151 Toxic Alcohols
• Toxic alcohol poisoning may initially be mistaken for simple inebriation.
• Untreated ethylene glycol poisoning may result in renal failure.
• Untreated methanol poisoning may result in blindness.
• Acidosis from ethylene glycol or methanol may not be evident until several hours after exposure.
• An osmol gap may not be evident in patients whose presentation to the emergency department is delayed, and these patients benefit from early dialysis.
• An osmol gap measurement is only a screening test and is never diagnostic like a quantitative alcohol measurement.
• Isopropanol poisoning classically results in an elevated osmol gap without significant acidosis.
• Treatment with ethanol or fomepizole should be considered in cases of a witnessed ingestion of a toxic alcohol, when such an ingestion is highly suspected from the history, in the presence of an enlarged osmol gap alone with appropriate clinical suspicion, in the patient with both an enlarged osmol gap and anion gap acidosis, or when the serum concentration of a toxic alcohol exceeds 20 mg/dL.
• Early alcohol dehydrogenase inhibition minimizes metabolism of the toxic alcohol to organic toxic acids and reduces alcohol-specific complications.
Epidemiology
Except for ethanol, no other alcohols are fit for human consumption, and they are properly termed toxic alcohols. Ethylene glycol, methanol, and isopropanol are the most common toxic alcohols associated with human poisoning.1 Less commonly reported but still clinically important toxic alcohols are propylene glycol, diethylene glycol, and other glycol ethers. Unintentional ingestion from a mislabeled or contaminated container occurs commonly in children, whereas intentional ingestion of a toxic alcohol as an ethanol substitute or for self-harm occurs more commonly in adults. If untreated, toxic alcohol poisoning can result in metabolic acidosis, renal failure, blindness, central nervous system (CNS) injury, pulmonary edema, or death.
Ethylene glycol is present in antifreeze solutions, deicing solutions, foam stabilizers, and chemical solvents.2 Methanol is a component of windshield-washing solutions, gas-line antifreeze solutions, solvents, and brake cleaners.3 Isopropanol is found in rubbing alcohol, aerosols, and other cosmetic products. Propylene glycol is commonly found as a diluent in parenteral medications such as phenytoin, diazepam, and lorazepam.4 The other toxic glycols can be found in various household and industrial cleaners, paints, resins, and solvents.
More than 35,000 toxic alcohol exposures are reported yearly to the American Association of Poison Control Centers.1 Most cases are individual poisonings. However, contamination of beverages or pharmaceutical products has resulted in epidemic poisonings, including two significant outbreaks, in India and in Haiti in the 1990s, from diethylene glycol that affected hundreds of victims.5
Definitive laboratory confirmation of toxic alcohol poisoning is usually not immediately available to the emergency physician. However, early recognition of poisoning and emergency department (ED)–initiated interventions significantly improve patient outcomes and reduce the occurrence of alcohol-specific complications.6
Pathophysiology
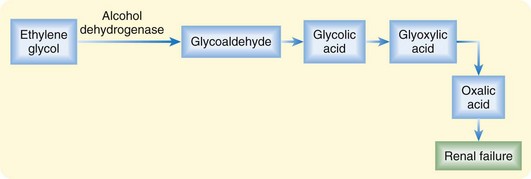
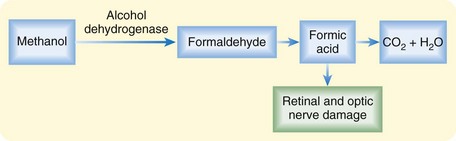
Most toxic alcohol poisonings occur by oral ingestion. Significant methanol poisoning has also been reported to occur by inhalation of brake cleaning products, and isopropanol poisoning has occurred through transcutaneous absorption in children treated for fevers at home with rubbing alcohol baths.7,8 Complete absorption is rapid by any route, each alcohol has a small volume of distribution (0.5 to 0.8 L/kg), and metabolism to toxic organic by-products occurs through hepatic alcohol dehydrogenase (ADH) (Figs. 151.1 and 151.2).
Toxicity from the parent products is limited to local mucous membrane irritation and CNS depression. The term toxic is specifically related to the production of different toxic by-products (oxalic acid and formic acid) by each of these alcohols. Ethylene glycol metabolism results in renal failure from deposition of oxalic acid in renal tubules.2 Methanol metabolism to formic acid results in blindness from direct injury to the retinal and optic nerves.3
Because the rate of metabolism through ADH varies by alcohol type and by individual variability in cytochrome P-450 genetic expression, clinical onset of worrisome symptoms can be delayed by 1 to 36 hours.2,3 Furthermore, the concomitant presence of ethanol may delay metabolism to the toxic by-products because ethanol has a higher affinity for ADH than the toxic alcohols and competitively inhibits metabolism of these alcohols to toxic by-products until the serum ethanol concentration drops to less than 100 mg/dL.
