Muscles of the pectoral region
Movements of the thoracic wall and diaphragm during breathing
 Image Library—illustrations of thoracic anatomy, Chapter 3
Image Library—illustrations of thoracic anatomy, Chapter 3
 Self-Assessment (scored)—National Board style multiple-choice questions, Chapter 3
Self-Assessment (scored)—National Board style multiple-choice questions, Chapter 3
 Short Questions (not scored)—National Board style multiple-choice questions, Chapter 3
Short Questions (not scored)—National Board style multiple-choice questions, Chapter 3
 Interactive Surface Anatomy—interactive surface animations, Chapter 3
Interactive Surface Anatomy—interactive surface animations, Chapter 3
Regional anatomy
The thorax is an irregularly shaped cylinder with a narrow opening (superior thoracic aperture) superiorly and a relatively large opening (inferior thoracic aperture) inferiorly (Fig. 3.1). The thorax consists of:
Fig. 3.1 Thoracic wall and cavity.
The thorax:
 houses and protects the heart, lungs, and great vessels,
houses and protects the heart, lungs, and great vessels,
 acts as a conduit for structures passing between the neck and the abdomen,
acts as a conduit for structures passing between the neck and the abdomen,
 plays a principal role in breathing, and
plays a principal role in breathing, and
 provides support for the upper limbs.
provides support for the upper limbs.
The thorax also provides support for the upper limb. Muscles anchored to the anterior thoracic wall provide some of this support, and together with their associated connective tissues, nerves, and vessels, and the overlying skin and superficial fascia, define the pectoral region.
PECTORAL REGION
The pectoral region is external to the anterior thoracic wall and anchors the upper limb to the trunk. It consists of:
 a superficial compartment containing skin, superficial fascia, and breasts; and
a superficial compartment containing skin, superficial fascia, and breasts; and
 a deep compartment containing muscles and associated structures.
a deep compartment containing muscles and associated structures.
Breast
The breasts consist of mammary glands, associated skin, and connective tissues. The mammary glands are modified sweat glands in the superficial fascia anterior to the pectoral muscles and the anterior thoracic wall (Fig. 3.2).
Fig. 3.2 Breasts.
The mammary glands consist of ducts and associated secretory lobules. These converge, forming 15 to 20 lactiferous ducts, which open independently onto the nipple. The nipple is surrounded by a circular pigmented area of skin, the areola (Fig. 3.2).
A well-developed, connective tissue stroma surrounds the ducts and lobules of the mammary gland. In certain regions, this stroma condenses, forming well-defined ligaments, the suspensory ligaments of breast, which are continuous with the dermis of the skin and support the breast.
In nonlactating women, the predominant component of the breasts is fat, whereas glandular tissue is more abundant in lactating women.
The breast lies on the deep fascia of the pectoralis major muscle and other surrounding muscles. A layer of loose connective tissue (the retromammary space) separates the breast from the deep fascia and provides some degree of movement over underlying structures.
Clinical app
Axillary process of breast
It is important for clinicians to remember when evaluating the breast for pathology that the upper lateral region of the breast can project around the lateral margin of the pectoralis major muscle and into the axilla. This axillary process (axillary tail) may perforate deep fascia and extend as far superiorly as the apex of the axilla (Fig. 3.2).
Arterial supply
The breast is related to the thoracic wall and to structures associated with the upper limb; therefore, vascular supply and drainage can occur by multiple routes (Fig. 3.2):
 medially, branches from the internal thoracic artery; and
medially, branches from the internal thoracic artery; and
Venous drainage
Veins draining the breast parallel the arteries and ultimately drain into the axillary, internal thoracic, and intercostal veins.
Innervation
Innervation of the breast is via anterior and lateral cutaneous branches of the second to sixth intercostal nerves. The nipple is innervated by the fourth intercostal nerve.
Lymphatic drainage
Lymphatic drainage of the breast is as follows:
 Approximately 75% is via lymphatic vessels that drain laterally and superiorly into axillary nodes (see Fig. 3.2).
Approximately 75% is via lymphatic vessels that drain laterally and superiorly into axillary nodes (see Fig. 3.2).
Axillary nodes drain into the subclavian trunks, parasternal nodes drain into the bronchomediastinal trunks, and intercostal nodes drain either into the thoracic duct or into the bronchomediastinal trunks.
Breast in men
The breast in men is rudimentary and consists only of small ducts, often composed of cords of cells, which normally do not extend beyond the areola. Breast cancer can occur in men.
Clinical app
Breast cancer
Breast cancer is one of the most common malignancies in women. Breast cancer develops in the cells of the acini, lactiferous ducts, and lobules of the breast. Tumor growth and spread depend on the exact cellular site of origin of the cancer. Breast tumors spread via the lymphatics and veins or by direct invasion.
Subcutaneous lymphatic obstruction and tumor growth pull on connective tissue ligaments, the suspensory ligaments, in the breast resulting in the appearance of an orange peel texture (peau d’orange) on the surface of the breast. Further subcutaneous spread can induce a rare manifestation of breast cancer that produces a hard, woody texture to the skin (cancer en cuirasse).
Surface anatomy
The breast in women
Although breasts vary in size, they are normally positioned on the thoracic wall between ribs II and VI and overlie the pectoralis major muscles. Each mammary gland extends superolaterally around the lower margin of the pectoralis major muscle and enters the axilla (Fig. 3.3). This portion of the gland is the axillary tail or axillary process. The positions of the nipple and areola vary relative to the chest wall depending on breast size.
Muscles of the pectoral region
Each pectoral region contains the pectoralis major, pectoralis minor, and subclavius muscles (Table 3.1, Fig. 3.4). All originate from the anterior thoracic wall and insert into bones of the upper limb.
Table 3.1 Muscles of the pectoral region
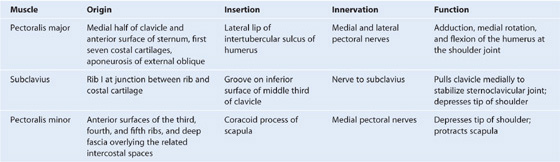
Fig. 3.4 Muscles and fascia of the pectoral region.
A continuous layer of deep fascia, clavipectoral fascia, encloses the subclavius and pectoralis minor and attaches to the clavicle above and to the floor of the axilla below.
The muscles of the pectoral region form the anterior wall of the axilla, a region between the upper limb and the neck through which all major structures pass.
THORACIC WALL
The thoracic wall consists of skeletal elements and muscles (see Fig. 3.1):
 Posteriorly, it is made up of 12 thoracic vertebrae and their intervening intervertebral discs.
Posteriorly, it is made up of 12 thoracic vertebrae and their intervening intervertebral discs.
The thorax wall extends between:
 the superior thoracic aperture bordered by vertebra TI, rib I, and the manubrium of sternum; and
the superior thoracic aperture bordered by vertebra TI, rib I, and the manubrium of sternum; and
Superior thoracic aperture
The superior thoracic aperture (Fig. 3.5; also see Fig. 3.1) consists of:
 the body of vertebra TI posteriorly,
the body of vertebra TI posteriorly,
 the medial margin of rib I on each side, and
the medial margin of rib I on each side, and
Fig. 3.5 Superior thoracic aperture.
The superior margin of the manubrium is in approximately the same horizontal plane as the intervertebral disc between vertebrae TII and TIII.
The first ribs slope inferiorly from their posterior articulation with vertebra TI to their anterior attachment to the manubrium. Consequently, the plane of the superior thoracic aperture is at an oblique angle, facing somewhat anteriorly (Fig. 3.5).
At the superior thoracic aperture, the superior aspects of the pleural cavities, which surround the lungs, lie on either side of the entrance to the mediastinum (Fig. 3.6).
Fig. 3.6 Superior thoracic aperture and axillary inlet.
Structures that pass between the upper limb and thorax pass over rib I and the superior part of the pleural cavity as they enter and leave the mediastinum (Fig. 3.6). Structures that pass between the neck and head and the thorax pass more vertically through the superior thoracic aperture (Fig. 3.5).
Inferior thoracic aperture
The inferior thoracic aperture is large and expandable, and bone, cartilage, and ligaments form its margin (Fig. 3.7). It is closed by the diaphragm (3.7B), and structures passing between the abdomen and thorax pierce or pass posteriorly to this structure. Skeletal elements of the inferior thoracic aperture are:
 the body of vertebra TXII posteriorly,
the body of vertebra TXII posteriorly,
 rib XII and the distal end of rib XI posterolaterally,
rib XII and the distal end of rib XI posterolaterally,
 the xiphoid process anteriorly.
the xiphoid process anteriorly.
Fig. 3.7 A. Inferior thoracic aperture. B. Diaphragm.
Clinical app
Thoracic outlet syndrome
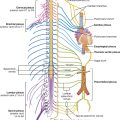
Clinically, thoracic outlet syndrome is used to describe symptoms resulting from abnormal compression of the brachial plexus of nerves as it passes over the first rib and through the axillary inlet into the upper limb. The anterior ramus of T1 passes superiorly out of the superior thoracic aperture to join and become part of the brachial plexus. A connective tissue band that can extend from the tip of a cervical rib to rib I is one cause of thoracic outlet syndrome by putting upward stresses on the lower parts of the brachial plexus as they pass over the first rib.
Joints between the costal margin and sternum lie roughly in the same horizontal plane as the intervertebral disc between vertebrae TIX and TX. Thus, the posterior margin of the inferior thoracic aperture is inferior to the anterior margin.
Skeletal framework
The skeletal elements of the thoracic wall consist of thoracic vertebrae, intervertebral discs, ribs, and sternum.
Thoracic vertebrae
There are 12 thoracic vertebrae, each of which is characterized by articulations with the ribs.
A typical thoracic vertebra
A typical thoracic vertebra has a heart-shaped vertebral body, with roughly equal dimensions in the transverse and anteroposterior directions, and a long spinous process (Fig. 3.8). The vertebral foramen is generally circular and the laminae are broad and overlap with those of the vertebra below. The superior articular processes are flat, with their articular surfaces facing almost directly posteriorly, whereas the inferior articular processes project from the laminae and their articular facets face anteriorly. The transverse processes are club shaped and project posterolaterally.
Fig. 3.8 Typical thoracic vertebra.
Articulation with ribs
A typical thoracic vertebra has three sites for articulations with ribs on each side (Fig. 3.8).
Not all vertebrae articulate with ribs in the same fashion (Fig. 3.9):
Fig. 3.9 Thoracic vertebrae.
Ribs
There are 12 pairs of ribs, each terminating anteriorly in a costal cartilage (Fig. 3.10).
Fig. 3.10 Ribs.
Although all ribs articulate with the vertebral column, only the costal cartilages of the upper seven ribs, known as true ribs, articulate directly with the sternum. The remaining five pairs of ribs are false ribs:
A typical rib consists of a curved shaft with anterior and posterior ends (Fig. 3.11). The anterior end is continuous with its costal cartilage. The posterior end articulates with the vertebral column and is characterized by a head, neck, and tubercle.
Fig. 3.11 A typical rib. A. Anterior view. B. Posterior view of proximal end of rib.
The head is somewhat expanded and typically presents two articular surfaces separated by a crest. The smaller superior surface articulates with the inferior costal facet on the body of the vertebra above, whereas the larger inferior facet articulates with the superior costal facet of its own vertebra.
The neck is a short flat region of bone that separates the head from the tubercle.
The tubercle projects posteriorly from the junction of the neck with the shaft and consists of two regions, an articular part and a nonarticular part:
 The raised nonarticular part is roughened by ligament attachments.
The raised nonarticular part is roughened by ligament attachments.
The shaft is generally thin and flat with internal and external surfaces.
The superior margin is smooth and rounded, whereas the inferior margin is sharp. The shaft bends forward just laterally to the tubercle at a site termed the angle. It also has a gentle twist around its longitudinal axis so that the external surface of the anterior part of the shaft faces somewhat superiorly relative to the posterior part. The inferior margin of the internal surface is marked by a distinct costal groove.
Distinct features of upper and lower ribs
The upper and lower ribs have distinct features (Fig. 3.12).
Fig. 3.12 Atypical ribs.
Rib I is flat in the horizontal plane and has broad superior and inferior surfaces. From its articulation with vertebra TI, it slopes inferiorly to its attachment to the manubrium of sternum. The head articulates with the body of vertebra TI and has only one articular surface. The tubercle has a facet for articulation with the transverse process. The superior surface of the rib is characterized by a distinct tubercle, the scalene tubercle, which separates two smooth grooves that cross the rib approximately midway along the shaft. The anterior groove is caused by the subclavian vein, and the posterior groove is caused by the subclavian artery. Anterior and posterior to these grooves, the shaft is roughened by muscle and ligament attachments.
Rib II, like rib I, is flat but twice as long. It articulates with the vertebral column in a way typical of most ribs.
The head of rib X has a single facet for articulation with its own vertebra.
Ribs XI and XII articulate only with the bodies of their own vertebrae and have no tubercles or necks. Both ribs are short, have little curve, and are pointed anteriorly.
Clinical apps
Cervical ribs
Cervical ribs are present in approximately 1% of the population.
A cervical rib is an accessory rib articulating with vertebra CVII; the anterior end attaches to the superior border of the anterior aspect of rib I.
Plain radiographs may demonstrate cervical ribs as small hornlike structures.
It is often not appreciated by clinicians that a fibrous band commonly extends from the anterior tip of the small cervical ribs to rib I, producing a “cervical band” that is not visualized on radiography. In patients with cervical ribs and cervical bands, structures that normally pass over rib I are elevated by, and pass over, the cervical rib and band.
Clinical apps
Rib fractures
Single rib fractures are of little consequence, though extremely painful.
After severe trauma, ribs may be broken in two or more places. If enough ribs are broken, a loose segment of chest wall, a flail segment (flail chest), is produced. When the patient takes a deep inspiration, the flail segment moves in the opposite direction to the chest wall, preventing full lung expansion and creating a paradoxically moving segment. If a large enough segment of chest wall is affected, ventilation may be impaired and assisted ventilation may be required until the ribs have healed.
Sternum
The adult sternum consists of three major elements: the broad and superiorly positioned manubrium of sternum, the narrow and longitudinally oriented body of sternum, and the small and inferiorly positioned xiphoid process (Fig. 3.13).
Fig. 3.13 Sternum.
Manubrium of sternum
The manubrium of sternum forms part of the bony framework of the neck and the thorax.
The superior surface of the manubrium is expanded laterally and bears a distinct and palpable notch, the jugular notch (suprasternal notch), in the midline (Fig. 3.13). On either side of this notch is a large oval fossa for articulation with the clavicle. Immediately inferior to this fossa, on each lateral surface of the manubrium, is a facet for the attachment of the first costal cartilage. At the lower end of the lateral border is a demifacet for articulation with the upper half of the anterior end of the second costal cartilage.
Body of the sternum
The body of the sternum is flat (Fig. 3.13).
The anterior surface of the body of the sternum is often marked by transverse ridges that represent lines of fusion between the segmental elements called sternebrae, from which this part of the sternum arises embryologically.
The lateral margins of the body of the sternum have articular facets for costal cartilages. Superiorly, each lateral margin has a demifacet for articulation with the inferior aspect of the second costal cartilage. Inferior to this demifacet are four facets for articulation with the costal cartilages of ribs III to VI.
At the inferior end of the body of the sternum is a demifacet for articulation with the upper demifacet on the seventh costal cartilage. The inferior end of the body of the sternum is attached to the xiphoid process.
Xiphoid process
The xiphoid process is the smallest part of the sternum (Fig. 3.13). Its shape is variable: it may be wide, thin, pointed, bifid, curved, or perforated. It begins as a cartilaginous structure, which becomes ossified in the adult. On each side of its upper lateral margin is a demifacet for articulation with the inferior end of the seventh costal cartilage.
Collection of sternal bone marrow
The subcutaneous position of the sternum makes it possible to place a needle through the hard outer cortex into the internal (or medullary) cavity containing bone marrow. Once the needle is in this position, bone marrow can be aspirated. Evaluation of this material under the microscope helps clinicians diagnose certain blood diseases such as leukemia.
Joints
Costovertebral joints
A typical rib articulates with:
 the bodies of adjacent vertebrae, forming a joint with the head of the rib; and
the bodies of adjacent vertebrae, forming a joint with the head of the rib; and
 the transverse process of its related vertebra, forming a costotransverse joint (Fig. 3.14).
the transverse process of its related vertebra, forming a costotransverse joint (Fig. 3.14).
Fig. 3.14 Costovertebral joints.
Together, the costovertebral joints and related ligaments allow the necks of the ribs either to rotate around their longitudinal axes, which occurs mainly in the upper ribs, or to ascend and descend relative to the vertebral column, which occurs mainly in the lower ribs. The combined movements of all of the ribs on the vertebral column are essential for altering the volume of the thoracic cavity during breathing.
The two facets on the head of the rib articulate with the superior facet on the body of its own vertebra and with the inferior facet on the body of the vertebra above (Fig. 3.14). This joint is divided into two synovial compartments by an intra-articular ligament, which attaches the crest to the adjacent intervertebral disc and separates the two articular surfaces on the head of the rib. The two synovial compartments and the intervening ligament are surrounded by a single joint capsule attached to the outer margins of the combined articular surfaces of the head and vertebral column.
Costotransverse joints are synovial joints between the tubercle of a rib and the transverse process of the related vertebra (Fig. 3.14). The joint is stabilized by two strong extracapsular ligaments that span the space between the transverse process and the rib on the medial and lateral sides of the joint:
A third ligament, the superior costotransverse ligament, attaches the superior surface of the neck of the rib to the transverse process of the vertebra above.
Slight gliding movements occur at the costotransverse joints.
Sternocostal joints
The sternocostal joints are joints between the upper seven costal cartilages and the sternum (Fig. 3.15).
Fig. 3.15 Sternocostal joints.
The joint between rib I and the manubrium is not synovial and consists of a fibrocartilaginous connection between the manubrium and the costal cartilage. The joints between rib II through VII and the sternum are synovial and have thin capsules reinforced by surrounding sternocostal ligaments.
Interchondral joints
Interchondral joints occur between the costal cartilages of adjacent ribs (Fig. 3.15), mainly between the costal cartilages of ribs VII to X, but may also involve the costal cartilages of ribs V and VI.
Interchondral joints provide indirect anchorage to the sternum and contribute to the formation of a smooth inferior costal margin. They are usually synovial, and the thin fibrous capsules are reinforced by interchondral ligaments.
Manubriosternal and xiphisternal joints
The joints between the manubrium and body of sternum and between the body of sternum and the xiphoid process are usually symphyses (Fig. 3.15). Only slight angular movements occur between the manubrium and body of sternum during respiration. The joint between the body of sternum and the xiphoid process often becomes ossified with age.
Additionally, the sternal angle is on a horizontal plane that passes through the intervertebral disc between vertebrae TIV and TV (Fig. 3.16). This plane separates the superior mediastinum from the inferior mediastinum and marks the superior border of the pericardium. It also separates the end of the ascending aorta from the beginning of the arch of the aorta, the end of the arch of the aorta from the beginning of the thoracic aorta, and passes through the bifurcation of the trachea just superior to the pulmonary trunk.
Fig. 3.16 Vertebral level TIV/V.
Clinical app
The manubriosternal joint as reference
A clinically useful feature of the manubriosternal joint is that it can be palpated easily. This is because the manubrium normally angles posteriorly on the body of sternum, forming a raised feature referred to as the sternal angle. This elevation marks the site of articulation of rib II with the sternum. Rib I is not palpable because it lies inferior to the clavicle and is embedded in tissues at the base of the neck. Therefore, rib II is used as a reference for counting ribs and can be felt immediately lateral to the sternal angle.
How to count ribs
Knowing how to count ribs is important because different ribs provide palpable landmarks for the positions of deeper structures. To determine the location of specific ribs, palpate the jugular notch at the superior extent of the manubrium of the sternum. Move down the sternum until a ridge is felt. This ridge is the sternal angle, which identifies the articulation between the manubrium of sternum and the body of sternum. The costal cartilage of rib II articulates with the sternum at this location. Identify rib II. Then continue counting the ribs, moving in a downward and lateral direction (Fig. 3.17).
Surface anatomy
Visualizing structures at the TIV/V vertebral level
The TIV/V vertebral level is a transverse plane that passes through the sternal angle on the anterior chest wall and the intervertebral disc between TIV and TV vertebrae posteriorly. This plane can easily be located, because the joint between the manubrium of sternum and the body of sternum forms a distinct bony protuberance that can be palpated. At the TIV/V level (Fig. 3.18):
 The costal cartilage of rib II articulates with the sternum.
The costal cartilage of rib II articulates with the sternum.
 The superior mediastinum is separated from the inferior mediastinum.
The superior mediastinum is separated from the inferior mediastinum.
 The ascending aorta ends and the arch of aorta begins.
The ascending aorta ends and the arch of aorta begins.
Intercostal spaces
Intercostal spaces lie between adjacent ribs and are filled by intercostal muscles (Fig. 3.19).
Fig. 3.19 Intercostal space. A. Anterolateral view. B. Details of an intercostal space and relationships.
Intercostal nerves and associated major arteries and veins lie in the costal groove along the inferior margin of the superior rib and pass in the plane between the inner two layers of muscles (Fig. 3.19). In each space, the vein is the most superior structure and is therefore highest in the costal groove. The artery is inferior to the vein, and the nerve is inferior to the artery and often not protected by the groove. Therefore, the nerve is the structure most at risk when objects perforate the upper aspect of an intercostal space.
Deep to the intercostal spaces and ribs, and separating these structures from the underlying pleura, is a layer of loose connective tissue called endothoracic fascia, which contains variable amounts of fat (Fig. 3.19B).
Muscles
Muscles of the thoracic wall include those that fill and support the intercostal spaces (external, internal, and innermost intercostal muscles [Table 3.2, Figs. 3.19, 3.20]); those that cross several ribs between the costal attachments (subcostal muscles [Table 3.2, Fig. 3.21A]); and those that pass between the sternum and the ribs (transversus thoracis muscles [Table 3.2, Fig. 3.21B]).
Table 3.2 Muscles of the thoracic wall
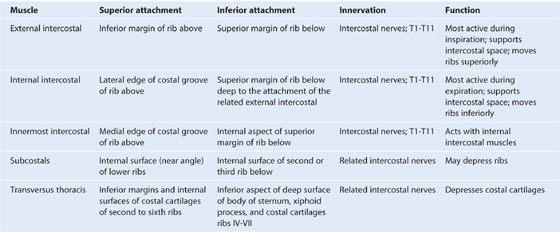
Fig. 3.20 Intercostal muscles.
Fig. 3.21 A. Subcostal muscles. B. Transversus thoracis muscles.
The muscles of the thoracic wall, together with muscles between the vertebrae and ribs posteriorly (i.e., the levatores costarum, and serratus posterior superior and serratus posterior inferior muscles), alter the position of the ribs and sternum changing the thoracic volume during breathing. They also reinforce the thoracic wall.
Intercostal muscles
The intercostal muscles are three flat muscles found in each intercostal space that pass between adjacent ribs (Figs. 3.19B, 3.20). Individual muscles in this group are named according to their positions:
The intercostal muscles are innervated by the related intercostal nerves. As a group, the intercostal muscles provide structural support for the intercostal spaces during breathing. They can also move the ribs.
Arterial supply
Vessels that supply the thoracic wall consist mainly of posterior and anterior intercostal arteries, which pass around the wall between adjacent ribs in intercostal spaces (Fig. 3.19A). These arteries originate from the aorta and internal thoracic arteries, which in turn arise from the subclavian arteries in the root of the neck. Together, the intercostal arteries form a basket-like pattern of vascular supply around the thoracic wall.
Posterior intercostal arteries
Posterior intercostal arteries originate from vessels associated with the posterior thoracic wall. The upper two posterior intercostal arteries on each side come from the supreme intercostal artery, which descends into the thorax as a branch of the costocervical trunk in the neck. The costocervical trunk is a posterior branch of the subclavian artery (Fig. 3.22).
Fig. 3.22 Arteries of the thoracic wall.
The remaining nine pairs of posterior intercostal arteries arise from the posterior surface of the thoracic aorta. Because the aorta is on the left side of the vertebral column, those posterior intercostal vessels passing to the right side of the thoracic wall cross the midline anterior to the bodies of the vertebrae and therefore are longer than the corresponding vessels on the left.
In addition to having numerous branches that supply various components of the wall, the posterior intercostal arteries have branches that accompany lateral cutaneous branches of the intercostal nerves to the superficial regions.
Anterior intercostal arteries
The anterior intercostal arteries originate directly or indirectly as lateral branches from the internal thoracic arteries (Fig. 3.22).
Each internal thoracic artery arises as a major branch of the subclavian artery in the neck. It passes anteriorly over the cervical dome of pleura and descends vertically through the superior thoracic aperture and along the deep aspect of the anterior thoracic wall. On each side, the internal thoracic artery lies posterior to the costal cartilages of the upper six ribs and about 1 cm lateral to the sternum. At approximately the level of the sixth intercostal space, it divides into two terminal branches (Fig. 3.22):
 the superior epigastric artery, which continues inferiorly into the anterior abdominal wall;
the superior epigastric artery, which continues inferiorly into the anterior abdominal wall;
Anterior intercostal arteries that supply the upper six intercostal spaces arise as lateral branches from the internal thoracic artery, whereas those supplying the lower spaces arise from the musculophrenic artery.
In each intercostal space, the anterior intercostal arteries usually have two branches:
 One passes below the margin of the upper rib.
One passes below the margin of the upper rib.
In addition to anterior intercostal arteries and a number of other branches, the internal thoracic arteries give rise to perforating branches that pass directly forward between the costal cartilages to supply structures external to the thoracic wall. These vessels travel with the anterior cutaneous branches of the intercostal nerves.
Venous drainage
Venous drainage from the thoracic wall generally parallels the pattern of arterial supply (Fig. 3.23).
Fig. 3.23 Veins of the thoracic wall.
Centrally, the intercostal veins ultimately drain into the azygos system of veins or into internal thoracic veins, which connect with the brachiocephalic veins in the neck.
Often the upper posterior intercostal veins on the left side come together and form the left superior intercostal vein, which empties into the left brachiocephalic vein.
Similarly, the upper posterior intercostal veins on the right side may come together and form the right superior intercostal vein, which empties into the azygos vein.
Lymphatic drainage
Lymphatic vessels of the thoracic wall drain mainly into lymph nodes associated with the internal thoracic arteries (parasternal nodes), with the heads and necks of ribs (intercostal nodes), and with the diaphragm (diaphragmatic nodes) (Fig. 3.24). Diaphragmatic nodes are posterior to the xiphoid and at sites where the phrenic nerves penetrate the diaphragm. They also occur in regions where the diaphragm is attached to the vertebral column.
Fig. 3.24 Major lymphatic vessels and nodes of the thoracic wall.
Parasternal nodes drain into bronchomediastinal trunks. Intercostal nodes in the upper thorax also drain into bronchomediastinal trunks, whereas intercostal nodes in the lower thorax drain into the thoracic duct.
Nodes associated with the diaphragm interconnect with parasternal, prevertebral, juxtaesophageal nodes, brachiocephalic (anterior to the brachiocephalic veins in the superior mediastinum), and lateral aortic/lumbar nodes (in the abdomen).
Superficial regions of the thoracic wall drain mainly into axillary lymph nodes in the axilla or parasternal nodes.
Innervation
Intercostal nerves
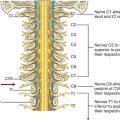
Innervation of the thoracic wall is mainly by the intercostal nerves, which are the anterior rami of spinal nerves T1 to T11 and lie in the intercostal spaces between adjacent ribs. The anterior ramus of spinal nerve T12 (the subcostal nerve) is inferior to rib XII (Fig. 3.25).
Fig. 3.25 Intercostal nerves.
A typical intercostal nerve passes laterally around the thoracic wall in an intercostal space. The largest of the branches is the lateral cutaneous branch, which pierces the lateral thoracic wall and divides into an anterior branch and a posterior branch that innervate the overlying skin.
The intercostal nerves end as anterior cutaneous branches, which emerge either parasternally, between adjacent costal cartilages, or laterally to the midline, on the anterior abdominal wall, to supply the skin.
In addition to these major branches, small collateral branches can be found in the intercostal space running along the superior border of the lower rib.
In the thorax, the intercostal nerves carry:
 somatic sensory innervation from the skin and parietal pleura, and
somatic sensory innervation from the skin and parietal pleura, and
 postganglionic sympathetic fibers to the periphery.
postganglionic sympathetic fibers to the periphery.
Sensory innervation from the skin overlying the upper thoracic wall is supplied by cutaneous branches (supraclavicular nerves), which descend from the cervical plexus in the neck.
In addition to innervating the thoracic wall, intercostal nerves innervate other regions:
 The anterior ramus of T1 contributes to the brachial plexus.
The anterior ramus of T1 contributes to the brachial plexus.
 The lower intercostal nerves supply muscles, skin, and parietal peritoneum of the abdominal wall.
The lower intercostal nerves supply muscles, skin, and parietal peritoneum of the abdominal wall.
Clinical app
Surgical access to the chest
Surgical access is challenging in the chest given the rigid nature of the thoracic cage. Moreover, the access site is also dependent on the organ that is operated upon and its relationships to subdiaphragmatic structures and structures in the neck.
Minimally invasive thoracic surgery (video-assisted thoracic surgery [VATS]) involves making small (1 cm) incisions in the intercostal spaces, inserting a small camera, and manipulating other instruments through additional small incisions. A number of procedures can be performed in this manner, including lobectomy, lung biopsy, and esophagectomy. A standard incision site would include a median sternotomy to obtain access to the heart, including the coronary arteries and the cardiac valves.
A left lateral thoracotomy or a right lateral thoracotomy is an incision through an intercostal space to access the lungs and the more lateral mediastinal structures.
Thoracostomy (chest) tube insertion
Insertion of a chest tube is a commonly performed procedure and is indicated to relieve air or fluid trapped in the thorax between the lung and the chest wall (pleural cavity). This procedure is done for pneumothorax, hemothorax, hemopneumothorax, malignant pleural effusion empyema, hydrothorax, chylothorax, and also after thoracic surgery.
The position of the thoracostomy tube should be between the anterior axillary and midaxillary anatomical lines from anterior to posterior and either the fourth or fifth intercostal space from cephalad to caudad. The position of the ribs in this region should be clearly marked. Anesthetic should be applied to the superior border of the rib and the inferior aspect of the intercostal space, including one rib and space above and one rib and space below. The neurovascular bundle runs in the neurovascular plane, which lies in the superior aspect of the intercostal space (just below the rib), hence the reason for positioning the tube on the superior border of a rib (i.e., at the lowest position in the intercostal space).
Clinical app
Intercostal nerve block
Local anesthesia of intercostal nerves produces excellent analgesia in patients with chest trauma and those patients requiring anesthesia for a thoracotomy, mastectomy, and upper abdominal surgical procedures.
The intercostal nerves are situated inferior to the rib borders in the neurovascular bundle. Each neurovascular bundle is situated deep to the external and internal intercostal muscle groups.
The nerve block may be undertaken using a “blind” technique or under direct imaging guidance.
The patient is placed in the appropriate position to access the rib. Typically, under ultrasound guidance, a needle may be advanced into the region of the subcostal groove followed by an injection with local anesthesia. Depending on the type of local anesthetic, analgesia may be short- or long-acting.
Given the position of the neurovascular bundle and the subcostal groove, complications may include puncture of the parietal pleura and an ensuing pneumothorax. Bleeding may also occur if the artery or vein is damaged during the procedure.
DIAPHRAGM
The diaphragm is a thin musculotendinous structure that fills the inferior thoracic aperture and separates the thoracic cavity from the abdominal cavity (Figs. 3.26, 3.27, and see Chapter 4). It is attached peripherally to the:
 xiphoid process of the sternum,
xiphoid process of the sternum,
 costal margin of the thoracic wall,
costal margin of the thoracic wall,
 ligaments that span across structures of the posterior abdominal wall, and
ligaments that span across structures of the posterior abdominal wall, and
 vertebrae of the lumbar region.
vertebrae of the lumbar region.
Fig. 3.26 Major structures passing between abdomen and thorax.
Fig. 3.27 Diaphragm.
