Chapter 32 Thoracoscopic Surgery for Thoracic Spinal Tumors
INTRODUCTION
For the surgery of thoracic spinal tumors, open thoracotomy is usually performed. However, morbidity associated with conventional open thoracotomy often limits the application of anterior approaches to the thoracic spine. Most of the vertebral body tumors of the thoracic spine involve the anterior column, and these tumors require rib removal and parietal pleura opening, which can cause the complications, such as post-thoracotomy syndromes and intercostal neuralgia.1 Open thoracotomy also requires extensive exposures with incisions measuring up to 20 cm. In recent years minimally invasive open microscopic approaches using special retractors have been developed that can minimize the size of the operative access to 6–10 cm. These “mini-approaches” are quite feasible in surgery on the upper and mid-thoracic spine. The limited working space available through small incisions may block the microscopic view and result in difficult manipulation of instruments. However, video assisted thoracic surgery (VATS) using four portals in the chest wall permits surgery that is as effective as the open approaches, and it provides a better view than the microscopic approach. Unlike the open approaches, the surgeon’s operative view is not obscured by hands or operative instruments, and high morbidity associated with open procedures can be avoided. Biomechanically, the VATS approach, like any other anterior approach to the spine for reconstruction, significantly increases the axial load-bearing capabilities of the spine.2
THORACOSCOPIC CORPECTOMY AND STABILIZATION
ENDOSCOPIC INSTRUMENTS
POSITIONING
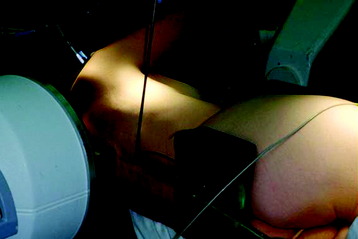
The patient is placed in a stable lateral decubitus position and fixed with a four-point-support at the symphysis, sacrum, and scapula, as well as with arm rests (Fig. 32-1). A left-sided position is preferred for the treatment of lesions from T4 to T8. A right-sided position is preferred for the approach to the thoracolumbar junction (T9–L3). The upper arm should be abducted and elevated to prevent disturbing the placement and manipulation of the endoscope.
LOCALIZATION
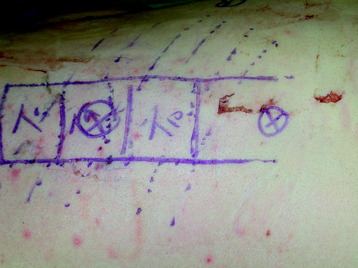
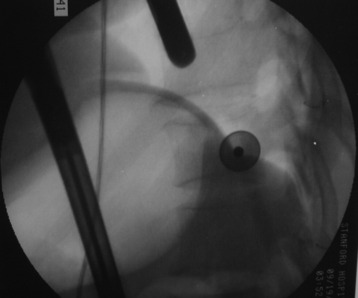
The target area is projected onto the skin level under fluoroscopic control. The borders of the lesion vertebra are marked on the skin, indicating the line of the anterior and posterior edges as well as the endplates of the affected segments (Fig. 32-2). The working channel is centered over the target vertebra (12.5 mm).
PLACEMENT OF PORTALS
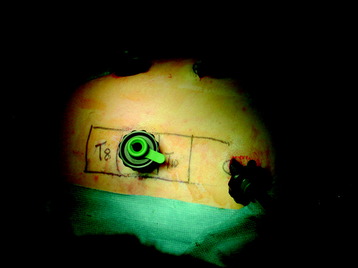
Through a 1.5-cm skin incision above the intercostal space, small Langenbeck hooks are inserted (Fig. 32-3) The muscles of the thoracic wall are crossed in a blunt, muscle-splitting technique, and the intercostal space is opened by blunt dissection, thus exposing the pleura and creating an opening to enter the thoracic cavity. The 10-mm trocar is inserted and one-lung ventilation is started. The 30-degree scope is inserted at a flat angle in the direction of the second trocar. Perforation of the thoracic wall to insert the second, third, and fourth trocars is performed under visual control through the scope, and the other trocars are inserted as shown (Fig. 32-4).