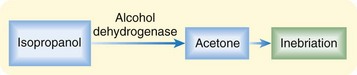
Isopropanol is unlike ethylene glycol and methanol in that it is not metabolized to an organic acid. Instead, it is metabolized to acetone, an osmotically active CNS depressant, which leads to profound inebriation (Fig. 151.3).8
Presenting Signs and Symptoms
Classic or Typical
Most patients poisoned with a toxic alcohol demonstrate some level of CNS depression consistent with inebriation (Table 151.1). Patients who arrive in the ED either shortly after a large ingestion or later after an ingestion, such that systemic accumulation of toxic metabolites has occurred, may be obtunded on presentation or may become obtunded during ED evaluation. The level of inebriation does not correlate with peak serum concentrations of the parent product or the accumulation of metabolic by-products.
| Symptoms |
Differential Diagnosis and Medical Decision Making
The differential diagnosis mnemonic “A CAT MUD PILES” should be used for any patient in whom ED evaluation demonstrates an anion gap acidosis (Box 151.1). Many of the possible conditions in the list can be easily excluded with a basic metabolic profile (e.g., uremia) and rapidly obtainable serum quantitative tests (e.g., salicylate). Although alcoholic ketoacidosis looks like toxic alcohol poisoning, it improves rapidly with only intravenous fluids and dextrose supplementation, whereas acidosis from a significant toxic alcohol exposure does not improve without antidotal treatment or enhanced elimination with hemodialysis, or both. In children, disorders of organic acid metabolism should be considered when poisoning is unlikely or has been excluded.
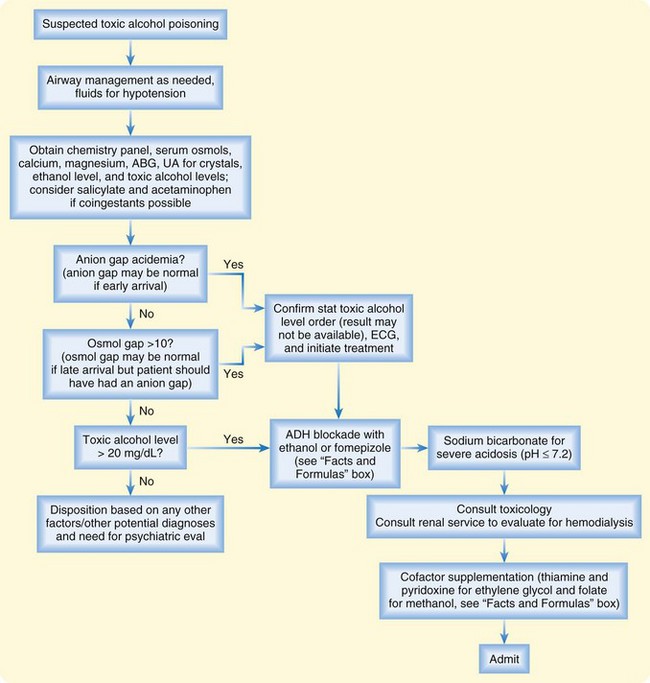
Laboratory testing in all patients with potential poisoning should include a basic metabolic profile to determine baseline renal function and acid-base status and to calculate the anion gap and osmol gap. In calculation of the osmol gap, the measured osmolality must be obtained at the same time as the basic metabolic panel (see “Facts and Formulas” box). The osmol gap value should be interpreted with caution because it is an imperfect screening test. The traditionally accepted normal value for an osmol gap is less than 10 mOsm/L. Unfortunately, an individual’s normal gap may range between −14 and +10 mOsm/L, so the osmol gap by itself is imperfect and not diagnostic.9–11
![]() Facts and Formulas
Facts and Formulas
Anion Gap
Urinalysis should be performed to look for crystals: monohydrate (spindle-like) or dihydrate (envelope-shaped) crystals are present in 50% to 60% of cases of ethylene glycol poisoning and suggest significant poisoning. However, the absence of these crystals does not reliably exclude poisoning. Some authorities have suggested examining the urine under a Wood lamp for fluorescence because antifreeze is commonly mixed with fluorescein. Unfortunately, urine may fluoresce in the presence or absence of ethylene glycol, so this maneuver has limited clinical utility.12
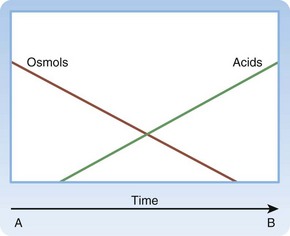
Although most clinicians are comfortable considering a diagnosis of ethylene glycol or methanol poisoning in patients with concomitant anion gap acidosis and an enlarged osmol gap, many patients do not arrive with those classic findings, and delays to definitive diagnosis and treatment are common.6,11 Ethylene glycol and methanol are osmotically active and contribute to the enlarged osmol gap; the toxic metabolites are minimally osmotically active and primarily contribute to the anion gap. The Mountain schematic is useful in helping one understand the time course of acidosis and an osmol gap without needing to memorize the kinetics of each intermediate step (Fig. 151.4).11
The Early Arrival
In a patient who arrives immediately after ingestion, as commonly occurs in children with an unintentional ingestion, anion gap acid acidosis is not present until enough time has elapsed for the parent product to be metabolized through ADH to produce laboratory evidence of acidosis. An enlarged osmol gap may be the only clue to potentially significant poisoning early after ingestion. In these cases, ADH inhibition with fomepizole or ethanol should be considered before acidosis develops (see Fig. 151.4, A).
