21 Thoracic Aorta
Thoracic aortic diseases typically require surgical intervention (Table 21-1). Acute aortic dissections, rupturing aortic aneurysms, and traumatic aortic injuries are surgical emergencies. Subacute aortic dissection and expanding aortic aneurysms require urgent surgical intervention. Stable thoracic or thoracoabdominal aortic aneurysms (TAAAs), aortic coarctation, or atheromatous disease causing embolization may be addressed surgically on an elective basis. The volume of thoracic aortic procedures has grown steadily because of factors such as increased public awareness, an aging population, earlier diagnosis, multiple advances in imaging, and advances in surgical techniques including endovascular stenting. Medical centers have emerged that specialize in thoracic aortic diseases, resulting in improved management and survival. This progress has created a set of patients who later require reoperation for long-term complications such as valve or graft failure, pseudoaneurysm at anastomotic sites, endocarditis, and/or progression of the original disease process into residual native aorta.
TABLE 21-1 Thoracic Aortic Diseases Amenable to Surgical Treatment
| Aneurysm |
| Congenital or developmental |
| Marfan syndrome, Ehlers–Danlos syndrome |
| Degenerative |
| Cystic medial degeneration |
| Annuloaortic ectasia |
| Atherosclerotic |
| Traumatic |
| Blunt and penetrating trauma |
| Inflammatory |
| Takayasu’s arteritis, Behçet syndrome, Kawasaki disease |
| Microvascular diseases (polyarteritis) |
| Infectious (mycotic) |
| Bacterial, fungal, spirochetal, viral |
| Mechanical |
| Poststenotic, associated with an arteriovenous fistula |
| Anastomotic (postarteriotomy) |
| Pseudoaneurysm |
| Aortic dissection |
| Stanford type A |
| Stanford type B |
| Intramural hematoma |
| Penetrating atherosclerotic ulcer |
| Atherosclerotic disease |
| Traumatic aortic injury |
| Aortic coarctation |
Data from Kouchoukos NT, Dougenis D: Surgery of the aorta. N Engl J Med 336:1876, 1997.
The anesthetic management of thoracic aortic diseases has unique considerations including the temporary interruption of blood flow, often resulting in ischemia of major organ systems. Critical components of anesthetic management include the maintenance of organ perfusion, the protection of vital organs during ischemia, and the monitoring and management of end-organ ischemia. As a result, the vigilant and skillful anesthesiologist contributes importantly to the overall success of these operations. The procedures performed by the thoracic aortic team for organ protection, such as partial left-heart bypass (PLHB) for distal aortic perfusion, cardiopulmonary bypass (CPB) with deep hypothermic circulatory arrest (DHCA), selective cerebral perfusion, and lumbar cerebrospinal fluid (CSF) drainage, are practiced routinely in no other area of medicine. The recently published multisociety guidelines represent a contemporary evidence-based, consensus-driven approach to thoracic aortic diseases. Their recommendations are referred to throughout this chapter, based on the well-known classification of recommendations and levels of evidence (Tables 21-2 and 21-3) by the American College of Cardiology (ACC) and American Heart Association (AHA).1
TABLE 21-2 Classification Scheme for Clinical Recommendations
| Clinical Recommendations | Definition of Recommendation Class |
|---|---|
| Class I | The procedure/treatment should be performed (benefit far outweighs the risk). |
| Class IIa | It is reasonable to perform the procedure/treatment (benefit still clearly outweighs risk). |
| Class IIb | It is not unreasonable to perform the procedure/treatment (benefit probably outweighs the risk). |
| Class III | The procedure/treatment should not be performed as it is not helpful and may be harmful (risk may outweigh benefit). |
Data from Hiratzka LF, Bakris GL, Beckman JA, et al: 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: Executive summary. A report of the American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine. Circulation 121:e266–e369, 2010.
TABLE 21-3 Classification Scheme for Supporting Evidence for Clinical Recommendations
| Supporting Evidence | Estimate of Certainty |
|---|---|
| Level A | Data derived from multiple randomized clinical trials (RCT) or meta-analysis. |
| Level B | Data derived from a single RCT or nonrandomized studies. |
| Level C | Only consensus opinions of experts, case studies, or standard of care. |
Data from Hiratzka LF, Bakris GL, Beckman JA, et al: 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: Executive summary. A report of the American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine. Circulation 121:e266–e369, 2010.
Anatomy of the aorta
During fetal development, the ductus arteriosus diverts blood from the pulmonary artery into the distal aortic arch. After birth, lung expansion and constriction of the ductus arteriosus because of increased blood oxygen content drives the blood from the pulmonary artery into the pulmonary circulation. Typically, the ductus arteriosus is functionally closed by 48 hours and is permanently closed by 3 weeks after birth.2 It subsequently fibroses to become the “ligamentum arteriosum.” Occasionally, this process fails and the ductus arteriosus remains patent. Furthermore, occasionally a ductal diverticulum may persist and be confused in later life with an aortic injury during aortic imaging. The pathogenesis of aortic coarctation may be related to residual ductal tissue that narrows the aorta as it constricts.
The coronary arteries are the first branches of the aorta. The aortic arch subsequently gives origin to the innominate, left carotid, and left subclavian arteries that supply the head, neck, and arms. The innominate artery (brachiocephalic trunk) is the first branch of the aortic arch, followed by the left common carotid artery, and, finally, the left subclavian artery. There are multiple aortic arch anatomic variations, including vascular rings, right-sided aortic arch, and branching anomalies. A right-sided aortic arch is found in about 0.1% of the population. A relatively common aortic arch branch anomaly with a 4% prevalence rate is an isolated left vertebral artery, so named because it arises directly from the aortic arch.3
The aortic arch also modulates blood pressure via baroreceptors within its outer wall. The aortic bodies are located inferior to the aortic arch. The aortic baroreceptors respond to a greater threshold pressure and thus are less sensitive when compared with the carotid sinus receptors. These receptors send impulses to the brainstem that interact with the medullary cardiovascular center for modulation of autonomic nervous system activity.4
General considerations for the perioperative care of aortic surgical patients
Patients undergoing thoracic aortic surgery share common considerations for the safe conduct of anesthesia and perioperative care that are addressed in this section (Table 21-4). The unique considerations and care that apply to specific diseases and procedures are addressed in subsequent sections devoted to their management.
TABLE 21-4 Anesthetic Considerations for the Care of Thoracic Aortic Surgical Patients
| Preanesthetic Assessment |
| Urgency of the operation (emergent, urgent, or elective) |
| Pathology and anatomic extent of the disease |
| Median sternotomy vs. thoracotomy vs. endovascular approach |
| Mediastinal mass effect |
| Airway compression or deviation |
| Preexisting or Associated Medical Conditions |
| Aortic valve disease |
| Cardiac tamponade |
| Coronary artery stenosis |
| Cardiomyopathy |
| Cerebrovascular disease |
| Pulmonary disease |
| Renal insufficiency |
| Esophageal disease (contraindications to TEE) |
| Coagulopathy |
| Prior aortic operations |
| Preoperative Medications |
| Warfarin (Coumadin) |
| Antiplatelet therapy |
| Antihypertensive therapy |
| Anesthetic Management |
| Hemodynamic monitoring |
| Proximal aortic pressure |
| Distal aortic pressure |
| Central venous pressure |
| Pulmonary artery pressure and cardiac output |
| TEE |
| Neurophysiologic monitoring |
| Electroencephalography |
| Somatosensory-evoked potentials |
| Motor-evoked potentials |
| Jugular venous oxygen saturation |
| Lumbar cerebrospinal fluid pressure |
| Body temperature |
| Single-lung ventilation for thoracotomy |
| Double-lumen endobronchial tube |
| Endobronchial blocker |
| Potential for bleeding |
| Large-bore intravenous access |
| Blood product availability |
| Antifibrinolytic therapy |
| Antibiotic prophylaxis |
| Postoperative Care Considerations and Complications |
| Hypothermia |
| Hypotension |
| Hypertension |
| Bleeding |
| Spinal cord ischemia |
| Stroke |
| Renal insufficiency |
| Respiratory insufficiency |
| Phrenic nerve injury |
| Diaphragmatic dysfunction |
| Recurrent laryngeal nerve injury |
| Pain management |
Preanesthetic Assessment
The second consideration is to determine the aortic diagnosis because its extent and physiologic consequences dictate both anesthetic management and surgical approach. Aortic diseases proximal to the left carotid artery typically are approached via a median sternotomy, whereas aortic diseases distal to this point usually are approached via a left thoracotomy or thoracoabdominal incision. Although an aortic diagnosis often is established in advance, at times a definitive diagnosis must be verified after OR admission by direct review of diagnostic studies or by subsequent TEE. In every case, a review of the operative plan with the surgical team facilitates thorough anesthetic preparation. Direct review of adequate aortic diagnostic imaging studies not only verifies the operative diagnosis but also determines the surgical possibilities (ACC/AHA Class I recommendation; level of evidence C).1 The anatomic details of an aortic disease permit the anesthesiologist to anticipate potential perioperative difficulties, including likely postoperative complications.
The systematic assessment of each organ system in the aortic surgical patient should focus on how it will affect the conduct of anesthesia and surgery. The baseline functional reserve of each organ system determines the likely perioperative complications and allows ranking of organ-protective strategies. It is reasonable to obtain further tests to quantitate the functional reserve of affected organ systems, for example, neurocognitive testing, brain imaging, noninvasive carotid artery imaging, pulmonary function testing, echocardiography, and cardiac catheterization (ACC/AHA Class IIa recommendation; level of evidence C).1 For example, significant cerebrovascular disease affects blood pressure management to ensure adequate cerebral perfusion. Significant cardiac compromise typically increases the risks for heart failure, myocardial ischemia, and arrhythmias. Significant lung disease often is predictive for postoperative respiratory failure, pneumonia, or both. Significant renal insufficiency affects fluid management, triggers the avoidance of nephrotoxic drugs, and customizes the dosing of renally cleared drugs. Hepatic disease and hematologic dysfunction are risk factors for perioperative bleeding and transfusion. Severe aortic atheroma is a major risk factor for atheroembolism and consequent stroke and limb ischemia.
Because myocardial ischemia is an important predictor of perioperative outcome, it has featured prominently in the guidelines for thoracic aortic diseases. Patients with evidence of myocardial ischemia should undergo further evaluation to determine the extent and severity of coronary artery disease (CAD; ACC/AHA Class I recommendation; level of evidence C).1 If significant CAD is responsible for an acute coronary syndrome, then coronary revascularization is indicated before or concomitant with the thoracic aortic procedure (ACC/AHA Class I recommendation; level of evidence C).1 Concomitant coronary artery bypass grafting (CABG) is reasonable in patients who have not only stable but significant CAD, but who are also scheduled to undergo surgery for diseases of the ascending aorta or aortic arch, or both (ACC/AHA Class IIa recommendation; level of evidence C).1 In contrast, the benefit of coronary revascularization is less clear in patients who have stable but significant CAD and who are scheduled to undergo surgical intervention for descending thoracic aortic disease (ACC/AHA Class IIb recommendation; level of evidence C).1
Preoperative Medications
Preoperative medications typically provide detailed information about concomitant medical conditions. As a general rule, all cardiac, pulmonary, and antiseizure medications should be continued up until the morning of surgery. Angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers should be discontinued the day before surgery to minimize the risk for perioperative vasoplegia and adverse outcomes.5,6 All oral hypoglycemic agents should be discontinued the night before surgery to avoid hypoglycemia. If possible, metformin should be discontinued the day before surgery to minimize the risks for severe lactic acidosis associated with exposure to iodinated contrast agents or perioperative hypovolemia. If a patient is receiving insulin, up to 50% of the typical morning dose should be given the day of surgery with subsequent close glucose monitoring. Warfarin (Coumadin) should be discontinued for approximately 5 days before surgery for full recovery of coagulation function as verified by a normalized international normalized ratio.7 If this is not possible, then the patient should be admitted for heparinization until shortly before surgery. Patients chronically exposed to low-molecular-weight heparin, aspirin, adenosine diphosphate platelet-receptor inhibitors (clopidogrel and prasugrel), and platelet glycoprotein IIb/IIIa inhibitors (abciximab, eptifibatide, tirofiban) are typically at increased risk for perioperative bleeding. Optimally, aspirin and clopidogrel should be discontinued at least 5 to 7 days before surgery to allow adequate recovery of platelet function for perioperative hemostasis.7
Anesthetic Management
Overall, the anesthetic plan including techniques, drugs, and monitoring should be individualized to enhance the conduct of the procedure including perfusion technique, hemodynamic monitoring, and preservation of organ function (ACC/AHA Class I recommendation; level of evidence C).1 Because thoracic aortic procedures may result in massive bleeding and cardiovascular collapse, it is essential to have immediate availability of packed red blood cells, large-bore vascular access, invasive blood pressure monitoring, and central venous access. Pulmonary artery catheterization assists in the management of cardiac dysfunction associated with CPB, DHCA, and PLHB. Intraoperative TEE is indicated in thoracic aortic procedures, including endovascular interventions, in which it assists in hemodynamic monitoring, procedural guidance, and endoleak detection (ACC/AHA Class IIa recommendation; level of evidence B).1
Large-bore peripheral intravenous cannulation (e.g., two 16-gauge catheters) secures vascular access for rapid intravascular volume expansion. Rapid transfusion is desirable via an intravenous set with a fluid warming device. Alternatively, large-bore central venous cannulation can be utilized for volume expansion. If a pulmonary artery catheter (PAC) is required, a second introducer sheath dedicated to volume expansion also can be placed in the same central vein. Central venous cannulation with ultrasound guidance often increases speed and safety, especially in emergencies.8 Both a urinary and a nasopharyngeal temperature probe are required for monitoring the absolute temperature of the peripheral and core, as well as the rates of change during deliberate hypothermia and subsequent rewarming. The rectum is an alternative site for monitoring peripheral temperature, and the PAC can provide core temperature monitoring.
General anesthetic maintenance is typically with a balanced technique, and neuromuscular blockade is achieved by titration of a nondepolarizing muscle relaxant. Anesthetics can be reduced during moderate hypothermia and then discontinued during deep hypothermia. With concomitant electroencephalographic (EEG) and/or somatosensory-evoked potential (SSEP) monitoring, anesthetic signal interference is minimized with the avoidance of barbiturates, bolus propofol, and doses of inhaled anesthetic greater than 0.5 minimum alveolar concentration. Propofol infusion, narcotics, and neuromuscular blocking drugs do not interfere with SSEP monitoring. With intraoperative motor-evoked potential (MEP) monitoring, high-quality signals are obtained when the anesthetic technique comprises total intravenous anesthesia with propofol and a narcotic such as remifentanil without neuromuscular blockade. Neuromonitoring (EEG, SSEP, MEP) in thoracic aortic procedures is not only compatible with contemporary anesthetic techniques but is also reasonable when the resulting data will guide perioperative management (ACC/AHA Class IIa recommendation; level of evidence B).1 The decision to utilize this monitoring modality should be based on procedural urgency, institutional resources, patient needs, and planned operative technique (ACC/AHA Class IIa recommendation; level of evidence B).1
In most cases, the duration of general anesthesia continues for several hours after admission to the intensive care unit (ICU) to permit a controlled anesthetic emergence. If epidural analgesia is used intraoperatively, a dilute solution of local anesthetic and narcotic is preferred to minimize postoperative hypotension from a concomitant sympathectomy and to minimize motor blockade to permit serial neurologic assessment of lower extremity function. Neuraxial anesthetic techniques are not recommended in patients at risk for neuraxial hematoma in the setting of concomitant thienopyridine antiplatelet therapy, low-molecular-weight heparins, and clinically significant anticoagulation (ACC/AHA Class III recommendation; level of evidence C).1,7
The potential for significant bleeding and rapid transfusion is always relevant in thoracic aortic procedures. Consequently, it is prudent to have fresh frozen plasma and platelets available for ongoing replacement during massive red blood cell transfusion. The time delay associated with standard laboratory testing severely limits the intraoperative relevance of these data to guide transfusion. Strategies to decrease bleeding and transfusion in these procedures include timely preoperative cessation of anticoagulants and platelet blockers, antifibrinolytic therapy, intraoperative cell salvage, biologic glue, activated factor VII, and avoidance of perioperative hypertension. It is reasonable to have an institutional algorithmic approach to the management of bleeding and transfusion for thoracic aortic surgery (ACC/AHA Class IIa recommendation; level of evidence C).1 This algorithm will depend significantly on institutional variations in point-of-care coagulation testing, blood component availability, and access to recombinant factor VII (ACC/AHA Class IIa recommendation; level of evidence C).1
The antifibrinolytic lysine analogs, ε-aminocaproic acid or tranexamic acid, are commonly utilized in thoracic aortic surgery with and without DHCA. The concerns with aprotinin are now historical because it was widely withdrawn from clinical practice after a large randomized trial demonstrated its significant mortality risk in the setting of high-risk cardiac surgery, including thoracic aortic surgery with DHCA.9,10 Even more recently, high-dose tranexamic acid has been correlated with a significantly increased risk for seizures after cardiac surgery.11,12 Based on the lessons from aprotinin, further adequately powered trials should examine the safety of lysine analogs in thoracic aortic surgery.9–12 Recombinant activated factor VII is a synthetic agent that accelerates hemostasis by binding with tissue factor at the site of tissue injury. Although this agent has demonstrated efficacy for hemostatic rescue in massive bleeding during thoracic aortic surgery, recent meta-analysis suggest further study for adequate delineation of its perioperative safety.13,14
Postoperative Care
After completion of the operation, the patient should be transported directly from the OR to the ICU. The continuation of care from the OR to the ICU should be seamless and protocol based.15 In the absence of complications, early anesthetic emergence is preferable for early assessment of neurologic function. If delayed anesthetic emergence is indicated, then sedation and analgesia can be provided. Common early complications include hypothermia, coagulopathy, delirium, stroke, hemodynamic lability, respiratory failure, metabolic disturbances, and renal failure. Frequent clinical and laboratory assessment are essential to manage this dynamic postoperative recovery, including the safe conduct of tracheal extubation. The management of blood glucose levels has been standardized with a recent guideline from the Society of Thoracic Surgeons (STS).16 The chest roentgenogram allows confirmation of endotracheal tube and intravascular catheter position, as well as the diagnosis of acute intrathoracic pathologies. Antibiotic prophylaxis is continued for 48 hours after surgery to minimize surgical infection risk.
Thoracic aortic aneurysm
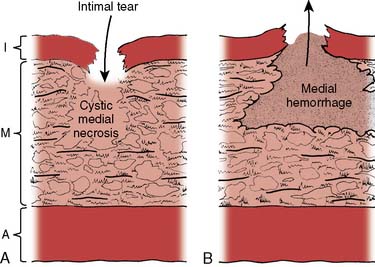
A thoracic aortic aneurysm is a permanent localized thoracic aortic dilatation that has at least a 50% diameter increase and three aortic wall layers.1 Localized dilatation of the thoracic aorta less than 150% of normal is termed ectasis. Annuloaortic ectasia is defined as isolated dilatation of the ascending aorta, aortic root, and aortic valve annulus. Pseudoaneurysm or a false aneurysm is a localized dilation of the aorta that does not contain all three layers of the vessel wall and instead consists of connective tissue and clot. Pseudoaneurysms are caused by a contained rupture of the aorta or arise from intimal disruptions, penetrating atheromas, or partial dehiscence of the suture line at the site of a previous aortic prosthetic vascular graft.
