CHAPTER 17 TOURETTE’S SYNDROME, TICS AND OBSESSIVE-COMPULSIVE DISORDERS
TICS AND GILLES DE LA TOURETTE SYNDROME
Once considered a rarity, Gilles de la Tourette syndrome is now recognized to be a relatively common disorder, which may be associated with considerable psychiatric comorbidity and impaired psychosocial functioning. Gilles de la Tourette syndrome is defined by the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR) as a combination of multiple motor tics and at least one vocal tic that cannot be explained by another cause and has persisted for at least 1 year.1,2 The current criteria no longer require that these tics produce significant distress or impairment, and a large proportion of people who fulfill these criteria are not aware of their tics or are not disturbed by them. However, in the tertiary referral setting and in patients with more severe or frequent tics or with associated comorbidity, there are both considerable psychosocial impairment and reduced quality of life.3 Gilles de la Tourette syndrome is frequently undiagnosed; tics may be misinterpreted as a “nervous habits” or, particularly in children, as inattention or inability to sit still. On occasion, tics are mistaken for another movement disorder or a psychiatric disease.
Differential Diagnosis of Tics
Other brief movement disorders may be difficult to distinguish from tics. Tics may particularly resemble dystonia, tremor, myoclonus, chorea, and akathisia (for a review of hyperkinetic movement disorders, see Chapters 33 to 37 Chapter 34 Chapter 35 Chapter 36 Chapter 37). They also need to be distinguished from mannerisms (bizarre execution of purposeful acts), stereotypies (purposeless, repetitive movements often over long periods of time, as in a learning disability), and other medical conditions, such as coughing or sniffing in upper respiratory tract infections or eye blinking in allergy or blepharospasm. Particularly difficult may be the distinction between tics and other features of Gilles de la Tourette syndrome, such as obsessive-compulsive behaviors (OCBs), attention deficit/hyperactivity disorder (ADHD), antisocial behaviors and movement disorders associated with treatment.4 These differentiations are particularly important for appropriate pharmacological management.
Epidemiology
Tics occur in 3% to 22% of children at some stage during their development5 but are transient in the majority. The more severe Gilles de la Tourette syndrome affects approximately 1% of chil dren, and prevalence rates range from 0.4 to 1.8%.6–13 However, although the disorder typically starts in childhood, on average between the ages of 5 and 7 years,14,15 and typically increases until the age of 13 years, it often improves in adolescence so that by the age of 18 years, 50% of those affected are virtually free of tics.16 The prevalence rate is higher in special educational populations, such as those with learning difficulties17 or autism.18 About three to four times as many boys as girls are affected.14 Prevalence rates and clinical characteristics are broadly similar across countries.14
In rare cases, tic disorders with both motor tics and verbalizations begin in adulthood. Some of these patients have been described to have had compulsive tendencies in childhood or a family history of tics or OCB. In comparison with patients with Gilles de la Tourette syndrome that started in childhood, patients with adult-onset tic disorder more often had a potential trigger event, have more severe symptoms and greater social morbidity, and increased sensitivity and poorer response to neuroleptics.19
Diagnosis
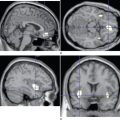
Tics and Gilles de la Tourette syndrome are clinical diagnoses. In cases of classic Gilles de la Tourette syndrome, no diagnostic tests are required. However, atypical cases, such as those without waxing and waning over time, those with adult onset, and particularly those with abnormalities on neurological examination, should be further investigated, including measurements of copper and ceruloplasmin for Wilson’s disease, full blood count for acanthocytes, and magnetic resonance imaging of the brain. Neuroimaging has also provided insight in the pathophysiology of Gilles de la Tourette syndrome: reduced volumes and abnormal asymmetry as well as altered dopamine metabolism of the basal ganglia, particularly the caudate20,21, and frontal lobe abnormalities,22 all of which implicate the frontal-striatal-thalamic-frontal circuitry.
Etiology
Suggestions for the etiology of Gilles de la Tourette syndrome have included genetic influences, infections, and perinatal difficulties. There is a wealth of evidence pointing toward genetic causes, including family studies, which suggested an autosomal dominant inheritance pattern with variable expression and penetrance.23–25 There is also growing evidence for bilineal transmission, with the father typically affected by childhood tics and the mother by symptoms of obsessive-compulsive disorder (OCD).26–28 Genetic studies have led to the identification of several regions of interest on chromosomes 2, 4, 8, and 1129–32 and, more recently regions of interest on the chromosomes 5, 10, 13,33 7,34 and 18.35 In addition, the DRD4 and MOA-A genes have been implicated,34,36 and linkage to chromosome 17 has been demonstrated.37 However, despite many years of research by a number of groups worldwide, no single genetic cause has been found for Gilles de la Tourette syndrome. This suggests that other factors also play a role in the etiology of this disorder.
