Chapter 217 Thoracic and Thoracolumbar Spine Fractures with Ventral Mass Lesion
Ventral versus Dorsal Operation
Ventral Decompression and Stabilization
Management of thoracic and thoracolumbar spine fractures continues to evolve, but remains controversial in regard to nonoperative treatment, the role of surgery, and what type of surgery is best. Nonoperative management has been shown to be effective if spinal canal compromise is minimal, dorsal elements are intact, and no neurologic deficits are present.1 More severe fractures also can be effectively treated nonoperatively.2 Patients with fractures and loss of more than 50% of vertebral body height, spinal canal stenosis greater than 50%, major kyphotic deformity in excess of the Cobb angle of 20 degrees, neurologic deficit (including sphincter dysfunction), intractable pain, and instability despite bedrest or sufficient bracing are generally considered candidates for surgery.
Dorsal decompression and stabilization techniques remain the most commonly used procedures for thoracic and thoracolumbar fractures. Ventral decompression and stabilization can be accomplished with a dorsal approach via either a unilateral transpedicular costotransversectomy or a lateral extracavitary approach.3–7 A bilateral microsurgical transpedicular approach without costotransversectomy also can be used. Visualization of the ventral dural surface and contralateral nerve root during removal of retropulsed bone fragments can be limited, however. Application of a ventral plating system can be difficult, if not impossible. Insertion of an anterior vertebral body prosthesis can be accomplished by unilateral transverse process resection and lateral rib transection for release to allow extrapleural lung retraction during cage placement. In addition, long constructs of dorsal instrumentation with pedicle screw implant two levels above and two levels below the fracture site often are necessary after extensive corpectomy to ensure stability. Percutaneous pedicle screw insertion is now possible. This limits the extent of posterior surgical dissection necessary for spine exposure. Placement of an expandable cage as a vertebral body prosthesis after corpectomy is now feasible with appropriate implant instrumentation to accomplish distraction, restore alignment, and maintain anterior column structural integrity. The complication rate with the lateral extracavitary approach, which allows for the most extensive ventral canal decompression, can be significant—even for experienced spine surgeons.8
Indirect decompression with distraction using pedicle screws can result in a satisfactory ventral decompression via ligamentotaxis. However, canal clearance of large retropulsed fragments can be incomplete, and anterior middle column reconstruction is limited. Moreover, the load-bearing capacity of the injured vertebral body may not be sufficient.9,10 The addition of transpedicular interbody grafting to posterior stabilization has not been shown to uniformly prevent loss of initial correction.11,12
The role of ventral thoracotomy approaches for decompression and stabilization in the treatment of thoracolumbar spine fractures has significantly advanced during the last 20 years,13–21 primarily due to the refinement of surgical approaches and the use of minimal access spine surgery, including thoracoscopy.22–24 Improved CT imaging permits more detailed analysis of fractures and selection of the optimum spinal approach. The goals of ventral surgeries are decompression of neural elements under direct vision as well as restoration of spinal alignment and stability without immobilizing intact motion segments. Ventral surgery can be accomplished either as a single-stage, stand-alone procedure with a plate or as combined posterior-anterior surgery in conjunction with a posterior short-segment pedicle screw fixation.16,19–21,23
Biomechanical studies indicate that ventral instrumentation systems, in combination with a ventral strut graft, restore spinal stability after vertebral body resection. In a porcine corpectomy model, ventral strut grafting with instrumentation was 15% stiffer during axial loading than was the intact spine.25 In comparison, dorsal instrumentation alone was 76% less stiff than the intact spine. Gurr et al.26 studied the ventrally placed Kaneda vertebral body prosthesis device and compared it with pedicle screw systems in a bovine model. Equivalent biomechanical stiffness after corpectomy and reconstruction with the Kaneda system over three spinal segments was achieved only if dorsal instrumentation spanned at least five levels.26 Thus, it would be necessary to include several healthy, asymptomatic spinal segments in such a fusion and stabilization procedure.
