16. Total Parenteral Nutrition*
Howard W. Kilbride, Mary Kay Leick-Rude, Steven L. Olsen and Jill Stiens
Total parenteral nutrition (TPN) support for critically ill newborns was first reported four decades ago. 28 However, in the modern era of neonatal care, TPN continues to be a critical aspect of intensive newborn care. Availability of TPN has been one of the developments responsible for improved outcome of neonatal surgical patients. 77,81,84 Increased survival of extremely preterm infants has provided new challenges for neonatal parenteral nutrition. 21 Current evidence would suggest that early nutritional support is important to prevent postnatal growth restriction, which has been commonly recognized in these infants. 29
This chapter discusses the nutritional needs of the high-risk newborn, specific indications for TPN, and guidelines for formulation and administration of intravenous (IV) nutritional solutions. It also provides an overview of mechanical, infectious, and metabolic complications, with emphasis on prevention and early identification.
PHYSIOLOGY
Fuel Stores
During periods of fasting, tissue stores of energy provide the major source of fuel for the body. Carbohydrate is stored in the liver and muscle as glycogen. Stable blood sugar levels are maintained by hormonal regulation of glycogen production (glycogenesis) and break down to glucose (glycogenolysis). Newborns, particularly those who are growth retarded or preterm, have low glycogen stores and often have insufficient regulatory mechanisms. 96
The body’s greatest energy stores are in the form of fat, which provides a calorie yield of 9 kcal/g when metabolized. In addition to normal deposits of adipose tissue, newborns (and hibernating adult animals) have unique stores called brown fat. These stores, which are anatomically located between the scapulae, in the axillae and mediastinum, and around the adrenal glands, protect the body from hypothermia through nonshivering thermogenesis70 (see Chapter 6).
Protein makes up lean body mass. Although protein generally is not used as an energy source postnatally, in fetal life, amino acids are oxidized apparently for energy. 100 This may be true for brief periods postnatally, but extended periods of protein catabolism (breakdown of endogenous substrates), such as during times of starvation, may lead to body dysfunction, as noted later.
The Effects of Insufficient Nutrition
The last trimester of gestation is a time of rapid fetal growth, with active transplacental transport of most nutritional substrates. Preterm delivery interrupts the nutritional supply and abruptly results in a catabolic state, which, if prolonged, may alter growth potential. It is unclear whether it is possible or desirable to achieve in utero growth rates for the postnatal preterm infant, but reestablishment of an anabolic state and maintenance of micronutrient sufficiency are necessary. 100 During this period of neonatal life, the rapidly growing brain is responsible for much of the nutritional requirements. Inadequate early nutrition may have irreversible effects on later neurodevelopmental outcome.62
Postnatal growth retardation also is associated with neonatal medical complications, including apnea, ventilator dependence, and chronic lung disease. 21 Immune responses may be depressed with increased susceptibility to infection (see Chapter 22). Protein malnutrition is most frequently seen in extreme preterms and may contribute to poor growth potential and long-term morbidity in these infants. 21,100,101 Poor postnatal growth for most extremely-low-birth-weight (ELBW) infants has emphasized the need for additional strategies to improve nutrition for this population. 25,26
Nutritional Requirements of the Neonate
CALORIC
Caloric requirements for term or near-term infants are 105 to 120 kcal/kg/day. These estimates are based on enteral intake (seeChapter 17). Parenteral requirements are about 20% less, or approximately 85 to 100 kcal/kg/day. Requirements are greater for very-low-birth-weight (VLBW) infants, whether extremely preterm or small for gestational age (SGA), but optimal intakes have not yet been determined.89
Factors affecting caloric requirements include the infant’s activity level, body temperature, and degree of stress. Nosocomial infections may also contribute to additional caloric needs. 102 Physical activity, which usually is infrequent in preterm infants, contributes less than 10% to the energy needs. 60 However, in pathologic states, such as with repetitious seizures or neonatal abstinence syndrome, increased activity may increase caloric needs. An elevation of body temperature increases caloric expenditure by approximately 12% for each degree Celsius above 37.8° C (100° F). Metabolic demands of surgery or severe cardiac or pulmonary distress may increase caloric requirements by 30% and chronic failure to thrive by 50% to 100%. In addition, postnatal dexamethasone therapy may slow weight and linear growth rates and potentially may affect brain growth. 21,68,94
WATER
Water requirements vary with gestational and postnatal age (post-conceptual age) and environmental conditions (e.g., care in an incubator versus radiant heat warmer, use of phototherapy) (see Chapter 14).
MINERAL
Sodium requirements are minimal for the first days of life. After 1 week, the average requirement is 3 to 4 mEq/kg/day. Large renal losses (>5 mEq/kg/day) may occur in very immature infants (<28 weeks’ gestation) in the first weeks of life. Potassium and chloride requirements are approximately 2 mEq/kg/day and 3 to 4 mEq/kg/day, respectively. Glucosuria with resulting osmotic diuresis may increase sodium and potassium urinary losses.32
Calcium is an important cofactor in hemostasis, enzyme function, muscle contraction, and cell membrane stability. In the newborn, 98% of calcium is stored in the bone. The initial calcium requirement is 1 mEq/kg/day to maintain calcium homeostasis and to avoid irritability and tetany associated with low serum ionized calcium levels. In utero, the accretion rate is 4 to 5 mEq/kg/day, which the growing preterm infant should receive in addition to adequate phosphorus and vitamin D to avoid osteopenia, rickets, and bone fractures.109 Excess calcium intake may cause central nervous system (CNS) depression or signs of renal toxicity.
The phosphorus requirement for the growing preterm infant is 40 to 60 mg/kg/day (31 mg = 1 mmol). Bone contains 80% of the body’s phosphorus. Low phosphorus intake causes increased renal calcium excretion and a depletion of bone calcium phosphate. Low phosphorus intake or chronic furosemide diuretic therapy also may lead to hypercalciuria and nephrolithiasis. 27 Because phosphorus is a major constituent of cellular energy function (adenosine triphosphate, 2,3-diphosphoglycerate, creatinine phosphate), severe depletion may result in muscle paralysis, respiratory failure, and interruption of important cellular functions, such as the hemoglobin-oxygen dissociation curve and leukocyte activity.
Magnesium is essential for intracellular enzyme systems. The requirement is 0.25 to 0.5 mEq/kg/day.4 Magnesium deficiency states mimic hypocalcemia, manifesting as irritability, tremulousness, tetany, and cardiac dysrhythmias. Magnesium excess may manifest as lethargy, hypotonia, and delayed stooling.
CARBOHYDRATE
During fetal life, glucose is the primary source of energy. 100 At birth, the preterm infant has only a small supply of glycogen, the storage form of glucose (equivalent to about 200 kcal of energy). Glucose is particularly important for the CNS, because other substrates are not available. Initially, a glucose infusion rate (GIR) of 6 mg/kg/min is sufficient to meet metabolic needs of the newborn infant. Requirements are greater for infants who are stressed (e.g., from sepsis or hypothermia) or hyperinsulinemic (e.g., infants of diabetic mothers or infants with Beckwith-Wiedemann syndrome). With long-term parenteral nutrition, at least 50% of total caloric requirement should be provided as carbohydrate (GIR 8 to 10 mg/kg/min), generally as dextrose (calculated as 3.4 kcal/kg of hydrated carbohydrate). To avoid metabolic consequences of excessive glucose loads, a GIR of more than 12 mg/kg/min (18 g/kg/day of glucose) should be avoided.
PROTEIN
The quantity of daily nitrogen required by a term newborn infant, based on estimates from breast milk intake, is approximately 325 mg/kg/day (approximately 2 g/kg/day of protein). 5,35 Requirements for preterm infants are much higher, as indicated by in utero accretion rates during the latter half of pregnancy. At 28 weeks’ gestation, the fetus requires 350 mg/kg/day of nitrogen. This figure declines to 150 mg/kg/day by term gestation. When the estimated accretion rate is added to the obligatory postnatal nitrogen excretion, the requirement for a 28-weeks’ gestation preterm may be calculated to be approximately 495 mg/kg/day (3.1 g/kg/day of protein). If one assumes parenterally administered amino acids are converted to body proteins at 75% efficiency, the estimated parenteral amino acid requirement would be as high as 3.7 g/kg/day.31,40,101
In fetal life, protein is actively transported from mother’s circulation across the placenta in quantities greater than needed for accretion, with the excess being oxidized for energy. 99 Clinicians have found that increasing protein intake postnatally at all energy intake levels above 40 kcal/kg/day results in increased protein accretion. Current evidence indicates that protein intake up to 4 g/kg/day is safe with no clinically significant increase in azotemia, acidosis, or hyperaminoacidemia.79 Further investigations are needed to determine safe upper limits for maximum protein administration beyond that level.
Studies have shown that administration of amino acids shortly after birth decreases protein catabolism, which is extremely important particularly for VLBW infants. 31,99 Based on the current evidence, providing VLBW infants with 3 g/kg/day of protein on the first day of life is safe. 25Many units have created a “stock” or “starter TPN (protein-containing) solution” to achieve the goal of providing 2 to 3 g/kg/day of protein immediately after admission to the neonatal intensive care unit (NICU) to promote anabolism. Although current studies overwhelmingly support the early use of parenteral protein nutrition, further investigation is needed to document the effect of this supplementation on long-term growth and development.
