Chapter 50C Trauma of the Nervous System
Spinal Cord Trauma
Transient Spinal Cord Syndromes
Mechanisms and Types of Injuries
Management of Acute Spinal Cord Injuries
Treatment of Spinal Cord Injuries in the Acute Setting
Long-Term Management of Spinal Cord Injuries
Delayed Posttraumatic Spinal Cord Syndromes
Sexual Dysfunction, Sexuality, and Fertility in Spinal Cord Injuries
Deep Vein Thrombosis and Thromboembolism in Spinal Cord Injury
Epidemiology
The annual incidence of SCI worldwide is between 11.5 and 57.8 cases per million persons (Ackery et al., 2004). In the United States, the annual incidence is approximately 40 cases per million, with approximately 12,000 new cases diagnosed a year. There is a bimodal age distribution, with the highest frequency occurring between 15 and 29 years of age and the second occurring at 65 years of age and older (van den Berg et al., 2010b). On a global scale, inconsistencies in reporting and failure to include patients who died before entering a hospital lead to wide discrepancies in the actual incidence. The mortality rate associated with SCIs between the time of the event and the time of presentation to the hospital is between 48.3% and 79% (Kraus et al., 1975). Once admitted to a hospital, the mortality rate decreases and is reported to be between 4.4% and 16.7% (Kraus, 1980). The leading cause of death in patients with SCI relates to respiratory complications (van den Berg et al., 2010). In North American trauma centers, approximately 1 in 40 patients admitted suffers from an acute SCI (Burney et al., 1993). The present estimation of SCI victims is reported to be 259,000 (Spinal Cord Injury Information Network, 2009). The two most common causes of SCIs are motor vehicle collisions and falls (van den Berg et al., 2010). Other causes of SCI include work-related injuries, sports and recreational injuries, and violence. Spinal cord injuries predominantly occur in the younger population (20-40 years of age); age 35 is the worldwide average, and age 28.7 is the U.S. average. Typically, SCIs occur more commonly in males than females by a factor of 3 or 4 (Sekhon and Fehlings, 2001).
Pathophysiology
Secondary injuries are divided into acute, intermediate, and chronic stages. The acute phase is divided into an early acute phase and a subacute phase. The biochemical processes occurring in the early acute phase of injury are targeted for neuroprotective therapies. Ionic homeostasis is desynchronized during this period and contributes to apoptosis and necrotic cell death. In particular, Ca2+ deregulation leads to a variety of damaging processes such as mitochondrial dysfunction. This in turn leads to low adenosine triphosphate (ATP) levels. Without enough ATP to sustain energy-dependent transporters such as the Na+/K+-ATPase membrane transporter, ionic homeostasis is further disrupted. This disruption of ionic homeostasis leads to failure of the Na+/K+/glutamate pump, which conceivably leads to elevated levels of glutamate. Glutamate in turn acts on a variety of glutamate receptors such as N-methyl-d-aspartate (NMDA), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainite receptors, leading to an influx of Na+ and Ca2+. Free radical reactions create membrane damage via lipid peroxidation, further promoting cell lysis, dysfunction of organelles, and calcium deregulation. Free radical production peaks at 12 hours post injury and continues to have an active presence for another week before returning to preinjury levels at 4 to 5 weeks. Recently it has been found that the primary mediator of free radical injury is the peroxynitrite radical (Xiong et al., 2007). In rats, the peroxynitrite radical has been shown to cause apoptosis (Bao and Liu, 2003). Antioxidants and inhibitors of peroxynitrite radicals have shown promise as neuroprotective elements. One such compound, methylprednisolone, is commonly used because of its suspected role in the inhibition of lipid peroxidation.
Following injury to the spinal cord, the blood-brain barrier has a higher permeability due to injured endothelial cells and astrocytic processes and inflammatory mediators that increase vascular permeability. Animal studies show the peak vascular permeability occurring at 24 hours and tapering off over a 2-week period (Noble and Wrathall, 1989). In humans, the time course is suspected to be the same. Two mediators upregulated to increase vascular permeability are TNF-α and IL-1β. Other compounds found to have negative effects on the permeability of the blood-brain barrier include reactive oxygen species (ROS; e.g., nitric oxide), histamine, matrix metalloproteineases, and elastase.
Despite the inflammatory response exerting deleterious effects, it is crucial in maintaining an environment for regenerative growth and removing cellular debris. Animal studies have been used to study the changes that occur on the cellular level. Recently, spinal cords taken from autopsy specimens of patients suffering from SCIs were used to study the changes occurring at the cellular level (Fleming et al., 2006). This study showed the presence of neutrophils at the injured sites within 4 hours post injury. After peaking between 1 and 3 days post injury, they remained present for as long as 10 days. Microglial cells were also shown to be an important component of the early inflammatory process. They became activated and increased in number during the first 3 days post injury. Like the neutrophils, their presence correlated with areas of increased tissue damage. During the following 5- to 10-day period post injury, the predominant cell population transitioned to the activated microglia and macrophages. Macrophages with a phagocytic phenotype were noted to be CD68 reactive (Schmitt et al., 2000). Over months to years after injury, most of the foamy macrophages seen were no longer CD68 reactive (Fleming et al., 2006). This is thought to be due to decreased phagocytic activity of existing macrophages, leading to reduced expression of the lysosomal protein. Currently it is felt that macrophages have a lifespan of 4 weeks but do not express a phagocytic phenotype for this entire duration (Ross and Auger, 2002). Furthermore, macrophages that arrive in chronic lesions may not be influenced by the local environment to produce CD68. Recently it has been felt that the secretion of oxidative and proteolytic enzymes by neutrophils, activated microglia, and macrophages during the first 3 days post injury imparts a high degree of secondary injury to the spinal cord. Noncellular mediators that contribute to this process include TNF-α, interferons, and interleukins, as discussed. Inhibition of TNF-α has been found to promote recovery following SCI (Bethea et al., 1999). However, TNF-α has been found to be neuroprotective in vitro (Cheng et al., 1994) and in studies with TNF-α–deficient mice (Kim et al., 2001). Thus, the exact role of TNF-α in SCI must be better defined before future therapeutic modalities can capitalize on the manipulation of this mediator.
Cell death following SCI occurs by one of two mechanisms: apoptosis or necrosis. Potentially, a newly discovered mechanism of cell death known as necroptosis can cause a programmed necrotic event to occur (Galluzzi and Kroemer, 2008). Apoptosis has not been well documented in human SCIs, but there is a substantial amount of literature on the topic in animal SCIs. Following SCI, there is expression of Fas ligand by microglia and lymphocytes and FasR by oligodendrocytes (Casha et al., 2001, 2005; Nagata et al., 1995). It is well acknowledged that one potential method of initiating the caspase cascade is through the interaction of the Fas ligand and Fas receptor. Proteolysis and deoxyribonucleic acid (DNA) cleavage are part of apoptosis. Blocking the caspase cascade blocks apoptosis. In animal studies, apoptosis readily occurs in oligodendrocytes following ischemic injury, thus resulting in axonal demyelination (Totiu et al., 2005). This phenomenon is not clearly witnessed in postmortem examination of human SCI (Kakulas, 2004; Norenberg et al., 2004).
The subacute period lasts from 2 days to 2 weeks. It is during this time that the phagocytic response is responsible for removing cellular debris. The removal of growth-inhibiting compounds found in myelin debris can potentially have some beneficial effects on the efforts of axonal recovery (Donnelly and Popovich, 2007). Astrocytes also reach peak numbers in the subacute period. They form a scar that prevents axonal regeneration in rodent studies. The presence of the astroglial scar is less obvious in humans (Hagg and Oudega, 2006). Despite suspected negative effects on healing, they have important roles in ionic homeostasis and reestablishing the blood-brain barrier, thus limiting the immigration of immune cells and edema.
Clinical Presentation
The majority of SCIs occur in the cervical spine (55%) (Sekhon and Fehlings, 2001). Other injuries are evenly divided among the thoracic, thoracolumbar, and lumbar regions. The most frequent injuries suffered are incomplete tetraplegia followed by complete paraplegia, complete tetraplegia, and incomplete paraplegia.
In general, SCIs can be categorized into complete injuries and incomplete injuries. In complete injuries, there is an absence of motor, sensory, and bowel and bladder function below the level of injury. There is some preservation of neurological function with incomplete injuries. At present, SCIs are graded using the American Spinal Injury Association/International Medical Society of Paraplegia (ASIA/IMSOP) Impairment Scale (Box 50C.1) in conjunction with motor grading provided by the Medical Research Council Muscle Grading System (Table 50C.1). This grading system provides a standard method by which clinicians and researchers can classify SCIs. In defining the level of the injury, the most caudal segment at which there is normal motor and sensory function is taken into account. This may differ from the level in the vertebral column where the injury occurred.
| Grade | Physical Examination Finding |
|---|---|
| 5 | Full ROM against full resistance |
| 4+ | Full ROM against nearly full resistance |
| 4 | Full ROM against moderate resistance |
| 4− | Full ROM against some resistance |
| 3 | Full ROM against gravity |
| 2 | Full ROM with gravity eliminated |
| 1 | Partial or trace muscle contraction |
| 0 | No muscular contraction |
ROM, Range of motion.
Modified from Aids to the Examination of the Peripheral Nervous System, 1986. Baillière Tindall on behalf of the Guarantors of Brain, London.
Spinal Cord Injury Syndromes
Central Cord Syndrome
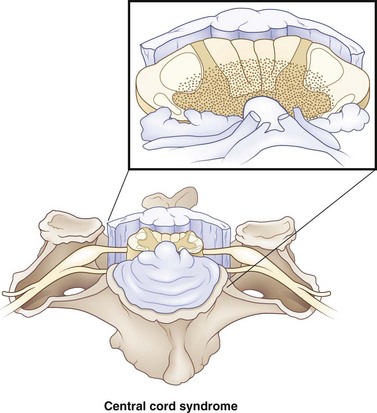
Central cord syndrome is present in 9% of all traumatic cord injuries and is the most common of the spinal cord syndromes. This is a condition first reported by Thornburn in 1887 and then popularized by Schneider et al. in 1954. Hyperextension in the cervical spine, with some preexisting cervical spondylosis, is usually responsible for this type of injury. Imaging the cervical spine in patients with central cord syndrome will reveal stenosis from spondylosis, fracture subluxation, or sequestered disk, with no spinal stenosis. Schneider proposed that these injuries resulted from acute compression from preexisting bone spurs anteriorly and hypertrophied ligamentum flavum posteriorly, and contributed to hematomyelia and central cord necrosis (Fig. 50C.1). Schneider witnessed weakness in the upper extremities greater than the lower extremities, as well as a variable degree of sensory disturbances and loss of bladder control. It was proposed that pyramidal signs due to involvement of the anterior horn cells led to weakness in the arms greater than the legs, secondary to the topography of the corticospinal tracts. Because of their good recovery, Schneider was in favor of taking a more conservative approach toward treating these patients. In his case series, he did note that the majority of his patients had permanent disability in their hands that rarely recovered. An alternative explanation takes into account the belief that a greater focus of the corticospinal tracts is dedicated to supplying the distal musculature in the upper extremity (Levi et al., 1996). Thus, injury to the corticospinal tract produces more significant weakness in the upper extremity, with concentration in the hands. Correlations of magnetic resonance imaging (MRI) (Quencer et al., 1992) and histopathology (Martin et al., 1992; Jimenez et al., 2000) fail to suggest hematomyelia from Schneider’s hypothesis. There is in fact minimal disruption of the central gray matter. Axonal disruption and swelling is more widespread in the white matter.
Anterior Cord Syndrome
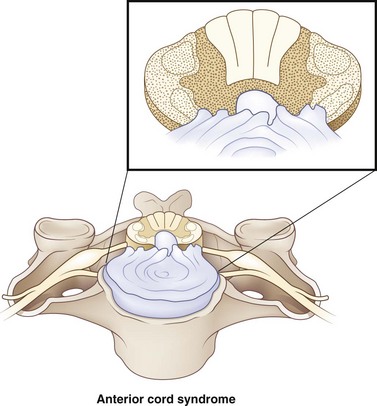
Anterior cord syndrome occurs with injuries to the ventral two-thirds of the cord, while sparing the posterior column (Fig. 50C.2). It is present in 2.7% of all traumatic SCIs (McKinley et al., 2007). Motor function is lost distal to the site of the injury. Spinothalamic function may be disrupted, leading to hyperesthesia and hypoalgesia below the level of the lesion. Though this syndrome is classically described for anterior spinal artery compromise, in the setting of trauma, it is due to flexion injuries or retropulsed disk or bone. Anterior cord syndrome carries a worse prognosis than other cord syndromes.
Posterior Column Syndrome
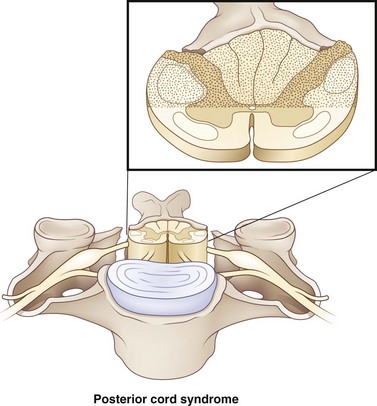
Posterior column syndrome is a rare condition with an incidence of less than 1%. This syndrome has been linked to neck hyperextension injuries. Injuries occur to the posterior aspect of the cord (Fig. 50C.3). Since the posterior columns are injured, there is usually a loss of position sense, with retained spinothalamic function. Motor function can be affected as well. Although this syndrome has been mentioned in the literature, it has been recently omitted from the International Standards for Neurological and Functional Classification of SCI (revised 2006) and is not currently recognized as a separate syndrome.
