Chapter 5 Head and Neck Blocks
 SPG block and RFA are very useful in the management of patients with intractable cluster headaches, migraine, trigeminal autonomic cephalalgias, and persistent idiopathic facial pain (“atypical facial pain”).
SPG block and RFA are very useful in the management of patients with intractable cluster headaches, migraine, trigeminal autonomic cephalalgias, and persistent idiopathic facial pain (“atypical facial pain”). Temporary diplopia is very common after SPG block because of spread of the injectate to the abducent nerve in the inferior orbital fissure. Diplopia may be used as a surrogate for a successful needle placement inside the pterygopalatine fossa (PPF).
Temporary diplopia is very common after SPG block because of spread of the injectate to the abducent nerve in the inferior orbital fissure. Diplopia may be used as a surrogate for a successful needle placement inside the pterygopalatine fossa (PPF). Practitioners should be familiar with the possible stimulation scenarios during SPG RFA to avoid lesioning the nearby maxillary nerve or branches. The optimal stimulation paresthesias should be behind the root of the nose.
Practitioners should be familiar with the possible stimulation scenarios during SPG RFA to avoid lesioning the nearby maxillary nerve or branches. The optimal stimulation paresthesias should be behind the root of the nose. Percutaneous radiofrequency thermocoagulation of the Gasserian ganglion is very successful in the management of patients with intractable trigeminal neuralgia when the pharmacological treatment is either ineffective or intolerable.
Percutaneous radiofrequency thermocoagulation of the Gasserian ganglion is very successful in the management of patients with intractable trigeminal neuralgia when the pharmacological treatment is either ineffective or intolerable. Percutaneous radiofrequency thermocoagulation of the Gasserian ganglion is usually considered for elderly patients at high risk for surgical microvascular decompression (MVD). The outcome may be less favorable than MVD, but it is less invasive with lower morbidity and mortality rates.
Percutaneous radiofrequency thermocoagulation of the Gasserian ganglion is usually considered for elderly patients at high risk for surgical microvascular decompression (MVD). The outcome may be less favorable than MVD, but it is less invasive with lower morbidity and mortality rates. The maxillary nerve exits through the foramen rotundum, which is located at the roof of the PPF. Hence, its block is essentially a modified SPG block.
The maxillary nerve exits through the foramen rotundum, which is located at the roof of the PPF. Hence, its block is essentially a modified SPG block. The mandibular nerve exits through the foramen ovale. The mandibular division (V3) is the most caudad and lateral part of the Gasserian ganglion.
The mandibular nerve exits through the foramen ovale. The mandibular division (V3) is the most caudad and lateral part of the Gasserian ganglion. The mandibular nerve can be blocked with either the lateral pterygoid approach or the foramen oval approach.
The mandibular nerve can be blocked with either the lateral pterygoid approach or the foramen oval approach. When performing SPG RFA, first look for inducing paresthesia at the root of the nose only to avoid injuring the maxillary nerve.
When performing SPG RFA, first look for inducing paresthesia at the root of the nose only to avoid injuring the maxillary nerve. When performing Gasserian ganglion RFA, try to be as selective as possible to the division affected to minimize potential adverse events.
When performing Gasserian ganglion RFA, try to be as selective as possible to the division affected to minimize potential adverse events. The approach to maxillary nerve block is essentially the same approach to SPG block; however, the needle is advanced more cephalad towards the foramen rotundum.
The approach to maxillary nerve block is essentially the same approach to SPG block; however, the needle is advanced more cephalad towards the foramen rotundum.Sphenopalatine Ganglion Block and Radiofrequency Ablation
Indications
Cluster headache involves activation of the parasympathetic outflow from the superior salivary nucleus of the facial nerve, predominantly through the sphenopalatine ganglion (SPG).1 The SPG is a large extracranial structure that has rich autonomic innervation (both sympathetic and parasympathetic), which explains the autonomic features associated with cluster headache. SPG block and radiofrequency ablation (RFA) are indicated in the management of intractable medically-resistant cluster headaches, migraines, and other trigeminal autonomic cephalalgias, and intractable orofacial pain syndromes after exhausting other conservative treatment options (e.g., persistent idiopathic facial pain, “atypical facial pain”).