The quality of the amino acid mixture infused is important for efficacy and safety. 1Although there is no formulation specifically for preterm infants, pediatric solutions provide greater quantities of essential amino acids and result in plasma amino acid levels similar to that of postprandial breast-fed infants. An essential amino acid is one that cannot be synthesized in adequate quantity to meet the requirements for normal growth and development. The differentiation between essential and nonessential amino acids is not clear in newborn infants, because the ability to synthesize some amino acids may vary with the clinical situation or stage of maturity. Lysine and threonine are essential in their entirety. There is a high requirement for branched-chain amino acids (e.g., leucine, isoleucine, valine) in the growing newborn. These are metabolized primarily in skeletal muscle. 41
Methionine is an essential sulfur-containing amino acid that is metabolized to cysteine and taurine. For preterm infants of less than 32 weeks’ gestation, cystathionase activity is insufficient for cysteine synthesis.108Some investigators have found cysteine supplementation results in greater nitrogen retention, and for this reason it is recommended for short-term supplementation for high-risk preterms, although the effects of prolonged use have not been fully investigated.92Cysteine is not stable in amino acid solutions, so cysteine hydrochloride supplements must be added separately to the parenteral nutrition. Taurine is a nonprotein amino sulfonic acid that is converted from cysteine by cysteine sulfonic acid decarboxylase. Taurine concentrations are low in infants who have received nonsupplemented TPN infusions. Taurine deficiency may have a detrimental effect on the developing nervous system . It is a general practice to add taurine to TPN for VLBW infants because this may prevent cholestasis in some newborns by more effectively conjugating bile salts and creating soluble end-products.42,93,107
Tyrosine is another amino acid that appears to be essential in the newborn period. It is present in small amounts in most amino acid solutions, although one manufacturer uses a soluble form, N-acetyl- l-tyrosine, which infants slowly metabolize to tyrosine. 82 Tyrosine is a by-product of phenylalanine metabolism, so supplementation has an effect on the phenylalanine requirement. Histidine is considered to be an essential amino acid for newborns, with the lowest levels evident in preterm infants. Arginine may be essential only for the newborn with reduced arginine synthetase activity. This amino acid is thought to facilitate clearance of nitrogenous waste products by “priming the urea cycle.” Use of amino acid infusate with insufficient arginine has been associated with hyperammonemia. 39 Glutamine also has been considered a conditionally essential amino acid; however, in a randomized trial, no benefit was shown for parenteral glutamine in relation to days to enteral feedings, incidence of necrotizing enterocolitis (NEC), or growth rates. 78
Nonessential amino acids make up the largest percentage of the amino acid pool in the fetal body. The desired quantities of these amino acids for parenteral solutions are not known. It is thought they should be provided in a balanced formulation. Pediatric solutions differ from adult solutions by providing glutamic acid and aspartic acid with lower glycine concentrations. 1,104
FAT
Long-chain fatty acids are essential in the newborn for brain development and appear to be important for gene expression and other molecular mechanisms. 106 Essential fatty acids (EFAs) include linoleic and linolenic, and in the newborn, arachidonic acid. 4 Biochemical evidence of EFA deficiency may be seen in less than 1 week in VLBW infants receiving a deficient diet, and the administration of parenteral glucose and amino acids may accelerate these abnormalities. 100 EFA deficiency results in an imbalance in fatty acid production with an overproduction of nonessential fatty acids. Clinical manifestations appearing at variable times after biochemical changes of EFA deficiency include scaly dermatitis, poor hair growth, thrombocytopenia, failure to thrive, poor wound healing, and increased susceptibility to bacterial infection. Clinical manifestations of EFA deficiency can be avoided if 3% to 4% of caloric intake is supplied as linoleic acid (approximately 0.5 g/kg/day of intravenous [IV] lipid). 33
In addition to preventing EFA deficiency, lipid emulsion is a concentrated source of nonprotein calories, which promotes nitrogen retention. Preterm infants appear to have limited capability to oxidize fatty acids. This limitation may be related to deficiency of carnitine, which, in the form of acylcarnitine, promotes transfer of fatty acids into mitochondria, where oxidative metabolism occurs. However, a systematic review of randomized studies found no benefit for carnitine supplementation on weight gain, lipid utilization, or ketogenesis, so routine supplementation is not recommended. 15
VITAMINS
The biologic role of vitamins, signs and symptoms of deficiency states, and recommended oral requirements are available in Chapter 17. Although there is not a multivitamin formulation specifically for preterm infants, the American Society for Clinical Nutrition (ASCN) has suggested that preterm infants receive 40% to 65% of the daily recommended vitamin doses for term infants and children. 89 These guidelines may result in excessive intakes of some water-soluble vitamins, particularly pyridoxine and riboflavin. Although preterm infants have limited stores of lipid-soluble vitamins because of low body fat, potential toxicity from excess administration is a concern. Vitamin A is a lipid-soluble vitamin important for tissue growth, protein synthesis, and epithelial differentiation. Vitamin A may be administered more effectively in lipid emulsion rather than dextrose amino acid solutions. 4,24 However, vitamin A supplementation has been proven to be effective in lowering chronic lung disease rates only when given by intramuscular (IM) injections three times per week. 105
Vitamin E is a lipid-soluble biologic antioxidant that is deficient in preterm infants. However, daily parenteral intake of 2 to 3 mg/kg has been associated with serum levels generally in the recommended range of 1 to 2 mg/dL. Pharmacologic doses have been tried unsuccessfully for prevention of bronchopulmonary dysplasia and retinopathy of prematurity, and IV high-dose vitamin E may increase risk for sepsis. 14 Therefore aiming for tocopherol levels greater than 3.5 mg/dL is not recommended. Vitamin K production by intestinal flora is impaired by insufficient enteral feedings and use of broad-spectrum antibiotics in infants on long-term TPN. Vitamin K is provided at the recommended dosage through parenteral pediatric multivitamin solutions. 4
TRACE MINERALS
Although trace minerals are relatively scarce (<0.01% of the weight of the human body by definition), they play an important role in normal growth and development. 111 Deficiencies of both zinc and copper have been identified in infants on long-term TPN not supplemented with trace minerals. Postsurgical infants with ongoing gastrointestinal losses may have negative zinc balance even if given usual zinc replacement in TPN. 88
Manifestations of deficiency and recommendations for intake are provided in Chapter 17. Parenteral recommendations are lower than enteral, which are based on physiologic requirements. For infants not receiving frequent blood transfusions, iron therapy may be necessary by 2 months of age. Infants receiving erythropoietin therapy need additional iron supplementation, given either enterally or parenterally. 66
INDICATIONS
Parenteral nutrition, including protein supplementation and carbohydrate at basal levels, should begin on the first day of life for preterm infants not being fed, as well as for other newborns who are not likely to tolerate enteral feedings within a few days. A preterm infant has limited nutritional stores and quickly develops negative protein balance without early supplementation. TPN continues to be a critical aspect of long-term management for neonatal surgical patients. 84When parenteral nutrition solutions are administered through a peripheral vein, caloric intake is limited because the fluid osmolarity should not exceed 900 mOsm/L, which results in relatively limited concentrations of carbohydrate (<12.5% dextrose) and amino acids (<3%). Some recommend even more conservative limits on osmolarity for peripheral lines (500 mOsm/L).44 When used with lipid emulsions, peripheral parenteral nutrition (PPN) allows caloric intake of about 70 to 80 kcal/kg/day and protein intake of 2.5 to 3.0 g/kg/day. This level of nutritional intake prevents catabolism and, in some cases, results in moderate growth. PPN usually is adequate for term newborns with transient bowel disease (such as may be seen after the repair of a small omphalocele) or for larger preterm infants whose enteral feedings are delayed for a few days. PPN is used commonly to supplement nutrition in newborns who are receiving partial enteral feedings. When caloric needs can be met by PPN, this route is preferred to the central route, because the catheter insertion risks are avoided and generally the risk for infection is less.
If parenteral nutritional duration is longer than 1 week, administration of TPN solution through a central line is recommended. The placement of a central line for parenteral nutrition allows a higher carbohydrate load to be used, giving more calories with less fluid. In preterm infants at risk for a patent ductus arteriosus and pulmonary edema, diminishing fluid intake and improving nutritional status may be important aspects of management. Specific indications for TPN by a central catheter include the following:
• ELBW infants (<1000 g birth weight) and others who do not tolerate a significant volume of enteral feeding within the first week of age or who cannot receive adequate caloric intake by PPN
• Infants who have had gastrointestinal surgery and will have a significant delay in enteral nutrition, such as those with a gastroschisis, bowel resection after NEC, or meconium peritonitis
• Infants with chronic gastrointestinal dysfunction, such as intractable diarrhea
DATA COLLECTION
Monitoring Growth
Weight loss or insufficient weight gain is the initial effect of inadequate caloric intake. Linear growth, although less affected, is diminished after long periods of poor nutrition. Because of “brain-sparing,” head circumference growth is the least affected. Measurements should be obtained in a standardized fashion and recorded weekly.
Fetal weight gain in utero at each week of gestation is currently used as the standard to assess adequacy of postnatal growth. In the midtrimester (24 to 27 weeks’ gestation), expected weight gain is 1.5% of body weight. 100 Charts are available to monitor postnatal growth rates based on data from a large preterm population, although for long-term monitoring, use of growth curves from normal populations may be more appropriate, as available from the Centers for Disease Control and Prevention (CDC) (www.cdc.gov/growthcharts/).34
Minimum monitoring of growth should consist of the following:
• Weigh daily, or more frequently in ELBW infants with rapidly changing extracellular fluid status. Maintenance of a thermostable environment with minimal handling of ELBW infants can be achieved through the use of in-bed scales. Strict attention to consistency of technique during the weighing process is essential to obtain accurate, reliable measurements. 103 Monitoring weight gain on a weekly basis in grams per kilogram of weight gained daily (g/kg/day) may help in reducing postnatal growth restriction and positively impact long-term neurodevelopmental outcome. An ideal rate of weight gain for ELBW infants appears to be 18 to 21 g/kg/day. 30
• Measure length weekly.
• Head circumference measured weekly.
Biochemical Monitoring
In addition to anthropometric measurements, biochemical parameters may be monitored to assess nutritional adequacy. Periodic assessment of calcium, phosphorus, and alkaline phosphatase levels is important to detect metabolic disturbances associated with osteopenia. 109 Tests for protein malnutrition include serum total protein, albumin, transferrin, retinol-binding protein, and transthyretin (prealbumin), the latter two suggested primarily for preterm infants. 5,35 Routine clinical use of these measurements awaits greater definition of normal variation and independent effects of systemic illness and medications.
