Chapter 2 Therapeutic Agents for Spine Injection
Local Anesthetics, Steroids, and Contrast Media
 Local anesthetics are rarely associated with immediate hypersensitivity (allergic) reactions, and the amide-type local anesthetics are particularly safe.
Local anesthetics are rarely associated with immediate hypersensitivity (allergic) reactions, and the amide-type local anesthetics are particularly safe. Local anesthetic systemic toxicity is an emergency that requires additional help and may be ameliorated with lipid emulsion therapy.
Local anesthetic systemic toxicity is an emergency that requires additional help and may be ameliorated with lipid emulsion therapy. Corticosteroid agents for spinal injection have significant dose-related complications with repetitive use. Users should be aware that multiple practitioners may be using these agents, with no consistent dosing guidelines.
Corticosteroid agents for spinal injection have significant dose-related complications with repetitive use. Users should be aware that multiple practitioners may be using these agents, with no consistent dosing guidelines. Particulate steroids have been associated with catastrophic spinal cord and brainstem infarcts, presumably from embolization into critical arteries, and caution suggests that nonparticulate agents may be more appropriate in specific procedures (e.g., cervical transforaminal epidurals).
Particulate steroids have been associated with catastrophic spinal cord and brainstem infarcts, presumably from embolization into critical arteries, and caution suggests that nonparticulate agents may be more appropriate in specific procedures (e.g., cervical transforaminal epidurals). Patients with iodine or shellfish allergies are not at greater risk for adverse reactions to contrast agents.
Patients with iodine or shellfish allergies are not at greater risk for adverse reactions to contrast agents. In cases of previous reaction to iodinated contrast, gadolinium is an acceptable alternative with extremely low incidence of adverse reactions.
In cases of previous reaction to iodinated contrast, gadolinium is an acceptable alternative with extremely low incidence of adverse reactions. Patients with previous anaphylactoid reactions, those with asthma, and certain food allergies may benefit from pretreatment with histamine receptor 1 and 2 antagonists and systemic steroids.
Patients with previous anaphylactoid reactions, those with asthma, and certain food allergies may benefit from pretreatment with histamine receptor 1 and 2 antagonists and systemic steroids. In patients who have had prior anaphylaxis due to contrast dyes, one might consider not injecting contrast agent if it is not critical to the outcome.
In patients who have had prior anaphylaxis due to contrast dyes, one might consider not injecting contrast agent if it is not critical to the outcome. Because local anesthetic injection does not contribute to the long-term therapeutic outcome in most spinal injections, and may cause toxicity or fall risk, its use should be limited.
Because local anesthetic injection does not contribute to the long-term therapeutic outcome in most spinal injections, and may cause toxicity or fall risk, its use should be limited. Doses of corticosteroid are not standardized, and thus dose selection should always be the lowest possible to achieve a therapeutic effect.
Doses of corticosteroid are not standardized, and thus dose selection should always be the lowest possible to achieve a therapeutic effect.Introduction
Therapeutic spinal injections date back to the early part of the twentieth century before World War II. Over the past several decades, corticosteroids have emerged as the preferred class of spinally administered drugs for presumed inflammatory causes of pain. Common usage has been based largely on case series demonstrating efficacy and select double-blind placebo controlled trials (see Chapter 1). Surface landmark–based technique has also changed over time, with most authors currently endorsing very specific fluoroscopically controlled and contrast-proven injections. Despite these proscriptions, there have been no large-scale head-to-head trials that demonstrate outcome-based superiority of the more technologically advanced techniques. This chapter focuses on the agents that are commonly being used to perform therapeutic epidural and spinal injections, as well as the appropriate and safe use of contrast media (e.g., iohexol or gadodiamide agents) and some of the experimental agents being considered for future use.
History
One of the first reports of spinal injections for pain control was trans-sacral (caudal) injections of the local anesthetic procaine.1 The first use of epidural corticosteroids came out of the European literature.2 One of the first large series was published in 1961, in which Goebert and colleagues3 administered 121 injections to 113 patients spanning a 5-year period. The majority of these injections were caudal epidural injections with only three cervical epidural injections. Large-volume injections of a mixture of 1% procaine with 125 mg of hydrocortisone acetate in 30-mL volumes were administered over a consecutive 3-day period.3 Subsequently, in the modern era after the 1970s, interlaminar epidural injections became standard. Winnie and colleagues4 published recommendations that are still popular today, including corticosteroid dose limitations, 2-week dosing intervals, and three epidural procedures in series. Later, many physicians adopted a transforaminal fluoroscopically guided approach.
Local Anesthetics
Local anesthetics block voltage-gated sodium channels and interrupt propagation of axonal impulses, but their action is not only limited to those biological actions. There are two classes of local anesthetics based on structure–activity relationships: amino esters and amino amides. Because of the popularity and much more common use of amide-type local anesthetics for spinal diagnostic and therapeutic procedures, the focus of this review is mainly on the amide-type local anesthetics lidocaine and bupivacaine.5,6
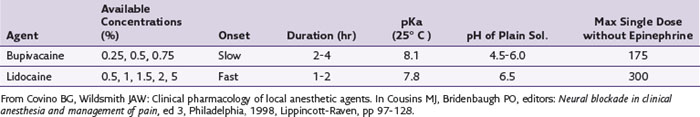
Important properties of local anesthetics that pertain to clinical use include potency, speed of onset, and duration of action. The potency of a local anesthetic is related to its lipid solubility, which is usually defined by the octanol-buffer coefficient. The molecule must diffuse into the nerve membrane and bind at a partially hydrophobic site on the sodium channel.7 The more hydrophobic or lipophilic the local anesthetic, the more quickly it will permeate neuronal membranes, which increases its sodium channel binding affinity. Bupivacaine, for example, is many times more lipophilic than lidocaine (Table 2-1).
