Chapter 76E Transjugular intrahepatic portosystemic shunting
Indications and technique
Indications
Variceal Bleeding
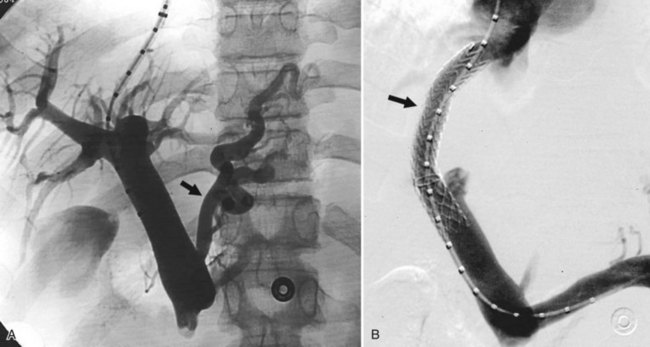
Bleeding gastroesophageal varices not controlled by medical or endoscopic means was the original indication for which TIPS was devised, and this is still the primary indication. TIPS can routinely decompress the portal system to drop the portosystemic gradient (PSG) and will lead to immediate cessation of bleeding in 95% of patients (Fig. 76E.1). A number of randomized trials have compared TIPS to best medical management (Cabrera et al, 1996; Cello et al, 1997; Garcia-Villarreal et al, 1999; Gulberg et al, 2002b; Jalan et al, 1997; Merli et al, 1998; Pomier-Layrargues et al, 2001; Rössle et al, 1997; Sanyal et al, 1997; Sauer et al, 1997). These have demonstrated that TIPS provides better long-term control of variceal bleeding. In these studies, recurrent bleeding occurred in 18% to 56% of patients in the sclerotherapy cohorts versus only 9% to 23% of patients in the TIPS arms. Not only were these differences statistically significant for the primary outcome, the crossover rate from endoscopy to TIPS was 5% to 28%, but only 0% to 7% of the TIPS patients crossed over to endoscopic treatment.
Better stratification of patients might also have yielded different results, as evidenced by one randomized trial (Monescillo et al, 2004) that stratified patients according to early measurement of their hepatic venous pressure gradient (HVPG) and then randomized high-risk patients (HVPG >20 mm Hg) to medical management or early TIPS placement. Not only did the TIPS group have fewer episodes of recurrent bleeding, both the in-hospital and 1 year mortality rates (11% and 38%) were significantly lower than in the non-TIPS cohort (38% and 65%).
One caveat regarding many of the randomized trials is that most were done using bare metal stents to create the shunt. Thus, within 6 to 12 months, the majority of the shunts in the TIPS group had stenosed. The current standard of care is to use stent grafts (polytetrafluoroethylene-covered stents) for TIPS procedures. These newer stent grafts have much improved patency and yield even better control of variceal bleeding than the bare metal TIPS (Angeloni et al, 2004; Angermayr et al, 2003; Bureau et al, 2007; Tripathi et al, 2006). Although more studies need to be done with stent grafts, at least one randomized trial (Garcia-Pagan et al, 2010) showed significantly better 1-year survival for TIPS performed with these improved stents compared with medical management (86% vs. 60%, P < .01).
The main downfall to TIPS is the development of new hepatic encephalopathy or exacerbation of existing encephalopathy, which occurs in 20% to 31% of cases (Boyer & Haskal, 2010). It is for this reason that TIPS is still recommended only after failure of medical management and not as front-line therapy for variceal bleeding. Interestingly, despite improved patency, the stent-graft TIPS has actually been associated with a lower incidence of encephalopathy compared with bare-stent TIPS (Bureau et al, 2007; Tripathi et al, 2006). This is another reason why the randomized trials comparing TIPS to medical management need to be repeated with the newer stent grafts.
Gastric Varices and Gastropathy
Gastric varices are also frequently seen in patients with portal hypertension and are actually associated with a higher rate of hemorrhage-related mortality compared with bleeding from esophageal varices (Garcia-Tsao & Bosch, 2010). TIPS can also provide good control of bleeding related to this condition, although the efficacy compared to endoscopic management is less clear. One large retrospective study comparing TIPS to endoscopic cyanoacrylate injection failed to demonstrate a benefit for TIPS in either control of bleeding or survival (Procaccini et al, 2009). However, another trial (Lo et al, 2007) that randomized patients to either TIPS or variceal obliteration with endoscopic cyanoacrylate injection showed that rebleeding was significantly less frequent in the TIPS group (11% vs. 38%, P = .014).
TIPS has also been used to treat portal hypertensive gastropathy, but data are sparse, and no randomized trials have evaluated the use of TIPS for this condition. Some investigators believe that TIPS is less effective for this condition, and several studies have a shown a poor correlation between HVPG and the presence or severity of gastropathy (Bellis et al, 2007; Shimizu et al, 2002). Given that the function of TIPS is to reduce portal pressure, this lack of correlation between the severity of gastropathy and portal pressures may account for the more variable results seen with TIPS in this condition.
Ectopic Varices
The effective portal decompression achieved by TIPS has led to expansion of the indication to include bleeding from ectopic varices, which most often arise in enteric stomas and are caused by portal hypertension transmitted to the peripheral tributaries of the mesenteric veins. Several small series have described successful treatment of varices in these locations by decompression with TIPS (Spier et al, 2008; Kochar et al, 2008; Han et al, 2007; Vidal et al, 2006; Macedo et al, 2005; Alkari et al, 2005; Vangeli et al, 2004; Ryu et al, 2000). The rebleeding rate in this indication is higher than expected after decompression of the portal system, which has led several authors (Kochar et al, 2008; Vangeli et al, 2004) to suggest that the ectopic varices should also be embolized at the time of TIPS.
