CHAPTER 385 Therapeutic Decision Making
The complexity of decision making has increased significantly since Walter Dandy declared that to “extirpate one of these aneurysmal angiomas in its active state would be unthinkable”1; Norlen stated that that “probably most, if not all, patients die of hemorrhage or are completely incapacitated”2; and Olivecrona and Riives concluded that there is no “proof that Roentgen treatment … in any way alters the spontaneous course of the illness.”3 Perhaps these statements could be considered “true” within the context that these great men were treating AVMs, but they are untrue in the context of the current understanding of the natural history and treatment of AVMs. Greater understanding of the natural history and innovations in surgery, perioperative management, diagnostic radiology, interventional radiology, and the application of focused ablative energy has created a radical change in decision making. It would be appropriate to consider that further development would continue to make management paradigms established today obsolescent for treatments hitherto unimagined. The aim of this chapter is to offer a template of relevant information to underpin management decisions. However, it is assumed that context-specific data, known only to local management teams, will be required to form the basis for informed management recommendations.
Management by Nonintervention
Risk for Intracranial Hemorrhage
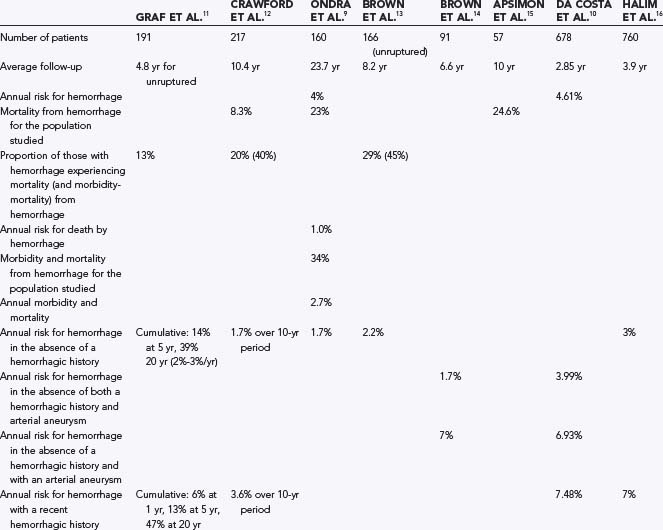
It is important to inform patients with AVMs of what their future is likely to be if untreated. Such information is complicated by the difficulty associated with understanding a disease that is diagnosed annually in just 0.9 to 1.5 per 100,000 population,4,5 the complexity of identifying important angioarchitectural features such as aneurysms,6,7 and the biases inherent in database research.8 Despite these difficulties, some information is available to help patients make an informed opinion on management. The literature would suggest that on diagnosis, the risk for future hemorrhage would be on the order of 4% per year with an annual mortality from hemorrhage of 1% (selected series results are reported in Table 385-1).9–16 Based on patients prospectively observed until hemorrhage, further stratification into higher risk for hemorrhage (recent hemorrhage, presence of arterial or intranidal aneurysms, exclusive deep venous drainage, deep location, and increasing age) and lower risk for hemorrhage (asymptomatic lesions in the absence of a hemorrhagic history) is possible.10–14,16,17 Stratification of risk beyond this point may be inappropriate because of the small number of patients in any series, the nature of referral bias, the noted complexity in assigning angioarchitectural features, and the absence of trial results. It has been argued that we do not have enough information to predict the natural history of AVMs with accuracy and that randomized control trials would be necessary to provide an informed opinion.18 However, the obstacles and ethics of mounting such a trial, which would require sufficient length of follow-up to predict a course, often in excess of 20 years, would be extremely challenging for this rare and complex disease for which treatment is possible and outcomes with nonintervention can be catastrophic. Furthermore, there is sufficient concordance among studies (in different centers and time periods) to have some confidence that for the purpose of informed consent, a reasonable opinion of the natural history can be made.
What assumptions can be made regarding the information that we have regarding the natural history? Although AVMs are congenital lesions, their risk for rupture is probably not constant. This is reflected in prospective studies that have indentified increased risk for hemorrhage after recent hemorrhage,17 with associated aneurysms (usually acquired),14,17,19 and with increasing age.11,12,20 Moreover, retrospective data suggest that acquired venous outflow stenosis (a condition that is more likely to produce a change in pressure within the AVM nidus where such venous outflow is limited, e.g., exclusive deep venous drainage) is also associated with increased risk.21–23 A unifying theme for these risk-altering characteristics is the wear-and-tear changes occurring over time with the obligate high shear stress associated with AVMs. Therefore, predicting the risk for hemorrhage should be considered both from the short-term point of view (based on the history and angioarchitectural features present at diagnosis) and from the long-term point of view (where wear-and-tear changes have yet to develop but may do so in the future). Having both short-term and long-term conservative management outcomes in management templates is important for AVMs because the expected hemorrhage rate during the latency period (i.e., short term) after focused irradiation will need to be considered when determining the risks associated with focused irradiation. A long-term perspective is also of importance given that the majority of AVMs are diagnosed in patients with an expected survival of more than 2 decades in the absence of an AVM. Although a hemorrhagic manifestation is known to increase the risk for rupture in the short term, this may not be of importance when considering the risks over decades. In the only long-term study, 40% of the AVM population suffered at least one new hemorrhage (mean follow-up of 23.7 years).9 In this Finnish study, in contrast to the results from studies with a mean follow-up of less than 10 years, an initial hemorrhagic manifestation was not seen to be an important predictor for future hemorrhage. This underscores the potential for wear-and-tear changes to be acquired over time such that a low-risk AVM can be changed into one with greater risk for rupture.
The short-term indicators of increased risk are unified by their relationship to hemodynamic “wear and tear” and fall into three categories: features demonstrating that the vasculature has had a history of being breached (history of hemorrhage), features suggesting that degeneration is occurring (presence of aneurysms, increasing length of time, increasing age),11,12,20,24,25 and those indicating increased vulnerability to breach because of limitations in potential alternative outflow in the event of acquired flow-related venous occlusion (exclusive deep venous drainage).25 Although patients with associated aneurysms and AVMs would expect to have a higher risk for hemorrhage, the magnitude of the increase in risk is significantly greater than the summation of the risks.10,14,25 The cause of the increased risk for hemorrhage from such an association is likely to be explained by vascular “wear and tear,” with the aneurysm being a marker for this progression in pathology.14
A relevant potential criticism of management decisions based on the natural history derived from clinical series is the potential for a significant number of AVMs to remain undiscovered throughout life. This would overestimate the true risk. However, the context in which we make management decisions is generally the same context in which the natural history data are derived. This allows assumptions derived from the known population with AVMs to be reasonably applied to all people with AVMs. Furthermore, some evidence exists that the pool of undiscovered AVMs remains small.15
In an effort to consolidate evidence presented in this section for use in constructing a simple paradigm of management, the following assumptions have been made (while recognizing the wide 95% confidence interval [CI] for most of the raw data) (Table 385-2):
TABLE 385-2 Chance of Remaining Hemorrhage Free from the Time of Diagnosis
| YEARS AFTER DIAGNOSIS | IN THE ABSENCE OF ANEURYSM AND HEMORRHAGIC MANIFESTATION (%)* | IN THE PRESENCE OF ANEURYSM OR HEMORRHAGIC MANIFESTATION (%)† |
|---|---|---|
| 2 | 96 | 86 |
| 5 | 90 | 74 |
| 10 | 78 | 64 |
| 15 | 67 | 55 |
| 20 | 57 | 47 |
| 25 | 49 | 40 |
| 30 | 42 | 35 |
* Presuming a 2% risk per year for the first 5 years and increasing to 3% per year after 5 years
† Presuming a 7% risk per year for the first 2 years and declining to 3% per year after 5 years)
Death as a Result of Hemorrhage from Arteriovenous Malformation
In the only long-term study, death from hemorrhage occurred in 23% of subjects over a 23.7-year period.9 The annual mortality from hemorrhage in this study was 1%, and 25% of hemorrhages from AVM resulted in death. On a hemorrhagic incidence basis, mortality of nearly 30% with each hemorrhage has been reported.13,24,25 In a population study, mortality secondary to hemorrhage from an AVM was 18% with a 95% CI of 3.8% to 43.3%.26 Although recurrent hemorrhage has been reported to have higher mortality than initial hemorrhage,24,27,28 this observation may reflect referral bias and sample size given that a patient sustaining a recurrent hemorrhage is more likely to be under observation with a correct diagnosis of AVM at the time of hemorrhage; in contrast, the source of a devastating intracranial hemorrhage may not be investigated at the initial evaluation of such a patient. The report of Fults and Kelly of 13.6% mortality for a first hemorrhage and 25% for a third or subsequent hemorrhage24 is a risk very close to that of the 29% reported by Brown and colleagues after a first hemorrhage from an AVM.13 Although evidence exists that recurrent hemorrhage may stereotypically mimic the first hemorrhage (and thus survival from hemorrhage would predict survival from further hemorrhage),29 this may be applicable only in the short term.29,30 An estimated 20% to 25% mortality from any hemorrhage secondary to an AVM is not unreasonable for the purposes of constructing a useful predictive model of outcomes after AVM.