Thoracic aortic aneurysms are common and are the 15th most common cause of death in people older than 65.16 This disease process is virulent (Box 21-1) but indolent because it typically grows slowly at an approximate rate of 0.1 cm/yr.16 The most common reason for more rapid degeneration is acute aortic dissection. Besides acquired risk factors such as hypertension, hypercholesterolemia, and smoking, current evidence points to the strong influence of genetic inheritance.17,18 Genetic analysis suggests that thoracic aortic aneurysms divide into two groups at the level of the ligamentum arteriosum. Above the ligamentum arteriosum, the disease is not related to typical arterial risk factors and has a smooth, noncalcified wall accompanied by no debris or clot. Below the ligamentum arteriosum, the disease process primarily is atherosclerotic, with an irregular calcified wall accompanied by copious debris and clot. This freedom from atheromatous disease in patients with thoracic aortic aneurysms of the ascending aorta has been called a “silver lining.”17 Inflammatory causes for thoracic aortic aneurysm include syphilis, mycotic aneurysm from endocarditis, giant-cell arteritis, and Takayasu arteritis.1
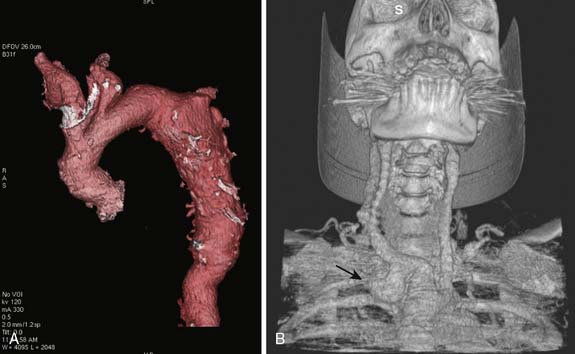
The aneurysm’s location and extent determine the operative strategy and related perioperative complications. Aneurysms of the aortic root and/or ascending aorta commonly are associated with a bicuspid aortic valve.19 Dilation of the aortic valve annulus, aortic root, and ascending aorta pulls the aortic leaflets apart and causes central aortic regurgitation (AR).18 Aneurysms involving the aortic arch require temporary interruption of cerebral blood flow to accomplish the operative repair. Endovascular stent repair is an established therapy for aneurysms isolated to the descending thoracic aorta.1,20 Repair of descending thoracic aortic aneurysms requires the sacrifice of multiple segmental intercostal artery branches that compromise spinal cord perfusion and results in a significant risk for postoperative paraplegia from spinal cord ischemia.21
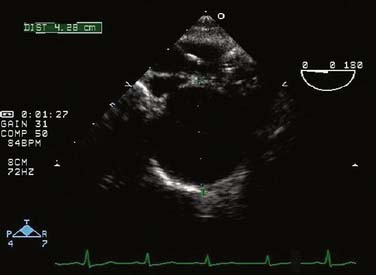
Thoracic aortic aneurysms mostly are asymptomatic and frequently are discovered incidentally.1,17 Common symptoms of thoracic aortic aneurysm include chest and back pain caused by aneurysmal dissection, rupture, or bony erosion. The intrathoracic “mass effect” from a large thoracic aortic aneurysm can compress local structures to cause hoarseness (recurrent laryngeal nerve), dyspnea (trachea, mainstem bronchus, pulmonary artery), central venous hypertension (superior vena cava syndrome), and/or dysphagia (esophagus). Rupture of thoracic aortic aneurysms is a surgical emergency and is often accompanied with acute pain with or without hypotension. Although rupture of an ascending aortic aneurysm may cause cardiac tamponade, rupture in the descending thoracic aorta may cause hemothorax, aortobronchial fistula, or aortoesophageal fistula.
Diagnostic Imaging for Thoracic Aortic Aneurysms
The chest radiograph may suggest a thoracic aortic aneurysm with features such as a widened mediastinum, enlarged aortic knob, dilated descending thoracic aorta, aortic calcifications, leftward tracheal deviation, upward deviation of the left mainstem bronchus, and/or new left pleural effusion. Typically, transthoracic echocardiography can provide a reasonable examination of the thoracic aorta, although the acoustic windows are limited by the lungs. The contemporary imaging modalities of choice are CT, MRI, and TEE. Computed tomographic angiography (CTA) images the thoracic aorta during the arterial phase of an intravenous radiocontrast agent injection. It defines vascular anatomy and surrounding nonvascular structures. Aneurysm leak is detected as extravascular contrast extravasation. This imaging modality has multiple advantages such as high resolution, wide availability, rapid acquisition, imaging in patients with metallic implants, and generation of volumetric aortic images for stent design. Because CTA requires iodinated contrast agents, it carries a risk for contrast nephropathy that can be attenuated by administration of acetylcysteine and sodium bicarbonate.22 (See Chapters 2 and 3.)
Contrast-enhanced magnetic resonance angiography with gadolinium also images the entire thoracic aorta in fine detail. Although the spatial resolution of magnetic resonance angiography is slightly inferior to CTA, it does allow for degrees of tissue and fluid characterization. The disadvantages of magnetic resonance angiography include its limited availability, lack of imaging in patients with metallic implants, imaging difficulty in the setting of continuous hemodynamic monitoring, and the time required for image acquisition. Its advantages are the avoidance of ionizing radiation and the lack of renal toxicity.1,17
TEE can image the thoracic aorta from the aortic valve to the distal ascending aorta and from the distal aortic arch to the proximal abdominal aorta. The distal ascending aorta and proximal aortic arch cannot be reliably imaged by TEE because the intervening trachea and left mainstem bronchus obstruct the acoustic window; this is known as the “blind spot” of TEE.23 It is possible to overcome this blind spot with modalities such as imaging across the trachea temporarily filled with a saline-filled balloon (the A-view) and utilizing an expanded aortic view.23,24 The advantages of TEE include its portability, its real-time interpretation, its compatibility at the bedside and in the OR, and its multiple imaging modalities for complete aortic and cardiac assessment. Its disadvantages include the requirement for sedation or general anesthesia and the risks for upper gastrointestinal injury.25
Surgical Considerations for Thoracic Aortic Aneurysms
Surgical repair aims to replace the aortic aneurysm with a tube graft to prevent further aneurysmal complications (Table 21-5). The first indication for thoracic aortic aneurysm resection is whenever the aneurysm is symptomatic regardless of size (ACC/AHA Class I recommendation; level of evidence C).1,17 Symptoms often herald the onset of rupture or dissection and should be interpreted as an urgent indication for surgery. A symptomatic presentation occurs in about 5% of patients. Unfortunately, the first symptom in the remaining 95% of patients often is death.
TABLE 21-5 Indications for Surgical Repair of Thoracic Aortic Aneurysms
| Atherosclerotic aneurysm diameter | |
| Ascending aorta | ≥5.5 cm |
| Descending aorta | ≥6.5 cm |
| Marfan’s or familial thoracic aneurysm diameter | |
| Ascending aorta | ≥5.0 cm |
| Descending aorta | ≥6.0 cm |
| Severe aortic regurgitation | |
| Aortoannular ectasia with aortic root aneurysm | |
| Rupture | |
| Refractory pain |
The second indication for resection is aortic diameter. In the ascending aorta, a diameter of 6.0 cm is the critical hinge point after which the risk for aneurysm rupture increases exponentially. Consequently, surgical resection is recommended in the ascending aorta when the diameter reaches 5.5 cm (ACC/AHA Class I recommendation; level of evidence C).1,17 In patients with genetically mediated aortopathies (Marfan syndrome, bicuspid aortic valve, familial thoracic aortic aneurysm or dissection; vascular Ehlers-Danlos syndrome; and Turner syndrome), surgical resection is recommended at a lower ascending aortic diameter of 5.0 cm (ACC/AHA Class I recommendation; level of evidence C).1,17 Ascending aortic aneurysms with diameters less than 5.5 cm but with an annual growth rate in diameter greater than 0.5 cm/yr qualify for surgical resection (ACC/AHA Class I recommendation; level of evidence C).1 It is reasonable to consider prophylactic replacement of the aortic root and ascending aorta in a woman with Marfan syndrome who is planning a pregnancy and who has an aortic root or ascending aortic diameter larger than 4.0 cm (ACC/AHA Class IIa recommendation; level of evidence C).1 In adults with the aggressive aortopathy of the Loeys-Dietz syndrome, it is reasonable to consider proximal thoracic aortic repair when the internal aortic diameter exceeds 4.2 cm (ACC/AHA Class IIa recommendation; level of evidence C).1,26 The ascending aortic aneurysm diameter also must be indexed to body size.27 For example, if the maximal cross-sectional area of the aortic root or ascending aorta (in square centimeters) divided by the patient’s height (in meters) exceeds a ratio of 10, then surgical repair is a reasonable option (ACC/AHA Class IIa recommendation; level of evidence C).1 The rationale behind indexing the aortic dimensions to body size is that shorter adults dissect and rupture their aortas at smaller diameters.1,27 Furthermore, those patients who are undergoing open aortic valve procedures and who have an aortic root or ascending aortic diameter larger than 4.5 cm should be considered for concomitant aortic replacement resection (ACC/AHA Class I recommendation; level of evidence C).1
The hinge point for rupture in the descending thoracic aorta is a diameter of 7.0 cm.17 Consequently, surgical resection is recommended in thoracoabdominal aneurysms when the aortic diameter exceeds 6.0 cm or less when it is associated with a connective tissue disorder such as Marfan syndrome (ACC/AHA Class I recommendation; level of evidence C).1 In patients who have aneurysmal degeneration of the descending thoracic aorta associated with prior dissection and/or a connective tissue disorder, surgical resection is recommended when the aortic diameter is more than 5.5 cm (ACC/AHA Class I recommendation; level of evidence B).1 Patients with aneurysms of the descending thoracic aorta should be considered for thoracic endovascular aortic repair (TEVAR) when technically feasible (ACC/AHA Class I recommendation; Level of Evidence B).1,20
Surgical Repair of Ascending Aortic and Arch Aneurysms
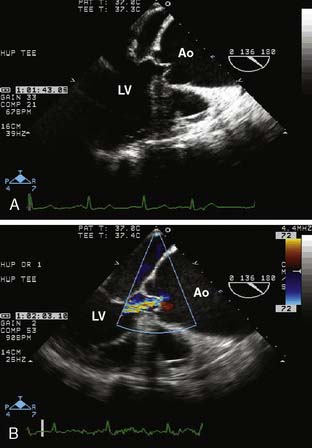
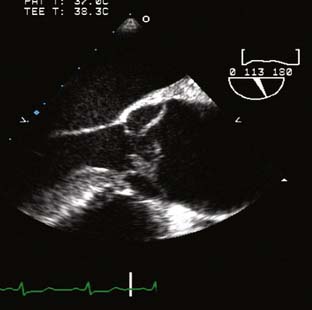
The type of surgical repair depends on aortic valve function and the aneurysm extent. Perioperative TEE can evaluate the aortic valve structure and function to guide and assess the surgical intervention (reimplantation, repair, replacement). Furthermore, TEE can assess the diameters of the aortic root, ascending aorta, and aortic arch to guide intervention. The most common aortic valve diseases associated with ascending aortic aneurysm are bicuspid aortic valve or AR caused by dilation of the aortic root (Figure 21-1). If the aortic valve and aortic root are normal, a simple tube graft can be used to replace the ascending aorta. If the aortic valve is diseased but the sinuses of Valsalva are normal, an aortic valve replacement combined with a tube graft for the ascending aorta without need for reimplantation of the coronary arteries can be performed (Wheat procedure; Figure 21-2; ACC/AHA class I recommendation; level of evidence C).1
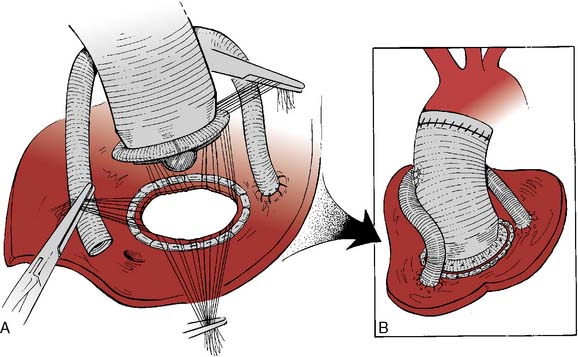
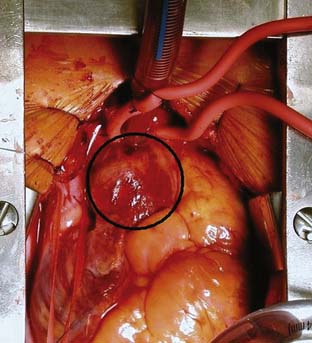
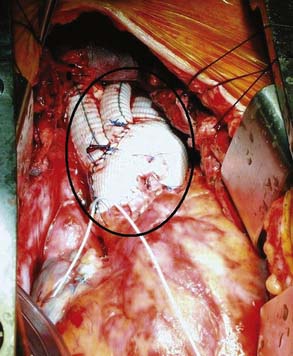
If disease also involves the aortic valve and the aortic root, the patient requires aortic root replacement and aortic valve intervention. If technically feasible, the aortic valve can be reimplanted with a modified David technique, which includes graft reconstruction of the aortic root with reimplantation of the coronary arteries (ACC/AHA Class I recommendation; level of evidence C).1,28 If not feasible, aortic root replacement with a composite valve-graft conduit is indicated (Bentall procedure; Figure 21-3; ACC/AHA Class I recommendation; level of evidence C).1 Aortic root replacement requires coronary reimplantation or aortocoronary bypass grafting (Cabrol technique; Figure 21-4).
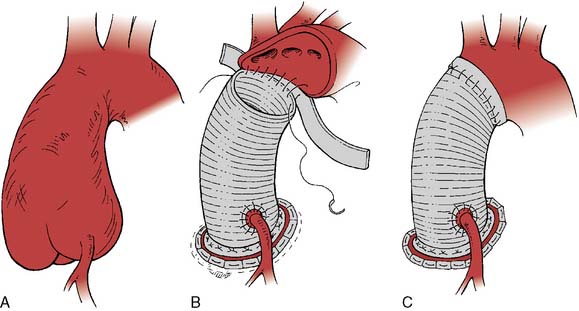
Figure 21-3 Replacement of the entire aortic root with a composite valved conduit for ascending aortic aneurysm.
(Adapted from Griepp RB, Ergin A: Aneurysms of the aortic arch. In Edmunds LH [ed]: Cardiac Surgery in the Adult. New York, McGraw-Hill, 1997, p 1209, by permission.)
Repairing aortic aneurysms that extend into or involve the aortic arch requires CPB with DHCA with or without perfusion adjuncts. For ascending aortic aneurysms that involve only the proximal aortic arch, partial arch replacement (hemiarch technique) is reasonable in which a tubular graft is interposed between the ascending aorta or aortic root and the underside of the aortic arch (ACC/AHA Class IIa recommendation; level of evidence B).1 Ascending aorta with hemiarch reconstruction often is performed using DHCA with or without ACP/RCP to make the distal anastomosis feasible without cross-clamping (“open technique”). In patients who have isolated aortic arch aneurysms and who have a low operative risk, arch replacement is reasonable when the arch diameter exceeds 5.5 cm (ACC/AHA Class IIa recommendation; level of evidence B).1 Total aortic arch replacement is reasonable in aneurysms that involve the entire arch (ACC/AHA Class IIa recommendation; level of evidence B).1 Ascending aortic aneurysms that extend through the aortic arch into the descending aorta can be repaired with the “elephant trunk” technique (Figure 21-5; ACC/AHA Class IIa recommendation; level of evidence B).1,29 Aortic arch aneurysms that extend into the aortic branch vessels may require repair with branched or trifurcated tube grafts to permit separate anastomosis to the innominate, left carotid, and left subclavian arteries.30 In patients with aortic arch aneurysms and concomitant severe comorbidity, recent guidelines support an endovascular repair technique (Class IIb recommendation; level of evidence C).1,20 However, in patients who have aortic arch aneurysms and who have reasonable surgical risk, the recent guidelines advise against an endovascular repair technique (Class III recommendation; level of evidence A).1,20
Anesthetic Management for Ascending Aorta and Arch Aneurysms
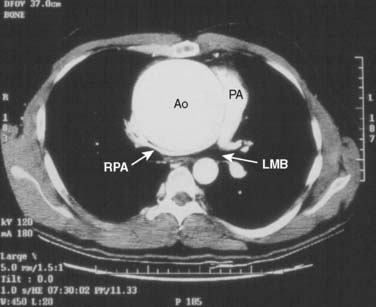
The conduct of general anesthesia in this setting has specific concerns. The imaging studies should be reviewed for aneurysm compression of mediastinal structures such as the right pulmonary artery and left mainstem bronchus (Figure 21-6). Prevention of hypertension increases forward flow in AR and minimizes the risk for aneurysm rupture. A right radial arterial catheter is preferred for most cases. If arterial cannulation of the right axillary, subclavian, or innominate artery is planned for CPB and ACP, bilateral radial arterial catheters often are required to measure cerebral and systemic perfusion pressures. Nasopharyngeal, tympanic, and bladder temperatures are important for estimating brain and core temperatures for monitoring the conduct of DHCA. Monitoring of jugular bulb venous oxygen saturation and the EEG may reflect cerebral metabolic activity to guide the conduct of DHCA. Intraoperative TEE is essential to guide and assess the surgical interventions. In patients with AR, TEE can assist in the conduct of CPB by guiding placement of cannulae such as the retrograde cardioplegia cannula (coronary sinus) and by monitoring left ventricular (LV) volume to ensure that the LV drainage cannula keeps the ventricle collapsed. Intraoperative TEE is reasonable in thoracic aortic procedures, including endovascular interventions, in which it assists in hemodynamic monitoring, procedural guidance, and endoleak detection (ACC/AHA Class IIa recommendation; level of evidence B).1
Neuroprotection Strategies for Temporary Interruption of Cerebral Blood Flow
The risk for stroke is substantial during the cerebral ischemia that accompanies aortic arch reconstruction.31 The first mechanism is cerebral ischemia due to hypoperfusion or temporary circulatory arrest during aortic arch repair. The second mechanism is cerebral ischemia due to embolization secondary to CPB and atheroma. Arterial emboli causes include air introduced into the circulation from open cardiac chambers, vascular cannulation sites, or arterial anastomosis. Atherosclerotic particulate debris may be released during clamping and unclamping of the aorta, the creation of anastomoses in the ascending aorta and aortic arch, or the excision of severely calcified and diseased cardiac valves. CPB may result in the microparticulate aggregates of platelets and fat. The turbulent high-velocity blood flow out of the aortic cannula used for CPB also may dislodge atherosclerotic debris within the aorta. Retrograde blood flow through a diseased descending thoracic aorta as a consequence of CPB conducted with femoral artery cannulation may cause retrograde cerebral embolization. For all these reasons, strategies to provide neurologic protection are essential in thoracic aortic operations (Box 21-2).