Brown-Séquard Syndrome
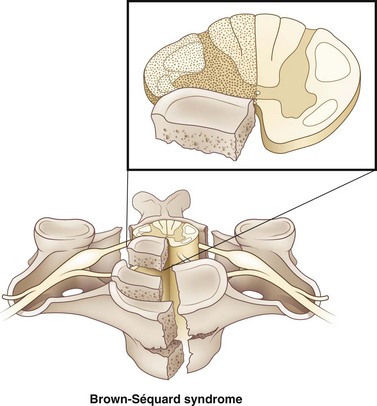
Brown-Séquard syndrome accounts for 1% to 4% of all traumatic SCIs. Injuries affect the lateral half of the cord (Fig. 50C.4). It occurs most frequently in the cervical spine and is usually due to penetrating injuries and (less commonly) blunt trauma including disk herniations. In cases of blunt trauma, Brown-Séquard syndrome usually occurs in the context of hyperextension injuries, though it has been observed in flexion injuries, locked facets, and compression-related injuries. Below the level of the lesion, it classically manifests with ipsilateral pyramidal deficit, loss of ipsilateral tactile discrimination, position sense, and vibratory sensation, and loss of pain and temperature sensation on the contralateral aspect of the body one to two dermatomes below the level of the injury.
There is rarely this classic presentation of Brown-Séquard syndrome, however. More frequently, patients presenting with Brown-Séquard syndrome present with a variation of the classic syndrome, termed Brown-Séquard plus (Taylor and Gleave, 1957). With Brown-Séquard plus, there is asymmetrical hemiplegia as well as hypalgesia more prominent on the less paretic side. Patients presenting with a clinical picture consistent with a classic Brown-Séquard syndrome injury have a worse prognosis than patients presenting with a variation of the syndrome, but the overall prognosis is good. Brown-Séquard has the best functional motor recovery when compared to other clinical spinal cord syndromes. Most subjects obtain bowel and bladder continence. Patient having predominantly more weakness in the upper extremities compared to the lower extremities have a favorable outcome in regard to ambulating. The symptoms of Brown-Séquard syndrome may appear instantaneously or in a delayed fashion. Furthermore, they may occur in conjunction with other spinal cord syndromes.
Conus Medullaris Syndrome
There is high probability that injuries to the thoracolumbar region can involve the conus medullaris. The conus medullaris represents the transition of the spinal cord from the central nervous system to the peripheral nervous system. The location of this region is highly variable—between the T12-L1 disk space to the middle third of L2 in the majority of the population (Fig. 50C.5). Thus, injury to the spinal column at the T12-L1 junction portrays inaccuracies regarding the exact injury to the neurological system. The lumbar parasympathetic fibers, sacral sympathetic fibers, and sacral somatic nerves originate in the conus medullaris. The classic presentation entails lower-extremity weakness, absent lower-limb reflexes, and saddle anesthesia. There is usually mixed upper motor neuron and lower motor neuron involvement. Loss of the bulbocavernosus and anal reflexes is permanent, differentiating conus medullaris syndrome from SCIs that have a return of these reflexes within 48 hours of the injury. Patients typically have an areflexic bowel and bladder (low-pressure, high-capacity bladder). The most common injuries to the vertebral column resulting in this condition are burst fractures or fracture-dislocation. There is no strong clinical evidence favoring surgical intervention over nonsurgical intervention for conus medullaris injuries. Furthermore, if surgical intervention is performed, there is no compelling evidence to suggest that earlier decompression affects functional outcome.
Cauda Equina Syndrome
The cauda equina is defined as the region of the neuroaxis occupied by the filum terminale. The only neurological structures in this region include the lumbar and sacral roots. Injuries in this location are typically a pure lower motor neuron injury (Fig. 50C.6). Findings often include absent bulbocavernosus reflex, absent deep tendon reflexes, flaccid urinary bladder, and reduced lower-extremity muscle tone. It is differentiated from conus medullaris syndrome by the presence of asymmetrical weakness and the absence of upper motor neuron involvement (Table 50C.2). Like conus medullaris syndrome, burst fracture and fracture-dislocation are the most common vertebral column injuries associated with this condition. Cauda equina injuries have better recoveries owing to the resiliency of the roots to injuries and the greater regeneration capacity of the roots compared to the spinal cord. The sacral roots, however, are very delicate, and injuries to them may be permanent. In general, cauda equina syndrome in the setting of herniated disk pathology is treated early (within 24 hours) if possible to prevent residual symptoms (Kennedy et al., 1999). Functional outcome in a traumatic setting, however, is similar to conus medullaris syndrome. There is no strong evidence correlating functional outcome to surgical decompression, nor is there any evidence that suggests cauda equina injuries fare better with early versus late decompression.
Table 50C.2 Similarities and Differences between Conus Medullaris Syndrome and Cauda Equina Syndrome
| Conus Medullaris Syndrome | Cauda Equina Syndrome |
|---|---|
| Upper and lower motor neuron involvement | Lower motor neuron involvement |
| Symmetrical motor impairment | Asymmetrical motor impairment |
| Vertebral column injuries between T12-L2 | Vertebral column injuries distal to L2 |
| Absent deep tendon reflexes | Absent deep tendon reflexes |
| Permanent areflexic bladder | Permanent areflexic bladder |
| Absent bulbocavernosus reflex | Absent bulbocavernosus reflex |
Transient Spinal Cord Syndromes
An estimated 7.3 out of 10,000 football participants suffer a cervical cord neuropraxia (Torg et al., 1997). Cervical cord neuropraxia is typically described as any motor or sensory complaints in any extremity lasting 15 to 30 minutes, but some cases can last up to 24 to 48 hours (Bailes, 2005). This injury is typically due to hyperextension, hyperflexion, or axial loading of the cervical spine. Cervical cord neuropraxia has been attributed to local anoxia and elevation of intracellular calcium (Torg et al., 1995). The description Bailes gave was “pathophysiologically similar to cerebral contusions, spinal cord concussion has become accepted to define those instances in which sufficient forces result in temporary inhibition of spinal cord impulse transmission without causing structural damage to the vertebral column or spinal cord, and is known to occur in athletes.” Bailes also concluded that “a single episode of temporary spinal cord dysfunction in an athlete with spinal stenosis will substantially increase the risk of future catastrophic SCI.” In his series, patients who returned to contact sport activities with no effacement of cerebrospinal fluid (CSF) around the cord or any radiographic abnormalities suggestive of cord damage encountered no further episodes of recurrent transient SCI with a mean follow-up period of 40 months.
Spinal Shock
Some characteristics of spinal shock have been noted. The severity of the injury correlates with the severity of spinal shock. An injury alters reflexes that occur closest to the insult first, with those more distal from the transection presenting later. Thus, high-level cervical injuries may have retention of sacral reflexes, such as a preserved bulbocavernosus and anal wink. The observation that a proximal-to-distal spread of reflex depression occurs on the order of minutes suggests a physiological explanation for these changes. It has been hypothesized that the loss of supraspinal input leading to hyperpolorization of neurons is responsible for this physiological change. There have been additional observations that an upward spread of reflex depression, the Schiff-Sherrington phenomenon, is not uncommon. It is important to delineate blood pressure drops from circulatory shocks from those of spinal shock (Table 50C.3). As there is loss of sympathetic tone, there is pooling of blood in the venous system and a loss of sympathetic tone in the cardiovascular system. On the one hand, circulatory shock requires volume replacement, and on the other hand, spinal shock requires vasopressors. As spinal shock resolves, muscle spindle reflexes return in a caudal-to-cranial direction, except at the level of injury. Over time, a spastic syndrome results.
Table 50C.3 Similarities and Differences between Neurogenic and Hypovolemic Shock
| Neurogenic Shock | Hypovolemic Shock |
|---|---|
| Hypotension | Hypotension |
| Bradycardia | Tachycardia |
| Areflexia | Normal reflexes |
| Responsive to pressors | Responsive to volume replacement |
There is no uniform consensus on what constitutes the cessation of spinal shock. Most references define the end of spinal shock with a return of certain reflexes. However, not all reflexes are uniformly depressed in each patient; reflexic changes are individualized. The resolution of spinal shock occurs over a period of days to months, so there is a slow transition from spinal shock to spasticity that occurs on a continuum. It has been proposed that this transition comprises four phases (Ditunno et al., 2004). The first phase occurs from 0 to 24 hours following the injury and is characterized by areflexia or hyporeflexia. Deep tendon reflexes are absent. During this period, the first pathological reflex to appear is the delayed plantar reflex, followed by a series of cutaneous reflexes such as the bulbocavernosus, abdominal wall, and cremasteric reflex. Impaired sympathetic control can lead to bradyarrhythmias, atrioventricular conduction block, and hypotension. Motor neuron hyperpolarization explains the changes that occur. Phase 2 occurs between day 1 and day 3 post injury. Cutaneous reflexes are more prominent during this period, but deep tendon reflexes remain mute. It is not unusual for elderly individuals and children to experience recovery of deep tendon reflexes during this time. The Babinski sign may become apparent in the elderly as well. Denervation supersensitivity and receptor up-regulation account for these changes in the second phase. The next phase occurs between 4 days to 1 month post injury. Deep tendon reflexes usually recuperate by day 30. There is great disagreement about when these reflexes appear. The recovery of the Babinski response closely parallels the return of the ankle jerk reflex. There is also diminution of the delayed plantar reflex. Autonomic changes such as bradyarrhythmias and hypotension begin to subside. This time period is reflected by axon-supported synapse growth. The fourth phase is dominated by hyperactive reflexes and occurs from 1 to 12 months after injury. Vasovagal hypotension and bradycardia generally resolve in 3 to 6 weeks, but orthostatic hypotension may take 10 to 12 weeks before it disappears. Episodes of malignant hypertension or autonomic dysreflexia begin to appear during this time period. Soma-supported synapse growth accounts for these findings.
Mechanisms and Types of Injuries
Cervical Spine Fractures
Atlanto-Occipital Dissociation
Atlanto-occipital dissociations occur from high-energy impact and frequently lead to death. When the diagnosis is missed, subjects can have poor outcomes. These injuries result in laceration of the pontomedullary or spinomedullary junctions and are more common in children because of the horizontal orientation of their atlanto-occipital joint. Atlanto-occipital dissociations are classified into three types based on the dislocation of the condyles in relation to the atlas: type I, anterior; type II, vertical; and type III, posterior (Traynelis et al., 1986). Patients who survive this injury can present with cranial neuropathy or weakness. The diagnosis in survivors can be made by measuring the dislocation on lateral cervical x-rays. Additional findings on x-rays include prevertebral soft-tissue swelling. However, studies with computed tomography (CT) and MRI are recommended in patients with suspected atlanto-occipital dissociation. When craniocervical subarachnoid blood is present, atlanto-occipital dissociation should be suspected. The diagnosis of this injury requires prompt reduction and stabilization in a halo vest, followed by fixation by occipital-cervical fusion. Traction can cause further deterioration and should be avoided.
Occipital Condyle Fractures
Occipital condyle fractures were first described by Bell in 1817 but have been more frequently diagnosed in head injuries since the introduction of CT. They tend to occur with high-energy compression shear forces. The frequency of occipital condyle fractures has been reported as high as 16.4% of patients who undergo high-energy blunt craniocervical trauma (Bloom et al., 1997). Patients can present with a complaint of subtle neck discomfort, but when associated with traumatic brain injury or atlanto-occipital dissociation, more severe presentation may occur. Thus, the variety of presentations seen include low Glasgow Comma Scale values, retropharyngeal soft-tissue swelling, occipitocervical tenderness, reduced craniocervical motion, and lower cranial neuropathy. Before the CT scan, the sensitivity of detection by plain radiography was approximately 3.2% (Hadley et al., 2002).
The classification described by Anderson and Montesano (1988) is still widely used today. According to their classification, there are three types of occipital condyle fractures: (1) a unilateral impacted fracture resulting in comminuted elements, (2) a linear basilar skull fracture that extends into the condyle, and (3) avulsion fractures of the condyle. Type III injuries warrant a high degree of caution, as they may have associated atlanto-occipital dissociation and can bear instability with alar and tectorial membrane disruption. For this reason, they are treated more aggressively with halo immobilization followed by occipital-cervical stabilization. External mobilization should be considered for type I and II injuries.
Atlantoaxial Injuries
Fractures of the axis occur in the odontoid process, pars interarticularis, vertebral body, lateral masses, or spinous process. Vertebral body and spinous process fractures are conservatively treated with external immobilization. The most common injuries to C2 involve the odontoid process. Injuries to the spinal cord result in instability from translational displacement of C1 on C2. Treatment strategies involve preserving axial rotation of the neck, which can be up to 60 degrees at this level. The most widely used classification of odontoid fractures is the Anderson and D’Alonzo classification. Type I fractures project into the upper portion of the odontoid. Type II fractures involve the base of the odontoid. A subgroup of these fractures is known as type IIA, which is a comminuted fracture at the base of the odontoid with associated free fragments. Type III fractures descend into the vertebral body. Type I and type III odontoid fractures are treated with external rigid immobilization with cervical collar for 8 to 12 weeks. There is less blood supply at the base of the odontoid, which factors into lower fusion rates and avascular necrosis than either type I or type III fractures. Furthermore, odontoid fractures occurring in patients older than 50 years of age have a 21-fold risk of nonunion (Lennarson et al., 2000). Thus, strong consideration should given to treating patients with type II odontoid fractures with halo bracing for 8 to 12 weeks. If no fusion is seen, surgical stabilization is necessary. For patients with type II fractures who are older than 50 years of age and have displacement greater than 5 mm, or with a comminuted component at the base of the odontoid (type IIA), surgical stabilization should be considered as the first treatment option. Usually, early surgical treatment with an odontoid screw can preserve C1/C2 rotation. Surgical stabilization following failed nonunion of odontoid fractures with nonoperative treatment requires a C1/C2 fusion limiting rotation.