An increasingly popular hypothesis suggests that Gilles de la Tourette syndrome is the product of an interaction between a genetic vulnerability and environmental factors. Stressors at various times of the life cycle have been implicated, particularly perinatal injury, but also stressors during pregnancy, such as severe nausea, vomiting, and antiemetic medication, which may alter dopaminergic receptors.16,38–41
Particularly intriguing has been the association of group A β-hemolytic streptococcal infections with a syndrome of sudden-onset neuropsychiatric disturbances, including OCD, tics, and other psychopathology in children. This syndrome has been termed pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS).42 More recently, laboratory evidence of streptococcal infection in patients with Gilles de la Tourette syndrome has been reported, including elevated antistreptolysin O titers and anti–basal ganglia antibodies in up to 25% of patients with Gilles de la Tourette syndrome.43,44 An autoimmune mechanism has therefore been suggested as a contributor to the development of tics in this syndrome.44,45 Although the majority of studies have supported this notion,43–46 others have disputed this association,47,48 and whether anti–basal ganglia antibodies play a role in the development of Gilles de la Tourette syndrome currently remains a controversy.17
Psychopathology
Gilles de la Tourette syndrome is associated with increased rates of a number of comorbid psychiatric conditions (see Robertson, 200049 and 200350). Although some of these are likely to represent a manifestation of or be integral to Gilles de la Tourette syndrome, others may be the consequence of the social and emotional consequences of this disorder. An investigation of 3500 patients with Gilles de la Tourette syndrome worldwide demonstrated that across all ages, 88% of individuals had associated psychiatric comorbidity, and male patients were more likely to have comorbid disorders.14 Only those with comorbid disorders had more severe behavioral problems such as anger control problems and self-injurious behavior, as well as sleep difficulties and coprolalia. The presence of such behavioral problems should therefore alert the clinician to the possible presence of comorbidity, the management of which is often at least as important as tic reduction.
The spectrum of comorbid disorders includes OCD, other anxiety disorders, mood disorders, ADHD, and other behavior disorders, including self-injurious behavior.51 Although the exact relationship of these to Gilles de la Tourette syndrome is unclear, a strong association exists between Gilles de la Tourette syndrome and OCD and OCB, and a number of studies have suggested that these reflect the variable expression of a single disorder.24,26,27,50 OCD in patients with Gilles de la Tourette syndrome has, however, been found to differ in clinical manifestation from primary OCD, with predominant checking, counting, and symmetry obsessions and less frequent obsessions with contamination and violence.52–57 The rate of ADHD is also increased in patients with Gilles de la Tourette syndrome, and although the relationship between Gilles de la Tourette syndrome and ADHD is less clear, it has been suggested that some types are related genetically to Gilles de la Tourette syndrome. Depending on whether or not the International Classification of Diseases and Health-Related Problems, 10th Revision, or DSM-IV-TR criteria are used, the prevalence of ADHD in youngsters is between 1% and 9%.58 In patients with Gilles de la Tourette syndrome, the prevalence of ADHD is much greater and may be as high as 60%.14 This is not confined to chronic populations: Even in an epidemiological study conducted on members of the Israeli Defense Force, the prevalence of ADHD in Gilles de la Tourette syndrome was 8%, in comparison with 4% in the population without Gilles de la Tourette syndrome, a difference that was statistically significant.59 Personality disorders in adulthood in patients with Gilles de la Tourette syndrome are likely to be related to comorbid ADHD in childhood rather than to the syndrome itself. Whether other psychiatric comorbid conditions such as conduct disorder, oppositional-defiant disorder, personality disorder, rage, and impulsivity are clearly more prevalent in patients with Gilles de la Tourette syndrome or their apparent prevalence is a result of referral bias in Gilles de la Tourette syndrome clinics is currently unknown. The multiple medications used for Gilles de la Tourette syndrome (see later discussion) may lead to increase. Anxiety and cognitive disorders, and anxiety may occur as a result of having Gilles de la Tourette syndrome and its social and personal consequences. The rate of depression, on the other hand, is clearly increased among patients with Gilles de la Tourette syndrome and is likely to be multifactorial in origin.60–63
Prognosis
Clearly, for symptomatic tics in the context of another neurological disorder or as a drug effect, the prognosis is associated with the underlying disorder. The prognosis of individuals with Gilles de la Tourette syndrome varies widely; whereas those with mild tics without coprolalia or associated comorbidity mostly do not suffer impairment of social or personal function, those at the other end of the spectrum can be severely disabled. Children may be disadvantaged in school, particularly if comorbidity is present,64,65 but, as mentioned previously, tics often improve in adolescence.15,57 When the affected individuals and their environments receive appropriate explanation of this disorder and understand it, most do not need regular follow-up. Adult-onset cases appear to have worse morbidity and worse response to treatment, but this is rare. Overall, health-related quality of life has been shown to be worse in patients with Gilles de la Tourette syndrome than in controls, although it is better than in patients with intractable epilepsy.3 Factors associated with poorer health-related quality of life in this study in a tertiary referral center were employment status, tic severity, obsessive-compulsive symptoms, anxiety, and depression.