Sphenopalatine Ganglion Anatomy
The SPG is located in the pterygopalatine fossa (PPF), which is a small, upside-down pyramidal space 2 cm high and 1 cm wide. The PPF is located behind the posterior wall of the maxillary sinus and is bordered posteriorly by the medial plate of the pterygoid process, superiorly by the sphenoid sinus, and medially by the perpendicular plate of the palatine bone; laterally, it communicates with the infratemporal fossa. Superolaterally lies the foramen rotundum with the exiting maxillary nerve, and inferomedially, there is the vidian nerve (greater petrosal and deep petrosal nerves) within the pterygoid canal. The PPF contains the internal maxillary artery and its branches, the maxillary nerve, and the SPG and its afferent and efferent branches. The SPG is located posterior to the middle turbinate and is a few millimeters deep to the lateral nasal mucosa. It is suspended from the maxillary nerve by the pterygopalatine nerves; inferiorly, it is connected to the greater and lesser palatine nerves; and posteriorly, it is connected to the vidian nerve. Efferent branches of the SPG form the posterior lateral nasal and pharyngeal nerves.2,3
Approaches to the Sphenopalatine Ganglion
Transnasal Approach
Transnasal blockade of the SPG was first reported using topical cocaine.4 Currently, lidocaine 4% is usually used.
Transnasal Endoscopic Approach
This endoscopic technique for transnasal injection and blockade of the SPG was first described by Prasanna and Murthy in 1993.5 This technique allows a needle to be inserted transnasally under vision through the sphenopalatine foramen into the PPF.
Transoral Approach
The PPF can also be accessed transorally by placing a 27-gauge needle inside the greater palatine foramen. This approach is usually used by dentists to block the palatine nerves.6
Infrazygomatic Approach
Neuroablation techniques are only feasible with this infrazygomatic approach. Needle placement is usually guided by fluoroscopy; however, computed tomography guidance is reported as well.7
Anterior Approach
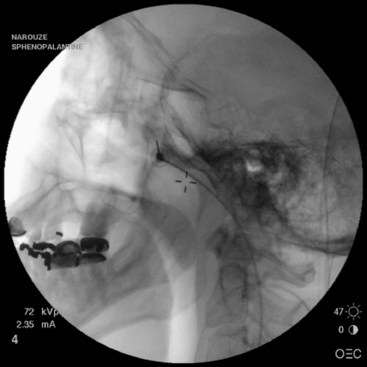
The needle entry is inferior to the zygomatic arch, just anterior to the mandible, between the mandibular ramus and the posterior border of the zygomatic bone. The author prefers this approach because the needle can be advanced in a target view toward the PPF without the need to walk the needle off the lateral pterygoid plate (which is usually very painful) (Fig. 5-1). Also, it is much easier to steer the needle (cephalad–caudad or anterior–posterior) within the fossa to selectively target different structures within the fossa. However, this approach is not feasible in all patients because there might not be enough room between the mandible and the zygoma to insert the needle.
Coronoid Approach
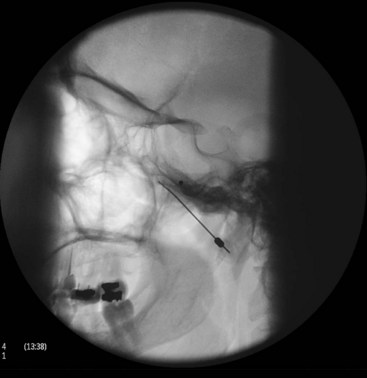
The needle entry is through the coronoid notch of the mandible. The needle is usually advanced to target the lateral pterygoid plate first and then walked off the bone interiorly to enter the PPF. By that time, the needle usually acquires certain direction, and it is hard to manipulate the needle after it is inside the fossa (Fig. 5-2).
Technique of Sphenopalatine Ganglion Block (Infrazygomatic Approach)
With the patient in the supine position and the head inside the C-arm, a lateral view is obtained and either the C-arm or the head of the patient is rotated until both pterygoid plates are superimposed on each other to better visualize the PPF. The skin entry site overlying the fossa is marked just inferior to the zygomatic arch either anterior to the mandible or through the coronoid notch. A 22-gauge, 3.5-inch blunt needle with a slight bend at the tip is used. The needle is first introduced in the lateral view and advanced medially and superiorly toward the PPF using real-time fluoroscopy. When in a proper direction, an anteroposterior (AP) view is obtained, and the tip of the needle is advanced to be just lateral to the nasal wall (Fig. 5-3). If the lateral pterygoid plate is encountered, the needle should be walked off the bone anteriorly and cephalad to slip into the fossa (the curved tip will help to guide the needle). A total of 0.1 to 0.2 mL of contrast agent is injected under real-time fluoroscopy to rule out intravascular spread because the PPF contains the maxillary artery and its branches (mainly the sphenopalatine artery). After negative aspiration of blood or air (the needle tip is too advanced into the nasal cavity or the maxillary sinus), 1 to 2 mL of 0.5% bupivacaine with or without steroids is injected slowly.
