Chapter 42 Thyroid and Antithyroid Drugs
| Abbreviations | |
|---|---|
| cAMP | Cyclic adenosine monophosphate |
| D1 | Iodothyronine deiodinase type 1 |
| D2 | Iodothyronine deiodinase type 2 |
| D3 | Iodothyronine deiodinase type 3 |
| DIT | Diiodotyrosine |
| MIT | Monoiodotyrosine |
| NADPH | Reduced nicotinamide adenine dinucleotide phosphate |
| PTU | Propylthiouracil |
| rT3 | Reverse T3 |
| T3 | L isomer of triiodothyronine |
| T4 | L isomer of thyroxine, tetraiodothyronine |
| TBPA | Transthyretin, thyroxine-binding prealbumin |
| TBG | Thyroxine-binding globulin |
| Tg | Thyroglobulin |
| TR | Thyroid hormone receptor |
| TRH | Thyrotropin-releasing hormone |
| TSH | Thyroid-stimulating hormone, thyrotropin |
Therapeutic Overview
| Therapeutic Overview |
|---|
| Hypothyroidism |
| Replacement therapy with synthetic thyroxine (T4) |
| Hyperthyroidism |
| Thioureylene drugs |
| β Adrenergic receptor antagonists |
| Glucocorticoids |
| Radioactive iodine |
| Surgery |
Mechanisms of Action
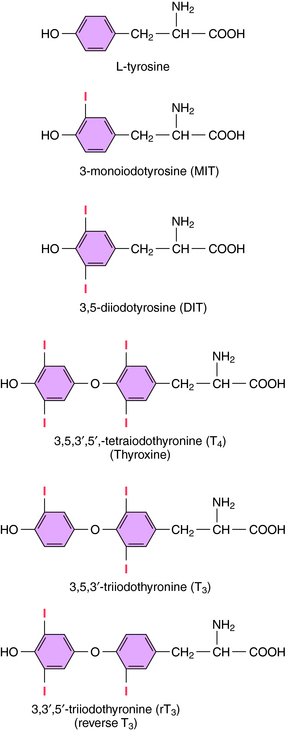
The iodinated compounds in thyroid follicular cells (thyrocytes) are derived by enzymatic condensation of the iodinated tyrosyl residues in thyroglobulin (Fig. 42-1). Among these are the two major thyroid hormones, L-isomers of T4 and T3. All of the T4 that is made is derived from the thyroid. In contrast, only a small fraction of the most biologically active form, T3, is produced and released from the thyroid. Most T3 is produced by peripheral tissues by removing an iodide from the outer ring of T4 through the action of 5’-deiodinase.
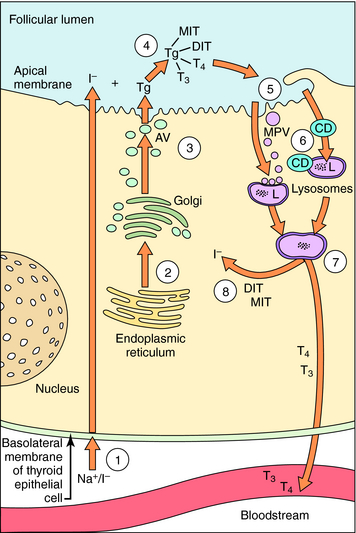
The synthesis and release of T3 and T4 are shown in Figure 42-2. Thyrocytes concentrate iodide from the circulation via a symporter present on their basolateral surface that admits Na+ down its electrochemical gradient. This sodium/iodide symporter, which is also present in salivary glands, breast, and stomach, can transport other anions, such as pertechnetate and perchlorate, which can competitively inhibit iodide transport. Once iodide enters the thyrocyte, it is transported to the follicular lumen by an anion transporter in the apical membrane termed pendrin. As iodide reaches the follicular lumen, it is oxidized by a mechanism involving thyroperoxidase, H2O2, and two reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidases. Activated iodide forms covalent links with specific tyrosyl residues on thyroglobulin (Tg), which is present in the follicular lumen, producing the thyroid hormone precursors monoiodotyrosine (MIT) and diiodotyrosine (DIT). This process is referred to as organification of iodide. MIT or DIT can donate their iodinated phenolic rings to acceptor iodotyrosyl residues on DIT in the Tg backbone and form an ether linkage with the acceptor phenolic ring to form T4 and T3 covalently bound to Tg. This process is called coupling. Iodinated Tg is transported to and stored associated with the apical membrane. When thyroid hormone is required, iodinated Tg is transported into follicular cells by endocytosis, where it undergoes proteolysis, releasing T4 and T3 into the circulation. MIT and DIT are deiodinated and reused.