From these peripheral attachments, muscle fibers converge to join the central tendon. The pericardium is attached to the middle part of the central tendon.
In the median sagittal plane, the diaphragm slopes inferiorly from its anterior attachment to the xiphoid, approximately at vertebral level TVIII/IX, to its posterior attachment to the median arcuate ligament, crossing anteriorly to the aorta at approximately vertebral level TXII.
Structures traveling between the thorax and abdomen pass through the diaphragm or between the diaphragm and its peripheral attachments (Figs. 3.26, 3.27):
 The inferior vena cava passes through the central tendon at approximately vertebral level TVIII.
The inferior vena cava passes through the central tendon at approximately vertebral level TVIII.
 The vagus nerves pass through the diaphragm with the esophagus.
The vagus nerves pass through the diaphragm with the esophagus.
 The aorta passes behind the posterior attachment of the diaphragm at vertebral level TXII.
The aorta passes behind the posterior attachment of the diaphragm at vertebral level TXII.
 The thoracic duct passes behind the diaphragm with the aorta.
The thoracic duct passes behind the diaphragm with the aorta.
Other structures outside the posterior attachments of the diaphragm lateral to the aortic hiatus include the sympathetic trunks and the least splanchnic nerves. The greater and lesser splanchnic nerves penetrate the crura.
Arterial supply
The arterial supply to the diaphragm is from vessels that arise superiorly and inferiorly to it (see Fig. 3.27). From above, pericardiacophrenic and musculophrenic arteries supply the diaphragm. These vessels are branches of the internal thoracic arteries. Superior phrenic arteries, which arise directly from lower parts of the thoracic aorta, and small branches from intercostal arteries contribute to the supply. The largest arteries supplying the diaphragm arise from below it. These arteries are the inferior phrenic arteries, which branch directly from the abdominal aorta.
Venous drainage
Venous drainage of the diaphragm is by veins that generally parallel the arteries. The veins drain into:
 the brachiocephalic veins in the neck,
the brachiocephalic veins in the neck,
 the azygos system of veins, or
the azygos system of veins, or
 the abdominal veins (left suprarenal vein and inferior vena cava).
the abdominal veins (left suprarenal vein and inferior vena cava).
Innervation
The diaphragm is innervated by the phrenic nerves (C3, C4, and C5), which penetrate the diaphragm and innervate it from its abdominal surface (Fig. 3.27).
Contraction of the domes of the diaphragm flattens the diaphragm, increasing thoracic volume. Movements of the diaphragm are essential for normal breathing.
MOVEMENTS OF THE THORACIC WALL AND DIAPHRAGM DURING BREATHING
During breathing, the dimensions of the thorax change in the vertical, lateral, and anteroposterior directions. Elevation and depression of the diaphragm significantly alter the vertical dimensions of the thorax. Depression results when the muscle fibers of the diaphragm contract. Elevation occurs when the diaphragm relaxes.
Changes in the anteroposterior and lateral dimensions result from elevation and depression of the ribs (Fig. 3.28). The posterior ends of the ribs articulate with the vertebral column, whereas the anterior ends of most ribs articulate with the sternum or adjacent ribs.
Because the anterior ends of the ribs are inferior to the posterior ends, when the ribs are elevated, they move the sternum upward and forward. Also, the angle between the body of the sternum and the manubrium may become slightly less acute. When the ribs are depressed, the sternum moves downward and backward. This “pump handle” movement changes the dimensions of the thorax in the anteroposterior direction (Fig. 3.28A).
As well as the anterior ends of the ribs being lower than the posterior ends, the middles of the shafts tend to be lower than the two ends. When the shafts are elevated, the middles of the shafts move laterally. This “bucket handle” movement increases the lateral dimensions of the thorax (Fig. 3.28B).
PLEURAL CAVITIES
Two pleural cavities, one on either side of the mediastinum, surround the lungs (Figs. 3.29, 3.30):
 superiorly, they extend above rib I into the root of the neck;
superiorly, they extend above rib I into the root of the neck;
 inferiorly, they extend to a level just above the costal margin; and
inferiorly, they extend to a level just above the costal margin; and
 the medial wall of each pleural cavity is the mediastinum (Fig. 3.31).
the medial wall of each pleural cavity is the mediastinum (Fig. 3.31).
Fig. 3.29 Pleural cavities.
Fig. 3.30 Pleural cavities.
Fig. 3.31 Cross-section of the thorax showing the position of the mediastinum.
Clinical app
The arrangement of pleural cavities is clinically significant
The pleural cavities are completely separated from each other by the mediastinum. Therefore, abnormal events in one pleural cavity do not necessarily affect the other cavity. This also means that the mediastinum can be entered surgically without opening the pleural cavities.
Another important feature of the pleural cavities is that they extend above the level of rib I. The apex of each lung actually extends into the root of the neck. As a consequence, abnormal events in the root of the neck can involve the adjacent pleura and lung, and events in the adjacent pleura and lung can involve the root of the neck.
Pleura
Each pleural cavity is lined by a single layer of flat cells, mesothelium, and an associated layer of supporting connective tissue; together, they form the pleura.
The pleura is divided into two major types, based on location:
 pleura associated with the walls of a pleural cavity is parietal pleura (Fig. 3.30); and
pleura associated with the walls of a pleural cavity is parietal pleura (Fig. 3.30); and
 pleura that reflects from the medial wall and onto the surface of the lung is visceral pleura (Fig. 3.30), which adheres to and covers the lung.
pleura that reflects from the medial wall and onto the surface of the lung is visceral pleura (Fig. 3.30), which adheres to and covers the lung.
Parietal pleura
The names given to the parietal pleura correspond to the parts of the wall with which they are associated (Fig. 3.32):
 Pleura related to the ribs and intercostal spaces is termed the costal part.
Pleura related to the ribs and intercostal spaces is termed the costal part.
 Pleura covering the diaphragm is the diaphragmatic part.
Pleura covering the diaphragm is the diaphragmatic part.
 Pleura covering the mediastinum is the mediastinal part.
Pleura covering the mediastinum is the mediastinal part.
Fig. 3.32 Parietal pleura.
Covering the superior surface of the cervical pleura is a distinct domelike layer of fascia, the suprapleural membrane (Fig. 3.32). This connective tissue membrane is attached laterally to the medial margin of the first rib and behind to the transverse process of vertebra CVII. Superiorly, the membrane receives muscle fibers from some of the deep muscles in the neck (scalene muscles) that function to keep the membrane taught. The suprapleural membrane provides apical support for the pleural cavity in the root of the neck.
In the region of vertebrae TV to TVII, the mediastinal pleura reflects off the mediastinum as a tubular, sleevelike covering for structures (i.e., airway, vessels, nerves, lymphatics) that pass between the lung and mediastinum. This sleevelike covering, and the structures it contains, forms the root of the lung. The root joins the medial surface of the lung at an area referred to as the hilum of lung. Here, the mediastinal pleura is continuous with the visceral pleura.
Clinical app
Innervation of parietal and visceral pleura
The parietal pleural is innervated by somatic afferent fibers. The costal pleura is innervated by branches from the intercostal nerves and pain would be felt in relation to the thoracic wall. The diaphragmatic pleura and the mediastinal pleura are innervated mainly by the phrenic nerves (originating at spinal cord levels C3, C4, and C5). Pain from these areas would refer to the C3, C4, and C5 dermatomes (lateral neck and the supraclavicular region of the shoulder).
The visceral pleural is innervated by visceral afferent fibers that accompany bronchial vessels and pain is generally not elicited from this tissue.
Peripheral reflections
The peripheral reflections of parietal pleura mark the extent of the pleural cavities (Fig. 3.33).
Fig. 3.33 Pleural reflections.
Superiorly, the pleural cavity can project as much as 3 to 4 cm above the first costal cartilage, but does not extend above the neck of rib I. This limitation is caused by the inferior slope of rib I to its articulation with the manubrium.
Anteriorly, the pleural cavities approach each other posterior to the upper part of the sternum. However, posterior to the lower part of the sternum, the parietal pleura does not come as close to the midline on the left side as it does on the right because the middle mediastinum, containing the pericardium and heart, bulges to the left.
Inferiorly, the costal pleura reflects onto the diaphragm above the costal margin. In the midclavicular line, the pleural cavity extends inferiorly to approximately rib VIII (Fig. 3.34). In the midaxillary line, it extends to rib X. From this point, the inferior margin courses somewhat horizontally, crossing ribs XI and XII to reach vertebra TXII. From the midclavicular line to the vertebral column, the inferior boundary of the pleura can be approximated by a line that runs between the rib VIII, rib X, and vertebra TXII.
Fig. 3.34 Parietal pleural reflections and recesses.
Visceral pleura
The visceral pleura is continuous with the parietal pleura at the hilum of each lung where structures enter and leave the organ. The visceral pleura is firmly attached to the surface of the lung, including both opposed surfaces of the fissures that divide the lungs into lobes.
Costomediastinal recesses
Anteriorly, a costomediastinal recess occurs on each side where the costal pleura is opposed to the mediastinal pleura. The largest is on the left side in the region overlying the heart (Fig. 3.34).
Costodiaphragmatic recesses
The largest and clinically most important recesses are the costodiaphragmatic recesses, which occur in each pleural cavity between the costal pleura and diaphragmatic pleura (Fig. 3.34). The costodiaphragmatic recesses are the regions between the inferior margin of the lungs and inferior margin of the pleural cavities. They are deepest after forced expiration and shallowest after forced inspiration.
During quiet respiration, the inferior margin of the lung crosses rib VI in the midclavicular line, rib VIII in the midaxillary line, and then courses somewhat horizontally to reach the vertebral column at vertebral level TX. From the midclavicular line and around the thoracic wall to the vertebral column, the inferior margin of the lung can be approximated by a line running between rib VI, rib VIII, and vertebra TX. The inferior margin of the pleural cavity at the same points is rib VIII, rib X, and vertebra TXII. The costodiaphragmatic recess is the region between the two margins.
During expiration, the inferior margin of the lung rises and the costodiaphragmatic recess becomes larger.
Clinical app
Pleural recesses
The lungs do not completely fill the anterior or posterior inferior regions of the pleural cavities (Fig. 3.34). This results in recesses in which two layers of parietal pleura become opposed. Expansion of the lungs into these spaces usually occurs only during forced inspiration; the recesses also provide potential spaces in which fluids can collect and from which fluids can be aspirated.
Pleural effusion
A pleural effusion occurs when excess fluid accumulates within the pleural space. As the fluid accumulates within the pleural space the underlying lung is compromised and may collapse as the volume of fluid increases. Once a pleural effusion has been diagnosed, fluid often will be aspirated to determine the cause, which can include infection, malignancy, cardiac failure, hepatic disease, and pulmonary embolism.
Clinical app
Pneumothorax
A pneumothorax is a collection of gas or air within the pleural cavity. When air enters the pleural cavity the tissue elasticity of the parenchyma causes the lung to collapse within the chest impairing the lung function. Occasionally, the gas within the pleural cavity may accumulate to such an extent that the mediastinum is “pushed” to the opposite side, compromising the other lung. This is termed a tension pneumothorax and requires urgent treatment.
Most pneumothoraces are spontaneous (i.e., they occur in the absence of no known pathology and no known lung disease.) Secondly, pneumothoraces may occur as a result of trauma, inflammation, smoking, and other underlying pulmonary diseases.
The symptoms of pneumothorax are often determined by the degree of air leak and the rate at which the accumulation of gas occurs and the ensuing lung collapse. They include pain, shortness of breath, and cardiorespiratory collapse if severe.
Lungs
The two lungs are organs of respiration and lie on either side of the mediastinum surrounded by the right and left pleural cavities. Air enters and leaves the lungs via the main bronchi, which are branches of the trachea.
The pulmonary arteries deliver deoxygenated blood to the lungs from the right ventricle of the heart. Oxygenated blood returns to the left atrium via the pulmonary veins.
The right lung is normally a little larger than the left lung because the middle mediastinum, containing the heart, bulges more to the left than to the right.
Each lung has a half-cone shape, with a base, apex, two surfaces, and three borders (Fig. 3.35).
Fig. 3.35 Lungs.
 The base sits on the diaphragm.
The base sits on the diaphragm.
 The apex projects above rib I and into the root of the neck.
The apex projects above rib I and into the root of the neck.
The lungs lie directly adjacent to, and are indented by, structures contained in the overlying area. The heart and major vessels form bulges in the mediastinum that indent the medial surfaces of the lung; the ribs indent the costal surfaces. Pathology, such as tumors, or abnormalities in one structure can affect the related structure.
Root and hilum
The root of each lung is a short tubular collection of structures that together attach the lung to structures in the mediastinum (Fig. 3.36). It is covered by a sleeve of mediastinal pleura that reflects onto the surface of the lung as visceral pleura. The region outlined by this pleural reflection on the medial surface of the lung is the hilum, where structures enter and leave.
Fig. 3.36 Roots and hila of the lungs.
A thin blade-like fold of pleura projects inferiorly from the root of the lung and extends from the hilum to the mediastinum. This structure is the pulmonary ligament (Fig. 3.36). It may stabilize the position of the inferior lobe and may also accommodate the down-and-up translocation of structures in the root during breathing.
In the mediastinum, the vagus nerves pass immediately posterior to the roots of the lungs, while the phrenic nerves pass immediately anterior to them.
Within each root and located in the hilum are:
Generally, the pulmonary artery is superior at the hilum, the pulmonary veins are inferior, and the bronchi are somewhat posterior in position.
On the right side, the lobar bronchus to the superior lobe branches from the main bronchus in the root, unlike on the left where it branches within the lung itself, and is superior to the pulmonary artery.
Right lung
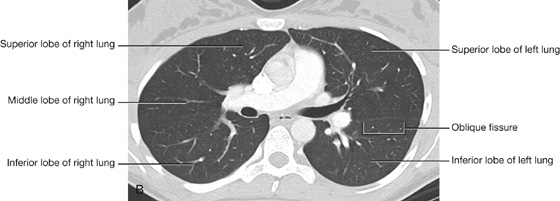
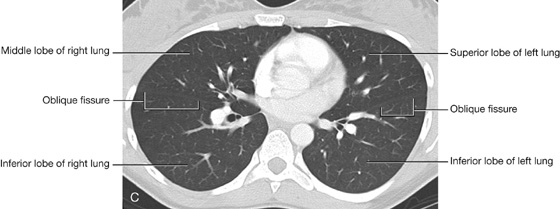
The right lung has three lobes and two fissures (Fig. 3.37A). Normally, the lobes are freely movable against each other because they are separated, almost to the hilum, by invaginations of visceral pleura. These invaginations form the fissures:
 the horizontal fissure separates the superior lobe (upper lobe) from the middle lobe.
the horizontal fissure separates the superior lobe (upper lobe) from the middle lobe.
Fig. 3.37 A. Right lung. B. Major structures related to the right lung.
The horizontal fissure follows the fourth intercostal space from the sternum until it meets the oblique fissure as it crosses rib V.
The largest surface of the superior lobe is in contact with the upper part of the anterolateral wall and the apex of this lobe projects into the root of the neck. The surface of the middle lobe lies mainly adjacent to the lower anterior and lateral walls. The costal surface of the inferior lobe is in contact with the posterior and inferior walls.
The medial surface of the right lung lies adjacent to a number of important structures in the mediastinum and the root of the neck (Fig. 3.37B). These include the:
The right subclavian artery and vein arch over and are related to the superior lobe of the right lung as they pass over the dome of cervical pleura and into the axilla.
Left lung
The left lung is smaller than the right lung and has two lobes separated by an oblique fissure (Fig. 3.38A). The oblique fissure of the left lung is slightly more oblique than the corresponding fissure of the right lung.
Fig. 3.38 A. Left lung. B. Major structures related to the left lung.
The largest surface of the superior lobe is in contact with the upper part of the anterolateral wall, and the apex of this lobe projects into the root of the neck. The costal surface of the inferior lobe is in contact with the posterior and inferior walls.
The inferior portion of the medial surface of the left lung, unlike the right lung, is notched because of the heart’s projection into the left pleural cavity from the middle mediastinum.
From the anterior border of the lower part of the superior lobe, a tonguelike extension (the lingula of left lung) projects over the heart bulge (Fig. 3.38A).
The medial surface of the left lung lies adjacent to a number of important structures in the mediastinum and root of the neck (Fig. 3.38B). These include the:
The left subclavian artery and vein arch over and are related to the superior lobe of the left lung as they pass over the dome of the cervical pleura and into the axilla.
Visualizing the pleural cavities and lungs, pleural recesses, and lung lobes and fissures
Palpable surface landmarks can be used to visualize the normal outlines of the pleural cavities and the lungs and to determine the positions of the lobes and fissures of each lung.
Superiorly, the parietal pleura projects above the first costal cartilage. Anteriorly, the costal pleura approaches the midline posterior to the upper portion of the sternum. Posterior to the lower portion of the sternum, the left parietal pleura does not come as close to the midline as it does on the right side. This is because the heart bulges onto the left side (Fig. 3.39A).
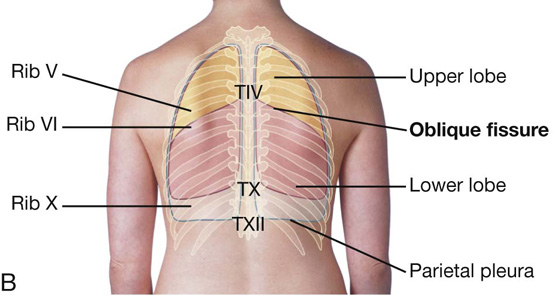
Inferiorly, the pleura reflects onto the diaphragm above the costal margin and courses around the thoracic wall following an VIII, X, XII contour (i.e., rib VIII in the midclavicular line, rib X in the midaxillary line, and vertebra TXII posteriorly).
The lungs do not completely fill the area surrounded by the pleural cavities, particularly anteriorly and inferiorly.
 Costomediastinal recesses occur anteriorly, particularly on the left side in relationship to the heart bulge (Fig. 3.39A).
Costomediastinal recesses occur anteriorly, particularly on the left side in relationship to the heart bulge (Fig. 3.39A).
 Costodiaphragmatic recesses occur inferiorly between the lower lung margin and the lower margin of the pleural cavity (Fig. 3.39A, B).
Costodiaphragmatic recesses occur inferiorly between the lower lung margin and the lower margin of the pleural cavity (Fig. 3.39A, B).
In the posterior view, the oblique fissure on both sides is located in the midline near the spine of vertebra TIV (Figs. 3.39B and 3.40A). It moves laterally in a downward direction, crossing the fourth and fifth intercostal spaces and reaches rib VI laterally.
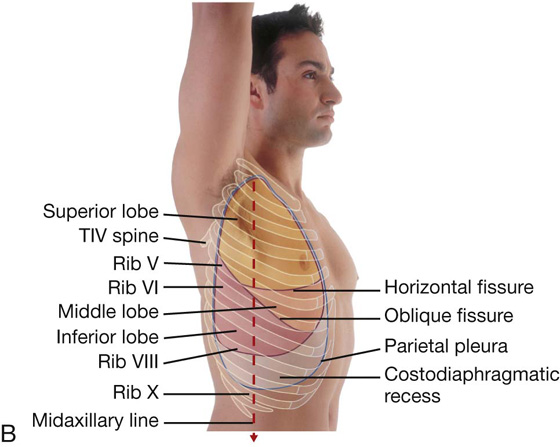
In the anterior view, the horizontal fissure on the right side follows the contour of rib IV and its costal cartilage and the oblique fissures on both sides follow the contour of rib VI and its costal cartilage (Fig. 3.40B).
Where to listen for lung sounds
The stethoscope placements for listening for lung sounds are shown in Figure 3.41.
Bronchial tree
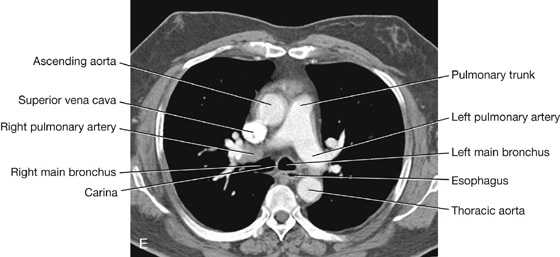
The trachea is a flexible tube that extends from vertebral level CVI in the lower neck to vertebral level TIV/V in the mediastinum, where it bifurcates into a right and a left main bronchus (Fig. 3.42). The trachea is held open by C-shaped transverse cartilage rings embedded in its wall—the open part of the C facing posteriorly. The lowest tracheal ring has a hook-shaped structure, the carina, that projects backward in the midline between the origins of the two main bronchi. The posterior wall of the trachea is composed mainly of smooth muscle.
Fig. 3.42 A. Bronchial tree. B. Bronchopulmonary segments.
Each main bronchus enters the root of a lung and passes through the hilum into the lung itself.
The main bronchus divides within the lung into lobar bronchi (secondary bronchi), each of which supplies a lobe. On the right side, the lobar bronchus to the superior lobe originates within the root of the lung.
The lobar bronchi further divide into segmental bronchi (tertiary bronchi), which supply bronchopulmonary segments (Fig. 3.42B).
Within each bronchopulmonary segment, the segmental bronchi give rise to multiple generations of divisions and, ultimately, to bronchioles, which further subdivide and supply the respiratory surfaces. The walls of the bronchi are held open by discontinuous elongated plates of cartilage, but these are not present in bronchioles.
Clinical app
Inhaled objects
The right main bronchus is wider and takes a more vertical course through the root and hilum than the left main bronchus (Fig. 3.42A). Therefore, inhaled foreign bodies tend to lodge more frequently on the right side than on the left.
Bronchopulmonary segments
A bronchopulmonary segment is the area of lung supplied by a segmental (tertiary) bronchus and its accompanying pulmonary artery branch.
Each bronchopulmonary segment is shaped like an irregular cone with the apex at the origin of the segmental (tertiary) bronchus and the base projected peripherally onto the surface of the lung.
A bronchopulmonary segment is the smallest, functionally independent region of a lung and the smallest area of lung that can be isolated and removed without affecting adjacent regions.
There are 10 bronchopulmonary segments in each lung (Fig. 3.43); some of them fuse in the left lung.
Pulmonary arteries
The right and left pulmonary arteries originate from the pulmonary trunk and carry deoxygenated blood to the lungs from the right ventricle of the heart (Fig. 3.44).
Fig. 3.44 Pulmonary vessels. Diagram of an anterior view.
The bifurcation of the pulmonary trunk occurs to the left of the midline just inferior to vertebral level TIV/V, and anteroinferiorly to the left of the bifurcation of the trachea.
Right pulmonary artery
The right pulmonary artery is longer than the left and passes horizontally across the mediastinum (Fig. 3.44). It passes:
 posteriorly to the ascending aorta, superior vena cava, and upper right pulmonary vein.
posteriorly to the ascending aorta, superior vena cava, and upper right pulmonary vein.
The right pulmonary artery enters the root of the lung and gives off a large branch to the superior lobe of the lung. The main vessel continues through the hilum of the lung, gives off a second (recurrent) branch to the superior lobe, and then divides to supply the middle and inferior lobes.
Left pulmonary artery
The left pulmonary artery is shorter than the right and lies anterior to the descending aorta and posterior to the superior pulmonary vein (Fig. 3.44). It passes through the root and hilum and branches within the lung.
Visualizing the pulmonary trunk by computed tomography
Fig. 3.45 Pulmonary vessels. A. Axial computed tomography image showing the left pulmonary artery branching from the pulmonary trunk. B. Axial computed tomography image (just inferior to the image in A) showing the right pulmonary artery branching from the pulmonary trunk.
Pulmonary veins
On each side a superior pulmonary vein and an inferior pulmonary vein carry oxygenated blood from the lungs back to the heart (Fig. 3.44). The veins begin at the hilum of the lung, pass through the root of the lung, and immediately drain into the left atrium.
Bronchial arteries and veins
The bronchial arteries (Fig. 3.44) and veins constitute the “nutritive” vascular system of the pulmonary tissues (bronchial walls and glands, walls of large vessels, and visceral pleura). They interconnect within the lung with branches of the pulmonary arteries and veins.
The bronchial arteries originate from the thoracic aorta or one of its branches:
The bronchial arteries run on the posterior surfaces of the bronchi and ramify in the lungs to supply pulmonary tissues.
The bronchial veins drain into:
 either the pulmonary veins or the left atrium; and
either the pulmonary veins or the left atrium; and
Innervation
Structures of the lung, and the visceral pleura, are supplied by visceral afferents and efferents distributed through the anterior pulmonary plexus and posterior pulmonary plexus (Fig. 3.46). These interconnected plexuses lie anteriorly and posteriorly to the tracheal bifurcation and main bronchi. The anterior plexus is much smaller than the posterior plexus.
Fig. 3.46 Pulmonary innervation.
Branches of these plexuses, which ultimately originate from the sympathetic trunks and vagus nerves, are distributed along branches of the airway and vessels.
Visceral efferents from:
 the vagus nerves constrict the bronchioles;
the vagus nerves constrict the bronchioles;
 the sympathetic system dilates the bronchioles.
the sympathetic system dilates the bronchioles.
Lymphatic drainage
Superficial, or subpleural, and deep lymphatics of the lung drain into lymph nodes called tracheobronchial nodes around the roots of lobar and main bronchi and along the sides of the trachea (Fig. 3.47). As a group, these lymph nodes extend from within the lung, through the hilum and root, and into the posterior mediastinum.
Fig. 3.47 Lymphatic drainage of lungs.
Imaging app
Visualizing the lungs
Medical imaging of the lungs is important because they are one of the commonest sites for disease in the body. While the body is at rest, the lungs exchange up to 5 L of air per minute, and this may contain pathogens and other potentially harmful elements (e.g., allergens).
Techniques to visualize the lung range from bronchoscopy, high-resolution computed tomography (CT), to plain chest radiographs.
Patients who have an endobronchial lesion (i.e., a lesion within a bronchus) may undergo bronchoscopic evaluation of the trachea and its main branches (Fig. 3.48). The bronchoscope is passed through the nose into the oropharynx and is then directed by a control system past the vocal cords into the trachea. The bronchi are inspected and, if necessary, small biopsies are obtained.
High-resolution lung computed tomography
High-resolution computed tomography (HRCT) is a diagnostic method for assessing the lungs but more specifically the interstitium of the lungs (Fig. 3.49). The technique involves obtaining narrow cross-sectional slices of 1 to 2 mm. These scans enable the physician and radiologist to view the patterns of disease and their distribution. Diseases that may be easily demonstrated using this procedure include emphysema, pneumoconiosis (coal worker’s pneumoconiosis), and asbestosis.
Plain chest radiograph
Plain chest radiographs are the most common method of visualizing the lungs (Fig. 3.50).
Fig. 3.50 Chest radiograph, AP view.
Lung cancer
It is important to stage lung cancer because the treatment depends on its stage.
If a small malignant nodule is found within the lung, it can sometimes be excised and the prognosis is excellent. Unfortunately, many patients present with a tumor mass that has invaded structures in the mediastinum or the pleurae or has metastasized. The tumor may then be inoperable and is treated with radiotherapy and chemotherapy.
Spread of the tumor is by lymphatics to lymph nodes within the hila, mediastinum, and root of the neck.
Imaging methods to assess spread include plain radiography, computed tomography (Fig. 3.51), and magnetic resonance imaging (MRI). Increasingly, radionuclide studies using fluorodeoxyglucose positron emission tomography (FDG PET) are being used.
Fig. 3.51 Axial CT image of lungs showing tumor (arrow) in right lung.
In FDG PET a gamma radiation emitter is attached to a glucose molecule. In areas of excessive metabolic activity (i.e., the tumor), excessive uptake occurs and is recorded by a gamma camera.
Clinical app
Pneumonia
Chest infection is a common disease. In most patients the infection affects the large airways and bronchi. If the infection continues, exudates and transudates are produced, filling the alveoli and the secondary pulmonary lobules. The diffuse, patchy nature of this type of infection is termed bronchial pneumonia.
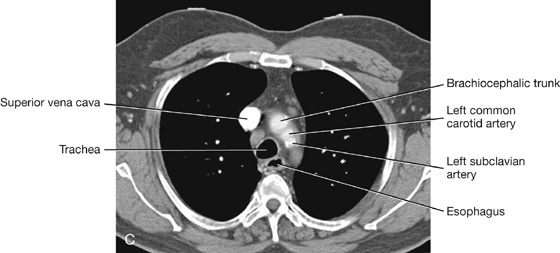
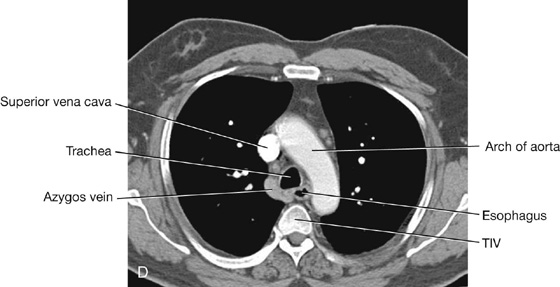
MEDIASTINUM
The mediastinum is a broad central partition that separates the two laterally placed pleural cavities. It extends (Fig. 3.52):
 from the sternum to the bodies of the vertebrae; and
from the sternum to the bodies of the vertebrae; and
 from the superior thoracic aperture to the diaphragm.
from the superior thoracic aperture to the diaphragm.
Fig. 3.52 Lateral view of the mediastinum.
The mediastinum contains the thymus gland, the pericardial sac, the heart, the trachea, and the major arteries and veins. It also serves as a passageway for structures such as the esophagus, thoracic duct, and various components of the nervous system as they traverse the thorax on their way to the abdomen.
For organizational purposes, the mediastinum is subdivided into several smaller regions. A transverse plane extending from the sternal angle (the junction between the manubrium and the body of the sternum) to the intervertebral disc between vertebrae TIV and TV separates the mediastinum into the (Fig. 3.53):
Fig. 3.53 Subdivisions of the mediastinum.
The area anterior to the pericardial sac and posterior to the body of the sternum is the anterior mediastinum. The region posterior to the pericardial sac and the diaphragm and anterior to the bodies of the vertebrae is the posterior mediastinum. The area in the middle, which includes the pericardial sac and its contents, is the middle mediastinum (Fig. 3.53).
Middle mediastinum
The middle mediastinum is centrally located in the thoracic cavity. It contains the pericardium, heart, origins of the great vessels, various nerves, and smaller vessels.
Pericardium
The pericardium is a fibroserous sac surrounding the heart and the roots of the great vessels. It consists of two components, the fibrous pericardium and the serous pericardium (Fig. 3.54).
Fig. 3.54 Sagittal section of the pericardium.
 The parietal layer lines the inner surface of the fibrous.
The parietal layer lines the inner surface of the fibrous.
The parietal and visceral layers of serous pericardium are continuous at the roots of the great vessels. The narrow space created between the two layers of serous pericardium, containing a small amount of fluid, is the pericardial cavity. This potential space allows for the relatively uninhibited movement of the heart.
Fibrous pericardium
The fibrous pericardium is a cone-shaped bag with its base attached to the central tendon of the diaphragm and a small muscular area on the left side of the diaphragm and its apex continuous with the adventitia of the great vessels (Fig. 3.54). Anteriorly, it is attached to the posterior surface of the sternum by sternopericardial ligaments. These attachments help to retain the heart in its position in the thoracic cavity. The sac also limits cardiac distention.
The phrenic nerves, which innervate the diaphragm and originate from spinal cord levels C3 to C5, pass through the fibrous pericardium and innervate this structure as they travel from their point of origin to their final destination (Fig. 3.55). Their location, within the fibrous pericardium, is directly related to the embryological origin of the diaphragm and the formation of the pericardial cavity. Similarly, the pericardiacophrenic vessels are also located within and supply the fibrous pericardium as they pass through the thoracic cavity.
Fig. 3.55 Phrenic nerves and pericardiacophrenic vessels.
Serous pericardium
The parietal layer of serous pericardium is continuous with the visceral layers of serous pericardium around the roots of the great vessels. These reflections of serous pericardium (Fig. 3.56) occur in two locations:
 one superiorly, surrounding the arteries, the aorta, and pulmonary trunk;
one superiorly, surrounding the arteries, the aorta, and pulmonary trunk;
Fig. 3.56 Posterior portion of pericardial sac showing reflections of serous pericardium.