Morbidity as a Result of Hemorrhage from Arteriovenous Malformations
Permanent new neurological deficits arising as a consequence of AVM hemorrhage are more difficult to estimate than the categorical outcome of death. Estimates for permanent adverse outcomes vary.29 However, there is some concordance among several series, with an approximately 50% risk for death and disability for each intraparenchymal hemorrhage from an AVM.13,24,25,30,31 The distribution of death and morbidity differs among series. There is a tendency for earlier series to report a greater proportion of cases resulting in death rather than morbidity. However, the combination of an approximately 40% to 50% risk for morbidity and mortality is consistent over time.
Nonhemorrhagic Decline in Health from Arteriovenous Malformations
Annually, 1.5% of patients with an AVM of the brain will undergo functional decline.13 The mechanism of the decline is usually either seizures (new or progression) or progressive neurological deficits caused by regional arterial hypotension or venous hypertension, or both.31 For such progression to take place it is likely that the AVMs would be large, supratentorial, and cortically based.
Management by Surgery
Risks associated with surgery have been found to relate to the size of the nidus,19,26,33,34 deep venous drainage,35 location in or adjacent to critical brain regions (eloquence),35 Spetzler-Martin grade (which combines size, eloquence, and deep venous drainage),36,37 lenticulostriate supply,38 deep meningeal supply39 and a diffuse nidus.35,40 It is important to remember that interobserver and intraobserver error is significant when examining these features within institutions,6,7,35 and one could only imagine that between institutions such error would be worse.6 Although it would be desirable to have models that can be simple to apply and are generalizable, this is not possible with the accuracy with which the different relevant angioarchitectural features are defined.
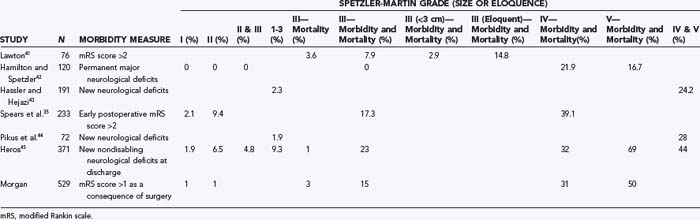
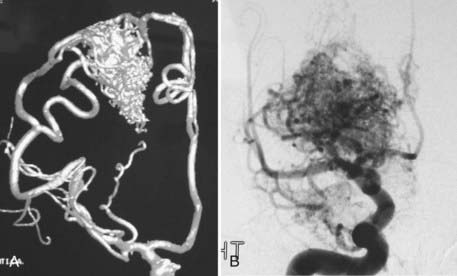
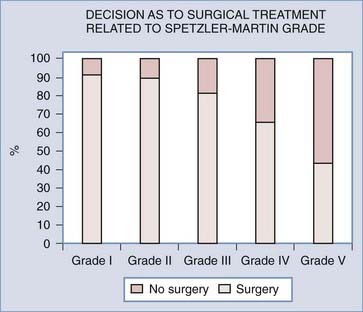
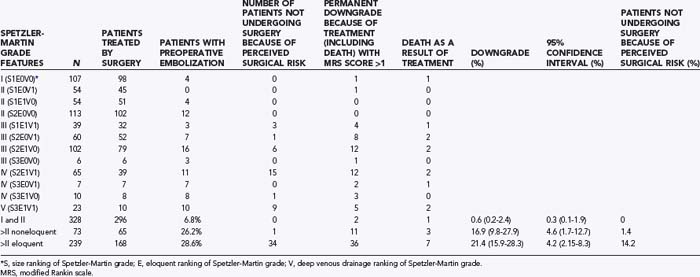
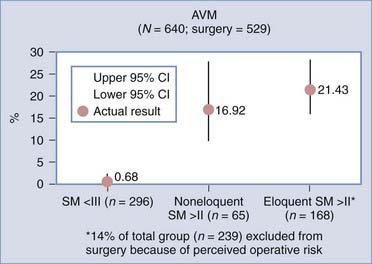
Analyses of series that have helped develop our concepts of risks related to surgery need to be understood in the context of their referral and selection biases (Table 385-3).35,41–45 A series in which there is a tendency toward conservative management of large AVMs may find that nidus size becomes irrelevant in predicting adverse outcomes after surgery. This may account for the discrepancy in the Spetzler-Martin grade variables that contribute to adverse surgical outcomes.35 Similarly, a variable threshold for deeming those with deep arterial supply inoperable may affect the interpretation of surgical series.35 Furthermore, if deep arterial supply is variably involved, those with single feeders may be more likely to have a good outcome with (and hence be considered for) surgery than those with a more complex deep supply (Fig. 385-1A and B). Frequently, a large number of patients are excluded from surgery for a variety of reasons, including surgical difficulty (a decision often made by considering some of the variables believed to be risk factors), thus distorting the applicability of results to all AVMs of similar grade. The Barrow Neurological Institute reported that only 5% of patients with Spetzler-Martin grades IV and V are recommended for surgery.46 This highly selected cohort would suggest that the results from surgery in their hands cannot be generalizable to the total population of patients with grade IV and V AVMs. Bias in selection may also account for the comparable outcomes between Spetzler-Martin grades I and II, with more grade II AVMs being recommended for conservative treatment.47 An example of the percentage of patients recommended for surgery for each of the Spetzler-Martin grades in a specific treating institution is presented in Figure 385-2. Therefore, the total population of patients (both those undergoing and those not undergoing surgery) needs to be examined with regard to the reasons for not recommending surgery before the paradigm that has been found applicable within a specific institution can be generalized for use in other contexts. That is, the lack of evidence of an effect of these variables is not evidence of a lack of effect.
Grading System
Grading systems attempting to estimate the risk associated with surgery have been used for more than 20 years.26,33–35,41,48–50 For grading systems to be of use, however, they must be applicable in the context in which the patient is to be managed. Although the Spetzler-Martin grading system can be criticized for lack of weighting of variables and lack of independence of variables,35 the need for further subclassification,41 intraobserver and interobserver error,6,7,35,40 and biasing from patient exclusion because of perceived risks,38,47 it is simple (grade I for a <3-cm nidus, grade II for 3 to 6 cm, grade III for >6 cm, with a point added for deep venous drainage and a point added if located in or adjacent to one of the following: sensorimotor cortex, language cortex, visual cortex, thalamus, hypothalamus, internal capsule, brainstem, cerebellar peduncles, or deep cerebellar nuclei), popular, reasonably robust, and a reliable discriminator of relative risk for patients undergoing surgery.49 Even though the relative risk between Spetzler-Martin grades is appropriately discriminatory, the absolute risk cannot be used without taking into consideration patients excluded from surgery because of perceived operative difficulty. With an increasing proportion of patients excluded with an increasing grade of AVM, estimation of risk becomes increasingly misleading. As the grade increases and the nonsurgical proportion increases (Fig. 385-2) (e.g., only 5% of patients with Spetzler-Martin grades IV and V undergo surgery at Barrow Neurological Institute), the absolute risk related to surgery from published series probably increasingly understates the true risk.46 This cannot be used as an argument against surgery because ancillary selection criteria can produce an informed decision and result in the reported permanent new neurological deficit rates in the literature. It is important to establish what selection criteria are being used before patients are selected for surgery. Biasing is likely to be specific to the institutional management norms, and this needs to be considered when informing patients what the risks associated with surgery for a specific grade may be. Biasing is evident in my experience (Table 385-4; also see Fig. 385-2). It is reasonable to conclude that for my series, risks associated with surgery for AVMs of grade I and II and for AVM grades greater than II in noneloquent brain regions are representative of all AVMs of their specific grade and type. However, the 21% (95% CI, 16% to 28%) of patients experiencing new permanent neurological deficits (modified Rankin score >1) as a result of surgery may seriously underestimate the risks for all eloquently located AVMs of Spetzler-Martin grades greater than II because there is a 14% incidence of surgery not being performed for risk-related reasons.
In conclusion, with regard to grading systems it is reasonable and appropriate to use Spetzler-Martin grading as the basis for stratifying and communicating surgical risks. It is also important to examine the Spetzler-Martin grades in the context in which the patient is to be managed and to incorporate additional variables demonstrated to have an impact on patient management (such as a diffuse nidus and lenticulostriate arterial supply)35,38,40 as second-tier criteria for decision making.