Biochemical monitoring of the infant’s physiologic status is necessary to avoid complications of TPN. Usefulness of the laboratory data should be balanced with the economic costs and risks from iatrogenic blood losses for the infant (Table 16-1).
| BUN, Blood urea nitrogen; PRN, as needed. | ||
| *When on lipid emulsion. |
||
| Variable | Frequency | |
|---|---|---|
| Acute | Stable | |
| Electrolytes, BUN | Daily | 2×/wk |
| Calcium, phosphorus | Weekly | Biweekly |
| Alkaline phosphatase | — | Biweekly |
| Serum glucose screen | q 8 hr | Daily |
| Urine glucose | q 8 hr | Daily |
| Hemoglobin/hematocrit | Daily | Weekly |
| Liver function: | ||
| Bilirubin | 2×/wk | PRN |
| Transaminase | Weekly | Biweekly |
| Triglyceride* | — | Weekly |
When serum electrolyte levels are abnormal, urinary electrolyte levels may be useful to clarify sodium and potassium requirements (e.g., if body sodium is depleted, low urine concentration would be expected).
TREATMENT
Vascular Access
UMBILICAL ARTERY CATHETERS AND UMBILICAL VEIN CATHETERS
Umbilical artery catheters (UACs) and umbilical vein catheters (UVCs) are commonly placed in sick newborns to provide vascular access for IV fluids, blood samplings, and blood pressure monitoring. Because of the risks for thromboembolic and infection complications, these lines generally are removed by 1 week of age. 22,36
PERIPHERAL AND MIDLINE CATHETERS
If continued venous access is necessary after this time, a peripheral, midline, or peripherally inserted central catheter (PICC) can be placed. The type of line used is determined by the anticipated length of time needed and the osmolarity of the substances to be infused. 44 Peripheral IVs are indicated for short-term IV access. A midline catheter, which is threaded to the proximal portion of an extremity or neck, can provide longer intravenous access than a peripheral IV when prolonged peripheral strength TPN is indicated. Midline catheters appear to be associated with lower rates of phlebitis than short peripheral catheters and with lower rates of infection and cost than central lines. 59
PERIPHERALLY INSERTED CENTRAL CATHETERS
A PICC line can provide maximal nutritional intake when long-term parenteral access is necessary. 2 Percutaneous placement of a 20- to 26-gauge (1.9- to 2.6-Fr) Silastic (silicone) or polyurethane catheter can be performed routinely in even the smallest of neonatal patients by trained nurses and physicians. 72,76 The catheter usually is placed in the antecubital or axillary veins in the arms; however, leg, scalp, or external jugular veins may be used to achieve central access. Veins that may be needed for percutaneous central line placement should not be sites for routine venipuncture (see Chapter 7).
Percutaneous line placement involves stabilization of the vein, maximum barrier precautions (sterile gloves, gown, large drape, masks), and antiseptic preparation of the skin with 2% chlorhexidine or povidone-iodine and alcohol product.16,54,87Fully equipped prepackaged kits are available for this procedure from a number of manufacturers. Most kits include an 18- or 19-gauge insertion needle that is used to puncture and tunnel through the subcutaneous tissue before entering the vein. Once the needle is within the vein, the catheter, which has been flushed with heparinized saline solution, is passed through the needle into the vein and advanced to a premeasured distance, which is the estimated location of the superior vena cava. 44(If the basilic vein is used, turn the infant’s head to face the insertion site to minimize the risk for the catheter entering neck vessels.) The catheter tip position should be documented radiographically. If the catheter is placed in the saphenous vein, obtain a lateral roentgenogram if the anteroposterior film suggests possible vertebral vessel cannulation rather than inferior vena cava placement.67Remove the needle carefully from the skin and discard it. A Steri-Strip should be placed over the catheter insertion site to maintain its position before the dressing is completed. The addition of heparin to IV fluids is commonly used by practitioners to prevent occlusion of vascular catheters. However, there is no indisputable evidence for this practice.86
The length of tubing outside the infant’s body should be measured and recorded. Excess may be carefully curled at the site of insertion and covered with a sterile, transparent dressing. If an arm board was used for stabilization, it may be removed. Arm restraints should not be necessary.
BROVIAC® CATHETER
Large-bore Silastic catheters (Broviac®) are placed surgically in infants in whom the percutaneous method is not successful and long-term access is anticipated. Generally, the catheters are placed in the internal or external jugular veins or common facial vein by cutdown and threaded to a central venous site. The distal end is tunneled subcutaneously and exited through the anterior chest wall. The catheter must be secured and dressed sterilely.
OTHER VASCULAR ACCESS OPTIONS
Other sites that may be used for TPN infusion on a short-term basis include subclavian, jugular, and femoral veins. Some centers use a UVC for short-term parenteral nutrition when another site is not feasible.
Composition of Infusate
CARBOHYDRATE
The prime source of calories for the neonate usually is dextrose. Peripherally, 10% to 12% solution is used. When central access is obtained, a 15% to 30% dextrose concentration may be used. The glucose load is increased if either the infusion rate or glucose concentration of the infusate is increased. Too rapid an increase in glucose load may exceed an infant’s carbohydrate tolerance and result in hyperglycemia. A rapid decrease in the infusion rate or the glucose concentration of the infusate may result in hypoglycemia.
When calculating caloric intake, use the following:
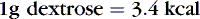
or
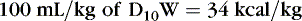
or
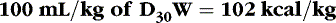
The glucose infusion rate (GIR) can be calculated:
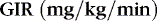
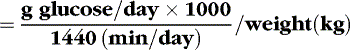
Generally, a newborn of 28 weeks’ gestation or more (>1000 g body weight) initially tolerates a GIR of about 6 mg/kg/min. Daily increases in dextrose concentration or fluid volume to increase carbohydrate administration by 2.0 mg/kg/min usually are tolerated. ELBW infants may be carbohydrate intolerant, and initial GIR should be lower (4 or 5 mg/kg/min) for these infants. An insulin infusion may be considered for ELBW infants experiencing persistent hyperglycemia with physiologic glucose infusion rates. 32
Blood glucose determinations and screening for glucosuria should be performed several times each day when glucose delivery is initiated or altered.
LIPIDS
Lipid emulsion at a rate of 0.5 to 1 g/day/100 kcal is sufficient to prevent EFA deficiency; however, additional lipids should be provided to supplement nonprotein caloric intake and support growth. 100Lipids should never make up more than 50% of total caloric intake. Fat emulsions should be given cautiously, beginning with 0.5 to 1 g/kg/day and advanced 0.5 g/kg every 1 to 2 days as tolerated to 3 g/kg/day maximum. Fat emulsions are available as either 10% or 20%, but the 20% concentration is universally used for VLBW infants, because its lower phospholipid concentration results in lower plasma levels of triglyceride and cholesterol and less fluid administration (Table 16-2). 80
| Composition | Intralipid (Clinitec) 20% | Liposyn II (Abbott) 20% |
|---|---|---|
| FATTY ACID DISTRIBUTION (%) | ||
| Linoleic acid | 50 | 54.5 |
| Oleic acid | 26 | 22.4 |
| Palmitic acid | 10 | 10.5 |
| Linolenic acid | 9 | 8.3 |
| Stearic acid | 3.5 | 4.2 |
| COMPONENTS (%) | ||
| Soybean oil | 20 | 20 |
| Safflower oil | — | — |
| Egg phospholipids | 1.2 | 1.2 |
| Glycerin | 2.25 | 2.5 |
| Caloric contents (kcal/dL) | 200 | 200 |
| Osmolarity (mOsm/L) | 260 | 292 |
Emulsified fat particles are similar in size and metabolic rate to naturally occurring chylomicrons. Most are cleared through passage in the adipose and muscle tissue. The capillary endothelial lipoprotein lipase hydrolyzes triglycerides and phospholipids, generating free fatty acids (FFAs), glycerol, and other glycerides. Most of the FFAs diffuse into the adipose tissue for re-esterification and storage. A small portion circulates to be used by other tissues for fuel or for conversion by the liver into very-low-density lipoprotein. Extremely preterm and SGA infants with decreased adipose tissue have prolonged clearance of fat emulsion. In general, because complications of lipids are related to delay in clearance, lipids should be infused over a 24-hour period to provide the lowest hourly rate.80 The rate-limiting step for lipid clearance is the metabolism by lipoprotein lipase. The use of heparin stimulates the release of this enzyme and may enhance clearance of IV lipids. Carbohydrate also must be administered with fat to facilitate fatty acid oxidation and to promote FFA clearance.
AMINO ACID SOLUTION
The compositions of two crystalline amino acid solutions available for neonatal parenteral use are presented in Table 16-3. The maximum concentration of the amino acid solution generally is 2% for peripheral use and 3% for central use. Each solution supplies an excess of nonessential amino acids, although more recently available solutions have sought to balance the nonessential amino acid profile.
| *Cysteine hydrochloride supplement may be added. |
||
| Amino Acid | Solutions | |
|---|---|---|
| Aminosyn-PF (Abbott) | Trophamine (Kendall McGaw) | |
| Essential | ||
| l-Leucine | 356 | 420 |
| l-Phenylalanine | 129 | 144 |
| l-Methionine | 54 | 102 |
| l-Lysine | 204 | 246 |
| l-Isoleucine | 228 | 246 |
| l-Valine | 194 | 234 |
| l-Histidine | 94 | 144 |
| l-Threonine | 154 | 126 |
| l-Tryptophan | 54 | 60 |
| NONESSENTIAL | ||
| l-Alanine | 210 | 162 |
| l-Arginine | 369 | 360 |
| l-Proline | 244 | 204 |
| l-Tyrosine | 19 | 69 |
| l-Cysteine | * | <10* |
| l-Serine | 149 | 114 |
| l-Glycine | 116 | 108 |
| l-Glutamine | — | — |
| l-Taurine | 21 | 7.5 |
Cysteine, which is an essential amino acid in preterm infants, is not stable for long periods in solution. This amino acid is commercially available to be added immediately before the solution is administered. TrophAmine and Aminosyn-PF include taurine, which is not available in other solutions.