The zone of reflection surrounding the veins is J-shaped, and the cul-de-sac formed within the J, posterior to the left atrium, is the oblique pericardial sinus.
A passage between the two sites of reflected serous pericardium is the transverse pericardial sinus. This sinus lies posteriorly to the ascending aorta and the pulmonary trunk, anteriorly to the superior vena cava, and superiorly to the left atrium.
When the pericardium is opened anteriorly during surgery, a finger placed in the transverse sinus separates arteries from veins. A hand placed under the apex of the heart and moved superiorly slips into the oblique sinus.
Vessels and nerves
Arteries supplying the pericardium are branches from the internal thoracic, pericardiacophrenic, musculophrenic, and inferior phrenic arteries, and the thoracic aorta.
Veins from the pericardium enter the azygos system of veins and the internal thoracic and superior phrenic veins.
Nerves supplying the pericardium arise from the vagus nerve [X], the sympathetic trunks, and the phrenic nerves.
Clinical app
Pericardial innervation
It is important to note that the source of somatic sensation (pain) from the parietal pericardium is carried by somatic afferent fibers in the phrenic nerves. For this reason, “pain” related to a pericardial problem may be referred to the supraclavicular region of the shoulder or lateral neck area, dermatomes for spinal cord segments C3, C4, and C5.
Clinical app
Pericarditis
Pericarditis is an inflammatory condition of the pericardium. Common causes are viral and bacterial infections, systemic illnesses (e.g., chronic renal failure), and postmyocardial infarction.
Clinical app
Pericardial effusion
Normally, only a tiny amount of fluid is present between the visceral and parietal layers of the serous pericardium. In certain situations, this space can be filled with excess fluid (pericardial effusion).
Because the fibrous pericardium is a “relatively fixed” structure that cannot expand easily, a rapid accumulation of excess fluid within the pericardial sac compresses the heart (cardiac tamponade), resulting in biventricular failure. Removing the fluid with a needle inserted into the pericardial sac can relieve the symptoms.
Clinical app
Constrictive pericarditis
Abnormal thickening of the pericardial sac (constrictive pericarditis) can compress the heart, impairing heart function resulting in heart failure.
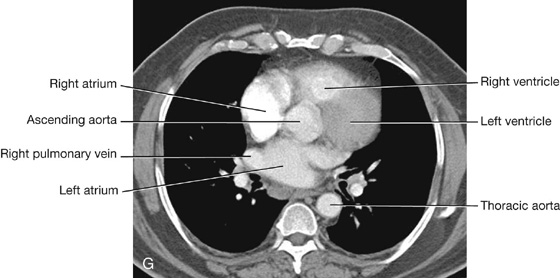
Heart
Cardiac orientation
The shape and orientation of the heart is that of a pyramid that has fallen over and is resting on one of its sides. Placed in the thoracic cavity, the apex of this pyramid projects forward, downward, and to the left, whereas the base is opposite the apex and faces in a posterior direction (Fig. 3.57). The sides of the pyramid consist of:
 a diaphragmatic (inferior) surface on which the pyramid rests,
a diaphragmatic (inferior) surface on which the pyramid rests,
 an anterior (sternocostal) surface oriented anteriorly,
an anterior (sternocostal) surface oriented anteriorly,
 a right pulmonary surface, and
a right pulmonary surface, and
Fig. 3.57 Schematic illustration of the heart showing orientation, surfaces, and margins.
Base (posterior surface) and apex
The base of the heart is quadrilateral and directed posteriorly (Fig. 3.58). It consists of:
 a small portion of the right atrium, and
a small portion of the right atrium, and
 the proximal parts of the great veins (superior and inferior venae cavae and the pulmonary veins).
the proximal parts of the great veins (superior and inferior venae cavae and the pulmonary veins).
Fig. 3.58 Base of the heart.
Because the great veins enter the base of the heart, with the pulmonary veins entering the right and left sides of the left atrium and the superior and inferior venae cavae at the upper and lower ends of the right atrium, the base of the heart is fixed posteriorly to the pericardial wall, opposite the bodies of vertebrae TV to TVIII (TVI to TIX when standing). The esophagus lies immediately posterior to the base.
From the base the heart projects forward, downward, and to the left, ending in the apex. The apex of the heart is formed by the inferolateral part of the left ventricle (Fig. 3.59) and is posterior to the left fifth intercostal space, 8 to 9 cm from the midsternal line.
Fig. 3.59 Anterior surface of the heart.
The anterior surface faces anteriorly and consists mostly of the right ventricle, with some of the right atrium on the right and some of the left ventricle on the left (Fig. 3.59).
The heart in the anatomical position rests on the diaphragmatic surface, which consists of the left ventricle and a small portion of the right ventricle separated by the posterior interventricular groove (Fig. 3.60). This surface faces inferiorly, rests on the diaphragm, is separated from the base of the heart by the coronary sinus, and extends from the base to the apex of the heart.
Fig. 3.60 Diaphragmatic surface of the heart.
The left pulmonary surface faces the left lung, is broad and convex, and consists of the left ventricle and a portion of the left atrium.
The right pulmonary surface faces the right lung, is broad and convex, and consists of the right atrium.
Some general descriptions of cardiac orientation refer to right, left, inferior (acute), and obtuse margins:
 The right and left margins are the same as the right and left pulmonary surfaces of the heart.
The right and left margins are the same as the right and left pulmonary surfaces of the heart.
 The inferior margin is defined as the sharp edge between the anterior and diaphragmatic surfaces of the heart (Figs. 3.57, 3.59)—it is formed mostly by the right ventricle and a small portion of the left ventricle near the apex.
The inferior margin is defined as the sharp edge between the anterior and diaphragmatic surfaces of the heart (Figs. 3.57, 3.59)—it is formed mostly by the right ventricle and a small portion of the left ventricle near the apex.
 The obtuse margin separates the anterior and left pulmonary surfaces (Fig. 3.57)—it is round and extends from the left auricle to the cardiac apex (Fig. 3.59), and is formed mostly by the left ventricle and superiorly by a small portion of the left auricle.
The obtuse margin separates the anterior and left pulmonary surfaces (Fig. 3.57)—it is round and extends from the left auricle to the cardiac apex (Fig. 3.59), and is formed mostly by the left ventricle and superiorly by a small portion of the left auricle.
Imaging app
Visualizing the heart
For radiological evaluations, a thorough understanding of the structures defining the cardiac borders is critical. The right border in a standard posterior–anterior view consists of the superior vena cava, the right atrium, and the inferior vena cava (Fig. 3.61A). The left border in a similar view consists of the arch of the aorta, the pulmonary trunk, and the left ventricle. The inferior border in this radiological study consists of the right ventricle and the left ventricle at the apex. In lateral views, the right ventricle is seen anteriorly, and the left atrium is visualized posteriorly (Fig. 3.61B).
Internal partitions divide the heart into four chambers (i.e., two atria and two ventricles) and produce surface or external grooves referred to as sulci.
 The coronary sulcus circles the heart, separating the atria from the ventricles (Fig. 3.62). It contains the right coronary artery, the small cardiac vein, the coronary sinus, and the circumflex branch of the left coronary artery.
The coronary sulcus circles the heart, separating the atria from the ventricles (Fig. 3.62). It contains the right coronary artery, the small cardiac vein, the coronary sinus, and the circumflex branch of the left coronary artery.
These sulci are continuous inferiorly, just to the right of the apex of the heart.
Cardiac chambers
Functionally, the heart consists of two pumps separated by a partition (Fig. 3.63).
 The right pump receives deoxygenated blood from the body and sends it to the lungs.
The right pump receives deoxygenated blood from the body and sends it to the lungs.
 The left pump receives oxygenated blood from the lungs and sends it to the body.
The left pump receives oxygenated blood from the lungs and sends it to the body.
Fig. 3.63 The heart has two pumps.
Each pump consists of an atrium and a ventricle separated by a valve.
The thin-walled atria receive blood coming into the heart, whereas the relatively thick-walled ventricles pump blood out of the heart. More force is required to pump blood through the body than through the lungs, so the muscular wall of the left ventricle is thicker than the right.
Interatrial, interventricular, and atrioventricular septa separate the four chambers of the heart (Fig. 3.64). The internal anatomy of each chamber is critical to its function.
Visualizing the chambers of the heart
Fig. 3.64 Magnetic resonance image of midthorax showing all four chambers and septa.
Surface anatomy
Visualizing the margins of the heart
Surface landmarks can be palpated to visualize the outline of the heart (Fig. 3.65).
In the anatomical position, the right atrium forms the right border of the heart and contributes to the right portion of the heart’s anterior surface (Fig. 3.66).
Fig. 3.66 Internal view of right atrium.
Blood returning to the right atrium enters through one of three vessels. These are:
 the superior and inferior venae cavae, which together deliver blood to the heart from the body; and
the superior and inferior venae cavae, which together deliver blood to the heart from the body; and
 the coronary sinus, which returns blood from the walls of the heart itself.
the coronary sinus, which returns blood from the walls of the heart itself.
The superior vena cava enters the upper posterior portion of the right atrium, and the inferior vena cava and coronary sinus enter the lower posterior portion of the right atrium (Fig. 3.66).
From the right atrium, blood passes into the right ventricle through the right atrioventricular orifice. This opening faces forward and medially and is closed during ventricular contraction by the tricuspid valve.
The interior of the right atrium is divided into two continuous spaces. Externally, this separation is indicated by a shallow, vertical groove (the sulcus terminalis cordis), which extends from the right side of the opening of the superior vena cava to the right side of the opening of the inferior vena cava. Internally, this division is indicated by the crista terminalis (Fig. 3.66), which is a smooth, muscular ridge that begins on the roof of the atrium just in front of the opening of the superior vena cava and extends down the lateral wall to the anterior lip of the inferior vena cava.
The space posterior to the crista is the sinus of venae cavae and is derived embryologically from the right horn of the sinus venosus. This component of the right atrium has smooth, thin walls, and both venae cavae empty into this space.
The space anterior to the crista, including the right auricle, is sometimes referred to as the atrium proper. This terminology is based on its origin from the embryonic primitive atrium. Its walls are covered by ridges called the musculi pectinati (pectinate muscles), which fan out from the crista like the “teeth of a comb.” These ridges are also found in the right auricle, which is an ear-like, conical, muscular pouch that externally overlaps the ascending aorta.
An additional structure in the right atrium is the opening of coronary sinus, which receives blood from most of the cardiac veins and opens medially to the opening of inferior vena cava. Associated with these openings are small folds of tissue derived from the valve of the embryonic sinus venosus (the valve of coronary sinus and the valve of inferior vena cava, respectively). During development, the valve of inferior vena cava helps direct incoming oxygenated blood through the foramen ovale and into the left atrium.
Separating the right atrium from the left atrium is the interatrial septum, which faces forward and to the right because the left atrium lies posteriorly and to the left of the right atrium. A depression is clearly visible in the septum just above the orifice of the inferior vena cava. This is the fossa ovalis (oval fossa), with its prominent margin, the limbus fossa ovalis (border of oval fossa) (Fig. 3.66).
The fossa ovalis marks the location of the embryonic foramen ovale, which is an important part of fetal circulation. The foramen ovale allows oxygenated blood entering the right atrium through the inferior vena cava to pass directly to the left atrium and so bypass the lungs, which are nonfunctional before birth.
Finally, numerous small openings—the openings of the smallest cardiac veins (the foramina of the venae cordis minimae)—are scattered along the walls of the right atrium. These are small veins that drain the myocardium directly into the right atrium.
In the anatomical position, the right ventricle forms most of the anterior surface of the heart and a portion of the diaphragmatic surface (Fig. 3.67). It is to the right of the right atrium and located in front of and to the left of the right atrioventricular orifice. Blood entering the right ventricle from the right atrium therefore moves in a horizontal and forward direction.
Fig. 3.67 Internal view of the right ventricle.
The outflow tract of the right ventricle, which leads to the pulmonary trunk, is the conus arteriosus (infundibulum) (Fig. 3.67). This area has smooth walls and derives from the embryonic bulbus cordis.
The walls of the inflow portion of the right ventricle have numerous muscular, irregular structures called trabeculae carneae (Fig. 3.67). Most of these are either attached to the ventricular walls throughout their length, forming ridges, or attached at both ends, forming bridges.
A few trabeculae carneae (papillary muscles) have only one end attached to the ventricular surface, whereas the other end serves as the point of attachment for tendon-like fibrous cords (the chordae tendineae), which connect to the free edges of the cusps of the tricuspid valve.
There are three papillary muscles in the right ventricle. Named relative to their point of origin on the ventricular surface, they are the anterior, posterior, and septal papillary muscles (Fig. 3.67).
 The septal papillary muscle is the most inconsistent papillary muscle, being either small or absent, with chordae tendineae emerging directly from the septal wall.
The septal papillary muscle is the most inconsistent papillary muscle, being either small or absent, with chordae tendineae emerging directly from the septal wall.
A single specialized trabeculum, the septomarginal trabecula (moderator band), forms a bridge between the lower portion of the interventricular septum and the base of the anterior papillary muscle. The septomarginal trabecula carries a portion of the cardiac conduction system, the right bundle of the atrioventricular bundle, to the anterior wall of the right ventricle.
The right atrioventricular orifice is closed during ventricular contraction by the tricuspid valve (right atrioventricular valve), so named because it usually consists of three cusps or leaflets (Fig. 3.67). The base of each cusp is secured to a fibrous ring surrounding the atrioventricular orifice. This fibrous ring helps maintain the shape of the opening. The cusps are continuous with each other near their bases at sites termed commissures.
The naming of the three cusps, the anterior, septal, and posterior cusps, is based on their relative position in the right ventricle (Fig. 3.67). The free margins of the cusps are attached to the chordae tendineae, which arise from the tips of the papillary muscles.
During filling of the right ventricle, the tricuspid valve is open, and the three cusps project into the right ventricle.
Without the presence of a compensating mechanism, when the ventricular musculature contracts, the valve cusps would be forced upward with the flow of blood and blood would move back into the right atrium. However, contraction of the papillary muscles attached to the cusps by chordae tendineae prevent the cusps from being everted into the right atrium.
Simply put, the papillary muscles and associated chordae tendineae keep the valves closed during the dramatic changes in ventricular size that occur during contraction.
In addition, chordae tendineae from two papillary muscles attach to each cusp. This helps prevent separation of the cusps during ventricular contraction. Proper closing of the tricuspid valve causes blood to exit the right ventricle and move into the pulmonary trunk.
Necrosis of a papillary muscle following a myocardial infarction (heart attack) may result in prolapse of the related valve.
At the apex of the infundibulum, the outflow tract of the right ventricle, the opening into the pulmonary trunk is closed by the pulmonary valve (Fig. 3.68), which consists of three semilunar cusps with free edges projecting upward into the lumen of the pulmonary trunk. The free superior edge of each cusp has a middle, thickened portion, the nodule of the semilunar cusp; and a thin lateral portion, the lunula of the semilunar cusp (Fig. 3.68).
Fig. 3.68 Posterior view of the pulmonary valve.
The cusps are named the left, right, and anterior semilunar cusps, relative to their fetal position before rotation of the outflow tracks from the ventricles is complete. Each cusp forms a pocket-like sinus (Fig. 3.68)—a dilation in the wall of the initial portion of the pulmonary trunk. After ventricular contraction, the recoil of blood fills these pulmonary sinuses and forces the cusps closed. This prevents blood in the pulmonary trunk from refilling the right ventricle.
Imaging app
Visualizing the right atrium and pulmonary veins
Fig. 3.69 Axial computed tomography image showing the pulmonary veins entering the left atrium.
The left atrium forms most of the base or posterior surface of the heart.
As with the right atrium, the left atrium is derived embryologically from two structures.
 The posterior half, or inflow portion, receives the four pulmonary veins (Fig. 3.70). It has smooth walls and derives from the proximal parts of the pulmonary veins that are incorporated into the left atrium during development.
The posterior half, or inflow portion, receives the four pulmonary veins (Fig. 3.70). It has smooth walls and derives from the proximal parts of the pulmonary veins that are incorporated into the left atrium during development.
Fig. 3.70 Internal view of left atrium
The interatrial septum is part of the anterior wall of the left atrium. The thin area or depression in the septum is the valve of the foramen ovale and is opposite the floor of the fossa ovalis in the right atrium.
During development, the valve of foramen ovale prevents blood from passing from the left atrium to the right atrium. This valve may not be completely fused in some adults, leaving a “probe patent” passage between the right atrium and the left atrium.
The left ventricle lies anterior to the left atrium. It contributes to the anterior, diaphragmatic, and left pulmonary surfaces of the heart, and forms the apex.
Blood enters the ventricle through the left atrioventricular orifice and flows in a forward direction to the apex (Fig. 3.71). The chamber itself is conical, is longer than the right ventricle, and has the thickest layer of myocardium. The outflow tract (the aortic vestibule) is posterior to the infundibulum of the right ventricle, has smooth walls, and is derived from the embryonic bulbus cordis.
Fig. 3.71 Internal view of the left ventricle.
The trabeculae carneae in the left ventricle are fine and delicate in contrast to those in the right ventricle. The general appearance of the trabeculae with muscular ridges and bridges is similar to that of the right ventricle (Fig. 3.71).
Papillary muscles, together with chordae tendineae, are also observed and their structure is as described above for the right ventricle. Two papillary muscles, the anterior and posterior papillary muscles, are usually found in the left ventricle and are larger than those of the right ventricle (Fig. 3.71).
In the anatomical position, the left ventricle is somewhat posterior to the right ventricle. The interventricular septum therefore forms the anterior wall and some of the wall on the right side of the left ventricle. The septum is described as having two parts:
The muscular part is thick and forms the major part of the septum, whereas the membranous part is the thin, upper part of the septum. A third part of the septum may be considered an atrioventricular part because of its position above the septal cusp of the tricuspid valve. This superior location places this part of the septum between the left ventricle and right atrium.
The left atrioventricular orifice opens into the posterior right side of the superior part of the left ventricle. It is closed during ventricular contraction by the mitral valve (left atrioventricular valve), which is also referred to as the bicuspid valve because it has two cusps, the anterior and posterior cusps (Fig. 3.71). At their base, the cusps are secured to a fibrous ring surrounding the opening, and are continuous with each other at the commissures. The coordinated action of the papillary muscles and chordae tendineae is as described for the right ventricle.
The aortic vestibule, or outflow tract of the left ventricle, is continuous superiorly with the ascending aorta. The opening from the left ventricle into the aorta is closed by the aortic valve. This valve is similar in structure to the pulmonary valve. It consists of three semilunar cusps with the free edge of each projecting upward into the lumen of the ascending aorta (Fig. 3.72).
Fig. 3.72 Anterior view of the aortic valve.
Between the semilunar cusps and the wall of the ascending aorta are pocket-like sinuses—the right, left, and posterior aortic sinuses. The right and left coronary arteries originate from the right and left aortic sinuses. Because of this, the posterior aortic sinus and cusp are sometimes referred to as the noncoronary sinus and cusp.
The functioning of the aortic valve is similar to that of the pulmonary valve with one important additional process: as blood recoils after ventricular contraction and fills the aortic sinuses, it is automatically forced into the coronary arteries because these vessels originate from the right and left aortic sinuses.
Where to listen for heart sounds
To listen for valve sounds, position the stethoscope downstream from the flow of blood through the valves (Fig. 3.73).
 The pulmonary valve is heard over the medial end of the left second intercostal space.
The pulmonary valve is heard over the medial end of the left second intercostal space.
 The aortic valve is heard over the medial end of the right second intercostal space.
The aortic valve is heard over the medial end of the right second intercostal space.
Clinical app
Valve disease
Valve problems consist of two basic types:
 incompetence (insufficiency), which results from poorly functioning valves, and
incompetence (insufficiency), which results from poorly functioning valves, and
 stenosis, a narrowing of the orifice, caused by the valve’s inability to open fully.
stenosis, a narrowing of the orifice, caused by the valve’s inability to open fully.
Mitral valve disease is usually a mixed pattern of stenosis and incompetence, one of which usually predominates. Both stenosis and incompetence lead to a poorly functioning valve and subsequent heart changes, which include:
 left ventricular hypertrophy (this is appreciably less marked in patients with mitral stenosis),
left ventricular hypertrophy (this is appreciably less marked in patients with mitral stenosis),
 increased pulmonary venous pressure,
increased pulmonary venous pressure,
 enlargement (dilation) and hypertrophy of the left atrium.
enlargement (dilation) and hypertrophy of the left atrium.
Aortic valve disease—both aortic stenosis and aortic regurgitation (backflow) can produce heart failure.
Valve disease in the right side of the heart (affecting the tricuspid or pulmonary valve) is most likely caused by infection. The resulting valve dysfunction produces abnormal pressure changes in the right atrium and right ventricle, and these can induce cardiac failure.
Common congenital heart defects
The most common abnormalities that occur during development are those produced by a defect in the interatrial and interventricular septa.
A defect in the interatrial septum allows blood to pass from one side of the heart to the other from the chamber with the higher pressure; this is clinically referred to as a shunt. An atrial septal defect (ASD) allows oxygenated blood to flow from the left atrium (higher pressure) across the ASD into the right atrium (lower pressure). Many patients with ASD are asymptomatic, but in some cases the ASD may need to be closed surgically or by endovascular devices. The most common of all congenital heart defects are those that occur in the interventricular septum—ventricular septal defect (VSD). These lesions are most frequent in the membranous portion of the septum and they allow blood to move from the left ventricle (higher pressure) to the right ventricle (lower pressure); this leads to right ventricular hypertrophy and pulmonary arterial hypertension. If large enough and left untreated, VSDs can produce marked clinical problems that might require surgery.
Occasionally, the ductus arteriosus, which connects the left branch of the pulmonary artery to the inferior aspect of the aortic arch, fails to close at birth. When this occurs, the oxygenated blood in the aortic arch (higher pressure) passes into the left branch of the pulmonary artery (lower pressure) and produces pulmonary hypertension. This is termed a patent or persistent ductus arteriosus (PDA).
Clinical app
Cardiac auscultation
Auscultation of the heart reveals the normal audible cardiac cycle, which allows the clinician to assess heart rate, rhythm, and regularity. Furthermore, cardiac murmurs that have characteristic sounds within the phases of the cardiac cycle can be demonstrated (Fig. 3.74).
Fig. 3.74 Heart sounds and how they relate to valve closure, the electrocardiogram, and ventricular pressure.
Cardiac skeleton
The cardiac skeleton consists of dense, fibrous connective tissue in the form of four rings with interconnecting areas between the atria and the ventricles (Fig. 3.75). The four rings surround the two atrioventricular orifices, the aortic orifice and opening of the pulmonary trunk. They are the anulus fibrosus. The interconnecting areas include:
 the right fibrous trigone—a thickened area of connective tissue between the aortic ring and right atrioventricular ring (Fig. 3.75); and
the right fibrous trigone—a thickened area of connective tissue between the aortic ring and right atrioventricular ring (Fig. 3.75); and
 the left fibrous trigone—a thickened area of connective tissue between the aortic ring and the left atrioventricular ring (Fig. 3.75).
the left fibrous trigone—a thickened area of connective tissue between the aortic ring and the left atrioventricular ring (Fig. 3.75).
Fig. 3.75 Cardiac skeleton (atria removed).
The cardiac skeleton helps maintain the integrity of the openings it surrounds and provides points of attachment for the cusps. It also separates the atrial musculature from the ventricular musculature. The atrial myocardium originates from the upper border of the rings, whereas the ventricular myocardium originates from the lower border of the rings.
The cardiac skeleton also serves as a dense connective tissue partition that electrically isolates the atria from the ventricles. The atrioventricular bundle, which passes through the anulus, is the single connection between these two groups of myocardium.
Coronary vasculature
Two coronary arteries arise from the aortic sinuses in the initial portion of the ascending aorta and supply the muscle and other tissues of the heart. They circle the heart in the coronary sulcus, with marginal and interventricular branches, in the interventricular sulci, converging toward the apex of the heart.
The returning venous blood passes through cardiac veins, most of which empty into the coronary sinus. This large venous structure is located in the coronary sulcus on the posterior surface of the heart between the left atrium and left ventricle. The coronary sinus empties into the right atrium between the opening of the inferior vena cava and the right atrioventricular orifice.
The right coronary artery branches from the right aortic sinus of the ascending aorta, passing anterior and to the right between the right auricle and the pulmonary trunk. It then descends vertically between the right atrium and right ventricle in the coronary sulcus (Fig. 3.76). Reaching the inferior margin of the heart, it turns posteriorly and continues in the sulcus onto the diaphragmatic surface and base of the heart. During this course, the following branches arise:
Fig. 3.76 Anterior view of coronary arterial system. Right dominant coronary artery.
The right coronary artery supplies the right atrium and right ventricle, the sinu-atrial and atrioventricular nodes, the interatrial septum, a portion of the left atrium, the posteroinferior one-third of the interventricular septum, and a portion of the posterior part of the left ventricle.
The left coronary artery branches from the left aortic sinus of the ascending aorta passing between the pulmonary trunk and the left auricle before entering the coronary sulcus. Posterior to the pulmonary trunk, the artery divides into its two terminal branches, the anterior interventricular and the circumflex (Fig. 3.76).
The left coronary artery supplies most of the left atrium and left ventricle, and most of the interventricular septum, including the atrioventricular bundle and its branches.
Variations in the distribution patterns of coronary arteries. Several major variations in the basic distribution patterns of the coronary arteries occur.
 In contrast, in hearts with a left dominant coronary artery, the posterior interventricular branch arises from an enlarged circumflex branch and supplies most of the posterior wall of the left ventricle (Fig. 3.77).
In contrast, in hearts with a left dominant coronary artery, the posterior interventricular branch arises from an enlarged circumflex branch and supplies most of the posterior wall of the left ventricle (Fig. 3.77).
Fig. 3.77 Left dominant coronary artery.
Clinical terminology for coronary arteries
In practice, physicians use alternative names for the coronary vessels. The short left coronary artery is referred to as the left main stem vessel. One of its primary branches, the anterior interventricular artery, is termed the left anterior descending artery (LAD). Similarly, the terminal branch of the right coronary artery, the posterior interventricular artery, is termed the posterior descending artery (PDA).
The coronary sinus receives four major tributaries: the great, middle, small, and posterior cardiac veins.
The great cardiac vein begins at the apex of the heart (Fig. 3.78A) and ascends in the anterior interventricular sulcus, where it travels with the anterior interventricular artery. In this location it may be referred to as the anterior interventricular vein. At the coronary sulcus, it turns to the left and continues onto the base/diaphragmatic surface of the heart and is associated with the circumflex branch of the left coronary artery. Continuing along its path in the coronary sulcus, the great cardiac vein gradually enlarges becoming the coronary sinus, which enters the right atrium (Fig. 3.78B).
The middle cardiac vein (posterior interventricular vein) begins near the apex of the heart and ascends in the posterior interventricular sulcus toward the coronary sinus (Fig. 3.78B). It is associated with the posterior interventricular branch of the right or left coronary artery throughout its course.
The small cardiac vein begins in the lower anterior section of the coronary sulcus, between the right atrium and right ventricle (Fig. 3.78A). It continues in this groove onto the base/diaphragmatic surface of the heart and enters the coronary sinus at its atrial end. It is a companion of the right coronary artery throughout its course and may receive the right marginal vein (Fig. 3.78A). This small vein accompanies the marginal branch of the right coronary artery along the acute margin of the heart. If the right marginal vein does not join the small cardiac vein, it enters the right atrium directly.
The posterior cardiac vein lies on the posterior surface of the left ventricle just to the left of the middle cardiac vein (Fig. 3.78B). It either enters the coronary sinus directly or joins the great cardiac vein.
Other cardiac veins. Two additional groups of cardiac veins are also involved in the venous drainage of the heart.
 The anterior veins of right ventricle (anterior cardiac veins) are small veins that arise on the anterior surface of the right ventricle (Fig. 3.78A). They cross the coronary sulcus and enter the anterior wall of the right atrium. They drain the anterior portion of the right ventricle. The right marginal vein may be part of this group if it does not enter the small cardiac vein.
The anterior veins of right ventricle (anterior cardiac veins) are small veins that arise on the anterior surface of the right ventricle (Fig. 3.78A). They cross the coronary sulcus and enter the anterior wall of the right atrium. They drain the anterior portion of the right ventricle. The right marginal vein may be part of this group if it does not enter the small cardiac vein.
The lymphatic vessels of the heart follow the coronary arteries and drain mainly into:
 brachiocephalic nodes, anterior to the brachiocephalic veins; and
brachiocephalic nodes, anterior to the brachiocephalic veins; and
 tracheobronchial nodes, at the inferior end of the trachea.
tracheobronchial nodes, at the inferior end of the trachea.
Cardiac conduction system
The cardiac conduction system initiates and coordinates contraction of the musculature of the atria and ventricles (Fig. 3.79). It consists of nodes and networks of specialized cardiac muscle cells organized into four basic components:
 the atrioventricular bundle with its right and left bundle branches, and
the atrioventricular bundle with its right and left bundle branches, and
 the subendocardial plexus of conduction cells (the Purkinje fibers).
the subendocardial plexus of conduction cells (the Purkinje fibers).
Fig. 3.79 Conduction system of the heart. A. Right chambers. B. Left chambers.
The unique distribution pattern of the cardiac conduction system is an important unidirectional pathway of excitation/contraction. Throughout its course, large branches of the conduction system are insulated from the surrounding myocardium by connective tissue. This tends to decrease inappropriate stimulation and contraction of cardiac muscle fibers.
Thus, a unidirectional wave of excitation and contraction is established, which moves from the papillary muscles and apex of the ventricles to the arterial outflow tracts.
Clinical app
Cardiac conduction system
The cardiac conduction system can be affected by coronary artery disease. The normal rhythm may be disturbed if the blood supply to the coronary conduction system is disrupted. If a dysrhythmia affects the heart rate or the order in which the chambers contract, heart failure and death may ensue.
Impulses begin at the sinu-atrial node, the cardiac pacemaker. This collection of cells is located at the superior end of the crista terminalis at the junction of the superior vena cava and the right atrium (Fig. 3.79A). This is also the junction between the parts of the right atrium derived from the embryonic sinus venosus and the atrium proper.
The excitation signals generated by the sinu-atrial node spread across the atria, causing the muscle to contract.
Concurrently, the wave of excitation in the atria stimulates the atrioventricular node, which is located near the opening of the coronary sinus, close to the attachment of the septal cusp of the tricuspid valve, and within the atrioventricular septum (Fig. 3.79A).
The atrioventricular bundle is a direct continuation of the atrioventricular node (Fig. 3.79A). It follows the lower border of the membranous part of the interventricular septum before splitting into right and left bundles.
The right bundle branch continues on the right side of the interventricular septum toward the apex of the right ventricle. From the septum it enters the septomarginal trabecula to reach the base of the anterior papillary muscle. At this point, it divides and is continuous with the final component of the cardiac conduction system, the subendocardial plexus of ventricular conduction cells or Purkinje fibers. This network of specialized cells spreads throughout the ventricle to supply ventricular musculature including the papillary muscles.
The left bundle branch passes to the left side of the muscular interventricular septum and descends to the apex of the left ventricle (Fig. 3.79B). Along its course it gives off branches that eventually become continuous with the subendocardial plexus of conduction cells (Purkinje fibers). As with the right side, this network of specialized cells spreads the excitation impulses throughout the left ventricle.
Cardiac innervation
The autonomic division of the peripheral nervous system is directly responsible for regulating:
 force of each contraction, and
force of each contraction, and
Branches from both the parasympathetic and sympathetic systems contribute to the formation of the cardiac plexus. This plexus consists of a superficial part, inferior to the aortic arch and between it and the pulmonary trunk (Fig. 3.80A), and a deep part, between the aortic arch and the tracheal bifurcation (Fig. 3.80B).
Fig. 3.80 Cardiac plexus. A. Superficial. B. Deep.
From the cardiac plexus, small branches that are mixed nerves containing both sympathetic and parasympathetic fibers supply the heart. These branches affect nodal tissue and other components of the conduction system, coronary blood vessels, and atrial and ventricular musculature.
Stimulation of the parasympathetic system:
 reduces force of contraction, and
reduces force of contraction, and
 constricts the coronary arteries.
constricts the coronary arteries.
