndrome (Table 27.4). For standardization, intra-abdominal pressure should be expressed in mm Hg and measured at end expiration with the patient supine after ensuring that abdominal muscle contractions are absent. The transducer is zeroed at the midaxillary line. The current reference standard for intra-abdominal pressure measurement is pressure measured via an indwelling urinary drainage catheter within the bladder. The recommended technique for measuring intra-abdominal pressure is to clamp the urinary catheter and instill a maximal volume of 25 mL of sterile, room-temperature saline into the bladder with the patient in the supine position. After zeroing a transducer and a stabilization period of at least 30 to 60 seconds, the mean intra-abdominal pressure can be read on a bedside monitor or as the height of the fluid column in urinary drainage tubing.
Table 27.4
| Definition 1 | IAP is the steady-state pressure concealed within the abdominal cavity. |
| Definition 2 | APP = MAP – IAP. |
| Definition 3 | FG = GFP – PTP = MAP – 2 × IAP. |
| Definition 4 | IAP should be expressed in mm Hg and measured at end expiration in the complete supine position after ensuring that abdominal muscle contractions are absent and with the transducer zeroed at the level of the midaxillary line. |
| Definition 5 | The reference standard for intermittent IAP measurement is via the bladder with a maximal instillation volume of 25 mL sterile saline. |
| Definition 6 | Normal IAP is approximately 5-7 mm Hg in critically ill adults. |
| Definition 7 | IAH is defined by a sustained or repeated pathologic elevation in IAP ≥12 mm Hg. |
| Definition 8 | IAH is graded as follows: grade I, IAH 12-15 mm Hg; grade II, IAP 16-20 mm Hg; grade III, IAP 21-25 mm Hg; grade IV, IAP > 25 mm Hg. |
| Definition 9 | ACS is defined as a sustained IAP >20 mm Hg (with or without an APP <60 mm Hg) that is associated with new organ dysfunction/failure. |
| Definition 10 | Primary ACS is a condition associated with injury or disease in the abdominopelvic region that frequently requires early surgical or interventional radiologic intervention. |
| Definition 11 | Secondary ACS refers to conditions that do not originate from the abdominopelvic region. |
| Definition 12 | Recurrent ACS refers to the condition in which ACS redevelops after previous surgical or medical treatment of primary or secondary ACS. |
From Malbrain ML, Cheatham ML, Kirkpatrick A, et al: Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome, I: Definitions. Intensive Care Med 2006;32:1722-1732.
Intra-abdominal hypertension is defined by a sustained or repeated intra-abdominal pressure greater than 12 mm Hg or an abdominal perfusion pressure less than 60 mm Hg, where abdominal perfusion pressure = mean arterial pressure − intra-abdominal pressure. Abdominal compartment syndrome is present when organ dysfunction occurs as a result of intra-abdominal hypertension. Abdominal compartment syndrome is further defined by sustained or repeated intra-abdominal pressure greater than 20 mm Hg or abdominal perfusion pressure less than 60 mm Hg in association with new-onset single or multiple organ system failure. In contrast to intra-abdominal hypertension, abdominal compartment syndrome is not graded but rather considered as an “all or none” phenomenon.213,214
Intra-abdominal hypertension has a variety of physiologic effects. In experimental preparations, animals die as a result of congestive heart failure as abdominal pressure passes a critical threshold. Increased intra-abdominal pressure decreases cardiac output and left and right ventricular stroke work, while increasing central venous pressure, pulmonary artery wedge pressure, and systemic and pulmonary vascular resistance. Abdominal decompression reverses these changes. As both hemidiaphragms are displaced upward with increased intra-abdominal pressure, decreased thoracic volume and compliance are seen. Decreased volume within the pleural cavity causes atelectasis and decreases alveolar clearance. Pulmonary infections also may result. Ventilated patients with abdominal hypertension require increased airway pressure to deliver a fixed tidal volume. As the diaphragm protrudes into the pleural cavity, intrathoracic pressure increases with reduction in cardiac output and increased pulmonary vascular resistance. Ventilation and perfusion abnormalities result, and blood gas measurements show hypoxemia, hypercarbia, and acidosis.215
Elevation in intra-abdominal pressure also causes renal dysfunction. Inadequate renal perfusion pressure and renal filtration gradient have been proposed as critical factors in the development of renal insufficiency associated with elevated intra-abdominal pressure. The filtration gradient is the mechanical force across the glomerulus and equals the difference between glomerular filtration pressure and proximal tubular pressure. In the presence of intra-abdominal hypertension, proximal tubular pressure may be assumed to equal intra-abdominal pressure. Glomerular filtration pressure may be estimated as mean arterial pressure minus intra-abdominal pressure. Changes in intra-abdominal pressure may have a greater impact on renal function and urine production than changes in mean arterial pressure. Oliguria is thought to be one of the first signs of intra-abdominal hypertension. Control of intra-abdominal pressure leads to reversal of renal impairment. Oliguria may be seen with intra-abdominal pressure of 15 to 20 mm Hg. Deterioration in cardiac output plays a role in diminished renal perfusion, but even with maintenance of cardiac output, impairment of renal function persists in intra-abdominal hypertension.211,216–218
Other organs affected by increased intra-abdominal pressure include the liver, where hepatic blood flow has been shown to decrease with abdominal hypertension.219,220 It may be assumed that hepatic synthesis of acute-phase proteins, immunoglobulins, and other factors of host defense may be impaired by reduced hepatic blood flow. Other gastrointestinal functions may be compromised by increased intra-abdominal pressure. Splanchnic hypoperfusion may begin with an intra-abdominal pressure of 15 mm Hg. Reduced perfusion may create changes in mucosal pH, translocation, bowel motility, and production of gastrointestinal hormones. Finally, intracranial hypertension is seen with chronically increased intra-abdominal pressure. Intracranial hypertension has been shown to decrease when intra-abdominal pressure is reduced in morbidly obese patients and in intracranial injury.
In a recent report, Cheatham and Safcsak review their experience with percutaneous, ultrasound-guided drainage of ascitic fluid contributing to abdominal hypertension and abdominal compartment syndrome.221,222 These investigators suggest that in patients with significant fluid accumulation creating abdominal hypertension, percutaneous drainage can avoid the need for decompressive laparotomy with its associated morbidity and occasional complications. Additional reports from other centers are required. To date, apart from the large experience described previously, percutaneous control of fluid accumulation to manage abdominal hypertension and compartment syndrome is limited to case reports.