A minimum quantity of energy substrates must be provided for effective utilization of parenteral protein. For ELBW infants, approximately 40 kcal/kg/day of carbohydrates or fat and 1.5 g/kg/day of protein are necessary for resting metabolic needs to prevent catabolism. However, urinary protein losses are greatest for preterm infants, so additional supplementation is needed to prevent protein deficits. For each gram of protein provided above the basal amount, approximately 10 kcal of nonprotein energy is needed. 25,31,100
ELECTROLYTES
Sodium and potassium may be supplied with chloride, acetate, or phosphate anions. The daily chloride requirement is approximately 3 mEq/kg/day and should be balanced with acetate to avoid alkalosis or acidosis (acetate is converted to bicarbonate). Amino acid preparations also supply anions that must be recognized to calculate a balanced anion solution. For example, TrophAmine supplies 1 mEq of acetate per gram of protein. On the other hand, cysteine addition to the TPN solution reduces the pH, necessitating buffering with acetate.
MINERALS
Phosphorus may be provided as sodium or potassium phosphate. Calcium may be provided as 10% calcium gluconate (9.7 mg of elemental calcium/100 mg of salt). Both calcium gluconate and potassium phosphate have relatively high levels of aluminum and should be used judiciously for chronic TPN in infants with renal dysfunction (see discussion of aluminum toxicity in the “Trace Elements” section).43When preparing a solution with both calcium and phosphate, care must be taken to avoid calcium phosphate precipitation, which may limit the intake of these important minerals. Magnesium is supplied as magnesium sulfate.
If one is using a potassium phosphate solution at pH 7.4, 4.4 mEq of potassium supplies 93 mg of elemental phosphorus (3 mM). When a solution of sodium phosphate is used at pH 7.4, 4.0 mEq of sodium is given with each 93 mg of elemental phosphorus.
CALCIUM
• Because of increased risk for precipitation, calcium chloride generally should not be used (but may be considered for an infant at risk for aluminum toxicity).
• An elevation in ambient temperature, increased storage time, rise in pH, and decrease in protein or glucose concentration may increase the likelihood of precipitation. The addition of cysteine, which lowers solution pH, may enhance calcium and phosphate solubility.104
• When one is preparing the solution, calcium and phosphate salts should be added separately, but not in sequence, during the last stages of solution mixing. The solubility of the added calcium should be calculated from the volume at the time the calcium is added, not the final volume.
VITAMINS
A preparation approximating the American Medical Association’s recommended formulation of IV vitamins is available (MVI-Ped). The daily recommended dose is one vial for infants weighing more than 3 kg, 65% vial for infants 1 to 3 kg, and 30% vial for infants less than 1 kg.4
TRACE ELEMENTS
Zinc is supplied as zinc sulfate. Serum zinc levels usually approximate the maternal levels at birth and decline over the first week of life. By the second week of life, neonates not receiving dietary zinc should have supplementation. It may be necessary to initiate zinc intake earlier in neonates with intestinal loss, such as after gastrointestinal surgery.
Copper is supplied as cupric sulfate. Approximately two thirds of stored copper is accumulated during the last trimester. Therefore a preterm infant may need early supplementation but a term infant has adequate hepatic stores for at least several weeks. Because copper is excreted through the biliary system, this mineral should be removed from parenteral fluids for infants with cholestasis. 111
Manganese, chromium, and selenium salts should be provided for long-term parenteral nutrition. Manganese supplementation should not be provided to infants with cholestasis. The chromium dose may be reduced or discontinued in an infant with impaired renal function. A commercially available trace element solution is available that provides zinc, copper, manganese, and chromium.
Traces of aluminum are incorporated into parenteral solutions during processing.53 Although aluminum is not known to have a physiologic role in the body, high aluminum levels have been associated with bone disease, encephalopathy, anemia, and hepatic cholestasis and may contribute to neurodevelopmental damage in preterm infants on chronic parenteral nutrition. 12Infants with disturbance of renal clearance are at greatest risk for aluminum loading. The U.S. Food and Drug Administration requires manufacturers to report the aluminum content of parenteral products. 43
Definitions of safe and potentially toxic levels of contamination are available. 6Clinicians should attempt to reduce aluminum intake and should monitor levels for infants at highest risk.3
Table 16-4 outlines a suggested composition for a TPN solution (guideline only). Even in the most knowledgeable hands, accurate calculation and ordering of parenteral nutrition for preterm or ill infants is a complex task. Online TPN ordering programs are available in many units to assist the clinician with this task. Use of such programs has been shown to decrease order entry errors. 58 The Case Study on p. 388 illustrates considerations in writing orders for TPN solutions.
| *MVI Pediatric (Astra Pharmaceuticals), reduced amount provided for very-low-birth-weight infants (see text). |
|
| †As Multitrace-4 Neonatal (American Regent Laboratories, Inc.). |
|
| Component | Daily Amount |
|---|---|
| CALORIES | |
| Dextrose 3.4 kcal/g | 10-15 g/kg |
| Lipids 2.0 kcal/mL (20%) solution | 1-3 g/kg |
| Protein (6.25 g protein = 1 g N 2) | 3.5-4 g/kg |
| ELECTROLYTES | |
| Sodium | 3 mEq/kg |
| Potassium | 2-3 mEq/kg |
| Chloride | 3-4 mEq/kg |
| Acetate | 3 mEq/kg |
| Phosphate | 2 mM/kg |
| Calcium | 3 mEq/kg |
| Magnesium | 0.3 mEq (20 mg)/kg |
| VITAMINS | |
| MVI-Ped | 1 vial* |
| Vitamin A | 0.7 mg |
| Thiamine (B 1) | 1.2 mg |
| Riboflavin (B 2) | 1.4 mg |
| Niacin | 17 mg |
| Pyridoxine (B 6) | 1 mg |
| Ascorbic acid (C) | 80 mg |
| Ergocalciferol (D) | 10 mcg |
| Vitamin E | 7 mg |
| Pantothenic acid | 5 mg |
| Cyanocobalamin | 1 mcg |
| Folate | 140 mcg |
| Vitamin K | 200 mcg |
| TRACE ELEMENTS | |
| Zinc (zinc sulfate) † | 300 mcg/kg |
| Copper (cupric sulfate) † | 20 mcg/kg |
| Manganese sulfate† | 5 mcg/kg |
| Chromium chloride† | 0.2 mcg/kg |
| Selenium | 2 mcg/kg |
Preparing the Solution
Solutions should be prepared in the hospital pharmacy under a laminar flow hood in a work area isolated from traffic and contaminated supplies.
The following case example illustrates considerations in writing orders for total parenteral nutrition (TPN).
History
A male infant born at 26 weeks’ gestation at 900 g is now 10 days old and unable to be fed because he has developed necrotizing enterocolitis (NEC). Because there will be a prolonged delay in enteral alimentation, a central vein catheter is placed for TPN. He is currently receiving D 10W at 140 mL/kg with maintenance electrolytes. His current weight is 850 g. Serum electrolytes and blood glucose are normal. The approach to calculating TPN requirements is as follows.
Caloric Requirement
Because the patient has already had a significant postpartum period without adequate nutrition, achieving caloric intake necessary for growth is a very important part of his care. The infant will probably require 100 kcal/kg or more for tissue repair and growth. We will begin with approximately 60 to 70 kcal/kg (the birth weight is used until weight gain is established) and advance the intake daily to reach this level.
Carbohydrate
Initially, a dextrose load just above what has been previously tolerated should be used. Thus the patient may receive D 12.5W at approximately 140 mL/kg/day; the volume could vary depending on the infant’s fluid requirements.
This represents:
12.5 g glucose/dL × 140 mL/kg = 17.5 g glucose/kg
17.5 g glucose/kg × 3.4 kcal/g glucose = 60 kcal/kg
Fat
Lipid emulsion should be added to increase the caloric intake, starting with 1.0 g/kg/day.
5 mL/kg 20% lipid emulsion (1.0 g) × 2 kcal/mL = 10 kcal/kg/day
Thus the total non-nitrogen calories on the first day of TPN are 70 (60 + 10).
Protein
Provision of protein nutrition is critical to this preterm infant for growth and to repair damaged tissues. The initial amino acid replacement is 2.5 to 3 g/kg/day.
Electrolytes
The patient should receive maintenance sodium ion (approximately 3 mEq/kg) and potassium ion (2 to 3 mEq/kg) unless there are excessive renal or gastrointestinal losses.
Anions
Balancing anions is the next consideration. The 3 g/kg of amino acids, if given as TrophAmine, adds approximately 3 mEq/kg of acetate to the solution (1 mEq acetate/1 g amino acids). If 3 mEq/kg of potassium is provided as potassium chloride, the solution has balanced anions. Giving 3 mEq/kg of sodium as sodium phosphate provides approximately 2.2 mM/kg of elemental phosphorus:
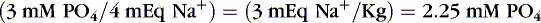
Minerals, Vitamins, and Trace Elements
Calcium, magnesium, phosphorus, vitamins, and trace elements should be ordered at this point. Calcium initially should be started at 2 to 3 mEq/kg/day but may be increased as tolerated with growth to 4 to 5 mEq/kg/day.