The preganglionic parasympathetic fibers reach the heart as cardiac branches from the right and left vagus nerves (Fig. 3.80). They enter the cardiac plexus and synapse in ganglia located either within the plexus or in the walls of the atria.
Stimulation of the sympathetic system:
 increases the force of contraction.
increases the force of contraction.
Sympathetic fibers reach the cardiac plexus through the cardiac nerves from the sympathetic trunk (Fig. 3.80). Preganglionic sympathetic fibers from the upper four or five segments of the thoracic spinal cord enter and move through the sympathetic trunk. They synapse in cervical and upper thoracic sympathetic ganglia, and postganglionic fibers proceed as bilateral branches from the sympathetic trunk to the cardiac plexus.
Visceral afferents from the heart are also a component of the cardiac plexus. These fibers pass through the cardiac plexus and return to the central nervous system in the cardiac nerves from the sympathetic trunk and in the vagal cardiac branches.
The afferents associated with the vagal cardiac nerves return to the vagus nerve [X]. They sense alterations in blood pressure and blood chemistry and are therefore primarily concerned with cardiac reflexes.
The afferents associated with the cardiac nerves from the sympathetic trunks return to either the cervical or the thoracic portions of the sympathetic trunk. If they are in the cervical portion of the trunk, they normally descend to the thoracic region where they reenter the upper four or five thoracic spinal cord segments along with the afferents from the thoracic region of the sympathetic trunk.
Heart attack
A heart attack occurs when the perfusion to the myocardium is insufficient to meet the metabolic needs of the tissue, leading to irreversible tissue damage. The most common cause is a total occlusion of a major coronary artery.
Occlusion of a major coronary artery, usually due to atherosclerosis, leads to inadequate oxygenation of an area of myocardium and cell death. The severity of the problem will be related to the size and location of the artery involved, whether or not the blockage is complete, and whether there are collateral vessels to provide perfusion to the territory from other vessels. Depending on the severity, patients can develop pain (angina) or a myocardial infarction (MI).
Percutaneous coronary intervention
This is a technique in which a long fine tube (a catheter) is inserted into the femoral artery in the thigh, passed through the external and common iliac arteries and into the abdominal aorta. It continues to be moved upward through the thoracic aorta to the origins of the coronary arteries. The coronaries may also be approached via the radial or brachial arteries. A fine wire is then passed into the coronary artery and is used to cross the stenosis. A fine balloon is then passed over the wire and may be inflated at the level of the obstruction, thus widening it; this is termed angioplasty. More commonly, this is augmented by placement of a fine wire mesh (a stent) inside the obstruction to hold it open. Other percutaneous interventions are suction extraction of a coronary thrombus and rotary ablation of a plaque.
If coronary artery disease is too extensive to be treated by percutaneous intervention, surgical coronary artery bypass grafting may be necessary. The great saphenous vein, in the lower limb, is harvested and used as a graft. It is divided into several pieces, each of which is used to bypass blocked sections of the coronary arteries. The internal thoracic and radial arteries can also be used.
Clinical app
Classic symptoms of heart attack
The typical symptoms are chest heaviness or pressure, which can be severe, lasting more than 20 minutes, and often associated with sweating. The pain in the chest (which may be described as an “elephant sitting on my chest” or by using a clenched fist to describe the pain [Levine sign]) often radiates to the arms (left more common than the right), and can be associated with nausea. The severity of ischemia and infarction depends on the rate at which the occlusion or stenosis has occurred and whether or not collateral channels have had a chance to develop.
Clinical app
Referred pain
When cardiac cells die during an MI, pain fibers (visceral afferents) are stimulated. They detect events at the cellular level as tissue-damaging events (i.e., cardiac ischemia). These visceral sensory fibers follow the course of sympathetic fibers that innervate the heart and enter the spinal cord between T1 and T4 spinal levels. At this level, somatic sensory fibers from spinal nerves T1 to T4 also enter the spinal cord via the posterior roots. Both types of fibers (visceral and somatic) synapse with interneurons, which then synapse with second neurons whose fibers pass across the cord and then ascend to the somatosensory areas of the brain that represent the T1 to T4 levels. The brain is unable to distinguish clearly between the visceral sensory distribution and the somatic sensory distribution, and therefore the pain is interpreted as arising from the somatic regions rather than the visceral organ (i.e., the heart; Fig. 3.81).
Fig. 3.81 Mechanism for perceiving heart pain in T1–T4 dermatomes.
Clinical app
Are heart attack symptoms the same in men and women?
Although men and women can experience the typical symptoms of severe chest pain, cold sweats, and pain in the left arm, women are more likely than men to have subtler, less recognizable symptoms. These may include abdominal pain, achiness in the jaw or back, nausea, shortness of breath and/or simply fatigue. The mechanism of this difference is not understood, but it is important to consider cardiac ischemia for a wide range of symptoms.
Pulmonary trunk
The pulmonary trunk is within the pericardial sac (Fig. 3.82A), covered by the visceral layer of serous pericardium and enclosed in a common sheath with the ascending aorta. Arising from the conus arteriosus of the right ventricle it is slightly anterior to the aortic orifice and ascends, moving posteriorly and to the left, lying initially anterior and then to the left of the ascending aorta. At approximately the level of the intervertebral disc between vertebrae TV and TVI, opposite the left border of the sternum and posterior to the third left costal cartilage, the pulmonary trunk divides into:
 the right pulmonary artery, which passes to the right, posterior to the ascending aorta and the superior vena cava, to enter the right lung (Fig. 3.82B); and
the right pulmonary artery, which passes to the right, posterior to the ascending aorta and the superior vena cava, to enter the right lung (Fig. 3.82B); and
 the left pulmonary artery, which passes inferiorly to the arch of the aorta and anteriorly to the descending aorta to enter the left lung (Fig. 3.82A, B).
the left pulmonary artery, which passes inferiorly to the arch of the aorta and anteriorly to the descending aorta to enter the left lung (Fig. 3.82A, B).
Fig. 3.82 Major vessels within the middle mediastinum. A. Anterior view. B. Posterior view.
Ascending aorta
The ascending aorta is within the pericardial sac and covered by a visceral layer of serous pericardium, which also surrounds the pulmonary trunk in a common sheath (Fig. 3.82A).
The origin of the ascending aorta is the aortic orifice at the base of the left ventricle, which is level with the lower edge of the third left costal cartilage and posterior to the left half of the sternum. Moving superiorly, slightly forward and to the right, the ascending aorta continues to the level of the second right costal cartilage. At this point, it enters the superior mediastinum and is then referred to as the arch of the aorta.
Immediately superior to the point where the ascending aorta arises from the left ventricle are three small outward bulges opposite the semilunar cusps of the aortic valve. These are the posterior, right, and left aortic sinuses. The right and left coronary arteries originate from the right and left aortic sinuses, respectively.
Other vasculature
Passing through the fibrous pericardium at approximately the level of the second costal cartilage, the inferior half of the superior vena cava is within the pericardial sac (Fig. 3.82B). It enters the right atrium at the lower level of the third costal cartilage. The portion within the pericardial sac is covered with serous pericardium except for a small area on its posterior surface.
After passing through the diaphragm, at approximately the level of vertebra TVIII, the inferior vena cava enters the fibrous pericardium. A short portion of this vessel is within the pericardial sac before entering the right atrium. While within the pericardial sac, it is covered by serous pericardium except for a small portion of its posterior surface (Fig. 3.82B).
A very short segment of each of the pulmonary veins is also within the pericardial sac. These veins, usually two from each lung, pass through the fibrous pericardium and enter the superior region of the left atrium on its posterior surface. In the pericardial sac, all but a portion of the posterior surface of these veins is covered by serous pericardium. In addition, the oblique pericardial sinus is between the right and left pulmonary veins, within the pericardial sac (Fig. 3.82B).
Superior mediastinum
Posterior to the manubrium of the sternum and anterior to the bodies of the first four thoracic vertebrae is the superior mediastinum (see Fig. 3.53).
 Lateral borders—the mediastinal part of the parietal pleura on either side.
Lateral borders—the mediastinal part of the parietal pleura on either side.
The superior mediastinum is continuous with the neck superiorly and with the inferior mediastinum inferiorly.
Major structures found in the superior mediastinum (Figs. 3.83, 3.84) include the:
 right and left brachiocephalic veins,
right and left brachiocephalic veins,
 left superior intercostal vein,
left superior intercostal vein,
 arch of the aorta with its three large branches,
arch of the aorta with its three large branches,
 left recurrent laryngeal branch of the left vagus nerve,
left recurrent laryngeal branch of the left vagus nerve,
 other small nerves, blood vessels, and lymphatics.
other small nerves, blood vessels, and lymphatics.
Fig. 3.83 Structures in the superior mediastinum.
Imaging app
Visualizing structures in the superior mediastinum
Fig. 3.84 Cross-section through the superior mediastinum at the level of vertebra TIII. A. Diagram. B. Axial computed tomography image.
Thymus
Lying immediately posterior to the manubrium of the sternum, the thymus, asymmetrical and bilobed, is the most anterior component of the superior mediastinum (Fig. 3.85).
Fig. 3.85 Thymus.
The upper extent of the thymus can reach into the neck as high as the thyroid gland and a lower portion typically extends into the anterior mediastinum over the pericardial sac.
Involved in the early development of the immune system, the thymus is a large structure in the child, begins to atrophy after puberty, and shows considerable size variation in the adult. In the elderly adult, it is barely identifiable as an organ, consisting mostly of fatty tissue that is sometimes arranged as two lobulated fatty structures.
Arteries to the thymus are small branches from the internal thoracic arteries. Venous drainage is usually into the left brachiocephalic vein and possibly into the internal thoracic veins.
Lymphatic drainage returns to multiple groups of nodes at one or more of the following locations:
 along the internal thoracic arteries (parasternal),
along the internal thoracic arteries (parasternal),
 at the tracheal bifurcation (tracheobronchial), and
at the tracheal bifurcation (tracheobronchial), and
Clinical app
Ectopic parathyroid glands in the thymus
The parathyroid glands develop from the third pharyngeal pouch, which also forms the thymus. The thymus is therefore a common site for ectopic parathyroid glands and, potentially, ectopic parathyroid hormone production.
Right and left brachiocephalic veins
The left and right brachiocephalic veins are located immediately posterior to the thymus and form on each side at the junction between the internal jugular and subclavian veins (see Fig. 3.83). The left brachiocephalic vein crosses the midline and joins with the right brachiocephalic vein to form the superior vena cava (Fig. 3.86).
Fig. 3.86 Superior mediastinum with thymus removed.
 The right brachiocephalic vein begins posterior to the medial end of the right clavicle and passes vertically downward, forming the superior vena cava when it is joined by the left brachiocephalic vein (Fig. 3.83). Venous tributaries include the vertebral, first posterior intercostal, and internal thoracic veins. The inferior thyroid and thymic veins may also drain into it.
The right brachiocephalic vein begins posterior to the medial end of the right clavicle and passes vertically downward, forming the superior vena cava when it is joined by the left brachiocephalic vein (Fig. 3.83). Venous tributaries include the vertebral, first posterior intercostal, and internal thoracic veins. The inferior thyroid and thymic veins may also drain into it.
 The left brachiocephalic vein begins posterior to the medial end of the left clavicle (Fig. 3.83). It crosses to the right, moving in a slightly inferior direction, and joins with the right brachiocephalic vein to form the superior vena cava posterior to the lower edge of the right first costal cartilage close to the right sternal border. Venous tributaries include the vertebral, first posterior intercostal, left superior intercostal, inferior thyroid, and internal thoracic veins. It may also receive thymic and pericardial veins.
The left brachiocephalic vein begins posterior to the medial end of the left clavicle (Fig. 3.83). It crosses to the right, moving in a slightly inferior direction, and joins with the right brachiocephalic vein to form the superior vena cava posterior to the lower edge of the right first costal cartilage close to the right sternal border. Venous tributaries include the vertebral, first posterior intercostal, left superior intercostal, inferior thyroid, and internal thoracic veins. It may also receive thymic and pericardial veins.
Left brachiocephalic vein
The left brachiocephalic vein crosses the midline posterior to the manubrium in the adult. In infants and children, the left brachiocephalic vein rises above the superior border of the manubrium and therefore is less protected.
Left superior intercostal vein
The left superior intercostal vein receives the second, third, and sometimes the fourth left posterior intercostal veins, usually the left bronchial veins, and sometimes the left pericardiacophrenic vein. It passes over the left side of the aortic arch, lateral to the left vagus nerve and medial to the left phrenic nerve, before entering the left brachiocephalic vein (Fig. 3.87). Inferiorly, it may connect with the accessory hemiazygos vein (superior hemiazygos vein).
Fig. 3.87 Left superior intercostal vein.
Superior vena cava
The vertically oriented superior vena cava begins posterior to the lower edge of the right first costal cartilage, where the right and left brachiocephalic veins join, and terminates at the lower edge of the right third costal cartilage, where it joins the right atrium (see Fig. 3.83).
The lower half of the superior vena cava is within the pericardial sac and contained in the middle mediastinum.
The superior vena cava receives the azygos vein immediately before entering the pericardial sac and may also receive pericardial and mediastinal veins.
Clinical app
Venous access for central and dialysis lines
Large systemic veins are used to establish central venous access for administering large amounts of fluid, drugs, and blood. Most of these lines (small bore tubes) are introduced through venous puncture into the axillary, subclavian, or internal jugular veins. The lines are then passed through the main veins of the superior mediastinum, with the tips of the lines usually residing in the distal portion of the superior vena cava or in the right atrium.
Clinical app
Using the superior vena cava to access the inferior vena cava
Because the superior and inferior vena cava are oriented along the same vertical axis, a guidewire, catheter, or line can be passed from the superior vena cava through the right atrium and into the inferior vena cava. This is a common route of access for such procedures as:
Arch of aorta and its branches
The thoracic portion of the aorta can be divided into ascending aorta, arch of aorta, and thoracic (descending) aorta. Only the arch of the aorta is in the superior mediastinum. It begins when the ascending aorta emerges from the pericardial sac and courses upward, backward, and to the left as it passes through the superior mediastinum, ending on the left side at vertebral level TIV/V. Extending as high as the midlevel of the manubrium of sternum, the arch is initially anterior and finally lateral to the trachea (Figs. 3.88, 3.89).
Fig. 3.88 Superior mediastinum with thymus and venous channels removed.
Imaging app
Visualizing structures at the TIV vertebral level
Fig. 3.89 Cross-section through the superior mediastinum at the level of vertebra TIV. A. Diagram. B. Axial computed tomography image.
Three branches arise from the superior border of the arch of the aorta and, at their origins, all three are crossed anteriorly by the left brachiocephalic vein.
The first branch
Beginning on the right, the first branch of the arch of aorta is the brachiocephalic trunk (Fig. 3.88). It is the largest of the three branches and, at its point of origin behind the manubrium of sternum, is slightly anterior to the other two branches. It ascends slightly posteriorly and to the right. At the level of the upper edge of the right sternoclavicular joint, the brachiocephalic trunk divides into:
 the right common carotid artery, and
the right common carotid artery, and
The arteries mainly supply the right side of the head and neck and the right upper limb, respectively.
Occasionally, the brachiocephalic trunk has a small branch, the thyroid ima artery, which contributes to the vascular supply of the thyroid gland.
The second branch
The second branch of the arch of aorta is the left common carotid artery (Fig. 3.88). It arises from the arch immediately to the left and slightly posterior to the brachiocephalic trunk and ascends through the superior mediastinum along the left side of the trachea.
The left common carotid artery supplies the left side of the head and neck.
The third branch
The third branch of the arch of the aorta is the left subclavian artery (Fig. 3.88). It arises from the arch of aorta immediately to the left of, and slightly posterior to, the left common carotid artery and ascends through the superior mediastinum along the left side of the trachea.
The left subclavian artery is the major blood supply to the left upper limb.
Ligamentum arteriosum
The ligamentum arteriosum is also in the superior mediastinum and is important in embryonic circulation, when it is a patent vessel (the ductus arteriosus). It connects the pulmonary trunk with the arch of aorta and allows blood to bypass the lungs during development (Fig. 3.88). The vessel closes soon after birth and forms the ligamentous connection observed in the adult.
Clinical app
Coarctation of the aorta
Coarctation of the aorta is a congenital abnormality in which the aortic lumen is constricted just distal to the origin of the left subclavian artery. At this point, the aorta becomes significantly narrowed and the blood supply to the lower limbs and abdomen is diminished. Over time, collateral vessels develop around the chest wall and abdomen to supply the lower body. The coarctation also affects the heart, which has to pump the blood at higher pressure to maintain peripheral perfusion. This in turn may produce cardiac failure.
Clinical app
Traumatic injury to the aorta
The aorta has three fixed points of attachment:
 the ligamentum arteriosum, and
the ligamentum arteriosum, and
 the point of entry behind the crura of the diaphragm.
the point of entry behind the crura of the diaphragm.
The rest of the aorta is relatively free from attachment to other structures of the mediastinum. A serious deceleration injury (e.g., in a traffic accident) is most likely to cause aortic trauma at these fixed points.
Aortic dissection
In certain conditions, such as in severe arteriovascular disease, the wall of the aorta can split longitudinally, creating a false channel, which may or may not rejoin into the true lumen distally. This aortic dissection occurs between the intima and media anywhere along its length. If it occurs in the ascending aorta or arch of the aorta, blood flow in the coronary and cerebral arteries may be disrupted, resulting in MI or stroke. In the abdomen the visceral vessels may be disrupted, producing ischemia to the gut or kidneys.
Clinical app
Aortic arch and its anomalies
A right-sided arch of aorta occasionally occurs and may be asymptomatic. It can be associated with dextrocardia (right-sided heart) and, in some instances, with complete situs inversus (left-to-right inversion of the body’s organs). It can also be associated with abnormal branching of the great vessels.
Clinical app
Abnormal origin of great vessels
Great vessels occasionally have an abnormal origin, including:
 a common origin of the brachiocephalic trunk and the left common carotid artery,
a common origin of the brachiocephalic trunk and the left common carotid artery,
 the left vertebral artery originating from the aortic arch, and
the left vertebral artery originating from the aortic arch, and
Trachea and esophagus
The trachea is a midline structure that is palpable in the jugular notch as it enters the superior mediastinum. Posterior to it is the esophagus, which is immediately anterior to the vertebral column (Fig. 3.89; also see Fig 3.83). Significant mobility exists in the vertical positioning of these structures as they pass through the superior mediastinum.
As the trachea and esophagus pass through the superior mediastinum, they are crossed laterally by the azygos vein on the right side and the arch of aorta on the left side.
The trachea divides into the right and left main bronchi at, or just inferior to, the transverse plane between the sternal angle and vertebral level TIV/V (Fig. 3.90), whereas the esophagus continues into the posterior mediastinum.
Fig. 3.90 Trachea in the superior mediastinum.
Nerves of the superior mediastinum
Vagus nerves
The vagus nerves [X] pass through the superior and posterior divisions of the mediastinum on their way to the abdominal cavity. As they pass through the thorax, they provide parasympathetic innervation to the thoracic viscera and carry visceral afferents from the thoracic viscera.
Visceral afferents in the vagus nerves relay information to the central nervous system about normal physiological processes and reflex activities. They do not transmit pain sensation.
The right vagus nerve enters the superior mediastinum between the right brachiocephalic vein and the brachiocephalic trunk. It descends in a posterior direction toward the trachea (Fig. 3.91), crosses the lateral surface of the trachea. and passes posteriorly to the root of the right lung to reach the esophagus. Just before the esophagus, it is crossed by the arch of the azygos vein.
Fig. 3.91 Right vagus nerve passing through the superior mediastinum.
As it passes through the superior mediastinum, branches are given off to the esophagus, cardiac plexus, and pulmonary plexus.
The left vagus nerve enters the superior mediastinum posterior to the left brachiocephalic vein between the left common carotid and left subclavian arteries (Fig. 3.92). It passes into the superior mediastinum just deep to the mediastinal part of the parietal pleura and crosses the left side of the arch of aorta. It descends in a posterior direction and passes posterior to the root of the left lung to reach the esophagus in the posterior mediastinum.
Fig. 3.92 Left vagus nerve passing through the superior mediastinum.
As the left vagus nerve passes through the superior mediastinum, branches go to the esophagus, cardiac plexus, and pulmonary plexus.
The left vagus nerve also gives rise to the left recurrent laryngeal nerve, which arises at the inferior margin of the arch of aorta just lateral to the ligamentum arteriosum (Fig. 3.92). The left recurrent laryngeal nerve passes inferior to the arch of aorta before ascending on its medial surface. Entering a groove between the trachea and esophagus, the left recurrent laryngeal nerve continues superiorly to enter the neck and terminate in the larynx (Fig. 3.93).
Fig. 3.93 Left recurrent laryngeal nerve passing through the superior mediastinum.
Phrenic nerves
The phrenic nerves arise in the cervical region from the third, fourth, and fifth cervical spinal cord segments. They descend through the thorax to supply motor and sensory innervation to the diaphragm and its associated membranes. As they pass through the thorax, they provide innervation through somatic afferent fibers to the mediastinal pleura, fibrous pericardium, and parietal layer of the serous pericardium.
The right phrenic nerve enters the superior mediastinum lateral to the right vagus nerve, and lateral and slightly posterior to the beginning of the right brachiocephalic vein (see Fig. 3.91). It continues inferiorly along the right side of this vein and the superior vena cava.
On entering the middle mediastinum, the right phrenic nerve descends along the right side of the pericardial sac, within the fibrous pericardium, anterior to the root of the right lung. The pericardiacophrenic vessels accompany it through most of its course in the thorax (see Fig. 3.55). It leaves the thorax by passing through the diaphragm with the inferior vena cava.
The left phrenic nerve enters the superior mediastinum in a position similar to the path taken by the right phrenic nerve. It lies lateral to the left vagus nerve and lateral and slightly posterior to the beginning of the left brachiocephalic vein (Fig. 3.92), and continues to descend across the left lateral surface of the arch of aorta, passing superficially to the left vagus nerve and the left superior intercostal vein.
On entering the middle mediastinum, the left phrenic nerve follows the left side of the pericardial sac, within the fibrous pericardium, anterior to the root of the left lung, and is accompanied by the pericardiacophrenic vessels (see Fig. 3.55). It leaves the thorax by piercing the diaphragm near the apex of the heart.
Surface anatomy
Visualizing structures in the superior mediastinum
A number of structures in the superior mediastinum in adults can be visualized based on their positions relative to skeletal landmarks that can be palpated through the skin (Fig. 3.94).
 The left brachiocephalic vein crosses from left to right behind the manubrium of sternum.
The left brachiocephalic vein crosses from left to right behind the manubrium of sternum.
Clinical app
The vagus nerves, recurrent laryngeal nerves, and hoarseness
The left recurrent laryngeal nerve is a branch of the left vagus nerve. It passes between the pulmonary artery and the aorta, a region known clinically as the aortopulmonary window and may be compressed in any patient with a pathological mass in this region. This compression results in vocal cord paralysis and hoarseness of the voice. Lymph node enlargement, often associated with the spread of lung cancer, is a common condition that may produce compression. Chest radiography is therefore usually carried out for all patients whose symptoms include a hoarse voice.
More superiorly, the right vagus nerve gives off the right recurrent laryngeal nerve, which “hooks” around the right subclavian artery at the superior sulcus of the right lung. If a patient has a hoarse voice and a right vocal cord palsy is demonstrated with a laryngoscopy, a chest CT should be obtained to assess for cancer in the right lung apex (Pancoast’s tumor).
Thoracic duct in the superior mediastinum
The thoracic duct, the major lymphatic vessel in the body, passes through the posterior portion of the superior mediastinum (see Figs. 3.84A, 3.89A). It:
Posterior mediastinum
The posterior mediastinum is posterior to the pericardial sac and diaphragm and anterior to the bodies of the mid and lower thoracic vertebrae (see Fig. 3.53).
 Its inferior boundary is the diaphragm.
Its inferior boundary is the diaphragm.
 Laterally, it is bordered by the mediastinal part of parietal pleura on either side.
Laterally, it is bordered by the mediastinal part of parietal pleura on either side.
 Superiorly, it is continuous with the superior mediastinum.
Superiorly, it is continuous with the superior mediastinum.
Major structures in the posterior mediastinum include the:
 esophagus and its associated nerve plexus,
esophagus and its associated nerve plexus,
 thoracic aorta and its branches,
thoracic aorta and its branches,
 thoracic duct and associated lymph nodes,
thoracic duct and associated lymph nodes,
Esophagus
The esophagus is a muscular tube passing between the pharynx in the neck and the stomach in the abdomen. It begins at the inferior border of the cricoid cartilage, opposite vertebra CVI, and ends at the cardiac opening of the stomach, opposite vertebra TXI.
The esophagus descends on the anterior aspect of the bodies of the vertebrae, generally in a midline position as it moves through the thorax (Fig. 3.95). As it approaches the diaphragm, it moves anteriorly and to the left, crossing from the right side of the thoracic aorta to a position anterior to it. It passes through the esophageal hiatus, an opening in the muscular part of the diaphragm, at vertebral level TX.
Fig. 3.95 Esophagus.
The esophagus has a slight anterior-to-posterior curvature that parallels the thoracic portion of the vertebral column, and is secured superiorly in the neck by its attachment to the pharynx and inferiorly in the thorax by its attachment to the diaphragm.
Relationships to important structures in the posterior mediastinum
In the posterior mediastinum, the right side of the esophagus is covered by the mediastinal part of the parietal pleura.
Posterior to the esophagus, the thoracic duct is on the right side inferiorly, but crosses to the left more superiorly. Also on the left side of the esophagus is the thoracic aorta.
Anterior to the esophagus, below the level of the tracheal bifurcation, are the right pulmonary artery and the left main bronchus. The esophagus then passes immediately posteriorly to the left atrium, separated from it only by pericardium. Inferior to the left atrium, the esophagus is related to the diaphragm.
Structures other than the thoracic duct posterior to the esophagus include portions of the hemiazygos veins, the right posterior intercostal vessels, and, near the diaphragm, the thoracic aorta.
Clinical app
Esophagus constrictions
The esophagus is a flexible, muscular tube that can be compressed or narrowed by surrounding structures at four locations (Fig. 3.96):
 the junction of the esophagus with the pharynx in the neck,
the junction of the esophagus with the pharynx in the neck,
 in the superior mediastinum where the esophagus is crossed by the arch of aorta,
in the superior mediastinum where the esophagus is crossed by the arch of aorta,
 in the posterior mediastinum where the esophagus is compressed by the left main bronchus,
in the posterior mediastinum where the esophagus is compressed by the left main bronchus,
 in the posterior mediastinum at the esophageal hiatus in the diaphragm.
in the posterior mediastinum at the esophageal hiatus in the diaphragm.
Fig. 3.96 Sites of normal esophageal constrictions.
These constrictions have important clinical consequences. For example, a swallowed object is most likely to lodge at a constricted area. An ingested corrosive substance would move more slowly through a narrowed region, causing more damage at this site than elsewhere along the esophagus. Also, constrictions present problems during the passage of instruments.
Arterial supply and venous and lymphatic drainage
The arterial supply and venous drainage of the esophagus in the posterior mediastinum involve many vessels. Esophageal arteries arise from the thoracic aorta, bronchial arteries, and ascending branches of the left gastric artery in the abdomen.
Venous drainage involves small vessels returning to the azygos vein, hemiazygos vein, and esophageal branches to the left gastric vein in the abdomen.
Lymphatic drainage of the esophagus in the posterior mediastinum returns to posterior mediastinal and left gastric nodes.
Innervation
Innervation of the esophagus, in general, is complex. Esophageal branches arise from the vagus nerves and sympathetic trunks.
Striated muscle fibers in the superior portion of the esophagus originate from the branchial arches and are innervated by branchial efferents from the vagus nerves.
Smooth muscle fibers are innervated by components of the parasympathetic part of the autonomic division of the peripheral nervous system, visceral efferents from the vagus nerves. These are preganglionic fibers that synapse in the myenteric and submucosal plexuses of the enteric nervous system in the esophageal wall.
Sensory innervation of the esophagus involves visceral afferent fibers originating in the vagus nerves, sympathetic trunks, and splanchnic nerves.
The visceral afferents from the vagus nerves are involved in relaying information back to the central nervous system about normal physiological processes and reflex activities. They are not involved in the relay of pain recognition.
The visceral afferents that pass through the sympathetic trunks and the splanchnic nerves are the primary participants in detection of esophageal pain and transmission of this information to various levels of the central nervous system.
Esophageal plexus
After passing posteriorly to the root of the lungs, the right and left vagus nerves approach the esophagus. As they reach the esophagus, each nerve divides into several branches that spread over this structure, forming the esophageal plexus (Fig. 3.97). There is some mixing of fibers from the two vagus nerves as the plexus continues inferiorly on the esophagus toward the diaphragm. Just above the diaphragm, fibers of the plexus converge to form two trunks:
Fig. 3.97 Esophageal plexus.
The vagal trunks continue on the surface of the esophagus as it passes through the diaphragm into the abdomen.
Clinical app
Esophageal cancer
When patients present with esophageal cancer, it is important to note which portion of the esophagus contains the tumor, because tumor location determines the sites to which the disease will spread.
Esophageal cancer spreads quickly to lymphatics, draining to lymph nodes in the neck and around the celiac artery in the abdomen. Endoscopy or barium swallow is used to assess the site. CT and MRI may be necessary to stage the disease.
Once the extent of the disease has been assessed, treatment can be planned.
Clinical app
Esophageal rupture
The first case of esophageal rupture was described by Herman Boerhaave in 1724. This case was fatal, but early diagnosis has increased the survival rate up to 65%. If the disease is left untreated, mortality is 100%.
Typically, the rupture occurs in the lower third of the esophagus with a sudden rise in intraluminal esophageal pressure produced by vomiting together with failure of the cricopharyngeus muscle in the lower neck to relax. Because the tears typically occur on the left, they are often associated with a large left pleural effusion that contains the gastric contents.
Thoracic aorta
The thoracic portion of the descending aorta (thoracic aorta) begins at the lower edge of vertebra TIV, where it is continuous with the arch of aorta. It ends anterior to the lower edge of vertebrae TXII, where it passes through the aortic hiatus posterior to the diaphragm. Situated to the left of the vertebral column superiorly, it approaches the midline inferiorly, lying directly anterior to the lower thoracic vertebral bodies (Fig. 3.98). Throughout its course, it gives off a number of branches, which are summarized in Table 3.3.
Fig. 3.98 Thoracic aorta and branches.
Table 3.3 Branches of the thoracic aorta
|
Branches |
Origin and course |
|
Pericardial branches |
A few small vessels to the posterior surface of the pericardial sac |
|
Bronchial branches |
Vary in number, size, and origin—usually two left bronchial arteries from the thoracic aorta and one right bronchial artery from the third posterior intercostal artery or the superior left bronchial artery |
|
Esophageal branches |
Four or five vessels from the anterior aspect of the thoracic aorta, which form a continuous anastomotic chain—anastomotic connections include esophageal branches of the inferior thyroid artery superiorly, and esophageal branches of the left inferior phrenic and the left gastric arteries inferiorly |
|
Mediastinal branches |
Several small branches supplying lymph nodes, vessels, nerves, and areolar tissue in the posterior mediastinum |
|
Posterior intercostal arteries |
Usually nine pairs of vessels branching from the posterior surface of the thoracic aorta—usually supply the lower nine intercostal spaces (first two spaces are supplied by the supreme intercostal artery—a branch of the costocervical trunk) |
|
Superior phrenic arteries |
Small vessels from the lower part of the thoracic aorta supplying the posterior part of the superior surface of the diaphragm—they anastomose with the musculophrenic and pericardiacophrenic arteries |
|
Subcostal artery |
The lowest pair of branches from the thoracic aorta located inferior to rib XII |
Azygos system of veins
The azygos system of veins consists of a series of longitudinal vessels on each side of the body that drain blood from the body wall and move it superiorly to the superior vena cava. Blood from some of the thoracic viscera may also enter the system, and there are anastomotic connections with abdominal veins.
The longitudinal vessels may or may not be continuous and are connected to each other from side to side at various points throughout their course (Fig. 3.99).
Fig. 3.99 Azygos system of veins.