PREVERTEBRAL DISSECTION
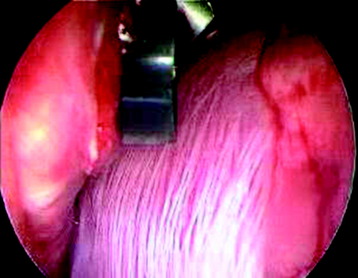
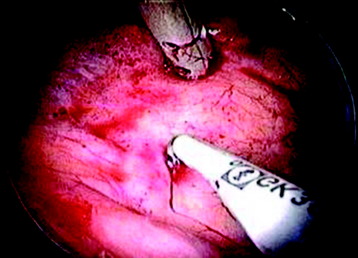
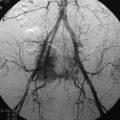
The target area can now be exposed with the help of a fan-retractor inserted through the anterior port. The retractor sweeps away lung tissue and holds down the insertion of the diaphragm on the spine. The anterior border of the vertebral body and disc, as well as the course of the aorta, is palpated with a blunt probe (Fig. 32-5).
The parietal pleura is widely incised with a hook diathermy electrode (Fig. 32-6). The incision starts from the anterior border of the vertebral body, continuing to the rib head and to the proximal rib segment. The incision line should be in the disc level, and care should be taken to not injure the segmental vessels that are located in the mid-portion of the vertebral body.
After the linear dissection of the pleura is done, the enlargement of the dissection continues from disc level to the adjacent vertebral bodies (Fig. 32-7).
The segmental vessels can be coagulated and divided with clips or cautery.
SCREW INSERTION
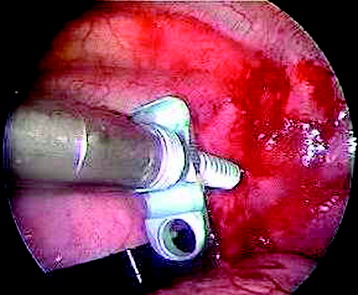
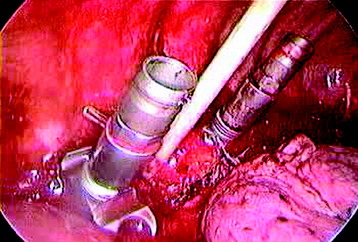
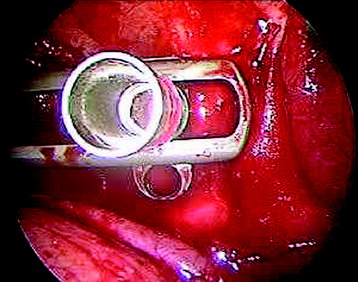
Before the cannulated screw is inserted into the adjacent vertebral body, K-wire is impacted first (Figs. 32-8 and 32-9). The insertion point is 1.5 cm from the posterior border of the vertebral body and 10 mm away from the endplate. The correct point should be confirmed with fluoroscopic guide. The insertion depth is about 20 mm. It is important to confirm the perpendicular position of the patient to the beam. The polyaxial, posterior screw is placed over the K-wire (Figs. 32-10 and 32-11). The direction of the polyaxial clamp can be controlled by the handle. The polyaxial clamp has to be orientated so that the hole for the anterior stabilization screw comes to lie anteriorly. After the first turns of the screw into the vertebral body, the K-wire has to be removed to prevent the risk of tissue perforation by pushing the K-wire forward during screw insertion. After partial insertion of the polyaxial screws, the K-wire has to be removed.
DISCECTOMY
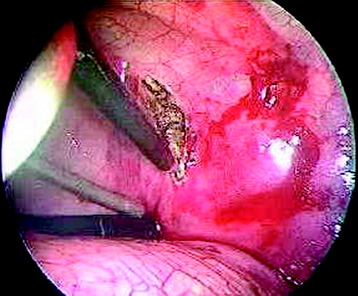
After the screw is inserted to the adjacent vertebral body, the disc is removed. The disc is shrunken with a monopolar coagulator and removed with a curette (Fig. 32-12). For the effective removal of the disc, the rib head should be drilled away. The proximal 2 cm of the rib is removed using a high-speed drill to expose the lateral surface of the pedicle and neural foramen.
