Thiopental
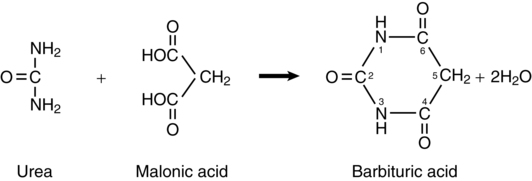
Barbituric acid (2,4,6,-trioxyhexahydropyrimidine) (Figure 71-1) was first synthesized by Adolph von Bäyer (founder of what was to become the Bayer chemical company) in 1864. Although this molecule is the structural framework from which barbiturates are derived, it is devoid of anesthetic properties. Structural modification at the number 2 and 5 carbon atoms results in barbiturate drugs that have sedative-hypnotic properties. For example, addition of a benzene ring at the C5 position results in phenobarbital, a drug first used for its sedative properties in the early 1900s and still one of the most widely used drugs in the world to treat seizure disorders. With the lack of thiopental in the United States, intensive care physicians are using phenobarbital to induce barbiturate coma in patients with raised intracranial pressure unresponsive to other therapies. Replacement of the oxygen atom with a sulfur atom at the C2 position and adding a large aliphatic carbon group to the C5 atom transforms barbituric acid into thiopental. Clinically, sodium thiopental was first administered in 1934 by Ralph Waters at the University of Wisconsin, Madison, and by John Lundy at the Mayo Clinic in Rochester, MN.
Pharmacokinetics
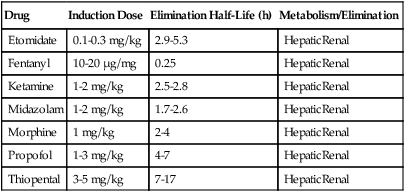
Distribution of intravenously administered drugs throughout body tissues is determined by tissue blood flow, blood-tissue concentration gradient, lipid solubility, extent of protein binding, and degree of ionization. Thiopental is very lipid soluble and, thus, readily crosses the blood-brain barrier. Other pharmacokinetic properties of thiopental are listed in Table 71-1. Decreased protein binding (e.g., in patients with uremia or cirrhosis) increases the free fraction of drug available to interact with GABA receptors in the brain.
Table 71-1
Induction Dose, Half-Life, and Metabolism/Route of Elimination for Commonly Used Intravenous Anesthetic Agents
| Drug | Induction Dose | Elimination Half-Life (h) | Metabolism/Elimination |
| Etomidate | 0.1-0.3 mg/kg | 2.9-5.3 | HepaticRenal |
| Fentanyl | 10-20 μg/mg | 0.25 | HepaticRenal |
| Ketamine | 1-2 mg/kg | 2.5-2.8 | HepaticRenal |
| Midazolam | 1-2 mg/kg | 1.7-2.6 | HepaticRenal |
| Morphine | 1 mg/kg | 2-4 | HepaticRenal |
| Propofol | 1-3 mg/kg | 4-7 | HepaticRenal |
| Thiopental | 3-5 mg/kg | 7-17 | HepaticRenal |
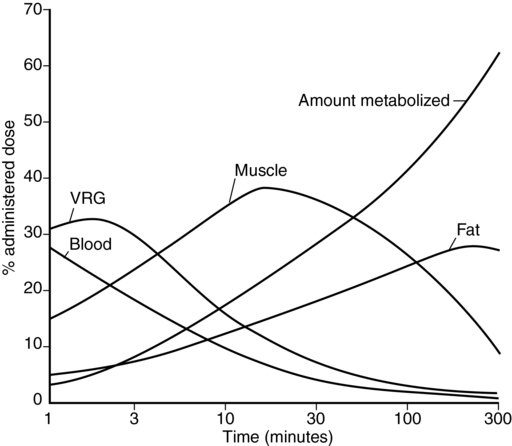
Because the brain is a highly perfused (i.e., belongs to the vessel-rich organ group) relatively low-volume organ, cerebral thiopental concentrations equilibrate rapidly with the central blood pool (Figure 71-2), resulting in depressed electroencephalographic activity—and induction of anesthesia—within 20 to 40 sec.