The azygos system of veins serves as an important anastomotic pathway capable of returning venous blood from the lower part of the body to the heart if the inferior vena cava is blocked.
The major veins in the system are:
 the azygos vein, on the right, and
the azygos vein, on the right, and
 the hemiazygos vein and the accessory hemiazygos vein, on the left.
the hemiazygos vein and the accessory hemiazygos vein, on the left.
There is significant variation in the origin, course, tributaries, anastomoses, and termination of these vessels.
Azygos vein
The azygos vein arises opposite vertebra LI or LII at the junction of the right ascending lumbar vein and the right subcostal vein (Fig. 3.99). It may also arise as a direct branch of the inferior vena cava, which is joined by a common trunk from the junction of the right ascending lumbar vein and the right subcostal vein.
The azygos vein enters the thorax through the aortic hiatus of the diaphragm, or through or posterior to the right crus of the diaphragm. It ascends through the posterior mediastinum, usually to the right of the thoracic duct. At approximately vertebral level TIV, it arches anteriorly, over the root of the right lung, to join the superior vena cava before the superior vena cava enters the pericardial sac.
Tributaries of the azygos vein include:
 fifth to eleventh right posterior intercostal veins,
fifth to eleventh right posterior intercostal veins,
 the accessory hemiazygos vein,
the accessory hemiazygos vein,
Hemiazygos vein
The hemiazygos vein (inferior hemiazygos vein) usually arises at the junction between the left ascending lumbar vein and the left subcostal vein (Fig. 3.99). It may also arise from either of these veins alone and often has a connection to the left renal vein.
The hemiazygos vein usually enters the thorax through the left crus of the diaphragm, but may enter through the aortic hiatus. It ascends through the posterior mediastinum, on the left side, to approximately vertebral level TIX. At this point, it crosses the vertebral column, posterior to the thoracic aorta, esophagus, and thoracic duct, to enter the azygos vein.
Tributaries joining the hemiazygos vein include:
 the lowest four or five left posterior intercostal veins,
the lowest four or five left posterior intercostal veins,
Accessory hemiazygos vein
The accessory hemiazygos vein (superior hemiazygos vein) descends on the left side from the superior portion of the posterior mediastinum to approximately vertebral level TVIII (Fig. 3.99). At this point, it crosses the vertebral column to join the azygos vein, or ends in the hemiazygos vein, or has a connection to both veins. Usually, it also has a connection superiorly to the left superior intercostal vein.
Vessels that drain into the accessory hemiazygos vein include:
 the fourth to eighth left posterior intercostal veins, and
the fourth to eighth left posterior intercostal veins, and
 sometimes, the left bronchial veins.
sometimes, the left bronchial veins.
Thoracic duct in the posterior mediastinum
The thoracic duct is the principal channel through which lymph from most of the body is returned to the venous system. It begins as a confluence of lymph trunks in the abdomen, sometimes forming a saccular dilation referred to as the cisterna chyli (chyle cistern), which drains the abdominal viscera and walls, pelvis, perineum, and lower limbs.
The thoracic duct extends from vertebra LII to the root of the neck.
Entering the thorax, posterior to the aorta, through the aortic hiatus of the diaphragm, the thoracic duct ascends through the posterior mediastinum to the right of midline between the thoracic aorta on the left and the azygos vein on the right (Fig. 3.100). It lies posterior to the diaphragm and the esophagus and anterior to the bodies of the vertebrae.
Fig. 3.100 Thoracic duct.
At vertebral level TV, the thoracic duct moves to the left of midline and enters the superior mediastinum. It continues through the superior mediastinum and into the neck.
After being joined, in most cases, by the left jugular trunk, which drains the left side of the head and neck, and the left subclavian trunk, which drains the left upper limb, the thoracic duct empties into the junction of the left subclavian and left internal jugular veins.
The thoracic duct usually receives the contents from:
 the confluence of lymph trunks in the abdomen,
the confluence of lymph trunks in the abdomen,
 descending thoracic lymph trunks draining the lower six or seven intercostal spaces on both sides,
descending thoracic lymph trunks draining the lower six or seven intercostal spaces on both sides,
 upper intercostal lymph trunks draining the upper left five or six intercostal spaces,
upper intercostal lymph trunks draining the upper left five or six intercostal spaces,
 ducts from posterior mediastinal nodes, and
ducts from posterior mediastinal nodes, and
 ducts from posterior diaphragmatic nodes.
ducts from posterior diaphragmatic nodes.
Sympathetic trunks
The sympathetic trunks are an important component of the sympathetic part of the autonomic division of the peripheral nervous system and are usually considered a component of the posterior mediastinum as they pass through the thorax (also see Chapter 1, pp. 23–26).
This portion of the sympathetic trunks consists of two parallel cords punctuated by 11 or 12 ganglia (Fig. 3.101). The ganglia are connected to adjacent thoracic spinal nerves by white and gray rami communicantes and are numbered according to the thoracic spinal nerve with which they are associated.
Fig. 3.101 Thoracic portion of sympathetic trunks.
In the superior portion of the posterior mediastinum, the trunks are anterior to the neck of the ribs. Inferiorly, they become more medial in position until they lie on the lateral aspect of the vertebral bodies. The sympathetic trunks leave the thorax by passing posterior to the diaphragm under the medial arcuate ligament or through the crura of the diaphragm. Throughout their course the trunks are covered by parietal pleura.
Branches from the ganglia
Two types of medial branches are given off by the ganglia:
 The first type includes branches from the upper five ganglia.
The first type includes branches from the upper five ganglia.
 The second type includes branches from the lower seven ganglia.
The second type includes branches from the lower seven ganglia.
The first type includes branches from the upper five ganglia and is mainly postganglionic sympathetic fibers, which supply the various thoracic viscera. These branches are relatively small, and also contain visceral afferent fibers.
The second type includes branches from the lower seven ganglia and is mainly preganglionic sympathetic fibers, which supply the various abdominal and pelvic viscera. These branches are large, carry visceral afferent fibers, and form the three thoracic splanchnic nerves referred to as the greater, lesser, and least splanchnic nerves (Fig. 3.101).
Anterior mediastinum
The anterior mediastinum is posterior to the body of the sternum and anterior to the pericardial sac (see Fig. 3.53).
 Its inferior boundary is the diaphragm.
Its inferior boundary is the diaphragm.
 Laterally, it is bordered by the mediastinal part of parietal pleura on either side.
Laterally, it is bordered by the mediastinal part of parietal pleura on either side.
The major structure in the anterior mediastinum is a portion of thymus, described previously (see Fig. 3.85). Also present are fat, connective tissue, lymph nodes, mediastinal branches of the internal thoracic vessels, and sternopericardial ligaments, which pass from the posterior surface of the body of the sternum to the fibrous pericardium.
Visualizing the mediastinum in the axial plane
This is a series of images that pass through the thorax from superior to inferior showing the various mediastinal structures and their relationships with each other. CT images, with contrast, in axial plane.
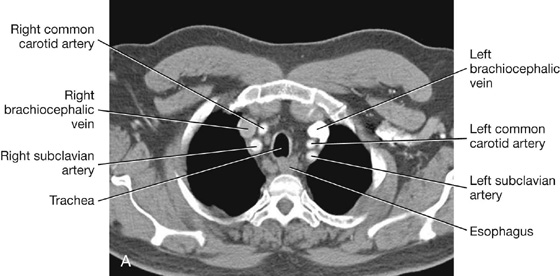
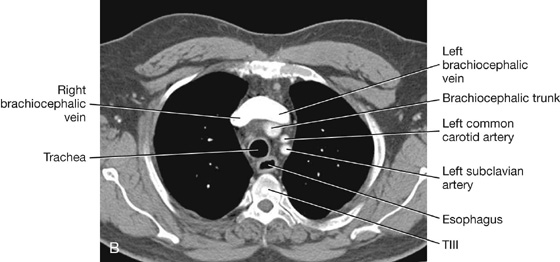
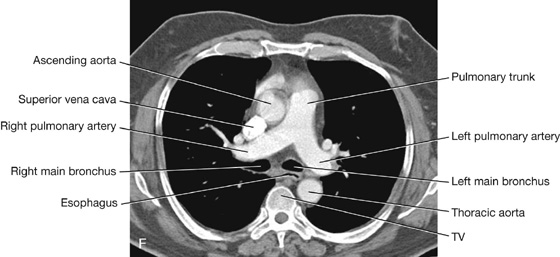
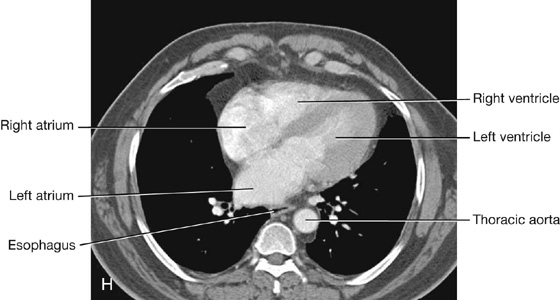
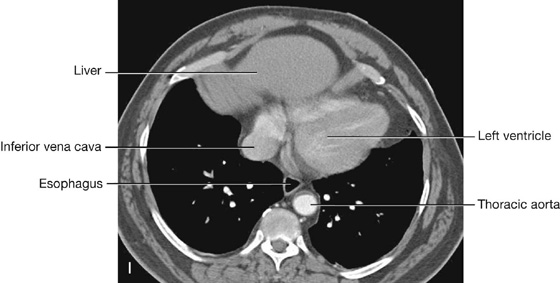
Fig. 3.102

 Medical Clinical Case Studies
Medical Clinical Case Studies Clinical Cases
Clinical Cases a wall,
a wall, two pleural cavities,
two pleural cavities, the lungs, and
the lungs, and the mediastinum.
the mediastinum.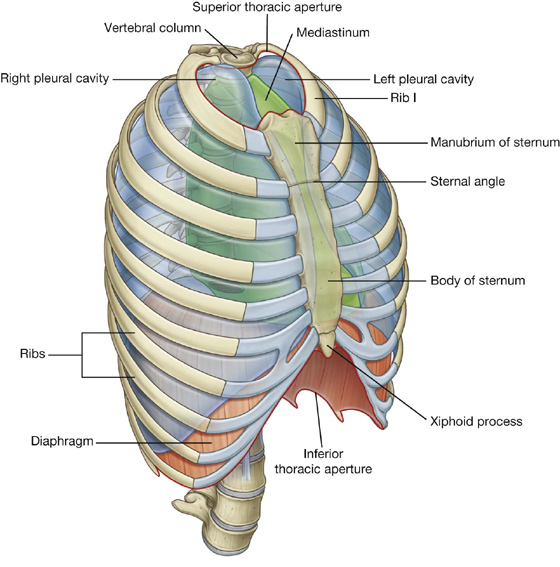
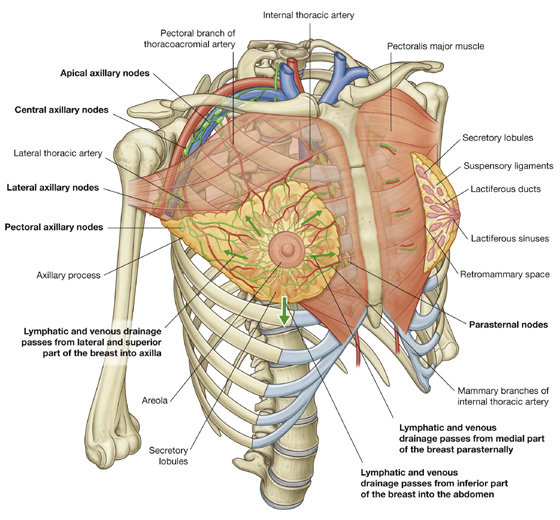
 laterally, vessels from the axillary artery—superior thoracic, thoracoacromial, lateral thoracic, and subscapular arteries;
laterally, vessels from the axillary artery—superior thoracic, thoracoacromial, lateral thoracic, and subscapular arteries; from the second to fourth intercostal arteries via branches that perforate the thoracic wall and overlying muscle.
from the second to fourth intercostal arteries via branches that perforate the thoracic wall and overlying muscle. Most of the remaining drainage is into parasternal nodes deep to the anterior thoracic wall and associated with the internal thoracic artery.
Most of the remaining drainage is into parasternal nodes deep to the anterior thoracic wall and associated with the internal thoracic artery. Some drainage may occur via lymphatic vessels that follow the lateral branches of posterior intercostal arteries and connect with intercostal nodes situated near the heads and necks of ribs.
Some drainage may occur via lymphatic vessels that follow the lateral branches of posterior intercostal arteries and connect with intercostal nodes situated near the heads and necks of ribs.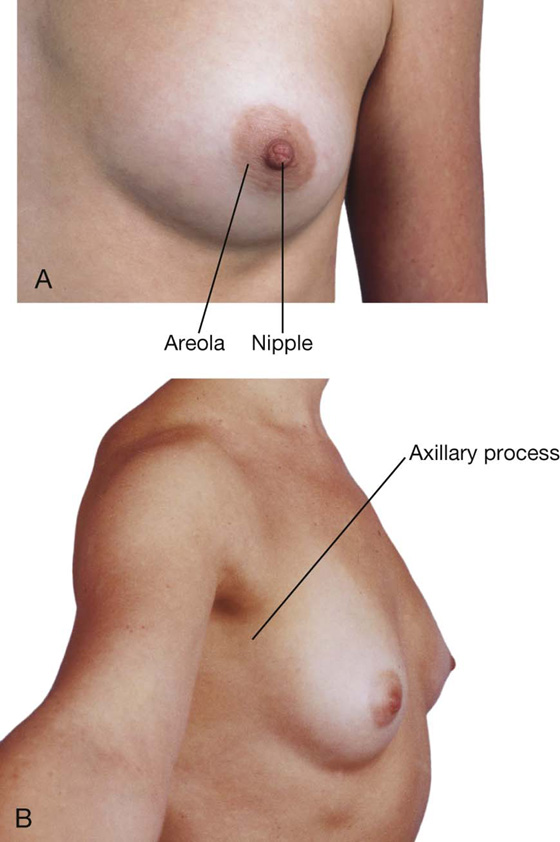
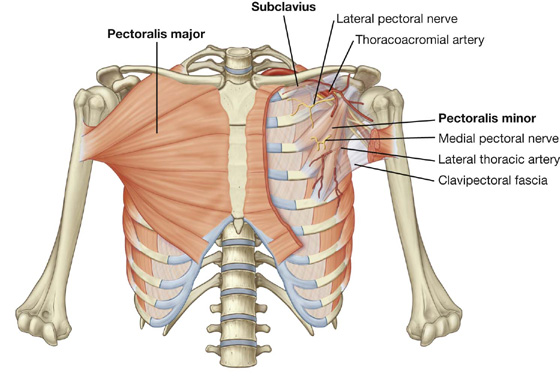
 Laterally, the wall is formed by ribs (12 on each side) and three layers of flat muscles, which span the intercostal spaces between adjacent ribs, move the ribs, and provide support for the intercostal spaces.
Laterally, the wall is formed by ribs (12 on each side) and three layers of flat muscles, which span the intercostal spaces between adjacent ribs, move the ribs, and provide support for the intercostal spaces. Anteriorly, the wall is made up of the sternum, which consists of the manubrium of sternum, body of sternum, and xiphoid process.
Anteriorly, the wall is made up of the sternum, which consists of the manubrium of sternum, body of sternum, and xiphoid process. the inferior thoracic aperture bordered by vertebra TXII, rib XII, the end of rib XI, the costal margin, and the xiphoid process of the sternum.
the inferior thoracic aperture bordered by vertebra TXII, rib XII, the end of rib XI, the costal margin, and the xiphoid process of the sternum. the manubrium anteriorly.
the manubrium anteriorly.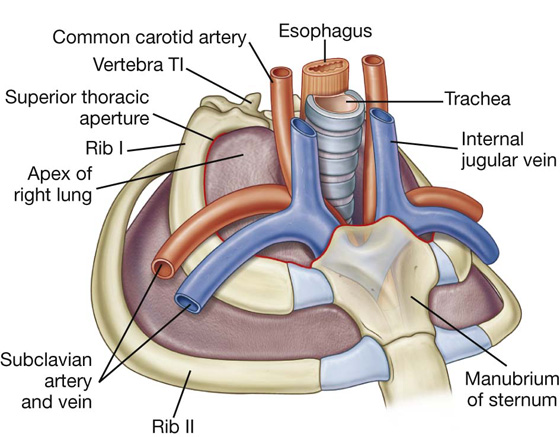
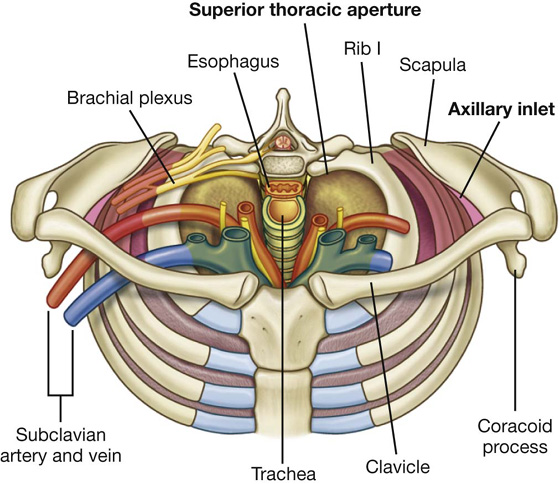
 the distal cartilaginous ends of ribs VII to X, which unite to form the costal margin anterolaterally, and
the distal cartilaginous ends of ribs VII to X, which unite to form the costal margin anterolaterally, and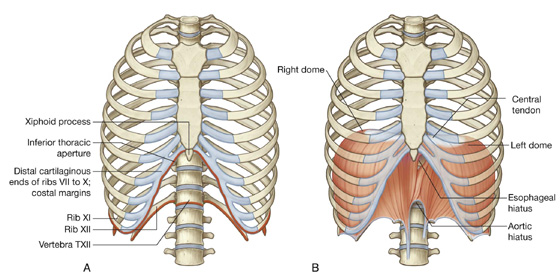
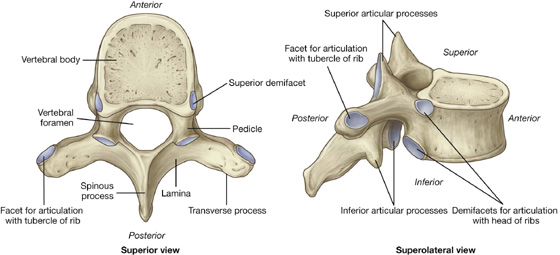
 Two demifacets (i.e., partial facets) located on the superior and inferior aspects of the body for articulation with corresponding sites on the heads of adjacent ribs. The superior costal facet articulates with part of the head of its own rib, and the inferior costal facet articulates with part of the head of the rib below.
Two demifacets (i.e., partial facets) located on the superior and inferior aspects of the body for articulation with corresponding sites on the heads of adjacent ribs. The superior costal facet articulates with part of the head of its own rib, and the inferior costal facet articulates with part of the head of the rib below. An oval facet (transverse costal facet) at the end of the transverse process articulates with the tubercle of its own rib.
An oval facet (transverse costal facet) at the end of the transverse process articulates with the tubercle of its own rib. The superior costal facets on the body of vertebra TI are complete and articulate with a single facet on the head of its own rib—in other words, the head of rib I does not articulate with vertebra CVII.
The superior costal facets on the body of vertebra TI are complete and articulate with a single facet on the head of its own rib—in other words, the head of rib I does not articulate with vertebra CVII. Similarly, vertebra TX (and often TIX) articulates only with its own ribs and therefore lacks inferior demifacets on the body.
Similarly, vertebra TX (and often TIX) articulates only with its own ribs and therefore lacks inferior demifacets on the body. Vertebrae TXI and TXII articulate only with the heads of their own ribs—they lack transverse costal facets and have only a single complete facet on each side of their bodies.
Vertebrae TXI and TXII articulate only with the heads of their own ribs—they lack transverse costal facets and have only a single complete facet on each side of their bodies.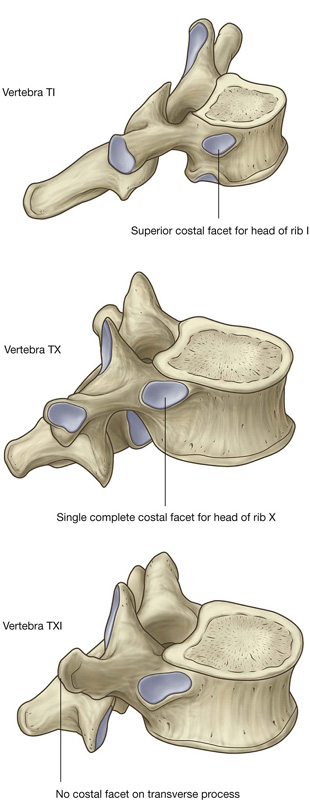
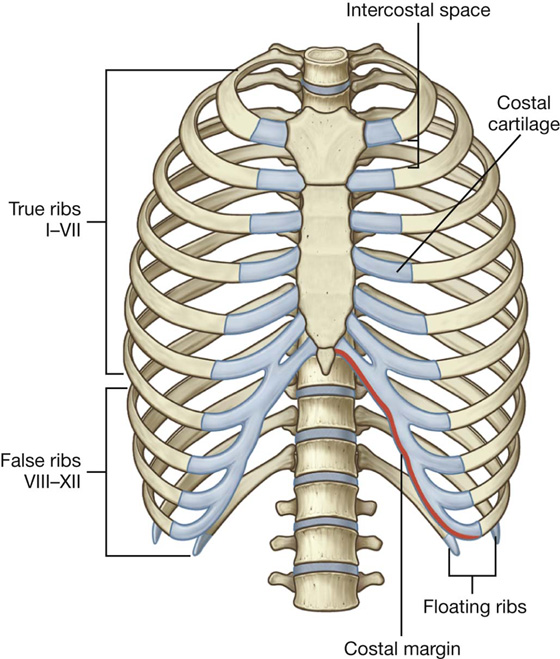
 The costal cartilages of ribs VIII to X articulate anteriorly with the costal cartilages of the ribs above.
The costal cartilages of ribs VIII to X articulate anteriorly with the costal cartilages of the ribs above. Ribs XI and XII have no anterior connection with other ribs or with the sternum and are often called floating ribs.
Ribs XI and XII have no anterior connection with other ribs or with the sternum and are often called floating ribs.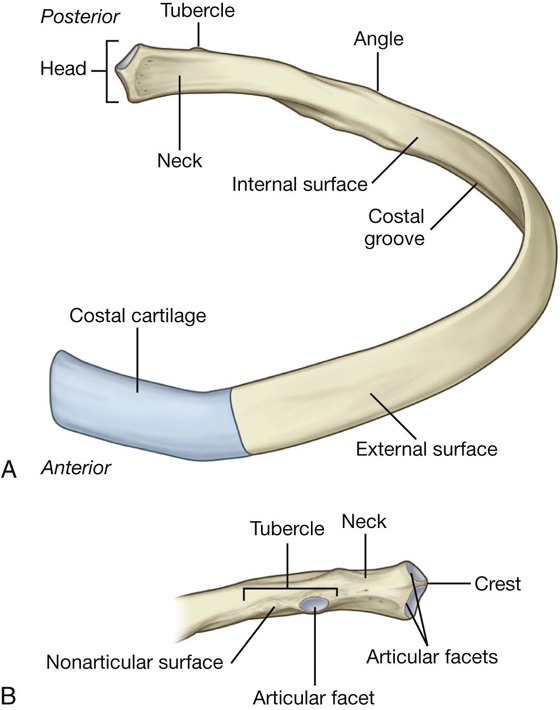
 The articular part is medial and has an oval facet for articulation with a corresponding facet on the transverse process of the associated vertebra.
The articular part is medial and has an oval facet for articulation with a corresponding facet on the transverse process of the associated vertebra.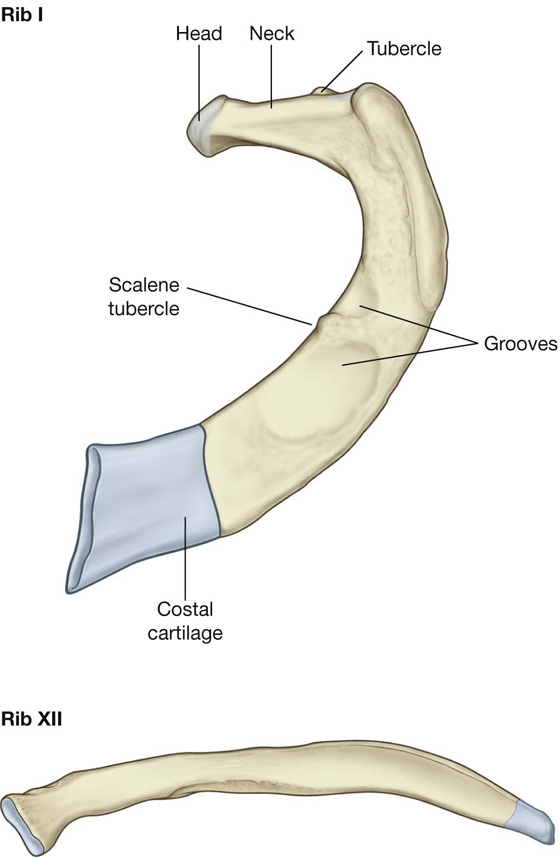
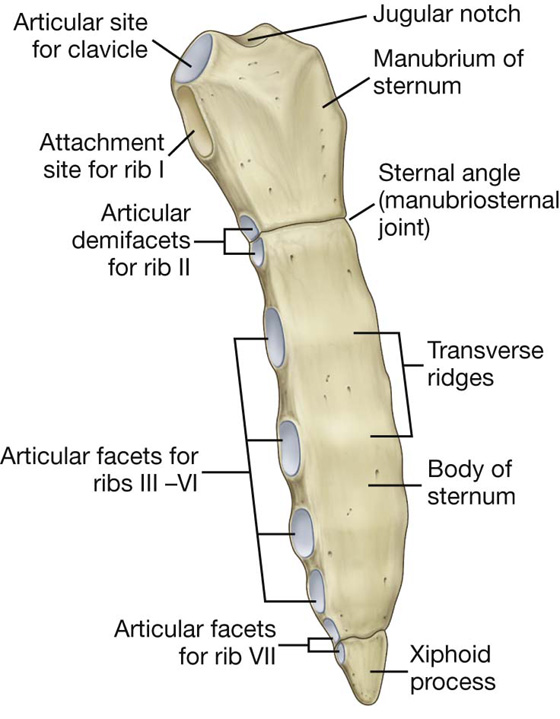
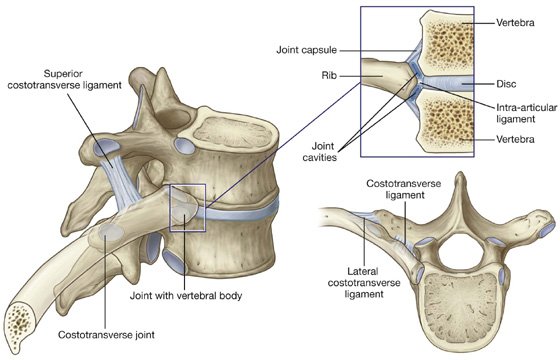
 The costotransverse ligament is medial to the joint and attaches the neck of the rib to the transverse process.
The costotransverse ligament is medial to the joint and attaches the neck of the rib to the transverse process. The lateral costotransverse ligament is lateral to the joint and attaches the tip of the transverse process to the roughened nonarticular part of the tubercle of the rib.
The lateral costotransverse ligament is lateral to the joint and attaches the tip of the transverse process to the roughened nonarticular part of the tubercle of the rib.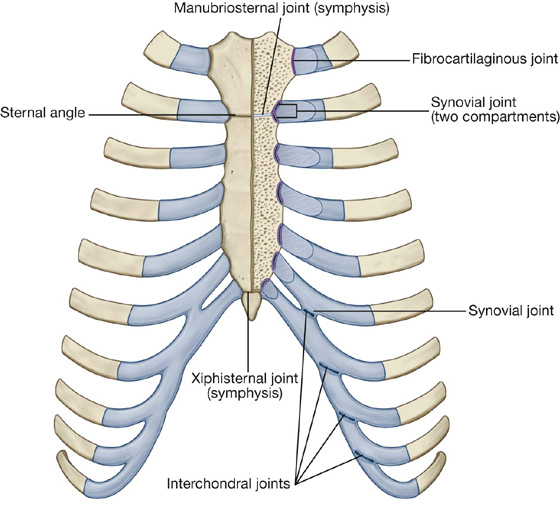
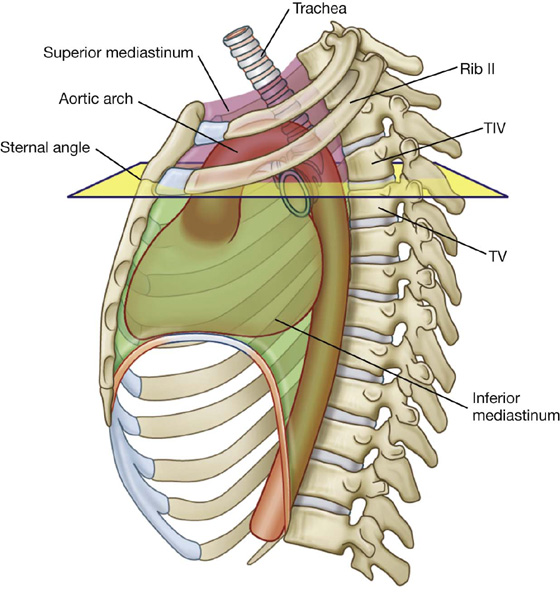
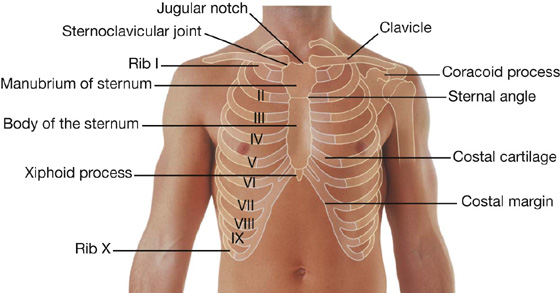
 The arch of aorta ends and the thoracic aorta begins.
The arch of aorta ends and the thoracic aorta begins. The trachea bifurcates.