Management
Nonpharmacological Treatment
Supportive psychotherapy and psychological education are very important for all patients and their families, particularly if the patients are young. More specific behavioral treatment has been shown to produce better results than psychotherapy in adult patients with Gilles de la Tourette syndrome and include habit reversal training, graded exposure, social skills training, imaginal exposure, massed negative practice, contingency management, relaxation training, and biofeedback.66,67
Pharmacological Treatment
The newer “atypical” antipsychotic agents have been demonstrated to be useful in treating patients with Gilles de la Tourette syndrome. Their chief advantage is the lower risk of extrapyramidal side effects. The main side effect is weight gain and, in some individuals, the precipitation of diabetes mellitus. It is therefore recommended that fasting glucose levels be checked in patients, particularly if they have put on weight. The atypical antipsychotic agents successfully used for treatment of Gilles de la Tourette syndrome have included risperidone,68 olanzapine,69 quetiapine,70 aripiprazole,71 and ziprasidone.72 It has also been suggested that quetiapine does not lead to hyperprolactinemia73 and may therefore merit further studies in patients with Gilles de la Tourette syndrome. In patients with severe vocal tics, which may not respond well to oral pharmacological treatment, botulinum toxin injections may be useful.74 Other suggested alternatives for the treatment of Gilles de la Tourette syndrome have included the neuroleptics amisulpride, aripiprazole, ziprasidone, fluphenazine, metoclopramide, piquindone, and tetrabenazine and agents from other substance groups, such as clonazepam, calcium channel antagonists, celecoxib, dopamine agonists, and selegiline. In severe, medically intractable cases, various surgical approaches have been tried with little success.75 However, in a literature review, Rauch and associates76 suggested that there is no compelling evidence that any neurosurgical procedure is superior to all others, and such surgery is not recommended outside specialist centers. Deep brain stimulation of the thalamus, which is largely reversible, is currently being explored as a treatment option for severe tics and OCD.77
The treatment of comorbid conditions requires additional drug choices. OCD and OCB often respond to selective serotonin reuptake inhibitors or the tricyclic antidepressant clomipramine, which inhibits both serotonin and noradrenaline uptake. In some countries, the use of some of these agents (e.g., paroxetine) is contraindicated in children. When ADHD exists comorbidly, the α2-adrenergic agonist clonidine and, in the United States, guanfacine can be useful for tics, impulse control, and ADHD, but electrocardiography and blood pressure control are recommended for patients taking these drugs. These agents must not be discontinued suddenly, because of rebound hypertension. Children with ADHD may require the addition of a psychostimulant such as methylphenidate. Previous concerns about exacerbation of tics with this medication have not been substantiated, and the management of ADHD may be more important than that of tics. An alternative may be the nonstimulant selective norepinephrine reuptake inhibitor atomoxetine.78,79 Depression in Gilles de la Tourette syndrome should be treated like primary depression or, for depression associated with other chronic disorders, by using cognitive-behavioral approaches, education, psychotherapeutic treatments, and pharmacotherapy.
OBSESSIVE-COMPULSIVE DISORDER
Obsessions are intrusive and recurrent thoughts, which include intrusive doubts, images, impulses, or ruminations (continuous pondering). They are recognized by affected individuals as their own thoughts, but they are characteristically egodystonic—that is, unwelcome and uncomfortable—to the individual, who usually tries to avoid or suppress them.52 Common obsessions are concerned with contamination, violence, sex, blasphemy, and numbers. Rarer obsessions are arithmomania (obsession with counting), onomatomania (the desire to utter a forbidden word), and folie de pourquoi (irresistible habit of repetitively asking the same banal question).