The Late Arrival
In a patient who arrives with severe symptoms and significantly after ingestion, an abnormal osmol gap may no longer be present despite profound acidosis and other clinical findings suggestive of treatable toxic alcohol ingestion (e.g., visual disturbances in methanol ingestion, renal failure in ethylene glycol ingestion). In these cases, the parent product has already been metabolized to the toxic organic acids through ADH, so the osmotically active parent product no longer contributes significantly to the osmol gap. The benefit of an ADH inhibitor (fomepizole or ethanol) in these cases is less important than the benefit achieved from rapid hemodialysis and cofactor supplementation (see Fig. 151.4, B).
Although ED clinical decisions depend mostly on clinical suspicion and interpretation of imperfect laboratory data such as the anion gap, osmol gap, and urinalysis, cases of suspected toxic alcohol poisoning require that measurements of serum concentrations of ethylene glycol, methanol, and isopropanol be ordered as soon as possible. These measurements are the only definitive means of confirming the diagnosis and guiding duration of both antidotal therapy and hemodialysis. Unfortunately, most hospital laboratories are not equipped to run these tests and must send the specimens to an off-site reference laboratory.11 Except when the patient arrives with the appropriately labeled product that was ingested in hand, a practical initial strategy is to order serum concentration measurements of all three of these alcohols because patients often do not know exactly what they ingested. Confirming the units of measure when these results are available is also important because different laboratories use different units, and management decisions may be significantly affected if test results are interpreted incorrectly. Serum concentration measurements for toxic alcohols other than ethylene glycol, methanol, and isopropanol are not routinely available; even if obtainable, test results do not guide management decisions.13
Treatment
Treatment decision making for the patient with toxic alcohol ingestion is easiest when the patient arrives with the ingested product in hand. Because this is an uncommon occurrence, definitive laboratory diagnosis is usually delayed by the need to send serum samples to off-site reference laboratories.6,11 ED treatment in these cases should not be delayed and must be based on a presumptive clinical diagnosis. Attention to the airway in cases of CNS depression should be the first priority. Intravenous fluids should be administered to treat hypotension and maintain renal perfusion in all cases of toxic alcohol poisoning.
In cases of suspected ethylene glycol or methanol poisoning, immediate ADH inhibition should be considered to block continued metabolism of the ethylene glycol to toxic acids.6 ADH inhibition is most effective when it is administered as early as possible after exposure and before significant acidosis develops.11 This treatment should be considered in cases of a witnessed ingestion, when this agent is highly suspected from the history, in the presence of an elevated osmol gap alone with appropriate clinical suspicion, in the patient with both an elevated osmol gap and anion gap acidosis, or when the serum concentration of a toxic alcohol exceeds 20 mg/dL.