Prevention of Hemorrhage
Delayed detection of a previously obliterated AVM also needs to be considered in management decisions. Errors can be made in interpretation of the postoperative angiogram. In addition, it is not always certain that the absence of early venous drainage on angiography confirms cure of the disease. It is possible, for example, that vasospasm, temporary occlusion of venous outflow (with unstable thrombus that may recanalize at some interval after angiography), or unobliterated angiomatous (or perinidal) feeding vessels may be complicit in the development of a future AVM nidus.51,52 Delayed angiography has identified new, residual, or recurrent AVMs in 3.5% of children whose AVMs were previously confirmed to be angiographically obliterated after surgery.53 This incidence of new, residual, or recurrent AVMs was not part of routine screening in the series of Kader and colleagues,53 and thus their results probably represent a minimal incidence. The occurrence of new, residual, or recurrent AVMs in adults cannot be excluded. Such findings have also been detected, after previously confirmed to be angiographically obliterated, after focused irradiation.54,55 Despite the suggestion that risk for late AVM recurrence is confined to children,53 this has not been my experience. In 101 patients with radiologic follow-up of more than 2 years after confirmed ablation, 5 were confirmed to have a new AVM (1 of whom was seen because of a late new hemorrhage) after an early postoperative angiogram confirmed “cure.” Only 1 patient was younger than 20 years at the initial surgery. This may over-represent the true risk for late new, residual, or recurrent AVMs because the discovery was made after a hemorrhagic event in 1 of these 5 patients, with just 4 new malformations being detected in 100 patients subjected to routine screening (i.e., 4% with a 95% CI of 1.6% to 9.8%). The optimal timing of delayed angiography has not yet been determined and the rate of late residual, recurrent, or new AVMs still has to be established, but in the absence of these details, for the purpose of decision making it is best to consider the initial surgery as being curative in 90% of patients.
Surgery and Epilepsy
New Seizures Arising As a Consequence of Surgery
Evidence from case series suggests that there is a risk for the development of new seizures after surgery for supratentorial AVMs in 6% to 15% of patients.56,57 New multiple seizures occur after 5% to 6% of all supratentorial AVM surgery.56,57 Overall, 68% of patients are seizure free without anticonvulsants more than 2 years after surgery.57
Outcome of Seizures after Surgery
Epilepsy accounts for approximately half of the nonhemorrhagic manifestations of AVMs. In these patients, approximately 80% experience an improvement in seizure management after surgery,52–54 and when multiple seizures have occurred preoperatively, 66% to 76% achieve freedom from disabling seizures after surgery (Engel Seizure Outcome Scale class 1).56,58,59 In patients with multiple seizures preoperatively, the incidence of deterioration in seizure frequency is less than 2%.56,59
Although postoperative seizures, when they occur, are likely to first appear in the initial 12 months after surgery, at least 25% of first postoperative seizures occur beyond this period.57 This has implications regarding the management decision for withdrawal of anticonvulsant medications after surgery.
New Neurological Deficits
The development of new neurological deficits related to surgery is a key factor in determining management. New neurological deficits account for nearly 80% of the complications of surgery and are present immediately on awakening from surgery in more than 80% of the patients in whom deficits will develop.60 Serious infections and complications of venous thrombosis should not be discounted as challenges to surgical management even though the focus is on neurological outcomes. However, the efficacy of management and the occurrence of new permanent neurological deficits are the most important determinants in decision making for AVM management. Examples of series reporting an incidence of new permanent neurological deficits are provided in Table 385-3.35,41,42–45
These series demonstrate the relationship between Spetzler-Martin grade and outcomes, and the results may be generalizable to all AVMs of a specific Spetzler-Martin grade if the number of patients excluded for reasons of surgical difficulty is low. In my retrospectively analyzed, prospectively collected database (which included the reason for management other than surgery), in patients with Spetzler-Martin grades I and II who underwent surgery, permanent new neurological deficits occurred in less than 2.5%, whereas in patients with Spetzler-Martin grades III and IV in noneloquent brain regions who underwent surgery, new permanent neurological deficits developed in 17% (see Table 385-4). In both these groups the results are generalizable, with very few patients being refused surgery because of perceived operative difficulty. However, the risk for new permanent neurological deficits in patients with AVMs of Spetzler-Martin grade III or greater that are located in eloquent brain regions does not reliably reflect the risk for all such AVMs (because 14% of all such AVMs were excluded as a result of perceived operative difficulty). These AVMs are, in general, likely to have a significantly greater risk associated with surgery than the actual demonstrated risk for the group (21%).
Management by Focused Irradiation
Balance between Cure and Risks Associated with Treatment by Focused Irradiation
Radiotherapy has been used for the management of AVMs for nearly as long as surgery has.3 However, the radical change with the advent of focused irradiation has resulted in it being recommended as the preferred treatment option for many AVMs. As with any of the treatment strategies, the local context has a significant impact on outcomes. This well-tolerated procedure needs to be considered in terms of the risks related to treatment, the risk associated with the unobliterated AVM nidus (between initiation of treatment and cure), and the efficacy of treatment. These considerations are interactive and relate to the dose of treatment and the size and location of the nidus. The risk for permanent deficits increases when the brain receives doses escalating above 10 Gy in a single fraction. To be considered at the same time is that the chance of cure of the AVM nidus decreases when marginal doses to the nidus decrease below 25 Gy. The smaller the volume of the AVM nidus to be covered, the steeper the gradient in dose at the margins of treatment. Hence, smaller lesions can be treated with a higher marginal dose to achieve a high probability of cure with an acceptably low risk for brain damage. From more than 1000 Gamma Knife procedures performed at the Karolinska Institute,61 the probability of obliteration was found to be 35.69 × ln (marginal dose) − 39.66. This model predicts that for a marginal dose of 22 Gy there is a 71% probability of cure, which decreases to 55% with a marginal dose of 14 Gy. This equation is clearly accurate only over the range of marginal doses normally considered in treating AVMs. Linear accelerator (LINAC)-based focused irradiation can achieve similar results. However, the probability of cure is clouded by the difference between those who have been demonstrated to be cured and those who have not yet been demonstrated to be cured. That there is a gap between our knowledge of patients with demonstrated failure and those who have not yet been demonstrated to have been cured is understandable because of the referral pattern for most focused irradiation services and the latent period between treatment and cure. This is illustrated by Friedman and colleagues’ report on LINAC-focused irradiation for Spetzler-Martin grade I and II AVMs.62 At 3 years, 66% of patients were demonstrated to have been cured and 21% were known to have failed to be cured. This discrepancy is common and has led some to suggest an approximately 30% lower rate of cure for focused irradiation than what has been reported.63 Thus, for an AVM nidus larger than 30 mm in diameter, the probability of obliteration at 2 years may be just 16% in highly regarded treatment institutions.
Innovative radiosurgical strategies (such as low-dose irradiation followed by repeated treatment) to increase the likelihood of obliteration continue to be developed in an attempt to deal with larger AVMs.64,65 However, fractionated treatments have a low chance of success,66 and any significant change in radiotherapy treatment protocols from single-fraction radiosurgery to multiple fractions will need to be validated with patient outcome data for these new protocols.
Another innovative combination is planned embolization to reduce the focused irradiation target. However, AVMs undergoing pretreatment embolization (in an attempt to reduce the focused irradiation volume) cannot be considered to be the equivalent of untreated AVMs of comparable volume. Postembolization AVMs are obliterated at 70% of the probability of obliteration of AVMs that have not undergone previous treatment when matched for AVM residual volume, marginal dose delivered, and location.67,68
Of some concern is the report of delayed hemorrhage after documented cure with focused irradiation.54,55 Delayed hemorrhage has also been reported after surgery on early postoperative angiography, but it is presumed that absence of the AVM on an angiogram performed several years after treatment would represent a cure with any form of therapy. The suggestion that there may be an ongoing small risk for hemorrhage after demonstrable occlusion assessed some years after treatment will require further study.
Radiosurgery for children can be performed without significant deviation from the results expected for adults.69,70
Although there are limitations in the potential for cure because of the dose that can safely be administered to normal brain, there are also limitations resulting from the accuracy of target definition. Variability among planners in determining contouring may lead to underdosing.71 This underscores the importance for radiosurgery institutions to provide outcome data to ensure that performance is known and optimal.
Time to Cure
Hemorrhage rates, as well as the morbidity and mortality from hemorrhage, remain unchanged after focused irradiation until obliteration occurs.55,72–77 Factors that increase the risk for hemorrhage (such as the presence of aneurysms) are unaffected by focused irradiation until obliteration of the nidus.72
In the absence of previous results provided by the specific focused irradiation center where the patient is to be managed, it is reasonable to assume that 70% of AVMs less than 3 cm in maximum diameter can be cured, with the average time of protection occurring after 2 years and with no less than a 5% permanent symptomatic radiation necrosis rate in eloquent brain regions (in the thalamus and brainstem the rate will be greater than 10%).78
Management by Embolization
Embolization has been incorporated in the management of AVMs for nearly 50 years.79 With the rapid advancement in endovascular innovation, it is difficult to predict the potential role for embolization even in the next few years. However, it is possible to make observations on its utility today. Embolization has been used successfully for cure, palliation, targeted hemorrhage risk reduction, and volume reduction as an adjunctive therapy to surgery and focused irradiation. Nonetheless, outcomes with its use are extremely variable because of the wide range of treatment paradigms used. Patient morbidity and mortality ranging from 2% to 11%80,81 obscure the complexity of compiling useful data. At the center of this difficulty is the goal of treatment by embolization. When the goal of embolization is preoperative volume reduction, cure rates and morbidity are often very low (because the goal is safety rather than cure). With an increase in attempts at cure by embolization alone, the cure rate, morbidity, and mortality are all increased. For this reason, the risks associated with its use need to be placed within the context for which it is being used.