Radiofrequency Ablation Technique
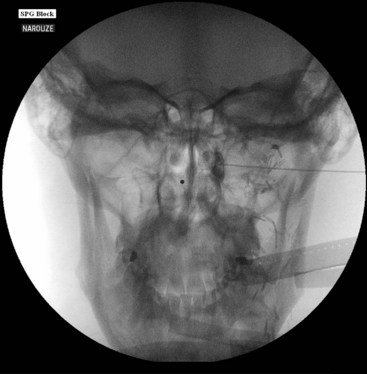
With the patient in the supine position and the head inside the C-arm, a lateral view is obtained and either the C-arm or the head of the patient is rotated until both pterygoid plates are superimposed on each other to better visualize the PPF. The skin entry site overlying the fossa is marked just inferior to the zygomatic arch either anterior to the mandible or through the coronoid notch. A 22-gauge, 10-cm, blunt RFA needle with a 2- or 5-mm active tip with a slight bend at the tip is used (Fig. 5-4). The needle is first introduced in the lateral view and advanced medially and superiorly toward the PPF using real-time fluoroscopy. When in a proper direction, an AP view is obtained, and the tip of the needle is advanced to be just lateral to the nasal wall. If the lateral pterygoid plate is encountered, the needle should be walked off the bone anteriorly and cephalad to slip into the fossa (the curved tip will help to guide the needle). Sensory stimulation is obtained with 50 Hz to look for deep paresthesias behind the root of the nose at less than 0.5 V (Table 5-1). After proper stimulation is achieved and before lesioning, 0.1 to 0.2 mL of contrast agent is injected under real-time fluoroscopy to rule out intravascular spread. Then 0.5 mL of lidocaine 2% is injected, and two radiofrequency lesions are carried out at 80° C for 60 seconds each. After lesioning, 0.5 mL of bupivacaine 0.5% and 5 mg of triamcinolone is injected with the aim of preventing postprocedure neuritis.8
Efficacy of Sphenopalatine Ganglion Radiofrequency Ablation
In a retrospective analysis of patients with refractory cluster headache treated by RFA of the SPG, 56 patients with episodic cluster headache and 10 patients with chronic cluster headache were followed over a period of 12 to 70 months.5 In the episodic cluster headache group, 60.7% experienced complete pain relief, but only three of 10 patients with chronic cluster headache had the same result. This report showed that RFA of the PPG may improve episodic cluster headache but not chronic cluster headache. Recently, however, Narouze and colleagues8 reported a favorable outcome after intractable chronic cluster headache as well. They reported significant improvement in both mean attack intensity and mean attack frequency for up to 18 months in 15 patients. Of these patients, 20% (three of 15) reported no change or increase in the headache intensity or frequency during the first few postprocedure weeks before noticing improvements in their headache pattern. However, 46.7% (seven of 15) of the patients reported a change in the headache pattern with return to the episodic form of cluster headache at a mean follow-up period of 18 months. Three patients remained headache free and off medications for the duration of the follow-up (18 to 24 months).
Two patients reported complete relief of their usual unilateral headache symptoms, and instead they developed episodic cluster headache on the contralateral side.8
Complications of Radiofrequency Ablation
Epistaxis is more frequent after the traditional intranasal topical application of local anesthetic; however, it can occur with this infrazygomatic approach if the needle is advanced too far medially through the lateral nasal wall.9
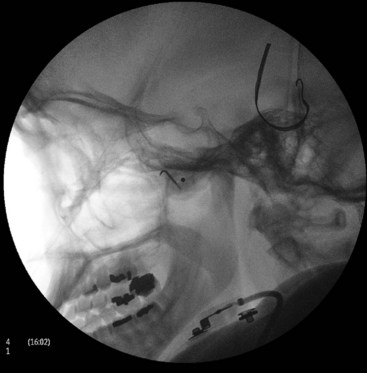
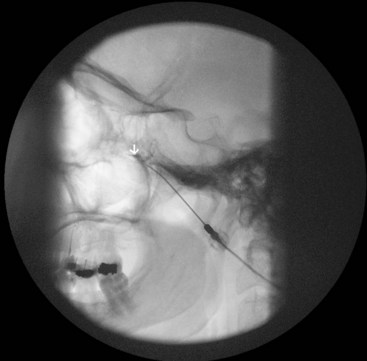
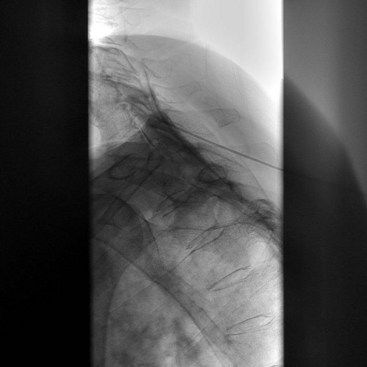
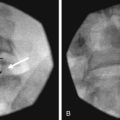
Intravascular injection and hematoma formation can occur after maxillary artery injury, which lies within the PPF (Fig. 5-5). Cheek hematoma is the most common complication. Infection is always a possibility, especially if the oral or nasal mucosa was accidentally penetrated.9
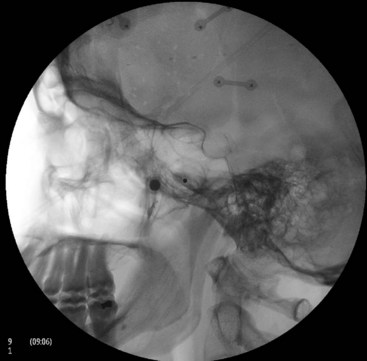
Fig. 5-5 Sphenopalatine ganglion block with the anterior approach showing intravascular spread.