Because many thoracolumbar spine fractures have significant involvement of both the anterior and middle column vertebral structures, spine surgeons have always found the ventral approach for decompression and stabilization challenging. In burst fractures, the epidural compression most commonly is ventral to the dura mater, making ventral surgery the most direct approach for decompression.20 However, in the past, because of the lack of appropriate ventral instrumentation and difficult, unfamiliar ventral exposures (i.e., thoracotomy or thoracolumbar exposures), the ventral route was considered less desirable by many spine surgeons. Recent advances in spinal instrumentation and refinement of ventral surgical approaches have changed this attitude. The indications for single-stage ventral decompression and stabilization have continued to expand, and several clinical studies have shown excellent results.16,19,20 The largest series, by Kaneda et al.,16 included 150 patients with thoracolumbar burst fractures treated with ventral decompression and stabilization. All of the patients had neurologic deficits after their injury. The mean follow-up was 8 years (range, 5–12 years), and the fusion rate was 93%. Only 10 patients developed nonunion, which was managed with secondary dorsal instrumentation and fusion. Postoperative spinal canal stenosis, measured by CT, ranged from 0% to 8% (mean, 2%). Neurologic function improved by at least one Frankel grade in 95% of patients, bladder dysfunction completely resolved in 72% of patients, and 86% of patients returned to work without restriction. This study demonstrates that a ventral approach alone can yield excellent results in restoration of neurologic functions and spinal stability.
More recently, Zdeblick et al.19 reviewed a 7-year experience with ventral spinal decompression and fusion at the University of Wisconsin. Surgical indications were incomplete neurologic deficit, segmental kyphotic deformity greater than 25 degrees, and significant comminution of the vertebral body/pedicle complex. Thirty-five patients with thoracolumbar burst fractures were treated with ventral surgery, strut grafting, and fixation using the Z plate. Thirty-three patients were treated only with anterolateral instrumentation. The mean surgical time was 295 minutes, and the mean estimated blood loss was 1750 mL, with a mean hospital stay of 9 days. All patients were followed for a minimum of 2 years with a mean follow-up of 27 months. Forty-six percent (16 out of 35 patients) presented with a neurologic deficit, and all 16 of those patients demonstrated at least one Frankel grade of improvement after surgery. Twenty-nine of 30 patients who underwent ventral spinal decompression and fusion demonstrated radiographic healing, and 5 patients were lost to follow-up. One patient required a subsequent posterior fusion for an increase in kyphotic deformity. There were no instances of hardware failure, and the sagittal alignment was improved from a mean preoperative kyphosis of 18 degrees to a mean of 6 degrees on final follow-up.
The results of both of these studies illustrate that stand-alone anterior decompression, fusion, and stabilization is an effective treatment with excellent clinical and radiographic results. However, blood loss can be high in these procedures, illustrating the increased morbidity of thoracotomy and thoracolumbar approaches. In addition, all patients in both series were required to wear a thoracolumbar orthosis after surgery. For instance, in the study by McDonough et al.,19 a thoracolumbar sacral orthosis was worn whenever the patient was out of bed for a minimum of 3 months.
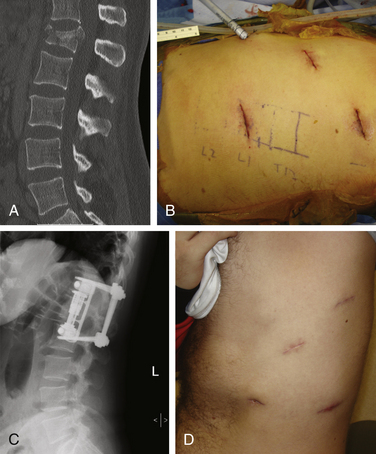
Because of the large incisions required for thoracotomy and thoracoabdominal approaches and their associated morbidities, and because of the difficulties of performing ventral spinal surgeries in the acute setting, Beisse et al.27–29 at the Trauma Center Murnau in Germany developed a minimally invasive thoracoscopic approach for the management of burst fractures using anterior column reconstruction and decompression (Fig. 217-1). The technique uses four small access portals along with a main working portal, which measures approximately 3 cm in diameter. Thoracic and thoracolumbar spine fractures from T4 through L1 can be treated using this technique. Between 1996 and 2004, these authors performed more than 800 endoscopic operations for spine fractures. To refine the technique, they developed an anterior plating system that can be placed thoracoscopically. Publications indicate that the rate of conversion to open surgery is less than 1%. There have been only four cases of hardware failure using the minimally invasive anterior implant, and no cases of misplaced hardware have been reported. Approach-related complications have been minimal (approximately 5.4%) in their series. In separate publications, the authors evaluated the results of the anterior decompression and illustrated the effectiveness of thoracoscopic spinal canal decompression for fractures.24 Using the method of Bradford and McBride,9 the average preoperative narrowing of the spinal canal reported by Beisse et al.24 was 55%. After thoracoscopic surgical decompression, fusion, and stabilization, the average spinal canal diameter was 110% of normal on postoperative CT scan. Neurologic improvement of incomplete paraplegia and paraparesis occurred in 13 out of 20 patients. The average stay in the intensive care unit was 1.4 days. Complications occurred in 36.7% of patients, but most were minor problems that were treated successfully. Correction of the posttraumatic deformity also was analyzed in 31 patients over a 2-year period. The average preoperative kyphotic deformity was 16 degrees, the average correction was 6 degrees, and at the 1-year follow-up there was no loss of correction.