Hormone synthesis in thyrocytes is regulated by TSH released from the pituitary. If the circulating level of thyroid hormone is abnormally elevated, pituitary sensitivity to thyrotropin-releasing hormone (TRH) is reduced, causing decreased production of TSH. Pituitary portal blood also carries counter-regulatory compounds that inhibit TSH release (i.e., dopamine and somatostatin) (see Chapter 38).
Thyroid hormones exert their major effects by binding to thyroid hormone receptors (TRs), members of the nuclear receptor superfamily (see Chapter 1). Two genes encode TRs, and each can be transcribed into alternatively spliced products. The relative proportions of each isoform expressed are developmentally dependent and tissue specific. TRs can homodimerize but are generally present as heterodimers, most commonly with the receptor for 9-cis-retinoic acid but also with other nuclear hormone receptors. TRs associate with DNA as part of a complex of transcription factors, even in the absence of thyroid hormone. When T3 binds to its receptor, the interactions of the receptor with transcription corepressors and coactivators change, and local chromatin structure is modified by changes in histone acetylation. Binding of T3 increases transcription of some genes and decreases the transcription of others. Thyroid hormones also regulate the processing of ribonucleic acid transcripts and the stability of specific messenger ribonucleic acids, and have other non-nuclear actions. Another potential regulatory process is the expression of the TR splice variant (TRα2), which interacts with thyroid hormone response elements but is not activated by T3. Consequently, expression of TRα2 can reduce sensitivity to thyroid hormone.
As shown in Table 42-1, antithyroid drugs can inhibit the synthesis, release, and metabolism of thyroid hormone and alter its peripheral effects.
| Compound | Mechanism of Action |
|---|---|
| Perchlorate | Inhibition of iodide transport |
| Thioureylenes | Inhibition of organification and coupling |
| Iodide, lithium | Inhibition of deiodination of T4 to T3 |
| Inhibition of hormone release | |
| β Adrenergic receptor blockers | Antagonize hypersensitivity for circulating catecholamines |
| Glucocorticoids | Treat potential adrenal crisis |
| Iopanoate (radiographic contrast agent) | Causes rapid decrease in serum T4 and T3 |
Drugs that Inhibit Thyroid Hormone Production
As depicted in Figure 42-2, thyroid hormone synthesis involves several processes including uptake, organification, and coupling of iodide, each of which can be inhibited. Perchlorate decreases thyroid hormone production by competing with iodide for the sodium/iodide symporter. Although perchlorate can be used briefly as a clinical antithyroid agent, cases of aplastic anemia have limited its usefulness. A single dose of perchlorate is used occasionally as a diagnostic agent after administration of a tracer dose of radioactive iodine to determine whether a defect exists in a patient’s ability to organify iodide.
Lithium, an element used for the treatment of bipolar (manic-depressive) disorder (see Chapter 30), suppresses the release of thyroid hormone, and when used chronically can lead to hypothyroidism and TSH-induced nontoxic goiters.
Drugs that Affect the Action of Thyroid Hormones
Some symptoms of hyperthyroidism, such as tachycardia, mimic overactivity of the sympathetic nervous system. This phenomenon is related to thyroid hormone-induced increased density of β adrenergic receptors, expression of G-protein subunits, cyclic adenosine monophosphate (cAMP) levels, and expression of proteins that are both T3-responsive and cAMP-responsive, such as uncoupling protein 1, which is involved in thermogenesis. In addition, activation of β adrenergic receptors apparently facilitates the conversion of T4 to T3. Consequently, β adrenergic receptor-blocking drugs can be used to reduce the clinical symptoms of hyperthyroidism such as tremor and tachycardia and are used as adjunct therapy.
Pharmacokinetics
The pharmacokinetic parameters for thyroid hormones and representative antithyroid drugs are listed in Table 42-2.