(Reproduced with permission from the Ohio Pain and Headache Institute.)
Reflex bradycardia was reported during radiofrequency lesioning, which could be explained by the rich parasympathetic connections to the SPG.10 Radiofrequency lesioning of the SPG may result in permanent or, more commonly, temporary hypesthesia or dysesthesia in the palate, maxilla, or posterior pharynx.2,3,8 Pulsed radiofrequency would seem to be safer; however, there are limited data for its efficacy.11
Dryness of the eye as a result of interruption of the parasympathetic supply is also common; however, it is usually only temporary. Temporary diplopia is more common after local anesthetic injections rather than RFA and can be explained by the spread of the injectate from the PPF to the inferior orbital fissure containing the abducent nerve.12 The author has noticed that most of the patients develop temporary diplopia if the needle tip is really inside the PPF, which is why the volume should be limited to only 1 to 2 mL (Fig. 5-6).
A thorough understanding of the anatomy allows the clinician to predict correct needle placement during RFA according to the result of the stimulation and hence can reduce the incidence of complications (Table 5-1).9
Trigeminal Nerve and Gasserian Ganglion Block and Neurolytic Procedures
Percutaneous Gasserian Ganglion Neurolytic Procedures
Gamma Knife and Stereotactic Radiation Therapy
This is a noninvasive treatment that allows high-dose irradiation of a small section of the trigeminal nerve. This leads to nonselective damage of the Gasserian ganglion.13
Percutaneous Balloon Microcompression
The Gasserian ganglion is compressed by a small balloon, which is percutaneously introduced through a needle into Meckel’s cavity. This leads to ischemic damage of the ganglion cells. The technique may be more suitable for treatment of V1 trigeminal neuralgia of the first branch because the corneal reflex tends to remain intact.14
Percutaneous Glycerol Rhizolysis
Under fluoroscopy in a sitting position with a flexed head, the needle is introduced into the trigeminal cistern visualized by radiography. Contrast agent is then injected to determine the size of the cistern before an equal volume of glycerol is injected after aspiration of the contrast agent.15
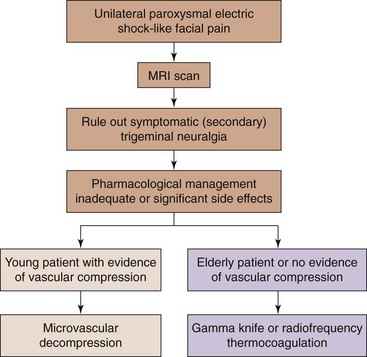
Choice of Percutaneous Gasserian Ganglion Neurolytic Treatment
Few systematic reviews have compared the various treatment approaches for trigeminal neuralgia.16,18,19 The first choice of treatment should be always conservative management. For those who fail pharmacological treatment, interventional management may be considered (Fig. 5-7).
Gasserian Ganglion Anatomy
The Gasserian ganglion lies within Meckel’s cavity in the middle cranial fossa close to the petrous bone. It is surrounded medially by the cavernous sinus, superiorly by the inferior surface of the temporal lobe, and posteriorly by the brainstem.20 Gasserian ganglion has three divisions with a characteristic somatotopic arrangement, in that the ophthalmic division (V1) is the most craniomedial and the mandibular division (V3) is the most caudolateral. The maxillary branch (V2) lies in between. The ophthalmic nerve exits through the superior orbital fissure, the maxillary nerve through the foramen rotundum, and the mandibular nerve through the foramen ovale.