The speed of onset of most local anesthetics directly relates to the dissociation constant, or pKa, of the compound as well as the pH of the local tissues. The pKa is the pH at which half of the compound is ionized or protonated; the other half is in the un-ionized or neutral form that more readily crosses the nerve membrane. This makes the local anesthetic with the pKa that is closest to physiological pKa of faster onset. The pH of the local anesthetic preparation also affects the onset time, and some commercially available preparations containing a vasoconstrictor (e.g., epinephrine) have an adjusted pH that is acidic because of the addition of hydrochloric salts, enhancing the stability of the vasoconstrictor (Table 2-2).8,9 In vivo, other factors such as dose or concentration can also affect the onset of action.7 If faster onset is desired, then the addition of a small amount of sodium bicarbonate (1 : 20 NaHCO3-to-anesthetic volume) can help adjust the pH closer to physiological conditions. Caution should be taken not to adjust the pH greater than 7 because of the possibility that drug precipitation will increase.
Defining the duration of action is somewhat more difficult because it depends on multiple variables such as the location of injection, the lipophilicity of the local anesthetic, the dose, and the presence or absence of a vasoconstrictor. Longer acting local anesthetics are more lipophilic and are more slowly “washed out” from the lipophilic membrane.10 In humans, the peripheral vascular effects of the local anesthetics themselves also affect duration. Many agents have a biphasic effect on vascular smooth muscle with a vasoconstrictive response at low concentrations and vasodilatation at higher concentrations. These effects are complex and vary according to the concentration, time, and location of injection.7 In general, the more vascular the location, the more rapidly the agent is absorbed, metabolized, and excreted.
Adverse Reactions
Reactions involving the use of local anesthetics can result from a number of different sources, including toxicity from the medication itself, a reaction to a preservative or added vasoconstrictor, or even an allergic reaction. Outside of the discussion of PABA as a potential allergen, immediate hypersensitivity reactions to amide local anesthetics and their pathomechanism remain largely unidentified. Clinically, the allergic response corresponds with anaphylaxis (manifesting as tachycardia; hypotension; and feelings of weakness, heat, or vertigo) even though immunologically mediated reactions have rarely been observed. Other ingredients in the local anesthetic commercial preparations such as certain preservatives or even anxiety need to be considered as potential sources of the adverse reaction as well.11
Epidemiological studies report a range of incidence of LAST that varies from 0 to 79 per 10,000.12–14 In general, the cardiac toxicity results from the binding and inhibition of sodium channels, however, and correlates with local anesthetic potency especially in regards to inhibition of cardiac conduction. In addition, there is a vast array of other inotropic and metabolic signaling systems as well as mitochondrial metabolism implicated as potential targets for local anesthetics that would help explain the local anesthetic agents variable role in LAST.15
One way to reduce the incidence of LAST is to prevent it. Unfortunately, there is no single intervention that has proven to prevent this potentially life-threatening response. Obviously, using the least amount of local anesthetic required, incremental injection, frequent aspiration looking for the return of blood, using an intravascular marker such as epinephrine, and the use of ultrasound guidance are all recommended to try to prevent LAST.15
Classically, LAST presents in a predictable sequence with subjective central nervous system (CNS) symptoms of auditory changes, circumoral numbness, and metallic taste. Signs can then progress to seizures, coma, respiratory arrest, and ultimately cardiac toxicity that include cardiac excitation followed by cardiac depression at greatly increased blood concentrations. Unfortunately, in reality, the presentation can be extremely variable and is atypical in about 40% of cases with LAST. Even with an atypical presentation, the first symptoms presented in less than 5 minutes after injection 75% of the time. Seizure was the most common presenting symptom, and fewer than 20% of these seizures presented without any of the classic prodromal symptoms.15
Treatment of LAST, given the possible serious morbidity, should be quick and aggressive. Priorities include obtaining an airway, circulatory support, and diminishing the systemic effects as much as possible. Seizures should be rapidly treated with benzodiazepines if possible. Initiating the clinical algorithm as part of advanced cardiac life support (ACLS) is also important, although LAST presents a very different clinical scenario than that usually addressed by ACLS. The cause of circulatory arrest in LAST means that vasopressin and epinephrine may have less of a role or not be recommended. In fact, animal studies indicate that lipid emulsion treatment had better outcomes than both epinephrine and vasopressin.15,16 (Please refer to the Practice Advisory sheets included in Table 2-3.)
Table 2-3 Practice Advisory on Treatment of Local Anesthetic Systemic Toxicity for Patients Experiencing Signs or Symptoms of Local Anesthetic Systemic Toxicity
IV, intravenous; LAST, local anesthetic systemic toxicity.
Neal JM, Bernards CM, Butterworth JF IV, et al: ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med 35:152-161, 2010.
Corticosteroids
Cortisone as a purified glucocorticoid preparation was first introduced in 1949; later, in 1952, its application was described for epidural use.17–19 Since then, the use of steroids has been applied in the field of interventional pain management with varying degrees of success and complications.
Glucocorticoids, of which the injectable corticosteroids are a part, are produced in the zona fasciculate of the adrenal cortex and function under negative feedback from the hypothalamus and pituitary gland as part of the hypothalamus–pituitary–adrenal axis.20 Glucocorticoids are used in interventional pain procedures because of their effects on inflammation. Glucocorticoids have significant inhibitory effects on cytokines and chemokines that are generated at sites of inflammation, as well as suppressive effects on leukocyte concentration, distribution, and function. Glucocorticoids have potential effects on most cells of the body through interactions with glucocorticoid receptors. Normally, intracellular glucocorticoid receptors are in a stabilized form coupled with two elements of heat-shock protein 90 (HSP90) and other proteins. Binding of glucocorticoid to its receptor allows the glucocorticoid to enter the cell where dissociation of the proteins occurs, and the glucocorticoid–receptor complex binds to the glucocorticoid response element of a target gene. Resultant transcription activity via RNA polymerase is thus altered, eventually leading to alterations in messenger RNA (mRNA) and new protein production, which leads to the hormonal response.21
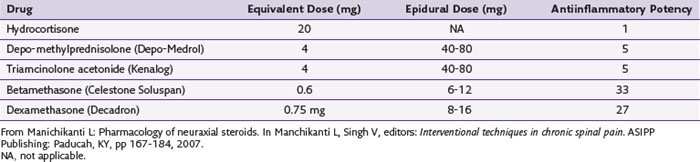
Table 2-4 lists the antiinflammatory potency of some of the more commonly used neuraxial steroids.