Although obsessions and compulsions are common in the general population, a diagnosis of OCD according to DSM-IV-TR requires that obsessions and compulsions cause marked distress or significantly interfere with a person’s functioning and do not occur in the context of a medical illness. There is considerable phenotypic variability of obsessions and compulsions, and the existence of specific subtypes has therefore been postulated: for example, familial and related to tic disorders, familial and unrelated to tics, and sporadic OCD.80 However, the existence of these different subtypes has been controversial.81
Epidemiology
OCD has a lifetime prevalence of 1.8% to 3.5% in the population with an onset in childhood or adolescence and a slight preponderance among girls.82–84 OCBs are much more common and may be part of the spectrum of normal behavior. OCD occurs worldwide with similar core features, but the content of the obsessions appears to be related to cultural context.85
Diagnosis
Obsessions and compulsions are also common in other psychiatric disorders, including depression, schizophrenia, and obsessional (anankastic) personality disorder, and they overlap with Gilles de la Tourette syndrome, as discussed previously. Segregation analysis in families with Gilles de la Tourette syndrome and OCD suggested that OCD and Gilles de la Tourette syndrome are variant expressions of the same syndrome. A concurrent obsessional (anankastic) personality is present in about 70% of cases.52 They may also occur in generalized anxiety disorder, puerperal illness (as a fear of harming the baby), anorexia nervosa, Huntington’s disease, encephalitis lethargica, PANDAS, manganese poisoning and after head injury. In these psychiatric and neurological disorders, other features are present, but even in pure OCD, soft neurological signs such as astereognosia or agraphesthesia may be present.52
Etiology
Obsessions were originally believed to be rooted in repressed impulses or in an aggressive or sexual nature,86 and other explanations have included obsessions as a result of aberrant learning.87 An increase in severity of OCD is also often seen when depression or stressful life events occur. However, obsessions and compulsions are seen in the context of a number of neurological disorders, such as Huntington’s disease or encephalitis lethargica, implicating underlying brain abnormalities, particularly in the frontal cortex and basal ganglia. Functional imaging studies and neuropsychological testing also provide increasing evidence that OCD is associated with abnormal functioning of the orbitofrontal cortex, the cingulate, and the caudate, and biochemical abnormalities, especially involving serotonin, are believed to be important in the pathophysiology of OCD.88
Increased rates of obsessions and compulsions in families of patients with OCD suggest that genetic factors play a role in the etiology of OCD, and twin studies with higher concordance rates in monozygous twins than in dizygous twins have supported the importance of genetic factors. In addition, abrupt onset or exacerbations of OCD or tics or both have been described after streptococcal infections (see previous discussion), suggestive of environmental causes. Neuroimaging studies reveal increased basal ganglia volumes, and the proposed cause involves the cross-reaction of streptococcal antibodies with basal ganglia tissue. A genetic susceptibility to PANDAS has been postulated.89
Prognosis
Mild cases of obsessions and compulsions are often self-limited within 1 year. OCD is a chronic disorder but typically runs a fluctuating course with periods of long remissions and the greatest prevalence in mid-adult life. A meta-analysis of studies with up to 16 years’ follow-up revealed persistence rates of 41% for full OCD and 60% for full or subthreshold OCD.90 Comorbid psychiatric illness and poor initial treatment response were poor prognostic factors.
Depression and abuse of alcohol and anxiolytics is common. Quality of life has been found to be significantly related to severity of obsessions, whereas the severity of compulsive rituals did not affect quality-of-life ratings. However, the single greatest predictor of poor quality of life was comorbid depression severity,91 and suicide rates are increased, particularly in patients with comorbid depression. This is contrary to previous notions that suicide is uncommon in patients with OCD.
Management
In many cases, obsessions and compulsions do not necessitate treatment, and the fluctuating course needs to be considered before treatment starts. In cases in which treatment is required, cognitive-behavioral therapy has been successful, including exposure and response prevention for compulsions, and habituation training and thought-stopping for obsessions.92 Psychoeducation can also be a valuable source.
In cases in which all other classic treatments have failed after a minimum of 5 years, psychosurgery is occasionally considered. Capsulotomy, cingulotomy, subcaudate tractotomy, and limbic leukotomy, performed by radiofrequency thermolesions or radiosurgery,93–96 and the largely reversible deep brain stimulation97 have all been used. These surgical approaches are aimed at altering the neural circuits between the frontal lobes and different structures of the limbic system, but they are used very rarely.
Conclusions and Recommendations
Husted DS, Shapira NA. A review of the treatment for refractory obsessive-compulsive disorder: from medicine to deep brain stimulation. CNS Spectr. 2004;9:833-847.
Leckman JF. Phenomenology of tics and natural history of tic disorders. Brain Dev. 2003;25(Suppl 1):S24-S28.