Use of an online TPN ordering program may assist the clinician by automating many of these calculations. 58
TPN Orders
Thus the TPN orders would be written for this patient as follows:
| 125 g Dextrose (D 12.5W) with the Following per Liter to Run 5.3 mL/hr | Quantity Provided per kg/day (in 140 mL): |
|---|---|
| 20 g amino acids | 2.8 g AA |
| 20 mEq potassium as potassium chloride |
2.8 mEq K +
2.8 mEq Cl 2
|
| 20 mEq sodium as sodium phosphate | 2.8 mEq Na + |
| 15 mM phosphate* | 2.1 mM Phos |
| 20 mEq calcium | 2.8 mEq Ca 2+ |
| 2 mEq magnesium | 0.3 mEq |
| 21.7 mL MVI-Ped | 3 mL/day |
| 1.3 mL trace element solution† | 0.18 mL |
| Run 20% lipid emulsion at 0.14 mL/hr for 24 hr (approximately 0.5 g/kg of lipids/day). | |
| *3 mM of sodium phosphate = 4 mEq sodium; if potassium phosphate is used, 3 mM of potassium phosphate = 4.4 mEq potassium. |
|
| †Commercially available trace element solution (Multitrace4—Neonatal, includes zinc, copper, manganese, and chromium). | |
Progression
On subsequent days, the dextrose concentration and lipids would be advanced slowly to increase the caloric intake to requirement as tolerated. The quantity of protein would also be increased to about 4 g/kg/day.
There should be quality control checks to monitor for sterility breaks in equipment, personnel, environment, and solutions.
Because many additives potentially can be insoluble in combination, a mixing sequence should be established that separates the most incompatible ingredients. Storage increases the risk for microbial contamination; therefore, TPN solutions should be prepared on the day they are needed. 64 However, to be able to provide an amino acid infusion to preterm infants immediately after admission, some units maintain a “stock” amino acid solution (10% dextrose with 3 g of amino acids per 100 mL). 97
Administering the Total Parenteral Nutrition Solution
Proper administration of the TPN solution is as important as its preparation in preventing complications. The label on the solution always should be checked to correctly identify the patient, using at least two identifiers, and to verify current formulation order.
Standardized procedures must be established to avoid infectious complications from solution contamination. Solutions on the nursing units may be returned to the pharmacy for additives before hanging, but no additives should be placed in the solution once it is hanging. The bag or bottle of TPN solution should be changed every 24 hours, and the tubing administration sets should be changed at least every 72 hours. Lipid emulsions and tubing should be changed every 24 hours.16,54,63Polyvinyl chloride (PVC) tubing and IV bags containing phthalates should be avoided to reduce potential toxicity from plasticizers.45,71
Exposure of TPN to light generates peroxides, which induce vasoconstriction and oxidant stress associated with bronchopulmonary dysplasia (BPD). Photoprotection of bags, syringes, and tubing used to deliver TPN and lipids may reduce the oxidant effect on the lungs and mesenteric blood flow. Light shielding also appears to diminish oxidative stress and alterations of lipid metabolism, resulting in lower levels of triglyceride and better substrate delivery. Amber-colored tubing may be used for this purpose.18,49,50
Changes in TPN infusion rates result in changes in glucose delivery to the newborn and may lead to hypoglycemia or hyperglycemia if the glucose homeostatic mechanisms do not adjust fast enough. Reactive hypoglycemia may occur if the glucose load is abruptly discontinued. 9Parenteral nutrition solutions must infuse at a constant rate via an infusion pump. Infusion rates should not be increased or decreased. If the parenteral nutrition infusion is suddenly discontinued because of a clotted catheter or accidental removal, an appropriate solution with dextrose should be infused via a peripheral vein and blood glucose should be monitored.
Use of parenteral nutrition may increase an infant’s risk for hyperglycemia during surgery. Because rapid fluid infusions may be necessary during operative procedures, the TPN solution should be discontinued and replaced with a physiologic infusate during the perioperative period. After surgery, TPN should be as when the patient is euglycemic, with recent evidence of early postoperative protein tolerance and improved protein balance. 81
Tapering of the TPN solution occurs as the infant begins to tolerate enteral feedings. When the patient is taking approximately two thirds of the necessary calories enterally, the central line may be removed.
Administering Fat Solution
Rapid infusion of the fat emulsion may exceed its clearance rate from the body and accentuate complications; therefore fat emulsions should not be infused faster than 0.15 g/kg/hr.4,80 Lipids generally are given through a Y-site connection to bypass the filter in the TPN line or may be given through a separate venous site. However, some hospitals use a combined dextrose, amino acid, and lipid solution known as three-in-one or total nutrient admixture (TNA).83,89 A 1.2-micron filter is used with this solution to remove certain drug precipitates (Ca/PO 4), air, and Candida, but it is not effective in removing bacteria. The decision to use TNA should be approached with caution in infants. Lipid emulsions increase the pH of the TPN solution, limiting the amount of calcium and phosphorus that can be delivered because of the risk for precipitation. Precipitates are particularly difficult to detect in TNA, which is a milky solution. High concentration of calcium and low pH of the solution also can disrupt TNA, causing it to “crack,” leading to separation of oil from the rest of the solution. One must store the admixture emulsion at an ambient temperature below 28° C to prevent coalescence. 56
When administering lipids to ill infants receiving other infusions, care must be taken to ensure that medications are compatible with lipids or medications must be provided by a separate intravenous route to prevent precipitation.
COMPLICATIONS
Mechanical Complications
Pneumothorax, hemothorax, hydrothorax, air embolism, thromboembolism, catheter misplacement, cardiac perforation, and tamponade are all recognized complications of Broviac®, subclavian, or jugular catheter insertions. Potential mechanical complications of percutaneous central lines include catheter occlusion, accidental dislodgement, erythematous tracking, phlebitis, thrombosis, superior vena cava syndrome, catheter migration, and catheter entrapment or breakage.69,72,74,75 A pleural or pericardial effusion may be blood or chyle or may be a signal that the catheter has eroded into the pleural or pericardial space. The effusion may be the infusate. Therefore chest roentgenogram examination is necessary to document correct catheter placement before a hypertonic solution is instilled.
The preceding complications may occur at any time while the catheter is present. Documentation of catheter position should be repeated if there is any history of pulling or tension on the catheter or any apparent change in its external position or change in the clinical condition associated with the preceding complications.
Any signs of catheter malfunction require troubleshooting and assessment for potential interventions to salvage the line. Some clinicians will flush a partially occluded line with a thrombolytic agent, such as recombinant tissue plasminogen activator (rt-PA). 48 The risk of this practice must be weighed against the benefits of maintaining the central line. In most cases, if the catheter is a temporary line, it may be better to remove it and place a new line in another site.
Infectious Complications
Infections associated with the central line may occur from contamination of the solution, tubing connections, or hubs. Although organisms may contaminate the solution during preparation, usually colonization occurs with entry into the line or bag. Intermittent administration of medications, removal of blood samples through the line, or multiple tubing changes provide opportunity for organisms to contaminate the solution.
Rigid criteria for sterile preparation of the solutions are mandatory (see “Preparing the Solution” section).
An in-line 0.22-μm membrane filter, which is incorporated into the IV tubing, is capable of trapping bacteria and fungi (although not endotoxin) and should help minimize the risk for septicemia from a contaminated IV bag. In addition, filters lessen the risk for an air embolism. An in-line filter setup is available that decreases the number of connections.
Nothing should be added to the TPN solution after it leaves the pharmacy.
AVOIDING LINE COLONIZATION
• When changing IV fluids, one should avoid bleed-back into the catheter.
• Line setups should be designed to minimize the number of ports and connections. 52
• Generally, medications should not be given into injection ports in the IV tubing but, rather, should be given into a dedicated heparin-locked Y-site entry port. Stopcocks are not recommended.
• The source of an infection is usually contamination with an organism that has colonized the hub or surrounding skin. Scrupulous attention to hand hygiene and disinfection of catheter tubing, hubs, ports, and connections by vigorous rubbing with 70% alcohol before tubing changes or entry are critical infection prevention strategies. 51,52,72
Dressings are not routinely changed on PICC lines. If the dressing becomes nonocclusive or moistened, the site should be cleaned according to hospital protocol and redressed with a sterile transparent dressing. 87 This should be performed using sterile gloves. The exposed catheter should be remeasured to ensure that it was not inadvertently moved during this process. Dressings are changed routinely on Broviac®, subclavian, jugular, and femoral catheters. Dressing changes are recommended at least weekly or more frequently if drainage is noted or the dressing is no longer occlusive.
EVALUATING INFANTS FOR INFECTIOUS DISEASE COMPLICATIONS
Central line–associated bacteremia represents an important source of nosocomial infections in the intensive care nursery. The prevalence of this complication varies by unit, based on patient demographics, including birth weight, gestational age, diagnoses (proportion of surgery and medicine), and care practices.
Bacteremia must be considered in a newborn with a central line in place who presents with signs of sepsis (e.g., temperature instability, lethargy, poor skin perfusion, increased cardiopulmonary distress, apnea). Some neonatal infections may be treated successfully with the line in place. However, if the infant remains systemically ill, even if the blood culture result is negative, the central line should be removed. 13
Altered immune function by lipid deposition in macrophages and the reticuloendothelial system must be considered in infants with sepsis. Malassezia furfur is a lipophilic, opportunistic fungal organism that may cause sepsis in infants receiving long-term lipid infusions. 90 This organism may contaminate the line and appear as a white film. This organism often will not grow in routine blood culture media. Specific culture techniques are necessary when Malassezia is suspected. 20
Guidelines for management of an infant with a central line in place with suspected sepsis are as follows:
• The infant should be evaluated for potential sources of infection, including a general physical examination looking for non–TPN-related sources and inspection of peripheral and central venous sites for erythema.
• Laboratory assessment should include (1) complete blood cell count with platelet count and (2) aerobic blood cultures. Other cultures, including urine, tracheal aspirate, and cerebrospinal fluid, may be indicated, based on clinical findings. A blood fungal culture should be considered if the infant has had preceding antibiotic treatment or signs of fungal infection. 10,11,46
• A chest x-ray evaluation should be performed if the infant demonstrates signs of respiratory distress or there is a need to reassess catheter position.