Ascites
Although TIPS was designed to treat variceal bleeding, early investigators recognized that the procedure also led to a reduction or resolution of ascites in many patients. A number of randomized controlled trials have compared TIPS to large-volume paracentesis (LVP) (Ginés et al, 2002; Lebrec et al, 1996; Rössle et al, 2000; Salerno et al, 2004; Sanyal et al, 2003). A meta-analysis of these trials (Deltenre et al, 2005) showed that at 4 and 12 months, ascites was controlled in 66% and 55% of the TIPS patients, respectively, compared with 24% and 19% for the LVP patients. Despite the improved control of ascites, survival was higher, but not statistically better, in the TIPS groups. Another meta-analysis of the data did show a slight survival benefit for the TIPS patients (Salerno et al, 2007). Again, these studies were all done with bare metallic stents, and because survival is better after TIPS with stent grafts, these studies bear repeating with the current technology. Data from trials of stent grafts show improved control of ascites compared to bare-stent TIPS. Jung and colleagues (2009) reported ascites control at 1 year in 64% of TIPS procedures performed with the Viatorr device (W.L. Gore, Flagstaff, AZ) versus only 33% in patients with bare metal stents.
Patients with intractable ascites tend to be a sicker population, and despite successful decompression of the portal system, survival in this cohort is likely to be lower compared with those who have had TIPS done for variceal hemorrhage (Wallace et al, 2004); however, TIPS can still lead to significant improvement in quality of life (Gulberg et al, 2002a). One caveat is that the physiologic effect is not always immediate, and some patients do not show resolution of ascites for 2 to 3 months after the TIPS; another is that the clinical response does not appear to be as good when TIPS is used to treat recurrent ascites in patients after liver transplantation. One small retrospective study (Saad et al, 2010) reported a clinical success rate of only 16% in patients after transplant.
Hepatic Hydrothorax
Based on the success with managing ascites, TIPS has also been applied to hepatic hydrothorax, which occurs in 5% to 10% of cirrhotic patients and can lead to significant shortness of breath and inability to perform normal daily activities. Similar to ascites, TIPS is not used for primary therapy but instead is reserved for patients who are refractory to medical management. Patients requiring multiple thoracenteses or chest tubes can benefit from TIPS, with a beneficial response reported in 74% to 79% of patients undergoing the procedure (Dhanasekaran et al, 2010; Gordon et al, 1997; Spencer et al, 2002). Of those who respond, about two thirds have complete resolution of the hydrothorax, and the remainder experience partial resolution of the effusion but are symptomatically improved, with either decreased or resolved dyspnea. In our experience (Spencer et al, 2002), the patients who showed no clinical response to TIPS were all critically ill with elements of multiorgan failure, and 30-day mortality was 83%. Thus TIPS is unlikely to be helpful as a last-ditch effort to improve pulmonary function in severely ill patients on mechanical ventilation.
Budd-Chiari Syndrome
Since the early 1990s, TIPS has also been used to relieve the portal hypertension and hepatic congestion associated with Budd-Chiari syndrome (Ochs et al, 1993). The results obtained depend on the patient’s physiologic status. Several studies (Khuroo et al, 2005; Blum et al, 1995) have shown that a more fulminant presentation correlates with decreased survival; however, patients with a more chronic form of Budd-Chiari syndrome have fairly good results with TIPS. The largest series of TIPS done for Budd-Chiari syndrome included 124 patients (Garcia-Pagan et al, 2008b). The 1- and 10-year transplant-free survival rates were 88% and 69%. This was significantly better than expected for this patient population and has led to a change in the guidelines so that TIPS is now a recommended treatment in patients who fail anticoagulation therapy (Boyer & Haskal, 2010). This study also demonstrated that patients with a greater degree of hepatic dysfunction did not benefit as much as those with better preserved hepatic function.
Portal Vein Occlusion
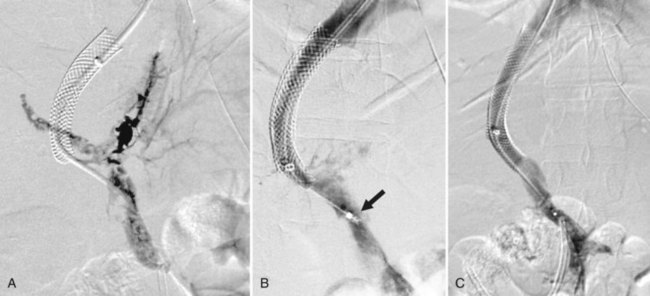
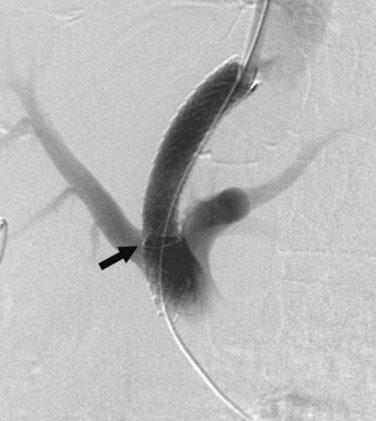
Portal vein (PV) occlusion is one indication for TIPS that sometimes arises even in patients without severe cirrhosis. The benefit of TIPS is that it provides a pathway into the portal system for introduction of mechanical thrombolytic devices (Fig. 76E.2) and also provides outflow to help maintain flow once portal patency is reestablished. TIPS has also been shown to be beneficial in treating portal hypertensive complications in patients with portal thrombosis who are awaiting liver transplantation (Bauer et al, 2006). The technical success rate for establishing a shunt and maintaining patency of the portal system is 70% to 100% (Bilbao et al, 2004; Radosevich et al, 1993), with the technical failures a result of the inability to traverse the portal occlusion. Clinical success—that is, termination of hemorrhage—was achieved in all patients in whom a TIPS was successfully created. This approach needs to be carefully considered in consult with a liver transplant surgeon and transplant hepatologist in transplant candidates, because TIPS placement in this setting can compromise access to the proximal PV, which has the potential to influence the outcome of liver replacement.