Complications
Multiple complications of corticosteroids are possible and largely relate to unwanted side effects (e.g., iatrogenic Cushing’s syndrome). Tuel and colleagues22 described a case of iatrogenic Cushing’s syndrome occurring in a 24-year-old man after a single dose of 60 mg of methylprednisolone. Laboratory evidence of suppression of the hypothalamic–pituitary axis, 20-lb weight gain, and cushingoid features (moon facies, stria) persisted for 12 months. In another report, two doses of 80 mg of methylprednisolone resulted in Cushing’s syndrome with peripheral edema, moon facies, a “buffalo hump,” and purpura in a 63-year-old woman.23 These cases illustrate that doses that are well within the normal guidelines for epidural steroid injections may still result in adverse consequences.
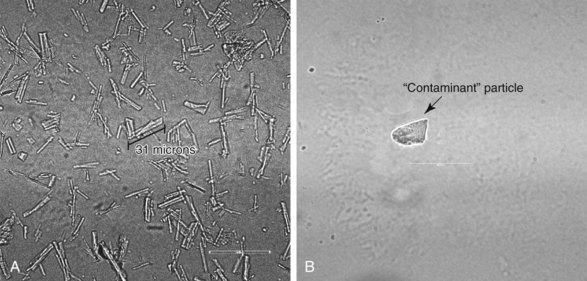
Perhaps the most dreaded recent complications have been attributed to particulate steroids. A large survey of members of the American Pain Society24 identified 78 cases of either spinal cord or brainstem infarcts that were known of by those members that responded to the survey. Of these, 14 were fatal cases, and all involved particulate steroids. Tiso et al25 and Benzon, et al26 both studied the microscopic appearances of commonly used steroids. Although discrepancies exist between the two studies, they are useful to any discussion of the potential pathophysiology of injury. One notable difference is that dexamethasone was found to be nonparticulate in the study by Benzon et al26 (Fig. 2-1). A subsequent animal study demonstrated that dexamethasone acted like a nonparticulate in that direct, intentional injection of the vertebral artery in a porcine model resulted in ischemic brain injury only in the animals receiving particulate steroids.27
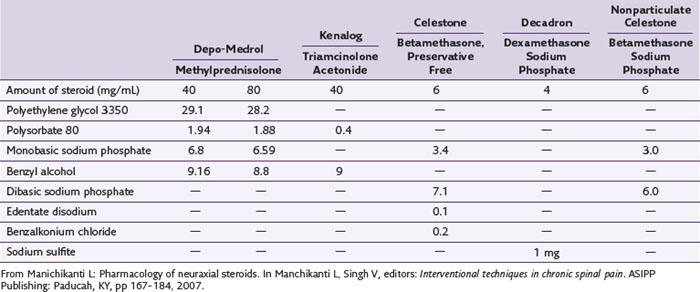
Although particulate size and aggregation have received much attention, other arguments exist regarding the etiology of steroid toxicity to the nervous system. One source of potential toxicity is the multiple chemical entities used in the formulation of epidural steroids, including benzyl alcohol, polyethylene glycol, and so on (Table 2-5). The ingredient with the most controversy is benzyl alcohol, which is used in Depo-Medrol, Aristocort, and Kenalog.20 Benzyl alcohol has been implicated in one case of flaccid paralysis of 16 months’ duration.28 Multiple other studies in different models on different steroids have been performed with variable results. Bogduk and Cherry,29 however, concluded that none of the literature provides direct evidence of the steroid itself or their preservatives causing neurotoxicity in the lumbar region.
Other side effects from the use of neuraxial steroids may be delayed for months or even years.30 The list of possible side effects not discussed above includes adrenal suppression, osteoporosis, avascular necrosis of the bone, fluid retention, adverse gastrointestinal effects, muscular effects, subcapsular cataracts, vision loss, dural puncture, and others. In-depth review of each of these is beyond the scope of this chapter, but essentially, all of these side effects can best be mitigated by minimizing the dose of steroids, using alternative therapies when able, and carefully monitoring the total dosage. Unfortunately, there is no consensus among pain practitioners regarding type, dosage, frequency, or total number of injections; however, a limitation of 3 mg/kg of body weight or 210 mg/year in the average patient with a total lifetime exposure of 420 mg of steroid has been advocated without any supporting scientific data.20
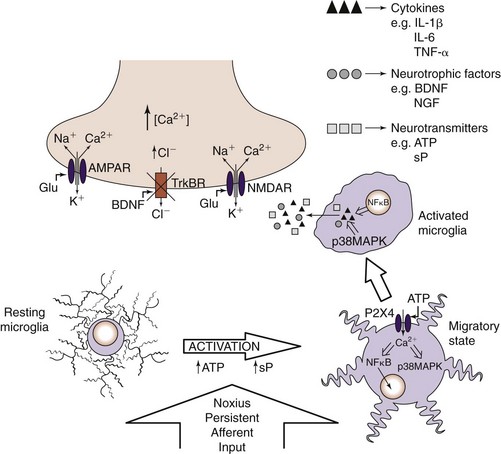
For many years, it was assumed that glia were simply structural supports for the varied neuronal cells in the CNS. In the past decade, it has become increasingly evident that glia are intricately involved in the development of persistent pain states and that glial expression of cytokines and other neurotransmitter substances play key roles. Sciatic constriction injury models in animals have provided significant clues as to the time course of glial activation. There are two main types of glial cells: microglia and macroglia (oligodendrocytes and astrocytes). Resting microglia become activated in the presence of injury and are drawn to the local source of adenosine triphosphate (Fig. 2-2). After being activated, microglia can produce various cytokines, neurotransmitters, and neurotrophins. Astrocytes become activated about 4 to 7 days after injury and are suspected to be involved in development of persistent pain (Fig. 2-3).31 Cytokines include tumor necrosis factor α, interleukin-1β (IL-1β), interleukin-6 (IL-6), and many others. Several cytokine antagonists exist, which include corticosteroids, disease-modifying anti-rheumatic drugs (DMARDs), and clonidine.
Initial studies of systemic infliximab and etanercept seemed to be promising, but randomized trials did not demonstrate a difference in the treatment of sciatica.32–34
Based on the failed randomized trial of infliximab, Cohen and colleagues35 suggested that precise local delivery to the area of nerve involvement might be necessary. In the first trial of perineural etanercept in humans, etanercept was injected via the transforaminal epidural route to three groups each of six patients with increasing doses of 2, 4, and 6 mg, respectively, and compared with a sham saline injection group in a 3 : 1 ratio. Because previous safety testing was incomplete, concurrent histological studies were performed in beagle dogs to evaluate for any functional deficits. Cohen and colleagues35 found etanercept to be effective compared with saline for several months in their study, and there were no histological trends or magnetic resonance imaging changes in human subjects that warranted study termination.