Summary
Ventral decompression and stabilization offers an effective and safe option in the treatment of thoracolumbar fractures, either as a single-stage stand-alone technique or as part of a combined posterior-anterior procedure.13,20,21,23 With the new minimally invasive thoracoscopic techniques and the addition of minimally invasive percutaneous posterior short-segment pedicle screw internal fixation, it is likely that more patients will benefit from these innovative surgical approaches. These methods can avoid a need for postoperative bracing, limit the number of spinal segments to be fused, reduce the need for iliac crest autograft, reduce postoperative pain, and improve neurologic as well as functional outcome.
Amini A., Beisse R., Schmidt M.H. Thoracoscopic spine surgery for decompression and stabilization of the anterolateral thoracolumbar spine. Neurosurg Focus. 2005;19:E4.
Beisse R. Endoscopic surgery on the thoracolumbar junction of the spine. Eur Spine J. 2006;15:687-704.
Beisse R. Video-assisted techniques in the management of thoracolumbar fractures. Orthop Clin North Am. 2007;38:419-429. abstract vii
Beisse R., Muckley T., Schmidt M.H., et al. Surgical technique and results of endoscopic anterior spinal canal decompression. J Neurosurg Spine. 2005;2:128-136.
Kaneda K., Taneichi H., Abumi K., et al. Anterior decompression and stabilization with the Kaneda device for thoracolumbar burst fractures associated with neurological deficits. J Bone Joint Surg [Am]. 1997;79:69-83.
McDonough P.W., Davis R., Tribus C., et al. The management of acute thoracolumbar burst fractures with anterior corpectomy and Z-plate fixation. Spine (Phila Pa 1976). 2004;29:1901-1908.
Payer M. Unstable burst fractures of the thoraco-lumbar junction: treatment by posterior bisegmental correction/fixation and staged anterior corpectomy and titanium cage implantation. Acta Neurochir (Wien). 2006;148:299-306.
1. Hitchon P.W., Torner J.C. Recumbency in thoracolumbar fractures. Neurosurg Clin North Am. 1997;8:509-517.
2. Wood K., Buttermann G., Mehbod A., et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study. J Bone Joint Surg [Am]. 2003;85:773-781.
3. Schmidt M.H., Larson S.J., Maiman D.J. The lateral extracavitary approach to the thoracic and lumbar spine. Neurosurg Clin North Am. 2004;15:437-441.
4. Maiman D.J., Larson S.J., Benzel E.C. Neurological improvement associated with late decompression of the thoracolumbar spinal cord. Neurosurgery. 1984;14:302-307.
5. Maiman D.J., Larson S.J., Luck E., et al. Lateral extracavitary approach to the spine for thoracic disc herniation: report of 23 cases. Neurosurgery. 1984;14:178-182.
6. Kaya R.A., Aydin Y. Modified transpedicular approach for the surgical treatment of severe thoracolumbar or lumbar burst fractures. Spine J. 2004;4:208-217.
7. Larson S.J., Holst R.A., Hemmy D.C., et al. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45:628-637.
8. Resnick D.K., Benzel E.C. Lateral extracavitary approach for thoracic and thoracolumbar spine trauma: operative complications. Neurosurgery. 1998;43:796-802.
9. Bradford D.S., McBride G.G. Surgical management of thoracolumbar spine fractures with incomplete neurologic deficits. Clin Orthop Relat Res. 1987;218:201-216.
10. Mueller L.A., Mueller L.P., Schmidt R., et al. The phenomenon and efficiency of ligamentotaxis after dorsal stabilization of thoracolumbar burst fractures. Arch Orthop Trauma Surg. 2006;126:364-368.