ADH inhibition can be achieved with either ethanol or fomepizole.2,3,14 Until 1999, ethanol was the only clinically available antidote. Its affinity for ADH is higher than that of ethylene glycol and methanol, it is inexpensive, and it is readily available. An ethanol level of 100 mg/dL has been the accepted goal for ensuring complete ADH inhibition; after intravenous loading, ethanol levels must be checked regularly until the measured ethylene glycol or methanol concentration is lower than 20 mg/dL. An ethanol load may be administered orally or with an intravenous infusion (see “Facts and Formulas” box). Treatment difficulties associated with ethanol therapy include (1) iatrogenic inebriation of the patient and inability to monitor mental status, (2) potential occurrence of hypoglycemia in pediatric patients, and (3) difficulty maintaining a therapeutic level because of individual variability in ethanol metabolism and clearance.14
The use of fomepizole, which was approved by the U.S. Food and Drug Administration in 1999, is an easier form of ADH inhibition than the use of ethanol.14–16 Dosing is weight based, and unlike ethanol, fomepizole does not require a constant infusion (see “Facts and Formulas” box). Fomepizole is given every 12 hours in patients not receiving hemodialysis or every 4 hours in patients undergoing hemodialysis, it does not cause inebriation, and it does not require monitoring of serum levels to ascertain therapeutic efficacy.14 Fomepizole should not be given when ethanol concentrations are significantly elevated because ethanol works as an ADH inhibitor, and fomepizole’s higher affinity for ADH will prolong the half-life and the clinical inebriation by the ethanol. Fomepizole is not available in all hospitals because of its high cost.6
Hemodialysis should be initiated in any patient with a significant ethylene glycol or methanol concentration and significant acidosis because it helps remove both the parent product and the resultant toxic acids.17–19 When a serum concentration is not immediately available, hemodialysis should be initiated in patients with clinical indicators of significant toxicity, such as pH less than 7.30 despite aggressive intravenous fluid resuscitation, creatinine concentration indicative of renal failure, or other electrolyte abnormalities unresponsive to conventional therapy.2,3,17 Hemodialysis should also be initiated soon after presentation in patients in whom ADH inhibition cannot be used because of antidote unavailability or a contraindication. Hemodialysis should also be considered to shorten the duration of antidote requirements and of hospitalization when acidosis has not occurred but the serum concentration of the toxic alcohol is extremely high.17,18 For example, the half-life of methanol has been reported to be as long as 54 hours in patients receiving ADH inhibition, and these patients may require several days of hospitalization and antidotal therapy if hemodialysis is not performed as well (Fig. 151.5).
Management of the other toxic alcohol poisonings requires aggressive supportive therapy, attention to the medical complications of acidosis, and hemodialysis only in the presence of significant renal insufficiency or other electrolyte abnormalities.13 Despite the similarity of its name to ethylene glycol, diethylene glycol does not produce oxalic acid, and any benefit from ADH inhibition in cases of diethylene glycol poisoning is uncertain.20
Tips and Tricks
• Consult your local poison center: 800-222-1222.
• Refer to the Mountain schematic (see Fig. 151.4) to help interpret anion gap and osmol gap results.
• If long delays until definitive laboratory confirmation of the ingested substance are expected, consider repeating the basic metabolic profile or measure arterial blood gases.
• If alcohol dehydrogenase has not been blocked by fomepizole or ethanol, acidosis should worsen despite standard intravenous fluid resuscitation if ethylene glycol or methanol is present.
• Treatment difficulties associated with ethanol therapy are (1) iatrogenic inebriation and inability to monitor mental status, (2) potential occurrence of hypoglycemia in children, and (3) difficulty maintaining a therapeutic level because of individual variability in ethanol metabolism and clearance.
Disposition
In cases of intentional ingestion, appropriate psychiatric evaluation is warranted after the patient’s medical issues are treated. In cases of unintentional poisoning, especially in children, appropriate poison prevention counseling for all caregivers is required before discharge.21
![]() Red Flags
Red Flags
• Urinalysis: The absence of crystals or fluorescence does not exclude poisoning.
• The absence of an osmol gap in patients with severe acidosis does not exclude toxic alcohol poisoning; it may be the result of a delayed presentation.
• The absence of an anion gap in patients who present early does not exclude toxic alcohol poisoning.
• Be prepared to have definitive laboratory confirmation delayed by 8 to 24 hours, depending on the individual hospital’s access to an outside reference laboratory. Empirical treatment should be started if indicated.
• Renal specialists should be consulted early, to prevent complications.
• Be wary of the potential for hypoglycemia in patients treated with intravenous ethanol.
• Do not give ethanol with fomepizole. Both agents compete for alcohol dehydrogenase, and their use together may reduce antidotal effectiveness.
• Because metabolism varies by alcohol type and by individual variability, clinical onset of symptoms can be delayed by 1 to 36 hours.