Etanercept has immune modulation properties that carry some risks. These risks include anaphylaxis, immune deficiency, sepsis, tuberculosis (reactivation or novel infection), and rarely lymphoma. A black box warning was submitted in May 2008 by the U.S. Food and Drug Administration to warn of these occurrences.36 Etanercept is an interesting therapy that seems to have some promise, but it should undergo further study before clinical use, due to potentially infectious outcomes. Another anticytokine drug with established safety profiles could emerge, however.
Clonidine is another drug that may have some promise for the treatment of sciatica.37 Clonidine has recently been studied in a randomized, controlled, double-blind study comparing it with the active control triamcinolone. Both drugs were equally effective in decreasing pain over the 6-week period of study, but functional improvement was more apparent with triamcinolone. It is possible that clonidine’s short duration of action might require longer exposure of drug at the dorsal root ganglion. Clonidine is an antagonist of IL-1β and other cytokines but for only a few days (Fig. 2-4).
Contrast Agents
The radiopaque nature of contrast media is one method to assist in confirming correct needle position. This in turn can improve the safety of the procedure. There continues to be an emerging number of case reports describing catastrophic injuries from presumed injection of medication in the intravascular, subdural, or even intrathecal (IT) space.24 By using a radiopaque contrast media before injection of local anesthetic or steroid, the risk of any procedure should be reduced.38 Because needle misplacement is a common complication associated with percutaneous spinal injections, utilization of a safe contrast medium is essential.39
Iodine is an element commonly used in contrast medium, and iodine-based contrast agents have proven to be satisfactory for visualization, yet provide no therapeutic effect. The iodine atom in contrast media provides its radiopaque aspect because iodine atoms provide more attenuation than the tissue surrounding them. The level of attenuation is known as the attenuation coefficient for that tissue.10 First-generation contrast agents loosely bound the iodine molecules, resulting in a highly osmolar compound that increased its toxicity. For this reason, they are referred to as high-osmolality contrast media (HOCM). Second-generation agents, referred to as nonionic or low-osmolality contrast media (LOCM), tightly bind the iodine atoms to a benzene ring but still provide the needed ratio of iodine to non-iodine particles that leads to an appropriate attenuation profile while maintaining an almost physiological osmolality.
Pharmacology
The different types of iodine-based contrast media can be placed into four different varieties, which are ionic monomers, nonionic monomers, ionic dimers, and nonionic dimers. All types are readily redistributed upon injection and rapidly excreted through the kidneys (90% within 12 hours of administration). These agents come in a range of concentrations that correlate with their radiopacity. The more concentrated the solution, the more iodine and the more radiopaque.38 Osmolality plays an important role in the safety of contrast media. Osmolality is the number of particles of solute in a solution and is highest in ionic contrast agents by way of a tri-iodinated benzoate anion. The hyperosmolality of some “ionic” agents directly relates to toxicity in the form of hemodynamic effects and patient discomfort.10 The nonionic agents contain iodine atoms tightly attached to a benzene ring, making the osmolality closer to physiological conditions, while at the same time preserving a high attenuation coefficient because of the amount of iodine atoms. The majority of pain clinicians now almost exclusively use the non-ionic monomers.
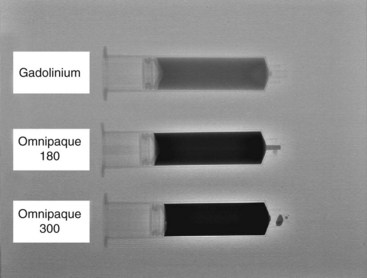
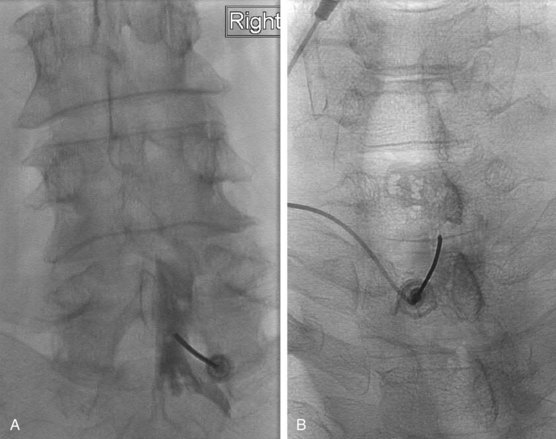
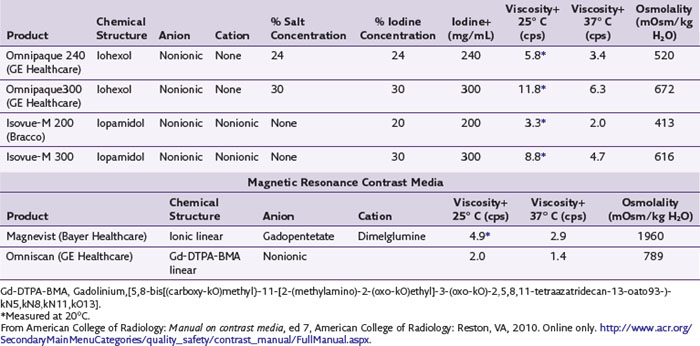
The most frequently used non-ionic monomers include iohexol (Omnipaque) and iopamidol (Isovue-M). Each agent is commercially available in varying concentrations and osmolality (Fig. 2-5). Each is rapidly absorbed into the bloodstream from paraspinal, epidural, and IT locations (Fig. 2-6). There is minimal deiodination, biotransformation, or metabolism.40,41
Adverse Events: Incidence
The actual incidence of adverse effects related to the use of iodinated contrast medium is difficult to quantify because of coadministration with other medications that may be responsible for an adverse reaction. The majority of the data regarding adverse events are related to intravascular injection of contrast medium, which usually involves larger doses of contrast medium compared with the amount injected for spinal procedures. There is even disagreement regarding how to classify adverse reactions. One method classifies reactions by their severity (Table 2-6). The incidence of adverse reactions was reported to be as high as 15% with the use of HOCM. The use of LOCM has a significantly lower incidence of adverse reactions, especially those of a non–life-threatening nature. In reports reviewing many contrast injections at multiple institutions in large numbers of patients, the incidence varied from 0.2% to 0.7%.42,43 Serious reactions occurred in one to two per 10,000 IV injections of LOCM. Fatalities associated with IV contrast media in the time when HOCM was being used were quoted as one per 40,000 IV injections. However, a Japanese study reported no fatality in more than 170,000 intravenous (IV) injections with the use of both LOCM and HOCM.44 As a result, the conservative estimate of incidence of one per 170,000 has been quoted, with the true incidence likely even less frequent, especially because of the use of LOCM and more effective treatment of reactions.