The absorption of orally administered T3 is virtually complete with a t1/2 of 24 hours in euthyroid subjects. Thus blood levels rise and fall appreciably after each dose. In contrast, oral absorption of T4 is incomplete and variable. Because T4 has a t1/2 of approximately 7 days in euthyroid subjects, its blood levels do not display substantial variations after a daily dose. Oral absorption of T4 can be impeded by several compounds, including dietary constituents such as ferrous sulfate, Ca++, and soy flour. Conjugated thyroid hormone metabolites are secreted in the bile, and there is substantial enterohepatic recirculation, which can be blocked by ingestion of drugs like cholestyramine (Chapter 25).
T4 is metabolized peripherally primarily by deiodination. Removal of an iodide from the outer ring of T4 produces T3, which is more biologically active than T4. However, removing an iodide from the inner ring of T4 (see Fig. 42-1) produces reverse T3 (rT3), which is biologically inactive. Similarly, removing an iodide from either ring inactivates T3.
Three iodothyronine deiodinases, which are intrinsic membrane selenoproteins, remove iodide from thyroid hormones. Deiodinase type 1 (D1) removes iodide from both rings, type 2 (D2) selectively deiodinates the outer ring, whereas type 3 (D3) selectively deiodinates the inner ring. D1 is most active in removing iodide from the inner ring of T3 sulfate and less active on T4; it is even less active in removing iodide from the outer ring of T3. D1 is the major deiodinase present in liver, kidney, and thyroid and is regulated by thyroid hormone levels in some tissues. D2 is the “activating” enzyme, selectively deiodinating the outer ring of T4, converting it to T3. D2 is the major enzyme in brown fat, heart, skeletal muscle, pituitary, and pineal, and thyroid hormone levels and adrenergic agents regulate D2 in some tissues. D3 deiodinates the inner ring of iodothyronines selectively, inactivating both T4 and T3. It is the major isoform in brain, fetal liver, and placenta.
Many factors influence the metabolism of thyroid hormones. Prolonged fasting reduces peripheral conversion of T4 to T3 by half while doubling the amount of T4 converted to rT3. The composition of a patient’s diet or nonthyroidal illness can also influence metabolism of thyroid hormones. Turnover of thyroid hormones is increased in hyperthyroidism and is slowed in hypothyroidism. Of the drugs that affect thyroid hormone metabolism, the iodine-rich antiarrhythmic agent amiodarone is the most egregious (Chapter 22). Amiodarone inhibits 5′-deiodinase activity, thus increasing serum T4 and rT3 levels while decreasing the level of T3. However, amiodarone has direct effects on the thyroid, promoting thyroiditis, and can cause hyperthyroidism or hypothyroidism as it releases iodide. One tablet of amiodarone contains 75 mg iodine.
Relationship of Mechanisms of Action to Clinical Response
Maintenance of a hypothyroid patient is most commonly accomplished with T4, which is the primary circulating form of thyroid hormone and has a duration of action that allows daily administration. Other commercially available forms include T3, T4 plus T3, desiccated thyroglobulin, and thyroid extract. To treat most hypothyroid patients, the thyroid hormone dose is increased gradually while the patient’s symptoms and serum levels of TSH are monitored. Because T4 has a long t1/2 in euthyroid individuals, which is even longer in hypothyroidism, it can take several months to establish the appropriate replacement dose for an individual patient. If a patient has a functioning remnant of thyroid tissue initially, that remnant can either hypertrophy or atrophy, affecting the replacement dose. In addition, the dose will change if drugs that alter thyroid hormone absorption or metabolism are prescribed or discontinued. Thus symptoms and TSH levels should be periodically monitored in hypothyroid patients.
Anonymous. Drugs for hypothyroidism and hyperthyroidism. Treat Guidel Med Lett. 2006;4:17-24.
Anonymous. Generic levothyroxine. Med Lett. 2004;46:77-78.
Anonymous. Potassium iodide for thyroid protection in a nuclear accident or attack. Med Lett. 2002;44:97-98.
Cooper D. Antithyroid drugs. N Engl J Med. 2005;352:905-917.
Duncan Bassett JH, Harvey CB, Williams GR. Mechanisms of thyroid hormone receptor-specific nuclear and extra nuclear actions. Mol Cell Endocrinol. 2003;213:1-11.
Shi etal 2002 Shi YB, Ritchie JWA, Taylor PM. Complex regulation of thyroid hormone action: Multiple opportunities for pharmacological intervention. Pharmacol Ther. 2002;94:235-251.