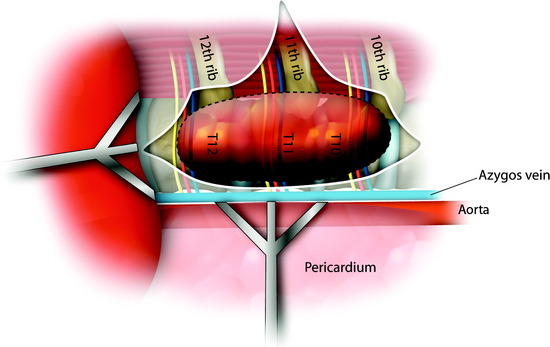
CORPECTOMY
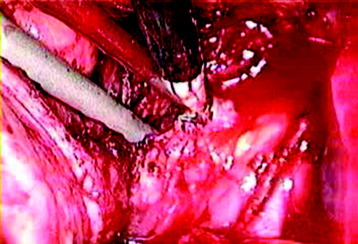
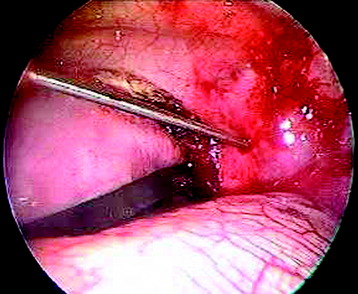
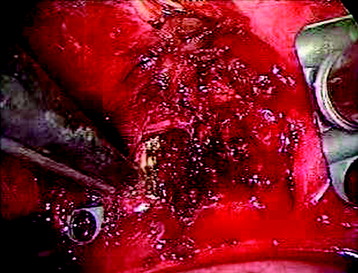
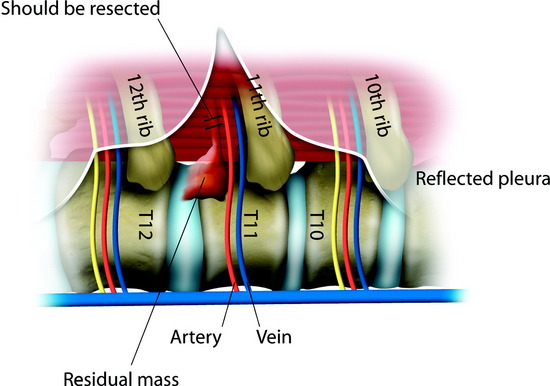
After the disc is removed, the corpectomy margin is prepared with a monopolar coagulator (Fig. 32-13). When the anterior margin is coagulated, the coagulation point should be 10 mm away from the great vessels (Fig. 32-14). After the parietal pleura is coagulated, the extent of the planned vertebrectomy is defined with an osteotome. The tumor mass is removed piece by piece with a curette and pituitary forceps (Fig. 32-15). For the complete decompression of the spinal cord, the pedicle should be removed. The lower border of the pedicle should first be identified with a blunt hook. The base of the pedicle is then resected in a cranial direction with a Kerrison rongeur, and the thecal sac can be identified from the lateral direction. The decompression is continued until the ventral surface of the dura is visualized (Fig. 32-16). The posterior longitudinal ligament can be removed with the Kerrison rongeur to expose the dura. Bone bleeding is controlled with bone wax, and epidural hemostasis is achieved with Gelfoam and bipolar cautery.
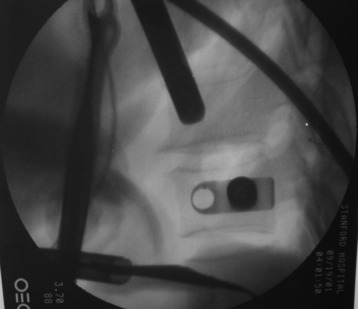
Reconstruction of the corpectomy site can be accomplished with either a bone graft or titanium mesh cage, depending on the pathology (Fig. 32-17). An autograft, allograft, and bone cement–filled titanium mesh are used. In cases of metastatic tumor, a bone cement graft is favored. Preparation of the graft bed is then completed, and the length and the depth of the bone graft are measured with a caliper. The bone graft is prepared for insertion and mounted on a graft holder. The cortical bone is perforated with several burr holes to facilitate vascular in-growth and new bone formation. The working portal is removed and a speculum is inserted. This allows the insertion of a bone graft up to 1.5 cm in length into the thoracic cavity.
PLATE OR ROD PLACEMENT
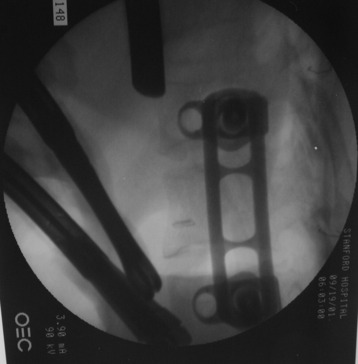
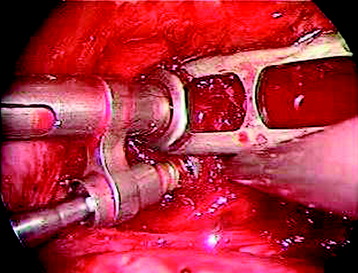
Using the measurement device, the distance between the polyaxial heads has to be measured. If the plates are used, 30 mm has to be added to select the proper plate length (Figs. 32-18 and 32-19). The stabilization plate is then placed onto the polyaxial heads. The rounded side with the markings is the upper side of the plate. In cases of multi-segmental assemblies, rod connection has to be chosen.