11. Knop C., Fabian H.F., Bastian L., et al. Late results of thoracolumbar fractures after posterior instrumentation and transpedicular bone grafting. Spine (Phila Pa 1976). 2001;26:88-99.
12. Knop C., Fabian H.F., Bastian L., et al. Fate of the transpedicular intervertebral bone graft after posterior stabilisation of thoracolumbar fractures. Eur Spine J. 2002;11:251-257.
13. Amini A., Beisse R., Schmidt M.H. Thoracoscopic spine surgery for decompression and stabilization of the anterolateral thoracolumbar spine. Neurosurg Focus. 2005;19:E4.
14. Dunn H.K. Anterior spine stabilization and decompression for thoracolumbar injuries. Orthop Clin North Am. 1986;17:113-119.
15. Ghanayem A.J., Zdeblick T.A. Anterior instrumentation in the management of thoracolumbar burst fractures. Clin Orthop Relat Res 335:. 1997:89-100.
16. Kaneda K., Taneichi H., Abumi K., et al. Anterior decompression and stabilization with the Kaneda device for thoracolumbar burst fractures associated with neurological deficits. J Bone Joint Surg [Am]. 1997;79:69-83.
17. Kostuik J.P. Anterior spinal cord decompression for lesions of the thoracic and lumbar spine, techniques, new methods of internal fixation results. Spine (Phila Pa 1976). 1983;8:512-531.
18. Kostuik J.P. Anterior fixation for fractures of the thoracic and lumbar spine with or without neurologic involvement. Clin Orthop Relat Res. 1984;189:103-115.
19. McDonough P.W., Davis R., Tribus C., et al. The management of acute thoracolumbar burst fractures with anterior corpectomy and Z-plate fixation. Spine (Phila Pa 1976). 2004;29:1901-1908.
20. Oskouian R.J.Jr., Shaffrey C.I., Whitehill R., et al. Anterior stabilization of three-column thoracolumbar spinal trauma. J Neurosurg Spine. 2006;5:18-25.
21. Payer M. Unstable burst fractures of the thoraco-lumbar junction: treatment by posterior bisegmental correction/fixation and staged anterior corpectomy and titanium cage implantation. Acta Neurochir (Wien). 2006;148:299-306.
22. Beisse R. Endoscopic surgery on the thoracolumbar junction of the spine. Eur Spine J. 2006;15:687-704.
23. Beisse R. Video-assisted techniques in the management of thoracolumbar fractures. Orthop Clin North Am. 2007;38:419-429.
24. Beisse R., Muckley T., Schmidt M.H., et al. Surgical technique and results of endoscopic anterior spinal canal decompression. J Neurosurg Spine. 2005;2:128-136.
25. Gurwitz G.S., Dawson J.M., McNamara M.J., et al. Biomechanical analysis of three surgical approaches for lumbar burst fractures using short-segment instrumentation. Spine (Phila Pa 1976). 1993;18:977-982.
26. Gurr K.R., McAfee P.C., Shih C.M. Biomechanical analysis of anterior and posterior instrumentation systems after corpectomy. A calf-spine model. J Bone Joint Surg [Am]. 1988;70:1182-1191.
27. Beisse R., Potulski M., Temme C., et al. Endoscopically controlled division of the diaphragm. A minimally invasive approach to ventral management of thoracolumbar fractures of the spine. Unfallchirurg. 1998;101:619-627.
28. Potulski M., Beisse R., Buhren V. Thoracoscopy-guided management of the “anterior column”. Methods and results. Orthopade. 1999;28:723-730.
29. Buhren V., Beisse R., Potulski M. Minimally invasive ventral spondylodesis in injuries to the thoracic and lumbar spine. Chirurgie. 1997;68:1076-1084.