Deep Hypothermic Circulatory Arrest
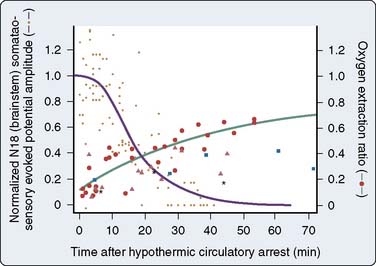
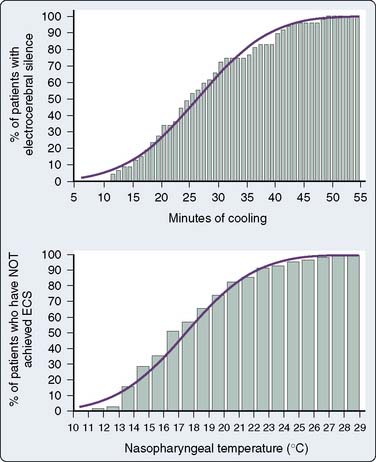
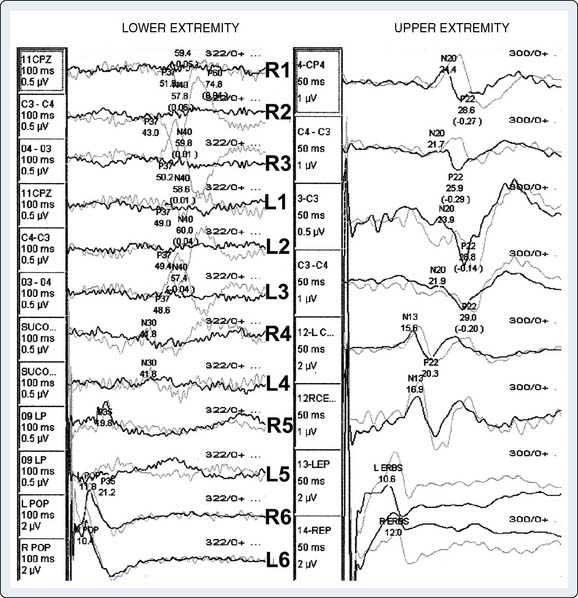
The brain is exquisitely susceptible to ischemic injury within minutes after the onset of circulatory arrest because it has a high metabolic rate, continuous requirement for metabolic substrate, and limited reserves of high-energy phosphates. The physiologic basis for deep hypothermia as a neuroprotection strategy is to decrease cerebral metabolic rate and oxygen demands to increase the period that the brain can tolerate circulatory arrest.32 Existing evidence indicates that autoregulation of cerebral blood flow is maintained during deliberate hypothermia with alpha-stat blood gas management without compromise of clinical outcome.33 Direct measurement of cerebral metabolites and brainstem electrical activity in adults undergoing DHCA with RCP at 14°C indicated the onset of cerebral ischemia after only 18 to 20 minutes (Figure 21-7).34 Despite this observation, the large body of experimental evidence and clinical experience with the deliberate hypothermia suggest that it is the single most important intervention for preventing neurologic injury in response to circulatory arrest.
Despite the proven efficacy of hypothermia for operations that require circulatory arrest, no consensus exists on an optimal protocol for the conduct of deliberate hypothermia for circulatory arrest. A strategy to protect the brain during aortic arch surgery must be a high priority in the perioperative management of these procedures to prevent stroke and optimize cognitive function (ACC/AHA Class I recommendation; level of evidence C).1 Although the average nasopharyngeal temperature for DHCA may be about 18° C, the optimal temperature for DHCA has not been established.31,32 A challenge in the selection of the ideal temperature for DHCA is the inability to directly measure the brain temperature. In an EEG-based approach to this question, the median nasopharyngeal temperature for electrocortical silence was 18° C, although a nasopharyngeal temperature of 12.5° C or cooling on CPB for at least 50 minutes achieved electrocortical silence in 99.5% of cases (Figure 21-8).35 Although the EEG functions well as a physiologic end point for cerebral metabolic suppression during cooling for DHCA as part of an institutional protocol, its outcome benefit remains to be demonstrated in a randomized trial.15,31,36 A jugular bulb venous oxygen saturation greater than 95% measured using an oximetric catheter represents an alternative physiologic end point to detect maximum cerebral metabolic suppression for DHCA.37 It is important to note that deep hypothermia to a set temperature (mean = 19°C) as an end point for DHCA without EEG or jugular bulb venous oxygen saturation has been associated with excellent neurologic outcomes in recent series.38,39 In addition to systemic hypothermia produced by extracorporeal circulation, topical hypothermia by packing the head in ice also has been incorporated in leading institutional DHCA protocols to minimize passive warming of the head.38,39 When providing topical hypothermia, care should be exercised to protect the eyes, nose, and ears from frostbite by protecting these vulnerable areas. Recent clinical studies support the practice of limiting the duration of straight DHCA to shorter than 45 minutes to avoid the associated significant increases in stroke and mortality risks.31 The technique of DHCA alone is a reasonable approach for neuroprotection during aortic arch surgery in the setting of adequate institutional experience (ACC/AHA Class IIa recommendation; level of evidence B).1
The conduct of DHCA extends CPB duration with consequent risks for coagulopathy and embolization. Rewarming increases cerebral metabolic rate and can aggravate neuronal injury during ischemia/reperfusion. Consequently, it is important to rewarm gradually by maintaining a temperature gradient of no more than 10° C in the heat exchanger and avoiding cerebral hyperthermia (nasopharyngeal temperature > 37.5°C). The current guidelines advise against cerebral hyperthermia in aortic arch procedures (ACC/AHA Class III recommendation; level of evidence B).1
Retrograde Cerebral Perfusion
RCP is performed by infusing cold oxygenated blood into the superior vena cava cannula at a temperature of 8° C to 14° C via CPB (Figure 21-9). The internal jugular venous pressure is maintained at less than 25 mm Hg to prevent cerebral edema. Internal jugular venous pressure is measured from the introducer port of the internal jugular venous cannula at a site proximal to the superior vena cava perfusion cannula and zeroed at the level of the ear. The patient is positioned in 10 degrees of Trendelenburg to decrease the risk for cerebral air embolism and prevent trapping of air within the cerebral circulation in the presence of an open aortic arch. RCP flow rates of 200 to 600 mL/min usually can be achieved. The potential benefits of RCP include partial supply of cerebral metabolic substrate, cerebral embolic washout, and maintenance of cerebral hypothermia.40 Although RCP has been associated with excellent clinical results in aortic arch repair, it has not become the standard technique for neuroprotection in DHCA.15,41 A recent large, single-center study (1991–2007; N = 1107; RCP in 82%) evaluated the role of RCP in proximal thoracic aortic repair. The perioperative rates for mortality and stroke in this series were 10.4% and 2.8%, respectively. The application of RCP was significantly protective against mortality (odds ratio, 0.42; 95% confidence interval, 0.25-0.70; P = 0.0009) and stroke (odds ratio, 0.35; 95% confidence interval, 0.15-0.81; P = 0.02).42 Despite the lack of randomized trials, RCP is safe and easily implemented in aortic arch repair as an adjunct to maintain cerebral hypothermia, provide partial metabolic substrate delivery, and decrease the risk for cerebral embolization.36–42 The technique of DHCA with RCP is a reasonable approach for neuroprotection during aortic arch surgery in the setting of adequate institutional experience (ACC/AHA Class IIa recommendation; level of evidence B).1
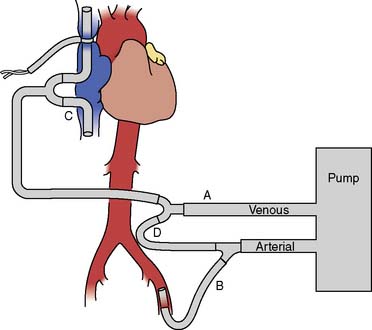
Figure 21-9 Extracorporeal perfusion circuit used to deliver retrograde cerebral perfusion.
(Adapted from Bavaria JE, Woo YJ, Hall RA, et al: Retrograde cerebral and distal aortic perfusion during ascending and thoracoabdominal aortic operations. Ann Thorac Surg 60:347, 1995, by permission of the Society of Thoracic Surgeons.)
Selective Antegrade Cerebral Perfusion
Selective ACP should be considered for aortic arch repairs longer than 45 minutes.31 ACP typically is initiated during DHCA by selective cannulation of the right axillary artery, right subclavian artery, innominate artery, or left common carotid artery (Figure 21-10).43 In transverse aortic arch reconstruction procedures, ACP can be accomplished by inserting individual perfusion cannulae into the open end of the aortic branch vessels after opening the aortic arch. After reattachment of the aortic arch branch vessels to the vascular graft, ACP can be provided through a separate arm of the vascular graft or by direct cannulation of the graft. A functional circle of Willis may provide contralateral brain perfusion during interruption of antegrade perfusion in the innominate, left carotid, or left subclavian arteries during construction of the vascular anastomoses. ACP with oxygenated blood at 10° C to 14° C at flow rates in the range of 250 to 1000 mL/min typically achieves a cerebral perfusion pressure in the range of 50 to 80 mm Hg.
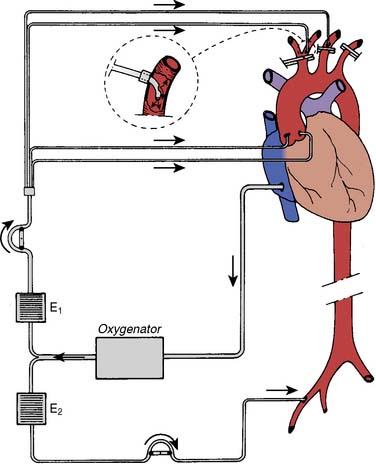
Figure 21-10 Extracorporeal perfusion circuit used for selective antegrade cerebral perfusion.
(From Bachet J, Teodori G, Goudot B, et al: Replacement of the transverse aortic arch during emergency operations for type A acute aortic dissection. J Thorac Cardiovasc Surg 96:878, 1988.)
Unilateral ACP via right axillary arterial cannulation is a popular technique for adult aortic repair.43 This technique assumes an adequate circle of Willis; however, the anatomic completeness of the circle of Willis does not guarantee adequate cerebral cross-perfusion during aortic arch repair.44,45 Consequently, it remains essential to monitor the contralateral hemisphere in unilateral ACP with modalities such as cerebral oximetry, carotid artery scanning, and transcranial Doppler.46–48
Given that ACP may be unilateral or bilateral, there remains controversy about which ACP technique is superior.49 A recent literature review combined 17 studies for a total sample size of 3548 patients; 83.1% with bilateral ACP and 16.9% with unilateral ACP.50 Although the stroke rates were less than 5% regardless of technique, the period of safe ACP was significantly prolonged with bilateral ACP (86–164 minutes) compared with unilateral ACP (30–50 minutes). The evidence favors bilateral ACP in the setting of aortic arch repair times longer than 60 minutes.50
Pilot clinical series in adult aortic arch repair also have been undertaken in the setting of ACP with moderate hypothermic circulatory arrest (MHCA; systemic temperature = 25° C).51,52 A large, single-center study (1999–2006; N = 501 [36.1% emergency cases]; median age, 64 years; 63.9% male sex) evaluated perioperative outcomes with this technique.53 With a perioperative mortality rate of 11.6%, multivariate predictors for mortality included age and CPB time. The stroke rate was 9.6% with operative time and renal dysfunction as its multivariate predictors for stroke. The rate of temporary neurologic dysfunction was 13.4%, its multivariate predictors including MHCA duration (odds ratio, 1.015; p = 0.01). Although MHCA with cold ACP appears to be an adequate technique for adult aortic arch repair, its safety is limited in the settings of the elderly, multiple comorbidities, and extended operative time. The safety of aortic arch repair recently was further demonstrated with bilateral ACP and a greater mean MHCA temperature of 28° C (2002–2008; N = 229; mean age, 70.8 ± 9.7 years; 68.1% male sex).54 Although MHCA with ACP appears to be a reasonable technique for adult aortic arch repair, its safety for ischemic protection of the spinal cord and kidney are still questioned.55,56 The technique of DHCA with ACP is a reasonable approach for neuroprotection during aortic arch surgery in the setting of adequate institutional experience (ACC/AHA Class IIa recommendation; level of evidence B).1
Pharmacologic Neuroprotection Strategies for Deep Hypothermic Circulatory Arrest
There are no proven pharmacologic regimens that have demonstrated effectiveness for decreasing the risk or severity of neurologic injury in the setting of thoracic aortic operations.36 The agents that have been reported in aortic arch series include thiopental, propofol, steroids, magnesium sulfate, and lidocaine.15,32,57 Furthermore, there is considerable variation in practice with these agents in aortic arch repair.57 In general, the existing evidence suggests that pharmacologic neuroprotection should be considered as a neuroprotective adjunct and not a substitute for hypothermia to protect against cerebral ischemia in the setting of hypoperfusion. The technique of DHCA with pharmacologic adjuncts is a reasonable approach for neuroprotection during aortic arch surgery in the setting of an institutional protocol and adequate institutional experience (ACC/AHA Class IIa recommendation; level of evidence B).1
Descending Thoracic and Thoracoabdominal Aortic Aneurysms
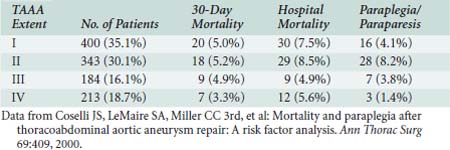
Surgical therapy for thoracic and TAAAs is to replace aneurysmal aorta with a prosthetic tube graft. Surgical access is via lateral thoracotomy or thoracoabdominal incision. Despite recent advances, major surgical challenges remain because the typical patient is elderly with multiple significant comorbidities (Table 21-6). The risks for spinal, mesenteric, renal, and lower extremity ischemia are significant due to thromboembolism, loss of collateral vascular networks, temporary interruption of blood flow, and reperfusion injury. The risks for wound dehiscence and respiratory failure remain significant because of the large incisions and diaphragmatic division, as well as injuries to the phrenic and recurrent laryngeal nerves. Consequently, TAAA repair is high risk (Table 21-7).58
TABLE 21-6 Preoperative Features in Patients with Thoracoabdominal Aortic Aneurysm (N = 1220)
| Characteristic | No. of Patients (%) |
|---|---|
| Crawford extent | |
| Extent I | 423 (34.7) |
| Extent II | 371 (30.4) |
| Extent III | 201 (16.5) |
| Extent IV | 225 (18.4) |
| Acute dissection | 46 (3.8) |
| Chronic dissection | 272 (22.3) |
| Marfan syndrome | 72 (5.9) |
| Symptomatic aneurysms | 855 (70.1) |
| Acute presentation | 112 (9.2) |
| Rupture | 76 (6.2) |
| Preoperative paraplegia or paraparesis | 16 (1.3) |
| Concurrent aneurysm | 224 (18.4) |
| Prior aneurysm repair | 502 (41.2) |
| Prior thoracic aortic aneurysm repair | 281 (23.0) |
| Diabetes | 69 (5.7) |
| Hypertension | 940 (77.1) |
| Coronary artery disease | 435 (35.7) |
| Prior coronary artery bypass or angioplasty | 202 (16.6) |
| Cerebrovascular disease | 135 (11.1) |
| Renal arterial occlusive disease | 312 (25.6) |
| Renal insufficiency | 151 (12.4) |
| Chronic obstructive lung disease | 491 (40.3) |
| Peptic ulcer disease | 83 (6.8) |
Data from Coselli JS, LeMaire SA, Miller CC 3rd, et al: Mortality and paraplegia after thoracoabdominal aortic aneurysm repair: A risk factor analysis. Ann Thorac Surg 69:409, 2000.
TABLE 21-7 Clinical Outcomes of Thoracoabdominal Aortic Aneurysm (TAAA) Repair (United States: 1988–1998)
| Complication | Intact TAAA (n = 1542) | Ruptured TAAA (n = 321) |
|---|---|---|
| Cardiac | 14.8% | 18.1% |
| Pulmonary | 19.0% | 12.7% |
| Hemorrhage | 12.4% | 10.9% |
| Acute renal failure | 14.2% | 28.0% |
| Paraplegia | —* | 3.4% |
| Any complication | 55.2% | 51.7% |
| In-hospital mortality | 22.3% | 53.8% |
TAAA, thoracoabdominal aortic aneurysm.
* Incidence of paraplegia not reported.
Data from Cowan JA, Dimick JB, Henke PK, et al: Surgical treatment of intact thoracoabdominal aortic aneurysms in the United States: Hospital and surgeon volume-related outcomes. J Vasc Surg 37:1169, 2003; and Cowan JA, Dimick JB, Wainess RM, et al: Ruptured thoracoabdominal aortic aneurysm treatment in the United States: 1988 to 1998. J Vasc Surg 38: 312, 2003.
Aneurysms of the descending thoracic aorta are classified by considering which third(s) of the descending thoracic aorta is (are) involved.1 Extent A involves the proximal third, extent B involves the middle third, and extent C involves the distal third. If more than one third is involved, then the extent is classified according to which thirds are involved, for example, an aneurysm involving the proximal two-thirds is classified as extent AB. Essentially, multisegment aneurysms can be classified as proximal or distal because these extents influence the risk for spinal cord ischemia after surgical repair, whether open or endovascular.
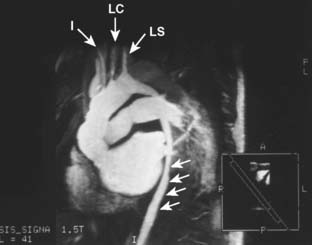
Aneurysms of the thoracoabdominal aorta typically are defined by the Crawford classification (Figure 21-11). Extent I TAAA begins at the left subclavian artery and ends below the diaphragm, but above the renal arteries. Extent II TAAA involves the entire descending thoracic aorta and ends below the diaphragm at the aortic bifurcation. Extent III TAAA begins in the lower half of the descending thoracic aorta and ends below the diaphragm at the aortic bifurcation. Extent IV TAAA is confined to the entire abdominal aorta. If an extent I or extent II TAAA involves the distal aortic arch, its surgical replacement often requires DHCA for the proximal anastomosis. The Crawford classification stratifies operative risk and guides perioperative management (Table 21-8). Open repair of TAAA typically is accomplished by one of three major techniques; (1) aortic cross-clamping, (2) aortic cross-clamping with a Gott shunt, and (3) aortic cross-clamping with PLHB or partial CPB (Figure 21-12).
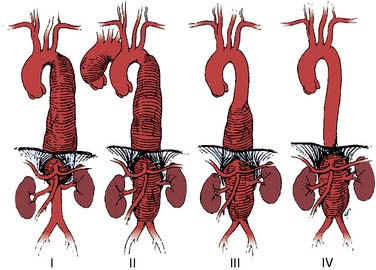
Figure 21-11 Crawford classification of thoracoabdominal aortic aneurysm extent.
(From Coselli JS: Descending thoracoabdominal aortic aneurysms. In Edmunds LH [ed]: Cardiac Surgery in the Adult. New York, McGraw-Hill, 1997, p 1232.)
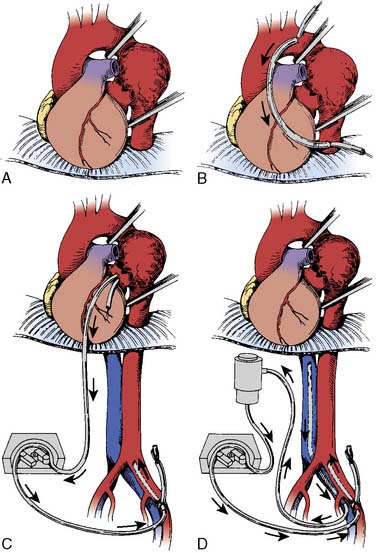
Figure 21-12 Operative techniques for repair of thoracic or thoracoabdominal aortic aneurysms.
(From O’Connor CJ, Rothenberg DM: Anesthetic considerations for descending thoracic aortic surgery: Part II. J Cardiothorac Vasc Anesth 9:734, 1995.)
Simple Aortic Cross-Clamp Technique
Although this technique was developed by Crawford, its major disadvantage is the concomitant vital organ ischemia below the aortic clamp. Consequently, surgical speed is critical to achieve an ischemic time less than 30 minutes to limit the risk for vital organ dysfunction.59 Its further disadvantages include proximal aortic hypertension, bleeding, and hemodynamic instability on reperfusion. Despite anesthetic interventions, this proximal aortic hypertension may induce LV ischemia.60 Blood loss can be minimized with intraoperative red blood cell salvaging. Hemodynamic instability during reperfusion can be minimized with correction of metabolic acidosis, rapid intravascular volume expansion, vasopressor therapy, and/or gradual clamp release. Mild systemic hypothermia and selective spinal cooling protect against the ischemia associated with this technique.61,62 Despite its physiologic consequences, this technique remains popular because it is simple and has proven clinical outcomes (Table 21-9).