Table 2-6 Classification of Severity and Manifestations of Adverse Reactions to Contrast Media*
| Mild | ||
|---|---|---|
| Signs and symptoms appear self-limited without evidence of progression (e.g., limited urticaria with mild pruritus, transient nausea, one episode of emesis) and include: | ||
| Moderate | |
|---|---|
| Signs and symptoms are more pronounced. Moderate degree of clinically evident focal or systemic signs or symptoms, including: | |
| Treatment: Clinical findings in moderate reactions frequently require prompt treatment. These situations require close, careful observation for possible progression to a life-threatening event. | |
| Severe | |
|---|---|
| Signs and symptoms are often life-threatening, including: | |
| Treatment: Requires prompt recognition and aggressive treatment; manifestations and treatment frequently require hospitalization. | |
* These classifications (mild, moderate, severe) do not attempt to distinguish between allergic-like and non–allergic-like reactions. Rather, they encompass the spectrum of adverse events that can be seen after the intravascular injection of contrast media.
From American College of Radiology: Manual on contrast media, ed 7, American College of Radiology: Reston, VA, 2010. Online only. http://www.acr.org/SecondaryMainMenuCategories/quality_safety/contrast_manual/FullManual.aspx.
Adverse Events
Importantly, the majority of adverse events are mild, not life-threatening, and easily treated with observation, reassurance, and support. Severe adverse events may have a mild presentation, but nearly all life-threatening reactions occur with the first 20 minutes of contrast injection.45
Unfortunately, predicting a contrast reaction with any great accuracy is not possible, although there are definitely patients who are at greater risk than others. An obvious risk factor for adverse reaction is a prior allergy-like reaction to contrast media, which increases the chance of subsequent reaction by five fold.44 In fact, any specific allergy may predispose the patient to an allergic-type reaction when exposed to contrast. Although difficult to explain, the proceduralist’s focus should be aimed at patients with a prior major anaphylactic response to other allergens. Allergies to shellfish or dairy products, which in the past have been purported to be a predictor of contrast media allergy, have proven to be unreliable.46,47 There is no evidence that the practice of questioning patients about this type of allergy provides any useful clinical information.48,49
A history of asthma may be indicative of an increased likelihood of reaction to contrast medium.44,50 Significant cardiac disease, including symptomatic angina and congestive heart failure as well as severe aortic stenosis and primary pulmonary hypertension or even well-compensated cardiomyopathy, may have an increased risk of adverse reaction. Care should be given to limiting the volume and osmolality of the contrast given to these patients.
Anxiety is another risk factor that can contribute to adverse reactions. Hopper et al51 studied the effect that informed consent had on anxiety level for patients undergoing injection of IV contrast media and found no significant difference in adverse reactions between the groups; the majority of patients in each group had an equally elevated level of anxiety when graded using a validated index of anxiety. Care should be taken to provide supportive measures to calm the patient as much as possible. In some cases, this may require mild IV sedation. However, in the name of patient safety, the patient should not be sedated to the point that he or she cannot provide feedback during the procedure.
Contrast-induced nephrotoxicity (CIN) is another concern when using iodine-based contrast (Table 2-7). In a patient with normal renal function, the risk of developing CIN is extremely low. In Byrd and Sherman’s52 review of significant risk factors for developing CIN, they highlighted preexisting renal insufficiency (serum creatinine level ≥1.5 mg/dL), diabetes mellitus, dehydration, cardiovascular disease with diuretic use, age older than 70 years, multiple myeloma, hypertension, and hyperuricemia. More recent studies have indicated that the patients at highest risk for CIN are those with a combination of diabetes mellitus and preexisting renal insufficiency.52–54 Other less common conditions that may put patients at risk for renal involvement include paraproteinemias, particularly multiple myeloma, which likely predispose patients to renal failure because of protein precipitation and aggregation; however, these data are based on HOCM-related data. Also, the use of β-blocking agents in some retrospective studies may lower the threshold, increase the severity, and reduce the response to treatment of contrast reactions with epinephrine.52 Additional even less commonly encountered clinical situations such as the use of papaverine or other intraarterial injections or patients with pheochromocytoma, hyperthyroidism, or carcinoma of the thyroid with possible iodine-131 treatment planned may require extra consideration of the risk-to-benefit ratio before an elective pain procedure is performed.
Delayed reactions to contrast media have also been described in the literature and have been reported with both iodinated and gadolinium-based contrast types. Concerns about gadolinium-based contrast adverse reactions are addressed elsewhere. Various types of signs and symptoms have been reported as delayed reactions, including nausea, vomiting, drowsiness, headaches, and pruritus, which are almost always self-limited and require no treatment other than reassurance. The delayed cutaneous reactions are the most important because they may recur (reported anecdotally ≤25%) and may have serious sequelae. The incidence of delayed adverse cutaneous reactions ranges from 0.5% to 9%, are more common in patients being treated with IL-2 therapy, and may present 3 hours to 7 days after contrast exposure. The cutaneous reactions are usually macular and self-limited; however, in rare instances, they have progressed to resemble Stevens-Johnson syndrome, toxic epidermal necrolysis, or even cutaneous vasculitis, with one fatality being reported.45
Premedication
“At-risk” patients who require contrast media may require premedication in an effort to avoid an adverse reaction. The exact mechanism by which the anaphylactoid reaction occurs is not completely understood; similarly, the role IV steroids play in reducing the risk of the reaction likewise is not completely understood. Evidence suggests that allergic contrast reactions are related to mediators released by basophils and that histamine and basophil counts are reduced by IV steroids as soon as 1 hour after administration with increasing effect at 4 and 8 hours (16 patients). Therefore, premedication is considered to be most effective at least 4 to 6 hours before injection.55–57 Premedication does not prevent all reactions, and given the possible risks, premedication should be reserved for those who have had moderately severe to severe contrast reactions in the past. No randomized controlled studies have demonstrated that premedication protects against severe life-threatening reactions, but it does reduce those that are less severe.57–59 Oral prednisone or methylprednisolone premedication regimens used with diphenhydramine, with or without a histamine-2 receptor blocker, have been described with approximately equal success.60,61 Alternatively, the use of a different contrast agent may also be protective (HOCM to LOCM), although changing from one LOCM to another has little, if any, benefit.62 Again, pretreatment does not prevent all breakthrough reactions, which most likely will present similarly to the original reaction,63 and the proceduralist should be prepared to treat that reaction.
