Transcranial doppler in aneurysmal subarachnoid hemorrhage
(CONSULTANT LEVEL EXAMINATION)
Overview
The management of aneurysmal subarachnoid hemorrhage (aSAH) and its accompanying sequelae integrates transcranial Doppler (TCD) ultrasonography for the surveillance of the emergence and course of vasospasm (VS) and delayed cerebral ischemia.1 TCD surveillance of patients after aSAH has been advocated by the two most recent aSAH treatment guidelines, and practice standards for its use are published.2–4 This disease is complex to manage, and TCD has emerged as an inexpensive, noninvasive tool for monitoring intracerebral hemodynamic changes seen with SAH as well as for evaluating other neurologic conditions, including intracranial arterial stenosis, arteriovenous malformations, emboli of different origin, venous sinus thrombosis, brain death, ischemic stroke and clot lysis, and sickle cell disease.5,6
Vasospasm after aneurysmal subarachnoid hemorrhage
The phenomenon of the diminution of blood flow transiting through the cerebral vasculature seen after aSAH or severe neurotrauma resulting from the decrease in the caliber of arteries is referred to as VS.7,8 Arterial spasm after aSAH was originally described by Ecker in 1951 and has since been the subject of decades of laboratory research and clinical investigations. The phenomena of “angiographic VS” and “clinical VS” are often discussed, both of which may culminate in eventual delayed cerebral ischemia (DCI) and permanent neurologic deficits. The exact cause of VS is not clearly understood.1,7 VS often occurs most intensely adjacent to the subarachnoid clot burden but can occur distant from the majority of the subarachnoid blood and is predicted by clot volume, age, location, and density of the SAH seen on the initial computed tomography (CT) scan.9,10 Because of more aggressive early surgical or endovascular treatment of ruptured aneurysms, fatality from early re-rupture has now been replaced by complications of hydrocephalus and VS as the most common and serious causes of morbidity and mortality after aSAH.11,12 Thus monitoring for VS and DCI with noninvasive techniques, such as TCD, remains a critical tool in the management of this disease.1
Angiographic VS is common after aSAH, occurring in as many as 67% of patients, with the highest incidence occurring between days 10 and 17 after aSAH.12 Classically, VS is reported to occur from day 4 to 14, but variations on this general rule are common.1,3,4,7,13 The incidence of early angiographic VS, detected within 48 hours of aneurysm rupture, occurs in 10% to 13% of aSAH patients and is associated with prior aSAH, large aneurysms, intraventricular hemorrhage, and worsened 3-month outcome and morbidity.14 It is now well recognized that VS is also seen after traumatic SAH.8
Transcranial doppler as a monitor for cerebral vasospasm
VS is a clinical diagnosis, and findings range from nonfocal neurologic signs, such as confusion, increasing somnolence, and combativeness, to focal and localizable neurologic deficits from stroke. In 1982, Aaslid provided the first descriptions of the use of TCD for early detection of VS.15 The gold standard for the diagnosis of cerebral VS has remained digital subtraction angiography (DSA), although this modality is not practical for use as a frequent monitor for VS. Computed tomography angiography (CTA) has emerged as a potentially helpful tool in the evaluation of VS and is often used along with TCD.3,4
Because of its portability for bedside testing, noninvasive nature, ease of repeated testing, and lack of known adverse side effects from its use, TCD has become one of the most used screening tools for monitoring patients with aSAH for the development of VS in practice currently. In common practice, TCD is performed daily to twice daily from the first day after presenting with aSAH until VS subsides. TCD is an operator-dependent examination, although up to 8% of patients may have skull insonation windows too thick to allow evaluation of cerebral blood flow velocities (CBFVs) in cm/sec.13
As described by Aaslid, TCD uses the underlying principle that the velocity of blood flow in a conduit is inversely related to the diameter of that conduit, and that as diameter is decreased, velocity will increase, and vice versa. An indirect evaluation of the vessel diameter is achieved by using the Doppler effect upon red blood cells by calculating the Doppler shift between the frequencies of the transmitted and received ultrasound waves.16
Indices and technical aspects of transcranial doppler ultrasonography
TCD provides several indices that assist in the clinical decision making for patients with aSAH. The CBFV is the most used metric and is also described by parameter of the mean CBFV (mCBFV), the peak systolic flow velocity (Vs), and the end diastolic flow velocity (Vd). In clinical practice, the mCBFV is typically reported, but the other parameters are used to calculate the resistance index (RI) and the pulsatility index (PI). Both RI and PI are presumptive measures of downstream vascular resistance and may predict intracranial compliance or intracranial pressure.17,18 Clinical decision-making based on this relationship of the PI currently remains controversial.19 Elevated RI and PI may also occur secondary to distal VS or intracranial stenosis. The derivation of RI and PI are shown in Figure 3-1.