TABLE 21-9 Advantages and Disadvantages of Distal Perfusion Techniques
| Potential Advantages |
| Control of proximal hypertension |
| Decrease left ventricular afterload |
| Less hemodynamic perturbations with aortic clamping and unclamping |
| Decrease duration of mesenteric ischemia |
| Decrease risk for paraplegia from spinal cord ischemia |
| Ability to control systemic temperature with heat exchanger |
| Vascular access for rapid volume expansion |
| Ability to oxygenate blood with extracorporeal oxygenator |
| Capability to selectively perfuse mesenteric organs or aortic branch vessels |
| Maintain lower extremity SSEPs and MEPs for neurophysiologic monitoring |
| Potential Disadvantages |
| Require greater level of systemic anticoagulation |
| Increase risk for vascular injury at cannulation sites |
| Increase risk for thromboembolic events. |
| Require perfusion team |
| Need to monitor and control upper and lower body arterial pressure and flow |
| Increase technical complexity of operation |
Gott Shunt
The Gott shunt allows passive shunting of blood from the proximal to distal aorta during aortic cross-clamping for thoracic aortic repair (see Figure 21-12B).63 Blood flow from the proximal to distal aorta through the Gott shunt depends on proximal aortic pressure, shunt length and diameter, and distal aortic pressure. Monitoring the femoral arterial pressure facilitates assessment of distal aortic perfusion and shunt flow. The advantages of the Gott shunt are its simplicity, its low cost, and its requirement for only partial anticoagulation. Its disadvantages include vessel injury, dislodgment, bleeding, and atheroembolism.
Partial Left-Heart Bypass
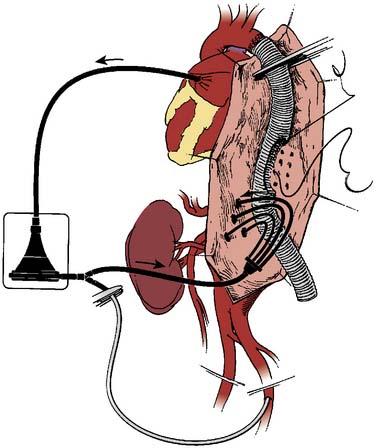
The control of both proximal and distal aortic perfusion during TAAA repair is achieved with PLHB. This technique requires left atrial cannulation, usually via a left pulmonary vein (see Figure 21-12C). Oxygenated blood from the left atrium flows through the CPB circuit into the distal aorta or a major branch via the arterial cannula.61 The CPB circuit can include a heat exchanger, membrane oxygenator, and/or a venous reservoir. The degree of heparinization for PLHB is minimal with heparin-coated circuits without an oxygenator. Full systemic anticoagulation with ACT greater than 400 seconds is required for CPB circuits with membrane oxygenators and heat exchangers.64 During PLHB, the proximal mean arterial pressure (MAP; radial artery) is generally maintained in the 80 to 90 mm Hg range. Flow rates in the range of 1.5 to 2.5 L/min typically maintain a distal aortic MAP in the 60 to 70 mm Hg range, monitored via a femoral arterial catheter.64 Sequential advancement of the aortic cross-clamp during PLHB permits segmental aortic reconstruction with a decrease in end-organ ischemia. The advantages of PLHB include control of aortic pressures and systemic temperature, reliable distal aortic perfusion, and selective antegrade perfusion of important branch vessels (Figure 21-13).61 Its disadvantages include increased expense, increased complexity, and requirement for systemic anticoagulation (see Table 21-9). An alternative technique uses partial CPB by femoral vein to femoral artery perfusion with or without an oxygenator. This can allow for distal perfusion without the need for cannulation of the heart or aorta. However, it does not offer the control that is achieved with proper PLHB.
Cardiopulmonary Bypass with Deep Hypothermic Circulatory Arrest
When a TAAA involves the distal aortic arch, CPB with DHCA is required to allow completion of the distal anastomosis. This technique has acceptable perioperative outcome for major reconstruction of the thoracoabdominal aorta because it also protects the spinal cord and mesenteric organs from ischemia.65 If CPB with DHCA is planned for TAAA repair through a left thoracotomy incision, TEE should monitor for AR so that any LV distention with the onset of asystole during deliberate hypothermia can be managed with insertion of a drainage cannula. The disadvantages of CPB with DHCA include the limited safe period for DHCA, risk for stroke from retrograde aortic perfusion, increased CPB duration, and bleeding. For TAAA with extension into the distal aortic arch, a two-stage elephant-trunk procedure can be performed instead of using CPB with DHCA.66 In the two-stage elephant-trunk procedure, the transverse aortic arch graft is performed first through a median sternotomy, leaving a short segment of graft extending into the descending aorta (see Figure 21-5). The second stage of the repair is performed through a left thoracotomy incision to access and anastomose the distal end of the transverse arch graft to the proximal end of the descending thoracic aortic graft. This two-stage repair avoids the need for retrograde CPB perfusion through the diseased descending thoracic aorta and decreases the risk for injury to the recurrent laryngeal nerve, esophagus, and pulmonary artery located in the proximity of the distal aortic arch.
Endovascular Stent Graft Repair of Thoracic Aortic Aneurysms
TEVAR was established for the management of thoracic aortic aneurysms and now has recent management guidelines.20 Endovascular stent grafts are tube grafts reinforced by a wire frame that are collapsed within a catheter for delivery and deployment within the aortic lumen. The principle of TEVAR is that the deployed stent complex spans the length of diseased aorta to exclude blood flow into the aneurysm cavity. TEVAR requires a landing zone for each end of the tubular graft. Endoleak is defined as blood flow within the aneurysm but outside the endovascular graft (Table 21-10).
| Type | Cause of Perigraft Flow | Consequences and Therapeutic Strategy |
|---|---|---|
| I | Inadequate seal at proximal and/or distal landing zone | Systemic blood pressure is transmitted to aneurysm with risk for rupture: timely repair is indicated. |
| II | Retrograde flow from aortic branches into aneurysm | It may thrombose. If aneurysm is expanding, aortic branch embolization is indicated. |
| III | Structural failure of stent, e.g., perforations, fractures | Systemic blood pressure is transmitted to aneurysm with risk for rupture: timely repair is indicated. |
| IV | Stent graft fabric porosity | This usually occurs at implantation and disappears with anticoagulation reversal. |
| V | Aneurysm expansion without obvious endoleak (“endodistention”) | The endovascular repair can be strengthened with a second stent. |
Data from Hiratzka LF, Bakris GL, Beckman JA, et al: 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: Executive summary. A report of the American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine. Circulation 121:e266–e369, 2010.
The current guidelines from the STS suggest TEVAR for aneurysms of the descending thoracic aorta when the aortic diameter is larger than 5.5 cm (Class IIa recommendation; level of evidence B, when the patient has significant comorbidity; Class IIb recommendation; level of evidence C, when the patient has no significant comorbidity).20 When the aortic diameter is less than 5.5 cm, the STS guidelines advise against TEVAR (Class III recommendation; level of evidence C).20 In the setting of TAAA, the STS guidelines support TEVAR in patients with severe comorbidity (Class IIb recommendation; level of evidence C).20 In patients with severe comorbidity and aortic arch aneurysm with distal extension, the STS guidelines support an endovascular repair technique (Class IIb recommendation; level of evidence C).1,20 In patients who have reasonable surgical risk and who have aortic arch aneurysms with distal extension, the STS guidelines advise against an endovascular repair technique (Class III recommendation; level of evidence A).1,20
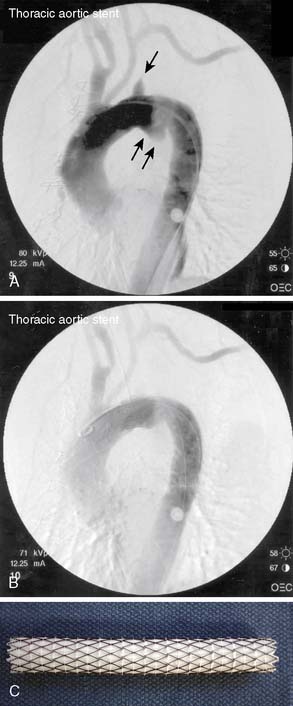
Endovascular stent graft repair for isolated descending thoracic aortic aneurysms with a proximal landing zone that involves the left subclavian artery can be accomplished using a two-stage procedure (Figure 21-14). In the first stage, the left subclavian artery can be divided and anastomosed onto the left common carotid artery. This first stage of the procedure provides a proximal landing zone, allowing the deployment of the endovascular stent graft over the left subclavian artery branch in the distal aortic arch in the second stage of the procedure without compromising flow through the vessel. Multiple recent meta-analyses have demonstrated the outcome importance of not sacrificing the left subclavian artery during TEVAR to avoid the risks for stroke, paraplegia, and left upper extremity ischemia.67–69 The recent guidelines from the Society of Vascular Surgery strongly support this principle but also recognize that in urgent TEVAR for life-threatening acute aortic syndromes, left subclavian artery coverage is unavoidable.70
There are currently two major options for endovascular TAAA repair, namely, total TEVAR and hybrid TEVAR. Total endovascular TAAA repair requires customized stents that preserve major aortic branches with fenestrations or side branches. Recent series have demonstrated the safety and efficacy of this TEVAR modality in high-risk TAAA patients.71–73 In hybrid TAAA repair, the landing zone for the nonfenestrated endovascular graft is created by aortic debranching procedures, for example, the renal and mesenteric arteries are anastomosed to the iliac arteries. Recent multiple series and meta-analyses have demonstrated the safety and efficacy of this TEVAR modality in high-risk patients.74–78 This hybrid approach also has been utilized in aortic arch reconstruction for high-risk patients with aortic arch aneurysms.79 Furthermore, TEVAR recently has extended proximally for therapy of select aneurysms of the ascending aorta.80,81 In summary, TEVAR for TAAA, whether wholly endovascular or hybrid, is in recent clinical development with an established niche in patients with excessive operative risk. It is likely that these technologies will mature further in the coming years. This maturation of TEVAR for diseases of the descending thoracic aorta likely will be rapid given that recent meta-analysis (N = 5888, 42 nonrandomized studies) demonstrated that TEVAR as compared with open aortic repair reduced perioperative mortality (odds ratio, 0.44; 95% confidence interval, 0.33–0.59), paraplegia (odds ratio, 0.42; 95% confidence interval, 0.28–0.63), pneumonia, cardiac complications, renal failure, bleeding, and transfusion, as well as length of hospital stay.82
Anesthetic Management for Thoracoabdominal Aortic Aneurysm Repair
Lung Isolation Techniques
Selective ventilation of the right lung with concomitant left lung collapse during TAAA repair enhances surgical access and protects the right lung from left lung bleeding. Collapse of the left lung typically is achieved when the left main bronchus is intubated either with a double-lumen endobronchial tube (DLT) or a bronchial blocker. Routine fiberoptic bronchoscopic guidance guarantees the effectiveness of either technique. The increased length of the left mainstem bronchus facilitates placement of a left-sided DLT and subsequently anchors it during surgery. Endobronchial blockade is achieved with one of the following devices: the Arndt blocker, the Cohen blocker, or the Univent tube (Figure 21-15).83 Wire-guided endobronchial blocking catheters permit the balloon-tipped catheter to be guided and positioned precisely in the left mainstem bronchus with a fiberoptic bronchoscope. The advantages of a left DLT include the ability to apply selective continuous positive airway pressure to the left lung. Its disadvantages include increased difficulty in difficult airways and bronchial injury in distorted endobronchial anatomy. The major advantage of endobronchial blockade is its compatibility with an existing standard 8.0-mm endotracheal tube. This is advantageous in emergencies and in difficult airways.83 The disadvantages of endobronchial blockade include increased time for left-lung collapse and dislodgement during surgery. The majority of patients will require temporary postoperative mechanical ventilation, usually via a single-lumen endotracheal tube. ICU personnel often are unaccustomed to managing patients with DLTs with their risks for malposition, airway obstruction, and difficulty with airway secretions. Endotracheal tube exchange may be challenging if there is airway edema. An endotracheal tube exchange catheter in combination with direct laryngoscopy often facilitates safe endotracheal tube exchange.84 It is recommended that in the setting of upper airway edema, DLTs are not routinely exchanged for single-lumen tubes (ACC/AHA Class III recommendation; level of evidence C).1
Paraplegia after Thoracoabdominal Aortic Aneurysm Repair
Paraplegia after TAAA repair is a devastating complication. The temporary interruption of distal aortic perfusion and sacrifice of spinal segmental arteries during TAAA repair are central events in the pathogenesis of spinal cord ischemia and paraplegia. There are multiple contributing factors (Table 21-11).85 The typical level of spinal cord ischemia after TAAA is midthoracic and is associated with a high perioperative mortality. There are many management strategies for prevention of this devastating complication after TAAA (Table 21-12).1,85
TABLE 21-11 Factors That Contribute to Paraplegia after Thoracic or Thoracoabdominal Aortic Procedures
| Thoracoabdominal aortic aneurysm extent |
| Hypotension or cardiogenic shock |
| Emergency surgery |
| Aortic rupture |
| Presence of aortic dissection |
| Duration of aortic cross-clamp |
| Sacrifice of intercostal or segmental artery branches |
| Prior thoracic or abdominal aortic aneurysm repair |
| Prior repair of type A aortic dissection |
| Occlusive peripheral vascular disease |
| Anemia |
TABLE 21-12 Minimizing Paraplegic Risk after Thoracic or Thoracoabdominal Aortic Procedures
| Minimize Aortic Cross-clamp Time |
| Distal aortic perfusion |
| Passive shunt (Gott) |
| Partial left heart bypass |
| Partial cardiopulmonary bypass |
| Deliberate Hypothermia |
| Mild-to-moderate systemic hypothermia (32° C to 35° C) |
| Deep hypothermic circulatory arrest (14° C to 18° C) |
| Selective spinal cord hypothermia (epidural cooling, 25° C) |
| Increase Spinal Cord Perfusion Pressure |
| Reimplantation of critical intercostal and segmental arterial branches |
| Lumbar cerebrospinal fluid (CSF) drainage (CSF pressure ≤ 10 mm Hg) |
| Arterial pressure augmentation (mean arterial pressure ≥ 85 mm Hg) |
| Intraoperative Monitoring of Lower Extremity Neurophysiologic Function |
| Somatosensory-evoked potentials |
| Motor-evoked potentials |
| Postoperative Neurologic Assessment for Early Detection of Delayed- Onset Paraplegia |
| Serial neurologic examinations |
| Pharmacologic Neuroprotection |
| Glucocorticoid |
| Barbiturate or central nervous system depressants |
| Magnesium sulfate |
| Mannitol |
| Naloxone |
| Lidocaine |
| Intrathecal papaverine |
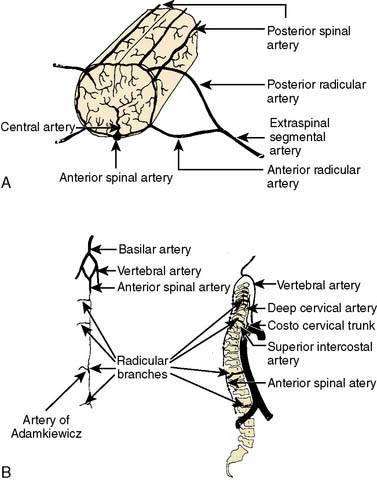
The spinal cord arterial supply provides a partial explanation for the clinical features of paraplegia after TAAA repair (Figure 21-16).85,86 The anterior spinal artery supplies the anterior two thirds of the spinal cord, and the posterior spinal arteries supply the posterior third. Branches from each vertebral artery join to form the anterior spinal artery that descends along the midline of the anterior surface of the spinal cord. The anterior spinal artery sometimes is discontinuous and fed in a variable extent by radicular arteries derived from ascending cervical, deep cervical, intercostal, lumbar, and sacral segmental arteries. The posterior spinal arteries also are derived from the vertebral arteries and receive collateral supply from posterior radicular arteries. The terminal cord segments are supplied by radicular arteries that arise from the internal iliac and sacral arterial network. The thoracolumbar spinal cord typically has multiple arterial sources with a clinical vulnerability to significant ischemia. In this watershed region, an important blood supply is derived from a large radicular artery (intercostal arteries T9-T12 in 75% of patients, T8-L3 in 15%, and L1-L2 in 10%).87,88 This important artery is known as the arteria magna or the artery of Adamkiewicz. Ischemia in the anterior spinal artery territory classically causes motor paralysis with preservation of proprioception.85 Clinical experience, however, has demonstrated that spinal cord ischemia after TAAA repair is variable, asymmetric, and can affect motor or sensory function, or both.85,89
Paraplegia is defined as lower extremity motor weakness with muscle strength weaker than gravity. Paraparesis is defined as lower extremity weakness with muscle power that allows movement at least against gravity (Table 21-13).89 Spinal cord ischemia may have an immediate onset, defined as lower extremity weakness on emergence from anesthesia within 24 hours of the procedure.85,89 Delayed-onset spinal cord ischemia is defined as lower extremity weakness that follows a normal postoperative neurologic examination after emergence from anesthesia. In the largest series of TAAA repairs ever reported (N = 2286; 1986–2006), the incidence rate of symptomatic spinal cord ischemia was 3.8%, with 63% of these cases having an immediate onset and 37% a delayed onset.61 Multiple series have indicated that delayed-onset spinal cord ischemia can present days, weeks, or even months after TAAA repair.61,85,89,90
TABLE 21-13 Description of Lower Extremity Weakness Caused by Spinal Cord Ischemia
| Score | Description |
|---|---|
| Paraplegia | Paraplegia |
| 0 | No movement of lower extremity |
| 1 | Minimal movement or flicker of lower extremity |
| 2 | Movement of the lower extremity but not against resistance or gravity (e.g., bend knee, move leg) |
| Paraparesis | Paraparesis |
| 3 | Movement of the lower extremity against resistance and gravity but without ability to stand or walk |
| 4 | Ability to stand and walk with assistance |
Data from Greenberg RK, Lu Q, Roselli E, et al: Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: A comparison of endovascular and open techniques. Circulation 118:808, 2008.
Immediate-onset paraplegia likely is a consequence of spinal cord ischemia, leading to infarction that occurred during surgery. In contrast with delayed-onset paraplegia, recovery with intervention in immediate-onset paraplegia has not been consistently demonstrated. This lack of therapeutic response likely indicates that irreversible spinal cord injury has occurred. Consequently, strategies to prevent immediate-onset paraplegia are directed toward intraoperative spinal cord protection (Box 21-3). The objective of intraoperative spinal cord monitoring is to detect spinal cord ischemia for immediate intervention to improve spinal cord perfusion. Distal aortic perfusion maintains spinal cord function during aortic cross-clamping and improves the ability to monitor spinal cord integrity during surgery with SSEP or MEPs.
Delayed-onset paraplegia indicates that, although the spinal cord was protected intraoperatively, it remains vulnerable to ischemia after surgery. Although the causes of this syndrome are incompletely understood, it often is preceded by hypotension.91 Strategies to minimize delayed-onset paraplegia concern the prevention of perioperative hypotension, early anesthetic emergence for early and subsequent serial neurologic assessment, and lumbar CSF drainage (Box 21-4). Given the catastrophic sequelae of permanent paraplegia after TAAA repair, all reasonable attempts to treat delayed-onset paraplegia can be justified.