The Technique of Gasserian Ganglion Block
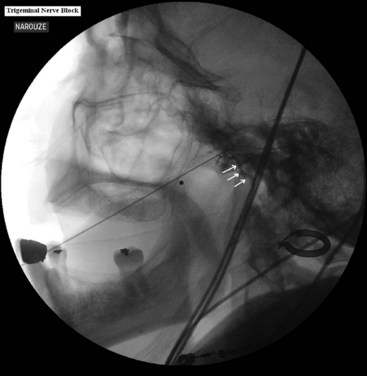
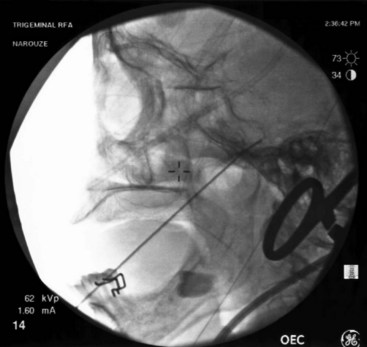
The procedure is usually performed with intravenous conscious sedation. The patient is in the supine position with the head inside the C-arm. After rotating the C-arm to obtain a submental view, the C-arm is slightly tilted to the affected side (oblique submental view) until the foramen ovale is best visualized (Fig. 5-8). It usually projects medially to the mandibular process. The point of the needle is about 2 cm lateral to the corner of the mouth on the ipsilateral side. A 3.5-inch, 22- to 25-gauge blunt nerve-stimulating needle is then advanced toward the foramen ovale under real-time fluoroscopy first in the AP submental view and then in the lateral view.
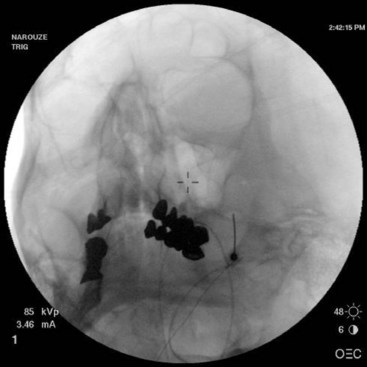
Fig. 5-8 Oblique submental view showing the needle tip inside the foramen ovale.
(Reproduced with permission from the Ohio Pain and Headache Institute.)
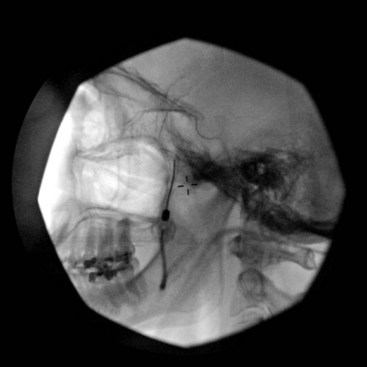
It is important to place a finger in the mouth to prevent or detect oral mucosa penetration. After the needle is inside the foramen ovale, motor stimulation is started, and muscle twitches should be observed in the mastication muscles (V3). There is no need to advance the needle farther inside the foramen ovale unless cerebrospinal fluid (CSF) will be aspirated from the needle. A total of 0.5 to 1 mL of contrast should be injected under real time fluoroscopy with digital subtraction, if available, to rule out intravascular spread (Fig. 5-9). After negative aspiration for CFS and blood, 1 to 2 mL of bupivacaine 0.5% with or without steroids can be injected slowly with close observation of the patient’s hemodynamics and vital signs.
The Technique of Gasserian Ganglion Radiofrequency Thermocoagulation
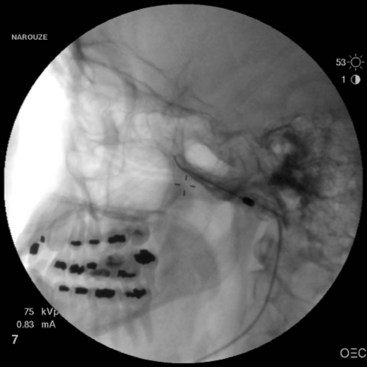
The procedure is usually performed under monitored anesthesia care with propofol or dexmedetomidine. The patient is in the supine position with the head inside the C-arm. After the C-arm is rotated so a submental view can be obtained, the C-arm is slightly tilted to the affected side (oblique submental view) until the foramen ovale is best visualized (Fig. 5-8). It usually projects medially to the mandibular process. The point of the needle is about 2 cm lateral to the corner of the mouth on the ipsilateral side. A 100-mm 22-gauge radiofrequency needle with a 2-mm active tip is then advanced toward the foramen under real-time fluoroscopy first in the AP submental view and then in the lateral view.