The Lindegaard (ratio) index (LI) is a method of correcting for increases in hyperdynamic systemic flow velocities that are either physiologically or medically induced in patients with aSAH (e.g., because of triple-H therapy: hypervolemia, hypertension, hemodilution). To calculate the LI, the mCBFV of the middle cerebral artery (MCA) is compared with an ipsilateral extracranial vessel, which is usually the proximal internal carotid artery (ICA) (Figure 3-2). This ratio helps to distinguish global hyperemia from VS, particularly when the LI is greater than 6.3,20 Others have suggested that an intracranial to extracranial ratio for the basilar artery (BA), for instance, comparison of the BA mCBFV to the extracranial vertebral artery (VA) mCBFV, of greater than 3 is highly sensitive and specific for posterior circulation VS.21 The extracranial/intracranial mCBFV ratio can be especially helpful in aSAH in the setting of the global hyperemia that accompanies the often-used triple-H therapy.
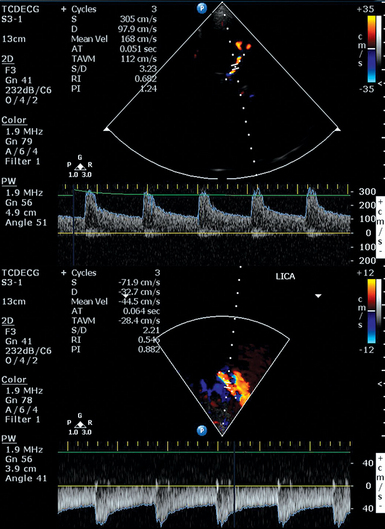
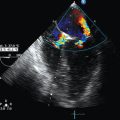
Figure 3-2 Moderate vasospasm in the left middle cerebral artery after aneurysmal subarachnoid hemorrhage as detected by real-time transcranial Doppler (TCD): mean flow velocity in the left middle cerebral artery is greater than130 cm/sec (top), and the LI calculated by the ratio of the latter to the mean flow velocity detected in the ipsilateral proximal internal carotid artery (LICA, bottom) is greater than 3. (Courtesy Dr. D. Karakitsos.)
TCD measurements alone may not correlate with angiographic or symptomatic VS. Differing hemodynamic states may alter the effects of decreased vessel lumen diameter on flow resistance as measured by TCD. It is postulated that with moderate VS, cerebral autoregulation compensates for perfusion pressure reduction in the region of spasm, if arterial blood pressure is above the lower limit of autoregulation. The flow velocity then increases as lumen area falls, yielding good correlation between angiographic and TCD measured spasm.22 As stenosis increases, the volume of flow is reduced, and velocity remains highly independent of diameter from ineffective attempts at cerebral autoregulation. When blood pressure is augmented with triple-H therapy, flow increases and ischemic symptoms may improve; mean flow velocity (MFV) may paradoxically have higher values than when normotensive. In this scenario, the correlation between TCD velocity and the degree of angiographic VS is likely to be poor. If vessel diameter worsens further, lower mCBFV and reduction of flow to critical values may manifest with ischemia.22
Interpretation of data from transcranial doppler
It is recommended that TCD studies be performed in the intensive care unit (ICU) for patients with aSAH.3,4 This data can be interpreted based on absolute criteria for VS or used to see trends in the tempo of VS over the course of several days.3 Some have proposed a point scoring system for VS, with reported sensitivity and specificity of both at 96%.23 The correlation between TCD mCBFV with decreases in vessel diameter on angiography have been most convincing for examinations of the MCA.1 Ideal placement of the reference standard for mCBFV and extracranial/intracranial mCBFV ratios is debated, although potential cutoff values where mild, moderate, and severe VS is likely present is presented in Table 3-1.