Gadolinium
Gadolinium-based contrast media (GBCM) has been in use since the 1980s. Osmolality, viscosity, and stability mark the differences among the different agents without any difference in their reported effectiveness. Currently, it is common for pain proceduralists to use GBCM in patients with previous allergic responses to iodinated contrast material.64 Shetty et al65 recently published a review of 2067 epidural steroid injections over a 25-month period in which 38 of those used GBCM to confirm needle placement. They found that, based on a radiologist’s review of the saved spot image, contrast spread as compared to iodinated contrast images resulted in significantly greater confidence of needle placement. In addition, they also found that GBCM was a useful confirmatory test to help localize the needle tip in patients in whom GBCM was used.65 This difference in visualization between iodinated contrast medium and GBCM would not be unexpected given the relatively low concentration of gadolinium in the available commercial preparations compared with the iodinated compounds. It is also important to note that similar to the use of intraarterial and power-injected gadolinium, the use of epidural gadolinium represents off-label use at this time.65 The authors of that study found and would suggest (which would be confirmed by our experience at our institution) that the use of digital subtraction would improve the visualization of gadolinium in the epidural space.
Adverse Reactions
The frequency of acute reactions of GBCM is eight times higher in those with a known previous reaction to GBCM. Second reactions tend to be more severe. Patients with asthma and other allergies, including those to certain foods, are also at greater risk, reported to be as high as 3.7%.45 In relation to patients with an allergy to iodinated contrast media, there is no known cross-reactivity, although a previous allergic-type reaction would place them in previously mentioned category of 3.7%. Similar recommendations for premedication and trying an alternative agent also apply to the patient with a previous allergic reaction to GBCM. Gadolinium has no known nephrotoxicity at the approved IV magnetic resonance dosing. It is also noted that initially there was some concern with GBCM’s promoting a sickle cell disease–related vaso-occlusive event66; however, at the approved dosage, there is no evidence to withhold GBCM from patients with sickle cell disease.
Nephrogenic systemic fibrosis (NSF) is a fibrosing disease of the skin and subcutaneous tissues and was first described in 2000 and noted to occur primarily in patients with end-stage chronic kidney disease, especially those on dialysis. In 2006, a number of reports emerged that noted a strong association with GBCM to patients with advanced renal disease and the development of NSF.67,68 Much about NSF and its development is unknown such as the causation, exact risk of developing NSF after GBCM, and why some at-risk patients develop NSF and others do not. It does appear that not all agents have the same risk of developing NSF. A recent report by the American College of Radiology Committee on Drugs and Contrast Media placed GBCM into three categories with gadodiamide (Omniscan) and gadopentetate dimeglumine (Magnevist) in the group associated with the greatest number of NSF cases, which likely reflects a number of different factors, including market share as well as agent toxicity.45,69–73 Risk factors include high cumulative dose or even a single dose of GBCM, although up to 50% of NSF occurred after one dose and usually occurred in days to 6 months after exposure. Patients with chronic kidney disease with a glomerular filtration rate (GFR) of less than 30 L/min/1.73 m2 and patients with acute kidney injury have a reported 1% to 7% risk45,67–69,73–76 of developing NSF.77 Recommendations include GFR screening within 6 weeks of the procedure and consideration of alternative procedures whenever possible in “at-risk” patients.
All of what has been reported above relates to the use of GBCM delivered in up to nine times the volume used in most spinal procedures, which likely reduces the practical risk of GBCM-related adverse events. In regard to diagnostic and therapeutic spine procedures performed by pain specialists, there are few reports that specifically report on the use of gadolinium and its safety. In a study by Safriel et al39 that reviewed 527 procedures in which GBCM was used and included a wide variety of procedures from cervical discograms to lumbar facet injections, they reported one documented IT injection without sequelae and two patients who required admission to the intensive care unit. These two patients who underwent cervical procedures (multilevel discogram and cervical epidural unspecified type) experienced headache, nausea, and seizures and required intensive care admission. In both cases, all symptoms completely resolved, and both were documented to have IT gadolinium observed on postprocedure imaging.39 In another report of four allergic reactions to gadolinium in patients with reported allergy to iodinated contrast, one underwent a lumbar facet injection, and the other three underwent lumbar transforaminal injections. Each of the four presented with a rash, and the fourth also experienced fever. Three of the four had also been exposed to gadolinium previously without difficulty. All four did not require hospitalization.64 The study mentioned earlier by Shetty et al65 reported no adverse reactions in the 38 patients who received GBCM. Overall, for patients with an known iodine contrast allergy and normal renal function, the use of GBCM appears to have a relatively low incidence of adverse events GBCM, but based on the small numbers, further study is warranted.
1 Evans W. Intrasacral epidural injections in the treatment of sciatica. Lancet. 1930:1225-1229.
2 Robecchi A, Capra R. [Hydrocortisone (compound F); first clinical experiments in the field of rheumatology]. Minerva Med. 1952;43:1259-1263.
3 Goebert HWJr, Jallo SJ, Gardner WJ, Wasmuth CE. Painful radiculopathy treated with epidural injections of procaine and hydrocortisone acetate: results in 113 patients. Anesth Analg. 1961;40:130-134.
4 Winnie AP, Hartman JT, Meyers HLJr, et al. Pain clinic. II. Intradural and extradural corticosteroids for sciatica. Anesth Analg. 1972;51:990-1003.