Subaxial Cervical Spine Injuries
Flexion-compression injuries are most often due to ventral axial loading. The prototypical cause of this injury is the classic diving injury. As a result of the forces directed on the body, there are compressive fractures seen in the anterior vertebral body. Posterior element fractures can occur in up to 50% of cases. There can be mild distraction of the facets and disruption of the posterior ligaments. With intact facets, these injuries are stable and can be treated with external immobilization. Vertical compression fractures lead to burst fractures. Surgery is warranted for retropulsion of bony elements into the canal leading to neurological deficits. Mechanical stability must be assessed for each of these cases. Teardrop fractures represent the extreme variant of these injuries and result from severe hyperflexion and axial forces. They appear as a fractured vertebral body with associated retrolisthesis and posterior ligamentous disruption and dislocation. These are highly unstable fractures and require surgical stabilization. Flexion-distraction injuries typically involve minimal osseous injury, with a predominance of ligamentous injury. They vary in severity from hyperflexion strain to bilateral jumped facets. Thus, MRI can typically be used to evaluate the extent of these injuries that is not obvious on lateral C-spine x-rays. A significant number of subjects who are diagnosed with jumped facets have neurological deficits; 21% of patients diagnosed with unilateral jumped facets are neurologically intact (Shapiro, 1993), compared to only 10% of patients diagnosed with bilateral jumped facets (Wolf et al., 1991). Treatment of locked facets begins with attempts at closed reduction with traction. Failed attempts warrant an open reduction. Once reduced, stabilization is required, with surgical fixation being the preferred method. Compressive-extension injuries can cause vertebral arch and laminar fractures and may lead to instability and a need for surgical stabilization. Extension-distraction injuries are common in falls and can produce central cord syndrome in the setting of trauma in an elderly patient with baseline cervical spondylosis. No fractures or ligamentous injuries may be present, but buckling of the ligamentum flavum is sufficient to damage the cord. Surgical decompression is beneficial to these patients, but the timing of such decompression is controversial. Lateral flexion injuries may lead to unilateral vertebral body or posterior arch injuries. They are usually stable and can be treated with external immobilization.
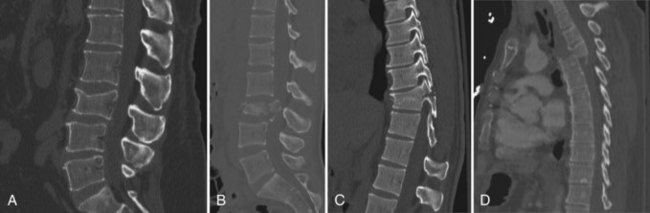
Thoracolumbar Injuries
In general, the thoracic and lumbar spine is divided into three segments: an anterior column, middle column, and posterior column. The anterior column extends from the anterior longitudinal ligament to the middle of the vertebral body. The middle column is defined as the portion between the middle of the vertebral body to the posterior longitudinal ligament. The posterior column is the remaining extent of the vertebrae. The classification of thoracolumbar fractures utilizes the three-column model of the spine. A CT scan can provide precise information regarding the extent of injury. There are four major categories of thoracolumbar spine fractures (Fig. 50C.7). Compression fractures involve compression of the anterior body, leading to wedging. Compression of the anterior and middle column is seen in burst fractures. With burst fractures, radiographic indications for surgery are typically loss of vertebral height of 50%, 30 degrees of kyphosis, or 50% canal compromise from retropulsion of elements. There may be neurological deficits associated with retropulsion. Seat belt injuries involve the middle and posterior column. Patients are generally neurologically intact. However, these injuries are typically deemed unstable fractures and should be treated with surgical stabilization. In fracture-dislocation injuries, involvement of all three columns is seen. The radiographic appearance of these injuries suggests a flexion-rotation, sheer, or flexion-distraction mechanism. These fractures are highly unstable and require surgical intervention.
Penetrating Spinal Cord Injuries
The majority of penetrating SCIs are due to gunshot wounds to the spine and second only to automobile accidents in causing spinal cord–related disability. Stab wounds to the spine are less commonly seen and present with Brown-Séquard features. When making an evaluation of gunshot injuries to the spine, the trajectory of the bullet must be considered in addition to the physical location of the bullet. It is important to assess whether bowel penetration and contamination is present. The destructive nature of a bullet is related to direct injury from the bullet itself, the shock waves it creates, and temporary cavitation. These factors are dependent on the size and velocity of the missile. On examination, injuries can present one level higher than the observed location of the bullet. CT scan can assess for instability and be more helpful than MRI, which can also be safely done. MRI will not typically influence acute treatment decisions unless an evolving hematoma is present. There has been debate over whether patients benefit from laminectomy and bullet removal with incomplete injuries. The National Institute of Disability and Rehabilitation Research suggests that there is no benefit from such heroic measures. However, patients with incomplete injuries with neurological deterioration should undergo decompression. The other indication for decompression includes injuries located at or below the level of the conus. Spinal nerve roots have a greater capacity to recover after decompression. There are cases of late neurological deterioration occurring as late as 17 years post injury (Ajmal et al., 2009). In these cases, improvement of symptoms can be seen after excision of the bullet and the surrounding reactive tissue. Spinal instability should warrant surgical stabilization. Infection can be common with a contaminated bullet causing viscus perforation and entering the spine. In a civilian population, long-term antibiotics (2-week course) have been shown to have favorable outcomes (Roffi et al., 1989). Steroids should be avoided for penetrating spine injuries.
Management of Acute Spinal Cord Injuries
Radiographic Evaluation
Plain Radiography
Initial radiographic evaluation of the spine should be done with plain x-rays. The decision to obtain cervical spine imaging is based on the NEXUS (National Emergency X-Radiography Utilization Study) criteria (Hoffman et al., 2000), a set of five screening assessments created to help guide physicians in making a decision to exclude low-risk/low-yield patients from undergoing cervical radiography. Patients meeting all the criteria in Box 50C.2 can bypass imaging. This study reported a 99% sensitivity and a 12.9% specificity in diagnosing SCIs when using these criteria. More recently, the Canadian C-Spine Rule was noted to be superior to the NEXUS criteria in terms of sensitivity and specificity for alert, stable patients in whom cervical spine injury is a concern (Stiell et al., 2003). Screening criteria include high-risk factors, low-risk factors, and ability to actively rotate the neck. Using the Canadian C-spine Rule, patients older than age 65 who are subject to a “dangerous mechanism” or experience paresthesias (high-risk criteria) should receive plain radiography. Those not meeting any of the high-risk criteria are assessed further. If this subgroup of patients have delayed pain in the neck, posterior neck tenderness, cannot tolerate sitting or ambulatory position at any time after injury, or are involved in an accident that is more than a simple rear-end motor vehicle collision (low-risk criteria), then imaging should be obtained. If they lack any of the listed findings, they are further assessed for their ability to rotate the head. Plain radiography should be obtained for patients incapable of rotating their head 45 degrees in either direction.
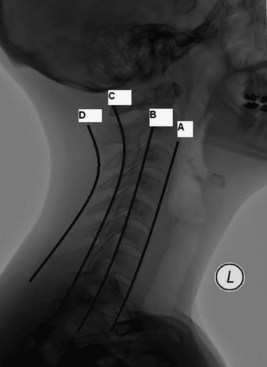
Typical plain radiography of the cervical spine should include anteroposterior (AP), open odontoid, and lateral views with flexion-extension. An adequate lateral C-spine x-ray should visualize the area between the occiput and the top of T1. A so-called swimmer’s view may be helpful to view the caudal portion of the cervical spine. Four lines should be drawn on a lateral C-spine x-ray to evaluate for subluxation or fractures: (1) anterior vertebral body line, (2) posterior vertebral body line, (3) spinal laminar line, and (4) posterior spinous line (Fig. 50C.8). Findings showing more than 3.5 mm of subluxation or kyphotic angulation greater than 11 degrees to adjacent vertebral body segments can imply instability. Subluxation on the order of 25% or 50%, respectively, suggests unilateral or bilateral jumped facets. Mild flexion distraction injuries can be suggested by enlargement of the interspinous distance. The atlantodental interval (ADI) is a measure taken from the anterior margin of the dens to the closest portion of the anterior arch of C1. A value greater than 3 mm can suggest transverse ligament disruption. The Powers ratio is defined as a ratio of the distance from the basion to the posterior arch of the atlas divided by the distance from the opisthion to the anterior arch of the atlas. Atlanto-occipital dissociation can be suggested with a Powers ratio greater than 1. Prevertebral soft-tissue swelling should also be noted on the lateral C-spine x-rays. The upper limit of normal at the level of C3 is 4 mm. The amount of lateral mass overhang of C1 on C2 seen on the odontoid views can be measured to assess the integrity of the transverse ligament. A sum of lateral mass overhang greater than 7 mm is suggestive of transverse ligament disruption.
Complete x-rays of the whole spine should performed if any abnormalities of the spine are detected on imaging, since noncontiguous spine injuries are seen in 10.5% of cases (Vaccaro et al., 1992). Plain radiographs of the thoracic and lumbar spine should include AP and lateral films to assess for alignment, kyphosis, disk height, and fractures. Thoracic fractures can be missed on x-rays and may require a CT or MRI if injury is suspected in this region. Scoliosis films can detect present deformity and sagittal imbalance but more importantly can be used as a baseline study for assessing progressive posttraumatic kyphosis.
SCIWORA and SCIWORET
Spinal cord injuries without radiographic abnormalities (SCIWORA) were first diagnosed in 1982 before the MRI era (Pang and Wilberger, 1982). Since the advent of MRI, there has been a broad spectrum of injuries present in SCIWORA patients that range from normal MRI imaging to complete cord disruption or abnormal disk pathology. SCIWORA are more commonly encountered in the pediatric population. The incidence of SCIWORA in cases of traumatic myelopathy of children between 1 and 17 years of age has been estimated at 34.8% (Pang, 2004). Reasons to suggest a higher incidence in the pediatric population center around the laxity of ligaments, expandability of the intervertebral disk, and the biological and anatomical differences noted in the spine between children and adults. Other considerations include the proportionally larger size of children’s heads and the lack of development of paravertebral muscles. Since the advent of the MRI, the definition of SCIWORA has been revised to exclude compressive lesions found on MRI, but not intraneuronal lesions. Some practitioners feel that any spinal cord lesions should be excluded in the definition. The acronym SCIWORET for spinal cord injury without radiographic evidence of trauma has recently been seen in the context of medical literature. SCIWORET was intended to refer to SCI without evidence of bony or ligamentous disruption in adults with baseline spondylotic changes, typically presenting with central cord syndrome. Thus, the definition of SCIWORA has been ambiguous depending on the strictness of the inclusion criteria.
Treatment of Spinal Cord Injuries in the Acute Setting
A significant reduction of morbidity and mortality for SCI patients receiving immediate care in an ICU setting is suggested by much of the relevant literature. Patients have improved neurological function, for example, if they receive care within 48 hours of suffering their injury (Zach et al., 1976). More aggressive monitoring and treatment of respiratory insufficiency have correlated with improved mortality for these patients (Hachen, 1977). With early management of hypotension and respiratory insufficiency, improved neurological function and overall mortality is noted (Gschaedler et al., 1979). Prospective analyses have reaffirmed these opinions by showing improved survival, reduced pulmonary complications, and reduced need of ventilatory support with aggressive pulmonary toilet in the ICU setting (McMichan et al., 1980). Tator reviewed his institutional experience with a specialized ICU center dedicated to spinal cord trauma (Tator et al., 1984). In this unit, they aggressively treated hypotension and reported a mean time of 4.9 hours between the time of injury to the time of admission and treatment. They were able to show that 43% of patients improved neurologically. In their analysis, they noted reduced morbidity and mortality, reduced length of hospital stay, and reduced cost of treatment.
Cardiovascular Management in the Intensive Care Unit Setting
There can be an impairment of sympathetic control with high thoracic or cervical cord injuries. In general, complete cervical injuries are associated with the highest risk of needing vasopressor support (Ploumis et al., 2010). With unopposed parasympathetic activity from the vagus nerve, bradycardia, hypotension, and other arrhythmias result. Lesions higher than T6 will also affect the supraspinal influence to the splanchnic bed, as well as the vascular supply to the lower extremity. As a result of the denervated sympathetic vascular bed, there is potential for up-regulation or hypersensitivity of denervated peripheral α-adrenoreceptors. Furthermore, there is evidence to suggest that there is decreased presynaptic noradrenaline uptake. Following the obvious disruption of descending cardiovascular pathways that takes place with acute injuries, there are a series of known changes in the autonomic system that contribute to abnormal cardiovascular control. They include (1) morphological changes in the preganglionic sympathetic neurons, (2) the formation of inappropriate connections, (3) altered responsiveness and transmission of signal to vascular smooth muscle, and (4) abnormal spinal efferents.
Studies have demonstrated that aggressive medical management of hypotension may be beneficial in improving neurological recovery. One particular study focused on keeping mean arterial pressure (MAP) elevated above 85 mm Hg and early open or closed reduction for bilateral jumped facets (Wolf et al., 1991). This study showed a favorable outcome with these measures. Another study has shown beneficial neurological outcome for patients receiving invasive hemodynamic monitoring and given volume and pressor support to maintain adequate cardiac output and MAP above 90 mm Hg (Levi et al., 1993). A study performed under a prospective setting tested the hypothesis that MAP parameters above 85 mm Hg during the first several days of injury were associated with better outcomes (Vale et al., 1997). This study’s authors noted that 9 of 10 ASIA A cervical injury patients required pressors versus 9 of 29 patients with complete thoracic injuries. In addition, 3 of 10 ASIA A cervical injury patients regained ambulatory capacity at the 1-year follow-up point, while 2/10 patients regained bowel and bladder control. The incomplete cervical injury group had 23 of 25 and 22 of 25 patients recover ambulatory capacity and bladder function, respectively. They further grouped patients into an early, middle, or late period when surgical intervention was performed and found no statistical correlation between timing of surgery and neurological outcome. Their study stressed the importance of aggressive volume resuscitation and blood pressure control in influencing outcome.
