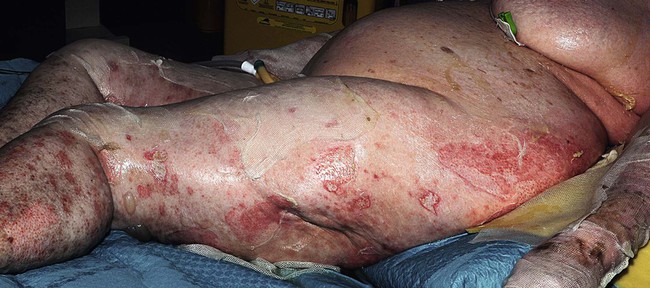
Toxic epidermal necrolysis and Stevens–Johnson syndrome
Specific investigations
Incidence of Stevens–Johnson syndrome and toxic epidermal necrolysis in patients with the acquired immunodeficiency syndrome in Germany.
Rzany B, Mockenhaupt M, Stocker U, Hamouda O, Schöpf E. Arch Dermatol 1993; 129: 1059.
HIV testing should be considered for high-risk patients presenting with TEN/SJS.
First-line therapies
Toxic epidermal necrolysis and Stevens–Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death?
Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Arch Dermatol 2000; 136: 323–7.
Early discontinuation of the causative drug improved prognosis in this study of 113 patients.
ALDEN, an algorithm for assessment of drug causality in Stevens–Johnson syndrome and toxic epidermal necrolysis: comparison with case-control analysis.
Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, et al. Clin Pharmacol Ther 2010; 88: 60–8.
A validated algorithm for assessing likelihood of causality of drugs in cases of SJS and TEN.




 Supportive measures
Supportive measures Withdrawal of culprit drug
Withdrawal of culprit drug Analgesia
Analgesia Bioengineered skin substitute
Bioengineered skin substitute Cyclosporine
Cyclosporine Systemic corticosteroids
Systemic corticosteroids Intravenous immunoglobulin
Intravenous immunoglobulin Plasmapheresis
Plasmapheresis Pentoxifylline
Pentoxifylline