It is important to place a finger in the mouth to prevent or detect oral mucosa penetration. After the needle is inside the foramen ovale, testing stimulation is started. When motor stimulation (masseter muscle, V3) is encountered, the needle needs to be slightly advanced into the foramen carefully about 2 mm to stimulate V2. V1 is even deeper. In the lateral view, the needle tip should be deep into the foramen, overlying the petrous bone (Fig. 5-10). Some practitioners advocate that the patient should be covered with a broad-spectrum antibiotic if CSF is aspirated from the needle. The patient is then allowed to wake up, and sensory stimulation can be carried out with 50 Hz. Paresthesia should be felt between 0.05 and 0.1 V in the painful areas. V3 stimulation is encountered superficial and lateral in the foramen, then V2, and V1 is the deepest toward the pons and more medially. After appropriate paresthesia is obtained at the desired sites, a 60° C thermo lesion is carried out for 60 seconds. The corneal reflex and the treated dermatome may be tested for hypoesthesia. If intact, a second lesion is made at 65° C for 60 seconds, and if there is still no hypoesthesia, then a third lesion can be made at 70° C for another 60 seconds.
Efficacy of Radiofrequency Thermocoagulation of the Gasserian Ganglion
The first application of radiofrequency in pain medicine was for the treatment of trigeminal neuralgia with reported excellent results. Kanpolat et al21 evaluated the effectiveness of percutaneous radiofrequency trigeminal rhizotomy in 1600 patients with idiopathic trigeminal neuralgia with a follow-up period of 1 to 25 years. Acute pain relief was accomplished in 97.6% of patients. Pain relief was reported in 92% of patients with a single procedure or with multiple procedures 5 years later. At 10-year follow-up, 52.3% of the patients who underwent a single procedure and 94.2% of the patients who underwent multiple procedures had experienced pain relief. At 20-year follow-up, 41% and 100% of these patients, respectively, had experienced pain relief. After the first procedure was performed, early pain recurrence (<6 mo) was observed in 123 patients (7.7%), and late pain recurrence was observed in 278 patients (17.4%). The authors concluded that percutaneous radiofrequency trigeminal rhizotomy is a minimally invasive, low-risk technique with a high rate of efficacy and the procedure may safely be repeated if pain recurs.
Wu et al22 also reported their results in 1860 patients with refractory trigeminal neuralgia. The outcome was excellent in 78.8%, good in 17.5%, and poor in 3.7% of cases. Pain recurrence was reported in 11.1% of cases during the first 12 months and 24.8% after 24 months.
Complications of Gasserian Ganglion Block and Neurolysis
The Gasserian (trigeminal) ganglion lies within Meckel’s cave, which is formed by a dura mater fold that surrounds the posterior two-thirds of the ganglion. Meckel’s cave contains CSF, so local anesthetic deposited in this area may spread to other cranial nerves and can potentially cause brainstem anesthesia.20 Meticulous attention should be paid to avoid intravascular injection.17 Negative aspiration is unreliable, and injection of the contrast agent should be performed under real-time fluoroscopy with digital subtraction, if available, before injection of the local anesthetic.
In a systematic review of ablative neurosurgical techniques for the treatment of trigeminal neuralgia, Lopez et al16 concluded that although radiofrequency thermocoagulation (RFT) seems to provide the highest rates of sustained complete pain relief, it is the technique that is associated with the greatest number of complications.
A total of 29.2% of the patients in the analyzed series developed some complications, mostly transient, with RFT, and the rate of complications from glycerol rhizolysis was 24.8%. Stereotactic radiosurgery was the safest technique; only 12.1% of the patients experienced complications, mostly dysesthesias (Table 5-2).
Table 5-2 Complications of Percutaneous Gasserian Ganglion Neuroablative Procedures
| Radiofrequency Thermocoagulation | Glycerol Rhizolysis | |
|---|---|---|
| Complications rate (%) | 29.2 | 24.8 |
| Masticatory weakness (%) | 11.9 | 3.1 |
| Dysesthesia (severe) (%) | 3.7 | 8.7 |
| Anesthesia dolorosa | 1.6 | 2.3 |
| Corneal numbness | 9.6 | 8.1 |
| Keratitis | 1.3 | 2.1 |
| Cranial nerve deficits | 0.9 | 0.2 |
| Meningitis | 0.2 | 0.7 |
Modified from Lopez BC, Hamlyn PJ, Zakrzewska JM: Systematic review of ablative neurosurgical techniques for the treatment of trigeminal neuralgia, Neurosurgery 54:973-982; discussion 982-983, 2004.
Postoperative trigeminal sensory loss affects virtually all patients treated with RFT, and it is considered a side effect rather than a complication. However, in a prospective study, 30% of these patients may experience permanent sensory loss.23
Maxillary Nerve Block and Mandibular Nerve Block
Maxillary Nerve Block
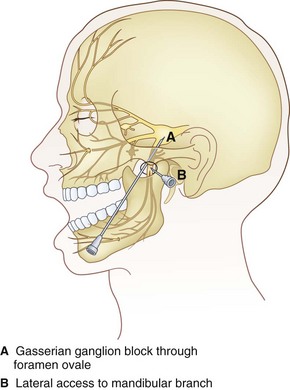
The maxillary nerve exits through the foramen rotundum, which is located at the roof of the PPF.