Pauls DL. An update on the genetics of Gilles de la Tourette syndrome. Psychosom Res. 2003;55:7-12.
Robertson MM. Tourette syndrome, associated conditions and the complexities of treatment. Brain. 2000;123(Pt 3):425-462.
Singer HS. Tourette’s syndrome: from behaviour to biology. Lancet Neurol. 2005;4:149-159.
1 American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, Text Revision. Washington, DC: American Psychiatric Association, 2000.
2 World Health Organization. Internation Classification of Diseases and Health-Related Problems, 10th revision. Geneva: World Health Organization, 1992.
3 Elstner K, Selai CE, Trimble MR, et al. Quality of life (QOL) of patients with Gilles de la Tourette’s syndrome. Acta Psychiatr Scand. 2001;103:52-59.
4 Kompoliti K, Goetz CG. Hyperkinetic movement disorders misdiagnosed as tics in Gilles de la Tourette syndrome. Mov Disord. 1998;13:477-480.
5 Gadow KD, Nolan EE, Sprafkin J, et al. Tics and psychiatric comorbidity in children and adolescents. Dev Med Child Neurol. 2002;44:330-338.
6 Kurlan R, McDermott MP, Deeley C, et al. Prevalence of tics in school children and association with placement in special education. Neurology. 2001;57:1383-1388.
7 Hornsey H, Banerjee S, Zeitlin H, et al. The prevalence of Tourette syndrome in 13–14-year-olds in mainstream schools. J Child Psychol Psychiatry. 2001;42:1035-1039.
8 Kadesjo B, Gillberg C. Tourette’s disorder: epidemiology and comorbidity in primary school children. J Am Acad Child Adolesc Psychiatry. 2000;39:548-555.
9 Khalifa N, von Knorring AL. Prevalence of tic disorders and Tourette syndrome in a Swedish school population. Dev Med Child Neurol. 2003;45:315-319.
10 Lanzi G, Zambrino CA, Termine C, et al. Prevalence of tic disorders among primary school students in the city of Pavia, Italy. Arch Dis Child. 2004;89:45-47.
11 Wang HS, Kuo MF. Tourette’s syndrome in Taiwan: an epidemiological study of tic disorders in an elementary school at Taipei County. Brain Dev. 2003;25(Suppl 1):S29-S31.
12 Jin R, Zheng RY, Huang WW, et al. [Study on the prevalence of Tourette syndrome in children and juveniles aged 7-16 years in Wenzhou area]. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:131-133.
13 Mason A, Banerjee S, Eapen V, et al. The prevalence of Tourette syndrome in a mainstream school population. Dev Med Child Neurol. 1998;40:292-296.
14 Freeman RD, Fast DK, Burd L, et al. An international perspective on Tourette syndrome: selected findings from 3,500 individuals in 22 countries. Dev Med Child Neurol. 2000;42:436-447.
15 Leckman JF, Zhang H, Vitale A, et al. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 1998;102:14-19.
16 Leckman JF, Dolnansky ES, Hardin MT, et al. Perinatal factors in the expression of Tourette’s syndrome: an exploratory study. J Am Acad Child Adolesc Psychiatry. 1990;29:220-226.
17 Eapen V, Robertson MM, Zeitlin H, et al. Gilles de la Tourette’s syndrome in special education schools: a United Kingdom study. J Neurol. 1997;244:378-382.
18 Baron-Cohen S, Scahill VL, Izaguirre J, et al. The prevalence of Gilles de la Tourette syndrome in children and adolescents with autism: a large scale study. Psychol Med. 1999;29:1151-1159.
19 Eapen V, Lees AJ, Lakke JP, et al. Adult-onset tic disorders. Mov Disord. 2002;17:735-740.
20 Peterson BS, Thomas P, Kane MJ, et al. Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. 2003;60:415-424.
21 Singer HS, Szymanski S, Giuliano J, et al. Elevated intrasynaptic dopamine release in Tourette’s syndrome measured by PET. Am J Psychiatry. 2002;159:1329-1336.
22 Kates WR, Frederikse M, Mostofsky SH, et al. MRI parcellation of the frontal lobe in boys with attention deficit hyperactivity disorder or Tourette syndrome. Psychiatry Res. 2002;116:63-81.
23 Curtis D, Robertson MM, Gurling HM. Autosomal dominant gene transmission in a large kindred with Gilles de la Tourette syndrome. Br J Psychiatry. 1992;160:845-849.
24 Eapen V, Pauls DL, Robertson MM. Evidence for autosomal dominant transmission in Tourette’s syndrome. United Kingdom cohort study. Br J Psychiatry. 1993;162:593-596.