Extremity Compartment Syndrome
The numerous causes of extremity compartment syndrome include complications of open and closed fractures, arterial injury, temporary vascular occlusion, snakebite, drug abuse, burns, physical exertion, and gunshot wounds. The most common cause of compartment syndrome is muscle injury leading to edema, which is correlated to the amount of tissue damage. Pressure is increased within the closed fascial space first by intracellular swelling followed by hematoma formation if a fracture is present. Because extremities, particularly at the calf, are composed of relatively unyielding fascial compartments, circulatory compromise occurs as tissue pressure increases with resulting ischemia and tissue damage. Leakage of intracellular fluid follows, and a further increase in intracompartmental pressure is seen.223
When extremity injuries produce complete ischemia, skeletal muscle that is deprived of oxygen may survive for 4 hours without irreversible damage. Total ischemia of 8 hours’ duration produces irreversible change. Peripheral nerves conduct for 1 hour after onset of total ischemia and can survive for 4 hours with only neurapraxic damage. After 8 hours, axonotmesis and irreversible damage occur. Ischemia caused by reduction or cessation of blood flow occurs when the perfusion gradient to a muscle compartment falls below a critical level. Perfusion is related to the compartment pressure. When intracompartmental blood pressure is 25 mm Hg, tissue perfusion in injured tissues is substantially decreased.223–225
Fasciotomy should be performed when intracompartmental pressure approaches 25 mm Hg, or if an extremity has been completely ischemic for 6 hours, the patient’s clinical condition is worsening, substantial tissue injury is present, or tissue pressure is increasing.225 Prophylactic treatment is valuable because fasciotomy does not reverse changes caused by initial extremity injury but can prevent changes resulting from secondary ischemic insults.
Pelvic Fractures
Substantial blunt force is required to disrupt the pelvic ring. The extent of injury is related to the direction and magnitude of force applied. Associated abdominal, thoracic, and head injuries are common. Force applied to the pelvis can cause rotational displacement with opening or compression of the pelvic ring. Other types of displacement seen with pelvic fractures are vertical with complete disruption of the ring and the posterior sacroiliac complex.226
Patients with pelvic ring injuries are easily divided into two groups on the basis of clinical presentation—patients who are hemodynamically stable and patients who are hemodynamically unstable.226 There is a dramatic difference in mortality rates between pelvic fracture patients who are hypotensive and patients who are hemodynamically stable. Hemodynamic stability and biomechanical pelvic instability are separate though related issues, which tends to confuse the clinical picture. The source of bleeding may be multifactorial and not directly related to the pelvic fracture itself. Blood loss secondary to pelvic fracture that contributes to hemodynamic instability is a significant risk factor, however. Early fracture diagnosis and stabilization using external skeletal fixation are crucial in the acute phase of patient management.227 Treatment of the patient also is directed by response to initial fluid resuscitation. Retroperitoneal bleeding in a pelvic fracture usually arises from a low-pressure source—the cancellous bone at the fracture site or adjacent venous injury. Significant retroperitoneal arterial bleeding occurs in approximately 10% of patients. Clinical evidence has suggested that provisional fracture stabilization using external fixation devices or even wrapping the fractured pelvis in a bed sheet can control low-pressure venous bleeding. Continued, unexplained bleeding after provisional fracture stabilization suggests an arterial source. Angiography with embolization of the involved vessel is indicated. Therapeutic angiography also may be required after abdominal exploration if a rapidly expanding or pulsatile retroperitoneal hematoma is encountered.228
References
1. Blalock, A. Principles of Surgical Care, Shock, and Other Problems. St. Louis: Mosby; 1940.
2. Peitzman, AB, Billiar, TR, Harbrecht, BG, et al. Hemorrhagic shock. Curr Probl Surg. 1995; 32:925–1002.
3. Spodick, DH. Acute cardiac tamponade. N Engl J Med. 2003; 349:684–690.
4. Moore, FA, Moore, EE. Initial management of life-threatening trauma. In: Souba WW, Fink MP, Jurkovich GJ, et al, eds. ACS Surgery: Principles and Practices 2006. New York: WebMD Professional Publishing; 2006:1125–1144.
5. Goris, RJ. Pathophysiology of shock in trauma. Eur J Surg. 2000; 166:100–111.
6. Horovitz, JH, Carrico, CJ, Shires, GT. Pulmonary response to major injury. Arch Surg. 1974; 108:349–355.
7. Ayala, A, Wang, P, Ba, ZF, et al. Differential alterations in plasma IL-6 and TNF levels after trauma and hemorrhage. Am J Physiol. 1991; 260:R167–R171.
8. Bitterman, H, Kinarty, A, Lazarovich, H, et al. Acute release of cytokines is proportional to tissue injury induced by surgical trauma and shock in rats. J Clin Immunol. 1991; 11:184–192.
9. Huynh, T, Currin, RT, Tanaka, Y, et al. Activation of Kupffer cells in vivo following femur fracture. Arch Surg. 1994; 129:1324–1329.
10. Rady, MY, Kirkman, E, Cranley, J, et al. A comparison of the effects of skeletal muscle injury and somatic afferent nerve stimulation on the response to hemorrhage in anesthetized pigs. J Trauma. 1993; 35:756–761.
11. Dries, DJ. The contemporary role of blood products and components used in trauma resuscitation. Scand J Trauma Resuscitation Emerg Med. 2010; 18:63.
12. Gann, DS, Cross, JS. The neuroendocrine response to critical illness. In: Barrie PS, Shires GT, eds. Surgical Intensive Care. Boston: Little Brown; 1993:93–134.
13. Jones, MT, Gillman, B. Factors involved in the regulation of adrenocorticotropic hormone/betalipotropic hormone. Physiol Rev. 1988; 68:743–818.
14. Bereiter, DA, Zaid, AM, Gann, DS. Effect of rate of hemorrhage on sympathoadrenal catecholamine release in cats. Am J Physiol. 1986; 250:E69–E75.
15. Bereiter, DA, Zaid, AM, Gann, DS. Effect of rate of hemorrhage on release of ACTH in cats. Am J Physiol. 1986; 250:E76–E81.
16. Hume, DM, Bell, CC, Bartter, F. Direct measurement of adrenal secretion during operative trauma and convalescence. Surgery. 1962; 52:174–187.
17. Chernow, B, Lake, CR, Barton, M, et al. Sympathetic nervous system sensitivity to hemorrhagic hypotension in the subhuman primate. J Trauma. 1984; 24:229–232.