The trachea bifurcates.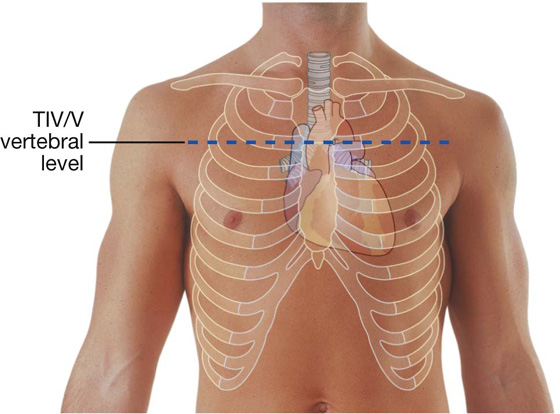
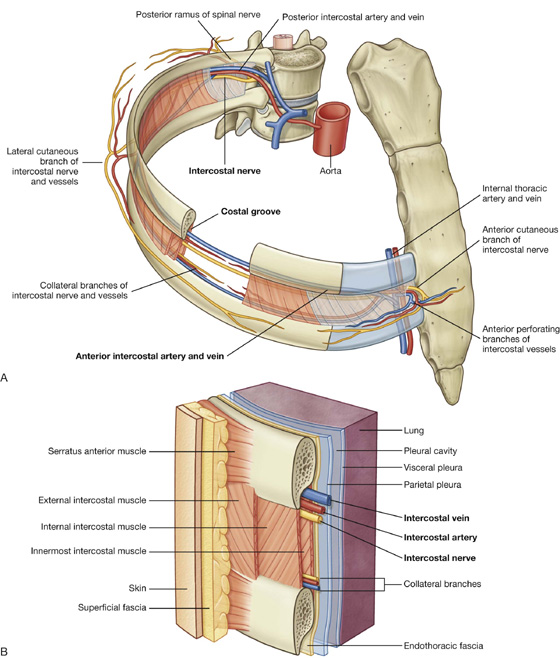
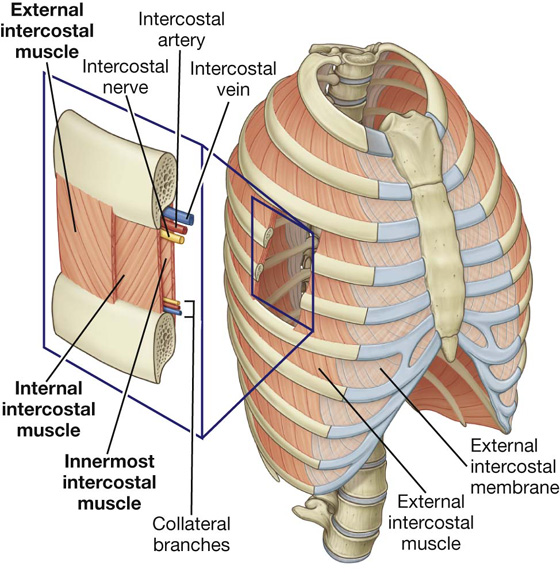
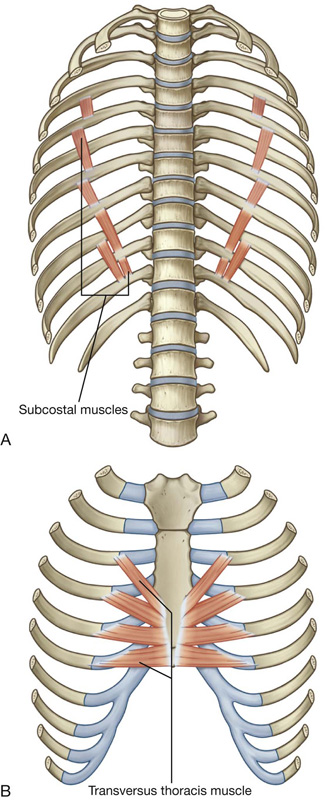
 The external intercostal muscles are the most superficial and their fibers pass from the rib above anteroinferiorly to the rib below (these muscles extend around the thoracic wall from the regions of the tubercles of the ribs to the costal cartilages, where each muscle continues as a thin connective tissue aponeurosis termed the external intercostal membrane).
The external intercostal muscles are the most superficial and their fibers pass from the rib above anteroinferiorly to the rib below (these muscles extend around the thoracic wall from the regions of the tubercles of the ribs to the costal cartilages, where each muscle continues as a thin connective tissue aponeurosis termed the external intercostal membrane). The internal intercostal muscles are sandwiched between the external and innermost muscles, and their fibers run in the opposite direction to those of the external intercostal muscles (the internal intercostal muscles extend around the thoracic wall from the sternum to the angles of the ribs, where each muscle continues as a thin connective tissue aponeurosis termed the internal intercostal membrane).
The internal intercostal muscles are sandwiched between the external and innermost muscles, and their fibers run in the opposite direction to those of the external intercostal muscles (the internal intercostal muscles extend around the thoracic wall from the sternum to the angles of the ribs, where each muscle continues as a thin connective tissue aponeurosis termed the internal intercostal membrane). The innermost intercostal muscles are the deepest of the three muscles and their fibers course in a similar direction as the internal intercostal muscles.
The innermost intercostal muscles are the deepest of the three muscles and their fibers course in a similar direction as the internal intercostal muscles.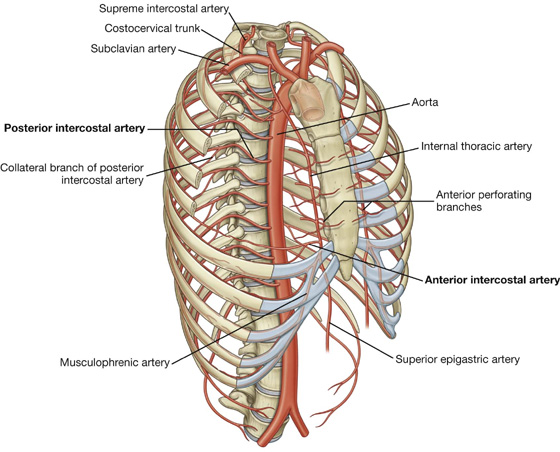
 the musculophrenic artery, which passes along the costal margin, goes through the diaphragm, and ends near the last intercostal space.
the musculophrenic artery, which passes along the costal margin, goes through the diaphragm, and ends near the last intercostal space. The other passes above the margin of the lower rib and meets a collateral branch of the posterior intercostal artery.
The other passes above the margin of the lower rib and meets a collateral branch of the posterior intercostal artery.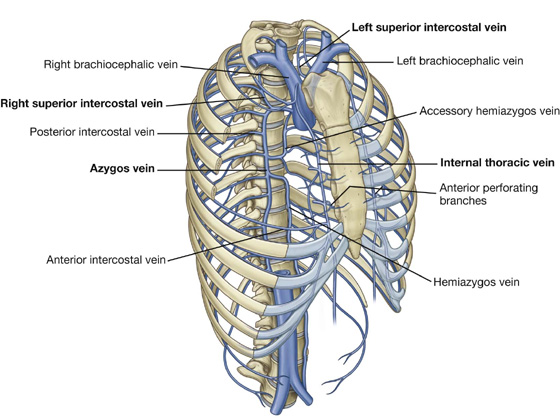
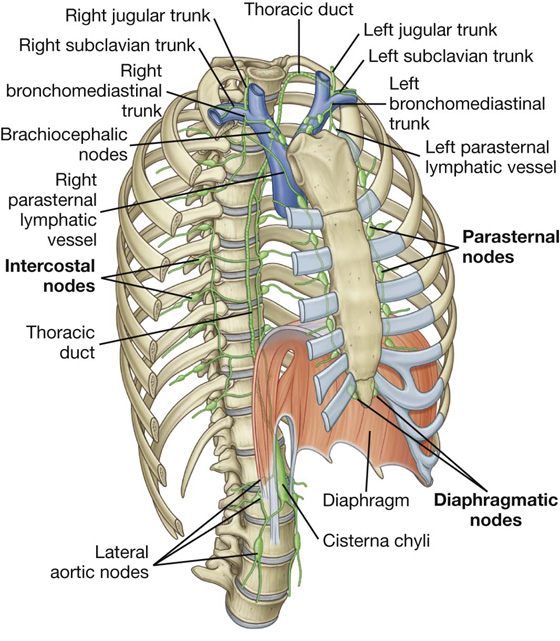
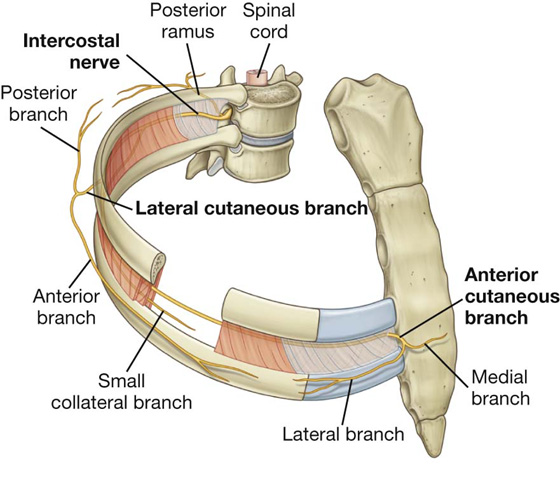
 somatic motor innervation to the muscles of the thoracic wall (intercostal, subcostal, and transversus thoracis muscles),
somatic motor innervation to the muscles of the thoracic wall (intercostal, subcostal, and transversus thoracis muscles), The lateral cutaneous branch of the second intercostal nerve (the intercostobrachial nerve) contributes to cutaneous innervation of the medial surface of the upper arm.
The lateral cutaneous branch of the second intercostal nerve (the intercostobrachial nerve) contributes to cutaneous innervation of the medial surface of the upper arm. ends of ribs XI and XII,
ends of ribs XI and XII,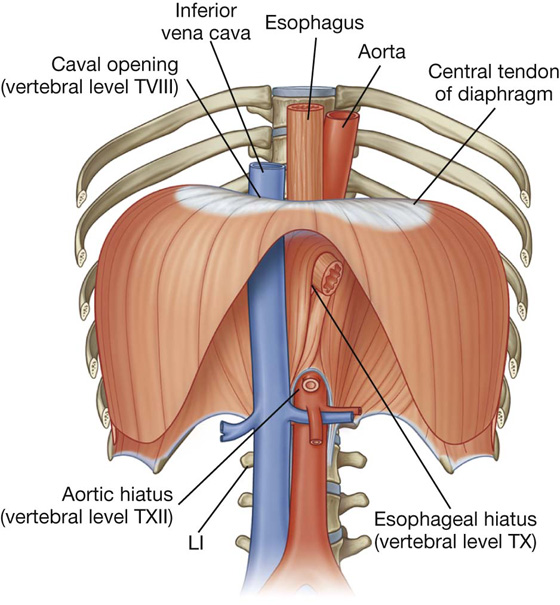
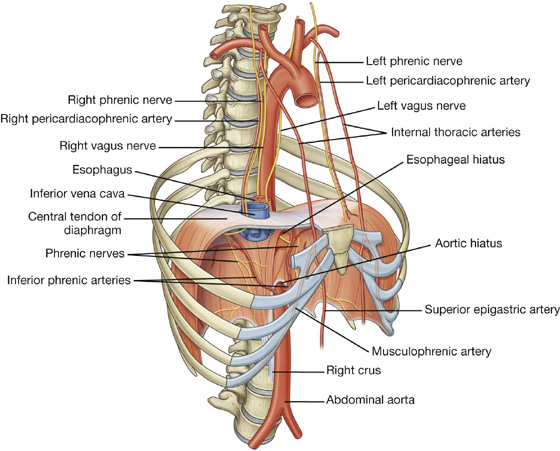
 The esophagus passes through the muscular part of the diaphragm, just to the left of midline, approximately at vertebral level TX.
The esophagus passes through the muscular part of the diaphragm, just to the left of midline, approximately at vertebral level TX. The azygos and hemiazygos veins may also pass through the aortic hiatus or through the crura of the diaphragm.
The azygos and hemiazygos veins may also pass through the aortic hiatus or through the crura of the diaphragm.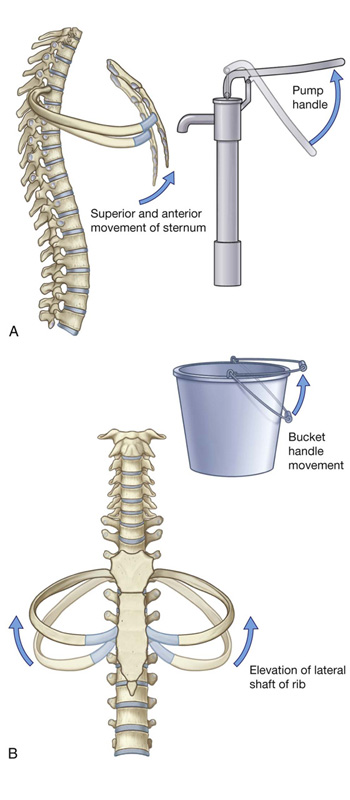
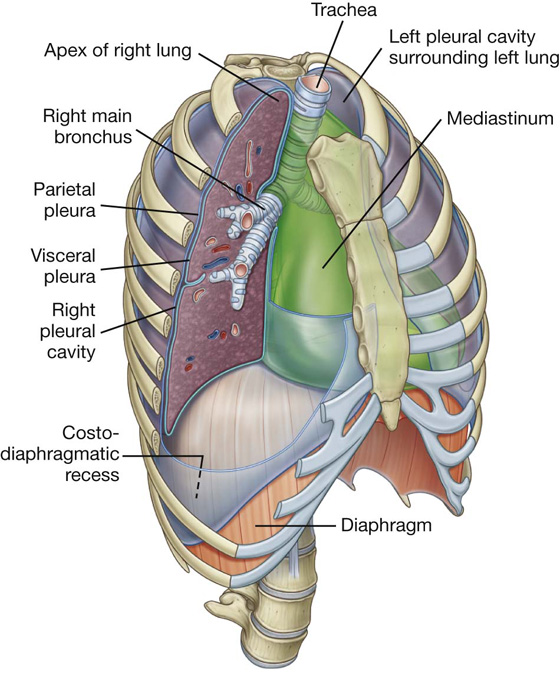
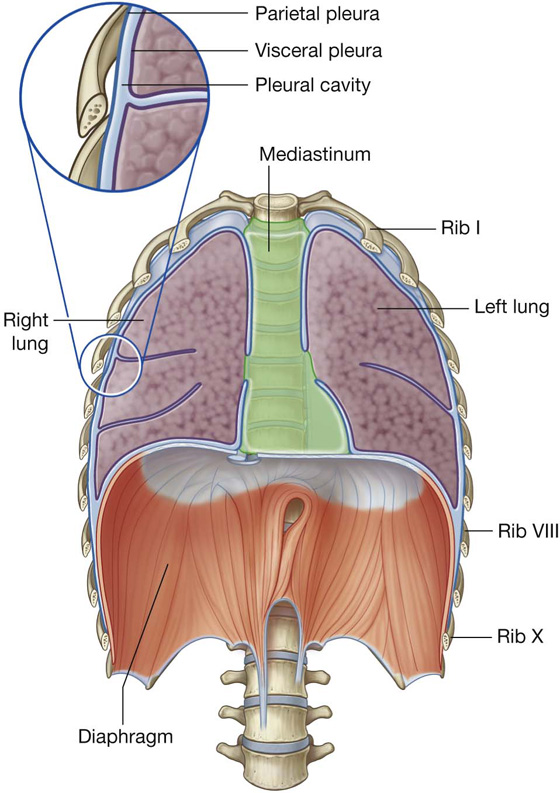
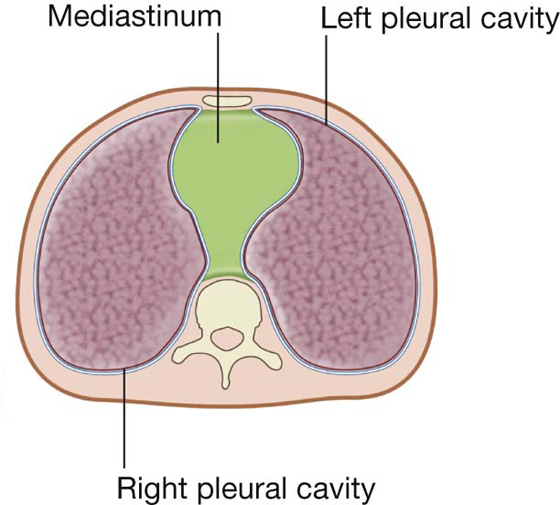
 The dome-shaped layer of parietal pleura lining the cervical extension of the pleural cavity is cervical pleura (dome of pleura or pleural cupola).
The dome-shaped layer of parietal pleura lining the cervical extension of the pleural cavity is cervical pleura (dome of pleura or pleural cupola).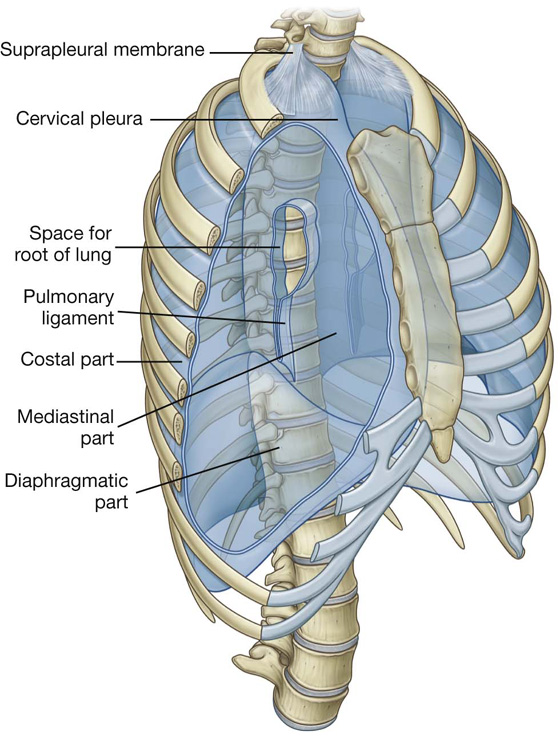
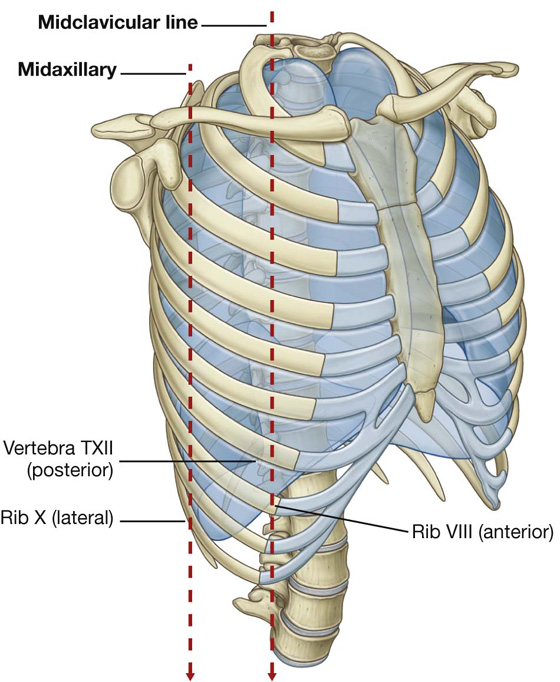
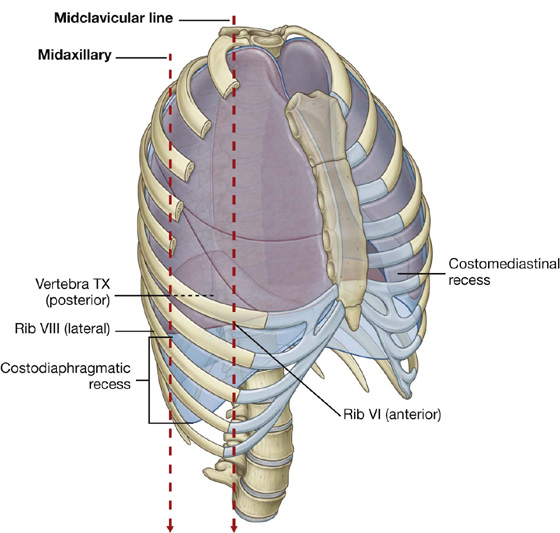
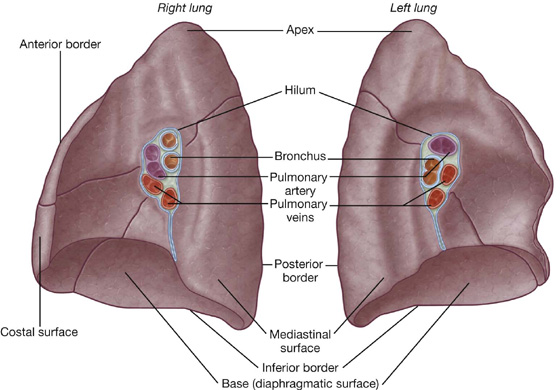
 The two surfaces—the costal surface lies immediately adjacent to the ribs and intercostal spaces of the thoracic wall. The mediastinal surface lies against the mediastinum anteriorly and the vertebral column posteriorly and contains the comma-shaped hilum of the lung through which structures enter and leave.
The two surfaces—the costal surface lies immediately adjacent to the ribs and intercostal spaces of the thoracic wall. The mediastinal surface lies against the mediastinum anteriorly and the vertebral column posteriorly and contains the comma-shaped hilum of the lung through which structures enter and leave. The three borders—the inferior border of the lung is sharp and separates the base from the costal surface. The anterior and posterior borders separate the costal surface from the medial surface. Unlike the anterior and inferior borders, which are sharp, the posterior border is smooth and rounded.
The three borders—the inferior border of the lung is sharp and separates the base from the costal surface. The anterior and posterior borders separate the costal surface from the medial surface. Unlike the anterior and inferior borders, which are sharp, the posterior border is smooth and rounded.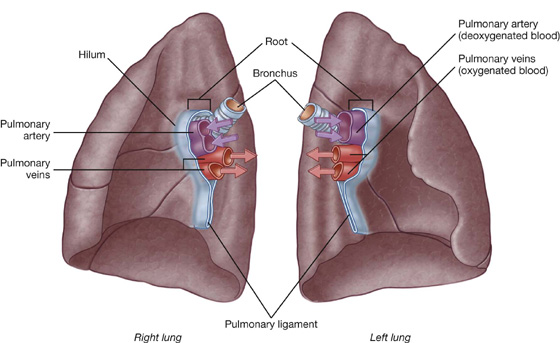
 a pulmonary artery,
a pulmonary artery, two pulmonary veins,
two pulmonary veins, a main bronchus,
a main bronchus, bronchial vessels,
bronchial vessels, nerves, and
nerves, and lymphatics.
lymphatics. the oblique fissure separates the inferior lobe (lower lobe) from the superior lobe and the middle lobe of the right lung;
the oblique fissure separates the inferior lobe (lower lobe) from the superior lobe and the middle lobe of the right lung;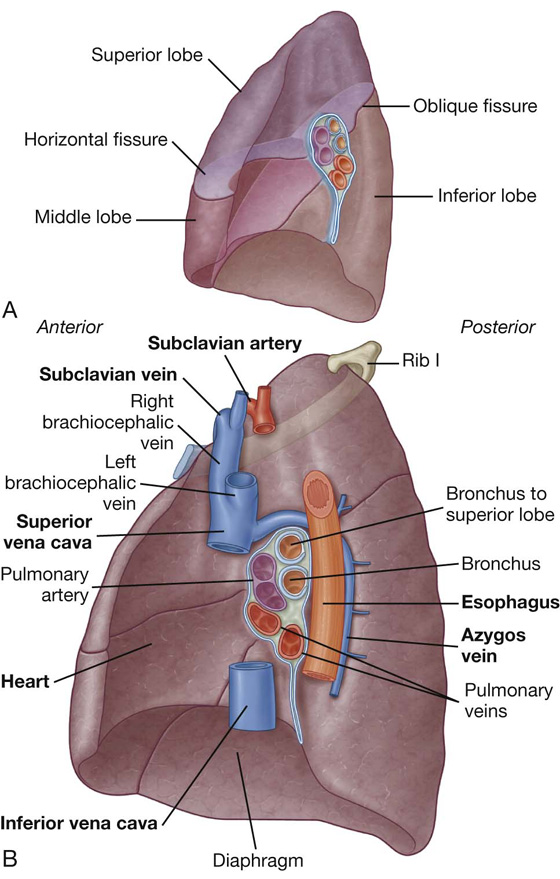
 heart,
heart, inferior vena cava,
inferior vena cava, superior vena cava,
superior vena cava, azygos vein, and
azygos vein, and esophagus.
esophagus.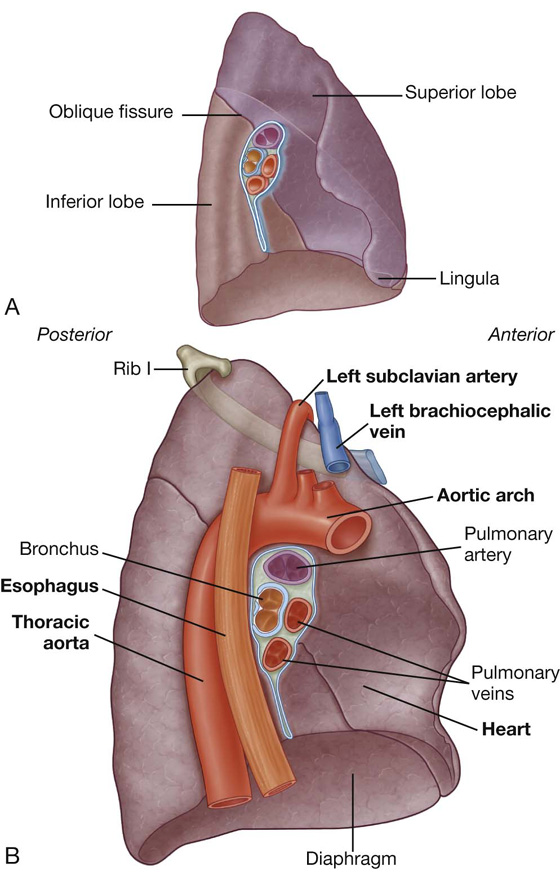
 heart,
heart, aortic arch,
aortic arch, thoracic aorta, and
thoracic aorta, and esophagus.
esophagus.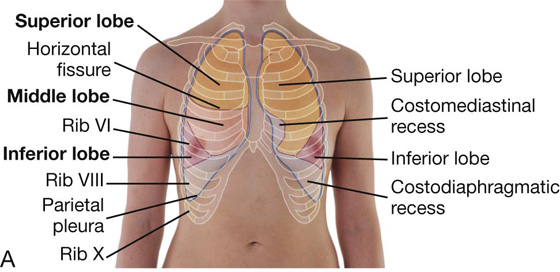
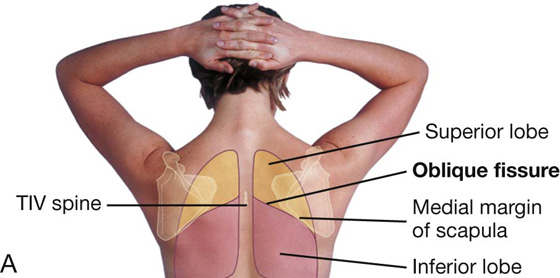
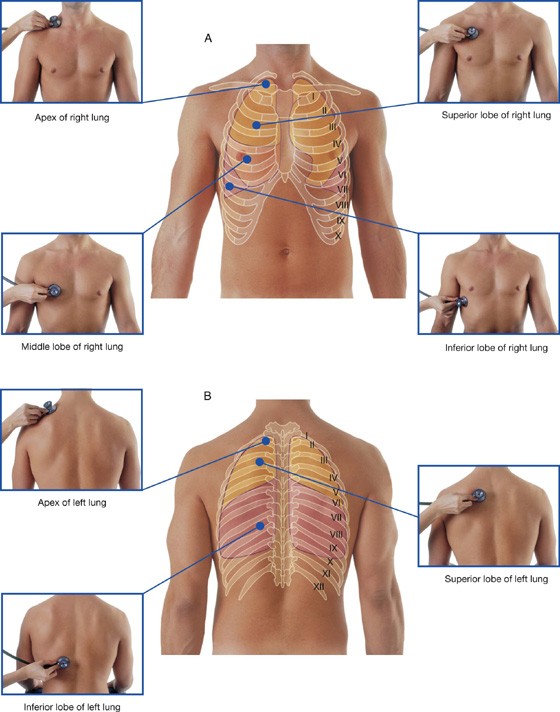
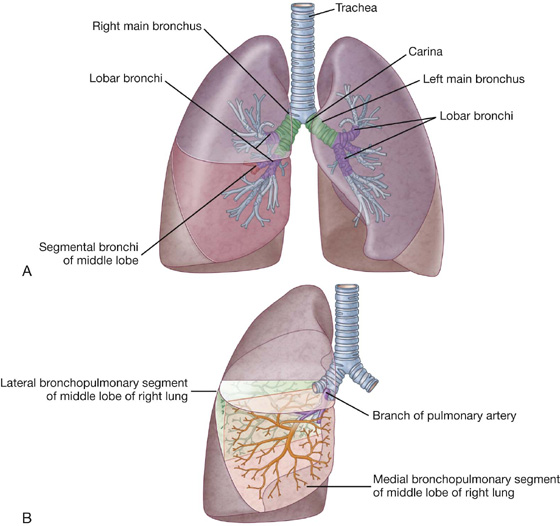
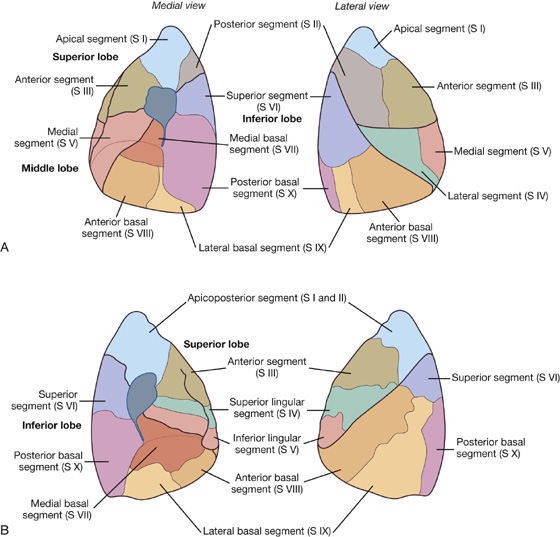
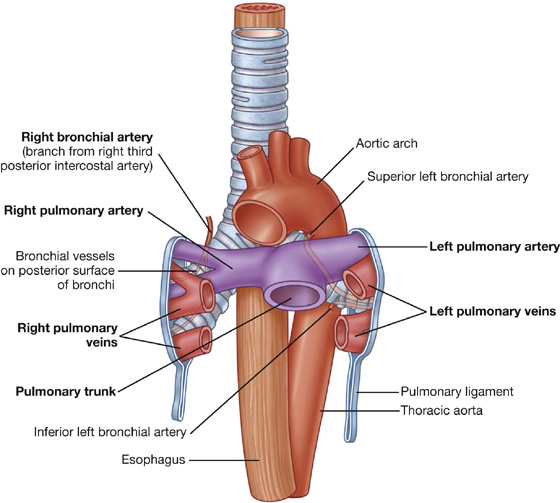
 anteriorly and slightly inferiorly to the tracheal bifurcation and anteriorly to the right main bronchus; and
anteriorly and slightly inferiorly to the tracheal bifurcation and anteriorly to the right main bronchus; and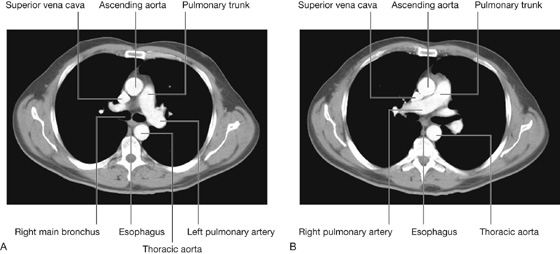
 A single right bronchial artery normally arises from the third posterior intercostal artery (but occasionally, it originates from the upper left bronchial artery).
A single right bronchial artery normally arises from the third posterior intercostal artery (but occasionally, it originates from the upper left bronchial artery). Two left bronchial arteries arise directly from the anterior surface of the thoracic aorta—the superior left bronchial artery arises at vertebral level TV, and the inferior one inferior to the left bronchus.