Lumbar Cerebrospinal Fluid Drainage
Lumbar CSF drainage is a strongly recommended spinal cord protective strategy for TAAA repair (ACC/AHA Class I recommendation; level of evidence B).1,92–95 The physiologic rationale is that reduction of CSF pressure improves spinal cord perfusion pressure (SCPP) and also may counter CSF pressure increases caused by aortic cross-clamping, reperfusion, increased central venous pressure, and/or spinal cord edema.85 Lumbar CSF drainage is performed by the insertion of a silicon elastomer ventriculostomy catheter via a 14-gauge Tuohy needle at the L3-L4 vertebral interspace. The catheter usually is advanced 10 to 15 cm into the subarachnoid space and securely fastened to the skin to prevent catheter movement while the patient is anticoagulated. The open end of the catheter is attached to a sterile reservoir, and CSF is drained when the lumbar CSF pressure exceeds 10 mm Hg. The lumbar CSF pressure is measured with a pressure transducer zero-referenced to the midline of the brain. Currently, the best strategy to manage a traumatic lumbar puncture or the drainage of blood-tinged CSF has not been determined.96,97 The lumbar CSF drainage catheter is inserted before or at the time of surgery for CSF drainage up to the first 24 hours after surgery. The lumbar drainage catheter subsequently can be capped and left in place for the next 24 hours. It then can be removed, assuming a normal neurologic examination and adequate coagulation.
The complications of lumbar CSF drainage include neuraxial hematoma, catheter fracture, meningitis, intracranial hypotension, and spinal headache. 64,85 Neuraxial hemorrhage after lumbar drain insertion remains a risk in patients subsequently subjected to systemic anticoagulation for CPB. Despite this risk, the overall safety of this technique has been established in multiple case series.64,85,98,99 Measures to minimize neuraxial hematoma include establishing normal coagulation for both CSF catheter insertion and removal, as well as allowing a few hours between its insertion and heparinization for CPB.85,98 In two large contemporary series (combined N = 2001), the complication rate associated with CSF drainage for thoracic aortic repair was about 1% with no spinal hematomas. Both series identified excessive CSF drainage as a principal risk factor for intracranial hypotension and subsequent subdural hematoma and emphasized the outcome benefit associated with a limited CSF drainage protocol.98,99 For routine use, CSF only should be drained, using a closed circuit reservoir, when the lumbar CSF pressure exceeds 10 mm Hg. Meningitis is characterized by high fever, altered mentation, and CSF pleocytosis often with bacteria. The risk for catheter fracture can be minimized by careful catheter removal.
Arterial Pressure Augmentation
The optimization of SCPP for spinal cord protection is recommended as part of an institutional perioperative protocol (ACC/AHA Class IIa recommendation; level of evidence B).1 This recommendation also recognized the variety of suitable techniques such as maintenance of proximal aortic pressure and distal aortic perfusion with the caveat that technique selection often is a function of institutional experience.1 In keeping with this recommendation are the principles of arterial pressure augmentation and CSF drainage for prevention and management of postoperative spinal cord ischemia (Figure 21-17).90 Spinal cord ischemia after TAAA repair is more likely in the setting of hypotension because the spinal arterial collateral network has been reduced due to factors such as intercostal artery sacrifice.91,100,101 Recent surgical techniques to preserve SCPP include selective intraoperative spinal cord perfusion and intercostal artery revascularization with interposition grafts.102,103
SCPP is estimated as the MAP minus the lumbar CSF pressure. In general, the SCPP should be maintained greater than 70 mm Hg after TAAA repair, that is, a MAP of 80 to 100 mm Hg.85,90,100 Because spinal cord ischemia often involves the thoracolumbar cord, it often is accompanied by a significant sympathectomy known as spinal vasodilatory shock.1,85,90 Early intervention to treat hypotension with vasopressor therapy may counter the autonomic nervous system dysfunction accompanying spinal cord ischemia and also augment SCPP. As in the treatment of neurogenic shock, high-dose vasopressor therapy with norepinephrine, phenylephrine, epinephrine, and/or vasopressin typically is required to restore systemic vascular resistance and spinal cord perfusion, with a MAP in the 80 to 100 mm Hg range.90 Recovery from spinal cord ischemia often is heralded by recovery of systemic vascular tone and a decreasing vasopressor requirement. Therapeutic hypertension, as discussed here, for the management of spinal cord ischemia after TAAA must be weighed against the risks for arterial bleeding.
Intraoperative Neurophysiologic Monitoring
Neurophysiologic monitoring of the spinal cord (SSEPs and/or MEPs) is recommended as a strategy for the diagnosis of spinal cord ischemia so as to allow immediate intraoperative neuroprotective interventions such as intercostal artery implantation, relative arterial hypertension, and CSF drainage (ACC/AHA Class IIb recommendation; level of evidence B).1 This management strategy may prevent immediate-onset postoperative paraplegia. SSEP monitoring is performed by applying electrical stimuli to peripheral nerves and recording the evoked potential that is generated at the level of the peripheral nerves, spinal cord, brainstem, thalamus, and cerebral cortex.104 Because SSEP monitors posterior spinal column integrity, MEPs have been advocated because they monitor the anterior spinal columns that are typically at risk during TAAA repair. MEP monitoring is performed by applying paired stimuli to the scalp and recording the evoked potential that is generated in the anterior tibialis muscle.105,106 (See Chapter 16.)
Paraplegia caused by spinal cord ischemia significantly dampens lower extremity evoked potentials as compared with the upper extremity (Figure 21-18). Intraoperative comparison of upper and lower extremity evoked potentials distinguishes spinal cord ischemia from the generalized effects of anesthetics, hypothermia, and/or electrical interference. As discussed earlier, the anesthetic must be designed for minimal interference with the selected neuromonitoring strategy. Although SSEPs can reliably exclude spinal cord ischemia with a negative predictive value of 99.2%, their sensitivity for its detection is only 62.5%, with no clinically useful predictive value for delayed-onset paraplegia.107 A recent study (N = 233) compared both MEPs and SSEPs for spinal cord monitoring in extensive descending thoracic and TAAA repairs.108 Both monitoring modalities had a nearly 90% correlation for spinal cord infarction (correlation statistic = 0.896; P < 0.0001), as well as a 98% negative predictive value for immediate-onset paraplegia. Furthermore, reversible changes in MEPs and SSEPs had no correlation with permanent paraplegia. In summary, despite the theoretic advantages of MEPs for monitoring the at-risk anterior spinal columns, in practice, this most recent data set suggests that SSEPs alone suffice for clinical purposes, and that MEPs did not add significantly to clinical management.108 This assessment also is reflected in the neuromonitoring recommendations of the latest thoracic aortic disease guidelines.1
Spinal Cord Hypothermia
Although DHCA is effective, moderate systemic hypothermia is also reasonable for spinal cord protection during TAAA repair (ACC/AHA Class IIa recommendation; level of evidence B).1 Furthermore, topical spinal cord hypothermia is possible with cold saline epidural infusion to avoid ischemia during TAAA repair.109,110 Although clinical experience with this technique has been limited to a few institutions, it has demonstrated clinical benefit as part of a multimodal spinal protection protocol.95,109,110 Epidural cooling is recommended as an adjunctive technique for spinal cord protection during major distal thoracic aortic reconstructions (ACC/AHA Class IIb recommendation; level of evidence B).1 This technique may disseminate further, given its adjunctive benefit and the recent clinical development of a specialized countercurrent closed-lumen epidural catheter for epidural cooling during major distal aortic reconstructions.111
Pharmacologic Protection of the Spinal Cord
Pharmacologic spinal cord protection with agents such as high-dose systemic glucocorticoids, mannitol, intrathecal papaverine, and anesthetic agents is recommended as an adjunctive technique in a multimodal neuroprotective protocol (ACC/AHA Class IIb recommendation; level of evidence B).1,95 Additional neuroprotective agents that have been studied in this regard include lidocaine, naloxone, and magnesium.95,112,113 Although there are multiple agents with potential benefit, only a few are utilized routinely in clinical practice.112
Renal Protection during Thoracoabdominal Aortic Aneurysm Repair
Even though there has been major progress in TAAA repair, renal dysfunction remains common and still independently predicts for adverse clinical outcomes.61,114 The thoracic aortic guidelines recommend preoperative hydration and intraoperative mannitol administration as reasonable nephroprotective strategies in extensive distal open thoracic aortic repairs, including TAAA repair (ACC/AHA Class IIb recommendation; level of evidence C).1 Furthermore, intraoperative cold renal perfusion with blood or crystalloid is recommended as a reasonable intraoperative nephroprotective strategy during TAAA repair (ACC/AHA Class IIb recommendation; level of evidence C).1,115,116 The administration of furosemide, mannitol, or dopamine for the sole purpose of renal preservation is not recommended in distal thoracic aortic repairs (ACC/AHA Class IIb recommendation; level of evidence B).1 Rhabdomyolysis from lower extremity ischemia was recently identified as a mechanism for renal dysfunction after TAAA repair.117–119 The maintenance of lower extremity perfusion bilaterally during distal aortic perfusion has been shown to ameliorate this rhabdomyolysis with a significant nephroprotective effect.
Postoperative Analgesia after Thoracoabdominal Aortic Aneurysm Repair
It is well recognized that the extensive thoracoabdominal incision is very painful. Because epidural analgesia has proved outcome utility in this type of extensive incision, it typically is part of the analgesic plan after TAAA repair.120 The timing of epidural catheter placement and analgesia must take into account the perioperative anticoagulation status of the patient to minimize the risk for neuraxial hematoma.7 Furthermore, the epidural analgesia regimen should be formulated for a predominantly sensory block to allow serial motor assessment of the lower extremities and to minimize systemic vasodilation from a sympathectomy. For example, bupivacaine, 0.05%, combined with fentanyl, 2 μg/mL, can be initiated at a basal rate of 4 to 8 mL/hr after the patient exhibits normal neurologic function.90 Bolus administration of concentrated local anesthetic through the epidural catheter should be discouraged to avoid sympathetic blockade and associated hypotension. The epidural catheter can be inserted before surgery, at the time of surgery, or in the postoperative period.
Anesthetic Management for Thoracic Endovascular Aortic Repair
TEVAR has revolutionized the management of descending thoracic and TAAAs with significant clinical outcome benefit.20,82 The anesthetic management is based on the principles of care for patients undergoing endovascular abdominal aortic repair, but with the additional concerns of spinal cord ischemia and stroke.121 Typically, these patients undergo a balanced general anesthetic with invasive blood pressure monitoring and central venous access. The right radial artery is preferred for blood pressure monitoring, given that the left subclavian artery frequently may be covered and/or the left brachial artery may be accessed as part of the procedure.67–70 PAC monitoring may be helpful in the setting of significant cardiac disease. TEE is reasonable in TEVAR in which it may assist in hemodynamic monitoring, procedural guidance, and endoleak detection (ACC/ACC Class IIa recommendation; level of evidence B).1 As discussed earlier, if spinal cord monitoring is planned (SSEPs and/or MEPs), the anesthetic technique must be designed not to interfere with their signal quality.
The risk factors for stroke after TEVAR include a history of prior stroke, mobile aortic arch atheroma, and TEVAR of the proximal or entire descending thoracic aorta.121,122 Therefore, the detection of mobile atheroma in the aortic arch is an important TEE finding in TEVAR because it predicts a greater stroke risk. The risk factors for spinal cord ischemia after TEVAR include perioperative hypotension (decreased SCPP), prior abdominal/descending thoracic aortic procedures (compromised spinal collateral arterial network), and coverage of the entire descending thoracic aorta (significant loss of intercostal arteries).121,123 Consequently, indications for CSF lumbar drainage in TEVAR include planned extensive coverage of the descending thoracic aorta, history of prior abdominal/descending thoracic aortic procedures, and postoperative paraparesis/paraplegia despite relative hypertension.121,123 Lumbar CSF drainage is a strongly recommended spinal cord protection strategy for TEVAR in patients with identified risk factors (ACC/AHA Class I recommendation; level of evidence B).1,121–123
Aortic dissection
Aortic dissection results from an intimal tear that exposes the media to the pulsatile force of blood within the aortic lumen (Figure 21-19).1,124 Blood may exit the true aortic lumen and dissect the aortic wall to create a false lumen. The aortic dissection may remain localized at the primary entry site at the original intimal tear, or it may extend proximally, distally, or both. It also may extend into the aortic branch vessels to cause branch occlusion, or intima may shear at the site of branch vessels to result in intimal fenestrations. Propagation of the dissection into the aortic root can cause AR.124 The weakened aortic wall often results in acute aortic dilation, which can progress to rupture resulting in pericardial tamponade, exsanguination, or both.
There are two generally accepted classifications of thoracic aortic dissections (Table 21-14).1,124 The DeBakey scheme classifies aortic dissections into three groups (Figure 21-20). (See Videos on the website.) DeBakey type I dissections originate from a primary entry site in the ascending aorta and extend to involve the entire aorta. DeBakey type II dissections originate from a primary entry site in the ascending aorta and are confined to the ascending aorta. DeBakey type III dissections originate from a primary entry site in the descending thoracic aorta and are confined to the descending thoracic aorta distal to the origin of the left subclavian artery. DeBakey type III dissections also can be subdivided into subtype IIIA that are confined to the descending thoracic aorta above the diaphragm and subtype IIIB that extend below the diaphragm into the abdominal aorta. The Stanford scheme classifies aortic dissections into two groups (Figure 21-21). Stanford type A dissections are aortic dissections that involve the ascending aorta regardless of extent, origin, or entry sites. Stanford type B dissections are confined to the descending thoracic aorta distal to the origin of the left subclavian artery regardless of the extent or entry sites. (See Videos 1 and 2.)
| DeBakey Classification |
| Type I: The entire aorta is involved (ascending, arch, and descending) |
| Type II: Confined to the ascending aorta |
| Type III: Intimal tear originating in the descending aorta with either distal or retrograde extension |
| Type IIIA: Intimal tear originating in the descending aorta with extension distally to the diaphragm or proximally into the aortic arch |
| Type IIIB: Intimal tear originating in the descending aorta with extension below the diaphragm or proximally into the aortic arch |
| Stanford Classification |
| Type A: Involvement of the ascending aorta or aortic arch regardless of the site of origin or distal extent |
| Type B: Confined to the descending aorta distal to the origin of the left subclavian artery |
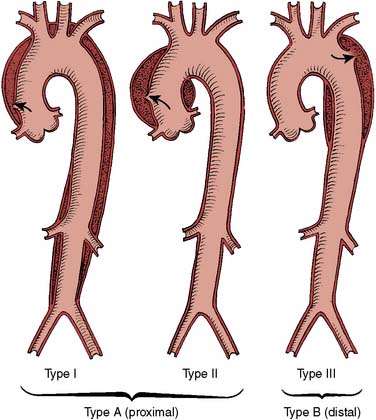
Figure 21-20 DeBakey classification of aortic dissections.
(Reprinted from Larson EW, Edwards WD: Risk factors for aortic dissection: A necropsy study of 161 cases. Am J Cardiol 53:849, 1984, by permission of Excerpta Medica, Inc.)
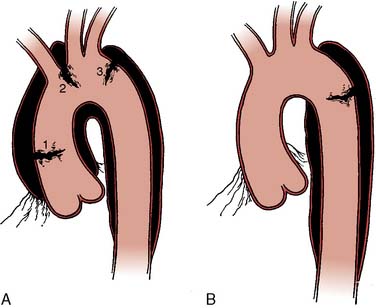
Figure 21-21 Stanford classification of aortic dissection.
(Reprinted from Daily PO, Trueblood HW, Stinson EB, et al: Management of acute aortic dissections. Ann Thorac Surg 10:237–247, 1970, by permission of Society of Thoracic Surgeons.)
Type A Aortic Dissection
Aortic dissections that involve the ascending aorta (Stanford type A) are considered surgical emergencies (Box 21-5) (ACC/AHA Class I recommendation; level of evidence B).1 The mortality rate without emergency surgery is about 1% per hour for the first 48 hours, 60% by about 1 week, 74% by 2 weeks, and 91% by 6 months (Figure 21-22).1,124,125 Immediate surgical intervention significantly improves the mortality rate (Table 21-15), especially in patients younger than 80 years.1,124–126 The principal causes of mortality include rupture, cardiac tamponade, myocardial ischemia from coronary dissection, severe acute AR, stroke caused by brachiocephalic dissection, and malperfusion syndromes including renal failure, ischemic bowel, and limb ischemia (Table 21-16). The type of preoperative presentation in type A dissection significantly determines operative mortality, prompting a clinical classification developed at the University of Pennsylvania (Penn classification: Table 21-17).127 An aortic dissection less than 2 weeks old is classified as acute and older than 2 weeks is classified as chronic. This distinction is clinically important because after 2 weeks, mortality risk has plateaued and thus emergency surgery is not necessarily indicated.
TABLE 21-15 Mortality in Acute Aortic Dissection According to Dissection Type and Management
| Dissection Type | N | Hospital Mortality (%) |
|---|---|---|
| Stanford type A | 289 | 101 (34.9) |
| Medical management | 81 | 47 (58.0) |
| Surgical management | 208 | 54 (26.0) |
| Stanford type B | 175 | 26 (14.9) |
| Medical management | 140 | 15 (10.7) |
| Surgical management | 35 | 11 (31.4) |
Data from Hagan PG, Nienaber CA, Isselbacher EM, et al: The International Registry of Acute Aortic Dissection (IRAD). New insights into an old disease. JAMA 283:897, 2000.
TABLE 21-16 Complications of Acute Stanford Type A Aortic Dissection (n = 513)
| Complications | Percentage |
|---|---|
| All neurologic defects | 18.0 |
| Coma/altered consciousness | 14.0 |
| Myocardial ischemia/infarction | 10.0 |
| Limb ischemia | 10.0 |
| Mesenteric ischemia/infarction | 4.0 |
| Acute renal failure | 6.2 |
| Hypotension | 26.0 |
| Cardiac tamponade | 17.0 |
| Mortality | 30.0 |
Data from Bossone E, Rampoldi V, Nienaber CA, et al: Usefulness of pulse deficit to predict in-hospital complications and mortality in patients with acute type A aortic dissection. Am J Cardiol 89:851, 2002.