18. Berne, RM, Levy, MN, Koeppen, BM, et al. Physiology, 5th ed. St. Louis: Mosby; 2004.
19. O’Regan, RG, Majcherczyk, S. Role of peripheral chemoreceptors and central chemosensitivity in the regulation of respiration and circulation. J Exp Biol. 1982; 100:23–40.
20. Holmes, CL, Patel, BM, Russell, JA, et al. Physiology of vasopressin relevant to management of septic shock. Chest. 2001; 120:989–1002.
21. Cryer, PE. Physiology and pathophysiology of the human sympathoadrenal neuroendocrine system. N Engl J Med. 1980; 303:436–444.
22. Jeremitsky, E, Omert, LA, Dunham, M, et al. The impact of hyperglycemia on patients with severe brain injury. J Trauma. 2005; 58:47–50.
23. Wiggers, CJ. Experimental hemorrhagic shock. In: Wiggers C, ed. Physiology of Shock. New York: Commonwealth Publications; 1950:121–146.
24. Bone, RC. Toward a theory regarding the pathogenesis of the systemic inflammatory response syndrome: What we do and do not know about cytokine regulation. Crit Care Med. 1996; 24:163–172.
25. Rivkind, AI, Siegel, JH, Guadalupi, P, et al. Sequential patterns of eicosanoid, platelet and neutrophil interactions in the evolution of the fulminant post-traumatic adult respiratory distress syndrome. Ann Surg. 1989; 210:355–373.
26. Waydhas, C, Nast-Kolb, D, Jochum, M, et al. Inflammatory mediators, infection, sepsis, and multiple organ failure after severe trauma. Arch Surg. 1992; 127:460–467.
27. Deitch, EA. Multiple organ failure: Pathophysiology and potential future therapy. Ann Surg. 1992; 216:117–134.
28. Nast-Kolb, D, Waydhas, C, Gippner-Steppert, C, et al. Indicators of the posttraumatic inflammatory response correlate with organ failure in patients with multiple injuries. J Trauma. 1997; 42:446–455.
29. Roumen, RM, Hendriks, T, van der Ven-Jongekrijg, J, et al. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma: Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg. 1993; 218:769–776.
30. van der Kleij, AJ, de Koning, J, Beerthuizen, G, et al. Early detection of hemorrhagic hypovolemia by muscle oxygen pressure assessment: Preliminary report. Surgery. 1983; 93:518–524.
31. Redl, H, Dinges, HP, Buurman, WA, et al. Expression of endothelial leukocyte adhesion molecule-1 in septic but not traumatic hypovolemic shock in the baboon. Am J Pathol. 1991; 139:461–466.
32. Anderson, BO, Brown, JM, Harken, AH. Mechanisms of neutrophil-mediated tissue injury. J Surg Res. 1991; 51:170–179.
33. Henson, PM, Johnston, RB, Jr. Tissue injury in inflammation: Oxidants, proteinases, and cationic proteins. J Clin Invest. 1987; 79:669–674.
34. Smedly, LA, Tonnesen, MG, Sandhaus, RA, et al. Neutrophil-mediated injury to endothelial cells: Enhancement by endotoxin and essential role of neutrophil elastase. J Clin Invest. 1986; 77:1233–1243.
35. Nathan, CF. Secretory products of macrophages. J Clin Invest. 1987; 79:319–326.
36. Gordon, S. Alternative activation of macrophages. Nat Rev Immunol. 2003; 3:23–35.
37. Molina, PE. Neurobiology of the stress response: Contribution of the sympathetic nervous system to the neuroimmune axis in traumatic injury. Shock. 2005; 24:3–10.
38. Selye, H. A syndrome produced by diverse nocuous agents—1936. J Neuropsychiatry Clin Neurosci. 1998; 10:230–231.
39. Chrousos, GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: Neuroendocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000; 24(Suppl 2):S50–S55.
40. Rozlog, LA, Kiecolt-Glaser, JK, Marucha, PT, et al. Stress and immunity: Implications for viral disease and wound healing. J Periodontol. 1999; 70:786–792.
41. Bjontorp, P. Stress and cardiovascular disease. Acta Physiol Scand Suppl. 1997; 640:144–148.
42. Felten, DL, Felten, SY, Carlson, SL, et al. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985; 135(Suppl 2):755S–765S.
43. Rivest, S. How circulating cytokines trigger the neural circuits that control the hypothalamic-pituitary-adrenal axis. Psychoneurology. 2001; 26:761–788.
44. Banks, WA, Kastin, AJ, Broadwell, RD. Passage of cytokines across the blood-brain barrier. Neuroimmunology. 1995; 2:241–248.
45. Goehler, LE, Gaykema, RP, Hansen, MK, et al. Vagal immune-to-brain communication: A visceral chemosensory pathway. Auton Neurosci. 2000; 85:49–59.
46. Kashuk, JL, Moore, EE, Sawyer, M, et al. Postinjury coagulopathy management: Goal directed resuscitation via POC thrombelastography. Ann Surg. 2010; 251:604–614.
47. Sauaia, A, Moore, FA, Moore, EE, et al. Epidemiology of trauma deaths: A reassessment. J Trauma. 1995; 38:185–193.
48. Kashuk, JL, Moore, EE, Millikan, JS, et al. Major abdominal vascular trauma—A unified approach. J Trauma. 1982; 22:672–679.
49. Cosgriff, N, Moore, EE, Sauaia, A, et al. Predicting life-threatening coagulopathy in the massively transfused trauma patient: Hypothermia and acidoses revisited. J Trauma. 1997; 42:857–862.
50. Simmons, RL, Collins, JA, Heisterkamp, CA, et al. Coagulation disorders in combat casualties. I. Acute changes after wounding. II. Effects of massive transfusion. III. Post-resuscitative changes. Ann Surg. 1969; 169:455–482.
51. Counts, RB, Haisch, C, Simon, TL, et al. Hemostasis in massively transfuse trauma patients. Ann Surg. 1979; 190:91–99.
52. Ciavarella, D, Reed, RL, Counts, RB, et al. Clotting factor levels and the risk of diffuse microvascular bleeding in the massively transfused patient. Br J Haematol. 1987; 67:365–368.
53. MacLeod, JB, Lynn, M, McKenney, MG, et al. Early coagulopathy predicts mortality in trauma. J Trauma. 2003; 55:39–44.