5 Abbott Laboratories. Product information: bupivacaine hydrochloride injection. North Chicago: Abbott Laboratories; 1999.
6 Abbott Laboratories: Product information: lidocaine hydrochloride injection, North Chicago, IL, Abbott Laboratories.
7 Miller RD. Miller’s anesthesia, ed 7. Philadelphia: Churchill Livingstone; 2010. pp 3084-3089
8 DiFazio CA, Carron H, Grosslight KR, et al. Comparison of pH-adjusted lidocaine solutions for epidural anesthesia. Anesth Analg. 1986;65:760-764.
9 Ririe DG, Walker FO, James RL, Butterworth J. Effect of alkalinization of lidocaine on median nerve block. Br J Anaesth. 2000;84:163-168.
10 Benzon HT. Essentials of pain medicine and regional anesthesia, ed 2. Philadelphia: Elsevier-Churchill Livingstone; 2005.
11 Ring J, Franz R, Brockow K. Anaphylactic reactions to local anesthetics. Chem Immunol Allergy. 2010;95:190-200.
12 Auroy Y, Benhamou D, Bargues L, et al. Major complications of regional anesthesia in France: the SOS Regional Anesthesia Hotline Service. Anesthesiology. 2002;97:1274-1280.
13 Brown DL, Ransom DM, Hall JA, et al. Regional anesthesia and local anesthetic-induced systemic toxicity: seizure frequency and accompanying cardiovascular changes. Anesth Analg. 1995;81:321-328.
14 Ireland PE, Ferguson JK, Stark EJ. The clinical and experimental comparison of cocaine and pontocaine as topical anesthetics in otolaryngological practice. Laryngoscope. 1951;61:767-777.
15 Neal JM, Bernards CM, Butterworth JFIV, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152-161.
16 Weinberg GL, Di Gregorio G, Ripper R, et al. Resuscitation with lipid versus epinephrine in a rat model of bupivacaine overdose. Anesthesiology. 2008;108:907-913.
17 Coursin DB, Wood KE. Corticosteroid supplementation for adrenal insufficiency. JAMA. 2002;287:236-240.
18 Hench PS, Slocumb CH, Polley HF, Kendal EC. Effect of cortisone and pituitary adrenocorticotropic hormone (ACTH) on rheumatic diseases. JAMA. 1950;144:1327-1335.
19 Williams RH, Wilson JD. Williams textbook of endocrinology, ed 9. Philadelphia: Saunders; 1998.
20 Manichikanti L. Interventional techniques in chronic spinal pain. In: Manchikanti L, Singh V, editors. Interventional Techniques in Chronic Spinal Pain. Paducah, KY: ASIPP Publishing; 2007:167-184.
21 Katzung BG. Basic & clinical pharmacology, ed 9. New York: Lange Medical Books/McGraw Hill; 2004.
22 Tuel SM, Meythaler JM, Cross LL. Cushing’s syndrome from epidural methylprednisolone. Pain. 1990;40:81-84.
23 Stambough JL, Booth REJr, Rothman RH. Transient hypercorticism after epidural steroid injection. A case report. J Bone Joint Surg Am. 1984;66:1115-1116.
24 Scanlon GC, Moeller-Bertram T, Romanowsky SM, Wallace MS. Cervical transforaminal epidural steroid injections: more dangerous than we think? Spine (Phila Pa 1976). 2007;32:1249-1256.
25 Tiso RL, Cutler T, Catania JA, Whalen K. Adverse central nervous system sequelae after selective transforaminal block: the role of corticosteroids. Spine J. 2004;4:468-474.
26 Benzon HT, Chew TL, McCarthy RJ, et al. Comparison of the particle sizes of different steroids and the effect of dilution: a review of the relative neurotoxicities of the steroids. Anesthesiology. 2007;106:331-338.
27 Okubadejo GO, Talcott MR, Schmidt RE, et al. Perils of intravascular methylprednisolone injection into the vertebral artery. An animal study. J Bone Joint Surg Am. 2008;90:1932-1938.
28 Craig DB, Habib GG. Flaccid paraparesis following obstetrical epidural anesthesia: possible role of benzyl alcohol. Anesth Analg. 1977;56:219-221.
29 Bogduk C, Cherry D. Report of the Working Party on Epidural Use of Steroids in the Management for Back Pain. Caberra, Australia: National Health and Medical Research Council; 1994. pp 1-76
30 Neal JM, Rathmell JP. Complications in regional anesthesia and pain medicine, ed 1. Philadelphia: Saunders/Elsevier; 2007.
31 Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010;10:167-184.
32 Genevay S, Stingelin S, Gabay C. Efficacy of etanercept in the treatment of acute, severe sciatica: a pilot study. Ann Rheum Dis. 2004;63:1120-1123.
33 Korhonen T, Karppinen J, Malmivaara A, et al. Efficacy of infliximab for disc herniation-induced sciatica: one-year follow-up. Spine (Phila Pa 1976). 2004;29:2115-2119.
34 Korhonen T, Karppinen J, Paimela L, et al. The treatment of disc herniation-induced sciatica with infliximab: results of a randomized, controlled, 3-month follow-up study. Spine (Phila Pa 1976). 2005;30:2724-2728.
35 Cohen SP, Bogduk N, Dragovich A, et al. Randomized, double-blind, placebo-controlled, dose-response, and preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica. Anesthesiology. 2009;110:1116-1126.
36 Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275-2285.
37 Romero-Sandoval A, Eisenach JC. Perineural clonidine reduces mechanical hypersensitivity and cytokine production in established nerve injury. Anesthesiology. 2006;104:351-355.
38 Benzon HT, Raj PP. Raj’s practical management of pain, ed 4. Philadelphia: Mosby-Elsevier; 2008.
39 Safriel Y, Ang R, Ali M. Gadolinium use in spine pain management procedures for patients with contrast allergies: results in 527 procedures. Cardiovasc Intervent Radiol. 2008;31:325-331.
40 Nycomed. Product information: Omnipaque, Iohexol. Princeton, NJ: Nycomed; 1996.
41 Bracco Diagnostics. Product information: Isovue iopamidol. Princeton, NJ: Bracco Diagnostics; 1999.
42 Cochran ST, Bomyea K, Sayre JW. Trends in adverse events after IV administration of contrast media. AJR Am J Roentgenol. 2001;176:1385-1388.