Current recommendations based on the 2002 American Association of Neurological Surgeons and the Congress of Neurological Surgeons (AANS/CNS) guidelines, as well as those developed by the Consortium for Spinal Cord Medicine, focus on providing acute critical care for patients who have suffered SCI. This entails transferring these patients to ICU centers, preferably with dedicated SCI units for cardiac and hemodynamic monitoring where cardiac arrhythmias and neurogenic shock can be detected in a timely fashion and treated appropriately. The primary treatment for hypotension is fluid resuscitation to restore preload. Once intravascular volume is restored, pressors should be initiated to keep MAP above 85 mm Hg for 1 week. Pressors should be used with invasive monitoring such as an arterial line and central venous catheters to allow for accurate readings. Dopamine has α-adrenergic, β-adrenergic, and dopaminergic agonist activity. It can counteract hypotension and bradycardia by increasing heart rate and contractility, thus increasing cardiac output. However, the α-mediated vasoconstriction effects are variable. Phenylephrine is a pure α-agonist useful in restoring systemic vascular resistance and increasing MAP. It is less potent than norepinephrine. With a lack of β-adrenergic activity, it can potentially cause reflex bradycardia due to increased end-systolic volume. Patients being treated with phenylephrine may have a difficult time with fluid resuscitation because of the increased partition coefficient of intravascular volume. Norepinephrine is a more logical choice than phenylephrine because of its combined α- and β-adrenergic agonist properties. Preload is restored through decreased venous capacitance. Norepinephrine has some inotropic activity to counteract hypotension and bradycardia. Epinephrine is used in refractory cases because of its potent effects in causing renal, splanchnic, and peripheral ischemia. Vasopressin is usually used in conjunction with norepinephrine and dopamine in septic patients; its role in SCI is yet to be defined. Milrinone and dobutamine can be used to promote cardiac output, but their vasodilatory effects make them less than ideal agents for treating hypotension. No individual pressor is considered the gold standard for treating hypotension in the setting of SCI, and each case should be considered individually (Ploumis et al., 2010).
Respiratory Management in the Intensive Care Unit Setting
Respiratory derangements in SCI patients depend on the extent and level of injury. The innervation of the diaphragm from the phrenic nerve is supplied by C3, C4, and C5. Injuries at or above the C2 level require immediate ventilatory support. Patients with injuries between C3 and C5 may need initial ventilatory support, but as inflammation subsides in the cord, they may regain ventilatory strength and have effective recruitment of accessory muscles. Age and preexisting comorbidities can greatly affect outcomes. Patients can still require ventilatory support with injuries below C5. There is a restrictive respiratory pattern encountered in SCI patients that can result from immobilization or additional physical injuries in the trauma patient such as contusion, pneumothorax, hemothorax, and flail chest; all these can lead to additional compromise. One study has shown a decrease in functional vital capacity (FVC) and expiratory flow rate immediately after injury (Ledsome and Sharp, 1981). Patients with FVC less than 25% had a greater chance of requiring ventilatory support. The authors attributed hypoxemia (Pao2 <80 mm Hg) to a ventilation/perfusion mismatch. They found supplemental oxygen to be beneficial in treating hypoxemia. Other challenges of the restrictive respiratory patterns associated with SCI are decreased compliance, increased effort in breathing, and difficulties in clearing secretions and producing an effective cough. Thus, additional complications such as retained secretions, atelectasis, and pneumonia occur. Complicating factors in the treatment of respiratory problems stem from pulmonary and fat emboli, which can contribute to poor respiratory function.
In acute SCI patients, atelectasis is the most common respiratory-related complication. It can progress to significant pneumonia and respiratory failure. Certain measures can prevent and treat atelectasis: intermittent positive-pressure breathing (IPPB), inflation by inflating bags, or the incorporation of sighs into mechanical ventilation settings. Bronchospasm can result from autonomic changes in acute injury. They are typically seen in the face if IPPB especially in asthmatics and bronchodilator use should be encouraged. Ipratropium has been shown to increase FVC in about half of patients with tetraplegia. Larger tidal volumes (>20 mL/kg) have been shown to decrease atelectasis. Pulmonary edema can occur from fluid overload; other less common causes include cardiogenic failure and pulmonary sources such as acute respiratory distress syndrome, infection, and trauma. Less obvious causes may be excessive antidiuretic hormone secretion or autonomic changes that exacerbate pulmonary edema. Secretions in the acute periods are usually excessive and have a different chemical content, suggesting a neuronal influence. The increase in secretions can be noted as early as the first hour. Mucus plugs can form in conjunction with decreased cough and bronchospasm. Aggressive measures taken by the respiratory therapist have been shown to decrease the incidence of pneumonia and bronchoscopy use in the acute period. Some clinicians have even advocated early fiberoptic bronchoscopy and bronchial lavage to promote clearance of secretions (McMichan et al.,1980). Warm air, bronchodilators, and mucolytics can help improve respiratory status. Intrapulmonary percussive ventilation is a device that delivers high-frequency pulsations to loosen secretions and provide aerosolized medications to the lungs. Manually assisted coughing with a provider or via a device can clear bronchopulmonary secretions. Additional measures include a rotational bed and postural drainage. Although suctioning is widely used and a mainstay of treatment, it can complicate matters by causing hypoxia, hypotension, infection, tracheal mucus drainage, vagus nerve stimulation, and increased mucus production. Its effectiveness can be limited by patient fears and anxieties.
Pneumonia is a common complication related to the use of mechanical ventilation, with a risk of 1% to 3% per day of mechanical ventilation (Ball et al., 2001). The culprits of ventilator-associated pneumonia occurring within the first 4 days are usually Haemophilus influenzae and Staphylococcus pneumoniae. Only when Pseudomonas aeruginosa is suspected should double coverage with antipseudomonal β-lactam agents and aminoglycosides be considered. Prevention of aspiration and monitoring for its effects are essential for optimal care. Patients with diabetes can have preexisting gastric atony. A nasogastric tube is needed to treat an ileus. Patients on tube feeds require monitoring of their gastric residual content after feeding.
Respiratory failure is defined as a Pco2 over 50 mm Hg or Po2 less than 50 mm Hg on room air, or the requirement of ventilatory support in the setting of a high cervical injury. It is prevalent in 40% of subjects with C1-C4 injuries, 25% of subjects with C5-C8 injuries, and 9.9% of subjects with thoracic injuries (Jackson and Groomers, 1994). Patients with injuries in the high cervical cord may suffer neurological deterioration in subsequent days as the injury ascends superiorly in the cord. There is no standard protocol in providing ventilatory support to this group of patients, but simple principles can be used. Patients with high cervical injuries will typically need full ventilatory support with controlled mechanical ventilation. SCI patients who are alert and maintain the capacity to initiate respiratory effort may require intubation, especially when FVC decreases to less than 10 to 15 cm3/kg. Patients with low FVC may have difficulties producing an effective cough and compromised bronchial hygiene. They do not require controlled ventilatory settings that promote ventilator dyssynchrony. Instead, they can benefit from pressure support modes to reduce ventilation/perfusion mismatches. Invasive respiratory support can incapacitate an individual’s defense mechanisms. The clearing mechanism of the cilia and the cough reflex become impaired. In addition, speech can be affected. Because of this, it may be beneficial to use noninvasive support. Patients with intact respiratory musculature but with decreased compliance from associated injuries may benefit from noninvasive support such as bilevel positive airway pressure (BiPAP) or continuous positive airway pressure (CPAP).
There is considerable debate over where to place tidal volume for SCI patients. Higher tidal volumes can be used to prevent atelectasis. Typically, tidal volume averages no higher than 6 to 8 mL/kg are used to avoid barotrauma, but one study suggested that tidal volumes as high as 20 mL/kg are needed to avoid atelectasis and prevent prolonged weaning times (Peterson et al., 1997). Regardless, tidal volumes can be increased in a manner to resolve atelectasis on chest x-ray. Peak pressure should never exceed 40 mm Hg.
Medical Management
There have been at least four prospective randomized trials studying steroid effects, with great controversy in interpretation of the methods and results from these studies. The National Acute Spinal Cord Injury Study (NASCIS) I trial compared two sets of patients receiving methylprednisolone (Bracken et al., 1985). One group received 100 mg of methylprednisolone followed by 25 mg every 6 hours for 10 days. The other arm used doses with 10 times that magnitude. Patients were followed up to 1 year after treatment, and results failed to show any significant difference between the two arms despite lacking a placebo. Following this study, NASCIS II was performed to include three groups: a placebo group, an opiate antagonist group using naloxone, and a methylprednisolone group that received a 30 mg/kg bolus over an hour, followed by 5.4 mg/kg/h for the next 23 hours (Bracken et al., 1990). Patients receiving methylprednisolone before 8 hours after injury were separated from patients receiving treatment after 8 hours following their injury. The authors suggested that patients with acute SCIs treated within 8 hours of the accident benefited from methylprednisolone. Furthermore, they pointed out that patients treated with methylprednisolone after 8 hours did significantly worse than the placebo group. The original author performed an ad hoc subgroup analysis to support his conclusions (Bracken et al., 1992). Although the entire patient sample consisted of 487 patients, this study showed encouraging results from a subgroup of patients receiving treatment within 8 hours of injury. This was not the case for patients receiving treatment 8 hours after injury. Since that time, there have been many critical analyses of the methods, statistical analysis, and scientific interpretation of the study. One of the most critical arguments stems from the lack of functional measures to assess outcome. Independent chart analysis shows that the placebo group treated within 8 hours of injury had similar recovery patterns to the corresponding methylprednisolone group for the first 6 weeks. Afterwards, the recovery plateaued. Most clinicians feel that incomplete injuries that show functional improvement at 6 months continue an ongoing trend of recovery. Chart analysis also showed that the placebo group treated within 8 hours of injury did worse than the group treated with placebo 8 hours after injury. In addition, the placebo group treated after 8 hours from the onset of injury had similar results to the methylprednisolone group treated within 8 hours of the injury. The study lacked any information regarding the timing or type of surgical interventions that were performed for these patients. Statistical tools have been criticized as being excessive, confusing, and difficult to replicate by professional statisticians. Furthermore, data from this study have never been made available for independent review. Only one study has attempted to model the NASCIS II study (Otani et al., 1994). Their results mirrored the NASCIS II data. Although results were published as Class I–level evidence, their methodology was not well reported, and they deviated from classical standards of a prospective randomized double-blinded study. NASCIS III attempted to evaluate the effects of methylprednisolone administered in a 24-hour versus 48-hour setting (Bracken et al., 1997). An added treatment arm received 48 hours of tirilazad, a medication with antioxidant properties. All patients received a bolus of 30 mg/kg followed by 5.4 mg/kg/h for the 24- and 48-hour methylprednisolone groups, or 2.5 mg of tirilazad every 6 hours. The authors noted neurological improvement at the 6-week and 6-month postinjury period for the 48-hour methylprednisolone group if medication was given between 3 and 8 hours post injury. Randomization bias was noted in NASCIS III, and there were no criteria established for a minimal motor deficit needed for participation in the study. Patients who had no motor deficits were included in the study, complicating matters.
Potential side effects related to methylprednisolone use in SCI patients include pulmonary embolism, sepsis, pneumonia, gastrointestinal hemorrhage, and wound infection. Recently, steroids have been demonstrated to increase the risk of major complications (Dimar et al., 2010). Because these side effects are substantial, methylprednisolone is used with reservations today.
Three additional agents considered to have neuroprotective effects were studied prospectively with randomized double-blinded clinical trials. Thyroid releasing hormone (TRH) has been tested in humans for its antagonistic effect on secondary injury mediators. Only one clinical trial tested this hypothesis in humans (Pitts et al., 1995). This study showed a statistically significant improvement for patients with incomplete SCI who received TRH, but the study was limited in statistical power by virtue of its small study size. Gacyclidine is an NMDA receptor antagonist known to compete against glutamate. Studies have not shown a significant benefit for incomplete cervical injuries at 1 year post injury (Tadie et al., 1999). Nimodipine has been studied for its ability to impede calcium-dependent injury in the secondary stages. Despite animal studies showing benefit, human controlled trials failed to show any benefit (Petitjean et al., 1998).
Nutritional Support
Compromised gastrointestinal and immune integrity alter normal physiology and factor into the timing of initiating a diet. It is generally acceptable to provide early nutritional support to meet caloric requirements, counteract losses of muscle mass, and maintain gastrointestinal and immune integrity. There has also been evidence to refute fears that early feeding leads to increased septic complications (Dvorak et al., 2004), but the clinician should always be cognizant of a paralytic ileus, which can promote aspiration in light of cervical immobilization and swelling.
Stabilization and Support
Nonsurgical Management
Closed reduction has been used to provide spinal realignment in the setting of facet fracture, jumped facets, subluxation, or spinal deformity as a primary means of realignment before open reduction is attempted. Successful reduction of jumped facets can be safely done. It has been shown that patients who undergo successful closed reduction can have better outcomes than those patients requiring surgery (Papadopoulos et al., 2002). Patients must be awake and cooperative and provide a reliable neurological exam. Traction is performed with the patient in supine position. The head is placed in Gardner Wells tongs or a halo ring attached to a set of weights by a rope suspended off the side of the bed via a pulley mechanism. A distracting force is applied with added weights. The initial weight for traction is usually 3 lbs multiplied to the level of injury in the cervical spine. Weight is added in 5- to 10-lb increments, spinal alignment is checked with fluoroscopy, and a neurological exam is performed at 10- to 15-minute intervals. In general, there is no defined upper limit of traction that should be applied. Subjective pain and neurological deficit should discourage any further traction. Furthermore, if the weight applied shifts the patient in bed, traction should be halted. Once spinal realignment is achieved, the patient may either be locked into the halo vest or taken to the operating room in traction, where surgical stabilization can be achieved.