Embolization as a Method of Cure
Cure by embolization remains uncommon in most, but not all institutions. Before the current catheters and embolic material became available, a cure rate of 5% was being achieved as attested to by analysis of the literature,82 and this came at the cost of an 8% permanent morbidity rate (including 1% mortality). AVMs best suited for intention to cure by embolization are those with a small nidus and a single feeding artery. These are also the lesions associated with lower risk at surgery. Surgery remains the preferred option for these AVMs given the greater chance of cure by this strategy.83 With the advent of improved catheters and the introduction of embolic material such as ethylene vinyl alcohol copolymer dissolved in dimethyl sulfoxide, an increase in the number of AVMs treated by intention to cure is evident from the rise in publications of case series on curative embolization. However, the reported experience remains limited and is likely to be confined to institutions with a bias toward endovascular management rather than surgery. Cure remains more elusive with endovascular treatment than with surgery for the equivalent lesion, and the complications of hemorrhage and infarction remain significant.84–87 Reports of mortality rates of 2% to 3%84,86 for this innovation in embolization suggest that continued development is warranted before it is widely introduced because its efficacy is limited (cures reported in less than 50% of patients with Spetzler-Martin grade I and II AVMs treated by embolization).85
Noncurative or Adjuvant Role for Embolization
Embolization to reduce blood loss as a prelude to surgery continues to remain an important role for this intervention.82,83,88 However, to achieve a reduction in risk for hemorrhage during surgery, it is estimated that 75% of the AVM needs to be obliterated.88 This level of obliteration is difficult to achieve and probably occurs in a minority of patients undergoing embolization.88 When assessing the potential benefits of embolization, the risks associated with embolization (8% morbidity rate, including 1% mortality) should be less than the reduction in risk for combined embolization and surgery when this strategy is used. An understanding of what can and cannot be easily achieved needs to be considered. Feeding arteries that are difficult to safely secure early in the surgery (such as posterior cerebral, lenticulostriate, and occasionally meningeal supply) are ideal candidates for targeted preoperative embolization. However, in many cases these arteries may prove to be difficult to embolize, particularly supply by lenticulostriate and deep meningeal arteries. If these arteries cannot be controlled by embolization, there may be little point in targeting other arteries. Furthermore, targeting convexity feeding arteries and leaving deep thin-walled perforating arteries may change the morphology of these unsecured arteries such that additional enlargement of the diameter and thinning of the walls can take place because of an increase in flow within these vessels if a reduction in the nidus has not been achieved.89 Embolization in this situation may actually increase the risks associated with surgery.
Embolization as a prelude to focused irradiation has been effective.90 By achieving a geographic reduction in volume (rather than a density volume reduction), an AVM may become a more favorable target for focused irradiation. However, as with any combined management strategy, consideration needs to be given to the risks related to each treatment regimen, as well as the risks associated with the natural history of the AVM until it is obliterated. Quantification of risk is extremely difficult because transfer of risk from one source to another is likely dependent on the individual treating center’s approach to AVMs. A conservative embolization approach may well lower the risks inherent with embolization, but at the expense of increasing risk to the patient from other interventions (or the natural history). If a conservative approach were to be used, the rate of morbidity with embolization would optimally be in the vicinity of 2% (as demonstrated by Hartmann and colleagues81), as opposed to Taylor and associates’ report of an 11% morbidity rate.80 This higher level of morbidity may be justifiable only for AVMs for which aggressive embolization has rendered a very difficult AVM resectable with a reduction in risk for complications exceeding 11%. The importance of treating centers to understand their own risks and expectations in their own treatment context and be able to communicate this with patients and colleagues is extremely important. Although it is appropriate to communicate risks associated with individual treatment episodes to patients, these risks must also be placed within the context of the entire management strategy.
Management by Combined Therapies
Combining therapies to manage complex lesions (e.g., embolization followed by focused irradiation and subsequently by surgery) can either be a planned strategy or be a consequence of failure to treat as effectively as planned. Steinberg and colleagues analyzed their experience with surgery performed at significant intervals (up to 11 years) after radiosurgery and concluded that radiosurgery did not have any negative impact on the technical ease of surgery.91 It is difficult to analyze this highly complex group of patients undergoing embolization, radiosurgery, and microsurgery in a way that resembles a normal intention-to-treat group or a retrospectively studied, prospectively entered database. This is partly related to an inability to plan for such a program of treatment in advance (because the intention would be to succeed with fewer management episodes for most patients whenever possible), and only patients well enough to continue with further treatment will complete a full program (thus having an impact on the retrospective analysis of combined treatments). Although combined treatment will be necessary for selected patients,92 it is unlikely to become the mainstay for treating most AVMs because of the commitment of time and resources required with such programs.
Individualizing Treatment Regimens
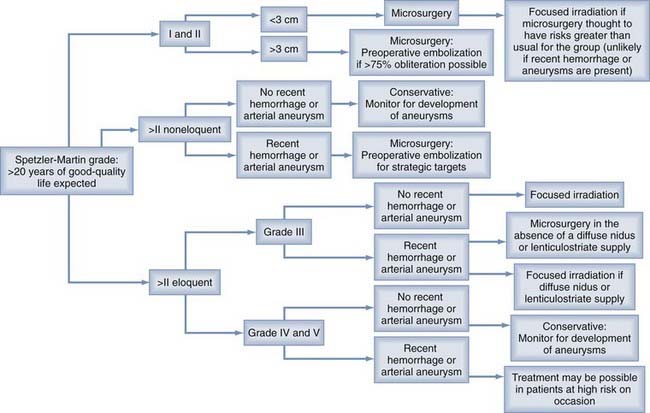
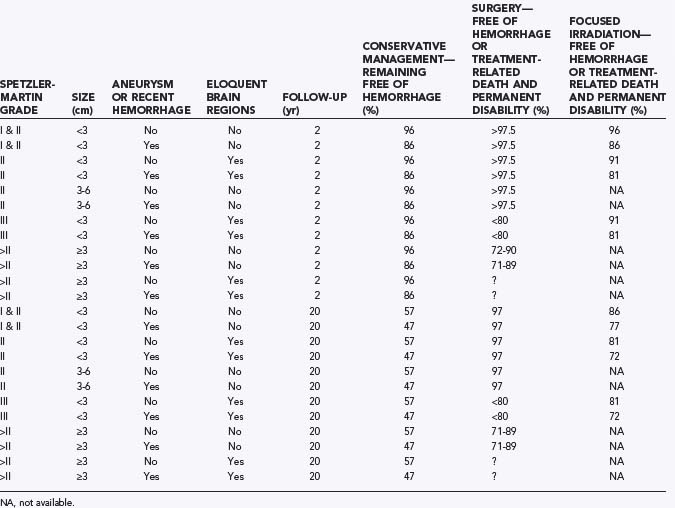
Patients often wish to know whether focused irradiation is an option. To incorporate focused irradiation into a treatment option protocol that is simple, it is reasonable to divide AVMs into those less than 3 cm in diameter (for which focused irradiation is likely to be effective) and those of a larger size (for which focused irradiation is unlikely to be effective). Grading AVMs with the Spetzler-Martin system is reasonable because of its wide adoption and ease of use (Table 385-5). In addition to the Spetzler-Martin grade, the presence of a diffuse nidus and lenticulostriate supply should be considered. These angioarchitectural features will probably have an adverse impact on outcomes with surgery irrespective of the grade assigned. Figure 385-3 has been constructed to facilitate discussion with patients with AVMs who are expected to survive more than 20 years in the absence of the AVM. In addition to providing such a paradigm, it would also be useful to disclose clinical outcomes for the specific treating institution where the patient is to be managed. Whenever possible, results from this treating institution should incorporate the confidence intervals for these results, and information should be provided that allows patients to understand whether the results are applicable to their individual management (Fig. 385-4).