TABLE 21-17 Penn Classification of Ischemic Presentations in Acute Stanford Type A Aortic Dissection
| Type A Dissection Presentation | Definition | Mortality Rate |
|---|---|---|
| Penn presentation a Type Aa |
Type A dissection with absence of ischemia | 3.1% |
| Penn presentation b Type Ab |
Type A dissection with branch vessel malperfusion producing clinical organ ischemia (e.g., stroke, renal failure, ischemic extremity, mesenteric ischemia) | 25.6% |
| Penn presentation c Type Ac |
Type A dissection with circulatory collapse (systolic blood pressure < 80 mm Hg and/or vasopressor therapy) with or without cardiac involvement | 17.6% |
| Penn presentation b + c Type A b + c |
Types Ab and Ac together | 40% |
Data from Augoustides JG, Geirsson A, Szeto W, et al: Observational study of mortality risk stratification by ischemic presentation in patients with acute type A aortic dissection: The Penn classification. Nat Clin Pract Cardiovasc Med 6:140, 2009
Type B Aortic Dissection
Aortic dissections confined to the descending thoracic aorta (Stanford type B) should be managed medically unless there are life-threatening complications present such as malperfusion, aortic rupture, as well as severe pain and/or hypertension despite aggressive medical therapy (ACC/AHA Class I recommendation; level of evidence B).1,124 Mortality with medical management in this type of aortic dissection is significantly lower than perioperative mortality (see Table 21-15).1,124,125 The greater operative mortality is due to the severe complications of type B aortic dissection and the operation itself. TEVAR for the therapy of complicated acute type B dissection is highly recommended (STS guideline: Class I recommendation; level of evidence A).1,20
Aortic Intramural Hematoma
Aortic intramural hematoma (IMH) is a variant of the classic aortic dissection (Table 21-18).1,124,125 IMH has no intimal flap with no obvious intimal tear on aortic imaging. This class of intimal tear comprises about 17% of all dissections, with a 30-day mortality rate of 24%: 36% in type A IMH and 12% with type B IMH (P < 0.05).126 Surgical management of type A IMH reduces mortality rate by 61.1% (14% vs. 36%; P = 0.02). Medical management for type B IMH reduced mortality fourfold (8% vs. 33%; P < 0.05).126 Surgical indications in type A IMH include ascending aortic diameter larger than 50 mm or IMH thickness more than 12 mm. Surgical indications in type B IMH include rapid progression, rupture, or severe clinical symptoms despite aggressive medical therapy. It is, therefore, reasonable to manage IMH similar to classic aortic dissection in the corresponding thoracic aortic segment (ACC/AHA Class IIa recommendation; level of evidence C).1 Although IMH may be caused by rupture of the vas vasorum in the aortic wall without intimal hematoma, there are frequently small intimal tears that are beyond the resolution of current aortic scanners and are only identifiable on close aortic inspection during surgery or autopsy.1,127–130
| Diagnostic Criteria |
| Crescent-shaped or circumferential thickening of aortic wall |
| Hematoma thickness > 7 mm |
| No dissection flap |
| No intimal tear |
| No blood flow within hematoma |
| Risk Factors for Mortality or Progression |
| Ascending aorta or arch involvement (type A) |
| Aortic diameter > 45 mm |
| Hematoma thickness > 11 mm |
Clinical Diagnosis and Imaging Studies for Aortic Dissection
Aortic dissection is more common in men and has a peak incidence in later life.1,124,125 It often has an earlier onset in the setting of Marfan syndrome, Ehlers-Danlos syndrome, Loeys-Dietz syndrome, bicuspid aortic valve, aortic coarctation, and familial aortic dissection.1,124,125 It also is commonly associated with hypertension but less so with atherosclerosis.17 Further predisposing factors include pregnancy, cocaine abuse, arteritis, aortic trauma, and aortic instrumentation.1
The pain of aortic dissection typically is severe, abrupt in onset, and has a ripping, tearing, or stabbing quality (ACC/AHA Class I recommendation; level of evidence B).1 Highly suggestive physical findings include a pulse deficit, a systolic blood pressure limb differential greater than 20 mm Hg, focal neurologic deficit, and a new murmur of AR (ACC/AHA Class I recommendation; level of evidence B).1 Besides a chest radiograph (ACC/AHA Class I recommendation; level of evidence C) and an electrocardiogram (ACC/AHA Class I recommendation; level of evidence B), urgent and definitive aortic imaging (TEE, CT, MRI) is strongly recommended in suspected aortic dissection (ACC/AHA Class I recommendation; level of evidence B).1 A negative chest radiograph should not delay aortic imaging, especially in patients suspected of presenting with aortic dissection (ACC/AHA Class I recommendation; level of evidence B).1 The selection of an aortic imaging modality should be guided by patient and institutional variables, including immediate test availability (ACC/AHA Class I recommendation; level of evidence C).1 If initial aortic imaging is negative in the setting of high clinical suspicion for dissection, a second imaging study should be arranged (ACC/AHA Class I recommendation; level of evidence C).1
The most common imaging study is contrast-enhanced spiral CT or CTA because it is widely available. Typical findings in acute aortic dissection include an intimal flap, luminal displacement of intimal calcifications, and aortic dilation (Figure 21-23).124,125 IMH appears as a crescent-shaped high-attenuation thickening of the aortic wall in noncontrast CT (Figure 21-24).124,125 CT can demonstrate rupture, branch-vessel involvement, and false lumen extent. Although MRI has a near 100% sensitivity and specificity, and is widely available, it also takes significantly longer than CT (Figure 21-25).1,124,125
At experienced centers, TEE provides high-resolution aortic images with comparable sensitivity and specificity to MRI and CT.1,124,125 Furthermore, TEE can look for complications by interrogating the aortic valve (AR severity and mechanism), assessing ventricular function including regional wall motion for coronary dissection, and diagnosing cardiac tamponade and pericardial effusion. Aortic dissection appears on TEE examination as an undulating intimal flap within the aorta separating a true and false lumen (Figure 21-26). It is sometimes difficult to distinguish the true lumen from the false lumen, but the true lumen tends to be smaller with pulsatile high-velocity flow in systole. Doppler color-flow imaging can detect flow communication between the true and false lumens at intimal tear sites. Aortic IMH appears as an echo-dense crescent-shaped thickening (>7 mm) of the aortic wall that may contain echolucent pockets of noncommunicating blood (see Figure 21-24). TEE also may distinguish clinical mimickers of aortic dissection and can assess the thoracic aortic anatomy in detail to guide surgical decision making.131,132
Anesthetic Management for Aortic Dissection
Acute aortic dissection is a medical emergency. Acute medical management is directed at treatment of pain and decreasing the arterial pressure with antihypertensive agents. Vasodilator therapy should be initiated to decrease wall stress with control of heart rate and blood pressure (ACC/AHA Class I recommendation; level of evidence C).1 In the presence of acute AR, β-blockers should be used with caution because they block the compensatory tachycardia (ACC/AHA Class I recommendation; level of evidence C).1 In the absence of contraindications, β-blockers should be titrated to a heart rate of 60 beats/min (ACC/AHA Class I recommendation; level of evidence C).1 Esmolol is a particularly useful β-blocker because it has a short pharmacologic half-life and can be rapidly titrated. Esmolol can be administered as an initial bolus of 5 to 25 mg intravenously, followed by a continuous infusion of 25 to 300 μg/kg/min. In patients with β-blocker contraindications, heart rate control should be gained with titration of nondihydropyridine calcium channel blockers such as verapamil or diltiazem (ACC/AHA Class I recommendation; level of evidence C).1 Alternatively, labetalol, a drug that has a 1:7 ratio of β-blocker to α-blocker activity, can be administered as a 20 to 80 mg intravenous bolus followed by an infusion at 0.5 to 2.0 mg/min. Metoprolol, a cardioselective β-blocker, may be advantageous in patients with reactive airway disease who are sensitive to β-adrenergic antagonists. Metoprolol is administered at a dose of 5 to 15 mg intravenously every 4 to 6 hours. If the systolic blood pressure remains greater than 120 mm Hg with adequate heart rate control, then vasodilators (e.g., nitroprusside at a dosage of 0.5 to 2.0 μg/kg/min or nicardipine at a dose of 1 to 15 mg/hr) should be titrated for further reductions of blood pressure while still maintaining adequate vital organ perfusion (ACC/AHA Class I recommendation; level of evidence C).1 Vasodilator therapy should not be initiated before heart rate control to avoid the associated reflex tachycardia that might aggravate the aortic dissection (ACC/AHA Class III recommendation; level of evidence C).1
Surgical Treatment of Stanford Type A Aortic Dissection
Surgical repair for type A aortic dissection requires resection of the proximal extent of the dissection. A partially dissected aortic root may be repaired with aortic valve resuspension. A destroyed aortic root must be replaced with a composite graft or a valve-sparing root replacement. In the setting of a DeBakey type II dissection, the entire dissected aorta merits replacement. CABG sometimes is necessary for aortic dissections that involve the coronary ostia. These surgical principles are all highly recommended (ACC/AHA Class III recommendation; level of evidence C).1
Although femoral arterial cannulation is popular for CPB, recent evidence suggests that it is associated with adverse clinical outcomes including death, stroke, and malperfusion syndromes.133,134 Cannulation of the distal ascending aorta or the axillary artery (ideally with an end-to-end graft) has been associated with significantly enhanced clinical outcome as compared with standard femoral arterial cannulation.133,134 It remains important to monitor cerebral perfusion throughout the operative procedure for detection and correction of acute malperfusion.46–48
In DeBakey type I dissections, the dissected descending thoracic aorta often undergoes aneurysmal degeneration and is responsible for significant aorta-related mortality in the long term.135 Consequently, long-term outcomes after extensive type A dissection would be significantly improved if this distal aortic degeneration could be prevented.135,136 Recent clinical series have demonstrated the efficacy and safety of anterograde stenting of the descending thoracic aorta during open aortic arch repair for DeBakey type 1 aortic dissection.137–139 This technique is also known as the endovascular stented elephant-trunk technique or the frozen elephant-trunk technique. The long-term aneurysmal degeneration of the descending thoracic aorta is prevented by immediate stenting in the acute dissection phase; thus, favorable long-term aortic remodeling is facilitated.
Integrated Management of Stanford Type B Aortic Dissection
Uncomplicated type B aortic dissection currently has the best clinical outcome when managed medically.20 Medical therapy for type B aortic dissection is directed at control of systemic hypertension to prevent aortic aneurysm formation, aortic rupture, and extension of the aortic aneurysm (ACC/AHA Class I recommendation; level of evidence C).1 Combination therapy with a diuretic, β-blocker, angiotensin-converting enzyme inhibitor, or other antihypertensive agents usually is necessary to achieve and maintain blood pressure less than 130/80 mm Hg. All patients after repair of type A aortic dissection also should be managed aggressively with antihypertensive therapy because many are left with a residual distal aortic dissection after repair. Serial imaging of the aorta is necessary to detect expansion of the aortic lumen and aneurysm development that may warrant surgical correction.
In the presence of life-threatening complications, TEVAR has emerged as a preferred alternative therapy to surgery.20,82,140,141 A recent landmark randomized trial demonstrated that, in the short term, TEVAR added no survival advantage over medical management for uncomplicated type B aortic dissection.142 However, because TEVAR did improve aortic remodeling in this trial, further adequately powered trials are indicated to test whether this translates into better aortic outcomes.143 Malperfusion syndromes associated with type B dissection also can be managed with intimal fenestration.144
Penetrating atherosclerotic ulcer
Penetrating atherosclerotic ulcer describes an isolated disruption of the intimal layer of the aortic wall at the site of atheromatous disease (Figure 21-27).1 This class of intimal tear may occur at any aortic locus but is most common in the descending thoracic aorta. It typically is associated with severe aortic atheroma in the elderly and penetrates through to the aortic adventitia.1 It may have associated IMH or pseudoaneurysm.145 Initial symptoms include chest and back pain similar to aortic dissection.1 Diagnosis typically is made with contrast-enhanced CT.146 Although patients may be managed medically, TEVAR has emerged as a major management strategy, especially in severely symptomatic or complicated presentations (STS Class IIa recommendation; level of evidence C).20,147,148 Furthermore, in asymptomatic presentations of this intimal syndrome, TEVAR currently is not recommended (STS Class IIa recommendation; level of evidence C).20
Traumatic aortic injury
The most common cause of traumatic aortic injury is blunt chest trauma or rapid deceleration injuries associated with motor vehicle accidents or falls. Although this injury may be fatal, the majority of patients have injuries in the region of the aortic isthmus.149 Patients with traumatic aortic injury commonly will have associated significant injuries. TEE is helpful in the management of traumatic aortic injury because it is portable, is often available in the OR, provides a rapid diagnosis, and does not require aortic instrumentation or radiographic contrast injection. TEE also can detect cardiac tamponade, left pleural effusion, hypovolemia, ventricular dysfunction from myocardial contusion, or vascular injuries from penetrating chest wounds.150 Its disadvantages include limited imaging in the setting of facial injuries, suspected cervical spine injuries, and lesions in the distal ascending aorta. The characteristic features of traumatic aortic injury detected by TEE are a mural flap at the site of intimal disruption and regional deformities of the aortic wall caused by the contained rupture (Figure 21-28). The mural flap commonly is limited to a 1- to 2-cm aortic segment located at the aortic isthmus just distal to the origin of the left subclavian artery. This mural flap usually is thick compared with the intimal flap seen in aortic dissection and is less mobile because it usually contains several layers of the vessel wall. Contrast-enhanced CT is frequently selected for aortic imaging because these patients typically require CT scanning as part of their initial diagnostic evaluation.151
Injuries to the ascending aorta or aortic arch typically require CPB with DHCA for repair. Injuries to the aortic isthmus can be repaired via a left thoracotomy. The descending thoracic aorta usually is repaired with an interposition graft with the aid of PLHB. The risk for perioperative spinal cord ischemia is minimal when distal aortic perfusion is provided (Table 21-19) because only a short segment of the thoracic aorta is replaced. Although open repair is possible, TEVAR has emerged as the preferred intervention whenever possible because of the well-documented outcome advantages (STS Class I recommendation; level of evidence B).20,151–153
TABLE 21-19 Incidence of Postoperative Paraplegia after Surgical Repair of Traumatic Aortic Injury in Relation to Operative Technique
| Operative Technique | No. of Patients | Paraplegia (%) |
|---|---|---|
| Clamp and sew | 73 | 16.4*† |
| Distal aortic perfusion | 134 | 4.5 |
| Gott shunt | 4 | 0 |
| Full CPB | 22 | 4.5 |
| Partial CPB | 39 | 7.7 |
| Centrifugal pump | 69 | 2.9* |
CPB, cardiopulmonary bypass.
* P < 0.004, distal aortic perfusion vs. clamp and sew.
† P < 0.01, centrifugal pump vs. clamp and sew.
Data from Fabian TC, Richardson JD, Croce MA, et al: Prospective study of blunt aortic injury: Multicenter trial of the American Association for the Surgery of Trauma. J Trauma 42:374, 1997.
Aortic atheromatous disease
Severe aortic atheroma is a major risk factor for stroke.1,20 Thoracic aortic replacement is indicated for serious embolism and to facilitate the conduct of concomitant cardiac procedures that require the safe cross-clamping of the aorta (see Figure 21-27). The anesthetic management of patients undergoing thoracic aortic reconstruction for atheromatous disease resembles the management of thoracic aortic aneurysms for corresponding aortic segments. Intraoperative epiaortic ultrasound imaging is superior to manual palpation or TEE for thoracic aortic atheroma.154,155 The epiaortic ultrasound is important for selecting the optimal site for aortic cannulation for CPB or placement of the aortic cross-clamp to minimize atheroembolic events such as stroke.154,155
Takayasu arteritis
Takayasu arteritis is a chronic vasculitis that affects primarily the thoracic aorta, its branches, and even the pulmonary artery (Figure 21-29).1 Takayasu arteritis occurs most frequently in young Asian women and occurs worldwide. Its onset is insidious with the gradual development of vascular insufficiency. Dilation and aneurysm formation also may occur in diseased aortic segments. Diagnostic criteria include onset of disease at age younger than 40 years, claudication of the extremities, decreased brachial pulses, a systolic blood pressure differential of 10 mm Hg between the arms, subclavian or abdominal aortic bruits, and angiographic demonstration of narrowing of the aorta and/or its primary branches.1
Initial therapy consists of high-dose corticosteroids (ACC/AHA Class I recommendation; level of evidence B).1 Elective revascularization should be postponed until the acute inflammatory state has been adequately treated (ACC/AHA Class I recommendation; level of evidence B).1 Anesthetic management of Takayasu arteritis is complicated by limited sites for arterial blood pressure measurement. The femoral artery may be the only site for accurate measurement of central aortic pressure in patients with stenosis of both subclavian arteries.
Aortic coarctation
Aortic coarctation is a common malformation that ranges from a discrete narrowing of the aorta to a long hypoplastic segment of the vessel to complete discontinuity of the aorta (Figure 21-30).156 The site of coarctation can be variable but is typically juxtaductal in location, affecting the proximal descending thoracic aorta just distal to the origin of the left subclavian artery. The distal descending aorta often is hypoplastic in severe cases. Conditions associated with aortic coarctation include Turner syndrome, bicuspid aortic valve, ventricular septal defect, patent ductus arteriosus, and intracerebral aneurysm.1,156
Figure 21-30 Three-dimensional reconstruction from a computed tomographic angiogram of a patient with aortic coarctation.
Adults with coarctation may present with headache, epistaxis, heart failure, or lower extremity claudication. Its typical hemodynamic profile is upper extremity hypertension combined with lower extremity hypotension and weak pulses. If the origin of the left subclavian artery is distal to the coarctation, blood pressure in the arms also may be diminished. The chest radiograph often shows rib notching caused by the enlarged intercostal arteries that serve as collateral vessels to supply the lower body. The electrocardiogram often shows left ventricular hypertrophy. The diagnosis is confirmed by definitive aortic imaging. Cardiac catheterization, MRI, and echocardiography are useful to detect associated cardiac abnormalities (see Chapter 20).
Balloon angioplasty with stenting is the preferred treatment when coarctation is limited to a discrete segment of the aorta.156,157 Although sedation often will suffice for the procedure, general anesthesia may be required when dilation of the coarctation is expected to be painful or when it is necessary to keep the patient immobile for the procedure. Complications of balloon angioplasty have included residual stenosis, recoarctation, paracoarctation aortic dissection or rupture, aortic aneurysm, and injury to the femoral artery. Aortic dissection or aneurysm at or near the angioplasty site may be a consequence of mechanical damage to the aortic wall or congenital defects of the aortic wall.
Operative repair in adults may involve interposition graft repair or extra-anatomic bypass grafting from the proximal aorta or left subclavian artery to the descending aorta. Extra-anatomic bypass is advantageous for reoperations or in adults when the distal aortic arch cannot be mobilized to perform an end-to-end anastomosis. Avoiding surgical dissection in the region of the distal aortic arch also decreases the risk for injury to the recurrent laryngeal and phrenic nerves. Although perioperative mortality is low, spinal cord ischemia is possible during repair if collateral circulation is inadequate or if distal aortic perfusion pressure is too low. Preventive strategies include intraoperative monitoring of distal aortic perfusion pressure, neuromonitoring with SSEPs and/or MEPs, deliberate hypothermia, and distal aortic perfusion with PLHB.158 Postoperative hypertension after repair may require aggressive monitoring and management.
Illustrative transesophageal echocardiography cases
Thoracic Aortic Atheroma
Data Collection
Severe thoracic aortic atheroma is characterized by atheroma protrusion larger than 5 mm into the aortic lumen or mobile atheroma, or both. Severe protruding and mobile atheroma, as depicted in Figures 21-31 to 21-35, disseminated throughout the descending thoracic aorta and aortic arch, make it likely that the ascending aorta also is involved with severe atheroma because advanced atheroma is a disseminated arterial process. The detection of severe thoracic aortic atheroma by TEE, therefore, represents a major opportunity to modify surgical decision making to minimize stroke risk in the setting of heavy aortic atheroma burden.
Figure 21-32 Short-axis view of the descending thoracic aorta.
In this aortic segment, there is also severe sessile aortic atheroma with a diameter greater than 5 mm. Notice the variation in morphology as compared with another descending thoracic aortic segment, as in Figure 21-31. When severe atheroma are detected, it is important to comprehensively evaluate the entire thoracic aorta as in this patient. Multiple thoracic aortic views demonstrate severe aortic atheroma. It is clear that this patient has a severe atheroma burden based not only on the severity but also on the extent.
Figure 21-33 Short-axis view of the descending thoracic aorta.
Again, there is severe aortic atheroma with variations in morphology. There is a pedunculated mobile atheroma that is at high risk for embolism downstream in the aorta. Taken together, Figures 21-31 through 21-33 show that this patient has a heavy atheroma burden throughout his descending thoracic aorta. Because atheroma is seldom a selective disease process, this atheroma burden is likely in the ascending aorta and aortic arch.