Surgical Management
Indications for surgery include decompression, stabilization, and correction of deformity. In a general sense, White and Panjabi define spinal stability as the “ability of the spine under physiological loads to limit patterns of displacement so as not to damage or irritate the spinal cord or nerve roots and, in addition, to prevent incapacitating deformity or pain due to structural changes” (White and Panjabi, 1990). The initial radiographic workup may suggest spinal instability, but more often, clinical judgment based on history and physical examination in conjunction with follow-up imaging can help establish a more definitive diagnosis of spinal instability. For acute fractures and dislocations, the timing of events in relation to the presentation and the completeness of the injury should be noted. Traditionally, complete injuries (ASIA A) were typically treated with the goal of surgical stabilization; there is definitely a stronger argument for early decompression for incomplete versus complete injuries. Prospective randomized trials done with animals show neurological improvement with early surgical decompression for SCI (Rabinowitz RS et al., 2008). Initial prospective randomized trials suggest that patients undergoing early surgical decompression (<72 hours post injury) do not fare better than patients undergoing late decompression (>5 days post injury) (Vaccaro et al., 1997). The benefit from surgical decompression is likely to be greater if done sooner than the 72-hour time window. In a systemic review of the literature, early decompression was found to have better neurological outcomes than late decompression if done within 24 hours of the injury (La Rosa et al., 2004). Surgery in the early period has been shown to be safe when there are stable hemodynamic parameters with monitoring and expert surgical and anesthesia staff are present (Fehlings and Perrin, 2006). Early results from the Surgical Treatment for Acute Spinal Cord Injury Study (STASCI) suggested improved neurological outcome for early decompression done within 24 hours post injury (Fehlings et al., 2008). Although traumatic central cord injuries are included in the STASCI study, the timing of surgery in traumatic central cord syndrome may be more controversial. Cervical pathology contributing to traumatic central cord syndrome are divided into one of three categories: (1) cervical spondylosis in the setting of segmental spinal stenosis or anterior pathology from disk/osteophyte complex; (2) fracture subluxations, and (3) disk sequestration with no evidence of spinal stenosis. Favorable results in motor recovery and cost effectiveness have been recorded for decompression of disk herniation and fractures causing central cord syndrome (Guest et al., 2002). A recent study showed no difference in outcome for patients with acute traumatic central cord syndrome treated with surgical decompression when comparing the timing of surgery, the surgical approach, or the type of cervical pathology (Chen et al., 2009). Currently, prospective randomized trials are underway to assess whether patients undergoing early decompression (<5 days) fare better than patients undergoing late decompression for traumatic central cord syndrome.
Recently the Spine Study Trauma Group attempted to provide a standard protocol to guide physicians in treating thoracolumbar fractures. As a result, the Thoracolumbar Injury Severity Score (TLISS) and Thoracolumbar Injury Classification and Severity Score (TLICS) were introduced (Tables 50C.4 and 50C.5). The TLISS is an algorithm that assigns a score based on mechanism of injury, posterior ligamentous injury, and neurological deficits (Vaccaro et al., 2005). There was concern that substantial variability existed among observers as they attempted to postulate the mechanisms of injury and assign an additive score for this category. The TLICS was then created to focused on fracture morphology (Lee et al., 2005). Insofar as the reliability of these two systems is untested and has never been clinically validated, clinicians should not rely solely on the TLICS and TLISS algorithms to guide their decision making.
| Parameter | Points |
|---|---|
| MECHANISM OF INJURY | |
| Compression: | |
| Simple compression | 1 |
| Lateral angulation >15 degrees | 1 |
| Burst | 1 |
| Translational/rotational | 3 |
| Distraction | 4 |
| NEUROLOGICAL INVOLVEMENT | |
| Intact | 0 |
| Nerve root | 2 |
| Cord, conus medullaris: | |
| Incomplete | 3 |
| Complete | 2 |
| Cauda equina | 3 |
| Posterior ligamentous complex: | |
| Intact | 0 |
| Injury suspected/indeterminate | 2 |
| Injured | 3 |
| Management | Points |
|---|---|
| Nonoperative | 0-3 |
| Nonoperative or operative | 4 |
| Operative | ≥5 |
Table 50C.5 Thoracolumbar Injury Classification and Severity Score
| Parameter | Points |
|---|---|
| MORPHOLOGY | |
| Compression fracture | 1 |
| Burst fracture | 2 |
| Translational/rotational | 3 |
| Distraction | 4 |
| NEUROLOGICAL INVOLVEMENT | |
| Intact | 0 |
| Nerve root | 2 |
| Cord, conus medullaris: | |
| Incomplete | 3 |
| Complete | 2 |
| Cauda equina | 3 |
| Posterior ligamentous complex: | |
| Intact | 0 |
| Injury suspected/indeterminate | 2 |
| Injured | 3 |
| Morphology | Points |
|---|---|
| Nonoperative | 0-3 |
| Nonoperative or operative | 4 |
| Operative | ≥5 |
Patients with compression fractures of the thoracolumbar spine not requiring open surgical intervention may qualify for vertebral augmentation procedures. Vertebroplasty is a percutaneous procedure that uses a specially formulated acrylic bone cement injected into a fractured vertebra to provide stabilization. Kyphoplasty is a procedure that uses an inflatable percutaneous balloon to restore height and reduce complications from cement leakage. There is a theoretical restoration of vertebral body height and reduction of kyphotic deformity with this procedure. In general, patients qualifying for vertebroplasty must have acute or subacute fractures and no posterior vertebral body breech. Two recent prospective randomized trials failed to show any improvement of pain with vertebroplasty (Buchbinder et al., 2009; Kallmes et al., 2009). Although kyphoplasty has been shown to reduce local kyphotic deformity, there does not seem to be a positive effect seen on a global scale (Korovessis et al., 2008; Pradhan et al., 2006).
Long-Term Management of Spinal Cord Injuries
Spinal Cord Injury and Bladder Function
In SCIs, neurogenic bladders are classified according to the location of the lesion. A lower motor neuron lesion is localized below the conus medullaris. In this situation, the bladder detrusor is areflexic or hyporeflexic but maintains a normal or underactive external sphincter. With this type of injury, there is still coordination between the bladder detrusor and the sphincter. Additional characteristics depend on the extent of involvement of the peripheral fibers and whether there is predominance of afferent or efferent fibers. With peripheral fiber loss, an absent sacral reflex (bulbocavernosus and cremasteric reflexes) may be observed. A purely motor neurogenic bladder will have preserved sensation. With afferent loss, there is impaired emptying consequent to diminished or absent sensation, and this can lead to chronic overdistention. Findings on urodynamic studies would reveal a low bladder pressure, absent electromyographic (EMG) activity, and altered functional bladder outlet mechanisms (Fig. 50C.9, A). As a result, a high postvoid residual would be expected. In upper motor neuron lesions, the sacral arc is preserved, but pontine modulation is disrupted. There is incoordination between the detrusor and sphincter, which is known as detrusor–external sphincter dyssynergia (DESD; Table 50C.6). With a preserved sacral reflex, there is urinary incontinence from reflexic contraction of the bladder when filling occurs and meets a certain threshold. Detrusor overactivity can been seen in suprasacral spinal lesion. When coupled with DESD, high intravesicular pressures result. As a result, urodynamic studies will confirm a baseline spontaneous activity of the bladder as well as simultaneous firing of the detrusor and external sphincter (see Fig. 50C.9, B). High intravesicular pressure and postvoid residuals occur from this abnormal activity. A highly compliant bladder, seen in lower motor injuries, can lead to overdistention injuries and require clean intermittent catheterization (CIC) to counteract the unwanted phenomenon. However, with a poorly compliant bladder, high pressures can lead to injuries in the upper urinary tract. A detrusor leak-point pressure (DLPP) determined from urodynamic studies will indicate the pressure at which leakage occurs in the bladder. This value is typically 40 cm H2O. Vesicoureteral reflux from high DLPP can also complicate matters by causing UTI, pyelonephritis, or ischemic injuries. Chronically, this can lead to renal scarring.
Table 50C.6 Differences between Atonic Bladder and Detrusor External Sphincter Dyssynergia (DESD)
| Atonic Bladder | DESD |
|---|---|
| Areflexic or hyporeflexic bladder | Detrusor overactivity |
| Localized below the conus medullaris | Occur from lesions above the conus medullaris |
| Coordination between bladder detrusor and sphincter | Incoordination between bladder detrusor and sphincter |
| Low bladder pressures | High bladder pressures |
Spinal Cord Injury and Bowel Function
Following SCI, there is a disruption of the extrinsic influences of the nervous system on the bowel. Initial studies evaluating bowel dysfunction in SCI patients showed decreased compliance and deficient postprandial motor and myoelectrical response in the colon (Glick et al., 1984). The term neurogenic bladder evolved to account for either a lower motor neuron (LMN) bowel syndrome producing areflexia or an upper motor neuron (UMN) syndrome producing hyperreflexia (Stiens et al., 1997). The LMN syndrome results from injury of the conus medullaris, cauda equina, or pelvic nerves, with decreased influence from the parasympathetic system. Thus, this injury pattern produces peristalsis leading to slow stool movement and constipation. Furthermore, a denervated EAS leads to fecal incontinence. A lesion proximal to the conus medullaris results in UMN bowel syndrome or hyperreflexic bowel, and this in turn leads to increased tone in the colonic wall, anus, and EAS. Reflex coordination and stool propulsion remain preserved from intact connections between the spinal cord and colon. Thus, with a tight EAS present, constipation predominates. Recently, this theory of UMN and LMN syndromes was tested as researchers characterized the motility of the bowel in more detail. Resting motility of the colon was present in lower levels of contractility than in normal subjects that was independent of the level or completeness of injury (Fajardo et al., 2003). Furthermore, a postprandial motor response was confined to the descending colon in SCI patients with lower levels of contractility than in normal subjects. Despite the efforts of research, there continues to be a great void in our understanding of the pathophysiological basis of bowel dysfunction in SCI patients.
The methods to treat constipation in this population are difficult, since the pathophysiological mechanisms are not well defined. Physical measures include manual disimpaction and anorectal stimulation. In terms of diet, a well-balanced meal should consist of a wide variety of ingredients and good proportions of carbohydrates, proteins, and fats. The use of laxatives should be limited. Fiber and probiotic supplements should be used to enhance stool consistency. Scheduled emptying and deposition of suppositories will also lead to a regular frequency of evacuation. If these methods do not work, then medications are supplemented to the existing regimen. This includes macrogol, high doses of psyllium, prokinetics, digestive enzymes, and many other agents combined to provide a customized treatment for each individual. Transanal irrigation can be used if medications are not effective. Randomized trials comparing this intervention to other traditional conservative bowel management programs showed transanal irrigation to be beneficial in reducing constipation, reducing fecal incontinence, and improving quality of life (Christensen et al., 2006). When conservative measures fail, an enterostomy should be considered. Presently, two other surgical interventions are gaining attention and have been noted to improve bowel function in the SCI population: the sacral anterior root stimulator and the Malone anterograde continence enema (MACE).
Delayed Posttraumatic Spinal Cord Syndromes
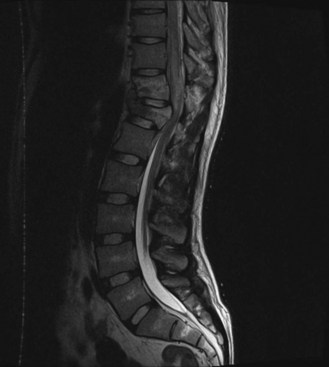
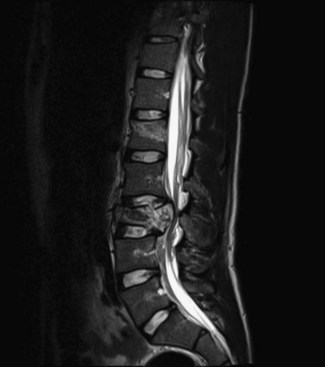
Posttraumatic Syringomyelia
Syringomyelia is found in 21% to 28% of SCI patients (Brodbelt and Stoodley, 2003). Cystic changes are found in approximately 30% to 50% of all SCI patients. There have been many proposed mechanisms but no unified theory behind the formation of the initial cystic structure. The formation has largely been attributed to hematomyelia, inflammatory responses leading to edema in the cord, ischemia, or arachnoiditis. Enlargement, on the other hand, is generally thought to be due to changes in the compliance of the subarachnoid space or from spinal stenosis, arachnoid adhesions, or persistent cord compression impairing CSF circulation. Correlations exist between the presence of uncorrected kyphosis and stenosis and severity of symptoms. The cystic cavity, acting as a one-way valve, creates imbalance of CSF flow into the cavity. The proposed “slosh mechanism” attributes the growing collections to be secondary to the influences of respirations and blood pressure on CSF pressure.
Neuropathic Pain
Neuropathic pain from SCI can be disabling. It can affect quality of life by limiting sleep and activities of daily living and causing functional disability. Furthermore, the prognosis for a resolution of the pain syndrome is poor. It is estimated that 65% to 85% of those suffering from SCI will suffer from neuropathic pain and that a third of these patients will suffer from severe pain (Siddall et al., 2003). The discrepancy in estimates appears to be related to the nonuniform nomenclature used in the literature. Only recently has the International Association for the Study of Pain (IASP) established a Spinal Injury Pain Task Force. In general, four types of pain are considered. Pain is divided into visceral and musculoskeletal, as well as two major neuropathic categories. Musculoskeletal pain is often related to mechanical instability of the spine or muscle spasm. The first type of neuropathic pain deals with a dermatomal pattern at the level of the injury, and the second type is more diffuse and occurs below the level of the injury. To date, only one prospective study shows that at 5 years after the injury, 41% of study participants had at-level neuropathic pain, and 34% had below-level neuropathic pain (Siddall et al., 2003). There appears to be no correlation between completeness or level of injury and the development of postoperative pain.