TABLE 385-5 Assessment of Outcomes of Conservative, Surgical, and Radiosurgical Treatment in Patients with Different Clinical Findings, Spetzler-Martin Grades, and Angioarchitectural Features
Perioperative Decision Making
Decision Making to Reduce the Incidence and Impact of Adverse Intraoperative Events
Of critical importance for most AVM surgery is avoidance of complications related to intraoperative events. Such events account for 80% of adverse outcomes.60
Avoidance of Resection of Functional Brain
Identifying the area of the brain that will be affected by surgery as a result of its proximity to the margin of the AVM is a crucial assessment that needs to be made before surgery. This is critical for application of the Spetzler-Martin grade and informed consent of the patient. The traditional method of radiologic-anatomic correlation can be challenging because of the existence of neuronal plasticity and the location of functionally critical brain regions.93 For these congenital lesions, neuronal functional reorganization will typically involve the homologous region in the contralateral hemisphere where similar local cortical organization typically exists.94 However, this cannot be assumed to have occurred, and the Spetzler-Martin grade is generally based on the usual anatomic location for function. The exception to this rule is when function has been lost before surgery. An example is homonymous hemianopia as a result of an occipital AVM. In such cases, the Spetzler-Martin grade of eloquence can be logically discounted if the visual cortex is the only eloquent cortex involved. The possibility of defining loss of function at the margins of the AVM nidus by functional imaging (including positron emission tomography, single-photon emission computed tomography, functional magnetic resonance imaging, magnetoencephalography, and selective amobarbital [Amytal] injection during angiography) is complicated by either the poor spatial resolution of the technique or the difficulty of assessing subtle changes in blood flow adjacent to a lesion where blood flow is very high.94 The potential for false-negative studies diminishes the usefulness of these techniques for reliably determining that function is unimportant adjacent to the AVM nidus. Awake craniotomy has been used, but for large AVM resections (where most of the concern rests), the duration of the procedure, the use of temporary clips, the need for hypotension, and the potential for intraoperative hemorrhage make this technique unsatisfactory.
Avoidance of Intraoperative Hemorrhage
Any arterial aneurysms identified need to be considered for preemptive obliteration to reduce the chance of rupture during surgery as a consequence of an increase in arterial pressure with ablation of the arteriovenous shunt.95–97 This has been reported to account for 7% of all complications leading to permanent deficits.98 For aneurysms on feeding arteries that cannot be exposed at the time of definitive AVM surgery, a separate procedure (either surgical or endovascular) preceding AVM ablation should be considered.95,97 Aneurysms located in the vicinity of the nidus can be addressed at the time of AVM surgery. However, for aneurysms that are proximal, judgment is required to incorporate what the probable change in pressure will be at the aneurysm location (this is dependent on the volume of arteriovenous shunting, as well as the local arteriovenous shunt flow that is carried by the artery with the aneurysm) and how fragile the wall of the aneurysm is likely to be (including size and wall irregularity). Round, small aneurysms proximal to a small AVM may be left with the expectation (requiring angiographic confirmation) that they will heal with ablation of the AVM.
Decision Making to Reduce the Incidence and Impact of Adverse Postoperative Events
Arterial-Capillary-Venous Hypertensive Syndromes
Hemorrhage (Fig. 385-5) developing as a delayed neurological deficit (with the exception of reports of extracerebral hemorrhage and hemorrhage associated with anticoagulant therapy) can be caused by rupture of a retained AVM, rupture of the feeding arterial system, normal perfusion pressure breakthrough, or venous occlusive hyperemia.38,60,98–106 First described by Spetzler and colleagues in 1978,104 normal perfusion pressure breakthrough accounts for some cases of hemorrhage complicating AVM resection. The underlying mechanism is loss of autoregulation induced by the long preoperative period of low pressure within the small arterial system surrounding the AVM. Because of the loss of autoregulation, the capillary bed is unable to be protected from restoration of normal arterial pressure after ablation of the arteriovenous shunt. This results in hyperemia or hemorrhage (or both). Hyperemia associated with intracerebral hemorrhage is seen in other clinical states (e.g., after carotid endarterectomy and high-flow extracranial-to-intracranial bypass).107–110 Experimental evidence suggests the cause to commonly be a sudden reversal of non–infarction-related cerebral hypoperfusion.111–113 Resetting of autoregulation to a lower blood pressure is an attractive hypothesis that can account for the clinical consequences seen in these states caused by a chronic alteration in cerebral perfusion pressure (CPP).32,104,114 In these conditions, chronic vasodilation of (what should be) cerebral resistance vessels results in a greater rise in pressure within them on reversal of the shunt than would have occurred had dilation not been present.32,115 This increase in pressure is transferred to the smaller and weaker vessels within the microcirculation and threatens their integrity.
Challenges to confirming the existence of hyperemia at surgery relate to selecting the site of measuring the changes in blood flow, provided that physiologic conditions are normal during surgery (such as normocapnia), and measuring pressure in both the relevant small pial artery and vein. Young and colleagues performed intraoperative studies to assess pressure autoregulation after resection of AVMs and found it to be intact.116
Despite evidence that hyperemia plays a role in delayed hemorrhage or edema after AVM resection, in many patients with hyperemia at the time of AVM excision, edema or hemorrhage does not develop.117–119 Hyperemia alone cannot account for the pathologic processes variously designated “normal perfusion pressure breakthrough,”104 “congestive apoplexy,”120 “overload,”95 or “vasogenic turgescence of the brain.”34 Furthermore, higher cerebral blood flow and more dramatic changes in cerebral blood flow have been found to occur during the study of seizures, a condition not characterized by brain swelling and hemorrhage.121–123
Stress on the circulation after AVM excision is not confined to the microcirculation. Excision of an AVM removes the low-resistance circulation existing in parallel with the normal circulation. This results in an increase in both mean and pulsatile pressure within the feeding artery toward the more central arterial pressure.32,117,124 Because the radii of arteries feeding the AVM do not immediately return to normal after ablation of the AVM, resistance to flow within these arteries will be lower than normal (even though their downstream resistance is higher), as predicted by the Poiseuille equation.32 This will result in a reduction in the arterial pressure gradient that normally occurs along the vessels before the arteriole. Thus, the pressure that occurs in these arteries, immediately after excision of the AVM, will be greater (approaching central aortic pressure) than normal (i.e., greater than the pressure would have been had the AVM never been present). In addition, the larger than normal diameter redundant feeding arterial system, with its normal downstream microcirculation, will generate a larger reflected wave than a normal-diameter vessel will and result in greater than normal pulsatility and even higher than normal peak pressure within the cardiac cycle.125 The resultant increase in pressure and pulsatility after AVM excision may challenge the integrity of the arterial wall proximal to the microcirculation, particularly at sites of aneurysmal weakness, or in vessels that are distal branches of the feeding artery that may have been extremely thin walled because of the chronic local hypotension.
In addition, the postsurgical state will produce vascular redundancy (in terms of vessel diameter), which may induce a propensity for stagnation and thrombosis.126 Thrombosis may develop in either arteries or veins (Fig. 385-6). Propagation of venous thrombosis of the major draining system is responsible for clinical deterioration in 1% to 3% of patients.38
Differentiating the pathophysiologic mechanisms (between the primary points of failure being the microcirculation, the more proximal arterial circulation, or thrombosis within a system with low shear) can be difficult, if not impossible, but they all have the common abnormality that local intravascular pressure rises to pathologic levels.38 Thus, the term arterial-capillary-venous hypertensive syndrome is appropriate to cover each of these processes (which may be interacting). Delayed ACVH has been reported to occur in 3% of operated patients (see Fig. 385-5A and B).98 It accounted for the majority of the delayed postoperative complications and is at greatest risk in patients with an AVM nidus size of at least 4 cm and postoperative hypertension or in whom markedly redundant draining veins are a feature of the AVM.98,102
Vasospasm
Vasospasm develops as a complication of AVM resection in just 2% of patients but is reported to occur in as many as 27% of procedures involving extensive dissection of the proximal middle and anterior cerebral arteries (Fig. 385-7).98 The degree of vasospasm may not correlate with the extent of subarachnoid blood, and the mechanism of its development may be related to altered vasoreactivity of these vessels at this time. The reduction in shear stress will lead to downregulation in the production of nitric oxide synthase, and an increase in pulsatility can lead to an increase in the release of endothelin. These reactions can produce vasoconstriction. Of importance is that management for prevention of ACVH syndrome with low blood pressure can exacerbate the consequences of the vasoconstriction.32,98 It can be difficult to distinguish between the narrowing that is appropriate and normal with vascular remodeling after AVM resection from narrowing that is excessive and has the potential for cerebral ischemia. The degree of narrowing is clearly the important discriminator in such differentiation.