Preoperative Decompression
A relatively new application of TIPS has been for preoperative portal decompression in patients who need to undergo major intraabdominal, nonhepatic operations (see Chapter 70B; Azoulay et al, 2001; Gil et al, 2004; Schlenker et al, 2009). For patients with severe portal hypertension that requires colonic resection, the operative mortality can be quite high. Portal hypertension poses a risk in terms of dilated collateral veins, increased risk of operative bleeding, and the possibility of portal hypertension–related ascites, causing infectious complications or incisional ascites leakage postoperatively. In the reports of TIPS for this indication, the operations were carried out without excessive bleeding or ascitic complications.
One unresolved question concerns how much time to allow between the TIPS and the operation to ensure optimal decompression of the dilated collateral veins. Although waiting a few weeks after portal decompression seems prudent, one group reported safely operating 2 days after TIPS (Theruvath & Adams, 2010). Given that TIPS can cause complications of its own, it should not be utilized casually for preoperative decompression.
Contraindications
When medical and endoscopic methods have failed to control massive variceal hemorrhage, TIPS may be the only potentially lifesaving option. Thus most of the contraindications typically cited are only relative contraindications; however, if the indication for TIPS is nonemergent, these contraindications assume greater importance. Right heart failure is one of the most significant contraindications, because TIPS can precipitate acute death from total heart failure (Peron et al, 2000), but this complication has been reported so infrequently that it is impossible to define an absolute threshold for right heart pressures above which TIPS should not be performed.
Various authors have proposed schemes to decide prospectively who should be treated with TIPS (Harrod-Kim et al, 2006; Malinchoc et al, 2000; Montgomery et al, 2005; Pan et al, 2008). Of these, the MELD score tends to correlate best with 1-year survival (Chalasani et al, 2000). Different MELD scores have been proposed as the cutoff above which patients should not undergo TIPS, but these figures must be weighed against the risk inherent in the patient’s condition. In situations of life-threatening variceal hemorrhage, TIPS can often be life saving, even in a patient with a high MELD score.
Cavernous transformation is generally considered a contraindication, because it greatly increases the difficulty of gaining access to the portal system. Furthermore, even if a portal branch is accessed, it may be impossible to negotiate across the main PV occlusion to a portion of the portal system that is patent. Cases have been described in which a TIPS was created to a dilated periportal collateral with successful decompression of the portal hypertension (Brountzos et al, 2004; Wils, et al, 2009). Detailed preprocedure cross-sectional imaging can be useful to assess for the presence of a large, dominant collateral that can act as a target for the shunt.
Polycystic liver disease has been listed as an absolute contraindication because of potential intracystic hemorrhage, although successful TIPS in polycystic livers has been reported by us and others (Bahramipour et al, 2000; Shin & Darcy, 2001; Sze et al, 2006); some of these were even done with bare metallic stents without significant bleeding despite needle entry into the cyst cavity during creation of the parenchymal tract. The reason for the lack of intracystic hemorrhage would appear to be that the shunt provides a low-resistance pathway to the right atrium, and there is little reason for blood to flow into the contained space of a cyst. Although technically challenging, evidence is insufficient to suggest that polycystic liver disease should continue to be listed as a contraindication.
Technique
Patient preparation is similar to other procedures that require heavy sedation or general anesthesia, and preprocedure imaging of the PV is helpful to ensure a patent PV. Although TIPS can be performed in the face of portal thrombosis that has not progressed to cavernous transformation, the procedure is more difficult in this setting and may require percutaneous transhepatic access. Puncture of the liver capsule can occur during TIPS, resulting in peritoneal hemorrhage; assessment and optimization of the patient’s platelet count and coagulation parameters are therefore critical. Prophylactic antibiotics should be administered, because a permanent stent graft is being implanted, and cases of TIPS infection have been described (Mizrahi et al, 2009; Sanyal & Reddy, 1998; Suhocki et al, 2008). In patients with massive ascites, a peritoneal drain should be placed at the start of the case. As the ascites is drained, it helps with fluoroscopic visualization of the device being used, and it also gives the patient a head start at resolving the ascites. Additionally, drainage can be a useful intraprocedural way to monitor any intraperitoneal hemorrhage that may develop.
The procedure is started by accessing the jugular vein. The right internal jugular is the preferred access, because it provides a straight path into the inferior vena cava (Baijal et al, 1996) and hepatic veins. When the right internal jugular vein is occluded, the left internal jugular vein can be utilized. Despite the seemingly tortuous course from that access to the hepatic veins, use of stiff guidewires and long sheaths allows a high rate of technical success when starting from the left jugular (Hausegger et al, 1998). If both internal jugular veins are occluded, collateral veins or external jugular veins can sometimes be used for access. Alternatively, if the jugular vein is only partially thrombosed, the occlusion can be probed with a catheter and guidewire. If the obstruction can be traversed, a sheath may be placed to allow use of this access.
At this point, the angiographic catheter should be exchanged for a balloon occlusion catheter. Through this catheter, wedged venography can be done to localize the PV prior to making needle passes. Although a nonballoon endhole catheter can be used for wedged venography, all cases of hepatic capsular rupture during wedged venography have occurred with very peripherally positioned endhole catheters (Semba et al, 1996; Theuerkauf et al, 2001). A more centrally positioned balloon occlusion catheter allows the forceful injection to diffuse through a larger number of HV branches, thus decreasing the chance of rupture. Carbon dioxide (CO2) gas is the preferred contrast agent for wedged venography. The lower viscosity compared with liquid iodinated contrast allows better flow through the sinusoids and better opacification of the PV (Krajina et al, 2002).