The mechanism behind the development of pain is unclear. Traditional efforts to understand the cause of neuropathic pain have focused on the region in close proximity to the level of injury. However, contemporary views suggest that more “downstream” or “upstream” changes occur in the nerve roots or the brain. When incomplete injuries occur, transmission of nociceptive impulses can theoretically still occur through various residual pathways. Even in complete injuries, residual sensory pathways exist that may not be evaluated with standard physical examination and may serve as these residual pathways. Studies have shown that injections of local anesthetic at the rostral end of the injuries can bring pain relief (Davis et al., 1954; Pollock et al., 1951). More recent investigations have shown an increased sensitivity of nerve cells close to the level of injury. These nerve cells have a higher amount of background activity, greater amount of responsiveness to stimuli, and a longer duration of firing. Additional changes are suggested to occur at the molecular level and include changes to the level and function of neurotransmitters and their receptors. Glial activation occurs following injury, leading to increased secretion of prostaglandins and various cytokines. Structural changes are known to occur, with restructuring of connections in the dorsal horn. More recent work has focused on thalamocortical dysrhythmia and the plasticity and reorganizational changes that occur in the brain.
The methods of treating neuropathic pain are broad, but principles center around treating the underlying causes of pain. If this is not possible, treatment should focus on palliative measures. Symptomatic treatment is necessary for at-level and below-level neuropathic pain. Surgical options should always focus first on decompressing nervous tissue, untethering the spinal cord, or dealing with a syrinx formation. If these measures do not provide a satisfactory remedy to the pain, then disconnecting the site of abnormal activity is the next appropriate step. Lesions in the dorsal root entry zone can be used to destroy an area of hyperactive nerve cells in the dorsal horn cells in close proximity to the level of the injury. Most studies suggest that 50% to 85% of patients have a good amount of relief of pain from this procedure (Siddall, 2009). Electrophysiological techniques such as intramedullary recording of C-fiber evoked electrical hyperactivity may aide in targeting the lesion. Finally, some patients may benefit from cordotomy or cordomyelotomy.
Pharmacological agents are at best a modest means of controlling pain. Only a third of patients have 50% pain reduction. Local and parenteral administration of lidocaine has been shown to be beneficial in treating neuropathic pain, but local administration is generally not long-lasting, and parenteral administration is typically impractical. NMDA antagonists such as ketamine work by reducing excitability, but ketamine, like lidocaine, confers no long-term relief and is not administered orally. Opioids, antiepileptics, and antidepressants increase inhibitory signals and limit excitability of neurons transmitting pain signals. Two randomized controlled studies show improvement of neuropathic pain following administration of parenteral morphine (Attal et al., 2002) and alfentanil (Elde et al., 1995). The evidence for using oral and transdermal varieties of narcotics is limited. Current recommendations for pharmacological treatment are directed at symptomatic relief and include first-line agents such as systemic lidocaine, gabapentin, and pregabalin. Although a recent randomized study failed to show benefit from the use of gabapentin in treating neuropathic pain (Rintala et al., 2007), pregabalin has been shown to be of benefit (Vraken et al., 2008). Other new agents that may be considered include lamotrigine and topiramate; topiramate in particular has had encouraging results (Harden et al., 2002). Second-line agents include TCAs, alone or in combination with antiepileptics. TCAs have been shown to have benefit in a subgroup of patients with depression. Both valproate and carbamazepine have been used to treat this condition as well. Third-line treatment options include ketamine, opioids, intrathecal baclofen and morphine, selective serotonin reuptake inhibitors (SSRIs), clonidine, or other antiepileptics. SSRIs are not proven to work. In patients with localized symptoms, topical lidocaine therapy may provide relief. Intrathecal medications have shown some promise. Intrathecal morphine and clonidine has provided short-term pain relief by acting on the opioid receptors of the dorsal horn of the spinal cord. Although intrathecal baclofen works well to treat spasticity, its role in neuropathic pain is still to be determined. A variety of neurostimulation modalities have been used but without good data proving efficacy. These include transcutaneous electrical nerve stimulation (TENS) and acupuncture, which have been shown to help with below-level neuropathic pain (Norrbrink, 2009). Spinal cord stimulators have been shown to be beneficial for at-level neuropathic pain and incomplete injuries (Ciono et al., 1995). Deep brain stimulators do not show good long-term results (Finnerup et al., 2001), while transcranial and epidural stimulation have mixed results (Nguyen et al., 1999).
Spasticity
Spasticity has been shown to affect more than 80% of SCI patients (Levi et al., 1995). It typically takes several months after the injury for symptoms to become obvious. The initial signs of spasticity include some early reflexes such as tendon reflex, flexor withdrawal reflex, and a Babinski sign. Over time, the threshold to bring about a reflex response decreases, so that even ephemeral stimuli can produce long-lasting flexor contraction. Extensor reflexes present later and co-contraction of combined flexor/extensor response becomes prominent. Spasms triggered by bladder distention or heat/cold sensations can cause severe pain and create enough intensity to expel an individual from their wheelchair. The symptoms of spasticity are debilitating and lead to a reduced quality of life by limiting activities of daily living, effecting sleep, causing pain, predisposing to unnecessary decubitus ulcers, infections, and contractures, creating a burden on caregivers, and limiting rehabilitation efforts. Although there are multiple definitions for spasticity, its characteristics were best expressed by Lance in 1980. He states that spasticity “is a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflex, as one component of the upper motor neuron syndrome” (Lance, 1980). To understand spasticity, it is necessary to understand the physiology behind motor activity. The afferent pathways rely on type Ia (muscle spindles) and type II (secondary spindle endings) fibers. Type Ia fibers synapse directly with alpha motor neurons or with interneurons. Type II synapse with interneurons of the ventral horn of the spinal cord. Interneurons receiving signals from afferent fibers fire when the muscle is being stretched. Upper motor neurons terminate on lower motor neurons, mainly the alpha motor neurons which provide innervation to the extrafusal fibers and gamma motor neurons which provide innervation to the intrafusal fibers.
Sexual Dysfunction, Sexuality, and Fertility in Spinal Cord Injuries
Sexual dysfunction poses serious quality-of-life issues in individuals suffering SCIs. Early studies have focused on subjective quantification of orgasms in this population. Early self-reported questionnaires indicated that following SCI, women had the potential to have orgasms. After early reports favoring orgasmic potential in female patients with SCI, laboratory controlled tests were conducted to assess orgasmic potential in females. Orgasms have been noted to occur in 44% of cord-injured subjects versus 100% of able-bodied control subjects (Sipski et al., 2001). Latency times to orgasm are reported to be less in able-bodied controls compared to SCI patients. Measured variables such as respiratory rate, heart rate, and systolic blood pressure have been seen to be higher in able-bodied subjects compared to the SCI population. Also, this study did not reveal a significant influence from the completeness of injury or the involvement of upper or lower motor neurons on orgasm in the laboratory. The self-reported investigation portion of the study verified that complete lower motor neuron SCI had a lower chance of orgasm than other injuries. It has been hypothesized that individuals with intact vagal nerve response are able to achieve orgasms from afferent signal from the vagina and cervix traveling through the vagus nerve. Functional MRI and positron emission tomography (PET) have recently been used to test this hypothesis by recording vagus nerve stimulation to the nucleus solitarius but have not produced any convincing results (Komisaruk et al., 2004; Whipple and Komisaruk, 2002).
As in females, surveys of males have shown that 42% to 47% of postinjury subjects experience orgasm (Alexander et al., 1993; Phelps et al., 1983). However, these studies lack important data regarding the types of injury or expression of orgasm.
To study orgasm in a controlled study with laboratory data, physiological measures for orgasm must be defined in relation to men. The relationship of ejaculation to orgasm is unclear in the population and has to be further clarified. Both historical and laboratory assessment of orgasm in SCI males report rates of 64.4% and 50%, respectively, compared to able-bodied male controls reporting rates of 100% (Sipski et al., 2006). Latency period was also increased in these subjects. Laboratory analysis differed from females in showing no comparable differences in heart rate or blood pressure during orgasm. Incomplete injuries had higher rates of orgasm compared to complete injuries (84.2% versus 50% in self-reports and 78.9% versus 28% in laboratory reporting). Complete LMN injuries affecting the sacral cord were found to have a worse outcome.
Spinal cord injuries arise in a substantial number of females who are capable of childbirth. There are currently 20,000 women between the ages of 16 and 30 dealing with chronic SCIs (Go et al., 1995). Each year, there are approximately 2000 additional women of childbearing age who suffer SCI. Females with SCI are still able to bear children, but only 14% of women in this category will eventually undergo at least one pregnancy. Among the factors responsible for a low rate of pregnancy are hormonal imbalances, autonomic dysreflexia, feelings of being overwhelmed with challenges of motherhood, discomfort or physical limitations during intercourse, side effects of medications, and psychological barriers. About one-third to one-half of women in this category have no desire to have children. Age was found to be the main factor in the decision-making process regarding pregnancy (Ghidini et al., 2008). There is a transient hypothalamic pituitary hypogonadism that translates into amenorrhea in this population. Transient amenorrhea rarely becomes permanent and affects 41.4% of this population. This phenomenon lasts on average 8 months and can take up to 18 months to resolve (Bughi et al., 2008). This is typically related to the “stress response” leading to increased corticotropin and decreased luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Increases in prolactin during this period can also contribute to amenorrhea. This is not affected by the level or extent of injury.
Autonomic Dysreflexia
The paucity of controlled trials in the prevention and management of autonomic dysreflexia prevent meaningful recommendations from being formulated. Since bladder distention and irritation are the most common stimulators of AD, good preventive medicine requires routine urological follow-up and enrollment in bladder management programs. A bladder management program that focuses on intermittent catheterization or indwelling catheter insures proper emptying measures. Urological examinations with cystoscopy or urodynamic studies may be done annually. Ultimately, measures to reduce bladder afferent stimulation may be necessary. The effects of botulinum toxin on bladder detrusor to increase bladder capacity and reduce bladder sphincter dyssynergia, facilitating emptying, has been studied and noted to have positive effects for up to 9 months (Schurch et al., 2000). This can be a safe and effective option for patients who use clean intermittent self-catheterization and do not respond to anticholinergic medications. Recently, capsaicin, an extract from red peppers, gained attention for reducing episodes of AD during catheterization in SCI. An analog of capsaicin, resiniferatoxin, had compelling evidence of reducing episodes of AD and was noted to be superior to capsaicin for patients receiving treatment within 60 days of injury (Giannantoni et al., 2002). Capsaicin and resiniferatoxin work by desensitizing sensory fibers. The long-term benefit of these medications has not been studied. Bladder and sphincter augmentation procedures target detrusor sphincter dyssynergia. Detrusor sphincter dyssynergia is believed to be the source of high intravesicular and urethral pressure, and surgery has been shown to decrease intravesical and urethral pressure. Anticholinergics and sacral denervation are unproven therapies with no strong evidence to support efficacy. Examinations of the colorectal system frequently lead to pain and irritation which are contributors to AD. Intersphincter anal blocks and lidocaine have class I evidence in preventing AD in SCI patients undergoing anorectal procedures (Cosman and Vu, 2005). Women with SCI who become pregnant have a high risk of developing episodes of AD during labor and delivery that occur with uterine contractions. In these cases, it is recommended that an epidural analgesia be placed to control the AD. Patients undergoing general surgery also have the potential to experience somatic and visceral noxious or non-noxious stimuli below the level of injury. It is recommended that patients undergo either general anesthesia or spinal anesthesia to reduce intraoperative hypertension.
Deep Vein Thrombosis and Thromboembolism in Spinal Cord Injury
Deep vein thrombosis (DVT) and pulmonary embolism (PE) continue to be major contributors to significant morbidity and mortality in this population. SCI patients are prone to develop DVT because of stasis, hypercoagulable state, and intimal injury. The incidence today is noted to be less than the period before the early 1990s when widespread use of subcutaneous heparin was not widely used. Prospective analysis of DVT in SCI before the use of prophylactic anticoagulation for a 21-day postinjury period was measured to be 62% (Geerts et al., 1994). Despite widespread use of DVT prophylaxis, screening for DVT in this population still detects a significant number of positive findings. In one prospective study screening prophylactic anticoagulation, the incidence of DVT during a 21-day period was 45.3% (Germing et al., 2009). PE is reported in 8% to 14% of all SCI patients (Geerts et al., 2004; Chiou-Tan et al., 2003).
The clinical manifestations of DVT and PE are not always present or obvious, so screening by diagnostic testing is the preferred method to detect these lesions. Screening for DVT in SCI patients can significantly reduce morbidity and mortality. Currently there are no formal guidelines to dictate when to obtain screening for DVTs, although the optimal period of testing is thought to be during the first 13 weeks post SCI (Furlan and Fehlings, 2007). Venography is an invasive test not widely used, while D-dimers are a sensitive yet nonspecific tool. Both venography and D-dimer testing have fallen out of favor; venous sonography, which is cheap and noninvasive, is the preferred modality. The sensitivity of venous sonography is 73% for more distal clots and 95% for proximal clots (Teasell et al., 2009). Distal clots that do not extend proximally are rarely worrisome because they are not a frequent cause of PE (Kakkar et al.,1969). V/Q scans and CTA are diagnostic tools used to detect PE. V/Q scans rely on the size, number, and shape of the clot to strengthen diagnostic yield. The sensitivity of CTA is related to the size of the lesion.
Current guidelines from the American College of Chest Surgeons favor the immediate use low-molecular-weight heparin in acute SCI patients (Geerts et al., 2004). Investigational prospective studies of unfractionated heparin in SCI subjects show no benefit between doses of 5000 units every 12 hours versus a placebo (Frisbie et al., 1981; Merli et al., 1988). Benefit from unfractionated heparin has been shown in SCI when an adjusted dose determined by partial thromboplastin time (PTT) is used. However, bleeding complications are higher (Green et al., 1988). Compelling class 1 data confirm that low-molecular-weight heparin is more effective and has fewer bleeding complications than unfractionated heparin for the prophylaxis of thromboembolic events (Green et al., 1990; Spinal Cord Injury Thromboprophylaxis Investigators, 2003). More recent findings have demonstrated that dalteparin is equivalent to enoxaparin in bleeding risk and DVT prevention (Chiou-Tan et al., 2003). Pneumatic compression stockings and gradient elastic stockings have been shown to reduce the incidence of DVTs in SCI patients (Winemiller et al., 1999). Rotating beds, commonly used to prevent decubitus ulcers and provide even distribution of respiration, have also proven effective in preventing DVT (Becker et al., 1987). Inferior vena cava filters have shown a benefit in reducing the risk of PE, but there has also been some suggestion that they may increase the risk of DVT (Gorman et al., 2009).