• Consider decreasing or discontinuing lipid infusion until the infection has been treated for 24 to 48 hours. 7
• If the infant is critically ill, the central line should be removed immediately. If the infant is stable, treatment may be considered through the line.
• A positive blood culture generally is considered to indicate bacteremia or sepsis in a newborn with a central line in place. However, the coagulase-negative Staphylococcus, an opportunistic organism that is a common cause of catheter-related sepsis, also is normal skin flora and frequently contaminates blood cultures. Use of ancillary diagnostic tools, such as the C-reactive protein levels and complete blood counts, are helpful to distinguish false-positive results from true infections. Some clinicians also recommend obtaining two cultures (two peripheral, or one peripheral and one from the line) before starting antibiotics. If both yield positive results, catheter-related sepsis is confirmed. 65
• If bacteremia is documented but the sepsis signs are improved, the catheter may remain in place while being used for antibiotic treatment. One should be sure that the antibiotics are compatible with the TPN solution (to avoid stopping the TPN during the antibiotic infusion). A follow-up blood culture and close clinical monitoring are necessary to document that the infection has been treated adequately.
If a central line is pulled because of sepsis, a new central line should not be placed for 48 to 72 hours.
Metabolic Complications
GLUCOSE METABOLISM
Hyperglycemia may occur with increased carbohydrate load, especially in ELBW infants who may have inadequate endogenous insulin production or decreased sensitivity to insulin. Elevated blood sugar may lead to hyperosmolality and osmotic diuresis, resulting in dehydration. Manifestations include polyuria, glucosuria, and excessive weight loss. Serum sodium is not a reliable measure of serum osmolality if there is hyperglycemia. Direct measurement or estimate by use of the following formula is necessary:
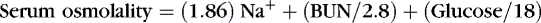
Transient glucose intolerance may be seen with stress. If hyperglycemia occurs without apparent change in glucose infusion, the possibility of sepsis, pain, hypoxemia, intraventricular hemorrhage (especially if the infant is <34 weeks’ gestation), or inadvertent increase in carbohydrate administration (mistake in preparation or rate of infusion) should be considered. Glucose intolerance also may be accentuated during infusions of lipid emulsion, especially in an ELBW infant. Discontinuation of the lipid infusion without alteration of the carbohydrate load will often eliminate hyperglycemia in this situation. Some ELBW infants remain hyperglycemic even on reduced carbohydrate intakes. These infants may benefit from a continuous insulin infusion to attain adequate caloric intake. Treatment varies, but the usual infant dose is 1 unit/kg/min. 32 Routine use of insulin to promote growth in the preterm infant is not advised because of side effects. 8
Hypoglycemia may result from an abrupt interruption of glucose infusion or excessive exogenous insulin administration. Manifestations of hypoglycemia include apnea, lethargy, jitteriness, and seizures. If these signs occur immediately after an interruption of the TPN infusion, an IV glucose infusion must be initiated at once, followed by close monitoring of the blood glucose to allow appropriate glucose administration. The glucose concentration of the infusate may usually be safely decreased by 5 g/dL every 12 hours. Blood glucose values should be monitored hourly until stable after each change.
AMINO ACID METABOLISM
Hyperammonemia may be seen in preterm infants given excessive protein loads. Hyperammonemia will occur also in an infant with a congenital metabolic disturbance, such as a urea cycle defect, when challenged with an amino acid load. Hyperammonemia may be manifested as somnolence, lethargy, seizures, and coma. Biochemical screening is necessary to identify this complication before symptoms appear.
Azotemia may occur before hyperammonemia, but blood urea nitrogen (BUN) elevation in the first week of life of a preterm infant is usually associated with dehydration and has not been a reliable marker of protein excess. 25 Therefore, although daily monitoring is common in the first week, rising BUN is not an indication by itself to decrease the protein load.
CHOLESTASIS
Infants receiving TPN for more than 2 weeks frequently develop cholestatic jaundice (direct bilirubin >2 mg/dL).19,95,98The risk appears greatest for the least mature infants and those receiving the longest period of TPN without enteral feeding. The cause appears to be multifactorial, including lack of bile flow stimulation, delayed enteral feedings, malnutrition, or inflammation after localized or generalized infection and may be influenced by the amino acid composition of the TPN. 110 Serum amino transferases often are normal early in the clinical course. Serum albumin and prealbumin levels usually remain normal. An abnormality in hepatic synthetic function or early rise in isoenzyme levels should lead the clinician to investigate other forms of liver disease. The differential of cholestatic jaundice includes the following:
• Bacterial sepsis
• Congenital viral infection
• Postpartum acquisition of cytomegalovirus
• Neonatal hepatitis
• Bile duct obstruction, such as biliary atresia or choledochal cyst
• Galactosemia
• Cystic fibrosis
• Alpha 1-antitrypsin deficiency
Management of cholestatic jaundice should include (when possible) the following:
• Increase enteral feedings as tolerated and decrease proportionately the parenteral nutrition
• Eliminate copper and manganese from trace minerals in TPN
• For infants with short bowel syndrome, control intestinal bacterial overgrowth47
LIPID METABOLISM
High-risk infants, including preterm and SGA low-birth-weight infants, may demonstrate intolerance to fat emulsion infusions. Hyperlipidemia may result, causing elevation of triglyceride, FFA, and lipoprotein levels. In extreme cases, lactescence may be visible in serum on a spun blood specimen (increased plasma turbidity). For screening, a triglyceride level should be checked after initiation of therapy and then weekly and doses adjusted based on results. Steroid therapy may elevate the triglyceride level. 85 Transient hyperglycemia may result from lipid infusion. This complication is usually dose related and rarely requires treatment. 23
Competitive displacement of bilirubin by FFA theoretically may increase the risk for kernicterus in preterm infants with hyperbilirubinemia. However, studies of preterm infants have indicated lipid infusions may be used in jaundiced infants but attention to the infusion rate and monitoring of FFAs are necessary. 80
PARENT TEACHING
In-Hospital Total Parenteral Nutrition
Clinicians caring for an ill newborn must be attentive to the involvement and emotional state of the parents. There remain a number of concerns for child abuse, foster placement, and relinquishment among infants who have been cared for in the NICU compared with healthy term newborns, especially when care has been prolonged and complex.
Clinical conditions or policies that promote separation of parents from their infant increase the risk for bonding problems. When a newborn infant cannot be fed orally, an important, normal part of the infant’s care is no longer available for the parents. The placement of a central line may be frightening to parents and result in less handling and caregiving . Infants requiring continuous care, including TPN, should have primary nursing (one regular nurse), and the parents should have regular and consistent communication with a primary physician. Care providers should attempt to keep the parents involved in other parts of the infant’s care, because the parents are unable to feed the infant. Parents should be fully informed about the purpose and appropriate care of the infant’s central line so they will feel comfortable handling their infant with the line in place.
A neonatal service that uses TPN has the best results if it includes an experienced “nutrition team,” comprising a neonatologist, surgeon, nutrition support nurse, pharmacist, dietitian, and social worker, with each member playing a vital role to make TPN a safe and effective therapy.
Home Total Parenteral Nutrition
Home parenteral nutrition has been used in infants with congenital intestinal anomalies or after massive bowel resection for NEC. TPN is initiated in the hospital. If growing and otherwise well, the infant may be a candidate for TPN at home. Issues to be addressed include ability and willingness of parents to care for the infant at home, available financial support, adequate home setting, pharmacy support services, and additional skilled nursing care needed. The infant should have a more permanent central line placed as early in the discharge process as possible. Parent teaching should begin early, including verbal and written instruction and hands-on practice and return demonstrations (see the Parent Teaching box below.)
Administration of TPN at home is different from hospital administration of TPN and is typically managed by a pediatric gastroenterology service in conjunction with a home infusion therapy or pharmacy service. Infants often go home on a cyclic TPN regimen (12 hr/day). An ambulatory pump improves the mobility and flexibility of the parent and infant and allows a more normal life.
Compliance and success with home TPN are greatly increased when the parents understand the need for and the appropriate way to administer TPN and how to troubleshoot and care for the catheter. 37
Home Administration of Parenteral Nutrition
• Strict handwashing and aseptic handling of tubing connections and hubs
• Use of infusion pump
• Monitoring of site for signs of infection, phlebitis, or leaking
• Troubleshooting for occlusion, leaking, extravasation
• Evaluation for signs of systemic infection
• Emergency response to broken or dislodged catheter, loss of electrical power
• Developmental care: oral stimulation, holding, appropriate play activities
• Dressing care and changes
• Monitoring for signs and symptoms of hypoglycemia
• Securing or taping of line to avoid dislodgement with positioning and handling
REFERENCES
1. Adamkin, D.D.; Radmacher, P.; Rosen, P., Comparison of a neonatal versus general-purpose amino acid formulation in preterm neonates, J Perinatol 15 (1995) 108.
2. Ainsworth, S.B.; Clerihew, L.; McGuire, W., Percutaneous central venous catheters versus peripheral cannulae for delivery of parenteral nutrition in neonates, Cochrane Database Syst Rev 2 (2004); CD004219, Accessed August 24, 2009, fromwww.nichd.nih.gov.
3. American Academy of Pediatrics, Committee on Nutrition, Aluminum toxicity in infants and children, Pediatrics 97 (1996) 413.
4. American Academy of Pediatrics, Committee on Nutrition, Parenteral nutrition, In: (Editor: Kleinman, R.E.) Pediatric nutrition handbooked 6 ( 2008)The Academy, Elk Grove Village, Ill.
5. American Academy of Pediatrics, Committee on Nutrition, Protein, In: (Editor: Kleinman, R.E.) Pediatric nutrition handbooked 6 ( 2008)The Academy, Elk Grove Village, Ill.
6. ASCN/ASPEN Working Group on Standards for Aluminum Content of Parenteral Nutrition Solutions, JPEN J Parenter Enteral Nutr 15 (1991) 194.