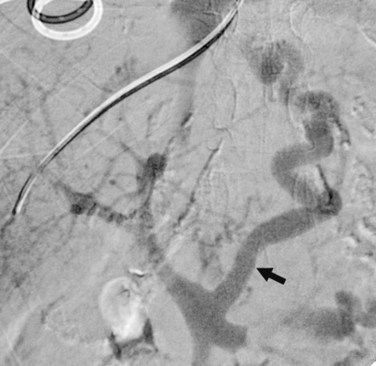
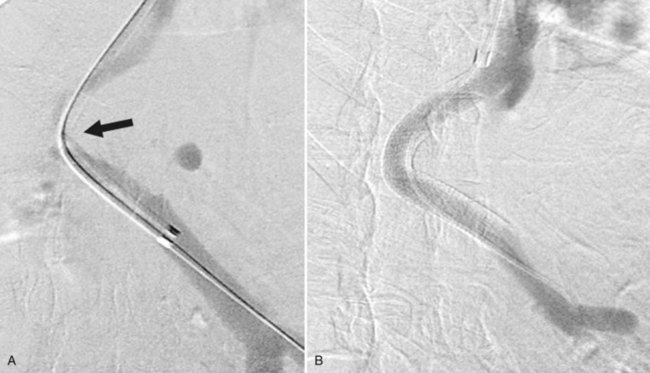
While the balloon catheter is in place, some recommend doing free and wedged HV pressures to determine the HVPG and confirm the presence of portal hypertension before creating the TIPS. Monescillo and colleagues (2004) have demonstrated that stratifying patients into low-risk (HVPG <20 mm Hg) and high-risk (>20 mm Hg) categories predicts the risk of recurrent bleeding and the chance of medical treatment failure. However, these pressures can be misleading; PV thrombosis may artifactually lower the wedged pressure, because it is a presinusoidal obstruction. Also, some patients who have gigantic varices decompressing the PV may actually have normal portal pressures (Fig. 76E.3) even when measured directly in the PV.
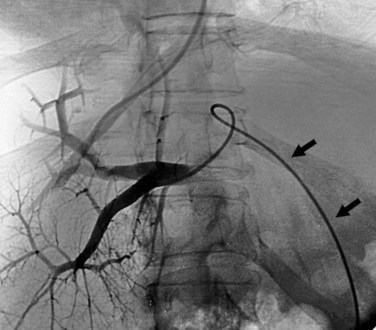
If the patient has a large umbilical vein collateral on the anterior abdominal wall, this can be punctured using ultrasound guidance and used as a pathway to feed a catheter into the portal system. Through this catheter, contrast can be injected to delineate the portal anatomy, plus the catheter can be positioned to act as a real-time target for the transhepatic needle passes (Fig. 76E.4). Unfortunately, such large umbilical collaterals are available in the minority of cases.
Next, a needle must be advanced into the portal system. Typically the preferred target is the trunk of the main right PV. Puncturing at the portal bifurcation is not recommended, because this is often extrahepatic, and access here increases the risk of intraabdominal hemorrhage. Access in more peripheral portal branches often leads to a tortuous tract that is more difficult to properly dilate and stent. Occasionally, the right portal trunk is a less optimal target than the left PV. This occurs when the right PV is thrombosed, or when the right lobe is shrunken and elevated, as can occur in patients with severe cirrhosis and massive ascites. In the later setting, the angle from the IVC to the right PV is often too acute to allow easy passage of the rigid transjugular needles. Middle HV to left PV tracts are often straighter and easier to create in this situation. A recent study (Chen et al, 2009) also found that TIPS created through the left PV were associated with a significantly lower rate of encephalopathy than right PV TIPS, but this has not been validated in larger studies.
An interesting variation on TIPS is the direct intrahepatic portosystemic shunt (DIPS) procedure (Hoppe et al, 2008). With this technique, intravascular ultrasound is used to guide a needle puncture directly from the IVC into the PV through the caudate lobe. The reported advantages of this are that the needle puncture is continuously monitored and guided into the IVC, in contrast to the relatively blind needle passes done during a TIPS procedure. In addition, the intrahepatic tract is relatively shorter than for a TIPS. This theoretically should lead to better patency, and in fact, primary patency at a mean of 256 days follow-up was 100% (Hoppe et al, 2008). Despite these potential advantages, DIPS has not been widely adopted.
Because accessing the PV is the most difficult part of a TIPS procedure, many investigators have tried to develop new guidance mechanism that will aid this portion of the procedure. One promising approach is the use of magnetic resonance (MR) guidance (Arepally et al, 2006; Kee et al, 2005). Although MR guidance allowed the PV to be accessed with relatively few needle passes, it did seem to add to the complexity of the cases. In the study by Kee and colleagues (2005), the average procedure time was 2.5 hours, whereas in general practice, a TIPS can often be performed within 60 to 90 minutes.
Immediately after advancing a catheter into the PV, it is necessary to confirm its position with contrast venography. During the transhepatic puncture, it is possible to access a hepatic arterial branch instead of the PV. If this is not recognized, and the operator proceeds with the case, a hepatic artery to HV shunt may be created with disastrous results (Kerlan et al, 1994). Initial venography also serves to identify the point of entry into the portal system. This has implications for the safety of completing the procedure, because access into the main PV or the portal bifurcation is often an extrahepatic segment of the vein, and balloon dilating such a tract may lead to intraperitoneal hemorrhage (Kim et al, 2001).