Management Decisions to Reduce the Potential Risk for Arterial-Capillary-Venous Hypertensive Syndrome and Vasospasm
Perioperative management is important to avoid the complications of ACVH syndrome and vasospasm. A protocol for managing Spetzler-Martin grade III or greater AVMs with diameters of 3.5 cm or larger can be used to reduce the incidence of postoperative hemorrhage from ACVH syndrome (smaller and lower Spetzler-Martin grades are unlikely to have delayed postoperative hemorrhage).32,98 Such a protocol involves intensive continuous monitoring and care for a minimum of 7 days (because delayed hemorrhage can occur within this period after surgery), with strict control of arterial blood pressure to achieve a mean arterial pressure (MAP) at the brain level of no greater than 70 mm Hg.98 In patients in whom clinical assessment is not possible, intracranial pressure can be measured with either a subdural or ventricular catheter (in this case it is important that the pressure within the choroid plexus be normal if a blind pass is to be made) to recognize dangerously low CPP. Blood pressure control can be initiated by preoperative loading with a β-adrenoceptor blocker (to reduce the rate of change of the upslope in pressure during systole) and continuation in the postoperative period. After surgery, a fall in CPP below 60 mm Hg (at the MAP maximum limit of 70 mm Hg) should prompt the performance of computed tomography to exclude a space-occupying lesion. If a hemorrhage is identified, surgery should be performed to ensure that management can continue safely with MAP being no greater than 70 mm Hg. In the event that a minimum CPP of 60 mm Hg cannot be achieved (in the absence of surgically treatable space-occupying lesions), the patient can be treated with therapeutic sodium thiopental coma to a burst suppression pattern on the electroencephalogram. Once burst suppression is achieved, a CPP of 50 mm Hg can be accepted. An escalating drug regimen can be used to achieve the target blood pressure. Such a regimen includes the use of sodium nitroprusside (for rapid titratability), β-adrenoceptor blockade, intravenous magnesium and nimodipine (additionally facilitating prevention of vasospasm), and angiotensin-converting enzyme inhibition (which assists in protection of the brain because it can reduce the lower limit of autoregulation in a normal brain).32 During invasive or potentially painful procedures, maintenance of blood pressure restrictions should be ensured with appropriate sedation or analgesia. Anticonvulsants are usually indicated for AVM surgery, but the need for seizure prevention with these larger lesions is more acute because of the potential for sudden increases in blood pressure during seizures. Fluid balance needs to be monitored carefully and loop diuretics used in the event that a significant positive balance develops. Such intervention can reduce the risk for complications.125
Vasospasm in the presence of a recently resected AVM is complicated by the blood pressure restrictions and the uncertain consequences of angioplasty.127,128 It may well prove that the combination of nimodipine and magnesium is the treatment of choice for the prevention of vasospasm. This is complementary to the requirement for low blood pressure.
The use of anticoagulant or antiplatelet medications is controversial. However, with lesions thought to be especially prone to propagated venous thrombosis (e.g., an extensive redundant venous network on the convexity surface of the brain) but less prone to hemorrhage (e.g., small and superficial), consideration should be given to the early introduction of these agents at a level that is appropriate for the surgery.98
Brown RD, Wiebers DO, Forbes GS. Unruptured intracranial aneurysms and arteriovenous malformations: frequency of intracranial hemorrhage and relationship of lesions. J Neurosurg. 1990;73:859-863.
Brown RD, Wiebers DO, Forbes G, et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988;68:352-357.
Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery. 1994;34:2-7.
Karlsson B, Lax I, Soderman M. Can the probability for obliteration after radiosurgery for arteriovenous malformations be accurately predicted? Int J Radiat Oncol Biol Phys. 1999;43:313-319.
Lawton MT. Spetzler-Martin Grade III arteriovenous malformations: surgical results and a modification of the grading scale. Neurosurgery. 2003;52:740-748.
Morgan MK, Drummond KJ, Sorby W, et al. Cerebral AVM surgery: risks related to lenticulostriate arterial supply. J Neurosurg. 1997;86:801-805.
Morgan MK, Rochford AM, Tsahtsarlis A, et al. Surgical risks associated with management of grade 1 and 2 brain arteriovenous malformations. Neurosurgery. 2004;54:832-837.
Morgan MK, Winder M, Little NS, et al. Delayed hemorrhage following brain AVM resection. J Neurosurg. 2003;99:967-971.
Ondra SL, Troupp H, George ED, et al. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73:387-391.
Piepgras DG, Sundt TMJr, Ragoowansi AT, et al. Seizure outcome in patients with surgically treated cerebral arteriovenous malformations. J Neurosurg. 1993;78:5-11.
Redekop G, TerBrugge K, Montanera W, et al. Arterial aneurysms associated with cerebral arteriovenous malformations: classification, incidence, and risk of hemorrhage. J Neurosurg. 1998;89:539-546.
Spears J, TerBrugge KG, Moosavian M, et al. A discriminative prediction model of neurological outcome for patients undergoing surgery of brain arteriovenous malformations. Stroke. 2006;37:1457-1464.
Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483.
1 Dandy WE. Arteriovenous aneurysm of the brain. Arch Surg. 1928;17:190-243.
2 Norlen G. Arteriovenous aneurysms of the brain: report of ten cases of total removal of the lesion. J Neurosurg. 1949;6:475-494.
3 Olivecrona H, Riives J. Arteriovenous aneurysms of the brain. Their diagnosis and treatment. Arch Neurol Psychiatry. 1948;59:567-602.
4 Al-Shahi R, Bhattacharya JJ, Currie DG, et al. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke. 2003;34:1163-1169.
5 Stapf C, Mast H, Sciacca RR, et al. The New York Islands AVM Study: design, study progress and initial results. Stoke. 2003;34:e29-e33.
6 Al-Shahi R, Pal N, Lewis SC, et al. Observer agreement in the angiographic assessment of arteriovenous malformations of the brain. for the AVM Observer Agreement Study Group. Stroke. 2002;33;:1501-1508.
7 Iancu-Gontard D, Guilbert F, Nguyen T, et al. Inter- and intraobserver variability in the assessment of brain arteriovenous malformation angioarchitecture and endovascular treatment results. AJNR Am J Neuroradiol. 2007;28:524-527.
8 Al-Shahi R, Warlow C. Arteriovenous malformations of the brain: ready to randomise? J Neurol Neurosurg Psychiatry. 2005;76:1327-1329.
9 Ondra SL, Troupp H, George ED, et al. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73:387-391.
10 da Costa LB, Wallace MC, TerBrugge K, et al. A single-center, prospective analysis of the natural history of hemorrhage from brain arteriovenous malformations with or without associated aneurysms: 805. Neurosurgery. 2005;57:396-397.
11 Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58:331-337.
12 Crawford PM, West CR, Chadwick DW, et al. Arteriovenous malformations of the brain: the natural history in unoperated patients. J Neurol Neurosurg Psychiatry. 1986;49:1-10.
13 Brown RD, Wiebers DO, Forbes G, et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988;68:352-357.
14 Brown RD, Wiebers DO, Forbes GS. Unruptured intracranial aneurysms and arteriovenous malformations: frequency of intracranial hemorrhage and relationship of lesions. J Neurosurg. 1990;73:859-863.
15 ApSimon HT, Reef H, Phadke RV, et al. A population-based study of brain arteriovenous malformation. Long-term treatment outcomes. Stroke. 2002;33:2794-2800.
16 Halim AX, Johnston SC, Singh V, et al. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke. 2004;35:1697-1702.
17 Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350-1355.
18 Wedderburn CJ, van Beijnum J, Bhattacharya JJ, et al. Outcome after interventional or conservative management of unruptured brain arteriovenous malformations: a prospective, population-based cohort study. for the SIVMS Collaborators. Lancet Neurol. 2008;7;:223-230.
19 Redekop G, TerBrugge K, Montanera W, et al. Arterial aneurysms associated with cerebral arteriovenous malformations: classification, incidence, and risk of hemorrhage. J Neurosurg. 1998;89:539-546.
20 Stapf C, Khaw AV, Sciacca RR, et al. Effect of age on clinical and morphological characteristics in patients with brain arteriovenous malformation. Stroke. 2003;34:2664-2670.
21 Dobbelaere P, Jomin M, Clarrisse J, et al. Interet pronostique de letude du drainage verneux de aneurysms arterio-veneux cerebraux. Neurochirugie. 1979;25:178-184.
22 Vinuela F, Nombela L, Roach MR, et al. Stenotic and occlusive disease of the venous drainage system of deep brain AVMs. J Neurosurg. 1985;63:180-184.
23 Stefani MA, Porter PJ, TerBrugge KG, et al. Angioarchitectural factors present in brain arteriovenous malformations associated with hemorrhagic presentation. Stroke. 2002;33:920-924.
24 Fults D, Kelly DL. Natural history of arteriovenous malformations of the brain: a clinical study. Neurosurgery. 1984;15:658-662.
25 Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350-1355.
26 Luessenhop AJ, Rosa L. Cerebral arteriovenous malformations. Indications for and results of surgery, and the role of intravascular techniques. J Neurosurg. 1984;60:14-22.
27 Brown RDJr, Wiebers DO, Torner JC, et al. Frequency of intracranial hemorrhage as a presenting symptom and subtype analysis: a population-based study of intracranial vascular malformations in Olmsted County, Minnesota. J Neurosurg. 1996;85:29-32.
28 Luessenhop AJ. Natural history of cerebral arteriovenous malformations. In: Wilson CB, Stein BM, editors. Intracranial Arteriovenous Malformations. Baltimore: Williams & Wilkins; 1984:12-23.
29 Hartmann A, Mast H, Mohr JP, et al. Morbidity of intracranial hemorrhage in patients with cerebral arteriovenous malformation. Stroke. 1998;29:931-934.
30 Morgan MK, Sekhon L, Rahman Z, et al. Morbidity of intracranial hemorrhage in patients with cerebral arteriovenous malformation. Stroke. 1998;29:2001-2003.