25 Pauls DL, Leckman JF. The inheritance of Gilles de la Tourette’s syndrome and associated behaviors. Evidence for autosomal dominant transmission. N Engl J Med. 1986;315:993-997.
26 Kurlan R, Eapen V, Stern J, et al. Bilineal transmission in Tourette’s syndrome families. Neurology. 1994;44:2336-2342.
27 McMahon WM, van de Wetering BJ, Filloux F, et al. Bilineal transmission and phenotypic variation of Tourette’s disorder in a large pedigree. J Am Acad Child Adolesc Psychiatry. 1996;35:672-680.
28 Hanna PA, Janjua FN, Contant CF, et al. Bilineal transmission in Tourette syndrome. Neurology. 1999;53:813-818.
29 Simonic I, Nyholt DR, Gericke GS, et al. Further evidence for linkage of Gilles de la Tourette syndrome (GTS) susceptibility loci on chromosomes 2p11, 8q22 and 11q23–24 in South African Afrikaners. Am J Med Genet. 2001;105:163-167.
30 Simonic I, Gericke GS, Ott J, et al. Identification of genetic markers associated with Gilles de la Tourette syndrome in an Afrikaner population. Am J Hum Genet. 1998;63:839-846.
31 A complete genome screen in sib pairs affected by Gilles de la Tourette syndrome. The Tourette Syndrome Association International Consortium for Genetics. Am J Hum Genet. 1999;65:1428-1436.
32 Merette C, Brassard A, Potvin A, et al. Significant linkage for Tourette syndrome in a large French Canadian family. Am J Hum Genet. 2000;67:1008-1013.
33 Curtis D, Brett P, Dearlove AM, et al. Genome scan of Tourette syndrome in a single large pedigree shows some support for linkage to regions of chromosomes 5, 10 and 13. Psychiatr Genet. 2004;14:83-87.
34 Diaz-Anzaldua A, Joober R, Riviere JB, et al. Association between 7q31 markers and Tourette syndrome. Am J Med Genet A. 2004;127:17-20.
35 Cuker A, State MW, King RA, et al. Candidate locus for Gilles de la Tourette syndrome/obsessive compulsive disorder/chronic tic disorder at 18q22. Am J Med Genet A. 2004;130:37-39.
36 Diaz-Anzaldua A, Joober R, Riviere JB, et al. Tourette syndrome and dopaminergic genes: a family-based association study in the French Canadian founder population. Mol Psychiatry. 2004;9:272-277.
37 Paschou P, Feng Y, Pakstis AJ, et al. Indications of linkage and association of Gilles de la Tourette syndrome in two independent family samples: 17q25 is a putative susceptibility region. Am J Hum Genet. 2004;75:545-560.
38 Santangelo SL, Pauls DL, Goldstein JM, et al. Tourette’s syndrome: what are the influences of gender and comorbid obsessive-compulsive disorder? J Am Acad Child Adolesc Psychiatry. 1994;33:795-804.
39 Lees AJ, Robertson M, Trimble MR, et al. A clinical study of Gilles de la Tourette syndrome in the United Kingdom. J Neurol Neurosurg Psychiatry. 1984;47:1-8.
40 Burd L, Severud R, Klug MG, et al. Prenatal and perinatal risk factors for Tourette disorder. J Perinat Med. 1999;27:295-302.
41 Burnstein MH. Tourette’s syndrome and neonatal anoxia: further evidence of an organic etiology. J Psychiatry Neurosci. 1992;17:89-93.
42 Swedo SE, Leonard HL, Garvey M, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998;155:264-271.
43 Cardona F, Orefici G. Group A streptococcal infections and tic disorders in an Italian pediatric population. J Pediatr. 2001;138:71-75.
44 Church AJ, Dale RC, Lees AJ, et al. Tourette’s syndrome: a cross sectional study to examine the PANDAS hypothesis. J Neurol Neurosurg Psychiatry. 2003;74:602-607.
45 Muller N, Riedel M, Straube A, et al. Increased antistreptococcal antibodies in patients with Tourette’s syndrome. Psychiatry Res. 2000;94:43-49.
46 Pavone P, Bianchini R, Parano E, et al. Anti-brain antibodies in PANDAS versus uncomplicated streptococcal infection. Pediatr Neurol. 2004;30:107-110.
47 Singer HS, Loiselle CR, Lee O, et al. Anti-basal ganglia antibodies in PANDAS. Mov Disord. 2004;19:406-415.
48 Singer HS. PANDAS and immunomodulatory therapy. Lancet. 1999;354:1137-1138.
49 Robertson MM. Tourette syndrome, associated conditions and the complexities of treatment. Brain. 2000;123(Pt 3):425-462.