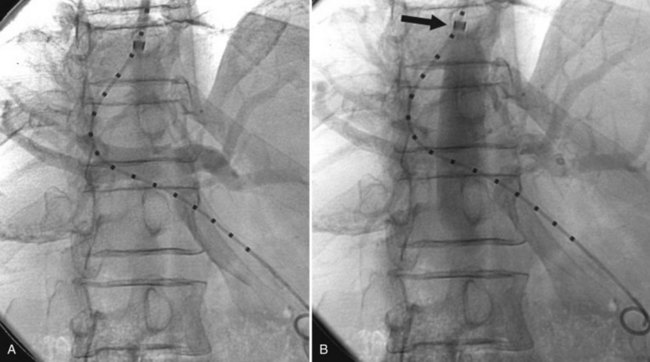
The next step is to measure the length of the tract to determine what size device to implant. This is done with a catheter with radiopaque markers on it. The tract is measured from the entry point in the PV back to the HV-IVC junction. It has been shown that patency is lower if the stent does not extend all the way to this point (Clark et al, 2004), because stenoses can form above a TIPS even in short segments of uncovered HV. Identification of the HV-IVC junction can be challenging, because the rapid inflow of unopacified IVC blood may hinder accurate localization of HV insertion. Positioning the sheath near the HV-IVC junction and injecting forcefully into the sheath to reflux contrast down the IVC is a useful maneuver to help define the HV-IVC junction (Fig. 76E.5).
Next, the tract has to be held open by deploying a stent. Although TIPS was first done with bare metal stents, restenosis became a significant problem. Primary patency decreased to 50% within 6 to 12 months, and many of the stenoses were due to pseudointimal hyperplasia in the shunt, as it coursed through the liver parenchyma (Saxon et al, 1998; Sterling & Darcy, 1997). Pathologic and histologic studies suggested that this pseudointimal hyperplasia was stimulated by communication with biliary epithelium, resulting from traumatic entry into the liver parenchyma during tract formation (LaBerge et al, 1993; Stout et al, 1995). To correct this problem, investigators started using stent grafts to exclude the liver parenchyma and bile from the shunt lumen.
One stent-graft device specifically designed for TIPS that has FDA approval for that application, the Viatorr device, has a 2-cm long, bare stent component that sits in the PV and a stent-graft segment—lengths vary from 4 to 8 cm—covering the parenchymal tract (Fig. 76E.6). Studies to date have shown significantly improved patency compared with stent grafts created with bare metal stents (Bureau et al, 2007; Tripathi et al, 2006; Vignali et al, 2005). An Italian multicenter study (Vignali et al, 2005) reported primary patency of TIPS created with Viatorr to be 76% at 2 years. Similarly, a randomized trial between Viatorr and bare-stent TIPS reported 2-year primary patency rates of 76% and 36% respectively (Bureau et al, 2007).
One concern that arose when stent grafts were first used was an increased potential for encephalopathy; however, for reasons that are not obvious, encephalopathy rates were actually lower with stent grafts than with bare metallic stents (Bureau et al, 2007; Tripathi et al, 2006). Along with the improved patency, a stent-graft TIPS provided better control of variceal hemorrhage in several studies (Angeloni et al, 2004; Bureau et al, 2007; Tripathi et al, 2006), although differences in survival did not reach statistical significance. Angermayr and colleagues (2003) did, however, show a significant improvement in survival: 76% at 3 years versus 62% for bare-stent TIPS.
In order to deploy a Viatorr, a delivery sheath must be advanced into the PV. This is because the bare portion of the stent, the caudal 2 cm of the Viatorr, is constrained only by the delivery sheath: as soon as it exits the sheath, that portion of the device expands. The need to have the sheath in the PV is occasionally a problem. If the tract is tightly curved, the sheath may kink once the inner dilator is removed. If the kink is significant, the sheath may not be able to pass across the Viatorr device. Switching to a self-constrained stent graft, such as the Fluency (Bard Peripheral Vascular, Tempe, AZ), allows passage of the device without needing to get the sheath through the tract into the PV (Fig. 76E.7). One theoretical disadvantage to this stent graft is the potential to occlude flow into the portal branches, because it is totally covered by PTFE, although this feature does not appear to increase the complication rate.
After stent-graft deployment, repeat portal venogram is performed, and pressure measurements are taken to confirm proper placement of the device, assess for complications related to stent placement, and ensure that the portal system has been adequately decompressed. The venogram should show good flow through the shunt and no further flow in varices or collateral pathways. Generally, the flow in the portal branches will also become hepatofugal toward the shunt, even if good hepatopetal flow was present prior to shunt creation. Pressure measurements should show a successful reduction in the portosystemic gradient. Typically, the target is to get the portosystemic gradient below 12 mm Hg, the commonly accepted threshold below which variceal bleeding is unlikely. Some speculate that resolution of ascites may require a slightly lower portosystemic gradient (PSG), down around 8 mm Hg (Boyer & Haskal, 2010). If the PSG is too high, careful venography and a pull-back pressure measurement are necessary to ensure that the tract is fully stented and that there are no kinks or thrombi that might be limiting flow through the shunt.
Special Technical Considerations
Budd-Chiari Syndrome
When TIPS is being considered in patients with Budd-Chiari syndrome, the hepatic veins may be extensively thrombosed such that catheterization is not possible. In this situation, the needle that passes across the liver parenchyma may need to start from the HV stump, if it can be engaged, or directly from the IVC itself (Gasparini et al, 2002). The retrohepatic segment of the IVC is approximately 5 to 6 cm in length; to avoid creating a tract with a greater chance of hemorrhage, the needle passes should start from within this segment of the vein. Whereas a normal puncture from the right HV involves anterior and medial direction of the needle, a needle pass directly from the IVC must be directed more laterally. As with other indications, use of graft-covered stents improves outcomes. In a recent study looking specifically at Budd-Chiari patients (Murad et al, 2008), the 2-year primary patency was 12% for bare stents versus 56% for stent grafts.