TABLE 3-1
TCD Parameters Indicating Vasospasm after Aneurysmal SAH3,21,27
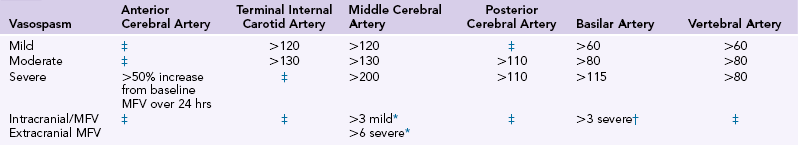
*Middle cerebral artery/extracranial internal carotid artery.
TCD monitoring is typically used after an aneurysm is secured. A role for early monitoring to establish increased risk of delayed cerebral ischemia (DCI) may be emerging. In a study of 199 patients with TCD examinations within 48 hours of SAH onset, 38% of patients had MCA MFV elevation greater than 90 cm/sec, which was associated with younger age, angiographic VS on admission, and elevated white blood cell count.24
Anterior circulation
Anatomic factors and insonation windows may make TCD more reliable for some vessels than for others. Prior work in aSAH showed a correlation between elevated TCD CBFVs and symptomatic VS that was improved in the intracranial ICA (sensitivity, 80%; specificity, 77%) and MCA (64% and 78%, respectively) distributions compared with the anterior cerebral artery (ACA) (45% and 84%, respectively).25 To improve the sensitivity of TCD of the ACA, Lindegaard had suggested that clinicians use both ACAs to access VS on either side, because of collateralization of the ACAs by the anterior communicating artery (ACom). Older data suggest that TCD studies of this vessel may be more useful to detect VS with trend analysis rather than absolute velocity thresholds, and that VS may be heralded by a relative increase in mCBFV of greater than 50% change over subsequent examinations or a change of 50 cm/sec in MFV over a 24-hour period.26 Likewise, the posterior cerebral artery (PCA) offers challenges to the diagnosis of VS with TCD. Prior work reported sensitivity of 48% and specificity of 69% in technically adequate TCDs with a mCBFV cutoff value of 90 cm/sec, with increased specificity of 93% but low sensitivity if this value was increased to 110 cm/sec.27 The PCA, similar to the ACA, has proven to be a difficult vessel for which to reliably establish TCD criteria for VS (see Table 3-1).
Increased mCBFV within the MCA is most correlated with angiographic VS. Various cutoff values of mCBFV have been proposed, and others rely more heavily on the intracranial to extracranial velocity ratio. Proposed velocity criteria are presented in Table 3-1. A LI of greater than 6 has been shown to reliably predict VS in patients with clinical findings possibly indicating ischemia.28 Systemic factors such as hematocrit and mean arterial pressure must be taken into account when interpreting the LI.
Terminal ICA VS is well described, with retrospective data showing sensitivities of 95% for the detection of VS with an ICA aneurysm, if the MCA (M1—sphenoidal segment of the MCA) and the ICA are successfully insonated.29 Other work with 49 patients and TCDs of 90 intracranial ICAs reported a specificity and positive predictive value of 100% when mCBFV values exceeded 130 cm/sec in the intracranial ICA.30
Posterior circulation
TCD detection of vertebral artery (VA) or BA VS differs from the anterior circulation. A cutoff velocity of approximately 80 to 85 cm/sec in the BA predicts more frequent progression to cerebral ischemia, which could influence clinical decision-making.21,31 An intracranial to extracranial ratio for the BA greater than 3 is highly sensitive and specific for posterior circulation VS (see Table 3-1). Further work on modifications of the LI for the posterior circulation showed normative values for the intracranial/extracranial VA mCBFV ratio (IVA/EVA) and BA/extracranial VA mCBFV ratio (BA/EVA), and evaluated aSAH patients with TCD and CT angiography (CTA).31 A BA/EVA ratio greater than 2 was 100% sensitive and 95% specific for detection of BA VS. In addition, the BA/EVA ratio showed close correlation with BA diameter and was greater than 3 in all patients with severe VS.