Future Directions
Therapies today target SCIs from a multitude of perspectives. Prospective trials to assess early versus late decompression in SCI are ongoing. Researchers have been investigating the use of an oscillating field stimulator in neural regeneration. This investigational device is built on the observation that neurons migrate toward the negative pole in an electrical field. Oscillation in the polarity of the electrical field promotes growth in both directions. The device has been assessed for safety in phase I trials and awaits further trials to test its efficacy (Shapiro et al., 2005). CSF drainage as a method of reducing ischemic paraplegia in patients undergoing thoracoabdominal aortic aneurysm surgery has been shown to improve outcomes (Coselli et al., 2002). Recently the safety profile of CSF drainage in SCI patients has been tested in a prospective controlled trial. Although not intended to assess efficacy, this study showed no improvement in motor scores. Hypothermia as a neuroprotective therapy has been studied extensively in animals and recently in human trials. On a biochemical, histological, and molecular level, hypothermia has been shown to reduce apoptosis, damage from oxidative stress, vasogenic edema, neutrophil and macrophage invasion, and extracellular glutamate concentration. However, the risks associated with hypothermia include unwanted cardiac arrhythmias, sepsis and infection, and coagulopathy. Retrospective analysis has been done showing no additional risks in patients undergoing 48 hours of hypothermia to a target temperature of 33°C (Levi et al., 2009).
New pharmacological arenas are currently being explored to expand upon the available treatment options for SCIs. One area of interest in SCI research deals with the regenerative capacity of neurons. It is well recognized that neurons possess the capacity to regenerate, but the presence of inhibitory molecules prevents regeneration from occurring. Of interest is a molecule contained within myelin, known as Nogo, which blocks axonal growth. Anti-Nogo antibodies have been used in animal studies to show functional recovery and axonal sprouting. Additional targets of therapy include the Rho GTPase. Rho GTPase has been found to be the central mediator of axonal growth inhibitors. BA-210 is a bacterial toxin known to inhibit Rho GTPase. It is added with a fibrin sealant to form a compound called Cethrin. Cethrin has been designed to be delivered to the dura at the time of spinal surgery. Initial clinical trials have shown promise for motor improvement in SCI, without significant side effects (Onose et al., 2009).
The latest addition to SCI therapies is cell transportation therapy. Transplantation of activated autologous macrophages has been considered a potential therapeutic modality, based on the knowledge that macrophages are present in abundance following nerve injury in the peripheral nervous system. Macrophages are thought to be essential in clearing cellular debris and secreting growth factors and cytokines that may promote axonal growth. A nonrandomized study has shown it to be safe, with an encouraging efficacy profile (Knoller et al., 2005). Schwann cells have also been targeted for their potential to encourage axonal growth and provide a myelin highway to reestablish axonal conduction. Researchers have also attempted to capitalize on the regenerating properties of olfactory ensheathing cells. Recently, transplantation of adult neural precursor cells in rats was seen to promote remyelination and functional recovery (Karimi-Abdolrezaee et al., 2006). Currently, multiple centers are also performing trials of bone marrow stromal cell transplantation and reporting a benefit from its use (Syková et al., 2006; Yoon et al., 2007). Stem cells offer the capability to differentiate into oligodendrocytes. The FDA has approved a phase I trial to study the safety and efficacy of oligodendrocyte progenitor cells in humans. With a number of clinical trials underway, researchers and clinicians feel hopeful that innovative treatments will be available in the future to provide new life to SCI victims.
Ackery A., Tator C., Krassioukov A. A global perspective of spinal cord injury epidemiology. J Neurotrauma. 2004;21(10):1355-1370.
Ajmal S., Enam S.A., Shamim M.S. Neurogenic claudication and radiculopathy as delayed presentations of retained spinal bullets. Spine J. 2009. (Epub ahead of print)
Alexamder C.J., Sipski M., Finley T.W. Sexual activities, desire, and satisfaction in males pre- and post-spinal cord injury. Arch Sex Behav. 1993;22:217-228.
Anderson P.A., Montesano P.X. Morphology and treatment of occipital condyle fractures. Spine. 1988;13:731-736.
Attal N., Guirimand F., Brasseur L., et al. Effects of IV morphine in central pain-a randomized placebo- controlled study. Neurology. 2002;58:554-563.
Bailes J.E. Experience with cervical stenosis and temporary paralysis in athletes. J Neurosurg Spine. 2005;2:11-16.
Ball P.A. Critical care of spinal cord injury. Spine. 2001;26(Suppl 24):S27-S30.
Bao F., Liu D. Peroxynitrite generated in the rat spinal cord induces apoptotic death and activates caspase-3. Neuroscience. 2003;116:59-70.
Becker D.M., Gonzalez M., Gentili A., et al. Prevention of deep venous thrombosis in patients with acute spinal cord injuries: use of rotating treatment tables. Neurosurgery. 1987;20:675-677.
Bethea J.R., Nagashima H., Acosta M.C., et al. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16(10):851-863.
Bloom A.I., Neeman Z., Slasky B.S., et al. Fractures of the occipital condyles and associated craniocervical ligament injury: incidence, CT imaging and implications. Clin Radiol. 1997;52(3):198-202.
Bracken M.B., Shepard M.J., Collins W.F.Jr., et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322(20):1405-1411.
Bracken M.B., Shepard M.J., Collins W.F.Jr., et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J Neurosurg. 1992;76(1):23-31.
Bracken M.B., Shepard M.J., Hellenbrand K.G., et al. Methylprednisolone and neurological function 1 year after spinal cord injury. Results of the National Acute Spinal Cord Injury Study. J Neurosurg. 1985;63(5):704-713.
Bracken M.B., Shepard M.J., Holford T.J., et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spine Cord Injury Study. JAMA. 1997;277(2):1597-1604.
Brodbelt A.R., Stoodley M.A. Post-traumatic syringomyelia: a review. J Clin Neurosci. 2003;10(4):401-408.
Buchbinder R., Osborne R.H., Ebeling P.R., et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361(6):557-568.
Bughi S., Shaw S.J., Mahmood G., et al. Amenorrhea, pregnancy, and pregnancy outcomes in women following spinal cord injury: a retrospective cross-section study. Endocr Pract. 2008;14(4):437-444.
Burney R.E., Mario R.F., Maynard F., et al. Incidence, characteristics, and outcome of spinal cord injury at trauma centers in North America. Arch Surg. 1993;128:596-599.
Casha S., Yu W.R., Fehlings M.G. FAS deficiency reduces apoptosis, spares axons and improves function after spinal cord injury. Exp Neurol. 2005;196:390-400.
Casha S., Yu W.R., Fehlings M.G. Oligodendroglial apoptosis occurs along degenerative axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neurosci. 2001;103:203-218.
Chen L., Yang H., Yang T., et al. Effectiveness of surgical treatment for traumatic central cord syndrome. J Neurosurg Spine. 2009;10:3-8.
Cheng B., Christakos S., Mattson M.P. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994:139-153.
Chiou-Tan F.Y., Graza H., Chan K.T., et al. Comparison of dalteparin and enoxaparin for deep vein thrombosis prophylaxis in patients with spinal cord injury. Am J Phys Med Rehabil. 2003;82:678-685.
Christensen P., Bazzocchi G., Coggrave M., et al. A randomized, controlled trial of transanal irrigation versus conservative bowel management in spinal cord-injured patients. Gastroenterology. 2006;131(3):738-747.
Ciono B., Meglio M., Pentimalli L, et al. Spinal cord stimulation in the treatment of paraplegic pain. J Neurosurg. 1995;82:35-39.
Coselli J.S., Lemaire S.A., Köksoy C., et al. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg. 2002;35:631-639.
Cosman B.C., Vu T.T. Lidocaine anal block limits autonomic dysreflexia during anorectal procedures in spinal cord injury: a randomized, double-blind, placebo-controlled trial. Dis Colon Rectum. 2005;48:1556-1561.
Davis L. Treatment of spinal cord injuries. AMA Arch Surg. 1954;69:488-495.
Dimar J.R., Fisher C., Vaccaro A., et al. Predictors of complications after spinal stabilization of thoracolumbar spine injuries. J Trauma. 2010;69:1497-1500.
Ditunno J.F., Little J.W., Tessler A., et al. Spinal shock revisited: a four-phase model. Spinal Cord. 2004;42(7):383-395.
Donnelly D.J., Popovich P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2007;209:378-388.
Dvorak M.F., Noonan V.K., Bélanger L., et al. Early versus late enteral feeding in patients with acute cervical spinal cord injury: a pilot study. Spine. 2004;29(9):E175-E180.
Elde P.K., Stubhaug A., Stenehjem A.E., et al. Central dysesthesia pain after traumatic spinal cord injury is dependent on N-methyl-d-aspartate receptor activation. Neurosurgery. 1995;37:1080-1087.
Fajardo N.R., Pasiliao R.V., Modeste-Duncan R., et al. Decreased colonic motility in persons with chronic spinal cord injury. Am J Gastroenterol. 2003;98(1):128-134.
Fehlings M.G., Perrin R.G. The timing of surgical intervention in the treatment of spinal cord injury: a systemic review of recent clinical evidence. Spine. 2006;31(Supp. l11):S28-35.
Fehlings, M.G., Vaccaro, A., Aarabi, B., et al., April 28, 2008. A prospective, multicenter trial to evaluate the role and timing of decompression in patients with cervical spinal cord injury: initial one-year results of the STASCIS Study. Oral Presentation at the 2008 American Association of Neurological Surgeons Annual Meeting. Chicago, IL.
Finnerup N.B., Yezierski R.P., Sang C.N., et al. Treatment of spinal cord injury pain. Pain Clin Updates. 2001;9:1-6.
Fleming C., Norenberg M.D., Ramsey D.A., et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249-3269.
Frisbie J.H., Sasahara A.A. Low dose heparin prophylaxis for deep venous thrombosis in acute spinal cord injury patients: a controlled study. Paraplegia. 1981;19:343-346.
Furlan J.C., Fehlings M.G. Role of screening tests for deep vein thrombosis in asymptomatic adults with acute spinal cord injury: an evidence-based analysis. Spine. 2007;32(17):1908-1916.
Galluzzi L., Kroemer G. Necroptosis: a specialized pathway of programmed necrosis. Cell. 2008;135(7):1161-1163.
Geerts W.H., Code K.I., Jay R.M., et al. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331(24):1601-1606.
Geerts W.H., Pineo G.F., Heit J.A., et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(Suppl 3):338S-400.
Germing A., Schakrouf M., Lindstaedt M., et al. Serial compression B-scan and Doppler sonography for the screening of deep vein thrombosis in patients with spinal cord injuries. J Clin Ultrasound. 2009;38:17-20.
Ghidini A., Healey A., Andreani M., et al. Pregnancy and women with spinal cord injuries. Acta Obstet Gynecol Scand. 2008;87(10):1006-1010.
Giannantoni A., Di Stasi S.M., Stephen R.L., et al. Intravesical capsaicin versus resiniferatoxin in patients with detrusor hyperreflexia: a prospective randomized study. J Urol. 2002;167:1710-1714.
Glick M.E., Meshkinpour H., Haldeman S., et al. Colonic dysfunction in patients with thoracic spinal cord injury. Gastroenterology. 1984;86(2):287-294.
Go B.K., DeVivo M.J., Richards J.S. The epidemiology of spinal cord injury. In: Stiver S.L., DeLisa J.A., Whiteneck C.G. Outcomes from the model systems. Gaithersburg: Aspen Publication; 1995:21-55.
Gorman P.H., Qadri S.F., Rao-Patel A. Prophylactic inferior vena cava (IVC) filter placement may increase the relative risk of deep venous thrombosis after acute spinal cord injury. J Trauma. 2009;66(3):707-712.
Green D., Lee M.Y., Ito V.Y., et al. Fixed- vs adjusted-dose heparin in the prophylaxis of thromboembolism in spinal cord injury. JAMA. 1988;260:1255-1258.
Green D., Lee M.Y., Lim A.C., et al. Prevention of thromboembolism after spinal cord injury using low-molecular-weight heparin. Ann Intern Med. 1990;113:571-574.
Gschaedler R., Dollfus P., Mole J.P., et al. Reflections on the intensive care of acute cervical spinal cord injuries in a general traumatology centre. Paraplegia. 1979;17:58-61.
Guest J., Eleraky M.A., Apostolides P.J., et al. Traumatic central cord syndrome: results of surgical management. J Neurosurg. 2002;97(Suppl 1):25-32.
Hachen H. Idealized care of the acute injured spinal cord in Switzerland. J Trauma. 1977;17(12):931-936.
Hadley M.N., Walters B.C., Grabb P.A., et al. Guidelines for the management of acute cervical spine and spinal cord injuries. Clin Neurosurg. 2002;49:407-498.
Hagg T., Oudega M. Degenerative and spontaneous regenerative processes after spinal cord injury. J Neurotrauma. 2006;23:264-280.
Harden R.N., Brenman E., Saltz S., et al. Topiramate in the management of spinal cord injury pain: a double-blind, randomized, placebo-controlled pilot study. Yezierski R.P., Burchiel K.J., editors. Spinal Cord Injury Pain: Assessment, Mechanisms, Management Progress in Pain Research and Management, vol. 23. 2002;393-407. Seattle
Hoffman J.R., Mower W.R., Wolfson A.B., et al. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. N Engl J Med. 2000;343:94-99.
Jackson A.B., Groomers T.E. Incidence of respiratory complications following SCI. Arch Phys Med Rehabil. 1994;75:270-275.
Jimenez O., Marcillo A., Levi A.D. A histopathological analysis of the human cervical spinal cord in patients with traumatic central cord syndrome. Spinal Cord. 2000;28:532-537.