7. Avila-Figueroa, C.; Goldmann, D.A.; Richardson, D.C.; et al., Intravenous lipid emulsions are the major determinant of coagulase-negative staphylococcal bacteremia in very low birth weight newborns, Pediatr Infect Dis J 17 (1998) 10.
8. Beardsall, K.; Vanhaesebrouck, S.; Ogilvy-Stuart, A.L.; et al., Early insulin therapy in very-low-birth-weight infants, N Engl J Med 359 (2008) 1873.
9. Bendorf, K.; Friesen, C.A.; Roberts, C.C., Glucose response to discontinuation of parenteral nutrition in patients less than 3 years of age, JPEN J Parenter Enteral Nutr 20 (1996) 120.
10. Benjamin Jr, D.K.; Miller, W.; Garges, H.; et al., Bactere-mia, central catheters, and neonates: when to pull the line, Pediatrics 107 (2001) 1272.
11. Benjamin Jr, D.K.; Ross, K.; McKinney Jr, R.E.; et al., When to suspect fungal infection in neonates: a clinical comparison of Candida albicans and Candida parapsilosis fungemia with coagulase-negative staphylococcal bacteremia, Pediatrics 106 (2000) 712.
12. Bishop, N.J.; Morley, R.; Day, J.P.; et al., Aluminum neurotoxicity in preterm infants receiving intravenous-feeding solutions, N Engl J Med 336 (1997) 1557.
13. Borghesi, A.; Stronati, M., Strategies for the prevention of hospital-acquired infections in the neonatal intensive care unit, J Hosp Infect 68 (2008) 293.
14. Brion, L.P.; Bell, E.F.; Raghuveer, T.S., Vitamin E supplementation for prevention of morbidity and mortality in preterm infants, Cochrane Database Syst Rev 3 (2003); CD003665.
15. Cairns, P.A.; Stalker, D.J., Carnitine supplementation of parenterally fed neonates, Cochrane Database Syst Rev 4 (2004); CD000950.
16. Centers for Disease Control and Prevention, Guidelines for the prevention of intravascular catheter-related infections, MMWR Recomm Rep 51 (RR-10) ( 2002) 1.
17. Chen, C.Y.; Tsao, P.N.; Chen, H.L.; et al., Ursodeoxycholic acid (UDCA) therapy in very-low-birth-weight infants with parenteral nutrition associated cholestasis, J Pediatr 145 (2004) 317.
18. Chessex, P.; Harrison, A.; Khashu, M.; et al., In preterm neonates, is the risk of developing bronchopulmonary dysplasia influenced by the failure to protect total parenteral nutrition from exposure to ambient light?J Pediatr 151 (2007) 213.
19. Christensen, R.D.; Henry, E.; Wiedmeier, S.E.; et al., Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease, J Perinatol 27 (2007) 284.
20. Chryssanthou, E.; Broberger, U.; Petrini, B., Malassezia pachydermatis fungaemia in a neonatal intensive care unit, Acta Paediatr 90 (2001) 323.
21. Clark, R.H.; Thomas, P.; Peabody, J., Extrauterine growth restriction remains a serious problem in prematurely born neonates, Pediatrics 111 (2003) 986.
22. Coleman, M.M.; Spear, M.L.; Finkelstein, M.; et al., Short-term use of umbilical artery catheters may not be associated with increased risk for thrombosis, Pediatrics 113 (2004) 770.
23. Cooke, R.J.; Yeh, Y.Y.; Gibson, D.; et al., Soybean oil emulsion administration during parenteral nutrition in the preterm infant: effect of essential fatty acid, lipid, and glucose metabolism, J Pediatr 111 (1987) 767.
24. Dahl, G.B.; Svensson, L.; Kinnander, N.J.; et al., Stability of vitamins in soybean oil fat emulsion under conditions simulating intravenous feeding of neonates and children, JPEN J Parenter Enteral Nutr 18 (1994) 234.
25. Denne, S.C.; Poindexter, B.B., Evidence supporting early nutritional support with parenteral amino acid infusion, Semin Perinatol 31 (2007) 56.
26. Dinerstein, A.; Nieto, R.M.; Solana, C.L.; et al., Early and aggressive nutritional strategy (parenteral and enteral) decreases postnatal growth failure in very low birth weight infants, J Perinatol 26 (2006) 436.
27. Downing, G.J.; Egelhoff, J.C.; Daily, D.K.; et al., Kidney function in very low birth weight infants with furosemide-related renal calcifications at ages 1 to 2 years, J Pediatr 120 (1992) 599.
28. Dudrick, S.J., Early developments and clinical applications of total parenteral nutrition, J Parenter Entera Nutr 27 (2003) 291.
29. Ehrenkranz, R.A., Early, aggressive nutritional management for very low birth weight infants: what is the evidence?Semin Perinatol 31 (2007) 48.
30. Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; et al., Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants, Pediatrics 117 (2006) 1253.
31. Embleton, N.D., Optimal protein and energy intakes in preterm infants, Early Hum Dev 83 (2007) 831.
32. Farrag, H.M.; Cowett, R.M., Glucose homeostasis in the micropremie, Clin Perinatol 27 (2000) 1.
33. Farrell, P.M.; Gutcher, G.R.; Palta, M.; et al., Essential fatty acid deficiency in premature infants, Am J Clin Nutr 48 (1988) 220.
34. Fenton, T.R., A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format, BMC Pediatr 3 (2003) 13.
35. Fomon, S.J., Requirements and recommended dietary intake of protein during infancy, Pediatr Res 30 (1991) 391.
36. Furdon, S.A.; Horgan, M.J.; Bradshaw, W.T.; et al., Nurses’ guide to early detection of umbilical arterial catheter complications in infants, Adv Neonatal Care 6 (2006) 242.
37. Grant, J., Recognition, prevention, and treatment of home total parenteral nutrition central venous access complications, JPEN J Parenter Enteral Nutr 26 (suppl 5) ( 2002) S21.
38. Gura, K.M.; Duggan, C.P.; Collier, S.B.; et al., Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management, Pediatrics 118 (2006) e197.
39. Hanning, R.M.; Zlotkin, S.H., Amino acid and protein needs of the neonate: effects of excess and deficiency, Semin Perinatol 13 (1989) 131.
40. Heird, W.C., Amino acid and energy needs of pediatric patients receiving parenteral nutrition, Pediatr Clin North Am 42 (1995) 765.
41. Heird, W.C., Amino acids in pediatrics and neonatal nutrition, Curr Opin Clin Nutr Metab Care 1 (1998) 73.
42. Howard, D.; Thompson, D.F., Taurine: an essential amino acid to prevent cholestasis in neonates?Ann Pharmacother 26 (1992) 1390.
43. Hubbard, W., Aluminum in large and small volume parenterals used in total parenteral nutrition, Fed Reg 63 (1998) 176.
44. Infusion Nurses Society, Infusion nursing standards of practice, J Infus Nurs 29 (suppl 1) ( 2006) S1.
45. Jaeger, R.J.; Weiss, A.L.; Brown, K., Infusion of di-2-ethylhexylphthalate for neonates: a review of potential health risk, J Infus Nurs 28 (2005) 54.
46. Karlowicz, M.G.; Hashimoto, L.N.; Kelly, R.E.; et al., Should central venous catheters be removed as soon as candidemia is detected in neonates?Pediatrics 106 (2000) e63.
47. Kaufman, S.S., Prevention of parenteral nutrition-associated liver disease in children, Pediatr Transplant 6 (2002) 37.
48. Kerner, J.A.; Garcia-Carenga, M.G.; Fisher, A.A.; et al., Treatment of catheter occlusion in pediatric patients, JPEN J Parenter Enteral Nutr 30 (2006) S73.
49. Khashu, M.; Harrison, A.; Lalari, V.; et al., Photoprotection of parenteral nutrition enhances advancement of minimal enteral nutrition in preterm infants, Semin Perinatol 30 (2006) 139.
50. Khashu, M.; Harrison, A.; Lalari, V.; et al., Impact of shielding parenteral nutrition from light on routine monitoring of blood glucose and triglyceride in preterm neonates, Arch Dis Child Fetal Neonatal Ed 94 (2) ( 2009) F111.
51. Kilbride, H.W.; Powers, R.; Wirtschafter, D.D.; et al., Evaluation and development of potential better practices to prevent neonatal nosocomial bacteremia, Pediatrics 111 (2003) e504.
52. Kilbride, H.W.; Wirtschafter, D.D.; Powers, R.J.; et al., Implementation of evidence-based potentially better practices to decrease nosocomial infections, Pediatrics 111 (2003) e519.
53. Klein, G.L., Aluminum in parenteral solutions revisited-again, Am J Clin Nutr 61 (1995) 449.
54. Kline, A.M., Pediatric catheter-related bloodstream infections: latest strategies to decrease risk, AACN Clin Issues 16 (2005) 185.
55. Laborie, S.; Lavoie, J.C.; Pineault, M.; et al., Protecting solutions of parental nutrition from peroxidation, JPEN J Parenter Enteral Nutr 23 (1999) 104.
56. Lee, M.D.; Yoon, J.F.; Kim, S.I.; et al., Stability of total admixtures in reference to ambient temperatures, Nutrition 19 (2003) 886.
57. Lee, S.; Gura, K.M.; Kim, S.; et al., Current clinical applications of omega-6 and omega-3 fatty acids, Nutr Clin Pract 21 (2006) 323.
58. Lehmann, C.U.; Conner, K.G.; Cox, J.M., Preventing provider errors: online total parenteral nutrition calculator, Pediatrics 113 (2004) 748.
59. Leick-Rude, M.K.; Haney, B., Midline catheter use in the intensive care nursery, Neonatal Netw 25 (2006) 189.
60. Leitch, C.A.; Denne, S.C., Energy expenditure in the extremely low-birth weight infant, Clin Perinatol 27 (2000) 181.