Barceloux DG, Krenzelok EP, Olson K, et al. American Academy of Clinical Toxicology practice guidelines on the treatment of ethylene glycol poisoning. Clin Toxicol. 1999;37:537–560.
Barceloux DG, Bond GR, Krenzelok EP, et al. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. Clin Toxicol. 2002;40:415–446.
Brent J. Fomepizole for ethylene glycol and methanol poisoning. N Engl J Med. 2009;360:2216–2223.
Hoffman RS, Smilkstein MJ, Howland MA, Goldfrank LR. Osmol gaps revisited: normal values and limitations. Clin Toxicol. 1993;31:81–93.
Mycyk MB, Aks SE. A visual schematic for clarifying the temporal relationship between the anion gap and the osmol gap in cases of toxic alcohol poisoning. Am J Emerg Med. 2003;21:333–335.
1 Bronstein AC, Spyker DA, Cantilena LR, Jr., et al. 2009 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 27th annual report. Clin Toxicol. 2010;48:979–1178.
2 Barceloux DG, Krenzelok EP, Olson K, et al. American Academy of Clinical Toxicology practice guidelines on the treatment of ethylene glycol poisoning. Clin Toxicol. 1999;37:537–560.
3 Barceloux DG, Bond GR, Krenzelok EP, et al. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. Clin Toxicol. 2002;40:415–446.
4 Barnes BJ, Gerst C, Smith JR, et al. Osmol gap as a surrogate marker for serum propylene glycol concentrations in patients receiving lorazepam for sedation. Pharmacotherapy. 2006;26:23–33.
5 Schier JG, Rubin CS, Miller D, et al. Medication-associated diethylene glycol mass poisoning: a review and discussion on the origin of contamination. J Public Health Policy. 2009;30:127–143.
6 Mycyk MB, DesLauriers C, Metz J, et al. Compliance with poison center fomepizole recommendations is suboptimal in cases of toxic alcohol poisoning. Am J Ther. 2006;13:485–489.
7 Frenia ML, Schauben JL. Methanol inhalation toxicity. Ann Emerg Med. 1993;22:1919–1923.
8 Dyer S, Mycyk MB, Ahrens WR, Zell-Kanter M. Hemorrhagic gastritis from topical isopropanol exposure. Ann Pharmacother. 2002;36:1733–1735.
9 Hoffman RS, Smilkstein MJ, Howland MA, Goldfrank LR. Osmol gaps revisited: normal values and limitations. Clin Toxicol. 1993;31:81–93.
10 Krahn J, Khajuria A. Osmolality gaps: diagnostic accuracy and long-term variability. Clin Chem. 2006;52:737–739.
11 Mycyk MB, Aks SE. A visual schematic for clarifying the temporal relationship between the anion gap and the osmol gap in cases of toxic alcohol poisoning. Am J Emerg Med. 2003;21:333–335.
12 Wallace KL, Suchard JR, Curry SC, Reagan C. Diagnostic use of physicians’ detection of urine fluorescence in a simulated ingestion of sodium fluorescein–containing antifreeze. Ann Emerg Med. 2001;38:49–54.
13 McKinney PE, Palmer RB, Blackwell W, Benson BE. Butoxyethanol ingestion with prolonged hyperchloremic metabolic acidosis treated with ethanol therapy. Clin Toxicol. 2000;38:787–793.
14 Brent J. Fomepizole for ethylene glycol and methanol poisoning. N Engl J Med. 2009;360:2216–2223.
15 Shannon M. Toxicology reviews: fomepizole—a new antidote. Pediatr Emerg Care. 1998;14:170–172.
16 Mycyk MB, Leikin JB. Antidote review: fomepizole for methanol poisoning. Am J Ther. 2003;10:68–70.
17 Rydel JJ, Carlson A, Sharma J, Leikin J. An approach to dialysis for ethylene glycol intoxication. Vet Hum Toxicol. 2002;44:36–39.
18 Moreau CL, Kerns W, Tomaszewski CA, et al. Glycolate kinetics and hemodialysis clearance in ethylene glycol poisoning. Clin Toxicol. 1998;36:659–666.
19 Poldelski V, Johnson A, Wright S, et al. Ethylene glycol–mediated tubular injury: identification of critical metabolites and injury pathways. Am J Kidney Dis. 2001;38:339–348.
20 Alfred S, Coleman P, Harris D, et al. Delayed neurologic sequelae resulting from epidemic diethylene glycol poisoning. Clin Toxicol. 2005;43:155–159.
21 Schnitzer PG. Prevention of unintentional childhood injuries. Am Fam Physician. 2006;74:1864–1869.