31 Choi JH, Mast H, Sciacca RR, et al. Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformation. Stroke. 2006;37:1243-1247.
32 Morgan MK, Winder M. Haemodynamics of arteriovenous malformation of the brain and consequences of resection. A review. J Clin Neurosci. 2001;8:216-224.
33 Luessenhop AJ. AVM grading in assessing surgical risk. J Neurosurg. 1987;66:637-638.
34 Pertuiset B, Ancri D, Kinuta Y, et al. Classification of supratentorial arteriovenous malformations. A score system for evaluation of operability and surgical strategy based on an analysis of 66 cases. Acta Neurochir (Wien). 1991;110:6-16.
35 Spears J, TerBrugge KG, Moosavian M, et al. A discriminative prediction model of neurological outcome for patients undergoing surgery of brain arteriovenous malformations. Stroke. 2006;37:1457-1464.
36 Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483.
37 Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery. 1994;34:2-7.
38 Morgan MK, Drummond KJ, Sorby W, et al. Cerebral AVM surgery: risks related to lenticulostriate arterial supply. J Neurosurg. 1997;86:801-805.
39 Ferch RD, Morgan MK. High grade arteriovenous malformations and their management. J Clin Neurosci. 2002;9:37-40.
40 Du R, Keyoung HM, Dowd CF, et al. The effects of diffuseness and deep perforating artery supply on outcomes after microsurgical resection of brain arteriovenous malformations. Neurosurgery. 2007;60:638-646.
41 Lawton MT. Spetzler-Martin grade III arteriovenous malformations: surgical results and a modification of the grading scale. Neurosurgery. 2003;52:740-748.
42 Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery. 1994;34:2-6.
43 Hassler W, Hejazi N. Complications of angioma surgery—personal experience in 191 patients with cerebral angiomas. Neurol Med Chir (Tokyo). 1998;38(suppl):238-244.
44 Pikus HJ, Beach ML, Harbaugh RE. Microsurgical treatment of arteriovenous malformations: analysis and comparison with stereotactic radiosurgery. J Neurosurg. 1998;88:641-646.
45 Heros RC. Prevention and management of therapeutic complications. In: Jafar JJ, Awad IA, Rosenwasser RH, editors. Vascular Malformations of the Central Nervous System. New York: Lippincott Williams & Wilkins; 1999:363-374.
46 Han PP, Ponce FA, Spetzler RF. Intention-to-treat analysis of Spetzler-Martin grades IV and V arteriovenous malformations: natural history and treatment paradigm. J Neurosurg. 2003;98:3-7.
47 Morgan MK, Rochford AM, Tsahtsarlis A, et al. Surgical risks associated with management of grade 1 and 2 brain arteriovenous malformations. Neurosurgery. 2004;54:832-837.
48 Shi YQ, Chen XC. A proposed scheme for grading intracranial arteriovenous malformations. J Neurosurg. 1986;65:484-489.
49 Steinmeier R, Schramm J, Muller HG, et al. Evaluation of prognostic factors in cerebral arteriovenous malformations. Neurosurgery. 1989;24:193-200.
50 Tamaki N, Ehara K, Lim TK, et al. Cerebral arteriovenous malformations: factors influencing the surgical difficulty and outcome. Neurosurgery. 1991;29:856-863.
51 Stapf C, Connolly ES, Schumacher HC, et al. Dysplastic vessels after surgery for brain arteriovenous malformations. Stroke. 2002;33:1053-1056.
52 Sato S, Kodama N, Sasaki T, et al. Perinidal dilated capillary networks in cerebral arteriovenous malformations. Neurosurgery. 2004;54:163-168.
53 Kader A, Goodrich JT, Sonstein WJ, et al. Recurrent cerebral arteriovenous malformations after negative postoperative angiograms. J Neurosurg. 1996;85:14-18.
54 Matsumoto H, Takeda T, Kohno K, et al. Delayed hemorrhage from completely obliterated arteriovenous malformation after gamma knife radiosurgery. Neurol Med Chir (Tokyo). 2006;46:186-190.
55 Maruyama K, Shin M, Tago M, et al. Radiosurgery to reduce the risk of first hemorrhage from brain arteriovenous malformations. Neurosurgery. 2007;60:453-458.
56 Piepgras DG, Sundt TMJr, Ragoowansi AT, et al. Seizure outcome in patients with surgically treated cerebral arteriovenous malformations. J Neurosurg. 1993;78:5-11.
57 Thorpe ML, Cordato DJ, Morgan MK, et al. Postoperative seizure outcome in a series of 114 patients with supratentorial arteriovenous malformations. J Clin Neurosci. 2000;7:107-111.
58 Hoh BL, Chapman PH, Loeffler JS, et al. Results of multimodality treatment for 141 patients with brain arteriovenous malformations and seizures: factors associated with seizure incidence and seizure outcomes. Neurosurgery. 2002;51:303-309.
59 Yeh H, Tew JM, Gartner M. Seizure control after surgery on cerebral arteriovenous malformations. J Neurosurg. 1993;78:12-18.
60 Morgan MK, Johnston IH, Hallinan JM, et al. Complications of surgery for arteriovenous malformations of the brain. J Neurosurg. 1993;78:176-182.
61 Karlsson B, Lax I, Soderman M. Can the probability for obliteration after radiosurgery for arteriovenous malformations be accurately predicted? Int J Radiat Oncol Biol Phys. 1999;43:313-319.
62 Friedman WA, Bova FJ, Bollampally SBS, et al. Analysis of factors predictive of success or complications in arteriovenous malformation radiosurgery. Neurosurgery. 2003;52:296-308.
63 Heffez DS, Osterdock RJ, Alderete L, et al. The effect of incomplete patient follow-up on the reported results of AVM radiosurgery. Surg Neurol. 1998;49:373-381.
64 Karlsson B, Jokura H, Yamamoto M, et al. Is repeated radiosurgery an alternative to staged radiosurgery for very large brain arteriovenous malformations? J Neurosurg. 2007;107:740-744.
65 Jones J, Jang S, Getch CC, et al. Advances in the radiosurgical treatment of large inoperable arteriovenous malformations. Neurosurg Focus. 2007;23(6):E7.
66 Karlsson B, Lindqvist M, Blomgren H, et al. Long-term results after fractionated radiation therapy for large brain arteriovenous malformations. Neurosurgery. 2005;57:42-49.
67 Andrade-Souza YM, Ramani M, Scora D, et al. Embolization before radiosurgery reduces the obliteration rate of arteriovenous malformations. Neurosurgery.. 2007;60:443-452.
68 Schlienger M, Atlan D, Lefkopoulos D, et al. Linac radiosurgery for cerebral arteriovenous malformations: results in 169 patients. Int J Radiat Oncol Biol Phys. 2000;46:1135-1142.
69 Buis DR, Dirven CM, Lagerwaard FJ, et al. Radiosurgery of brain arteriovenous malformations in children. J Neurol. 2008;255:551-560.
70 Kiran NA, Kale SS, Vaishya S, et al. Gamma Knife surgery for intracranial arteriovenous malformations in children: a retrospective study in 103 patients. J Neurosurg. 2007;107(6 suppl):479-484.
71 Buis DR, Lagerwaard FJ, Barkhof F, et al. Stereotactic radiosurgery for brain AVMs: role of interobserver variation in target definition on digital subtraction angiography. Int J Radiat Oncol Biol Phys. 2005;62:246-252.
72 Nataf F, Ghossoub M, Schlienger M, et al. Bleeding after radiosurgery for cerebral arteriovenous malformations. Neurosurgery. 2004;55:298-305.
73 Betti OO, Munari C, Rosier R. Stereotactic radiosurgery with the linear accelerator: treatment of arteriovenous malformations. Neurosurgery. 1989;24:311-321.
74 Pollock BE, Lunsford LD, Dondziolka D, et al. Patient outcomes after stereotactic radiosurgery for “operable” arteriovenous malformations. Neurosurgery. 1994;35:1-8.
75 Friedman WA, Blatt DL, Bova FJ, et al. The risk of hemorrhage after radiosurgery for arteriovenous malformations. J Neurosurg. 1996;84:912-919.
76 Pollock BE, Flickinger JC, Lunsford LD, et al. Hemorrhage risk after stereotactic radiosurgery of cerebral arteriovenous malformations. Neurosurgery. 1996;38:652-660.
77 Steiner L, Lindquist C, Adler JR, et al. Clinical outcome of radiosurgery for cerebral arteriovenous malformations. J Neurosurg. 1992;77:1-8.
78 Flickinger JC, Kondziolka D, Lunsford LD, et al. Development of a model to predict permanent symptomatic postradiosurgery injury for arteriovenous malformation patients. Int J Radiat Oncol Biol Phys. 2000;46:1143-1148.
79 Luessenhop AJ, Spence WT. Artificial embolization of cerebral arteries: report of use in a case of arteriovenous malformation. JAMA. 1960;172:1153-1155.