Technique of Maxillary Nerve Block
This uses the same approach as in SPG block; however, the needle is directed more cephalad toward the roof of the PPF to target the maxillary nerve as it exits the foramen rotundum on its way to enter the inferior orbital fissure (Figs. 5-11 to 5-13).
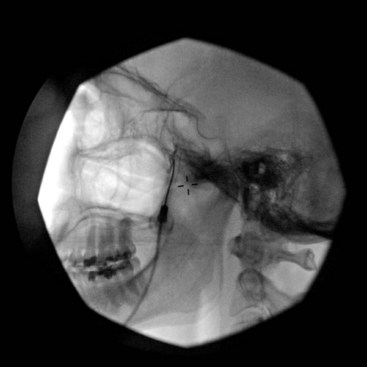
Fig. 5-12 Maxillary nerve block. The contrast agent is spreading cephalad through the foramen rotundum.
(Reproduced with permission from the Ohio Pain and Headache Institute.)
Mandibular Nerve Block
Technique of Mandibular Nerve Block
There are two approaches for blockade of the mandibular nerve.
Lateral “Pterygoid Plate” Approach
This uses the same approach as in SPG block; however, after the needle approaches the lateral pterygoid plate, it is then walked off posteriorly (contrary to SPG block) and advanced a couple of millimeters medially and cephalad to target the mandibular nerve as it exits the foramen ovale (Fig. 5-14).
Atlanto-Axial and Atlanto-Occipital Joint Injections
Atlanto-Axial Joint Injection
The lateral atlanto-axial joint (AAJ) may account for up to 16% of patients with occipital headache.24 Clinical presentations suggestive of pain originating from the lateral AAJ include occipital or suboccipital pain, focal tenderness over the suboccipital area, restricted painful rotation of C1 on C2, and pain provocation by passive rotation of C1. These clinical presentations are not specific and therefore cannot be used alone to establish the diagnosis.25 The only means of establishing a likely diagnosis is a diagnostic block with intraarticular injection of local anesthetic.24
Indications
The indications are as follows:
 Diagnostic: AAJ injection with local anesthetic is the only way to make a definitive diagnosis of pain stemming from the AAJ.
Diagnostic: AAJ injection with local anesthetic is the only way to make a definitive diagnosis of pain stemming from the AAJ. Therapeutic: AAJ injection with local anesthetic and steroids may be indicated in the management of AAJ pain. Intraarticular steroids are effective in short-term pain relief originating from the lateral AAJ.26,27
Therapeutic: AAJ injection with local anesthetic and steroids may be indicated in the management of AAJ pain. Intraarticular steroids are effective in short-term pain relief originating from the lateral AAJ.26,27 Prognostic: AAJ injection with local anesthetic and steroids may be used as a prognostic tool before AAJ radiofrequency lesioning or AAJ arthrodesis for intractable cases. One report showed favorable long-term outcome after both pulsed and thermal radiofrequency lesioning of the AAJ.28 In intractable cases not responsive to more conservative management, arthrodesis of the lateral AAJ may be indicated.29
Prognostic: AAJ injection with local anesthetic and steroids may be used as a prognostic tool before AAJ radiofrequency lesioning or AAJ arthrodesis for intractable cases. One report showed favorable long-term outcome after both pulsed and thermal radiofrequency lesioning of the AAJ.28 In intractable cases not responsive to more conservative management, arthrodesis of the lateral AAJ may be indicated.29Anatomy of the Atlanto-Axial and Atlanto-Occipital Joints
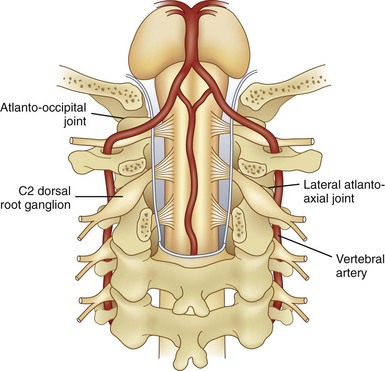
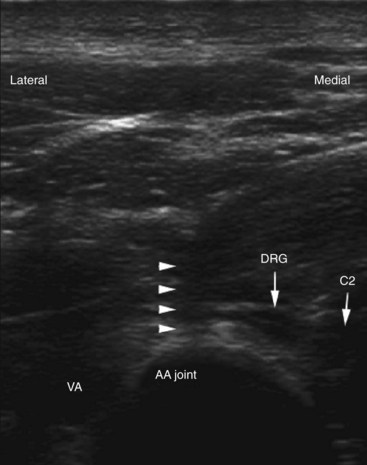
AAJ and atlanto-occipital joint (AOJ) intraarticular injections have the potential for serious complications, so it is crucial to be familiar with the anatomy of the joint in relation to the surrounding vascular and neural structures (Fig. 5-15). The vertebral artery is lateral to the AAJ as it courses through the C2 and C1 foramina. Then it curves medially to go through the foramen magnum, crossing the medial posterior aspect of the AOJ. The C2 dorsal root ganglion and nerve root with its surrounding dural sleeve crosses the posterior aspect of the middle of the joint. Therefore, during AAJ injection, the needle should be directed toward the posterolateral aspect of the joint. This will avoid injury to the C2 nerve root medially and the vertebral artery laterally (Fig. 5-15).24,27 On the other hand, the AOJ should be accessed from the most superior posterior lateral aspect to avoid the vertebral artery medially.
