Chapter 54 Transfusion-Transmitted Diseases
Blood Safety Decision Making
Not surprisingly, from the 1980s until now, donor deferrals and blood-testing interventions have been rapidly, successively, and additively implemented for emerging and theoretical risks. Collection facilities introduced anti-HBc and alanine aminotransferase (ALT) testing as surrogates for non-A, non-B hepatitis, HIV p24 antigen testing, then nucleic acid testing (NAT) for hepatitis C, HIV, extensive deferrals for the risks attending transmissible spongiform encephalopathies (TSEs,) NAT for West Nile virus (WNV) and hepatitis B, and antibody testing for Trypanosoma cruzi. The cost-benefit estimates for some of these interventions exceeded by orders of magnitude generally accepted thresholds but did not deter their adoption. For example, the costs per quality-adjusted life-year proximate to implementation include HIV NAT, $1,966,000; HCV NAT, $1,830,000; WNV NAT, $520,000 to $897,000; human T-lymphotropic virus (HTLV) antibody testing, $63,000,000; and T. cruzi antibody testing, $2,123,000 (Fig. 54-1). It is unlikely that this reactive approach can be sustained in the current health care–reform environment.
These systems provide an opportunity for monitoring the risks and benefits of new initiatives, (e.g., proactive pathogen reduction). Pathogen-reduction processes offer the opportunity to abrogate most of the residual risk for all of the historically important transfusion-transmitted infections, bacterial contamination, babesiosis, malaria, WNV, and Chagas disease. Pathogen reduction could eliminate the often lengthy, reactive, iterative paradigm of emergence of a new pathogen in the population, recognition of a material threat to transfusion recipients, development of donor-deferral strategies followed by development and refinement of test systems that has characterized our historical approach. Critically, broadly active pathogen-reduction processes offer a layer of protection against unsuspected emergence of new agents. If already in use, they would need only to be validated as active against a new agent or appropriate model agents. The challenge to precautionism is balancing the impact of pathogen reduction on product quality and the potential long-term toxicities that may not be apparent in premarketing clinical trials against the unquantified probability that new agents will emerge and threaten enough morbidity to make pathogen reduction clinically and economically feasible.1
The conference’s consensus statement potentially portends a paradigm change toward risk-based decision making: risk identification, risk assessment, risk management, and risk communication.2 Risk will never be zero, and there is now a realization that cost considerations,3 politics, ideology, and public opinion cannot be ignored. Further policy making considerations will likely take these factors into account, balancing risk, safety, stakeholder interest, and overall impact on public health.
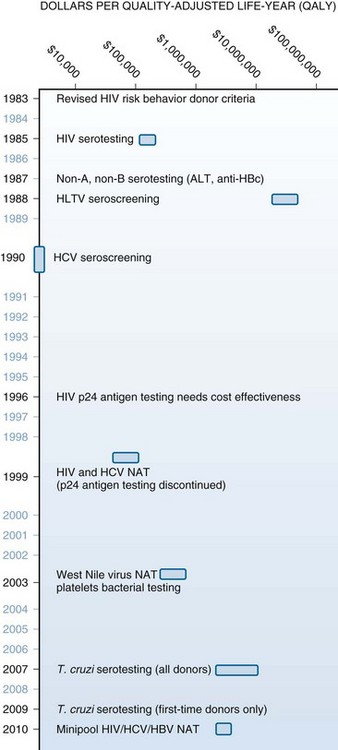
Figure 54-1 VARIOUS SAFETY MEASURES INTRODUCED DURING THE PAST THREE DECADES ENHANCED TRANSFUSION SAFETY.
Table 54-1 Patients Benefiting From Cytomegalovirus Risk–Reduced Blood Components*
CMV, Cytomegalovirus; HIV, human immunodeficiency virus.
*CMV risk–reduced components include CMV-seronegative and leukocyte-reduced components.
Pathogen Reduction
Our approach to new transfusion-transmitted infections is intrinsically reactive. We await disease emergence and identification of the new agent, develop an understanding of the infection’s risk factors, devise donor deferrals, and then implement screening assays. Before deploying a sensitive and specific test, morbidity and mortality will accumulate. This reactive strategy will continue unless more broad-reaching interventions are brought forward. Pathogen-reduction technology (PRT) offers a proactive strategy to address new threats; these technologies involve physical, chemical, and photochemical treatments of blood components to inactivate or decrease viral, bacterial, and parasite infectivity.4
Amotosalen/UVA and riboflavin/UVB also target nucleic acids. Treated plasma retains 72% to 77% of fibrinogen and factor VIII levels, greater than 80% of protein S and α2-antiplasmin, and 96% of ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin motifs 13). Platelets treated with these technologies have approximately 30% lower 1-hour posttransfusion corrected-count–increments than control platelets.5 In clinical trials, mild and moderate bleeding frequency is increased, but not severe bleeding complications; and the time between transfusions and total platelet transfusions has not generally been different. It is unclear whether a proportion of platelets is impaired (in which case, platelet dose escalation would ameliorate concerns about lower corrected count increment) or all platelets are affected (increasing the dose would be insufficient). Pulmonary toxicity similar to transfusion-related acute lung injury (TRALI) has been reported in clinical trials and in animal model experiments in which UV light has been implicated. Previous clinical trials in red blood cells (RBCs) were halted because of nonsymptomatic immunoreactivity against S303–induced red cell neoantigens and are being resumed with a reformulated process. Preliminary reports suggest riboflavin/UV causes functional impairment in red cells stored nearest the 42-day expiration date.
1 Kleinman S, Cameron C, Custer B, et al. Modeling the risk of an emerging pathogen entering the Canadian blood supply. Transfusion. 2010;50:2592.
2 Bennett JL, Blajchman MA, Delage G, et al. Proceedings of a Consensus Conference: Risk-Based Decision Making for Blood Safety. Transfus Med Rev. 2011;25:267.
3 Jackson BR, Busch MP, Stramer SL, et al. The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations. Transfusion. 2003;43:721.
4 Aubuchon JP, Prowse CV, eds. Pathogen inactivation: the penultimate paradigm shift. Bethesda Md: AABB Press, 2010.
5 Seghatchian J, Hervig T, Putter JS. Effect of pathogen inactivation on the storage lesion in red cells and platelet concentrates. Transfus Apher Sci. 2011;45:75.