Limitations of transcranial doppler in the detection of vasospasm
Several factors are known to affect TCD mCBFV measurements that may impact assessment during aSAH, including hematocrit, arterial carbon dioxide, episodic alterations in vasomotor status, the patient’s level of consciousness, and the observer’s level of experience. 2,32–34 At this time, there appears to be no particular advantage of continuous TCD monitoring, although further study into how moment-to-moment variability affects detection of VS is suggested.34 Same-day interobserver variability has been reported to be about 7.5% and about 13% on different days.35 Variations in MCA mCBFV have also been observed with age, pregnancy, menstruation, and arousal of individuals.36,37 Some vessels may be better assessed with TCD than are others because of anatomic features and individual patient characteristics.2,25,27 Temporal bone thickness is quite variable among individuals but has some relationship to age, sex, and race. Inability to insonate the MCA and some other anterior circulation vessels is more often associated with the elderly than the young, with women than men, and with nonwhites than whites.38
Management strategy in aneurysmal subarachnoid hemorrhage
Daily TCD ultrasonography examination should be considered in all patients with aSAH, along with correlation with serial clinical neurologic examinations and physiologic data. In our practice, patients admitted with aSAH are studied as soon as possible after DSA and securing the aneurysm with either surgical clipping or endovascular coiling. In the setting of clinical stability, TCDs are continued daily while patients are maintained in a state of normovolemia and normonatremia. When a patient without new examination findings enters the window for increased risk of developing VS, if TCD mCBFVs increase to generally accepted levels for VS for that vessel, fluid balance is shifted to maintaining a positive fluid state, and volume is augmented, along with serum sodium with hypertonic saline if cerebral salt wasting develops. Patients are allowed to autoregulate their blood pressure up to systolic pressures (SBP) of 200 mm/Hg or mean arterial pressures (MAP) of 120 to 140 mm/Hg, depending on the clinical status of the patient and other existing comorbidities. The application of a hemodynamic monitor may also be considered to optimize cardiopulmonary function and fluid management. If there is increased clinical suspicion for VS with either increasing TCD measured mCBFV values or clinical findings of potential ischemia, hypervolemic and hypertensive therapy is begun with either aggressive crystalloid or colloids, and phenylephrine or norephinephrine infusions, along with placement of an invasive hemodynamic monitor. Alternatively, dobutamine or milrinone may be used in the setting of neurogenic stunned myocardium to augment cardiac output (see Chapter 7). Based on the clinical status of the patient and the reliability of the neurologic examination, other diagnostic imaging may then be considered. The use of a perfusion study, such as CT-perfusion, may be helpful in these cases, but if the suspicion is strong for clinical worsening, then titration of MAP or SBP goals is warranted. DSA, as both a diagnostic and therapeutic intervention, may be performed at this stage. TCD follow-up is vital in assessing the results of therapy and, along with the clinical examination, will aid in the timing of repeat angiography and guide hemodynamic management.
Transcranial doppler ultrasonography for traumatic vasospasm
Several clinical applications of TCD currently exist in practice. TCD ultrasonography may be helpful in the setting of head trauma, as a marker of increased intracranial pressure (ICP), assessment of cerebral autoregulation, brain death, ischemic stroke, intraoperative monitoring, and assessment of right-to-left shunt, among other uses. Evidence has emerged regarding the incidence of VS assessed by TCD in the anterior and posterior circulation after traumatic SAH (tSAH) or blast-related head injury.8,39,40 The incidence of VS is reported to occur at a higher rate in some populations with tSAH than aSAH, a departure from prior teaching regarding VS. In a large prospective cohort study of traumatic brain injury, hemodynamically significant VS in the anterior circulation was found in 44.6% of the patients, whereas VS in the BA (BA FV > 90 cm/sec) or hemodynamically significant VS in the posterior circulation was found in 19% and 22.5% of patients, respectively. The most common day of VS onset was postinjury day 2. In this study, the VS resolved after 5 days in 50% of the patients with anterior circulation spasm and after 3.5 days in 50% of patients with posterior circulation spasm.41
Transcranial doppler as a marker of intracranial pressure and compliance