54. Hirshberg, A, Dugas, M, Banez, EI, et al. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: A computer simulation. J Trauma. 2003; 54:454–463.
55. Brohi, K, Singh, J, Heron, M, et al. Acute traumatic coagulopathy. J Trauma. 2003; 54:1127–1130.
56. Holcomb, JB, Jenkins, D, Rhee, P, et al. Damage control resuscitation: Directly addressing the early coagulopathy of trauma. J Trauma. 2007; 62:307–310.
57. Hess, JR, Brohi, K, Dutton, RP, et al. The coagulopathy of trauma: A review of mechanisms. J Trauma. 2008; 65:748–754.
58. MacLeod, JB. Trauma and coagulopathy. A new paradigm to consider. Arch Surg. 2008; 143:797–801.
59. Martini, WZ. Coagulopathy by hypothermia and acidosis: Mechanisms of thrombin generation and fibrinogen availability. J Trauma. 2009; 67:202–209.
60. Duchesne, JC, Islam, TM, Stuke, L, et al. Hemostatic resuscitation during surgery improves survival in patients with traumatic-induced coagulopathy. J Trauma. 2009; 67:33–39.
61. Brohi, K, Cohen, MJ, Ganter, MT, et al. Acute traumatic coagulopathy: Initiated by hypoperfusion: Modulated through the protein C pathway? Ann Surg. 2007; 245:812–818.
62. Niles, SE, McLaughlin, DF, Perkins, JG, et al. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008; 64:1459–1465.
63. Brohi, K, Cohen, MJ, Ganter, MT, et al. Acute coagulopathy of trauma: Hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008; 64:1211–1217.
64. Reed, RL, Bracey, AW, Jr., Hudson, JD, et al. Hypothermia and blood coagulation: Dissociation between enzyme activity and clotting factor levels. Circ Shock. 1990; 32:141–152.
65. Landis, RC. Protease activated receptors: Clinical relevance to hemostasis and inflammation. Hematol Oncol Clin North Am. 2007; 21:103–113.
66. Ganter, MT, Brohi, K, Cohen, MJ, et al. Role of the alternative pathway in the early complement activation following major trauma. Shock. 2007; 28:29–34.
67. Bernard, GR, Vincent, JL, Laterre, PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001; 344:699–709.
68. Cohen, MJ, Call, M, Nelson, M, et al. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg. 2012; 255:379–385.
69. American College of Surgeons Committee on Trauma. Advanced Trauma Life Support for Doctors, 8th ed. Chicago: American College of Surgeons; 2008.
70. Santry, HP, Alam, HB. Fluid resuscitation: Past, present, and the future. Shock. 2010; 33:229–241.
71. Demling, RH. The pathogenesis of respiratory failure after trauma and sepsis. Surg Clin North Am. 1980; 60:1373–1390.
72. Moon, PF, Hollyfield-Gilbert, MA, Myers, TL, et al. Effects of isotonic crystalloid resuscitation on fluid compartments in hemorrhaged rats. Shock. 1994; 2:355–361.
73. Madigan, MC, Kemp, CD, Johnson, JC, et al. Secondary abdominal compartment syndrome after severe extremity injury: Are early, aggressive fluid resuscitation strategies to blame? J Trauma. 2008; 64:280–285.
74. Lee, CC, Chang, IJ, Yen, ZS, et al. Effect of different resuscitation fluids on cytokine response in a rat model of hemorrhagic shock. Shock. 2005; 24:177–181.
75. Chen, H, Koustova, E, Shults, C, et al. Differential effect of resuscitation on toll-like receptors in a model of hemorrhagic shock without a septic challenge. Resuscitation. 2007; 74:526–537.
76. Chen, H, Inocencio, R, Alam, HB, et al. Differential expression of extracellular matrix remodeling genes in rat model of hemorrhagic shock and resuscitation. J Surg Res. 2005; 123:235–244.
77. Alam, HB, Sun, L, Ruff, P, et al. E- and P-selectin expression depends on the resuscitation fluid used in hemorrhaged rats. J Surg Res. 2000; 94:145–152.
78. Fluid Resuscitation. State of the Science for Treating Combat Casualties and Civilian Injuries. Washington, DC: Institute of Medicine; 1999.
79. Ayuste, EC, Chen, H, Koustova, E, et al. Hepatic and pulmonary apoptosis after hemorrhagic shock in swine can be reduced through modifications of conventional Ringer’s solution. J Trauma. 2006; 60:52–63.
80. Koustova, E, Stanton, K, Gushchin, V, et al. Effects of lactated Ringer’s solutions on human leukocytoses. J Trauma. 2002; 52:872–878.
81. Chen, H, Alam, HB, Querol, RI, et al. Identification of expression patterns associated with hemorrhage and resuscitation: Integrated approach to data analysis. J Trauma. 2006; 60:701–724.
82. Alam, HB, Stanton, K, Koustova, E, et al. Effect of different resuscitation strategies on neutrophil activation in a swine model of hemorrhagic shock. Resuscitation. 2004; 60:91–99.
83. Deb, S, Sun, L, Martin, B, et al. Lactated Ringer’s solution and hetastarch but not plasma resuscitation after rat hemorrhagic shock is associated with immediate lung apoptosis by the up-regulation of the Bax protein. J Trauma. 2000; 49:47–55.
84. Barron, ME, Wilkes, MM, Navickis, RJ. A systemic review of the comparative safety of colloids. Arch Surg. 2004; 139:552–563.
85. Vincent, JL, Navickis, RJ, Wilkes, MM. Morbidity in hospitalized patients receiving human albumin: A meta-analysis of randomized, controlled trials. Crit Care Med. 2004; 32:2029–2038.
86. Wilkes, MM, Navickis, RJ. Does albumin infusion affect survival? Review of meta-analytic findings. In: Vincent JL, ed. 2002 Yearbook of Intensive Care and Emergency Medicine. Berlin: Springer-Verlag; 2002:454–464.
87. Blunt, MC, Nicholson, JP, Park, GR. Serum albumin and colloid osmotic pressure in survivors and nonsurvivors of prolonged critical illness. Anaesthesia. 1998; 53:755–761.
88. Finfer, S, Bellomo, R, Boyce, N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. SAFE Study Investigators. N Engl J Med. 2004; 350:2247–2256.
89. Rocha-e-Silva, M, Negraes, GA, Soares, AM, et al. Hypertonic resuscitation from severe hemorrhagic shock: Patterns of regional circulation. Circ Shock. 1986; 19:165–175.
