Thermoregulation and perioperative hypothermia
Heat balance and thermoregulation
In unanesthetized patients, cold-induced autonomic defenses follow a hierarchical pattern that progresses from vasoconstriction to nonshivering thermogenesis and, finally, shivering thermogenesis (Figure 161-1). Vasoconstriction decreases cutaneous blood flow and heat loss, primarily in the fingers and toes. Although its effects are minimal in adults, nonshivering thermogenesis can double metabolic heat production in the mitochondria-rich brown fat of neonates and infants. Shivering thermogenesis results from involuntary skeletal muscle activity that increases metabolic rate and heat production.
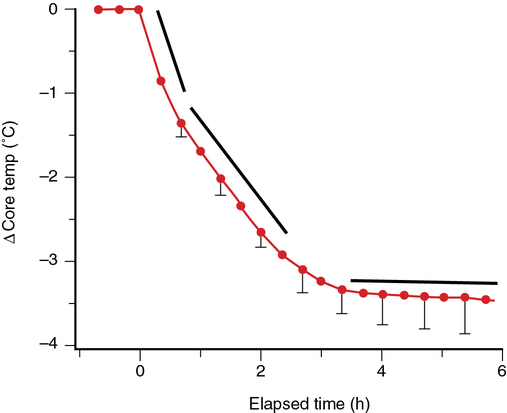
Mechanisms and prevention of perioperative hypothermia
Perioperative hypothermia occurs via several heat-loss mechanisms: redistribution, convection, radiation, conduction, and evaporation. Although all of these mechanisms are important to some extent, the initial drop in core temperature—and the most important cause of perioperative hypothermia—is predominantly due to redistribution (i.e., transfer) of heat from the core to peripheral tissues (see Figure 161-1). Rapid core-to-peripheral heat transfer produces hypothermia in nearly all patients regardless of the type of anesthesia delivered (e.g., general or regional).