The correlation of TCD PI (see Figure 3-1) and intracranial pressure is described. Early work by Klingelhöfer et al shows that ICP and RI share a direct correlation.42 Furthermore, the PI as a ratio is sensitive to changes in ICP because downstream compression of arterioles secondary to high ICP will decrease the denominator of this equation (CBFV), which is a surrogate measure of flow. Increased downstream vascular resistance created by compression of smaller arterioles occurs without effect on the larger insonated arteries of the circle of Willis. Also, as increased ICP reduces compliance of the entire system, velocity variations resulting from the rigidity of arteries and reduced Vd will increase the numerator of this relationship. In turn, both of these factors will increase the index and may indicate increasing ICP.18 Follow-up prospective studies of this relationship have shown this correlation to be significant in a mixed population of neurosurgical patients who underwent TCD evaluation with an extraventricular drain in place, but this relationship has also been questioned.43–46 TCD has not gained acceptance as a surrogate for invasive ICP monitoring, although information provided noninvasively by TCD ultrasonography may guide decisions to place invasive extraventricular drains, subdural monitors, or intraparenchymal monitors for suspicion of increased ICP in patients with severe neurologic illness or trauma.18 To the best of our knowledge, no one yet suggests using PI as an accurate method to assess quantitatively ICP in mm Hg. In numerous publications, however, it was shown that PI correlates well with ICP that was measured by invasive methods.17,47 More studies are clearly required before we understand the relationship of ICP and TCD. Nonetheless, even today quantitative and qualitative change in TCD values and waveform morphologies may persuade physicians to undertake other diagnostic steps and/or change medical treatment that will improve the care of these patients and their subsequent outcomes.
Pearls and highlights
• The utility of TCD in the ICU has grown substantially since its introduction 30 years ago.
• TCD currently maintains an important role in the day-to-day management and triage of more invasive and expensive diagnostic tests and subsequent intervention in the setting of vasospasm resulting from aneurysmal and traumatic SAH.
• Limitations currently exist to the use of TCD as an isolated marker of radiographic VS.
• Complete TCD evaluations, including calculation of the Lindegaard (ratio) index (LI) for the anterior circulation and a modified LI for the posterior circulation may increase the specificity for VS detected by TCD in the setting of cerebral hyperemia.
• Similar to many tools used in the ICU, TCD is best used as part of the multimodality environment that incorporates radiographic, metabolic, and clinical findings to better manage patients with SAH.
The views and opinions herein belong solely to the authors. They do not, nor should they be construed as belonging to, representative of, or being endorsed by the Uniformed Services University of the Health Sciences, the U.S. Army, the U.S. Air Force, the Department of Defense, or any other branch of the federal government of the United States.
References
1. Saqqur, M, Aygun, D, Demchuck, A. Role of transcranial Doppler in neurocritical care. Crit Care Med. 2007; 35(Suppl 5):S216–S223.
2. Alexandrov, A, Sloan, MA, Tegeler, CH, et al, Practice standards for transcranial Doppler ultrasound: part I—test performance. J Neuroimaging. 2007;17(1):11–18.
3. Diringer, M, Bleck, TP, Claude Hemphill, J, 3rd., et al, Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidiciplinary Consensus Conference. Neurocrit Care. 2011;15(2):211–240.
4. Connolly, EJ, Rabinstein, AA, Carhuapoma, JR, et al, Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711–1737.
5. Bassocchi, M, Quaia, E, Auiani, C, Moroldo, M, Transcranial Doppler: state of the art. Eur J Radiol. 1998;27(Suppl 2):S141–S148.
6. Lowe, L, Bulas, DI. Transcranial Doppler monitoring and clinical decision making after subarachnoid hemorrhage. J Stroke Cerebrovasc. 2003; 2003(35):54–64.
7. Rigamonti, A, Ackery, A, Baker, AJ, Transcranial Doppler monitoring in subarachnoid hemorrhage: a critical tool in critical care. Can J Anesth. 2008;55(2):112–123.
8. Armonda, RA, Bell, RS, Vo, AH, et al, Wartime traumatic cerebral vasospasm: recent review of combat casualties. Neurosurgery. 2006;59(6):1215–1225. [discussion 1225].
9. Reilly, C, Amidei, C, Tolentino, J, et al. Clot volume and clearance rate as independent predictors of vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004; 101(2):255–261.
10. Gonzalez, N, Boscardin, WJ, Glenn, T, et al, Vasospasm probability index: a combination of transcranial Doppler velocities, cerebral blood flow, and clinical risk factors to predict cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007;107(6):1001–1012.