61. Levine, A.; Maayan, A.; Shamir, R.; et al., Parenteral nutrition-associated cholestasis in preterm neonates: evaluation of ursodeoxycholic acid treatment, J Pediatr Endocrinol Metab 12 (1999) 549.
62. Lucas, A.; Morley, R.; Cole, T.J., Randomised trial of early diet in preterm babies and later intelligence quotient, BMJ 317 (1998) 1481.
63. Matlow, A.G.; Kitai, I.; Kirpalani, H.; et al., A randomized trial of 72- versus 24-hour intravenous tubing set changes in newborns receiving lipid therapy, Infect Control Hosp Epidemiol 20 (1999) 487.
64. McKinnon, B.T., FDA safety alert: hazards of precipitation associated with parenteral nutrition, Nutr Clin Pract 11 (1996) 59.
65. Mermel, L.A.; Farr, B.M.; Sherertz, R.J.; et al., Guidelines for the management of intravascular catheter-related infections, Infect Control Hosp Epidemiol 22 (2001) 222.
66. Meyer, M.P.; Haworth, C.; Meyer, J.H.; et al., A comparison of oral and intravenous iron supplementation in preterm infants receiving recombinant erythropoietin, J Pediatr 129 (1996) 258.
67. Mitsufuji, N.; Matsuo, K.; Kakita, S.; et al., Extravascular collection of fluid around the vertebra resulting from malpositioning of a peripherally inserted central venous catheter in extremely low birth weight infants, J Perinatal Med 30 (2002) 341.
68. Murphy, B.P.; Inder, T.E.; Huppi, P.S.; et al., Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease, Pediatrics 107 (2001) 217.
69. Nadroo, A.M.; Lin, J.; Green, R.S.; et al., Death as a complication of peripherally inserted central catheters in neonates, J Pediatr 138 (2001) 599.
70. Nedergaard, J.; Cannon, B., Brown adipose tissue: development and function, In: (Editors: Polin, R.A.; Fox, W.W.; Abman, S.) Fetal and neonatal physiologyed 3 ( 2003)Saunders, Philadelphia.
71. Pak, V.M.; Nailon, R.E.; McCauley, L.A., Controversy: neonatal exposure to plasticizers in the NICU, MCN Am J Matern Child Nurs 32 (2007) 244.
72. Paulson, P.R.; Miller, K.M., Neonatal peripherally inserted central catheters: recommendations for prevention of insertion and postinsertion complications, Neonatal Netw 27 (2008) 245.
73. Pelegano, J.F.; Rowe, J.C.; Carey, D.E.; et al., Simultaneous infusion of calcium and phosphorus in parenteral nutrition for premature infants: use of physiologic calcium/phosphorus ratio, J Pediatr 114 (1989) 115.
74. Pettit, J., Assessment of infants with peripherally inserted central catheters: Part 1. Detecting the most frequently occurring complications, Adv Neonatal Care 2 (2002) 304.
75. Pettit, J., Assessment of infants with peripherally inserted central catheters: Part 2. Detecting less frequently occurring complications, Adv Neonatal Care 3 (2003) 14.
76. Pettit, J., Technological advances for PICC placement and management, Adv Neonatal Care 7 (2007) 122.
77. Pierro, A.; Eaton, S., Metabolism and nutrition in the surgical neonate, Semin Pediatr Surg 17 (2008) 276.
78. Poindexter, B.B.; Ehrenkranz, R.A.; Stoll, B.J.; et al., Parenteral glutamine supplementation does not reduce the risk of mortality or late-onset sepsis in extremely low birth weight infants, Pediatrics 113 (2004) 1209.
79. Premji, S.; Fenton, T.; Sauve, R., Does amount of protein in formula matter for low-birthweight infants?JPEN J Parenter Enteral Nutr 30 (2006) 507.
80. Putet, G., Lipid metabolism of the micropremie, Clin Perinatol 27 (2000) 57.
81. Reynolds, R.M.; Bas, K.D.; Thureen, P.J., Achieving positive protein balance in the immediate postoperative period in neonates undergoing abdominal surgery, J Pediatr 152 (2008) 63.
82. Roberts, S.A.; Ball, R.O.; Moore, A.M.; et al., The effect of graded intake of glycly-L-tyrosine on phenylalanine and tyrosine metabolism in parenterally fed neonates with an estimation of tyrosine requirement, Pediatr Res 49 (2001) 111.
83. Rollins, C.J., Total nutrient admixtures: stability issues and their impact on nursing practice, J IV Nurs 20 (1997) 299.
84. Rowe, M.I.; Rowe, S.A., The last fifty years of neonatal surgical management, Am J Surg 180 (2000) 345.
85. Sentipal-Walerius, J.; Dollberg, S.; Mimouni, F.; et al., Effect of pulsed dexamethasone therapy on tolerance of intravenously administered lipids in extremely low birth weight infants, J Pediatr 134 (1999) 229.
86. Shah, P.S.; Kalyn, A.; Satodia, P.; et al., A randomized, controlled trial of heparin versus placebo infusion to prolong the usability of peripherally placed percutaneous central venous catheters (PCVCs) in neonates: the HIP (Heparin Infusion for PCVC) study, Pediatrics 119 (2007) e284.
87. Sharpe, E.L., Tiny patients, tiny dressings: a guide to the neonatal PICC dressing change, Adv Neonatal Care 8 (2008) 150.
88. Shulman, R.J., Zinc and copper balance studies in infants receiving total parenteral nutrition, Am J Clin Nutr 49 (1989) 879.
89. Shulman, R.J.; Phillips, S., Parenteral nutrition in infants and children, J Pediatr Gastroenterol Nutr 36 (2003) 587.
90. Sizun, J.; Karangwa, A.; Giroux, J.D.; et al., Malassezia furfur-related colonization and infection of central venous catheters: a prospective study in a pediatric intensive care unit, Intensive Care Med 20 (1994) 496.
91. So, K-W; Ng, P-C, Treatment and prevention of neonatal osteopenia, Curr Paediatr 15 (2005) 106.
92. Sogheir, L.M.; Brion, L.P., Cysteine, cystine or N-acetylcysteine supplementation in parenterally fed neonates, Cochrane Database Syst Rev 4 (2006); CD004869.
93. Spencer, A.U.; Yu, S.; Tracy, T.F.; et al., Parenteral nutrition–associated cholestasis in neonates: multivariate analysis of the potential protective effect of taurine, JPEN J Parenter Enteral Nutr 29 (2005) 337.
94. Stark, A.R.; Carlo, W.A.; Tyson, J.E.; et al., Adverse effects of early dexamethasone in extremely-low-birth-weight infants: National Institute of Child Health and Human Development Neonatal Research Network, N Engl J Med 344 (2001) 95.
95. Steinbach, M.; Clark, R.H.; Kelleher, A.S.; , for the Pediatrix Amino-Acid Study Group, Demographic and nutritional factors associated with prolonged cholestatic jaundice in the premature infant, J Perinatol 28 (2008) 129.
96. Sunehag, A.; Gustafsson, J.; Ewald, U., Very immature infants (<30 wk) respond to glucose infusion with incomplete suppression of glucose production, Pediatr Res 36 (1994) 550.
97. Te Braake, F.WJ.; Van Den Akker, C.HP.; Wattimena, D.JL.; et al., Amino acid administration to premature infants directly after birth, J Pediatr 147 (2005) 457.
98. Teitelbaum, D.H.; Tracy, T., Parenteral nutrition-associated cholestasis, Semin Pediatr Surg 10 (2001) 72.
99. Thureen, P.J.; Anderson, A.H.; Baron, K.A.; et al., Protein balance in the first week of life in ventilated neonates receiving parenteral nutrition, Am J Clin Nutr 68 (1998) 1128.
100. Thureen, P.J.; Hay Jr, W.W., Intravenous nutrition and postnatal growth of the micropremie, Clin Perinatol 27 (2000) 197.
101. Thureen, P.; Heird, W.C., Protein and energy requirements of the preterm/low birthweight (LBW) infant, Pediatr Res 95R (2005) 57.
102. Torine, I.J.; Denne, S.C.; Wright-Coltart, S.; et al., Effect of late-onset sepsis on energy expenditure in extremely premature infants, Pediatr Res 61 (2007) 600.
103. Torrence, C.R.; Horns, K.M.; East, C., Accuracy and precision of neonatal electronic incubator scales, Neonatal Netw 14 (1995) 35.
104. In: (Editor: Trissel, L.A.) Handbook on injectable drugsed 12 ( 2003)American Society of Health-System Pharmacists, Bethesda, Md.
105. Tyson, J.E.; Wright, L.L.; Oh, W.; et al., Vitamin A supplementation for extremely-low-birth-weight infants, N Engl J Med 340 (1999) 1962.
106. Uauy, R.; Hoffman, D.R., Essential fat requirements of preterm infants, Am J Clin Nutr 71 (suppl) ( 2000) S245.
107. Verner, A.; Craig, S.; McGuire, W., Effect of taurine supplementation on growth and development in preterm or low birth weight infants, Cochrane Database Syst Rev 4 (2007); CD006072.
108. Viña, J.; Vento, M.; Garcia-Sala, F.; et al., L-Cysteine and glutathione metabolism are impaired in premature infants due to cystathionase deficiency, Am J Clin Nutr 61 (1995) 1067.
109. Williford, A.L.; Pare, L.M.; Carlson, G.T., Bone mineral metabolism in the neonate: calcium, phosphorus, magnesium, and alkaline phosphate, Neonatal Netw 27 (2008) 57.
110. Wright, K.; Ernst, K.D.; Gaylord, M.S.; et al., Increased incidence of parenteral nutrition-associated cholestasis with Aminosyn PF compared to TrophAmine, J Perinatol 23 (2003) 444.
111. Zlotkin, S.H.; Atkinson, S.; Lockitch, G., Trace elements in nutrition for premature infants, Clin Perinatol 22 (1995) 223.