90. Chiara, O, Pelosi, P, Brazzi, L, et al. Resuscitation from hemorrhagic shock: Experimental model comparing normal saline, dextran, and hypertonic saline solutions. Crit Care Med. 2003; 31:1915–1922.
91. Dubick, MA, Bruttig, SP, Wade, CE. Issues of concern regarding the use of hypertonic/hyperoncotic fluid resuscitation of hemorrhagic hypotension. Shock. 2006; 25:321–328.
92. Shackford, SR, Schmoker, JD, Zhuang, J. The effect of hypertonic resuscitation on pial arteriolar tone after brain injury and shock. J Trauma. 1994; 37:899–908.
93. Wade, CE, Grady, JJ, Kramer, GC, et al. Individual patient cohort analysis of the efficacy of hypertonic saline/dextran in patients with traumatic brain injury and hypotension. J Trauma. 1997; 42(Suppl):S61–S65.
94. Rizoli, SB, Rhind, SG, Shek, PN, et al. The immunomodulatory effects of hypertonic saline resuscitation in patients sustaining traumatic hemorrhagic shock: A randomized, controlled, double-blinded trial. Ann Surg. 2006; 243:47–57.
95. Riddez, L, Drobin, D, Sjöstrand, F, et al. Lower dose of hypertonic saline dextran reduces the risk of lethal rebleeding in uncontrolled hemorrhage. Shock. 2002; 17:377–382.
96. Wade, CE, Kramer, GC, Grady, JJ, et al. Efficacy of hypertonic 7. 5% saline and 6% dextran-70 in treating trauma: A meta-analysis of controlled clinical studies. Surgery. 1997; 122:609–616.
97. Kempski, O, Obert, C, Mainka, T, et al. “Small volume resuscitation” as treatment of cerebral blood flow disturbances and increased ICP in trauma and ischemia. Acta Neurochir Suppl. 1996; 66:114–117.
98. Goulin, GD, Duthie, SE, Zornow, MH, et al. Global cerebral ischemia: Effects of pentastarch after reperfusion. Anesth Analg. 1994; 79:1036–1042.
99. Poole, GV, Meredith, JW, Pennell, T, et al. Comparison of colloids and crystalloids in resuscitation from hemorrhagic shock. Surg Gynecol Obstet. 1982; 154:577–586.
100. Hauser, CJ, Shoemaker, WC, Turpin, I, et al. Oxygen transport responses to colloids and crystalloids in critically ill surgical patients. Surg Gynecol Obstet. 1980; 150:811–816.
101. Carey, LC, Lowery, BD, Cloutier, CT. Hemorrhagic shock. Curr Probl Surg. 1971; 8:1–48.
102. Weaver, DW, Ledgerwood, AM, Lucas, CE, et al. Pulmonary effects of albumin resuscitation for severe hypovolemic shock. Arch Surg. 1978; 113:387–392.
103. Lowe, RJ, Moss, GS, Jilek, J, et al. Crystalloid vs. colloid in the etiology of pulmonary failure after trauma: A randomized trial in man. Surgery. 1977; 81:676–683.
104. Shah, DM, Browner, BD, Dutton, RE, et al. Cardiac output and pulmonary wedge pressure: Use for evaluation of fluid replacement in trauma patients. Arch Surg. 1977; 112:1161–1168.
105. Guyton, AC, Lindsey, AW. Effect of elevated left atrial pressure and decreased plasma protein concentration on the development of pulmonary edema. Circ Res. 1959; 7:649–657.
106. Zarins, CK, Rice, CL, Smith, DE, et al. Role of lymphatics in preventing hypooncotic pulmonary edema. Surg Forum. 1976; 27:257–259.
107. Demling, RH, Manohar, M, Will, JA, et al. The effect of plasma oncotic pressure on the pulmonary microcirculation after hemorrhagic shock. Surgery. 1979; 86:323–328.
108. Holcroft, JW, Trunkey, DD, Carpenter, MA. Excessive fluid administration in resuscitating baboons from hemorrhagic shock, and an assessment of the thermodye technic for measuring extravascular lung water. Am J Surg. 1978; 135:412–416.
109. Ledgerwood, AM, Lucas, CE. A review of studies on the effects of hemorrhagic shock and resuscitation on the coagulation profile. J Trauma. 2003; 54(Suppl):S68–S74.
110. Teixeira, PG, Inaba, K, Shulman, I, et al. Impact of plasma transfusion in massively transfused trauma patients. J Trauma. 2009; 66:693–697.
111. Kashuk, JL, Moore, EE, Johnson, JL, et al. Postinjury life threatening coagulopathy: Is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008; 65:261–271.
112. Cotton, BA, Gunter, OL, Isbell, J, et al. Damage control hematology: The impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008; 64:1177–1183.
113. Holcomb, JB, Wade, CD, Michalek, JE, et al. Increased plasma and platelets to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008; 248:447–458.
114. Hirshberg, A, Hoyt, DB, Mattox, KL. Timing of fluid resuscitation shapes the hemodynamic response to uncontrolled hemorrhage: Analysis using dynamic modeling. J Trauma. 2006; 60:1221–1227.
115. Dries, DJ. Hypotensive resuscitation. Shock. 1996; 6:311–316.
116. Tisherman, SA, Barie, P, Bokhari, F, et al. Clinical practice guideline: End points of resuscitation. J Trauma. 2004; 57:898–912.
117. Elliott, DC. An evaluation of the end points of resuscitation. J Am Coll Surg. 1998; 187:536–547.
118. Brain Trauma Foundation. Management and prognosis of severe traumatic brain injury. J Neurotrauma. 2000; 17:449–627.
119. Chesnut, RM, Marshall, LF, Klauber, MR, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993; 34:216–222.
120. Shoemaker, WC, Appel, P, Bland, R. Use of physiologic monitoring to predict outcome and to assist in clinical decisions in critically ill postoperative patients. Am J Surg. 1983; 146:43–50.
121. Shoemaker, WC, Appel, PL, Kram, HB, et al. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988; 94:1176–1186.
122. Bishop, MH, Shoemaker, WC, Appel, PL, et al. Relationship between supranormal circulatory values, time delays, and outcome in severely traumatized patients. Crit Care Med. 1993; 21:56–63.
123. Fleming, A, Bishop, M, Shoemaker, W, et al. Prospective trial of supranormal values as goals of resuscitation in severe trauma. Arch Surg. 1992; 127:1175–1181.