11. McLaughlin, N, Bojanowski, MW. Management of patients with aneurismal subarachnoid hemorrhage and associated symptomatic vasospasm on presentation. Neurochirugie. 2012; 58(2-3):160–169.
12. Dorsh, N, King, MT, A review of cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Part I: incidence and effects. J Clin Neurosci. 1997;1(1):19–26.
13. Zubkov, A, Rabinstien, AA, Medical management of cerebral vasospasm: present and future. Neurological Res. 2009;31(6):626–631.
14. Baldwin, M, Macdondald, RL, Huo, D, et al. Early vasospasm on admission angiography in patients with aneurysmal subarachnoid hemorrhage is a predictor for in-hospital complications and poor outcome. Stroke. 2004; 35(11):2506–2511.
15. Aaslid, R, Markwalder, TM, Nornes, H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982; 57(6):769–774.
16. Kassab, M, Majid, A, Farooq, MU, et al, Transcranial Doppler: an introduction for primary care physicians. J Am Board Fam Med. 2007;20(1):65–71.
17. Bellner, J, Romner, B, Reinstrup, P, et al. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP). Surg Neurol. 2004; 62(1):45–51.
18. Razumovsky, A, Armonda, RA. We still do not have a reliable and validated noninvasive technique that can provide an accurate quantitative measurement of intracranial pressure (ICP) that could replace invasive quantitative measurements of ICP. Neurosurgery. 2011; 68(1):E289–E292.
19. Zweifel, C, Czosnyka, M, Carrera, E, et al. Reliability of the blood flow velocity pulsatility index for assessment of intracranial and cerebral perfusion pressures in head-injured patients. Neurosurgery. 2012; 71(4):853–861.
20. Lindegaard, K, Nornes, H, Bakke, SJ, et al. Cerebral vasospasm after subarachnoid haemorrhage investigated by means of transcranial Doppler ultrasound. Acta Neurochir Suppl (Wien). 1988; 42(Suppl):81–84.
21. Sviri, G, Newell, DW, Lewis, DH, et al. Transcranial Doppler grading criteria for basilar artery vasospasm. Neurosurgery. 2006; 59(2):360–366.
22. Aaslid, R, Transcranial Doppler assessment of cerebral vasospasm. Eur J Ultrasound. 2002;16(1-2):3–10.
23. Sebastian, J, Derksen, C, Khan, K, et al. Derivation of transcranial Doppler criteria for angiographically proven middle cerebral artery vasospasm after aneurysmal subarachnoid hemorrhage, J Neuroimaging. dx.doi.org/10.1111/j.1552-6569.2012.00771.x, Nov 19, 2012. [[Epub ahead of print.]].
24. Carrera, E, Schmidt, JM, Oddo, M, et al. Transcranial Doppler ultrasound in the acute phase of aneurysmal subarachnoid hemorrhage. Cerebrovasc Dis. 2009; 27(6):579–584.
25. Suarez, J, Qureshi, AI, Yahia, AB, Syptomatic vasospasm diagnosis after subarachnoid hemorrhage: Evaluation of transcranial Doppler ultrasound and cerebral angiography as related to compromised vascular distribution. Crit Care Med. 2002;30(6):1348–1355.
26. Grosset, D, Straiton, J, McDonald, I, et al. Use of transcranial Doppler sonography to predict development of a delayed ischemic deficit after subarachnoid hemorrhage. J Neurosurg. 1993; 78(2):183–187.
27. Wozniak, M, Sloan, MA, Rothman, MI, et al. Detection of vasospasm by transcranial Doppler sonography. The challenges of the anterior and posterior cerebral arteries. J Neuroimaging. 1996; 6(2):87–93.
28. Ionita, C, Graffagnino, C, Alexander, MJ, Zaidat, OO. The value of CT angiography and transcranial Doppler sonography in triaging suspected cerebral vasospasm in SAH prior to endovascular therapy. Neurocrit Care. 2008; 9(1):8–12.
29. Creissard, P, Proust, F, Vasospasm diagnosis: theoretical sensitivity of transcranial Doppler evaluated using 135 angiograms demonstrating vasospasm. Practical consequences. Acta Neurochir (Wien). 1994;131(1-2):12–18.