Portal Thrombus
Performing TIPS in patients with portal thrombosis requires a moderate level of expertise. One of the major technical challenges is accessing the PV. Although this can often still be accomplished from a transjugular route, use of a percutaneous transhepatic tract is sometimes needed to initially recanalize the PV (Radosevich et al, 1993). Once access is established, it is usually beneficial to deploy the stents across the parenchymal tract even before attempting lysis of the portal thrombus. TIPS provides a great conduit for using mechanical thrombolytic devices in the PV. Often, the thrombus is more chronic in nature and does not easily lyse, thus extending stents down the main PV to a patent segment below the thrombus is frequently necessary to establish good flow through the shunt. Because these stents can complicate a subsequent liver transplant operation, it is important to discuss the likelihood and timing of transplantation prior to placing a stent in the main PV. When the patient is close to receiving a new liver, we have elected to not perform TIPS rather than risk increasing the complexity of the transplant operation.
Alkari B, et al. Transjugular intrahepatic porto-systemic shunt and variceal embolisation in the management of bleeding stomal varices. Int J Colorectal Dis. 2005;20(5):457-462.
Angeloni S, et al. Polytetrafluoroethylene-covered stent grafts for TIPS procedure: 1-year patency and clinical results. Am J Gastroenterol. 2004;99(2):280-285.
Angermayr B, et al. Survival in patients undergoing transjugular intrahepatic portosystemic shunt: ePTFE-covered stent grafts versus bare stents. Hepatology. 2003;38(4):1043-1050.
Arepally A, et al. Evaluation of MR/fluoroscopy-guided portosystemic shunt creation in a swine model. J Vasc Interv Radiol. 2006;17(7):1165-1173.
Azoulay D, et al. Neoadjuvant transjugular intrahepatic portosystemic shunt: a solution for extrahepatic abdominal operation in cirrhotic patients with severe portal hypertension. J Am Coll Surg. 2001;193(1):46-51.
Bahramipour PF, et al. Transjugular intrahepatic portosystemic shunt for the treatment of intractable ascites in a patient with polycystic liver disease. Cardiovasc Intervent Radiol. 2000;23(3):232-234.
Baijal SS, et al. Management of idiopathic Budd-Chiari syndrome with primary stent placement: early results. J Vasc Intervent Radiol. 1996;7(4):545-553.
Bauer J, et al. The role of TIPS for portal vein patency in liver transplant patients with portal vein thrombosis. Liver Transpl. 2006;12(10):1544-1551.
Bellis L, et al. Hepatic venous pressure gradient does not correlate with the presence and the severity of portal hypertensive gastropathy in patients with liver cirrhosis. J Gastrointestin Liv Dis. 2007;16(3):273-277.
Bilbao JI, et al. Transjugular intrahepatic portosystemic shunt (TIPS) in the treatment of venous symptomatic chronic portal thrombosis in non-cirrhotic patients. Cardiovasc Intervent Radiol. 2004;27(5):474-480.
Blum U, et al. Budd-Chiari syndrome: technical, hemodynamic, and clinical results of treatment with transjugular intrahepatic portosystemic shunt. Radiology. 1995;197(3):805-811.
Boyer TD, Haskal ZJ. AASLD practice guidelines: the role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension. Hepatology. 2010;51(1):1-16.
Brountzos EN, et al. Transjugular intrahepatic portosystemic shunt in cavernomatous portal vein occlusion. Hepatogastroenterology. 2004;51(58):1168-1171.
Bureau C, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre study. Liver Int. 2007;27(6):742-747.
Cabrera J, et al. Transjugular intrahepatic portosystemic shunt versus sclerotherapy in the elective treatment of variceal hemorrhage. Gastroenterology. 1996;110(3):832-839.
Cello JP, et al. Endoscopic sclerotherapy compared with percutaneous transjugular intrahepatic portosystemic shunt after initial sclerotherapy in patients with acute variceal hemorrhage: a randomized, controlled trial. Ann Intern Med. 1997;126(11):858-865.
Chalasani N, et al. Determinants of mortality in patients with advanced cirrhosis after transjugular intrahepatic portosystemic shunting. Gastroenterology. 2000;118(1):138-144.
Chen L, et al. Outcomes of transjugular intrahepatic portosystemic shunt through the left branch vs. the right branch of the portal vein in advanced cirrhosis: a randomized trial. Liver Int. 2009;29(7):1101-1109.
Clark TW, et al. The effect of initial shunt outflow position on patency of transjugular intrahepatic portosystemic shunts. J Vasc Intervent Radiol. 2004;15(2):147-152.
Deltenre P, et al. Transjugular intrahepatic portosystemic shunt in refractory ascites: a meta-analysis. Liver Int. 2005;25(2):349-356.
Dhanasekaran R, et al. Transjugular intrahepatic portosystemic shunt for symptomatic refractory hepatic hydrothorax in patients with cirrhosis. Am J Gastroenterol. 2010;105(3):635-641.
Garcia-Pagan JC, et al. TIPS for Budd-Chiari syndrome: long-term results and prognostics factors in 124 patients. Gastroenterology. 2008;135(3):808-815.
Garcia-Pagan JC, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362(25):2370-2379.
Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362(9):823-832.
Garcia-Villarreal L, et al. Transjugular intrahepatic portosystemic shunt versus endoscopic sclerotherapy for the prevention of variceal rebleeding after recent variceal hemorrhage. Hepatology. 1999;29(1):27-32.