124. Bishop, MH, Shoemaker, WC, Appel, PL, et al. Prospective, randomized trial of survivor values of cardiac index, oxygen delivery, and oxygen consumption as resuscitation end points in severe trauma. J Trauma. 1995; 38:780–787.
125. Moore, FA, Haenel, JB, Moore, EE, et al. Incommensurate oxygen consumption in response to maximal oxygen availability predicts postinjury multisystem organ failure. J Trauma. 1992; 33:58–67.
126. Durham, RM, Neunaber, K, Mazuski, JE, et al. The use of oxygen consumption and delivery as end points for resuscitation in critically ill patients. J Trauma. 1996; 41:32–40.
127. Velmahos, GC, Demetriades, D, Shoemaker, WC, et al. End points of resuscitation of critically injured patients: Normal or supranormal? A prospective randomized trial. Ann Surg. 2000; 232:409–418.
128. Mizock, BA, Falk, JL. Lactic acidosis in critical illness. Crit Care Med. 1992; 20:80–93.
129. Abramson, D, Scalea, TM, Hitchcock, R, et al. Lactate clearance and survival following injury. J Trauma. 1993; 35:584–589.
130. McNelis, J, Marini, CP, Jurkiewicz, A, et al. Prolonged lactate clearance is associated with increased mortality in the surgical intensive care unit. Am J Surg. 2001; 182:481–485.
131. Mikulaschek, A, Henry, SM, Donovan, R, et al. Serum lactate is not predicted by anion gap or base excess after trauma resuscitation. J Trauma. 1996; 40:218–224.
132. Davis, JW, Kaups, KL, Parks, SN. Base deficit is superior to pH in evaluating clearance of acidosis after traumatic shock. J Trauma. 1998; 44:114–118.
133. Eachempati, SR, Reed, RL, 2nd., Barie, PS. Serum bicarbonate as an endpoint of resuscitation in critically ill patients. Surg Infect (Larchmt). 2003; 4:193–197.
134. Davis, JW, Shackford, SR, Mackersie, RC, et al. Base deficit as a guide to volume resuscitation. J Trauma. 1988; 28:1464–1467.
135. Falcone, RE, Santanello, SA, Schulz, MA, et al. Correlation of metabolic acidosis with outcome following injury and its value as a scoring tool. World J Surg. 1993; 17:575–579.
136. Sauaia, A, Moore, FA, Moore, EE, et al. Early predictors of postinjury multiple organ failure. Arch Surg. 1994; 129:39–45.
137. Kincaid, EH, Miller, PR, Meredith, JW, et al. Elevated arterial base deficit in trauma patients: A marker of impaired oxygen utilization. J Am Coll Surg. 1998; 187:384–392.
138. Rixen, D, Raum, M, Bouillon, B, et al. Base deficit development and its prognostic significance in posttrauma critical illness: An analysis by the trauma registry of the Deutsche Gesellschaft für Unfallchirurgie. Shock. 2001; 15:83–89.
139. Davis, JW, Parks, SN, Kaups, KL, et al. Admission base deficit predicts transfusion requirements and risk of complications. J Trauma. 1996; 41:769–774.
140. Rixen, D, Siegel, JH. Metabolic correlates of oxygen debt predict posttrauma early acute respiratory distress syndrome and the related cytokine response. J Trauma. 2000; 49:392–403.
141. Dunham, CM, Watson, LA, Cooper, C. Base deficit level indicating major injury is increased with ethanol. J Emerg Med. 2000; 18:165–171.
142. Davis, JW, Kaups, KL, Parks, SN. Effect of alcohol on the utility of base deficit in trauma. J Trauma. 1997; 43:507–510.
143. Brill, SA, Stewart, TR, Brundage, SI, et al. Base deficit does not predict mortality when secondary to hyperchloremic acidosis. Shock. 2002; 17:459–462.
144. Dantzker, DR. The gastrointestinal tract: The canary of the body? JAMA. 1993; 270:1247–1248.
145. Harbrecht, BG, Alarcon, LH, Peitzman, AB. Management of shock. In: Moore EE, Feliciano DV, Mattox KL, eds. Trauma. 5th ed. New York: McGraw-Hill; 2004:201–226.
146. Roumen, RM, Vreugde, JP, Goris, RJ. Gastric tonometry in multiple trauma patients. J Trauma. 1994; 36:313–316.
147. Chang, MC, Cheatham, ML, Nelson, LD, et al. Gastric tonometry supplements information provided by systemic indicators of oxygen transport. J Trauma. 1994; 37:488–494.
148. Miller, PR, Kincaid, EH, Meredith, JW, et al. Threshold values of intramucosal pH and mucosal-arterial CO2 gap during shock resuscitation. J Trauma. 1998; 45:868–872.
149. Ivatury, RR, Simon, RJ, Havriliak, D, et al. Gastric mucosal pH and oxygen delivery and oxygen consumption indices in the assessment of adequacy of resuscitation after trauma: A prospective, randomized study. J Trauma. 1995; 39:128–136.
150. Ivatury, RR, Simon, RJ, Islam, S, et al. A prospective randomized study of end points of resuscitation after major trauma: Global oxygen transport indices versus organ-specific gastric mucosal pH. J Am Coll Surg. 1996; 183:145–154.
151. Wall, P, Henderson, L, Buising, C, et al. Monitoring gastrointestinal intraluminal PCO2: Problems with airflow methods. Shock. 2001; 15:360–365.
152. Soller, BR, Cingo, N, Puyana, JC, et al. Simultaneous measurement of hepatic tissue pH, venous oxygen saturation and hemoglobin by near infrared spectroscopy. Shock. 2001; 15:106–111.
153. McKinley, BA, Marvin, RG, Cocanour, CS, et al. Tissue hemoglobin O2 saturation during resuscitation of traumatic shock monitored using near infrared spectrometry. J Trauma. 2000; 48:637–642.
154. Cairns, CB, Moore, FA, Haenel, JB, et al. Evidence for early supply independent mitochondrial dysfunction in patients developing multiple organ failure after trauma. J Trauma. 1997; 42:532–536.
155. Keynes, G. The Apology and Treatise of Ambroise Paré Containing the Voyages Made Into Divers Places With Many of His Writings Upon Surgery. Chicago: University of Chicago Press; 1952.