Dorsal Decompression and Stabilization
The choice of the surgical approach to stabilizing thoracic and thoracolumbar spine fractures with ventral mass lesion (i.e., fractures with retropulsed bone fragments) remains debatable. In addition to multiple retrospective clinical studies that show the efficacy of both dorsal and ventral approaches,1–6 two randomized prospective studies comparing both procedures have been published. Esses et al.7 reported on 40 patients who were randomized into posterior and anterior decompression and instrumentation groups. Eighteen patients underwent anterior surgery with the use of the Kostuik-Harrington device, and 22 patients underwent posterior surgery with the use of the AO fixateur interne. The mean follow-up period was 20 months. The study showed no difference between the groups in terms of improvement in neurologic deficit or kyphotic deformity. However, the patients who underwent anterior decompression and stabilization achieved statistically significant improvement in canal decompression. There were two implant failures in each group, and the patients undergoing the anterior approach suffered more blood loss. The other study, by Wood et al.,8 included patients with T10-L2 burst fractures without neurologic deficit. Thirty-eight patients were randomized and completed a minimum of 2 years of follow-up. Eighteen patients underwent posterior fusion with pedicle screw-hook instrumentation and autologous bone graft, and 20 underwent anterior fusion with two-level fibular and rib-strut arthrodesis and instrumentation. The study showed no difference between the groups in terms of functional outcome measures that included Ronald and Morris functional disability, Oswestry Disability Index, visual analogue scale, and SF-36 scores. Moreover, there was no difference in kyphosis and residual canal between the groups at the 2-year follow-up. Blood loss was higher in the group treated anteriorly. However, there were 17 complications in the group treated posteriorly, including instrumentation removal, pseudarthrosis, wound dehiscence, and infection, as opposed to only 3 complications in the group treated anteriorly. Biomechanical studies on ventral and dorsal implants have been performed in the laboratory,9–11 and the stability of both constructs has been found to be comparable.
Dorsal Approach
Harrington rods, initially developed for the treatment of scoliosis,12 eventually came to be used in spinal fractures. Later, similar dorsal implants were used to manage thoracolumbar fractures. Dorsal instrumentation with rods, hooks, sublaminar wires, or pedicle screws has been used with favorable results. Retrospective studies have demonstrated neurologic improvement, correction of angulation, restoration of vertebral body height and spinal canal, as well as rapid mobilization.1,3,5,6 The introduction and advancement of transpedicular pedicle screw fixation has revolutionized the dorsal approach to the treatment of thoracic and thoracolumbar spine fractures. The advantage of the dorsal approach to the thoracolumbar spine is the familiarity of the spine surgeon with this access route. In case of a fracture-dislocation, with or without locked facets, the dorsal approach facilitates facet reduction and reconstitution of the spinal canal. Because of its simplicity and its ability to provide stabilization and rapid mobilization, the dorsal approach also is suited in cases of total paralysis when no recovery of function is anticipated. In case of a burst fracture with retropulsed bone in the canal, adequate decompression of the spinal canal can be performed by using the transpedicular or transfacet approach, either unilaterally or bilaterally. After removal of the medial facet joint and pedicle with a power drill, retropulsed bone fragments can be removed and the spinal canal decompressed using impactors or reversed curets. The adequacy of decompression can be confirmed by intraoperative ultrasound, which is a useful technique to evaluate spinal canal morphology.
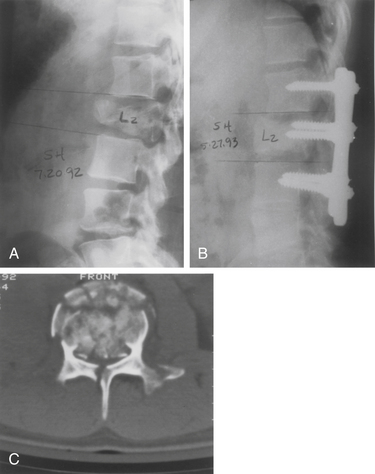
Long-segment constructs generally span two levels above and two levels below the fracture. Some clinical studies have shown posterior long-segment instrumentation to have an advantage over short-segment instrumentation in maintaining correction.13,14 On the other hand, short-segment constructs that span one level above and one level below the fracture have been shown to be successful, and have the advantage of limiting the disruption of lower motion segments.13,15,16 Multisegmental spinal stabilization is then accomplished by using pedicle screws solely, or in conjunction with hooks when the pedicles are small (Fig. 217-2). Unfortunately, in the presence of severe wedging and collapse of the vertebral body, dorsal instrumentation may fail in the absence of ventral load sharing. Thus, long multisegmental rostrocaudal fixation may be necessary to prevent construct failure. Long constructs, however, may result in stiffness and discomfort.
Summary
The dorsal approach allows adequate reconstruction of the canal and stabilization with pedicle screws and rods. The patient shown in Figure 217-2 is a 24-year-old man with a burst fracture at L2 with a conus injury. The preoperative CT scan shows severe spinal canal compromise from the retropulsed bone. The postoperative CT scan demonstrates the effectiveness of this approach in a select group of patients.