50 Robertson MM. The heterogeneous psychopathology of Tourette syndrome. In: Bedard MA, Agid Y, Chouinard S, et al, editors. Mental and Behavioral Dysfunction in Movement Disorders. Totowa, NJ: Humana Press; 2003:433-466.
51 Kurlan R, Como PG, Miller B, et al. The behavioral spectrum of tic disorders: a community-based study. Neurology. 2002;59:414-420.
52 Katona C, Robertson MM. Gilles de la Tourette Syndrome. Psychiatry at a Glance, 3rd ed. Oxford, UK: Blackwell Science, 2005.
53 Eapen V, Robertson MM, Alsobrook JP, et al. Obsessive compulsive symptoms in Gilles de la Tourette syndrome and obsessive compulsive disorder: differences by diagnosis and family history. Am J Med Genet. 1997;74:432-438.
54 Frankel M, Cummings JL, Robertson MM, et al. Obsessions and compulsions in Gilles de la Tourette’s syndrome. Neurology. 1986;36:378-382.
55 Leckman JF, Pauls DL, Zhang H, et al. Obsessive-compulsive symptom dimensions in affected sibling pairs diagnosed with Gilles de la Tourette syndrome. Am J Med Genet B Neuropsychiatr Genet. 2003;116:60-68.
56 Miguel EC, Leckman JF, Rauch S, et al. Obsessive-compulsive disorder phenotypes: implications for genetic studies. Mol Psychiatry. 2004;10:258-275.
57 Coffey BJ, Miguel EC, Biederman J, et al. Tourette’s disorder with and without obsessive-compulsive disorder in adults: are they different? J Nerv Ment Dis. 1998;186:201-206.
58 Swanson JM, Sergeant JA, Taylor E, et al. Attention-deficit hyperactivity disorder and hyperkinetic disorder. Lancet. 1998;351:429-433.
59 Apter A, Pauls DL, Bleich A, et al. An epidemiologic study of Gilles de la Tourette’s syndrome in Israel. Arch Gen Psychiatry. 1993;50:734-738.
60 Robertson MM, Trimble MR, Lees AJ. The psychopathology of the Gilles de la Tourette syndrome. A phenomenological analysis. Br J Psychiatry. 1988;152:383-390.
61 Robertson MM, Channon S, Baker J, et al. The psychopathology of Gilles de la Tourette’s syndrome. A controlled study. Br J Psychiatry. 1993;162:114-117.
62 Robertson MM, Banerjee S, Hiley PJ, et al. Personality disorder and psychopathology in Tourette’s syndrome: a controlled study. Br J Psychiatry. 1997;171:283-286.
63 Rickards H, Robertson M. A controlled study of psychopathology and associated symptoms in Tourette syndrome. World J Biol Psychiatry. 2003;4:64-68.
64 Carter AS, O’Donnell DA, Schultz RT, et al. Social and emotional adjustment in children affected with Gilles de la Tourette’s syndrome: associations with ADHD and family functioning. Attention Deficit Hyperactivity Disorder. J Child Psychol Psychiatry. 2000;41:215-223.
65 Brand N, Geenen R, Oudenhoven M, et al. Brief report: cognitive functioning in children with Tourette’s syndrome with and without comorbid ADHD. J Pediatr Psychol. 2002;27:203-208.
66 Wilhelm S, Deckersbach T, Coffey BJ, et al. Habit reversal versus supportive psychotherapy for Tourette’s disorder: a randomized controlled trial. Am J Psychiatry. 2003;160:1175-1177.
67 Piacentini J, Chang S. Behavioral treatments for Tourette syndrome and tic disorders: state of the art. Adv Neurol. 2001;85:319-331.
68 Scahill L, Leckman JF, Schultz RT, et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60:1130-1135.
69 Budman CL, Gayer A, Lesser M, et al. An open-label study of the treatment efficacy of olanzapine for Tourette’s disorder. J Clin Psychiatry. 2001;62:290-294.
70 Parraga HC, Parraga MI, Woodward RL, et al. Quetiapine treatment of children with Tourette’s syndrome: report of two cases. J Child Adolesc Psychopharmacol. 2001;11:187-191.
71 Kastrup A, Schlotter W, Plewnia C, et al. Treatment of tics in Tourette syndrome with aripiprazole. J Clin Psychopharmacol. 2005;25:94-96.
72 Sallee FR, Kurlan R, Goetz CG, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39:292-299.
73 Kunwar AR, Megna JL. Resolution of risperidone-induced hyperprolactinemia with substitution of quetiapine. Ann Pharmacother. 2003;37:206-208.