43 Wang CL, Cohan RH, Ellis JH, et al. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol. 2008;191:409-415.
44 Katayama H, Yamaguchi K, Kozuka T, et al. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175:621-628.
45 American College of Radiology. Manual on contrast media, ed 7. Reston, VA: American College of Radiology; 2010. Online only http://www.acr.org/SecondaryMainMenuCategories/quality_safety/contrast_manual/FullManual.aspx
46 Coakley FV, Panicek DM. Iodine allergy: an oyster without a pearl? AJR Am J Roentgenol. 1997;169:951-952.
47 Lieberman PL, Seigle RL. Reactions to radiocontrast material. Anaphylactoid events in radiology. Clin Rev Allergy Immunol. 1999;17:469-496.
48 Beaty AD, Lieberman PL, Slavin RG. Seafood allergy and radiocontrast media: are physicians propagating a myth? Am J Med. 2008;121:e151-e154. 158
49 Boehm I. Seafood allergy and radiocontrast media: are physicians propagating a myth? Am J Med. 2008;121:e19.
50 Shehadi WH. Adverse reactions to intravascularly administered contrast media. A comprehensive study based on a prospective survey. Am J Roentgenol Radium Ther Nucl Med. 1975;124:145-152.
51 Hopper KD, Houts PS, TenHave TR, et al. The effect of informed consent on the level of anxiety in patients given i.v. contrast material. AJR Am J Roentgenol. 1994;162:531-535.
52 Byrd L, Sherman RL. Radiocontrast-induced acute renal failure: a clinical and pathophysiologic review. Medicine (Baltimore). 1979;58:270-279.
53 Parfrey PS, Griffiths SM, Barrett BJ, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med. 1989;320:143-149.
54 Schwab SJ, Hlatky MA, Pieper KS, et al. Contrast nephrotoxicity: a randomized controlled trial of a nonionic and an ionic radiographic contrast agent. N Engl J Med. 1989;320:149-153.
55 Lasser EC. Pretreatment with corticosteroids to prevent reactions to i.v. contrast material: overview and implications. AJR Am J Roentgenol. 1988;150:257-259.
56 Lasser EC, Berry CC, Mishkin MM, et al. Pretreatment with corticosteroids to prevent adverse reactions to nonionic contrast media. AJR Am J Roentgenol. 1994;162:523-526.
57 Morcos SK. Review article: acute serious and fatal reactions to contrast media: our current understanding. Br J Radiol. 2005;78:686-693.
58 Brockow K, Christiansen C, Kanny G, et al. Management of hypersensitivity reactions to iodinated contrast media. Allergy. 2005;60:150-158.
59 Tramer MR, von Elm E, Loubeyre P, Hauser C. Pharmacological prevention of serious anaphylactic reactions due to iodinated contrast media: systematic review. BMJ. 2006;333:675.
60 Dunsky EH, Zweiman B, Fischler E, Levy DA. Early effects of corticosteroids on basophils, leukocyte histamine, and tissue histamine. J Allergy Clin Immunol. 1979;63:426-432.
61 Greenberger PA, Patterson R, Tapio CM. Prophylaxis against repeated radiocontrast media reactions in 857 cases. Adverse experience with cimetidine and safety of beta-adrenergic antagonists. Arch Intern Med. 1985;145:2197-2200.
62 Davenport MS, Cohan RH, Caoili EM, Ellis JH. Repeat contrast medium reactions in premedicated patients: frequency and severity. Radiology. 2009;253:372-379.
63 Freed KS, Leder RA, Alexander C, et al. Breakthrough adverse reactions to low-osmolar contrast media after steroid premedication. AJR Am J Roentgenol. 2001;176:1389-1392.
64 O’Donnell CJ, Cano WG. Allergic reactions to gadodiamide following interventional spinal procedures: a report of 4 cases. Arch Phys Med Rehabil. 2007;88:1465-1467.
65 Shetty SK, Nelson EN, Lawrimore TM, Palmer WE. Use of gadolinium chelate to confirm epidural needle placement in patients with an iodinated contrast reaction. Skeletal Radiol. 2007;36:301-307.
66 Brody AS, Sorette MP, Gooding CA, et al. AUR memorial Award. Induced alignment of flowing sickle erythrocytes in a magnetic field. A preliminary report. Invest Radiol. 1985;20:560-566.
67 Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104-1108.
68 Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359-2362.
69 Collidge TA, Thomson PC, Mark PB, et al. Gadolinium-enhanced MR imaging and nephrogenic systemic fibrosis: retrospective study of a renal replacement therapy cohort. Radiology. 2007;245:168-175.
70 Harrington RA, Chair, FDA Advisory Committee. Gadolinium-based contrast agents & nephrogenic systemic fibrosis. FDA Briefing Document. Joint Meeting of the Cardiovascular and Renal Drugs and Drug Safety and Risk Management Advisory Committee, 2009. Online at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM190850.pdf
71 Peak AS, Sheller A. Risk factors for developing gadolinium-induced nephrogenic systemic fibrosis. Ann Pharmacother. 2007;41:1481-1485.
72 Thomsen HS, Marckmann P, Logager VB. Nephrogenic systemic fibrosis (NSF): a late adverse reaction to some of the gadolinium based contrast agents. Cancer Imaging. 2007;7:130-137.
73 Wertman R, Altun E, Martin DR, et al. Risk of nephrogenic systemic fibrosis: evaluation of gadolinium chelate contrast agents at four American universities. Radiology. 2008;248:799-806.
74 Broome DR, Girguis MS, Baron PW, et al. Gadodiamide-associated nephrogenic systemic fibrosis: why radiologists should be concerned. AJR Am J Roentgenol. 2007;188:586-592.
75 Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243:148-157.
76 Shabana WM, Cohan RH, Ellis JH, et al. Nephrogenic systemic fibrosis: a report of 29 cases. AJR Am J Roentgenol. 2008;190:736-741.
77 Wahba IM, Simpson EL, White K. Gadolinium is not the only trigger for nephrogenic systemic fibrosis: insights from two cases and review of the recent literature. Am J Transplant. 2007;7:2425-2432.