30. Burch CM Wozniak, MA, Sloan, MA, et al. Detection of intracranial internal carotid artery and middle cerebral artery vasospasm following subarachnoid hemorrhage. J Neuroimaging. 1996; 6(1):8–15.
31. Soustiel, J, Shik, V, Shreiber, R, et al, Basilar vasospasm diagnosis: investigation of a modified “Lindegaard Index” based on imaging studies and blood velocity measurements of the basilar artery. Stroke. 2002;33(1):72–77.
32. Brass, L, Pavlakis, SG, DeVivo, D, et al. Transcranial Doppler measurements of the middle cerebral artery. Effect of hematocrit. Stroke. 1988; 19(12):1466–1469.
33. Maeda, H, Matsumoto, M, Handa, N, et al, Reactivity of cerebral blood flow to carbon dioxide in various types of ischemic cerebrovascular disease: evaluation by the transcranial Doppler method. Stroke. 1993;24(5):670–675.
34. Venkatesh, B, Shen, Q, Lipman, J. Continuous measurement of cerebral blood flow velocity using transcranial Doppler reveals significant moment-to-moment variability of data in healthy volunteers and in patients with subarachnoid hemorrhage. Crit Care Med. 2002; 30(3):563–569.
35. Moppett, I, Mahajan, RP. Transcranial Doppler ultrasonography in anaesthesia and intensive care. Br J Anaesth. 2004; 93(5):710–724.
36. Krejza, J, Mariak, Z, Walecki, J, et al, Transcranial color Doppler sonography of basal cerebral arteries in 182 healthy subjects: age and sex variability and normal reference values for blood flow parameters. Am J Roentgenol. 1999;172(1):213–218.
37. Belfort, M, Tooke-Miller, C, Allen, JC, et al. Changes in flow velocity, resistance indices, and cerebral perfusion pressure in the maternal middle cerebral artery distribution during normal pregnancy. Acta Obstet Gynecol Scand. 2001; 80(2):104–112.
38. Grolimund, P, Seiler, RW, Aaslid, R, et al. Evaluation of cerebrovascular disease by combined extracranial and transcranial Doppler sonography. Experience in 1,039 patients. Stroke. 1987; 18(6):1018–1024.
39. Razumovsky, A, Tigno, T, Hochheimer, SM, et al. Cerebral hemodynamic changes after wartime traumatic brain injury. Acta Neurochir Suppl. 2013; 115:87–90.
40. Soustiel, J, Shik, V, Feinsod, M, Basilar vasospasm following spontaneous and traumatic subarachnoid haemorrhage: clinical implications. Acta Neurochir (Wien). 2002;144(2):137–144.
41. Oertel, M, Boscardin, WJ, Obrist, WD, et al, Posttraumatic vasospasm: the epidemiology, severity, and time course of an underestimated phenomenona prospective study performed in 299 patients. J Neurosurg. 2005;103(5):812–824.
42. Klingelhofer, J, Conrad, B, Benecke, R, et al, Evaluation of intracranial pressure from transcranial Doppler studies in cerebral disease. J Neurol 1988;235(3):159–162.
43. Behrens, A, Lenfeldt, N, Ambarki, K, et al, Transcranial Doppler pulsatility index: not an accurate method to assess intracranial pressure. Neurosurgery. 2010;66(6):1050–1057.
44. Voulgaris, S, Partheni, M, Kaliora, H, et al. Early cerebral monitoring using the transcranial Doppler pulsatility index in patients with severe brain trauma. Med Sci Monit. 2005; 11(2):49–52.
45. Bellner, J, Romner, B, Reinstrup, P, et al. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP). Surg Neurol. 2004; 62(1):45–51.
46. de Riva, N, Budohoski, KP, Smielewski, P, et al, Transcranial Doppler pulsatility index: what it is and what it isn’t. Neurocrit Care. 2012;17(1):58–66.
47. Bor-Seng-Shu E, Hirsch, R, Teixeira, MJ, et al. Cerebral hemodynamic changes gauged by transcranial Doppler ultrasonography in patients with posttraumatic brain swelling treated by surgical decompression. J Neurosurg. 2006; 104(1):93–100.