80 Taylor CL, Dutton K, Rappard G, et al. Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg. 2004;100:810-812.
81 Hartmann A, Pile-Spellman J, Stapf C, et al. Risk of endovascular treatment of brain arteriovenous malformations. Stroke. 2002;33:1816-1820.
82 Frizzel RT, Fisher WSIII. Cure, morbidity, and mortality associated with embolization of brain arteriovenous malformations: a review of 1246 patients in 32 series over a 35-year period. Neurosurgery. 1995;37:1031-1040.
83 Morgan MK, Zurin AAR, Harrington T, et al. Changing role for preoperative embolisation in the management of arteriovenous malformations of the brain. J Clin Neurosci. 2000;7:527-530.
84 van Rooij WJ, Sluzewski M, Beute GN. Brain AVM embolization with Onyx. AJNR Am J Neuroradiol. 2007;28:172-177.
85 Weber W, Kis B, Siekmann R, et al. Endovascular treatment of intracranial arteriovenous malformations with onyx: technical aspects. AJNR Am J Neuroradiol. 2007;28:371-377.
86 Katsaridis V, Papagiannaki C, Aimar E. Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology. 2008;50:589-597.
87 Mounayer C, Hammami N, Piotin M, et al. Nidal embolization of brain arteriovenous malformations using Onyx in 94 patients. AJNR Am J Neuroradiol. 2007;28:518-523.
88 Vinuela F, Dion JE, Duckwiler G, et al. Combined endovascular embolization and surgery in the management of cerebral arteriovenous malformations: experience with 101 cases. J Neurosurg. 1991;75:856-864.
89 Morgan MK, Sundt TMJr. The case against staged operative resection of cerebral arteriovenous malformations. Neurosurgery. 1989;25:429-436.
90 Gobin YP, Laurent A, Merienne L, et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg. 1996;85:19-28.
91 Steinberg GK, Chang SD, Levy RP, et al. Surgical resection of large incompletely treated intracranial arteriovenous malformations following stereotactic radiosurgery. J Neurosurg. 1996;84:920-928.
92 Chang SD, Marcellus ML, Marks MP, et al. Multimodality treatment of giant intracranial arteriovenous malformations. Neurosurgery. 2000;53:1-11.
93 Lazar RM, Marshall RS, Pile-Spellman J, et al. Interhemispheric transfer of language in patients with left frontal cerebral arteriovenous malformation. Neuropsychologia. 2000;38:1325-1332.
94 Cannestra AF, Pouratian N, Forage J, et al. Functional magnetic resonance imaging and optical imaging for dominant-hemisphere perisylvian arteriovenous malformations. Neurosurgery. 2004;55:804-812.
95 Drake CG. Considerations for and experience with surgical treatment in 166 cases. Clin Neurosurg. 1979;26:145-206.
96 Batjer H, Suss RA, Samson D. Intracranial arteriovenous malformations associated with aneurysms. Neurosurgery. 1986;18:29-35.
97 Thompson RC, Steinberg GK, Levy RP, et al. The management of patients with arteriovenous malformations and associated intracranial aneurysms. Neurosurgery. 1998;43:202-212.
98 Morgan MK, Sekhon LHS, Finfer S, et al. Delayed neurological deterioration following resection of arteriovenous malformations of the brain. J Neurosurg. 1999;90:695-701.
99 Sundt TMJr, Piepgras DG, Stevens LN. Surgery for supratentorial arteriovenous malformations. Clin Neurosurg. 1990;37:49-115.
100 Yasargil MG, Curcic M, Kis M, et al. Microneurosurgery, vol 3 B. New York: Thieme. 1988.
101 Drake CG. Considerations for and experience with surgical treatment in 166 cases. Clin Neurosurg. 1979;26:145-206.
102 Al-Rodhan NRF, Sundt TMJr, Piepgras DG, et al. Occlusive hyperemia: a theory for the hemodynamic complications following resection of intracerebral arteriovenous malformations. J Neurosurg. 1993;78:167-175.
103 Batjer HH, Devous MDSr, Meyer YJ, et al. Cerebrovascular hemodynamics in arteriovenous malformation complicated by normal perfusion pressure breakthrough. Neurosurgery. 1988;22:503-509.
104 Spetzler RF, Wilson CB, Weinstein P, et al. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651-672.
105 Young WL, Kader A, Ornstein E, et al. Cerebral hyperemia after arteriovenous malformation resection is related to “breakthrough” complications but not to feeding artery pressure. Neurosurgery. 1996;38:1085-1095.
106 Sekhon LHS, Morgan MK, Spence I, et al. Normal perfusion pressure breakthrough: the role of capillaries. J Neurosurg. 1997;86:519-524.
107 Piepgras DG, Morgan MK, Sundt TMJr, et al. Intracerebral hemorrhage after carotid endarterectomy. J Neurosurg. 1988;68:532-536.
108 Powers AD, Smith RR. Hyperperfusion syndrome after carotid endarterectomy: a transcranial Doppler evaluation. Neurosurgery. 1990;26:56-60.
109 Schroeder T, Sillesen H, Sorensen O, et al. Cerebral hyperperfusion following carotid endarterectomy. J Neurosurg. 1987;66:824-829.
110 Sundt TMJr, Piepgras DG, Marsh WR, et al. Bypass vein grafts for giant aneurysms and severe intracranial occlusive disease in the anterior and posterior circulation. In: Sundt TMJr, editor. Occlusive Cerebrovascular Disease. Diagnosis and Surgical Management. Philadelphia: WB Saunders; 1987:439-464.
111 Morgan MK, Anderson RB, Sundt TMJr. A model of the pathophysiology of cerebral arteriovenous malformations by a carotid-jugular fistula in the rat. Brain Res. 1989;496:241-250.
112 Morgan MK, Anderson RE, Sundt TMJr. The effects of hyperventilation on cerebral blood flow in the rat with an open and closed carotid-jugular fistula. Neurosurgery. 1989;25:606-612.
113 Irikura K, Morii S, Miyasaka Y, et al. Impaired autoregulation in an experimental model of chronic cerebral hypoperfusion in rats. Stroke. 1996;27:1399-1404.
114 Spetzler RF, Hamilton MG. Pressure autoregulation is intact after arteriovenous malformation resection. Neurosurgery. 1993;33:772-773.
115 Postiglione A, Bobkiewicz T, Vinholdt-Pedersen E, et al. Cerebrovascular effects of angiotensin converting enzyme inhibition involve large artery dilatation in rats. Stroke. 1991;22:1363-1368.
116 Young WL, Kader A, Prohovnik I, et al. Pressure autoregulation is intact after arteriovenous malformation resection. Neurosurgery. 1993;32:491-497.
117 Barnett GH, Little JR, Ebrahim ZY, et al. Cerebral circulation during arteriovenous malformation operation. Neurosurgery. 1987;20:836-842.
118 Rosenblum BR, Bonner RF, Oldfield EH. Intraoperative measurement of cortical blood flow adjacent to cerebral AVM using laser Doppler velocimetry. J Neurosurg. 1987;66:396-399.
119 Young WL, Prohovnik I, Ornstein E, et al. Monitoring of intraoperative cerebral hemodynamics before and after arteriovenous malformation resection. Anesth Analg. 1988;67:1011-1014.
120 Gowers WR. A Manual of Disease of the Nervous System. Philadelphia: P. Blakiston; 1888. 764-773
121 Nilsson B, Rehncrona S, Siesjö BK. Coupling of cerebral metabolism and blood flow in epileptic seizures, hypoxia and hypoglycaemia. Ciba Found Symp. 1978;56:199-218.
122 Nitsch C, Suzuki R, Fujiwara K, et al. Incongruence of regional cerebral blood flow increase and blood-brain barrier opening in rabbits at the onset of seizures induced by bicuculline, methoxypyridoxine, and kainic acid. J Neurol Sci. 1985;67:67-79.
123 Tenny RT, Sharbrough FW, Anderson RE, et al. Correlation of intracellular redox states and pH. Ann Neurol. 1980;8:564-573.
124 Sorimachi T, Takeuchi S, Koike T, et al. Blood pressure monitoring in feeding arteries of cerebral arteriovenous malformations during embolization: a preventive role in hemodynamic complications. Neurosurgery. 1995;37:1041-1048.
125 Nichols WW, O’Rourke MF. McDonald’s Blood Flow in Arteries. Theoretic, Experimental and Clinical Principles, 3rd ed. London: Edward Arnold; 1990. 254
126 Hassler W, Steinmetz H. Cerebral hemodynamics in angioma patients: an intraoperative study. J Neurosurg. 1987;67:822-831.
127 Morgan MK, Winder M, Little NS, et al. Delayed hemorrhage following brain AVM resection. J Neurosurg. 2003;99:967-971.
128 Morgan MK, Day MJ, Little N, et al. The use of intra-arterial papaverine in the management of vasospasm complicating the resection of arteriovenous malformations of the brain: a report of two cases. J Neurosurg. 1995;82:296-299.