Gasparini D, et al. Transjugular intrahepatic portosystemic shunt by direct transcaval approach in patients with acute and hyperacute Budd-Chiari syndrome. Eur J Gastroenterol Hepatol. 2002;14(5):567-571.
Gil A, et al. The role of transjugular intrahepatic portosystemic shunt prior to abdominal tumoral surgery in cirrhotic patients with portal hypertension. Eur J Surg Oncol. 2004;30(1):46-52.
Ginés P, et al. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology. 2002;123(6):1839-1847.
Gordon FD, et al. The successful treatment of symptomatic, refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt. Hepatology. 1997;25(6):1366-1369.
Gulberg V, et al. Improved quality of life in patients with refractory or recidivant ascites after insertion of transjugular intrahepatic portosystemic shunts. Digestion. 2002;66(2):127-130.
Gulberg V, et al. Transjugular intrahepatic portosystemic shunting is not superior to endoscopic variceal band ligation for prevention of variceal rebleeding in cirrhotic patients: a randomized, controlled trial. Scand J Gastroenterol. 2002;37(3):338-343.
Han SG, et al. A case of successful treatment of stomal variceal bleeding with transjugular intrahepatic portosystemic shunt and coil embolization. J Korean Med Sci. 2007;22(3):583-587.
Harrod-Kim P, Saad W, Waldman D. Predictors of early mortality after transjugular intrahepatic portosystemic shunt creation for the treatment of refractory ascites. J Vasc Intervent Radiol. 2006;17(10):1605-1610.
Hausegger KA, et al. Use of the left internal jugular vein approach for transjugular portosystemic shunt. AJR Am J Roentgenol. 1998;171(6):1637-1639.
Hoppe H, Wang SL, Petersen BD. Intravascular US-guided direct intrahepatic portocaval shunt with an expanded polytetrafluoroethylene-covered stent-graft. Radiology. 2008;246(1):306-314.
Jalan R, et al. A randomized trial comparing transjugular intrahepatic portosystemic stent-shunt with variceal band ligation in the prevention of rebleeding from esophageal varices. Hepatology. 1997;26(5):1115-1122.
Jung HS, et al. TIPS: comparison of shunt patency and clinical outcomes between bare stents and expanded polytetrafluoroethylene stent-grafts. J Vasc Interv Radiol. 2009;20(2):180-185.
Kee ST, et al. MR-guided transjugular intrahepatic portosystemic shunt creation with use of a hybrid radiography/MR system. J Vasc Intervent Radiol. 2005;16(2 Pt 1):227-234.
Kerlan RKJr, et al. Inadvertent catheterization of the hepatic artery during placement of transjugular intrahepatic portosystemic shunts. Radiology. 1994;193(1):273-276.
Khuroo MS, et al. Budd-Chiari syndrome: long-term effect on outcome with transjugular intrahepatic portosystemic shunt. J Gastroenterol Hepatol. 2005;20(10):1494-1502.
Kim JK, et al. Extrahepatic portal vein tear with intraperitoneal hemorrhage during TIPS. Cardiovasc Intervent Radiol. 2001;24(6):436-437.
Kochar N, et al. Bleeding ectopic varices in cirrhosis: the role of transjugular intrahepatic portosystemic stent shunts. Aliment Pharmacol Therapeut. 2008;28(3):294-303.
Krajina A, et al. Wedged hepatic venography for targeting the portal vein during TIPS: comparison of carbon dioxide and iodinated contrast agents. Cardiovasc Intervent Radiol. 2002;25(3):171-175.
LaBerge JM, et al. Histopathologic study of stenotic and occluded transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 1993;4(6):779-786.
Lebrec D, et al. Transjugular intrahepatic portosystemic shunts: comparison with paracentesis in patients with cirrhosis and refractory ascites: a randomized trial. French Group of Clinicians and a Group of Biologists. J Hepatol. 1996;25(2):135-144.
Lo GH, et al. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy. 2007;39(8):679-685.
Macedo TA, Andrews JC, Kamath PS. Ectopic varices in the gastrointestinal tract: short- and long-term outcomes of percutaneous therapy. Cardiovasc Intervent Radiol. 2005;28(2):178-184.
Malinchoc M, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864-871.
Merli M, et al. Transjugular intrahepatic portosystemic shunt versus endoscopic sclerotherapy for the prevention of variceal bleeding in cirrhosis: a randomized multicenter trial. Gruppo Italiano Studio TIPS (GIST). Hepatology. 1998;27(1):48-53.
Mizrahi M, et al. Endotipsitis-persistent infection of transjugular intrahepatic portosystemic shunt: pathogenesis, clinical features and management. Liver Int. 2009;30(2):175-183.
Monescillo A, et al. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004;40:793-801.
Montgomery A, et al. MELD score as a predictor of early death in patients undergoing elective transjugular intrahepatic portosystemic shunt (TIPS) procedures. Cardiovasc Intervent Radiol. 2005;28(3):307-312.
Murad SD, et al. Long-term outcome of a covered vs. uncovered transjugular intrahepatic portosystemic shunt in Budd-Chiari syndrome. Liver Int. 2008;28(2):249-256.
Ochs A, et al. Transjugular intrahepatic portosystemic stent-shunt (TIPS) in the treatment of Budd-Chiari syndrome. J Hepatol. 1993;18(2):217-225.
Pan JJ, et al. Factors predicting survival after transjugular intrahepatic portosystemic shunt creation: 15 years’ experience from a single tertiary medical center. J Vasc Interv Radiol. 2008;19(11):1576-1581.
Peron JM, et al. Transjugular intrahepatic portosystemic shunts in the treatment of refractory ascites: results in 48 consecutive patients. J Vasc Interv Radiol. 2000;11(9):1211-1216.