74 Porta M, Maggioni G, Ottaviani F, et al. Treatment of phonic tics in patients with Tourette’s syndrome using botulinum toxin type A. Neurol Sci. 2004;24:420-423.
75 Robertson M, Doran M, Trimble M, et al. The treatment of Gilles de la Tourette syndrome by limbic leucotomy. J Neurol Neurosurg Psychiatry. 1990;53:691-694.
76 Rauch SL, Baer L, Cosgrove GR, et al. Neurosurgical treatment of Tourette’s syndrome: a critical review. Compr Psychiatry. 1995;36:141-156.
77 Temel Y, Visser-Vandewalle V. Surgery in Tourette syndrome. Mov Disord. 2004;19:3-14.
78 Newcorn JH, Spencer TJ, Biederman J, et al. Atomoxetine treatment in children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:240-248.
79 Castellanos FX, Acosta MT. [Tourette syndrome: an analysis of its comorbidity and specific treatment]. Rev Neurol. 2004;38(Suppl 1):S124-S130.
80 Pauls DL, Alsobrook JP, Goodman W, et al. A family study of obsessive-compulsive disorder. Am J Psychiatry. 1995;152:76-84.
81 McKay D, Abramowitz JS, Calamari JE, et al. A critical evaluation of obsessive-compulsive disorder subtypes: symptoms versus mechanisms. Clin Psychol Rev. 2004;24:283-313.
82 Angst J, Gamma A, Endrass J, et al. Obsessive-compulsive severity spectrum in the community: prevalence, comorbidity, and course. Eur Arch Psychiatry Clin Neurosci. 2004;254:156-164.
83 Mohammadi MR, Ghanizadeh A, Rahgozar M, et al. Prevalence of obsessive-compulsive disorder in Iran. BMC Psychiatry. 2004;4:2.
84 Cillicilli AS, Telcioglu M, Askin R, et al. Twelve-month prevalence of obsessive-compulsive disorder in Konya, Turkey. Compr Psychiatry. 2004;45:367-374.
85 Fontenelle LF, Mendlowicz MV, Marques C, et al. Trans-cultural aspects of obsessive-compulsive disorder: a description of a Brazilian sample and a systematic review of international clinical studies. J Psychiatr Res. 2004;38:403-411.
86 Freud S. Obsessions and phobias, their psychical mechanisms and their aetiology. In: Strachey J, editor. The Standard Edition of the Complete Psychological Works. London: Hogarth Press, 1895.
87 Rachman S, Hodgson RJ. Obsessions and Compulsions. Englewood Cliffs, NJ: Prentice-Hall, 1980.
88 Evans DW, Lewis MD, Iobst E. The role of the orbitofrontal cortex in normally developing compulsive-like behaviors and obsessive-compulsive disorder. Brain Cogn. 2004;55:220-234.
89 Arnold PD, Richter MA. Is obsessive-compulsive disorder an autoimmune disease? CMAJ. 2001;165:1353-1358.
90 Stewart SE, Geller DA, Jenike M, et al. Long-term outcome of pediatric obsessive-compulsive disorder: a meta-analysis and qualitative review of the literature. Acta Psychiatr Scand. 2004;110:4-13.
91 Masellis M, Rector NA, Richter MA. Quality of life in OCD: differential impact of obsessions, compulsions, and depression comorbidity. Can J Psychiatry. 2003;48:72-77.
92 Geffken GR, Storch EA, Gelfand KM, et al. Cognitive-behavioral therapy for obsessive-compulsive disorder: review of treatment techniques. J Psychosoc Nurs Ment Health Serv. 2004;42:44-51.
93 Kim MC, Lee TK, Choi CR. Review of long-term results of stereotactic psychosurgery. Neurol Med Chir (Tokyo). 2002;42:365-371.
94 Lippitz BE, Mindus P, Meyerson BA, et al. Lesion topography and outcome after thermocapsulotomy or Gamma knife capsulotomy for obsessive-compulsive disorder: relevance of the right hemisphere. Neurosurgery. 1999;44:452-458.
95 Mindus, Jenike MA. Neurosurgical treatment of malignant obsessive compulsive disorder. Psychiatr Clin North Am. 1992;15:921-938.
96 Mindus P, Rasmussen SA, Lindquist C. Neurosurgical treatment for refractory obsessive-compulsive disorder: implications for understanding frontal lobe function. J Neuropsychiatry Clin Neurosci. 1994;6:467-477.
97 Kopell BH, Greenberg B, Rezai AR. Deep brain stimulation for psychiatric disorders. J Clin Neurophysiol. 2004;21:51-67.