Dai L.Y., Jiang L.S., Jiang S.D. Posterior short-segment fixation with or without fusion for thoracolumbar burst fractures: a five to seven-year prospective randomized study. J Bone Joint Surg [Am]. 2009;91:1033-1041.
Gertzbein S.D. Scoliosis Research Society: multicenter spine fracture study. Spine (Phila Pa 1976). 1992;17:528-540.
Hitchon P.W., Torner J., Eichholz K.M., Beeler S.N. Comparison of anterolateral and posterior approaches in the management of thoracolumbar burst fractures. J Neurosurg Spine. 2006;5:117-125.
Kaneda K., Taneichi H., Abumi K., et al. Anterior decompression and stabilization with the Kaneda device for thoracolumbar burst fractures associated with neurological deficits. J Bone Joint Surg [Am]. 1997;79:69-83.
Wood K.B., Bohn D., Mehbod A. Anterior versus posterior treatment of stable thoracolumbar burst fractures without neurologic deficit: a prospective, randomized study. J Spinal Disord Tech. 2005;18:S15-S23.
1. Danisa O.A., Shaffrey C.I., Jane J.A., et al. Surgical approaches for the correction of unstable thoracolumbar burst fractures: a retrospective analysis of treatment outcomes. J Neurosurg. 1995;83:977-983.
2. Gertzbein S.D. Scoliosis Research Society: multicenter spine fracture study. Spine (Phila Pa 1976). 1992;17:528-540.
3. Hitchon P.W., Torner J., Eichholz K.M., Beeler S.N. Comparison of anterolateral and posterior approaches in the management of thoracolumbar burst fractures. J Neurosurg Spine. 2006;5:117-125.
4. Kaneda K., Taneichi H., Abumi K., et al. Anterior decompression and stabilization with the Kaneda device for thoracolumbar burst fractures associated with neurological deficits. J Bone Joint Surg [Am]. 1997;79:69-83.
5. Lemons V.R., Wagner F.C.Jr., Montesano P.X. Management of thoracolumbar fractures with accompanying neurological injury. Neurosurgery. 1992;30:667-671.
6. Stephens G.C., Devito D.P., McNamara M.J. Segmental fixation of lumbar burst fractures with Cotrel Dubousset instrumentation. J Spinal Disord. 1992;5:344-348.
7. Esses S.I., Botsford D.J., Kostuik J.P. Evaluation of surgical treatment for burst fractures. Spine (Phila Pa 1976). 1990;15:667-673.
8. Wood K.B., Bohn D., Mehbod A. Anterior versus posterior treatment of stable thoracolumbar burst fractures without neurologic deficit: a prospective, randomized study. J Spinal Disord Tech. 2005;18:S15-S23.
9. Dick J.C., Brodke D.S., Zdeblick T.A., et al. Anterior instrumentation of the thoracolumbar spine. A biomechanical comparison. Spine (Phila Pa 1976). 1997;22:744-750.
10. Eichholz K.M., Hitchon P.W., From A., et al. Biomechanical testing of anterior and posterior thoracolumbar instrumentation in the cadaveric spine: invited submission from the joint section meeting on disorders of the spine and peripheral nerves, March 2004. J Neurosurg Spine. 2004;1:116-121.
11. Hitchon P.W., Goel V.K., Rogge T.N., et al. In vitro biomechanical analysis of three anterior thoracolumbar implants. J Neurosurg. 2000;93:252-258.
12. Harrington P.R. Treatment of scoliosis. Correction and internal fixation by spine instrumentation. J Bone Joint Surg [Am]. 1962;44:591-610.
13. McLain R.F. The biomechanics of long versus short fixation for thoracolumbar spine fractures. Spine (Phila Pa 1976). 2006;31:S70-S79.
14. Tezeren G., Kuru I. Posterior fixation of thoracolumbar burst fracture: short-segment pedicle fixation versus long-segment instrumentation. J Spinal Disord Tech. 2005;18:485-488.
15. Dai L.Y., Jiang L.S., Jiang S.D. Posterior short-segment fixation with or without fusion for thoracolumbar burst fractures. A five to seven-year prospective randomized study. J Bone Joint Surg [Am]. 2009;91:1033-1041.
16. Parker J.W., Lane J.R., Karaikovic E.E., Gaines R.W. Successful short-segment instrumentation and fusion for thoracolumbar spine fractures: a consecutive 41/2-year series. Spine (Phila Pa 1976). 2000;25:1157-1170.