Two left bronchial arteries arise directly from the anterior surface of the thoracic aorta—the superior left bronchial artery arises at vertebral level TV, and the inferior one inferior to the left bronchus. into the azygos vein on the right or into the superior intercostal vein or hemiazygos vein on the left.
into the azygos vein on the right or into the superior intercostal vein or hemiazygos vein on the left.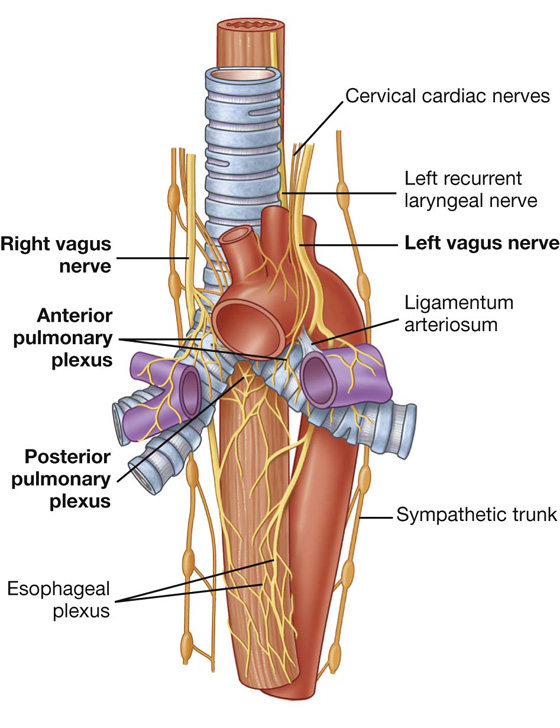
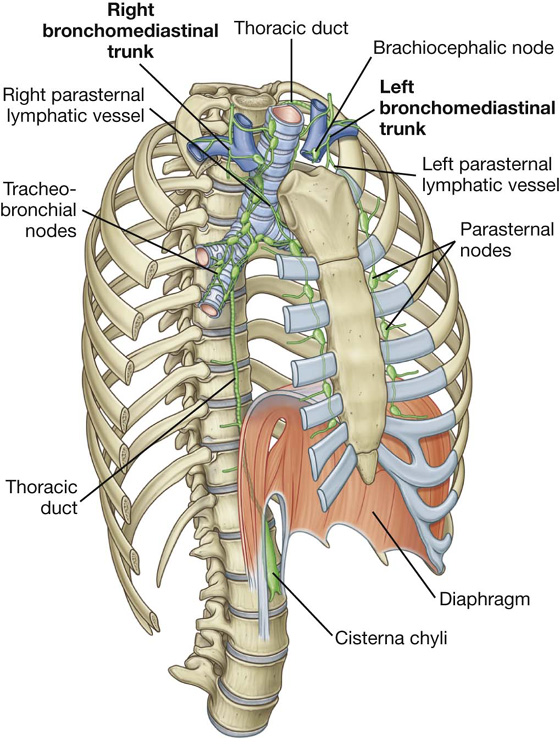
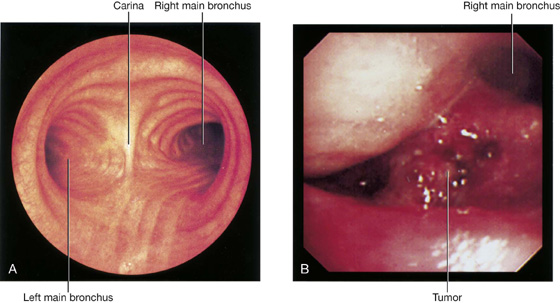
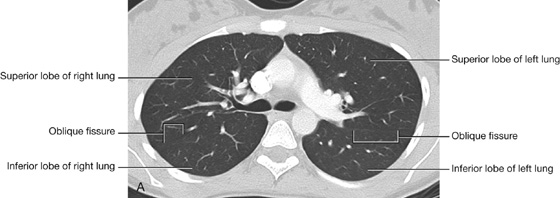
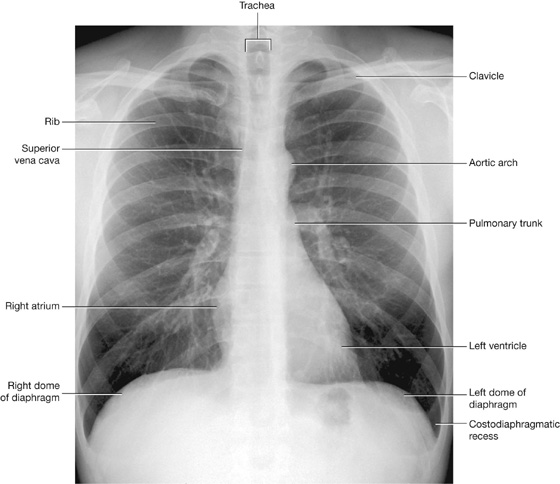
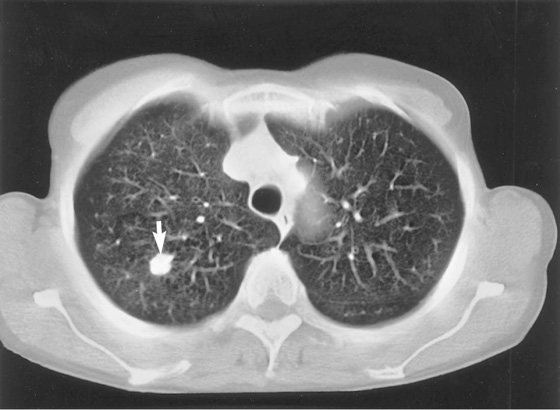
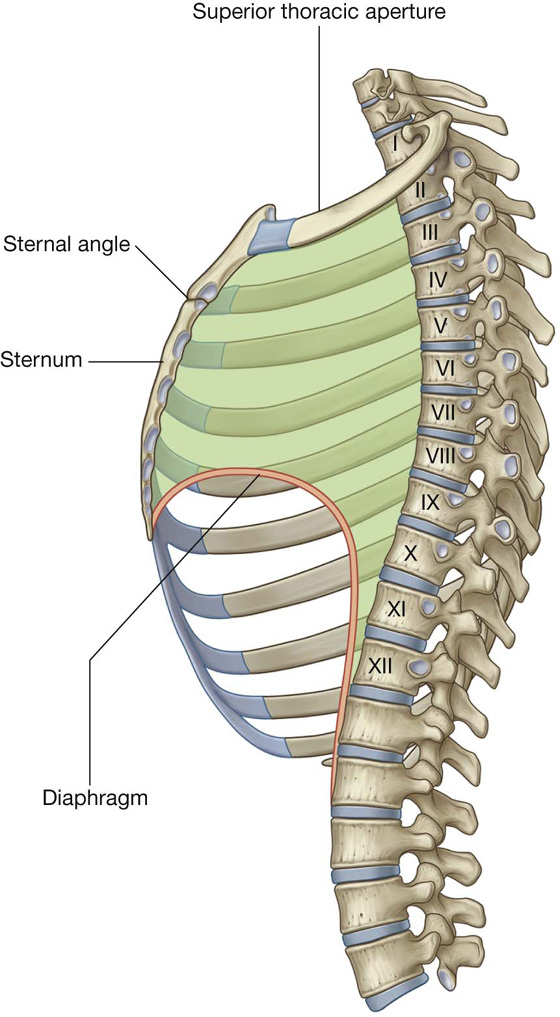
 superior mediastinum; and
superior mediastinum; and inferior mediastinum, which is further partitioned into the anterior, middle, and posterior mediastinum by the pericardial sac.
inferior mediastinum, which is further partitioned into the anterior, middle, and posterior mediastinum by the pericardial sac.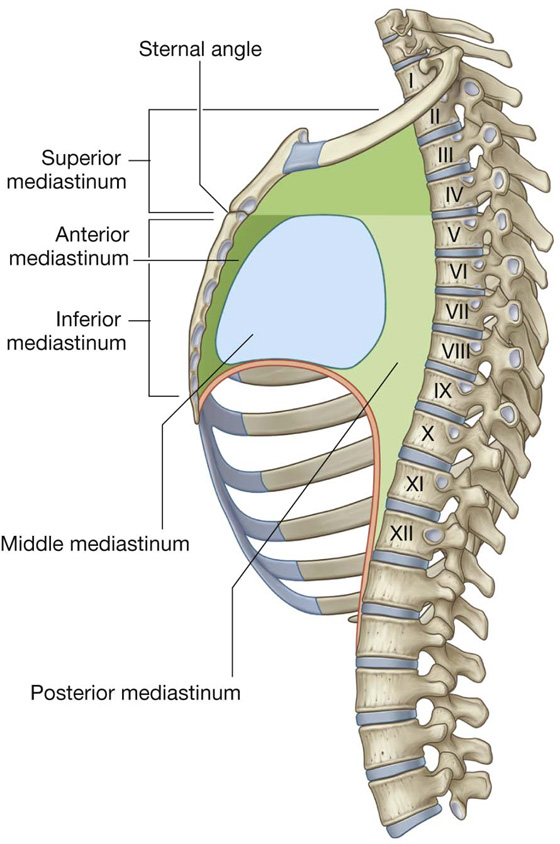
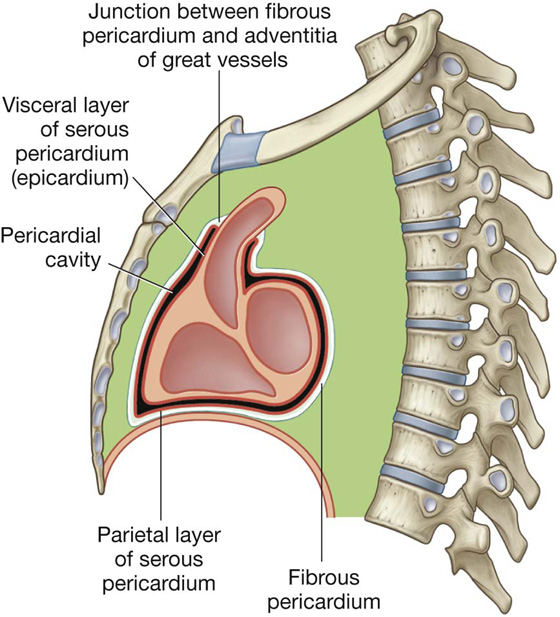
 The visceral layer (epicardium) of serous pericardium adheres to the heart and forms its outer covering.
The visceral layer (epicardium) of serous pericardium adheres to the heart and forms its outer covering.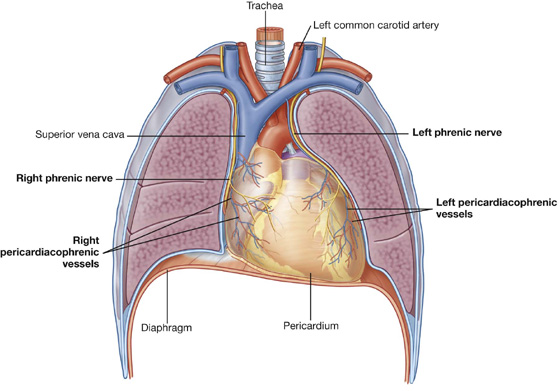
 the second more posteriorly, surrounding the veins, the superior and inferior vena cava, and the pulmonary veins.
the second more posteriorly, surrounding the veins, the superior and inferior vena cava, and the pulmonary veins.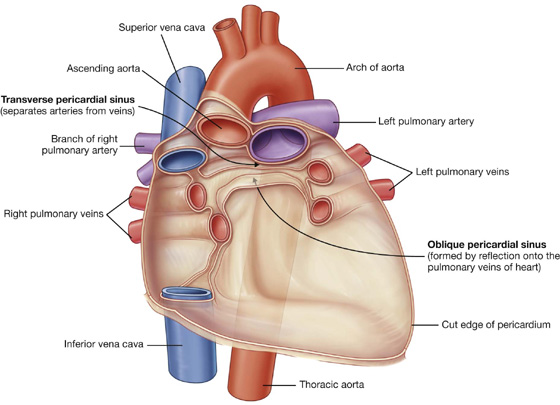
 a left pulmonary surface.
a left pulmonary surface.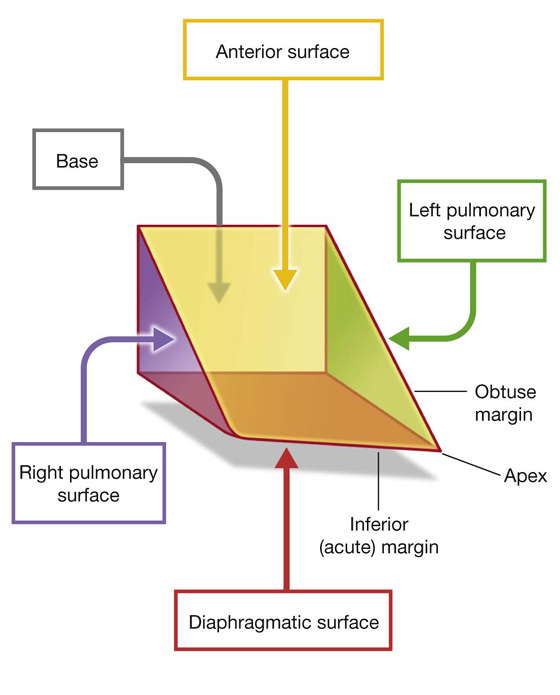
 the left atrium,
the left atrium,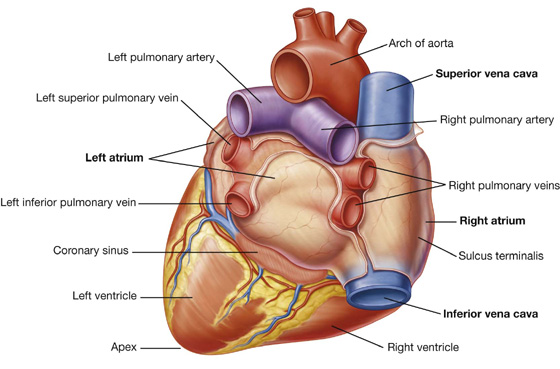
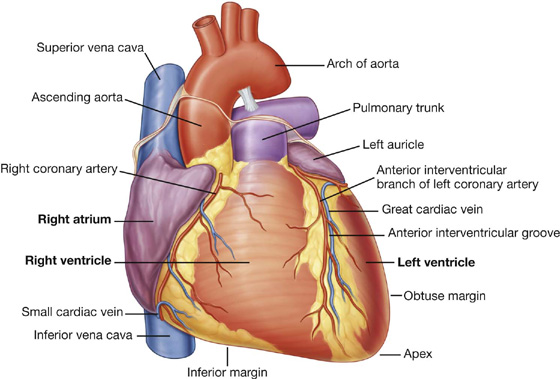
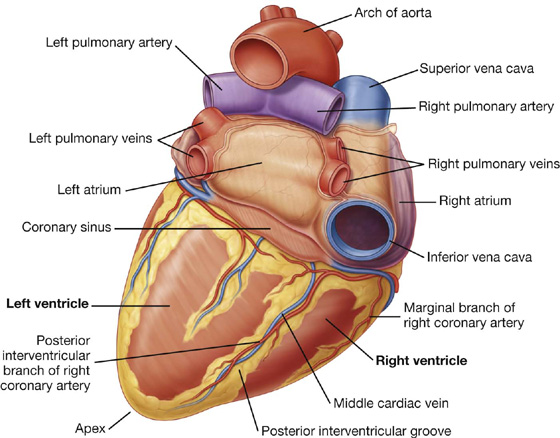
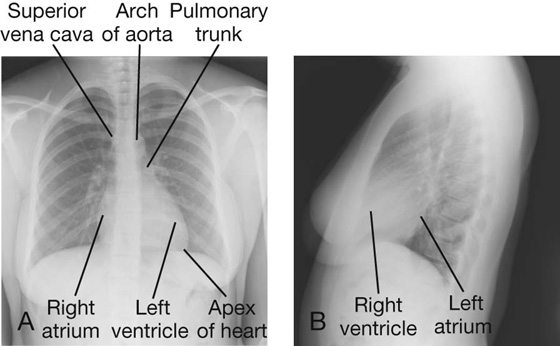
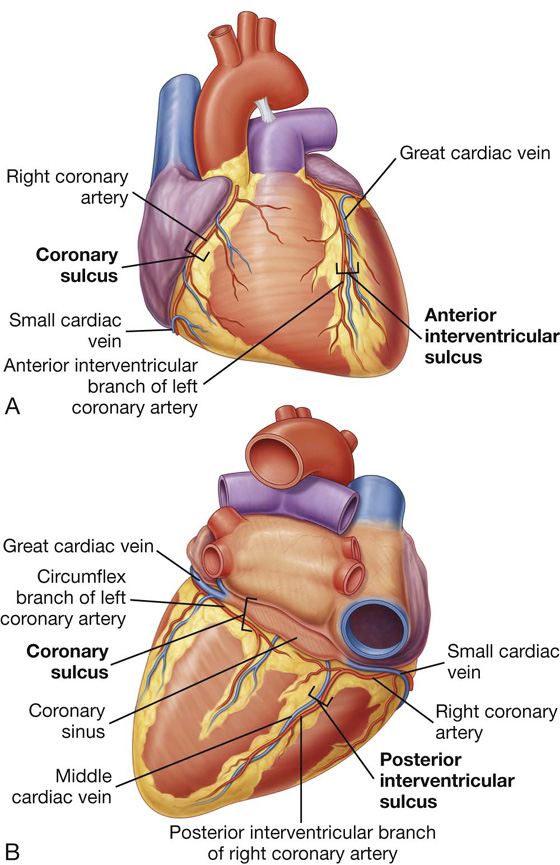
 The anterior and posterior interventricular sulci separate the two ventricles—the anterior interventricular sulcus on the anterior surface of the heart containing the anterior interventricular artery and the great cardiac vein, and the posterior interventricular sulcus on the diaphragmatic surface of the heart containing the posterior interventricular artery and the middle cardiac vein.
The anterior and posterior interventricular sulci separate the two ventricles—the anterior interventricular sulcus on the anterior surface of the heart containing the anterior interventricular artery and the great cardiac vein, and the posterior interventricular sulcus on the diaphragmatic surface of the heart containing the posterior interventricular artery and the middle cardiac vein.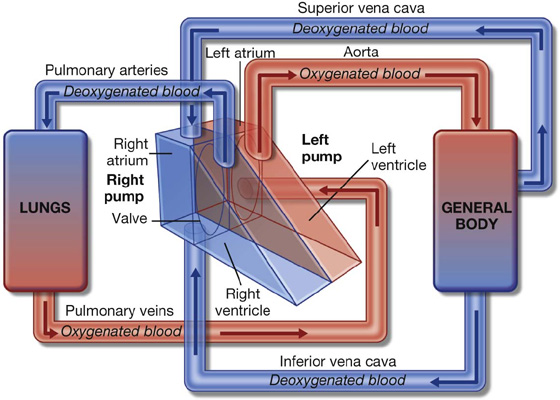
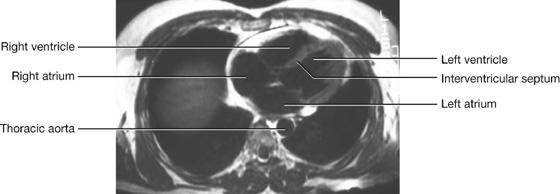
 The upper limit of the heart reaches as high as the third costal cartilage on the right side of the sternum and the second intercostal space on the left side of the sternum.
The upper limit of the heart reaches as high as the third costal cartilage on the right side of the sternum and the second intercostal space on the left side of the sternum. The right margin of the heart extends from the right third costal cartilage to near the right sixth costal cartilage.
The right margin of the heart extends from the right third costal cartilage to near the right sixth costal cartilage. The left margin of the heart descends laterally from the second intercostal space to the apex located near the midclavicular line in the fifth intercostal space.
The left margin of the heart descends laterally from the second intercostal space to the apex located near the midclavicular line in the fifth intercostal space. The lower margin of the heart extends from the sternal end of the right sixth costal cartilage to the apex in the fifth intercostal space near the midclavicular line.
The lower margin of the heart extends from the sternal end of the right sixth costal cartilage to the apex in the fifth intercostal space near the midclavicular line.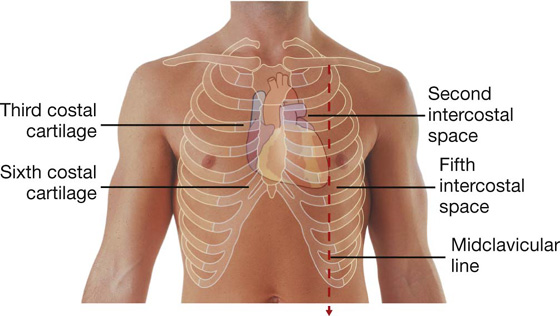
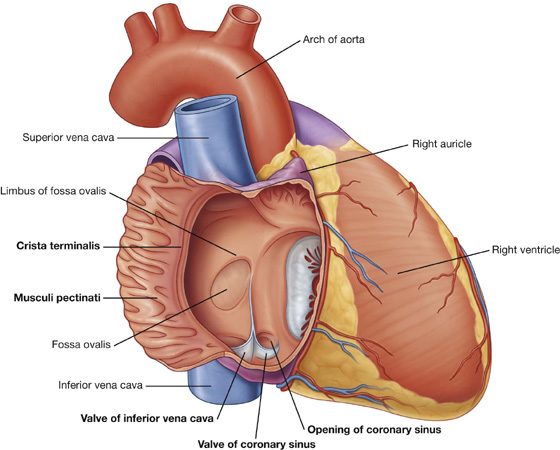
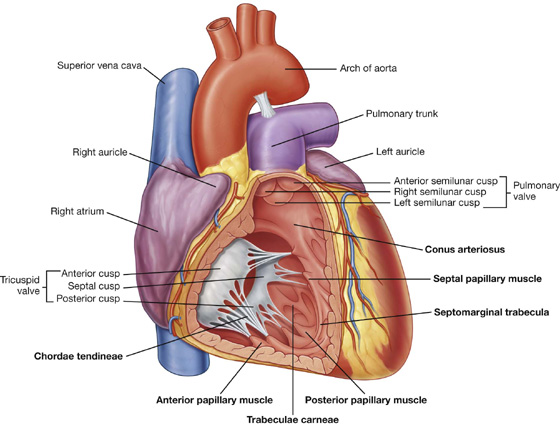
 The anterior papillary muscle is the largest and most constant papillary muscle, and arises from the anterior wall of the ventricle.
The anterior papillary muscle is the largest and most constant papillary muscle, and arises from the anterior wall of the ventricle. The posterior papillary muscle may consist of one, two, or three structures, with some chordae tendineae arising directly from the ventricular wall.
The posterior papillary muscle may consist of one, two, or three structures, with some chordae tendineae arising directly from the ventricular wall.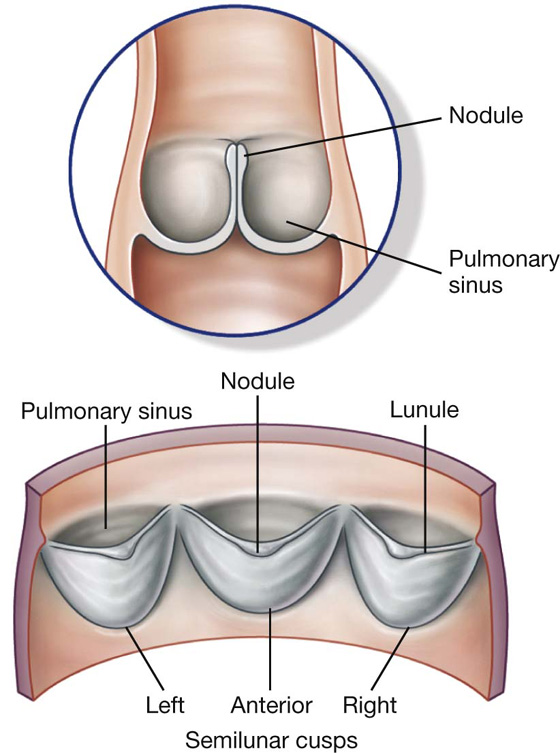
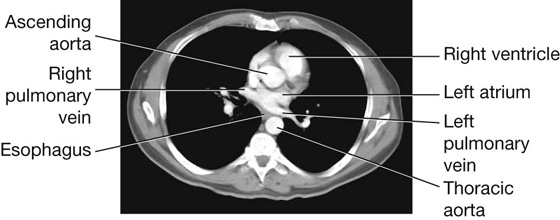
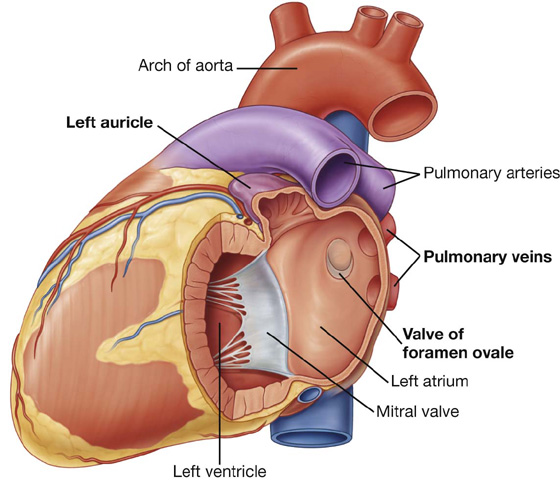
 The anterior half is continuous with the left auricle. It contains musculi pectinati and derives from the embryonic primitive atrium. Unlike the crista terminalis in the right atrium, no distinct structure separates the two components of the left atrium.
The anterior half is continuous with the left auricle. It contains musculi pectinati and derives from the embryonic primitive atrium. Unlike the crista terminalis in the right atrium, no distinct structure separates the two components of the left atrium.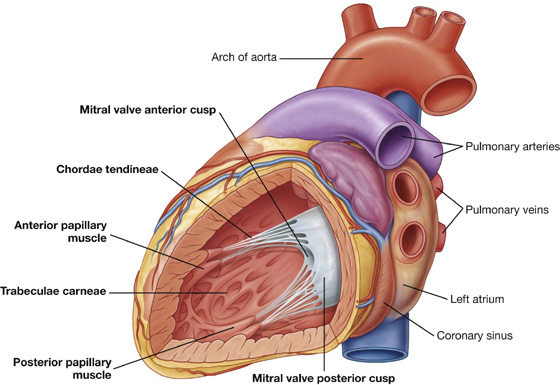
 a muscular part, and
a muscular part, and a membranous part.
a membranous part.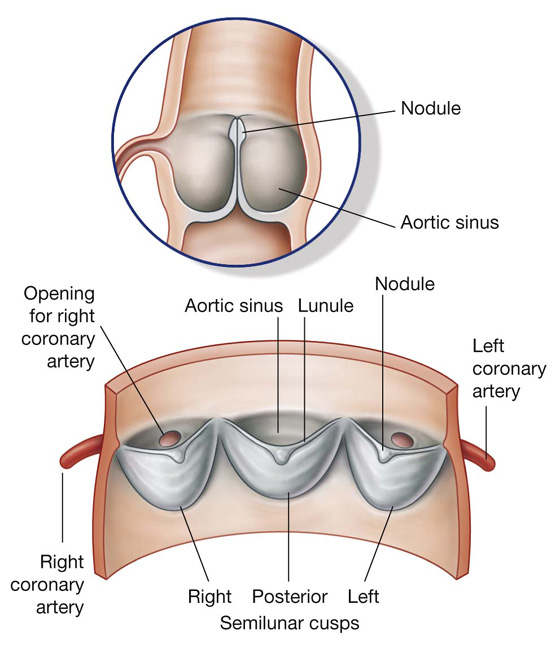
 The tricuspid valve is heard just to the left of the lower part of the sternum near the fifth intercostal space.
The tricuspid valve is heard just to the left of the lower part of the sternum near the fifth intercostal space. The mitral valve is heard over the apex of the heart in the left fifth intercostal space at the midclavicular line.
The mitral valve is heard over the apex of the heart in the left fifth intercostal space at the midclavicular line.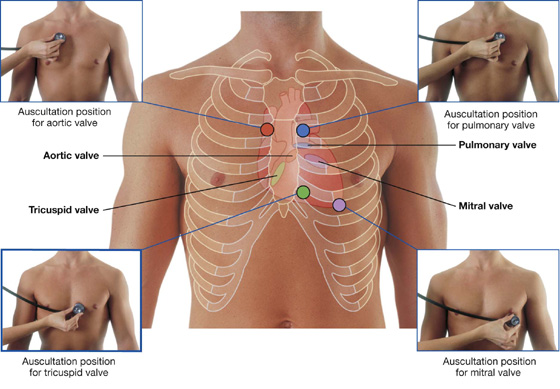
 pulmonary edema, and
pulmonary edema, and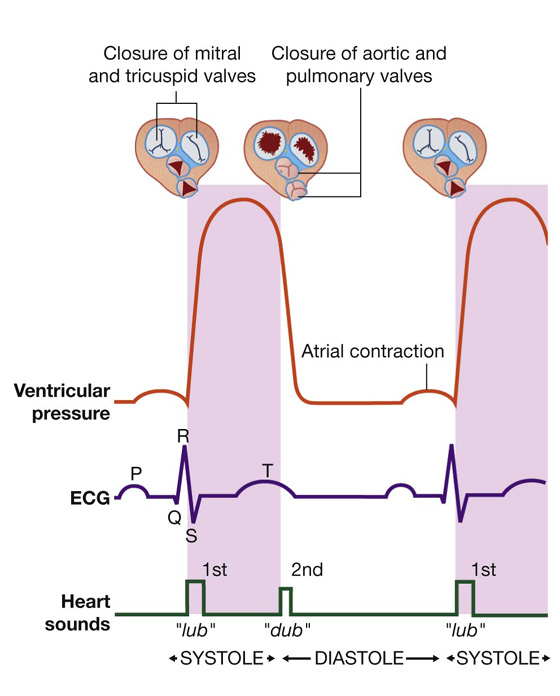
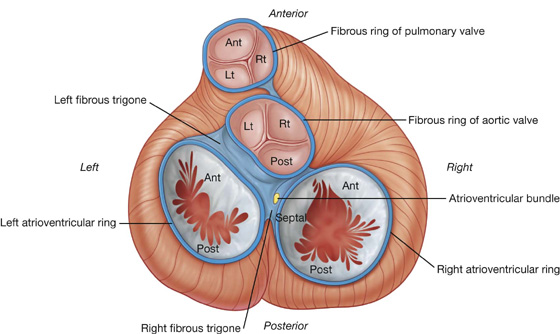
 an early atrial branch, passing between the right auricle and ascending aorta, gives off the sinu-atrial nodal branch, which passes posteriorly around the superior vena cava to supply the sinu-atrial node;
an early atrial branch, passing between the right auricle and ascending aorta, gives off the sinu-atrial nodal branch, which passes posteriorly around the superior vena cava to supply the sinu-atrial node; a right marginal branch arising as the right coronary artery approaches the inferior (acute) margin of the heart. This branch continues along this border toward the apex of the heart;
a right marginal branch arising as the right coronary artery approaches the inferior (acute) margin of the heart. This branch continues along this border toward the apex of the heart; a small branch to the atrioventricular node as the right coronary artery continues on the base/ diaphragmatic surface of the heart; and
a small branch to the atrioventricular node as the right coronary artery continues on the base/ diaphragmatic surface of the heart; and the posterior interventricular branch, its final branch, which lies in the posterior interventricular sulcus.
the posterior interventricular branch, its final branch, which lies in the posterior interventricular sulcus.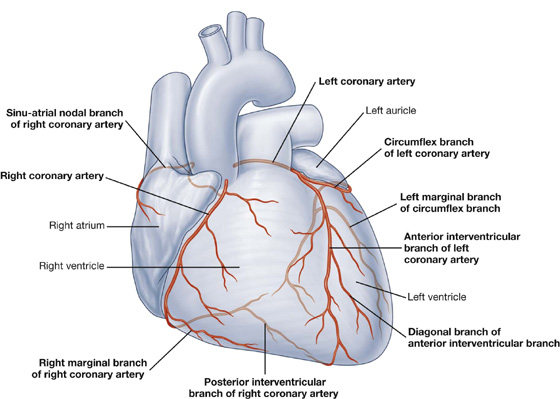
 The anterior interventricular branch (left anterior descending artery—LAD) continues around the left side of the pulmonary trunk and descends obliquely toward the apex of the heart in the anterior interventricular sulcus. During its course, one or two large diagonal branches may arise and descend diagonally across the anterior surface of the left ventricle.
The anterior interventricular branch (left anterior descending artery—LAD) continues around the left side of the pulmonary trunk and descends obliquely toward the apex of the heart in the anterior interventricular sulcus. During its course, one or two large diagonal branches may arise and descend diagonally across the anterior surface of the left ventricle. The circumflex branch continues to the left in the coronary sulcus and onto the base/diaphragmatic surface of the heart. It usually ends before reaching the posterior interventricular sulcus. A large branch, the left marginal artery, usually arises from it and continues across the rounded obtuse margin of the heart.
The circumflex branch continues to the left in the coronary sulcus and onto the base/diaphragmatic surface of the heart. It usually ends before reaching the posterior interventricular sulcus. A large branch, the left marginal artery, usually arises from it and continues across the rounded obtuse margin of the heart. The distribution pattern described above for both right and left coronary arteries is the most common and consists of a right dominant coronary artery. This means that the posterior interventricular branch arises from the right coronary artery. The right coronary artery therefore supplies a large portion of the posterior wall of the left ventricle, and the circumflex branch of the left coronary artery is relatively small.