156. Simeone, FA. Shock, trauma and the surgeon. Ann Surg. 1963; 158:759–774.
157. Cannon, WB. Traumatic Shock. New York: Appleton; 1923.
158. Mapstone, J, Roberts, I, Evans, P. Fluid resuscitation strategies: A systemic review of animal trials. J Trauma. 2003; 55:571–589.
159. Cannon, WB, Fraser, J, Cowell, EM. The preventive treatment of wound shock. JAMA. 1918; 70:618–621.
160. Capone, AC, Safar, P, Stezoski, W, et al. Improved outcome with fluid restriction in treatment of uncontrolled hemorrhagic shock. J Am Coll Surg. 1995; 180:49–56.
161. Shaftan, GW, Chiu, CJ, Dennis, C, et al. Fundamentals of physiologic control of arterial hemorrhage. Surgery. 1965; 58:851–856.
162. Milles, G, Koucky, CJ, Zacheis, HG. Experimental uncontrolled arterial hemorrhage. Surgery. 1966; 60:434–442.
163. Shires, T, Coln, D, Carrico, J, et al. Fluid therapy in hemorrhagic shock. Arch Surg. 1964; 88:688–693.
164. Traverso, LW, Lee, WP, Langford, MJ. Fluid resuscitation after an otherwise fatal hemorrhage, I: Crystalloid solutions. J Trauma. 1986; 26:168–175.
165. Baue, AE, Tragus, ET, Wolfson, SK, Jr., et al. Hemodynamic and metabolic effects of Ringer’s lactate solution in hemorrhagic shock. Ann Surg. 1967; 166:29–38.
166. Wiggers, CJ. Physiology of Shock. New York: Commonwealth Publications; 1950.
167. Gann, DS, Wright, PA, Shock—the final common pathway?. Advances in Trauma Critical Care. Maull, KI, eds. Advances in Trauma Critical Care; Vol. 10. Mosby-Year Book, St. Louis, 1995:43–59.
168. Bickell, WH, Bruttig, SP, Millnamow, GA, et al. The detrimental effects of intravenous crystalloid after aortotomy in swine. Surgery. 1991; 110:529–536.
169. Stern, SA, Dronen, SC, Birrer, P, et al. Effect of blood pressure on hemorrhage volume and survival in a near-fatal hemorrhage model incorporating a vascular injury. Ann Emerg Med. 1993; 22:155–163.
170. Kowalenko, T, Stern, S, Dronen, S, et al. Improved outcome with hypotensive resuscitation of uncontrolled hemorrhagic shock in a swine model. J Trauma. 1992; 33:349–353.
171. Novak, L, Shackford, SR, Bourguignon, P, et al. Comparison of standard and alternative prehospital resuscitation in uncontrolled hemorrhagic shock and head injury. J Trauma. 1999; 47:834–844.
172. Martin, RR, Bickell, WH, Pepe, PE, et al. Prospective evaluation of preoperative fluid resuscitation in hypotensive patients with penetrating truncal injury: A preliminary report. J Trauma. 1992; 33:354–362.
173. Bickell, WH, Wall, MJ, Jr., Pepe, PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994; 331:1105–1109.
174. Champion, HR, Sacco, WJ, Copes, WS, et al. A revision of the trauma score. J Trauma. 1989; 29:623–629.
175. The Abbreviated Injury Scale, 1990. Des Plaines, IL: Association for the Advancement of Automotive Medicine, 1990.
176. Wall, MJ, Jr., Granchi, T, Liscum, K, et al. Delayed versus immediate resuscitation in patients with penetrating trauma: Subgroup analysis. J Trauma. 1995; 39:173.
177. McSwain, NE, Champion, HR, Fabian, TC, et al. State of the art of fluid resuscitation 2010: Prehospital and immediate transition to the hospital. J Trauma. 2011; 70(Suppl):S2–S10.
178. Marr, AB, Moore, FA, Sailors, RM, et al. Preload optimization using “Starling curve” generation during shock resuscitation: Can it be done? Shock. 2004; 21:300–305.
179. McKinley, BA, Kozar, RA, Cocanour, CS, et al. Normal versus supranormal oxygen delivery goals in shock resuscitation: The response is the same. J Trauma. 2002; 53:825–832.
180. McKinley, BA, Marvin, RG, Cocanour, CS, et al. Blunt trauma resuscitation: The old can respond. Arch Surg. 2000; 135:688–695.
181. McKinley, BA, Kozar, RA, Cocanour, CS, et al. Standardized trauma resuscitation: Female hearts respond better. Arch Surg. 2002; 137:578–584.
182. McKinley, BA, Valdivia, A, Moore, FA. Goal-oriented shock resuscitation for major torso trauma: What are we learning? Curr Opin Crit Care. 2003; 9:292–299.
183. Scalea, TM, Simon, HM, Duncan, AO, et al. Geriatric blunt multiple trauma: Improved survival with early invasive monitoring. J Trauma. 1990; 30:129–136.
184. Balogh, Z, McKinley, BA, Cocanour, CS, et al. Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch Surg. 2003; 138:637–643.
185. Friese, RS, Shafi, S, Gentilello, LM. Pulmonary artery catheter use is associated with reduced mortality in severely injured patients: A National Trauma Data Bank analysis of 53,312 patients. Crit Care Med. 2006; 34:1597–1601.
186. Kauvar, DS, Lefering, R, Wade, CE. Impact of hemorrhage on trauma outcome: An overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006; 60:S3–S11.
187. Bouillon, B, Raum, M, Fach, H, et al. The incidence and outcome of severe brain trauma—Design and first results of an epidemiological study in an urban area. Restor Neurol Neurosci. 1999; 14:85–92.
188. Manley, G, Knudson, MM, Morabito, D, et al. Hypotension, hypoxia, and head injury: Frequency, duration, and consequences. Arch Surg. 2001; 136:1118–1123.
189. Chesnut, RM, Marshall, SB, Piek, J, et al. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir Suppl (Wien). 1993; 59:121–125.
190. Hoyt, DB, Bulger, EM, Knudson, MM, et al. Death in the operating room: An analysis of a multi-center experience. J Trauma. 1994; 37:426–432.
191. Como, JJ, Dutton, RP, Scalea, TM, et al. Blood transfusion rates in the care of acute trauma. Transfusion. 2004; 44:809–813.
192. Ketchum, L, Hess, JR, Hiippala, S. Indications for early fresh frozen plasma, cryoprecipitate, and platelet transfusion in trauma. J Trauma. 2006; 60:S51–S58.