FINAL FIXATION
After the plate or rod is placed and the polyaxial plate is well-aligned, the assembly can be closed by using a fixation nut (Fig. 32-20). The nut should be placed with the smooth part against the stabilization plate. Using the cannulated nut driver, the nut can be placed and tightened onto the screw. An anterior screw is added with the aid of the guiding instrument.
THORACOSCOPIC PARASPINAL TUMOR RESECTION
When an attempt is made to resect the paraspinal tumor thoracoscopically, significant bone removal is not required. The adjacent rib and vertebral body may be eroded. The opening of the parietal pleura can expose the tumor mass. The pleural incision is made over the center of the tumor mass. In cases of the cystic mass, fluid aspiration is performed before the pleural incision, which can facilitate the en bloc removal. The pleura is opened widely to expose and mobilize the margins of the tumor. The tumor is usually removed piece by piece. In cases of neurogenic tumor, the stalk of the tumor, proximally within the neural foramen, is resected after the tumor is debulked. The edges of the intrathoracic tumor are grasped and dissected from the adjacent vascular and visceral structures using blunt and sharp dissection. Traction should be avoided on the proximal tumor within the neural foramen to avoid avulsing the nerve root, which may cause the dura to tear and cerebrospinal fluid leakage.3,4 For patients with dumbbell tumors, a combined approach is used. The dissection of the intraspinal component must be performed first to free the tumor from its relation to the cord.4,5 Thus, traction on the spinal cord is avoided during the thoracic dissection. This step is performed via a posterior approach with laminectomy. Then the patient is placed in a lateral decubitus position for the thoracoscopic stage.
CASE ILLUSTRATION
A 19-year-old male musician was asymptomatic except for chronic coughing.
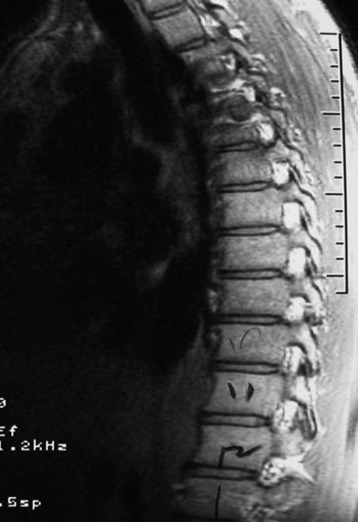
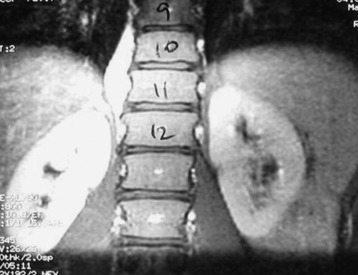
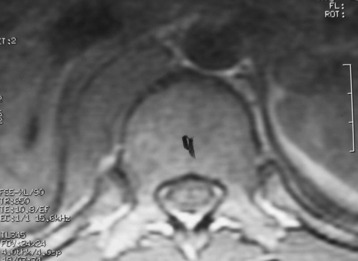
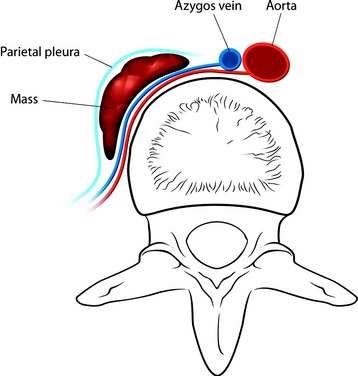
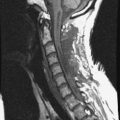
He was diagnosed with pneumonia. However, the sputum examination for bacteria and tuberculosis all turned out to be negative. Chest x-ray showed questionable paraspinal thickening. On physical examination, he showed no skin lesions, no palpable mass. and no deformity. Neurologically no abnormal finding was found. Computed tomography scan and magnetic resonance imaging (MRI) were performed. MRI showed the long, slender paraspinal mass along the right side of the thoracic spine from T10 to T12 (Fig. 32-21). The mass was adhered to the vertebral bodies on the right side and attached to the dome of the diaphragm (Fig. 32-22). The aorta was about 3 or 4 mm away from the mass (Fig. 32-23). The connection of the mass to the spinal cord was not found. The possibility of a schwannoma was low.