Discussion
Acute Thoracic Aortic Dissection
Data Collection
There is type A dissection that requires surgical intervention (Figures 21-36 to 21-41). The aortic root is dilated and dissected. The ascending aorta is dilated and dissected with extension into the aortic arch and descending thoracic aorta (DeBakey I extent) (See Videos 3 and 4). Although there is severe AR, the mechanisms are intimal prolapse and aortic root dilation without native aortic cusp destruction. There are no regional wall motion abnormalities to suggest myocardial ischemia from coronary dissection. There is a pericardial tamponade.
Figure 21-37 Midesophageal short-axis view of the descending thoracic aorta (at zero degree of rotation).
Figure 21-38 Intraoperative photograph of the ascending aorta after sternotomy and pericardiotomy.
The ascending aorta is dissected: The wall is hemorrhagic and in places transparent through the adventitia (area indicated within the black circle). Aortic rupture was imminent in this case. The decision to operate emergently and proceed with proximal thoracic aortic replacement on cardiopulmonary bypass with deep hypothermic circulatory arrest was based on the transesophageal echocardiographic findings, as shown in Figures 21-36 and 21-37.
Figure 21-39 Midesophageal short-axis view of the aortic valve in diastole (at 27 degrees of rotation).
The aortic leaflets appear normal. There is prolapse of the intimal dissection flap through the aortic valve, preventing diastolic coaptation and giving the appearance of a “double aortic valve.” Severe aortic regurgitation results from this mechanism, as shown in Figure 21-41. This view also allows for assessment of right ventricular function, in particular, right ventricular hypokinesis caused by ischemia from right coronary dissection. Color-flow Doppler interrogation in this view shows trace pulmonic insufficiency, a common incidental finding. There is also a small pericardial collection evident: This is the residual from the hemopericardium that was drained at pericardiotomy. Before pericardiotomy, the hemopericardium was under tension and had resulted in severe cardiac tamponade.
An intimal dissection flap in the ascending aorta has a large fenestration. The sinotubular junction is splayed and dilated because of the dissection. This is a second mechanism for aortic regurgitation, as the dilated aortic root will result in diastolic aortic cusp separation. In this case, the intimal flap is also responsible for the severe aortic insufficiency, as shown in Figure 21-41.
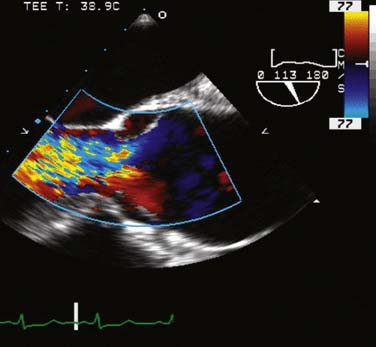
Color-flow Doppler interrogation reveals severe aortic regurgitation, as the diastolic jet visualized by color Doppler imaging fills the entire left ventricular outflow tract. The mechanisms of this severe aortic regurgitation, namely, intimal prolapse and aortic root dilation, are more fully explained in Figures 21-39 and 21-40.
Thoracic Aortic Transection
Data Collection
The TEE examination confirms thoracic aortic transection localized to the aortic arch with brachiocephalic involvement (Figures 21-42 to 21-48). The ascending aorta and descending thoracic aorta, including the isthmus, are not involved. In other words, there is no extension either proximal or distal to the aortic arch. This transection and associated IMH involve most of the aortic arch. There is contained rupture of the aortic arch.
Discussion
The TEE examination suggests that the aortic arch requires extensive acute repair. Although acute endovascular aortic arch repair may be possible in the future, acute total arch endovascular repair is not currently part of standard thoracic aortic endovascular intervention. The current endovascular technology still consists mainly of tubular components with no fenestrations or branches. Given that this is a young patient with minimal comorbidities, the thoracic aorta was accessed anteriorly via sternotomy, and a total aortic arch repair with CPB and DHCA was performed (Figures 21-49 and 21-50). If the patient was elderly with multiple comorbidities, a hybrid aortic arch repair might have been considered. In this procedure, the brachiocephalic arteries are transposed to the ascending aorta to create the landing zone for aortic arch stenting. This procedure is typically performed off-pump via sternotomy: Stent deployment can be anterograde through the ascending aorta or retrograde via the femoral artery.
Bicuspid Aortic Valve
A 43-year-old man presented with progressive heart failure. His father had undergone aortic valve replacement at 50 years of age. His physical examination was compatible with advanced AR. Transthoracic echocardiography showed severe AR and a possible bicuspid valve. Coronary catheterization excluded significant CAD. He was referred for aortic valve surgery. The patient expressed a strong preference to avoid chronic anticoagulation after surgery. He requested aortic valve repair (see Chapter 19).
Data Collection
The aortic valve is bicuspid (Figures 21-51 to 21-58). There is no detectable aortic valve calcification and no aortic stenosis. There is severe eccentric AR because of anterior leaflet prolapse in the region of the raphe. Although mildly dilated, the aortic root does not qualify for replacement at this point. The ascending aorta is not dissected but has a diameter at the level of the pulmonary artery of 43 mm.
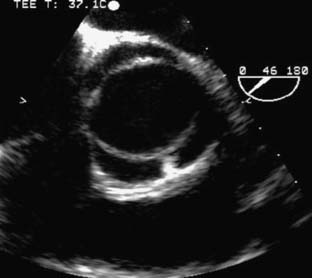
Figure 21-51 Midesophageal short-axis view of the aortic valve in systole (imaging angle of 46 degrees).
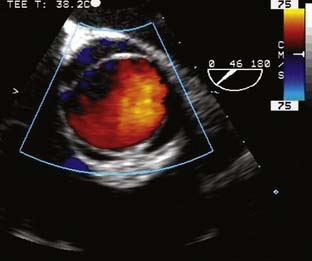
Figure 21-52 Midesophageal short-axis view of the aortic valve in systole (imaging angle of 46 degrees).
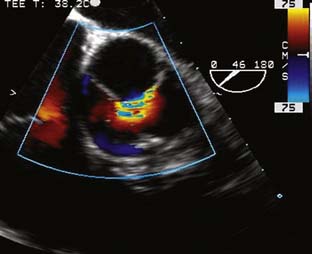
Figure 21-54 Midesophageal short-axis view of the aortic valve in diastole (imaging angle of 46 degrees).
Color-flow Doppler imaging shows central commissural aortic regurgitation at the midpoint of the anterior cusp. This location of the regurgitation suggests focal prolapse of an aortic cusp. When considered together with the posterior eccentric flow evident in Figure 21-53, the mechanism for the aortic regurgitation in this case is focal prolapse of the anterior cusp in the region of the raphe. This identification of this focal mechanism suggests the possibility of a focal aortic valve repair.
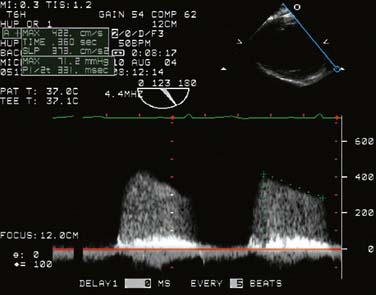
Figure 21-55 Transgastric long-axis view of the aortic valve (imaging angle of 123 degrees).
Continuous-wave Doppler interrogation of the aortic valve shows significant aortic regurgitation (flow above the baseline). The pressure half-time has been quantified at 331 milliseconds, consistent with moderate aortic insufficiency. However, the final grading of the aortic insufficiency was severe when considered together with color-flow Doppler in Figure 21-53 and effects on the left ventricle in Figure 21-56. Because the jet of insufficiency is eccentric, it is possible to underestimate the peak diastolic velocity with continuous-wave Doppler. This is the probable explanation for the underestimation of the aortic regurgitation by the pressure half-time method. This underlines the importance of quantifying the valve lesion by multiple methods.
Figure 21-56 Transgastric midpapillary short-axis view of the left ventricle at end-diastole (imaging angle of zero degree).
1 Hiratzka L.F., Bakris G.L., Beckman J.A., et al. ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: Executive summary. A report of the American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55:1509.
2 Corbet A.J. Medical manipulation of the ductus arteriosus. In: Garson A., Bricker T., Fisher D., Neish S., editors. The Science and Practice of Pediatric Cardiology. ed 2. Baltimore: Williams & Wilkins; 1997:2489.
3 Kern M.J., Serota H., Callicoat P., et al. Use of coronary arteriography in the preoperative management of patients undergoing urgent repair of the thoracic aorta. Am Heart J. 1990;119:143.
4 Sanders J.S., Ferguson D.W., Mark A.L. Arterial baroreflex control of sympathetic nerve activity during elevation of blood pressure in normal man: Dominance of aortic baroreflexes. Circulation. 1988;77:279.
5 Miceli A., Capoun R., Fino C., et al. Effects of angiotensin-converting enzyme inhibitor therapy on clinical outcome in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2009;54:1778.
6 Augoustides J.G. Should all antihypertensive agents be continued before surgery? In: Fleisher L.A., editor. Evidence-Based Practice of Anesthesiology. ed 2. Philadelphia: Saunders Elsevier; 2009:49.
7 Horlocker T.T., Wedel D.J., Rowlingson J.C., et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy. Reg Anesth Pain Med. 2010;35:64.
8 Augoustides J.G., Cheung A.T. Pro: Ultrasound should be the standard of care for central catheter insertion. J Cardiothorac Vasc Anesth. 2009;23:720.
9 Fergusson D.A., Herbert P.C., Mazer C.D., et al. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;358:2319.
10 Augoustides J.G. Aprotinin and renal dysfunction: What does history teach us? Expert Opin Drug Saf. 2009;8:5.
11 Hgaage D.L., Bland J.M. Lessons from aprotinin: Is the routine use and inconsistent dosing of tranexamic acid prudent? Meta-analysis of randomized and large matched observational studies. Eur J Cardiothorac Surg. 2010;37:1375-1383.
12 Murkin J.M., Falter F., Granton J., et al. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010;110:350.
13 Tritapepe L., De Santis V., Vitale D., et al. Recombinant activated Factor VII for refractory bleeding after acute aortic dissection surgery: A propensity score analysis. Crit Care Med. 2007;35:1685.
14 Zangrillo A., Mizzi A., Biondi-Zoccai G., et al. Recombinant activated factor VII in cardiac surgery: A meta-analysis. J Cardiothorac Vasc Anesth. 2009;23:34.
15 Appoo J.J., Augoustides J.G., Pochettino A., et al. Perioperative outcome in adults undergoing elective deep hypothermic circulatory arrest with retrograde cerebral perfusion in proximal aortic arch repair: Evaluation of protocol-based care. J Cardiothorac Vasc Anesth. 2009;20:3.
16 Lazar H.L., McDonnell M., Chipkin S.R., et al. The Society of Thoracic Surgeons practice guideline series: Blood glucose management during adult cardiac surgery. Ann Thorac Surg. 2009;87:663.
17 Elefteriades J.A., Farkas E.A. Thoracic aortic aneurysm. J Am Coll Cardiol. 2010;55:841.
18 Albornoz G., Coady M.A., Roberts M., et al. Familial thoracic aortic aneurysms and dissections—incidence, modes of inheritance, and phenotypic patterns. Ann Thorac Surg. 2006;82:1400.
19 Augoustides J.G., Wolfe Y., Walsh E.K., et al. Recent advances in aortic valve disease: Highlights from a bicuspid aortic valve to transcatheter aortic valve replacement. J Cardiothorac Vasc Anesth. 2009;23:569.
20 Svensson L.G., Kouchoukos N.T., Miller D.C., et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent grafts. Ann Thorac Surg. 2008;85:S1.
21 Sinha A., Cheung A.T. Spinal cord protection and thoracic aortic surgery. Curr Opin Anaesthesiol. 2010;23:195.
22 Brown J.R., Block C.A., Malenka D.J., et al. Sodium bicarbonate plus N-acetylcysteine prophylaxis: A meta-analysis. JACC Cardiovasc Interv. 2009;2:1116.
23 Mahajan A., Crowley R., Ho J.K., et al. Imaging the ascending aorta and aortic arch using transesophageal echocardiography: The expanded aortic view. Echocardiography. 2008;25:408.
24 Nierich A.P., van Zaane B., Buhre W.F., et al. Visualization of the distal ascending aorta with A-mode transesophageal echocardiography. J Cardiothorac Vasc Anesth. 2008;22:766.
25 Augoustides J.G., Hosalkar H.H., Milas B.L., et al. Upper gastrointestinal injuries related to perioperative transesophageal echocardiography: Index case, classification proposal, and call for a registry. J Cardiothorac Vasc Anesth. 2006;20:379.
26 Augoustides J.G., Plappert T., Bavaria J.E. Aortic decision-making in the Loeys-Dietz syndrome: Aortic root aneurysm and a normal-caliber ascending aorta and aortic arch. J Thorac Cardiovasc Surg. 2009;138:502.
27 Davies R.R., Gallo A., Coady M.A., et al. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg. 2006;81:169.
28 Song H.K., Bavaria J.E., Kindem M.W., et al. Surgical treatment of patients enrolled in the national registry of genetically triggered thoracic aortic conditions. Ann Thorac Surg. 2009;88:781.
29 Toda K., Taniguchi K., Masai T., et al. Arch aneurysm repair with long elephant trunk: A 10-year experience in 111 patients. Ann Thorac Surg. 2009;88:16.
30 Spielvogel D., Etz C.D., Silovitz D., et al. Aortic arch replacement with a trifurcated graft. Ann Thorac Surg. 2007;83:S791.
31 Stein L.H., Elefteriades J.A. Protecting the brain during aortic surgery: An enduring debate with unanswered questions. J Cardiothorac Vasc Anesth. 2010;24:316-321.
32 Harrington D.K., Fragomeni F., Bonser R.S. Cerebral perfusion. Ann Thorac Surg. 2007;83:S799.
33 Abdul Aziz K.A., Meduoye A. Is pH-stat or alpha-stat the best technique to follow in patients undergoing deep hypothermic circulatory arrest? Interact Cardiovasc Thorac Surg. 2010;10:271.
34 Cheung A.T., Bavaria J.E., Pochettino A., et al. Oxygen delivery during retrograde cerebral perfusion in humans. Anesth Analg. 1999;88:8.
35 Stecker M.M., Cheung A.T., Pochettino A., et al. Deep hypothermic circulatory arrest: I. Effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg. 2001;71:14.
36 Shuhaiber J.H. Evaluating the quality of trials of hypothermic circulatory arrest aortic surgery. Asian Cardiovasc Thorac Ann. 2007;15:449.
37 Reich D.L., Horn M., Hossain S., et al. Using jugular bulb oxyhemoglobin saturation to guide onset of deep hypothermic circulatory arrest does not predict post-operative neuropsychological function. Eur J Cardiothorac Surg. 2004;25:401.
38 Percy A., Widman S., Rizzo J.A., et al. Deep hypothermic circulatory arrest with high cognitive needs: Full preservation of cognitive abilities. Ann Thorac Surg. 2009;87:117.
39 Gega A., Rizzo J.A., Johnson M.H., et al. Straight deep hypothermic arrest: Experience in 394 patients supports its effectiveness as a sole means of brain preservation. Ann Thorac Surg. 2007;84:759.
40 Pochettino A., Cheung A.T. Pro: Retrograde cerebral perfusion is useful for deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth. 2003;17:764.
41 Augoustides J.G., Andritsos M. Innovations in aortic disease: The ascending aorta and aortic arch. J Cardiothorac Vasc Anesth. 2010;24:198-207.
42 Estrera A.L., Miller C.C., Lee T.Y., et al. Ascending and transverse aortic arch repair: The impact of retrograde cerebral perfusion. Circulation. 2008;118:S160.
43 Etz C.D., Plestis K.A., Kan F.A., et al. Axillary cannulation significantly improves survival and neurologic outcome after atherosclerotic aneurysm repair of the aortic root and ascending aorta. Ann Thorac Surg. 2008;86:441.
44 Urbanski P.P., Lenos A., Blume J.C., et al. Does anatomical completeness of the circle of Willis correlate with sufficient cross-perfusion during unilateral cerebral perfusion. Eur J Cardiothorac Surg. 2008;33:402.
45 Morita S., Yasaka M., Yasumori K., et al. Transcranial Doppler study to assess intracranial arterial communication before aortic arch operation. Ann Thorac Surg. 2008;86:448.
46 Murkin J.M. NIRS: A standard of care for CPB vs an evolving standard for selective cerebral perfusion? J Extra Corpor Technol. 2009;41:11.
47 Augoustides J.G., Kohl B.A., Harria H., et al. Color-flow Doppler recognition of intraoperative brachiocephalic malperfusion during operative repair of acute type A aortic dissection: Utility of transcutaneous carotid artery ultrasound scanning. J Cardiothorac Vasc Anesth. 2007;21:81.
48 Estrera A.L., Garami Z., Miller C.C., et al. Cerebral monitoring with transcranial Doppler ultrasonography improves neurologic outcome during repairs of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2005;129:277.
49 Kazui T. Which is more appropriate as a cerebral protection method—unilateral or bilateral perfusion? Eur J Cardiothorac Surg. 2006;29:1039.
50 Malvindi P.G., Scrascia G., Vitale N. Is unilateral antegrade cerebral perfusion equivalent to bilateral cerebral perfusion for patients undergoing aortic arch surgery? Interact Cardiovasc Thorac Surg. 2008;7:891.
51 Cook R.C., Goa M., Macnab A.J., et al. Aortic arch reconstruction: Safety of moderate hypothermia and antegrade cerebral perfusion during systemic circulatory arrest. J Card Surg. 2006;21:158.
52 Kamiya H., Hagl C., Kropivnitskaya I., et al. The safety of moderate hypothermic lower body circulatory arrest with selective perfusion: A propensity score analysis. J Thorac Cardiovasc Surg. 2007;133:501.
53 Khaladji N., Shrestha M., Meck S., et al. Hypothermic circulatory arrest with selective antegrade cerebral perfusion in ascending and aortic arch surgery: A risk factor analysis for adverse outcome in 501 patients. J Thorac Cardiovasc Surg. 2008;135:908.
54 Minatoya K., Ogino H., Matsuda H., et al. Evolving selective cerebral perfusion for aortic arch replacement: High flow rate with moderate hypothermic circulatory arrest. Ann Thorac Surg. 2008;86:1827.
55 Etz C.D., Luehr M., Kari F.A., et al. Selective cerebral perfusion at 28 degrees C—is the spinal cord safe? Eur J Cardiothoracic Surg. 2009;36:946.
56 Ohno M., Omoto T., Fukuzumi M., et al. Hypothermic circulatory arrest: Renal protection by atrial natriuretic peptide. Asian Cardioavsc Thorac Ann. 2009;17:401.
57 Dewhurst A.T., Moore S.J., Liban J.B. Pharmacological agents as cerebral protectants during deep hypothermic circulatory arrest in adult thoracic aortic surgery: A survey of current practice. Anaesthesia. 2002;57:1016.
58 Cowan J.A.Jr, Dimick J.B., Henke P.K., et al. Surgical treatment of intact thoracoabdominal aortic aneurysms in the United States: Hospital and surgeon volume-related outcomes. J Vasc Surg. 2003;37:1169.
59 Livesay J.J., Cooley D.A., Ventemiglia R.A., et al. Surgical experience in descending thoracic aneurysmectomy with and without adjuncts to avoid ischemia. Ann Thorac Surg. 1985;39:37.
60 Roizen M.F., Beaupre P.N., Alpert R.A., et al. Monitoring with two-dimensional transesophageal echocardiography: Comparison of myocardial function in patients undergoing supraceliac, suprarenal-infraceliac, or infrarenal aortic occlusion. J Vasc Surg. 1984;1:300.