193. Malone, DL, Hess, JR, Fingerhut, A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J Trauma. 2006; 60:S91–S96.
194. Vaslef, SN, Knudsen, NW, Neligan, PJ, et al. Massive transfusion exceeding 50 units of blood products in trauma patients. J Trauma. 2002; 53:291–296.
195. Hauser, CJ, Boffard, K, Dutton, R, et al. Results of the CONTROL Trial: Efficacy and safety of recombinant activated factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010; 69:489–500.
196. Nishijima, DK, Zehtabchi, S. The efficacy of recombinant activated factor VII in severe trauma. Ann Emerg Med. 2009; 54:737–744.
197. Al-Ruzzeh, S, Navia, JL. The “off-label” role of recombinant factor VII in surgery: Is the problem deficient evidence or defective concept? J Am Coll Surg. 2009; 209:659–667.
198. Levi, M, Levy, JH, Andersen, HF, et al. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010; 363:1791–1800.
199. Nunez, TC, Voskresensky, IV, Dossett, LA, et al. Early prediction of massive transfusion in trauma: Simple as ABC (assessment of blood consumption)? J Trauma. 2009; 66:346–352.
200. Cotton, BA, Dossett, LA, Haut, ER, et al. Multicenter validation of a simplified score to predict massive transfusion in trauma. J Trauma. 2010; 69(suppl):S33–S39.
201. Callcut, RA, Johannigman, JA, Kadon, KS, et al. All massive transfusion criteria are not created equal: Defining the predictive value of individual transfusion triggers to better determine who benefits from blood. J Trauma. 2011; 70:794–801.
202. The CRASH-2 CollaboratorsRoberts, I, Shakur, H, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: An exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011; 377:1096–1101.
203. CRASH-2 Collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomized, placebo-controlled trial. Lancet. 2010; 376:23–32.
204. Morrison, JJ, Dubose, JJ, Rasmussen, TE, et al. Military application of tranexamic acid in trauma emergency resuscitation (MATTERs) study. Arch Surg. 2012; 147:113–119.
205. Gruen, RL, Mitra, B. Tranexamic acid for trauma. Lancet. 2011; 377:1052–1054.
206. Napolitano, L. Cumulative risks of early red blood cell transfusion. J Trauma. 2006; 60:S26–S34.
207. Silliman, CC, Moore, EE, Johnson, JL, et al. Transfusion of the injured patient: Proceed with caution. Shock. 2004; 21:291–299.
208. Moore, FA, Moore, EE, Sauaia, A. Blood transfusion: An independent risk factor for postinjury multiple organ failure. Arch Surg. 1997; 132:620–625.
209. Dunne, JR, Malone, DL, Tracy, JK, et al. Allogenic blood transfusion in the first 24 hours after trauma is associated with increased systemic inflammatory response syndrome (SIRS) and death. Surg Infect. 2004; 5:395–404.
210. Blajchman, MA. The clinical benefits of the leukoreduction of blood products. J Trauma. 2006; 60:S83–S90.
211. Schein, M, Wittmann, DH, Aprahamian, CC, et al. The abdominal compartment syndrome: The physiological and clinical consequences of elevated intra-abdominal pressure. J Am Coll Surg. 1995; 180:745–753.
212. Chang, MC, Miller, PR, D’Agostino, R, Jr., et al. Effects of abdominal decompression on cardiopulmonary function and visceral perfusion in patients with intra-abdominal hypertension. J Trauma. 1998; 44:440–445.
213. Cheatham, ML, White, MW, Sagraves, SG, et al. Abdominal perfusion pressure: A superior parameter in the assessment of intra-abdominal hypertension. J Trauma. 2000; 49:621–627.
214. Malbrain, ML, Cheatham, ML, Kirkpatrick, A, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome, I: Definitions. Intensive Care Med. 2006; 32:1722–1732.
215. Ivatury, RR, Diebel, L, Porter, JM, et al. Intra-abdominal hypertension and the abdominal compartment syndrome. Surg Clin North Am. 1997; 77:783–800.
216. Sugrue, M, Buist, MD, Hourihan, F, et al. Prospective study of intra-abdominal hypertension and renal function after laparotomy. Br J Surg. 1995; 82:235–238.
217. Sugrue, M, Jones, F, Janjua, KJ, et al. Temporary abdominal closure: A prospective evaluation of its effects on renal and respiratory physiology. J Trauma. 1998; 45:914–921.
218. Sugrue, M, Jones, F, Deane, SA, et al. Intra-abdominal hypertension is an independent cause of postoperative renal impairment. Arch Surg. 1999; 134:1082–1085.
219. Diebel, LN, Dulchavsky, SA, Wilson, RF. Effect of increased intra-abdominal pressure on mesenteric arterial and intestinal mucosal blood flow. J Trauma. 1992; 33:45–49.
220. Diebel, LN, Wilson, RF, Dulchavsky, SA, et al. Effect of increased intra-abdominal pressure on hepatic arterial, portal venous, and hepatic microcirculatory blood flow. J Trauma. 1992; 33:279–283.
221. Cheatham, ML, Safcsak, K. Percutaneous catheter decompression in the treatment of elevated intra-abdominal pressure. Chest. 2011; 140:1428–1435.
222. Dries, DJ. Abdominal compartment syndrome: Toward less-invasive management (editorial). Chest. 2011; 140:1396–1398.
223. Mubarak, SJ, Hargens, AR. Acute compartment syndromes. Surg Clin North Am. 1983; 63:539–565.
224. Duis, HJ, Nijsten, MW, Klasen, HJ, et al. Fat embolism in patients with an isolated fracture of the femoral shaft. J Trauma. 1988; 28:383–390.
225. Feliciano, DV, Cruse, PA, Spjut-Patrinely, V, et al. Fasciotomy after trauma to the extremities. Am J Surg. 1988; 156:533–536.
226. Scalea, TM, Burgess, AR. Pelvic fractures. In: Moore EE, Feliciano DV, Mattox KL, eds. Trauma. 5th ed. New York: McGraw-Hill; 2004:779–807.
227. Gylling, SF, Ward, RE, Holcroft, JW, et al. Immediate external fixation of unstable pelvic fractures. Am J Surg. 1985; 150:721–724.
228. Ben-Menachem, Y, Coldwell, DM, Young, JW, et al. Hemorrhage associated with pelvic fractures: Causes, diagnosis, and emergent management. Am J Roentgenol. 1991; 157:1005–1014.