Kakkar V.V., Howe C.T., Flanc C., et al. Natural history of postoperative deep-vein thrombosis. Lancet. 1969;2:230-232.
Kakulas B.A. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord. 2004;42:549-563.
Kallmes D.F., Comstock B.A., Heagerty P.J., et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361(6):569-579.
Karimi-Abdolrezaee S., Eftekharpour E., Wang J., et al. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377-3389.
Kennedy J.G., Soffe K.E., McGrath A., et al. Predictors of outcome in cauda equina syndrome. Eur Spine J. 1999;8(4):317-322.
Kim G.M., Xu J., Xu K., et al. Tumor necrosis factor receptor deletion reduces nuclear factor-kappaB activation, cellular inhibition of apoptosis protein 2 expression, and functional recovery after traumatic spinal cord injury. J Neurosci. 2001;21:6617-6625.
Knoller N., Auerbach G., Fulga V. Clinical experience using incubated autologous macrophages as a treatment from complete spinal cord injury: phase I study results. J Neurosurg Spine. 2005;3:173-181.
Komisaruk B.R., Whipple B., Crawford A., et al. Brain activation during vaginocervical self-stimulation and orgasm in women with complete spinal cord injury: fMRI evidence of mediation by the vagus nerve. Brain Res. 2004;1024:77-88.
Korovessis P., Zacharatos S., Repantis T., et al. Evolution of bone mineral density after percutaneous kyphoplasty in fresh osteoporotic vertebral body fractures and adjacent vertebrae along with sagittal spine alignment. J Spinal Disord Tech. 2008;21(4):293-298.
Kraus J.F. Injury to the head and spinal cord: the epidemiological relevance of the medical literature published form 1960 to 1978. J Neurosurg. 1980;Suppl:S3-S10.
Kraus J.F., Franti C.E., Riggins R.S., et al. Incidence of traumatic spinal cord lesions. J Chronic Dis. 1975;28:471-492.
Lance J.W. The control of muscle tones, reflexes, and movement: Robert Wartenberg Lecture. Neurology. 1980;30:1303-1313.
La Rosa G., Conti A., Cardali S., et al. Does early decompression improve neurological outcome pf spinal cord inured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42(9):503-512.
Ledsome J.R., Sharp J.M. Pulmonary function in acute cervical cord injury. Am Rev Respir Dis. 1981;124(1):41-44.
Lee J.Y., Vaccaro A.R., Lim M.R., et al. Thoracolumbar injury classification and severity score: a new paradigm for the treatment of thoracolumbar spine trauma. J Orthop Sci. 2005;10:671-675.
Lennarson P.J., Mostafavi H., Traynelis V.C., et al. Management of type II dens fractures: a case-control study. Spine. 2000;25:1234-1237.
Levi A.D., Green B.A., Wang M.Y., et al. Clinical application of modest hypothermia after spinal cord injury. J Neurotrauma. 2009;26(3):407-415.
Levi A.D., Tator C.H., Bunge R.P. Clinical syndromes associated with disproportionate weakness of the upper versus lower extremities after cervical spinal cord injury. Neurosurg. 1996;38:179-185.
Levi L., Wolf A., Belzberg H. Hemodynamic parameters in patients with acute cervical cord trauma: Description, intervention, and predictor of outcome. Neurosurg. 1993;33(6):1007-1017.
Levi R., Hultling C., Seiger A. The Stockholm Spinal Cord Injury Study: 2. Associations between clinical patient characteristics and post-acute medical problems. Paraplegia. 1995;33:585-594.
Martin D., Schoenen J., Lenelle J., et al. MRI-pathological correlations in acute traumatic central cord syndrome: case report. Neuroradiol. 1992;34:262-266.
McKinley W., Santos K., Meade M., et al. Incidence and outcomes of spinal cord injury clinical syndromes. J Spinal Cord Med. 2007;30(3):215-224.
McMichan J.C., Michel L., Westbrook P.R. Pulmonary dysfunction following traumatic quadriplegia. JAMA. 1980;243(6):528-531.
Medical Research Council. Aids to the examination of the peripheral nervous system. Philadelphia: Saunders; 1986.
Merli G.J., Herbison G.J., Ditunno J.F., et al. Deep vein thrombosis prophylaxis in acute spinal cord injured patients. Arch Phys Med Rehabil. 1988;69:661-664.
Nagata S., Goldstein P. The Fas death factor. Science. 1995;267:1449-1456.
Nguyen J.P., Lefaucheur J.P., Decq P., et al. Chronic motor cortex stimulation in the treatment of central and neuropathic pain. Correlation between clinical, electrophysiological and anatomical data. Pain. 1999;82:245-251.
Noble L.J., Wrathall J.R. Distribution and time course of protein extravasation in the rat spinal cord after contusive injury. Brain Res. 1989;482:57-66.
Norenberg M.D., Smith J., Marcillo A. The pathology of human spinal cord injury; defining the problems. J Neurotrauma. 2004;21:429-440.
Norrbrink C. Transcutaneous electrical nerve stimulation for treatment of spinal cord injury neuropathic pain. J Rehabil Res Dev. 2009;46(1):85-93.
Onose G., Anghelescu A., Muresanu D.F. A review of published reports on neuroprotection in spinal cord injury. Spinal Cord. 2009;47:716-726.
Otani K., Abe H., Kadoya S., et al. Beneficial effects of methylprednisolone sodium succinate in the treatment of acute spinal cord injury. Sekitsui Sekizui J. 1994;7:633-647.
Pang D. Spinal cord injury without radiographic abnormality in children, 2 decades later. Neurosurg. 2004;55(6):1325-1343.
Pang D., Wilberger J.E.Jr. Spinal cord injury without radiographic abnormalities in children. J Neurosurg. 1982;57(1):114-129.
Papadopoulos S.M., Selden N.R., Quint D.J., et al. Immediate spinal cord decompression for cervical spinal cord injury: feasibility and outcome. J Trauma. 2002;52(2):323-332.
Peterson P., Brooks C.A., Mellick D., et al. Protocol for ventilator management in high tetraplegia. Top Spinal Cord Inj Rehabil. 1997;2:101-106.
Petitjean M.E., Pointilart V., Dixmerias F., et al. Medical treatment of spinal cord injury in the acute stage. Ann Fr Anesth Reanim. 1998:114-122.
Phelps G., Brown M., Chen J., et al. Sexual experience and plasma testosterone levels in male veterans after spinal cord injury. Arch Phys Med Rehabil. 1983;64:47-52.
Pitts L.H., Ross A., Chase G.A., et al. Treatment with thyrotropin-releasing hormone (TRH) in patients with traumatic spinal cord injuries. J Neurotrauma. 1995;12:235-243.
Ploumis A., Yadiapalli N., Fehlings M.D., et al. A systemic review of the evidence supporting a role for vasopressor support in acute SCI. Spinal Cord. 2010;48(5):356-362.
Pollock L.J., Brown M., Boshes B., et al. Pain below the level of injury of the spinal cord. Arch Neurol Psychiatry. 1951;65:319-322.
Pradhan B.B., Bae H.W., Kropf M.A., et al. Kyphoplasty reduction of osteoporotic vertebral compression fractures: correction of local kyphosis versus overall sagittal alignment. Spine. 2006;31(4):435-441.
Quencer R.M., Bunge R.P., Egnor M., et al. Acute traumatic central cord syndrome: MRI-pathological correlation. Neuroradiol. 1992;34:85-94.
Rabinowitz R.S., Eck J.C., Harper C.M.Jr., et al. Urgent surgical decompression compared to methylprednisolone for the treatment of acute spinal cord injury: a randomized prospective study in beagle dogs. Spine. 2008;33(21):2260-2268.
Rintala D.H., Holmes S.A., Courtade D., et al. Comparison of the effectiveness of amitriptyline and gabapentin on chronic neuropathic pain in persons with spinal cord injury. Arch Phys Med Rehabil. 2007;88:1547-1560.
Roffi R.P., Waters R.L., Adkins R.H. Gunshot wounds to the spine associated with a perforated viscus. Spine. 1989;14(8):808-811.
Ross J.A., Auger M.J. The biology of the macrophage. In: Burke B.L., editor. The Macrophage. New York: Oxford University Press; 2002:3-72.
Schmitt A.B., Buss A., Breuer S., et al. Major histocompatibility complex class II expression by activated microglia caudal to lesions of descending tracts in the human spinal cord is not associated with a T cell response. Acta Neuropathol (Berl). 2000;100:528-536.
Schneider R.C., Cherry G., Pantek H. The syndrome of acute central cervical spinal cord injury. J Neurosurg. 1954;11:546-577.
Schurch B., Stohrer M., Kramer G. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol. 2000;164:692-697.
Sekhon L.H., Fehlings M.G. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26(Suppl 24):S101-110.
Shapiro S. Management of unilateral locked facets of the cervical spine. Neurosurg. 1993;35(5):832-837.
Shapiro S., Borgens R., Pascuzzi R., et al. Oscillating field stimulation for complete spinal cord injury in humans: a phase I trial. J Neurosurg Spine. 2005;2:3-10.
Siddall P.J. Management of neuropathic pain following spinal cord injury: now and in the future. Spinal Cord. 2009;47:352-359.
Siddall P.J., McClelland J.M., Rutkowski S.B. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249-257.
Sipski M., Alexander C.J., Gomez-Marin O. Effects of level and degree of spinal cord injury on male orgasm. Spinal Cord. 2006;44:798-804.
Sipski M.L., Alexander C.J., Rosen R. Sexual arousal and orgasm in women: effects of spinal cord injury. Ann Neurol. 2001;49(1):35-44.
Spinal Cord Injury Information Network. Available at http://www.spinalcord.uab.edu, 2009. Accessed September 3
Spinal Cord Injury Thromboprophylaxis Investigators. Prevention of venous thromboembolism in the acute treatment phase after spinal cord injury: a randomized, multicenter trial comparing low-dose heparin plus intermittent pneumatic compression with enoxaparin. J Trauma. 2003;54:1116-1124.
Stiell I.G., Clement C.M., McKnight R.D., et al. The Canadian C-spine rule versus the NEXUS low-risk criteria in patients with trauma. N Engl J Med. 2003;349:2510-2518.
Stiens S.A., Bergman S.B., Goetz L.L. Neurogenic bowel dysfunction after spinal cord injury: clinical evaluation and rehabilitative management. Arch Phys Med Rehabil. 1997;78(Suppl 3):S86-S102.
Syková E., Homola A., Mazanec R., et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006;15:675-687.
Tadie M., D’Arbigny P., Mathé J., et al. Acute spinal cord injury: early care and treatment in a multicenter study with Gacyclidine. Soc Neurosci. 1999;25:1090.
Tator C.H., Rowed D.W., Schwartz M.I., et al. Management of acute spinal cord injuries. Can J Surg. 1984;27(3):289-293.
Taylor R.G., Gleave J.R. Incomplete spinal cord injuries. J Bone Joint Surg Br. 1957;39-B(3):438-450.
Teasell R.W., Hsieh J.T., Aubut J.L. Venous thromboembolism after spinal cord injury. Arch Phys Med Rehab. 2009;90:232-245.
Torg J.S., Corcoran T.A., Thibault L.E., et al. Cervical cord neuropraxia: classification, pathomechanics, morbidity, and management guidelines. J Neurosurg. 1997;87:843-850.
Torg J.S., Thibault L., Sennet B., et al. The Nicolas Andry Award: the pathomechanics and pathophysiology of cervical spine cord injury. Clin Orthop Relat Res. 1995;321:259-269.
Totoiu M.O., Keirstead H.S. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486:373-383.
Traynelis V.C., Marano G.D., Dunker R.O., et al. Traumatic atlanto-occipital dislocation. Case report. J Neurosurg. 1986;65(6):683-870.
Vaccaro A.R., An H.S., Lin A., et al. Noncontiguous injuries of the spine. J Spinal Disord. 1992;5(3):320-329.
Vaccaro A.R., Daugherty R.J., Sheehan T.P., et al. Neurological outcome of early versus late surgery for cervical spinal cord injury. Spine. 1997;22(22):2609-2613.
Vaccaro A.R., Zeiller S.C., Hulbert R.J., et al. The thoracolumbar injury severity score: a proposed treatment algorithm. J Spinal Disord Tech. 2005;18(3):209-215.
Vale F.L., Burns J., Jackson A.B., et al. Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87:239-246.
van den Berg M.E., Castellote J.M., Mahillo-Fernandez I., et al. Incidence of spinal cord injury worldwide: a systemic review. Neuroepidemiology. 2010;34(3):184-192.
van den Berg M.E., Castellote J.M., de Pedro-Cuesta J., et al. Survival after spinal cord injury: a systematic review. J Neurotrauma. 2010;27(8):1517-1528.
Vraken J.H., Dijkgraaf M.G.W., Kruis M.R., et al. Pregabalin in patients with central neuropathic pain: a randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain. 2008;136:150-157.
Whipple B., Komisaruk B.R. Brain (PET) responses to vaginal-cervical self-stimulation in women with complete spinal cord injury: Preliminary findings. J Sex Mar Ther. 2002;28:79-86.
White A.A., Panjabi M.M. Clinical Biomechanics of the Spine, second ed. Philadelphia: Lippincott; 1990.
Winemiller M.H., Stolp-Smith K.A., Silverstein M.D., et al. Prevention of venous thromboembolism in patients with spinal cord injury: effects of sequential pneumatic compression and heparin. J Spinal Cord Med. 1999;22:182-191.
Wolf A., Levi L., Mirvis S., et al. Operative management of bilateral jumped facets. J Neurosurg. 1991;75:883-890.
Xiong Y., Rabchevsky A.G., Hall E.D. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J Neurochem. 2007;100(3):639-649.
Yoon S.H., Shim Y.S., Park Y.H., et al. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor. Phase I/II clinical trial. Stem Cells. 2007;25:2066-2073.
Zach G.A., Seiler W., Dollfus P. Treatment results of spinal cord injuries in the Swiss Paraplegic Centre of Basle. Paraplegia. 1976;14:58-65.