61 Coselli J.S., Bozinovski J., LeMaire S.A. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg. 2007;83:S862.
62 Tabayashi K., Motoyoshi N., Saiki Y., et al. Efficacy of perfusion cooling of the epidural space and cerebrospinal fluid drainage during repair of extent I and extent II thoracoabdominal aneurysms. J Cardiovasc Surg (Torino). 2008;49:749.
63 Verdant A. Contemporary results of standard open repair of acute traumatic rupture of the thoracic aorta. J Vasc Surg. 2010;51:294.
64 Cheung A.T., Pochettino A., Guvakov D.V., et al. Safety of lumbar drains in thoracic aortic operations performed with extracorporeal circulation. Ann Thorac Surg. 2003;76:1190.
65 Kulik A., Castner C.F., Kouchoukos N.T. Replacement of the descending thoracic aorta: Contemporary outcomes using hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2010;139:249.
66 Etz C.D., Plestis K.A., Kari F.A., et al. Staged repair of thoracic and thoracoabdominal aortic aneurysms using the elephant trunk technique: A consecutive series of 215 first stage and 120 complete repairs. Eur J Cardiothorac Surg. 2008;34:605.
67 Dunning J., Martin J.E., Shennib H., et al. Is it safe to cover the left subclavian artery when placing an endovascular stent in the descending thoracic aorta? Interact Cardiovasc Thorac Surg. 2008;7:690.
68 Rizvi A.Z., Murad M.H., Fairman R.M., et al. The effect of left subclavian artery coverage on morbidity and mortality in patients undergoing thoracic aortic interventions: A systematic review and meta-analysis. J Vasc Surg. 2009;50:1159.
69 Cooper D.G., Walsh S.R., Sadat U., et al. Neurological complications after left subclavian artery coverage during thoracic endovascular aortic repair: A systematic review and meta-analysis. J Vasc Surg. 2009;49:1594.
70 Matsumara J.S., Lee W.A., Mitchell R.S., et al. The Society of Vascular Surgery practice guidelines: Management of the left subclavian artery with thoracic endovascular repair. J Vasc Surg. 2009;50:1155.
71 Gilling-Smith G.L., McWilliams R.G., Scurr J.R., et al. Wholly endovascular repair of thoracoabdominal aneurysm. Br J Surg. 2008;95:703.
72 D’Elia P., Tyrrell M., Sobocinski J., et al. Endovascular thoracoabdominal aortic aneurysm repair: A literature review of early and mid-term results. J Cardiovasc Surg (Torino). 2009;50:439.
73 Haulon S., D’Elia P., O’Brien N., et al. Endovascular repair of thoracoabdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2010;39:171.
74 Bakoyiannis C., Kalles V., Economopoulos K., et al. Hybrid procedures in the treatment of thoracoabdominal aortic aneurysms: A systematic review. J Endovasc Ther. 2009;16:443.
75 Drinkwater S.L., Bockler D., Eckstein H., et al. The visceral hybrid repair of thoracoabdominal aortic aneurysms—a collaborative approach. Eur J Vasc Endovasc Surg. 2009;38:578.
76 Patel R., Conrad M.F., Parachuri V., et al. Thoracoabdominal aneurysm repair: Hybrid versus open repair. J Vasc Surg. 2009;50:15.
77 Chiesa R., Tshiomba Y., Melissano G., et al. Is hybrid procedure the best treatment option for thoracoabdominal aortic aneurysm? Eur J Vasc Endovasc Surg. 2009;38:26.
78 Biasi L., Ali T., Loosemore T., et al. Hybrid repair of complex thoracoabdominal aortic aneurysms using applied endovascular strategies combined with visceral and renal revascularizations. J Thorac Cardiovasc Surg. 2009;138:133.
79 Szeto W.Y., Bavaria J.E. Hybrid repair of aortic arch aneurysms: Combined open arch reconstruction and endovascular repair. Semin Thorac cardiovasc Surg. 2009;21:1347.
80 Szeto W.Y., Moser W.G., Desai N.D., et al. Transapical deployment of endovascular thoracic aortic stent graft for an ascending aortic pseudoaneurysm. Ann Thorac Surg. 2010;89:616.
81 Szeto Wy, Fairman R.M., Acker M.A., et al. Emergency endovascular deployment of stent graft in the ascending aorta for contained rupture of innominate artery pseudoaneurysm in a pediatric patient. Ann Thorac Surg. 2006;81:1872.
82 Cheng D., Martin J., Shennib H., et al. Endovascular aortic repair versus open surgical repair for descending thoracic aortic disease: A systematic review and meta-analysis of comparative studies. J Am Coll Cardiol. 2010;55:986.
83 Campos J.H. Lung isolation techniques for patients with difficult airway. Curr Opin Anesthesiol. 2010;23:12.
84 Augoustides J.G. Esophageal placement of an airway exchange catheter. J Cardiothorac Vasc Anesth. 2007;21:773.
85 Sinha A.C., Cheung A.T. Spinal cord protection and thoracic aortic surgery. Curr Opin Anaesthesiol. 2010;23:95.
86 Backes W.H., Nijenhuis R.J., Mess W.H., et al. Magnetic resonance angiography of collateral blood supply to spinal cord in thoracic and thoracoabdominal aortic aneurysm patients. J Vasc Surg. 2008;48:261.
87 Nojiri J., Matsumoto K., Kato A., et al. The Adamkiewicz artery: Demonstration by intra-arterial computed tomographic angiography. Eur J Cardiothorac Surg. 2007;31:249.
88 Boll D.T., Bulow H., Blackham K.A., et al. MDCT angiography of the spinal vasculature and the artery of Adamkiewicz. AJR Am J Roentgenol. 2006;187:1054.
89 Greenberg R.K., Lu Q., Roselli E., et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: A comparison of endovascular and open techniques. Circulation. 2008;118:808.
90 Cheung A.T., Weiss S.J., McGarvey M.L., et al. Interventions for reversing delayed-onset postoperative paraplegia after thoracic aortic reconstruction. Ann Thorac Surg. 2002;74:413.
91 Kawanishi Y., Okada K., Matsumori M., et al. Influence of perioperative hemodynamics on spinal cord ischemia in thoracoabdominal aortic repair. Ann Thorac Surg. 2007;84:488.
92 Ling E., Arellano R. Systematic overview of the evidence supporting the use of cerebrospinal fluid drainage in thoracoabdominal aneurysm surgery for prevention of paraplegia. Anesthesiology. 2000;93:1115.
93 Khan S.N., Stansby G. Cerebrospinal fluid drainage in thoracic and thoracoabdominal aneurysm surgery. Cochrane Database Syst Rev. 1, 2004. CD003635
94 Cina C.S., Abouzahr L., Arena G.O., et al. Cerebrospinal fluid drainage to prevent paraplegia during thoracic and thoracoabdominal aneurysm surgery: A systematic review and meta-analysis. J Vasc Surg. 2004;40:36.
95 Acher C.W., Wynn M. A modern theory of paraplegia in the treatment of aneurysms of the thoracoabdominal aorta: An analysis of technique specific observed/expected ratios for paralysis. J Vasc Surg. 2009;49:1117.
96 Sethi M., Grigore A.M., Davison J.K. Pro: It is safe to proceed with thoracoabdominal aneurysm surgery after encountering a bloody tap during cerebrospinal fluid catheter placement. J Cardiothorac Vasc Anesth. 2006;20:269.
97 Wynn M.M., Mittnacht A., Norris E. Con: Surgery should not proceed when a bloody tap occurs during spinal drain placement for elective thoracoabdominal aortic surgery. J Cardiothorac Vasc Anesth. 2006;20:273.
98 Estrera Al R., Sheinbaum, Miller C.C.III, et al. Cerebrospinal fluid drainage during thoracic aortic repair: Safety and current management. Ann Thorac Surg. 2009;88:9.
99 Wynn M.M., Mell M.W., Tefera G., et al. Complications of spinal fluid drainage in thoracoabdominal aortic aneurysm repair: A report of 486 patients treated from 1987 to 2008. J Vasc Surg. 2009;49:29.
100 Griepp R.B., Griep E.B. Spinal cord perfusion and protection during descending thoracic and thoracoabdominal aortic surgery: The collateral network concept. Ann Thorac Surg. 2007;83:S865.
101 Etz C.D., Luehr M., Kari F.A., et al. Paraplegia after extensive thoracic and thoracoabdominal aortic aneurysm repair: Does critical spinal cord ischemia occur postoperatively? J Thorac Cardiovasc Surg. 2008;135:324.
102 Woo E.Y., McGarvey M., Jackson B.M., et al. Spinal cord ischemia may be reduced via a novel technique of intercostal artery revascularization during open thoracoabdominal aneurysm repair. J Vasc Surg. 2007;46:421.
103 Kawaharada N., Ito T., Koyanagi T., et al. Spinal cord protection with selective spinal perfusion during descending thoracic and thoracoabdominal aortic surgery. Interact Cardiovasc Thorac Surg. 2010;10:986-990. discussion 990–991
104 McGarvey M.I., Cheung A.T., Szeto W., et al. management of neurologic complications of thoracic aortic surgery. J Clin Neurophysiol. 2007;24:336.
105 Jacobs M.J., Mess W., Mochtar B., et al. The value of motor evoked potentials in reducing paraplegia during thoracoabdominal aneurysm repair. J Vasc Surg. 2006;43:239.
106 Sloan T.B., Jameson L.C. Electrophysiologic monitoring during surgery to repair the thoracoabdominal aorta. J Clin Neurophysiol. 2007;24:316.
107 Achouh P.E., Estrera A.L., Miller C.C.III, et al. Role of somatosensory evoked potentials during thoracic and thoracoabdominal aneurysm repair. Ann Thorac Surg. 2007;84:782.
108 Keyhani K., Miller C.C.III, Estrera A.L., et al. Analysis of motor and somatosensory evoked potentials during thoracic and thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2009;49:36.
109 Motoyoshi N., Takahashi G., Sakurai M., et al. Safety and efficacy of epidural cooling for regional spinal cord hypothermia during thoracoabdominal aneurysm repair. Eur J Cardiothorac Surg. 2004;25:139.
110 Tabayashi K., Motoyoshi N., Saiki Y., et al. Efficacy of perfusion cooling of the epidural space and cerebrospinal fluid drainage during repair of extent I and II thoracoabdominal aortic aneurysm. J Cardiovasc Surg (Torino). 2008;49:7499.
111 Shimizu H., Mori A., Yamada T., et al. Regional spinal cord cooling using a countercurrent closed-lumen epidural catheter. Ann Thorac Surg. 2010;89:1312.
112 Reece T.B., Kern J.A., Tribble C.G., et al. The role of pharmacology in spinal cord protection during thoracic aortic reconstruction. Semin Thorac Cardiovasc Surg. 2003;15:365.
113 Kohno H., Ishida A., Imamaki M., et al. Efficacy and vasodilatory benefit of magnesium prophylaxis for protection against spinal cord ischemia. Ann Vasc Surg. 2007;21:352.
114 Hagiwari S., Saima S., Negishi K., et al. High incidence of renal failure in patients with aortic aneurysms. Nephrol Dial Transplant. 2007;22:1361.
115 Koksoy C., Lemaire S.A., Curling P.E., et al. Renal perfusion during thoracoabdominal aortic operations: Cold crystalloid is superior to normothermic blood. Ann Thorac Surg. 2002;73:730-738.
116 Lemaire S.A., Jones M.M., Conklin L.D., et al. Randomized comparison of cold blood and cold crystalloid renal perfusion for renal protection during thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2009;49:11.
117 Miller C.C.III, Villa M.A., Sutton J., et al. Serum myoglobin and renal morbidity and mortality following thoracic and thoracoabdominal aortic repair: Does rhabdomyolysis play a role? Eur J Endovasc Surg. 2009;37:388.
118 Miller C.C.III, Villa M.A., Achouch P., et al. Intraoperative skeletal muscle ischemia contributes to risk of renal dysfunction following thoracoabdominal aortic repair. Eur J Cardiothorac Surg. 2008;33:691.
119 Miller C.C.III, Grimm J.C., Estrera A.L., et al. Postoperative renal function preservation with nonischemic femoral arterial cannulation for thoracoabdominal aortic repair. J Vasc Surg. 2010;51:38.
120 Popping D.M., Elia N., Marret E., et al. Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery: A meta-analysis. Arch Surg. 2008;143:990.
121 Gutsche J.T., Szeto W., Cheung A.T. Endovascular stenting of thoracic aneurysm. Anesthesiol Clin. 2008;26:481.
122 Gutsche J.T., Cheung A.T., McGarvey M.L., et al. Risk factors for perioperative stroke after thoracic endovascular aortic repair. Ann Thorac Surg. 2007;84:1195.
123 Cheung A.T., Pochettino A., McGarvey M.L., et al. Strategies to manage paraplegia risk after endovascular stent repair of descending thoracic aortic aneurysms. Ann Thorac Surg. 2005;80:1280-1288. discussion 1288–1289
124 Golledge J., Eagle K.A. Acute aortic dissection. Lancet. 2008;372:55.
125 Ramanath V.S., Oti J.K., Sundt T.M.3rd, et al. Acute aortic syndromes and thoracic aortic aneurysm. Mayo Clin Proc. 2009;84:465.
126 Trimarchi S., Eagle K.A., Nienaber C.A., et al. Role of age in acute type A aortic dissection outcome: Report from the International Registry of Acute Aortic Dissection (IRAD). J Thorac Cardiovasc Surg. 2010;140:784.
127 Augoustides J.G., Geirsson A., Szeto W., et al. Observational study of mortality risk stratification by ischemic presentation in patients with acute type A aortic dissection: The Penn classification. Nat Clin Pract Cardiovasc Med. 2009;6:140.
128 Attia R., Young C., Fallouh H.B., et al. In patients with acute aortic intramural haematoma is open surgical repair superior to conservative management? Interact Cardiovasc Thorac Surg. 2009;9:868.
129 Park K.H., Lim C., Chopi J.H., et al. Prevalence of aortic intimal defect in surgically treated type A intramural hematoma. Ann Thorac Surg. 2008;86:1494.
130 Chao CP, Walker T.G., Kalava S.P., et al. Natural history and CT appearances of aortic intramural hematoma. Radiographics. 2009;29:791.
131 Augoustides J.G., Floyd T.F., Kolansky D.M. Echocardiography in suspected acute type A aortic dissection: Detection and management of a false positive presentation. J Cardiothorac Vasc Anesth. 2006;20:912.
132 Augoustides J.G., Harris H., Pochettino A. Direct innominate artery cannulation in acute type A dissection and severe aortic atheroma. J Cardiothorac Vasc Anesth. 2007;21:727.
133 Kamiya H., Kallenbach K., Halmer D., et al. Comparison of ascending aorta versus femoral artery cannulation for acute aortic dissection type A. Circulation. 2009;120:S282.
134 Tiwari K.K., Murzi M., Bevilacqua S., et al. Which cannulation (ascending aortic cannulation or peripheral arterial cannulation) is better for acute type A aortic dissection surgery? Interact Cardiovasc Thorac Surg. 2010;10:797-802.
135 Geirsson A., Bavaria J.E., Swarr D., et al. Fate of the residual distal and proximal aorta after acute type A dissection repair using a contemporary surgical reconstruction algorithm. Ann Thorac Surg. 2007;84:1955.
136 Schoder M., Lammer J., Czerny M. Endovascular aortic arch repair: Hopes and certainties. Eur J Vasc Endovasc Surg. 2009;38:255.
137 Pochettino A., Brinkman W.T., Moeller P., et al. Antegrade thoracic stent grafting during repair of acute Debakey I dissection prevents development of thoracoabdominal aneurysms. Ann Thorac Surg. 2009;88:482.
138 Sun L., Oi R., Chang Q., et al. Surgery for acute type A dissection using total arch replacement combined with stented elephant trunk implantation: Experience in 107 patients. J Thorac Cardiovasc Surg. 2009;138:1358.
139 Tsagakis K., Kamler M., Kuehl H., et al. Avoidance of proximal endoleak using a hybrid stent graft in arch replacement and descending aorta stenting. Ann Thorac Surg. 2009;88:773.
140 Adams J.D., Garcia L.M., Kern J.A. Endovascular repair of the thoracic aorta. Surg Clin North Am. 2009;89:895.
141 Szeto W.Y., McGarvey M., Pochettino A., et al. Results of a new surgical paradigm: Endovascular repair for acute complicated type B aortic dissection. Ann Thorac Surg. 2008;85:S1.
142 Nienaber C.A., Rousseau H., Eggebrecht H., for the INSTEAD Trial. Randomized comparison of strategies for type B dissection. The Investigation of Stent grafts In Aortic Dissection (INSTEAD) trial. Circulation. 2009;120:2519.
143 Tang D.G., Dake M.D. TEVAR for acute uncomplicated aortic dissection: Immediate repair vs medical therapy. Semin Vasc Surg. 2009;22:145.
144 Pradhan S., Elefteriades J.A., Sumpio B.E. Utility of the aortic fenestration technique in the management of acute aortic dissections. Ann Thorac Cardiovasc Surg. 2007;13:296.
145 Lee S., Cho S.H. Huge ascending aortic pseudoaneurysm caused by penetrating atherosclerotic ulcer. Circ Cardiovasc Imaging. 2008;1:e19.
146 Yoo S.M., Lee H.Y., White C.S. MDCT evaluation of the acute aortic syndrome. Radiol Clin North Am. 2010;48:67.
147 Eggebrecht H., Plicht B., Kahlert P., et al. Intramural hematoma and penetrating ulcers: Indications to endovascular treatment. Eur J Endovasc Surg. 2009;38:659.
148 Brinster D.R. Endovascular repair of the descending thoracic aorta for penetrating atherosclerotic ulcer disease. J Card Surg. 2009;24:203.
149 Steenburg S.D., Ravenel J.G., Ikonomidis J.S., et al. Acute traumatic aortic injury: Imaging evaluation and management. Radiology. 2008;248:748.
150 Cinnella G., Dambrosio M., Brienza N., et al. Transesophageal echocardiography for diagnosis of traumatic aortic injury: An appraisal of the evidence. J Trauma. 2004;57:1246.
151 Demetriades D., Velmanos G.C., Scalea T.M., et al. Diagnosis and treatment of blunt thoracic injuries: changing perspectives. J Trauma. 2008;64:1415.
152 Akowuah E., Angelini G., Bryan A.J. Open versus endovascular repair of traumatic aortic rupture: A systematic review. J Thorac Cardiovasc Surg. 2009;138:768.
153 Barnard J., Humphreys J., Bittar M.N. Endovascular versus open repair for blunt thoracic aortic injury. Interact Cardiovasc Thorac Surg. 2009;9:506.
154 Van Zaane B., Zuithoff N.P., Reitsma J.B., et al. Meta-analysis of the diagnostic accuracy of transesophageal echocardiography for assessment of atherosclerosis in the ascending aorta in patients undergoing cardiac surgery. Acta Anesthesiol Scand. 2008;52:1179.
155 Royse A.G., Royse C.F. Epiaortic ultrasound assessment of the aorta in cardiac surgery. Best Practice Res Clin Anesthesiol. 2009;23:335.
156 Siversides C., Kiess M., Beauchesne L., et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: Outflow tract obstruction, coarctation of the aorta, tetralogy of Fallot, Ebstein anomaly and Marfan’s syndrome. Can J Cardiol. 2010;26:80.
157 Wheatley G.H.3rd, Koullias G.J., Rodrigues-Lopez J.A., et al. Is endovascular repair the new gold standard for primary adult coarctation? Eur J Cardiothorac Surg. 2010;38:305-310.
158 Flore A.C., Ruzmetov M., Johnson R.G., et al. Selective use of left heart bypass for aortic coarctation. Ann Thorac Surg. 2010;89:851.