Pomier-Layrargues G, et al. Transjugular intrahepatic portosystemic shunt (TIPS) versus endoscopic variceal ligation in the prevention of variceal rebleeding in patients with cirrhosis: a randomised trial. Gut. 2001;48(3):390-396.
Procaccini NJ, et al. Endoscopic cyanoacrylate versus transjugular intrahepatic portosystemic shunt for gastric variceal bleeding: a single-center U.S. analysis. Gastrointest Endosc. 2009;70(5):881-887.
Radosevich PM, et al. Transjugular intrahepatic portosystemic shunts in patients with portal vein occlusion. Radiology. 1993;186(2):523-527.
Rössle M, et al. Randomised trial of transjugular-intrahepatic-portosystemic shunt versus endoscopy plus propranolol for prevention of variceal rebleeding. Lancet. 1997;349(9058):1043-1049.
Rössle M, et al. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med. 2000;342(23):1701-1707.
Ryu RK, et al. Treatment of stomal variceal hemorrhage with TIPS: case report and review of the literature. Cardiovasc Intervent Radiol. 2000;23(4):301-303.
Saad WE, et al. Transjugular intrahepatic portosystemic shunts in liver transplant recipients for management of refractory ascites: clinical outcome. J Vasc Interv Radiol. 2010;21(2):218-223.
Salerno F, et al. Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology. 2004;40(3):629-635.
Salerno F, et al. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology. 2007;133(3):825-834.
Sanyal AJ, Reddy KR. Vegetative infection of transjugular intrahepatic portosystemic shunts. Gastroenterology. 1998;115(1):110-115.
Sanyal AJ, et al. Transjugular intrahepatic portosystemic shunts compared with endoscopic sclerotherapy for the prevention of recurrent variceal hemorrhage: a randomized, controlled trial. Ann Intern Med. 1997;126(11):849-857.
Sanyal AJ, et al. The North American Study for the Treatment of Refractory Ascites. Gastroenterology. 2003;124(3):634-641.
Sauer P, et al. Transjugular intrahepatic portosystemic stent shunt versus sclerotherapy plus propranolol for variceal rebleeding. Gastroenterology. 1997;113(5):1623-1631.
Saxon RS, et al. Transjugular intrahepatic portosystemic shunt patency and the importance of stenosis location in the development of recurrent symptoms. Radiology. 1998;207(3):683-693.
Schlenker C, Johnson S, Trotter JF. Preoperative transjugular intrahepatic portosystemic shunt (TIPS) for cirrhotic patients undergoing abdominal and pelvic surgeries. Surg Endosc. 2009;23(7):1594-1598.
Semba CP, et al. Hepatic laceration from wedged venography performed before transjugular intrahepatic portosystemic shunt placement. J Vasc Interv Radiol. 1996;7(1):143-146.
Shimizu T, et al. Bleeding portal-hypertensive gastropathy managed successfully by partial splenic embolization. Hepatogastroenterology. 2002;49(46):947-949.
Shin ES, Darcy MD. Transjugular intrahepatic portosystemic shunt placement in the setting of polycystic liver disease: questioning the contraindication. J Vasc Interv Radiol. 2001;12(9):1099-1102.
Spencer EB, Cohen DT, Darcy MD. Safety and efficacy of transjugular intrahepatic portosystemic shunt creation for the treatment of hepatic hydrothorax. J Vasc Interv Radiol. 2002;13(4):385-390.
Spier BJ, et al. Bleeding stomal varices: case series and systematic review of the literature. Clin Gastroenterol Hepatol. 2008;6(3):346-352.
Sterling KM, Darcy MD. Stenosis of transjugular intrahepatic portosystemic shunts: presentation and management. AJR Am J Roentgenol. 1997;168(1):239-244.
Stout LC, et al. Pseudointimal biliary epithelial proliferation and Zahn’s infarct associated with a 6-1/2-month-old transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. 1995;90(1):126-130.
Suhocki PV, et al. Treatment of TIPS/biliary fistula–related endotipsitis with a covered stent. J Vasc Interv Radiol. 2008;19(6):937-939.
Sze DY, et al. Transjugular intrahepatic portosystemic shunt creation in a polycystic liver facilitated by hybrid cross-sectional/angiographic imaging. J Vasc Interv Radiol. 2006;17(4):711-715.
Theruvath TP, Adams DB. Preoperative transjugular intrahepatic portosystemic shunt for extrahepatic surgery in cirrhosis. Am Surg. 2010;76(1):115-117.
Theuerkauf I, et al. Infarction and laceration of liver parenchyma caused by wedged CO(2) venography before tips insertion. Cardiovasc Intervent Radiol. 2001;24(1):64-67.
Tripathi D, et al. Improved clinical outcome with transjugular intrahepatic portosystemic stent-shunt utilizing polytetrafluoroethylene-covered stents. Eur J Gastroenterol Hepatol. 2006;18(3):225-232.
Vangeli M, et al. Bleeding ectopic varices: treatment with transjugular intrahepatic porto-systemic shunt (TIPS) and embolisation. J Hepatol. 2004;41(4):560-566.
Vidal V, et al. Usefulness of transjugular intrahepatic portosystemic shunt in the management of bleeding ectopic varices in cirrhotic patients. Cardiovasc Intervent Radiol. 2006;29(2):216-219.
Vignali C, et al. TIPS with expanded polytetrafluoroethylene-covered stent: results of an Italian multicenter study. AJR Am J Roentgenol. 2005;185(2):472-480.
Wallace MJ, et al. Transjugular intrahepatic portosystemic shunts: experience in the oncology setting. Cancer. 2004;101(2):337-345.
Wils A, et al. Transjugular intrahepatic portosystemic shunt in patients with chronic portal vein occlusion and cavernous transformation. J Clin Gastroenterol. 2009;43(10):982-984.