The distribution pattern described above for both right and left coronary arteries is the most common and consists of a right dominant coronary artery. This means that the posterior interventricular branch arises from the right coronary artery. The right coronary artery therefore supplies a large portion of the posterior wall of the left ventricle, and the circumflex branch of the left coronary artery is relatively small.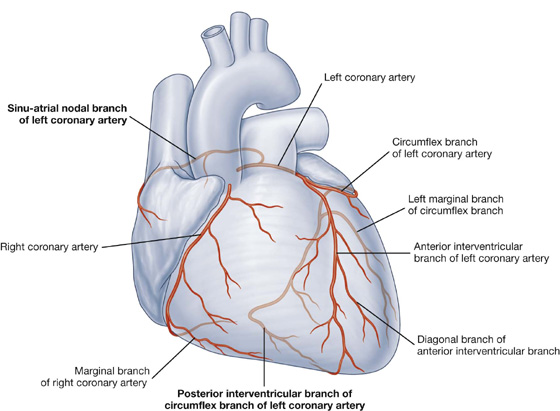
 Another point of variation relates to the arterial supply to the sinu-atrial and atrioventricular nodes. In most cases, these two structures are supplied by the right coronary artery. However, vessels from the circumflex branch of the left coronary artery occasionally supply these structures.
Another point of variation relates to the arterial supply to the sinu-atrial and atrioventricular nodes. In most cases, these two structures are supplied by the right coronary artery. However, vessels from the circumflex branch of the left coronary artery occasionally supply these structures.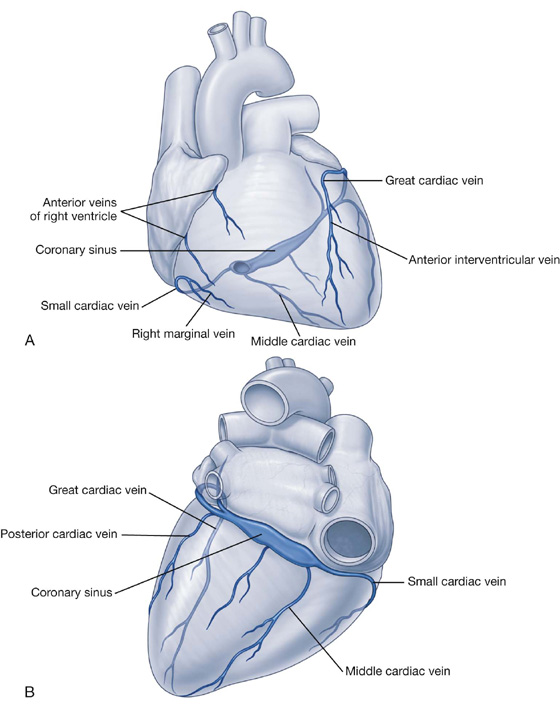
 A group of the smallest cardiac veins (venae cordis minimae or veins of Thebesius) have also been described. Draining directly into the cardiac chambers, they are numerous in the right atrium and right ventricle, are occasionally associated with the left atrium, and are rarely associated with the left ventricle.
A group of the smallest cardiac veins (venae cordis minimae or veins of Thebesius) have also been described. Draining directly into the cardiac chambers, they are numerous in the right atrium and right ventricle, are occasionally associated with the left atrium, and are rarely associated with the left ventricle. the sinu-atrial node,
the sinu-atrial node, the atrioventricular node,
the atrioventricular node,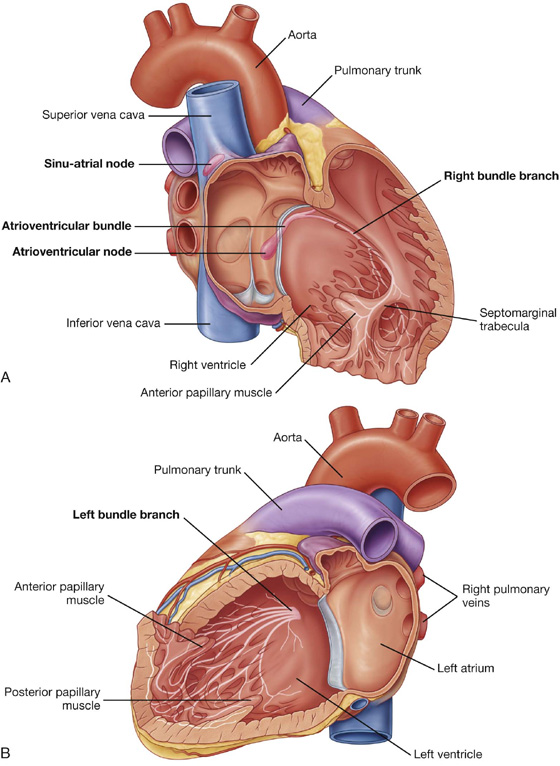
 heart rate,
heart rate, cardiac output.
cardiac output.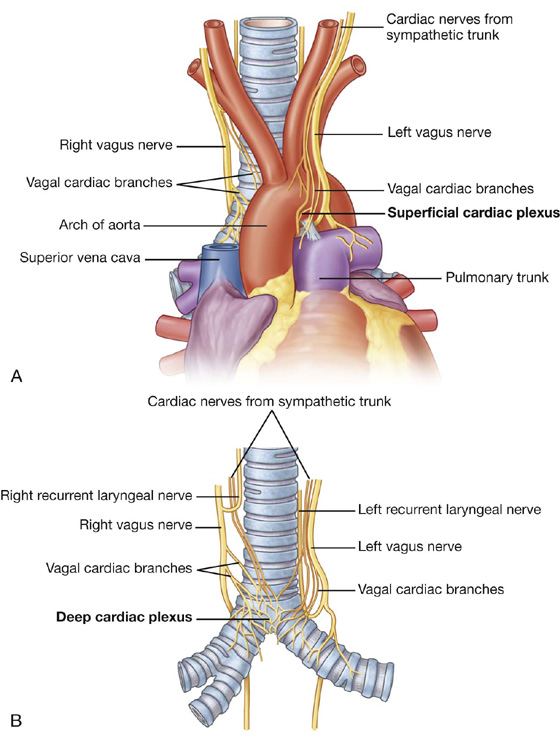
 decreases heart rate,
decreases heart rate, increases heart rate, and
increases heart rate, and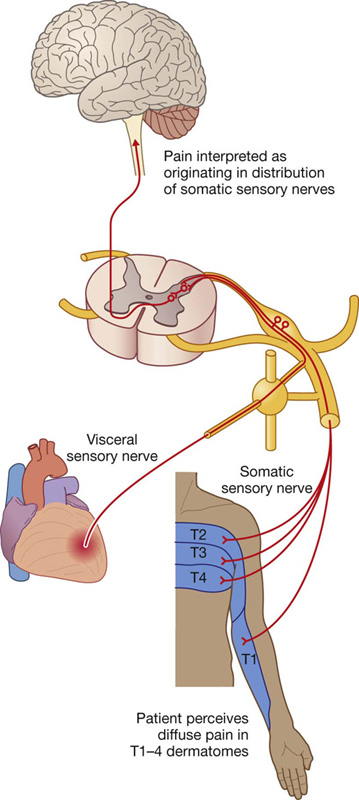
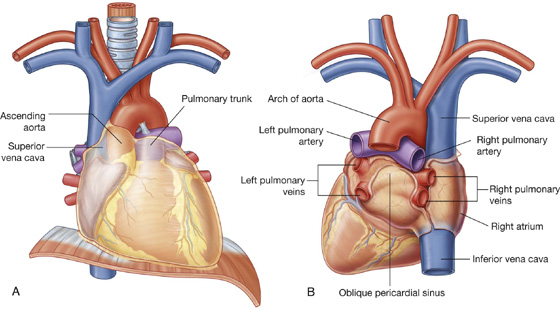
 Superior border—an oblique plane passing from the jugular notch upward and posteriorly to the superior border of vertebra TI.
Superior border—an oblique plane passing from the jugular notch upward and posteriorly to the superior border of vertebra TI. Inferior border—a transverse plane passing from the sternal angle to the intervertebral disc between vertebra TIV/V separates it from the inferior mediastinum.
Inferior border—a transverse plane passing from the sternal angle to the intervertebral disc between vertebra TIV/V separates it from the inferior mediastinum. thymus,
thymus, superior vena cava,
superior vena cava, trachea,
trachea, esophagus,
esophagus, phrenic nerves,
phrenic nerves, vagus nerves,
vagus nerves, thoracic duct, and
thoracic duct, and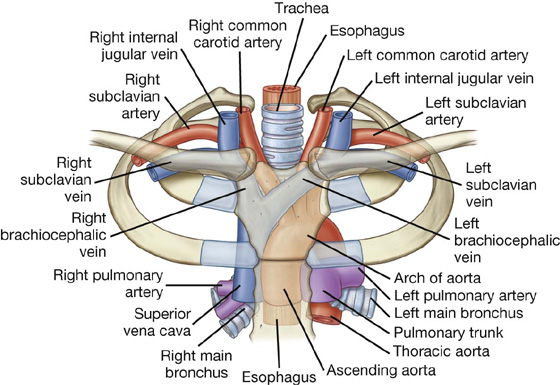
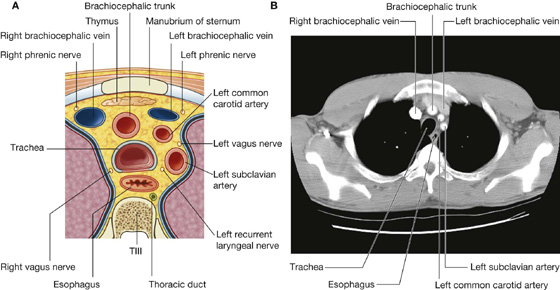
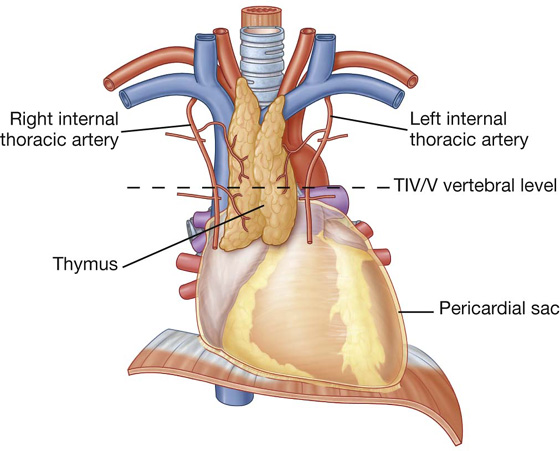
 in the root of the neck.
in the root of the neck.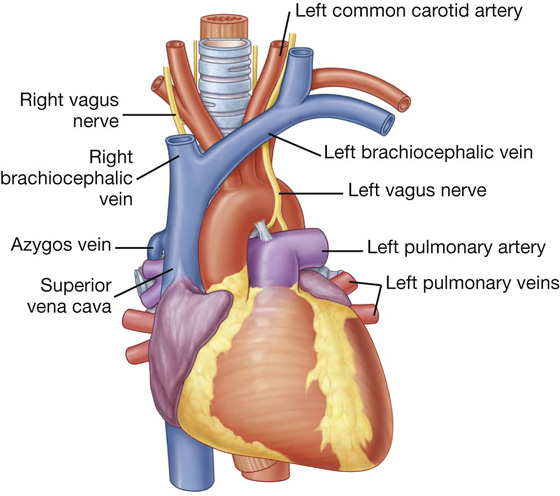
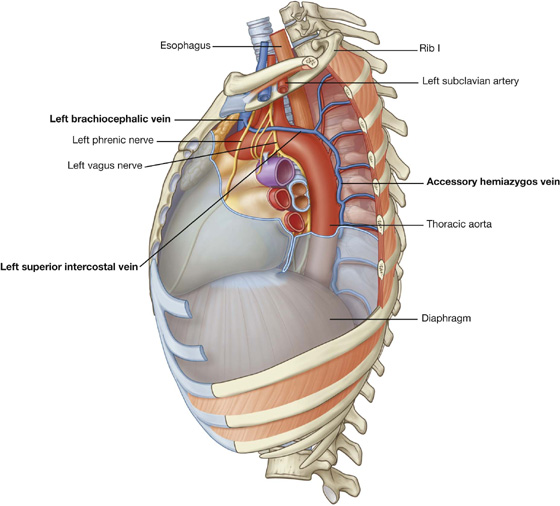
 transjugular liver biopsy,
transjugular liver biopsy, transjugular intrahepatic portosystemic shunts (TIPS), and
transjugular intrahepatic portosystemic shunts (TIPS), and insertion of an inferior vena cava filter to catch emboli dislodged from veins in the lower limb and pelvis (i.e., patients with deep vein thrombosis [DVT]).
insertion of an inferior vena cava filter to catch emboli dislodged from veins in the lower limb and pelvis (i.e., patients with deep vein thrombosis [DVT]).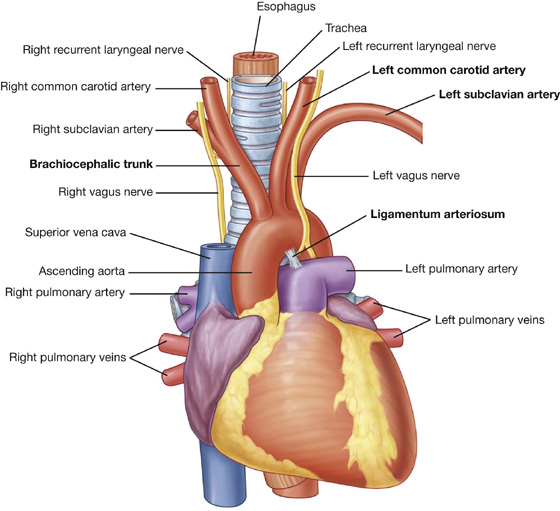
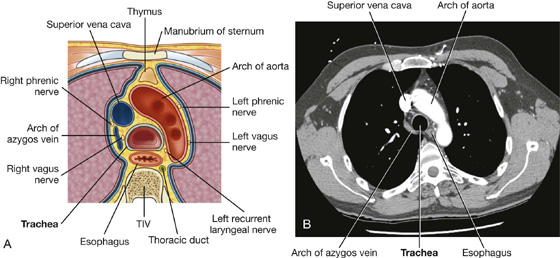
 the right subclavian artery.
the right subclavian artery. the aortic valve,
the aortic valve, the right subclavian artery originating from the distal portion of the aortic arch and passing behind the esophagus to supply the right arm—as a result, the great vessels form a vascular ring around the trachea and the esophagus, which can potentially produce difficulty swallowing.
the right subclavian artery originating from the distal portion of the aortic arch and passing behind the esophagus to supply the right arm—as a result, the great vessels form a vascular ring around the trachea and the esophagus, which can potentially produce difficulty swallowing.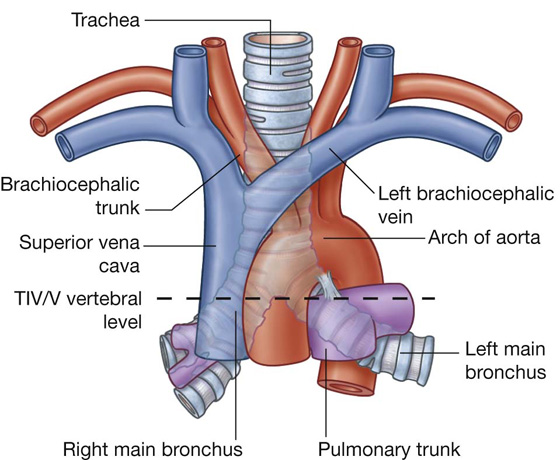
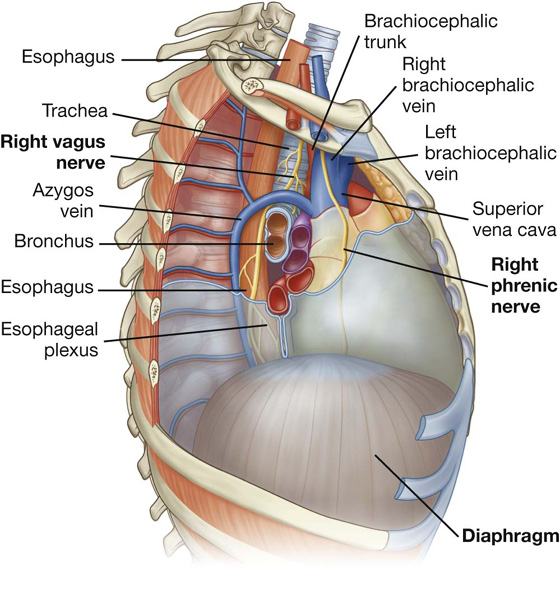
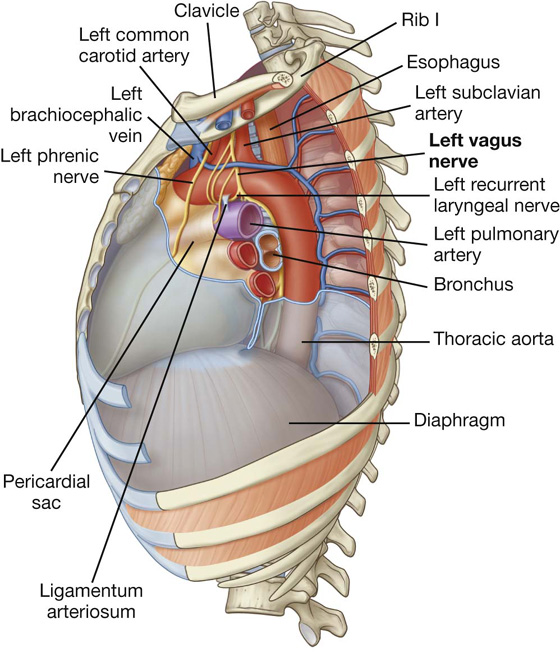
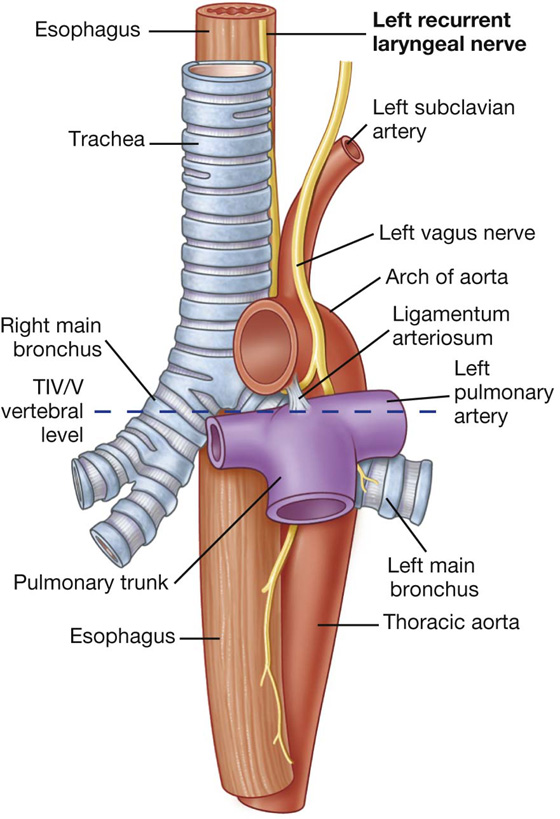
 On each side, the internal jugular and subclavian veins join to form the brachiocephalic veins behind the sternal ends of the clavicles near the sternoclavicular joints.
On each side, the internal jugular and subclavian veins join to form the brachiocephalic veins behind the sternal ends of the clavicles near the sternoclavicular joints. The brachiocephalic veins unite to form the superior vena cava behind the lower border of the costal cartilage of the right first rib.
The brachiocephalic veins unite to form the superior vena cava behind the lower border of the costal cartilage of the right first rib. The arch of aorta begins and ends at the transverse plane between the sternal angle anteriorly and vertebral level TIV/V posteriorly. The arch may reach as high as the midlevel of the manubrium of sternum.
The arch of aorta begins and ends at the transverse plane between the sternal angle anteriorly and vertebral level TIV/V posteriorly. The arch may reach as high as the midlevel of the manubrium of sternum.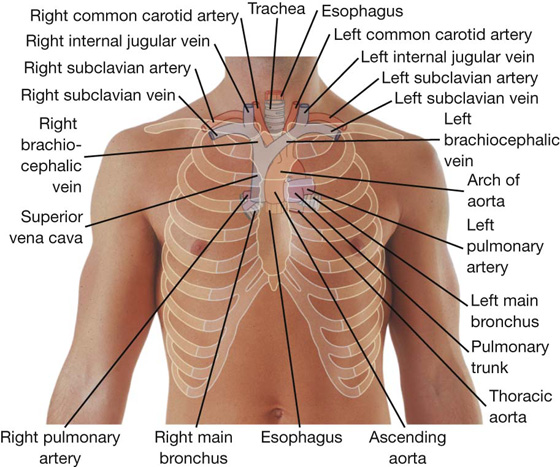
 enters the superior mediastinum inferiorly, slightly to the left of the midline, having moved to this position just before leaving the posterior mediastinum opposite vertebral level TIV/V; and
enters the superior mediastinum inferiorly, slightly to the left of the midline, having moved to this position just before leaving the posterior mediastinum opposite vertebral level TIV/V; and continues through the superior mediastinum, posterior to the arch of aorta, and the initial portion of the left subclavian artery, between the esophagus and the left mediastinal part of the parietal pleura.
continues through the superior mediastinum, posterior to the arch of aorta, and the initial portion of the left subclavian artery, between the esophagus and the left mediastinal part of the parietal pleura. Its superior boundary is a transverse plane from the sternal angle to the TIV and TV intervertebral disc.
Its superior boundary is a transverse plane from the sternal angle to the TIV and TV intervertebral disc. azygos system of veins,
azygos system of veins, sympathetic trunks, and
sympathetic trunks, and thoracic splanchnic nerves.
thoracic splanchnic nerves.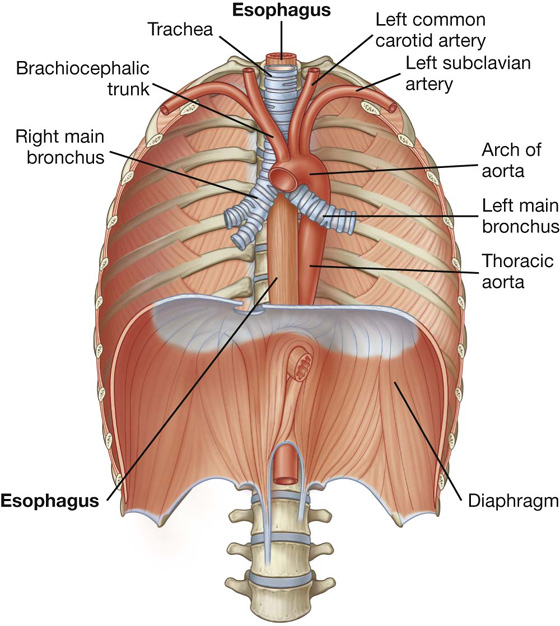
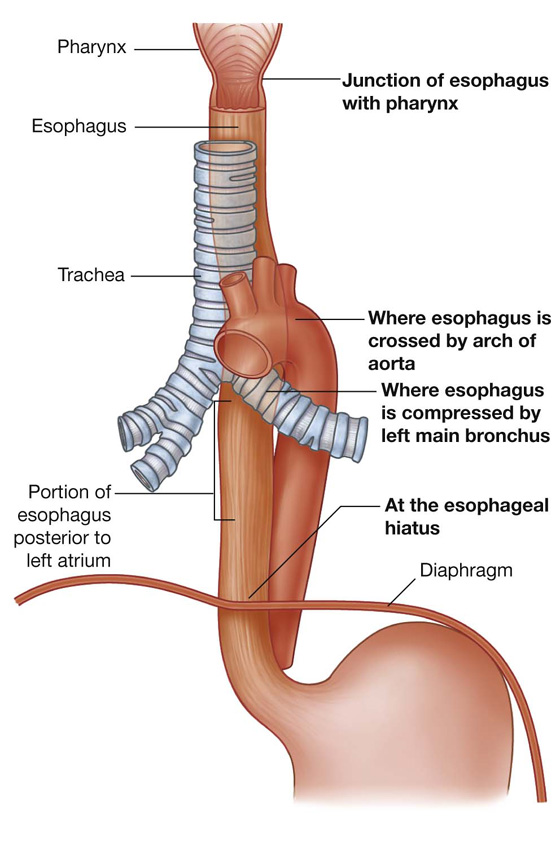
 the anterior vagal trunk on the anterior surface of the esophagus, mainly from fibers originally in the left vagus nerve;
the anterior vagal trunk on the anterior surface of the esophagus, mainly from fibers originally in the left vagus nerve; the posterior vagal trunk on the posterior surface of the esophagus, mainly from fibers originally in the right vagus nerve.
the posterior vagal trunk on the posterior surface of the esophagus, mainly from fibers originally in the right vagus nerve.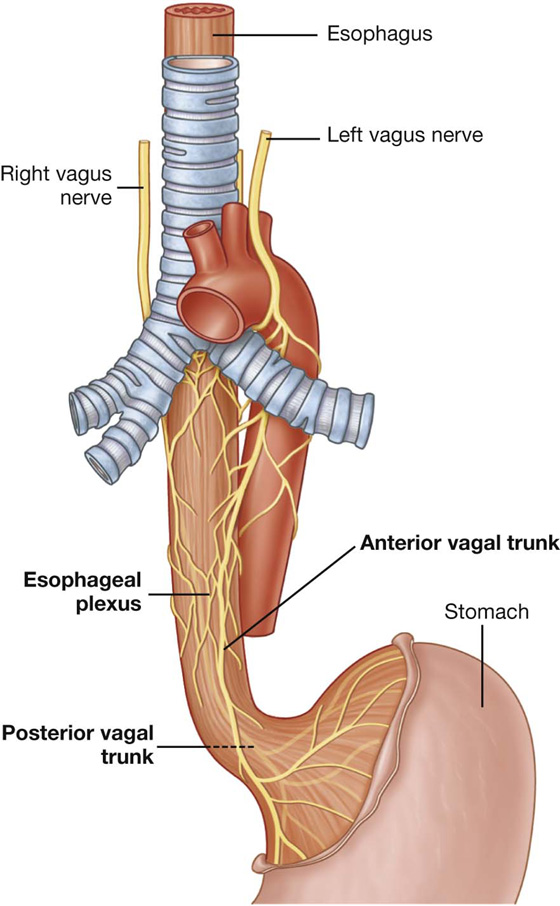
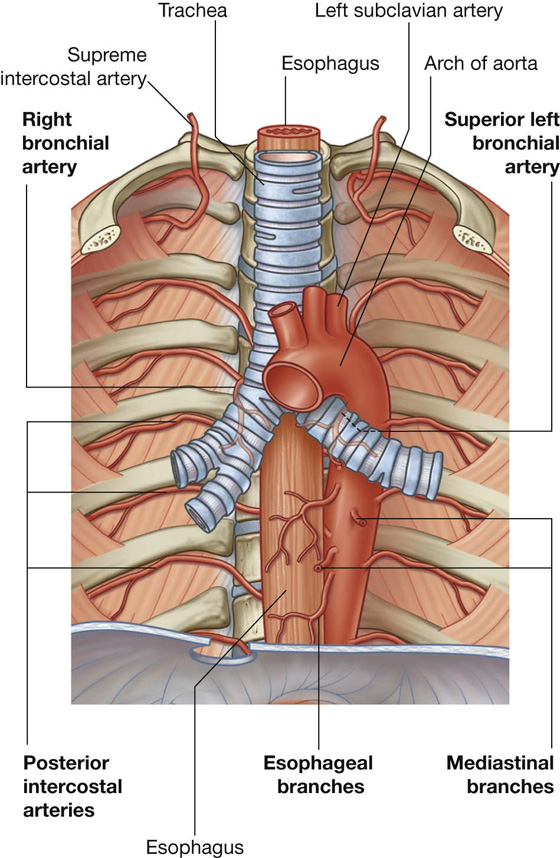
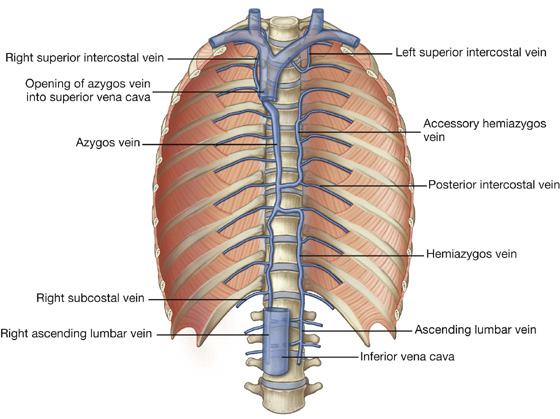
 the right superior intercostal vein (a single vessel formed by the second, third, and fourth intercostal veins),
the right superior intercostal vein (a single vessel formed by the second, third, and fourth intercostal veins), the hemiazygos vein,
the hemiazygos vein, esophageal veins,
esophageal veins, mediastinal veins,
mediastinal veins, pericardial veins, and
pericardial veins, and right bronchial veins.
right bronchial veins. esophageal veins, and
esophageal veins, and mediastinal veins.
mediastinal veins.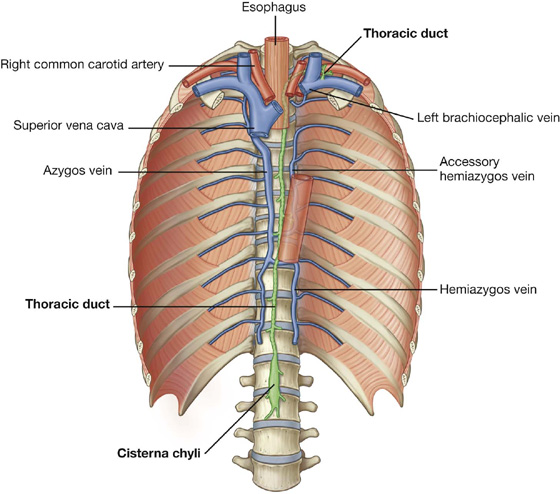
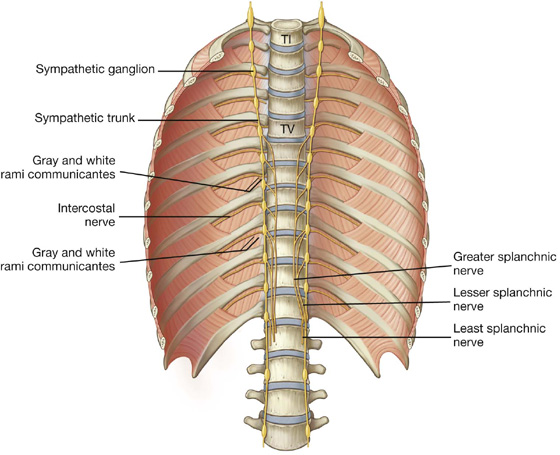
 The greater splanchnic nerve on each side usually arises from the fifth to ninth or tenth thoracic ganglia. It descends across the vertebral bodies moving in a medial direction, passes into the abdomen through the crus of the diaphragm, and ends in the celiac ganglion.
The greater splanchnic nerve on each side usually arises from the fifth to ninth or tenth thoracic ganglia. It descends across the vertebral bodies moving in a medial direction, passes into the abdomen through the crus of the diaphragm, and ends in the celiac ganglion. The lesser splanchnic nerve usually arises from the ninth and tenth or tenth and eleventh thoracic ganglia. It descends across the vertebral bodies moving in a medial direction, and passes into the abdomen through the crus of the diaphragm to end in the aorticorenal ganglion.
The lesser splanchnic nerve usually arises from the ninth and tenth or tenth and eleventh thoracic ganglia. It descends across the vertebral bodies moving in a medial direction, and passes into the abdomen through the crus of the diaphragm to end in the aorticorenal ganglion. The least splanchnic nerve (lowest splanchnic nerve), when present, usually arises from the twelfth thoracic ganglion. It descends and passes into the abdomen through the crus of the diaphragm to end in the renal plexus.
The least splanchnic nerve (lowest splanchnic nerve), when present, usually arises from the twelfth thoracic ganglion. It descends and passes into the abdomen through the crus of the diaphragm to end in the renal plexus. Its superior boundary is a transverse plane passing from the sternal angle to the intervertebral disc between vertebrae TIV and TV, separating it from the superior mediastinum.
Its superior boundary is a transverse plane passing from the sternal angle to the intervertebral disc between vertebrae TIV and TV, separating it from the superior mediastinum.