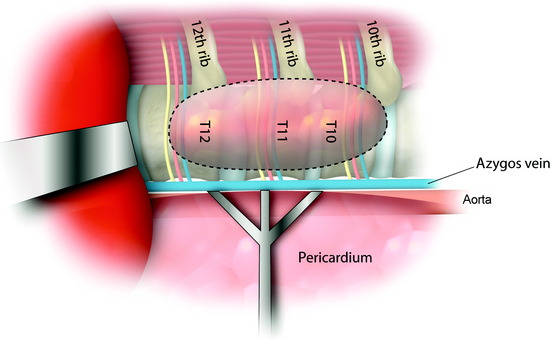
The operation was performed thoracoscopically from the right side. When the lower thoracic cavity is entered, the pulmonary ligament is seen.6 The pulmonary ligament represents the transition between the parietal and visceral pleural surfaces adjacent to the diaphragm posteroinferiorly. The lower thoracic spine is exposed after the pulmonary ligament is cut (Figs. 32-24 and 32-25). The diaphragm is carefully retracted caudally with an endoscopic fan retractor to expose the costodiaphragmatic recess. Within the thoracic cavity, the thoracolumbar junction can be visualized endoscopically as low as the T12–L1 disc space and L1 vertebral body.
The exposed lower thoracic vertebral bodies showed the elevated appearance. After the parietal pleura was incised enough to expose the whole length of the mass, marginal dissection was started (Fig. 32-26). The superficial vessels on the tumor were sealed with bipolar cauterization. The tumor mass was incised and divided into small sections with a scalpel.
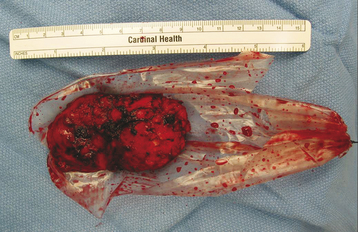
A large piece of tumor was grasped with a disc rongeur and removed through the endoscopic portal. The intercostal nerve from which the tumor originated was identified and sectioned distal to the tumor to mobilize the tumor mass (Fig. 32-27). The residual portion, which was located near the neural foramen, was removed using micro-dissection tools and a disc rongeur. The segmental vessels lay underneath the mass. With careful dissection, the lower surface of the mass could be easily dissected from the vertebral body. If the underlying segmental vessels are injured, the use of bipolar coagulation or hemoclip application can control the bleeding. The pathology was ganglioneuroma.
Ganglioneuromas are a subgroup of peripheral neuroblastic tumors that have been defined as childhood embryonal tumors of migrating neuroectodermal cells derived from the neural crest.7 Peripheral neuroblastic tumors are classified into three subgroups: neuroblastomas, ganglioneuroblastomas, and ganglioneuromas. Immature neuroblastic tumors are reportedly capable of maturing into more differentiated forms, such as spontaneous or therapy-induced maturation of neuroblastoma or maturation from ganglioneuroblastoma to ganglioneuroma. The final stage of maturation in the neuroblastic tumors is usually seen in patients more than 10 years old. Ganglioneuromas, a mature form of neuroblastic tumors, are rare, slow-growing benign tumors characterized by a firm, round or oval, and well-encapsulated mass with occasional lobulations, occurring most commonly in children or young adults (Fig. 32-28). They can arise from the sympathetic nervous system, extending from the skull base to the pelvis but mainly from the paraspinal sympathetic chain ganglia. Therefore, they are most commonly found in the posterior mediastinum and abdomen, where they usually arise from the adrenal medulla, and in the lumbar and pelvic retroperitoneal space. A very few may originate from other locations or heterotopic areas, as well as from peripheral nerves (sensory ganglia or nerves). Ganglioneuromas of sympathetic chain origin usually develop as a paravertebral mass and may infrequently become dumbbell tumors extending into the spinal canal through one or more intervertebral foramina. The pattern may depend on whether the tumor originates from within or outside the spinal canal or within the intervertebral foramen. In many cases of dumbbell ganglioneuroma, the intraspinal portion of the tumor extends extradurally, but intradural extension is extremely rare.