Technique of Atlanto-Axial Joint Injections
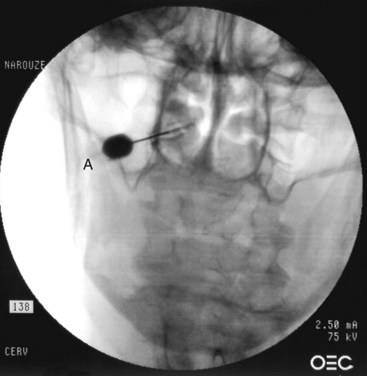
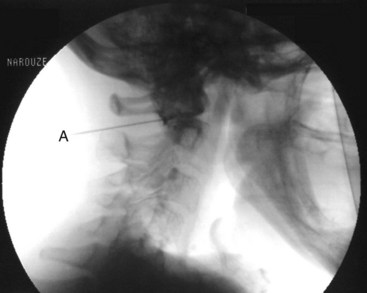
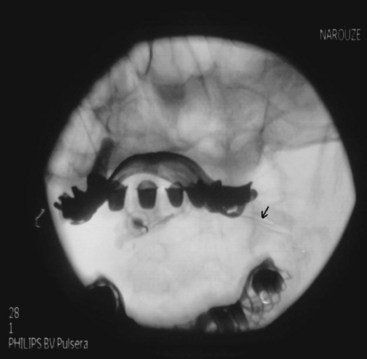
The patient is placed in the prone position with a pillow under the chest to allow for slight neck flexion. The fluoroscopy C-arm is brought to the head of the table in an AP direction. Under fluoroscopic guidance, the C-arm is rotated in the sagittal plane until the lateral atlanto-axial joint is better visualized. Using a marking pen, the needle insertion site is marked on the skin overlying the lateral third of the atlanto-axial joint. The skin is prepped and draped in the usual sterile fashion and a skin wheel is raised with local anesthetic at the insertion site. Then a 22- to 25-gauge, 3.5-inch blunt needle is advanced in anterior and medial direction toward the posterolateral aspect of the inferior margin of the inferior articular process of the atlas. This will avoid contact with the C2 nerve root and dorsal ganglion, which crosses the posterior aspect of the middle of the joint (Fig. 5-15). After touching the bone to safely establish the correct depth, the needle is withdrawn slightly; directed toward the posterolateral aspect of the lateral atlanto-axial joint; and advanced for couple of millimeters and usually a distinctive pop is felt, signaling entering the joint cavity. At this point, a lateral view is obtained, which shows the tip of the needle in the middle of the joint anterior to the posterior margin of the joint. Careful attention should be paid to avoid the vertebral artery that lies lateral to the lateral atlanto-axial joint as it courses through the C1 and C2 foramina. After careful negative aspiration for blood or cerebrospinal fluid, 0.2 mL of water-soluble nonionic contrast agent (Omnipaque 240) is injected to verify intraarticular placement of the tip of the needle.
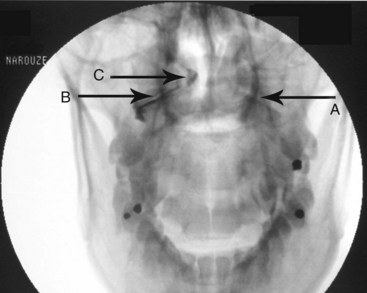
Injection of the contrast agent is done under direct real-time fluoroscopy to check for inadvertent intraarterial injection, which is manifest by rapid clearance of the contrast agent. AP and lateral views are obtained to ensure that the contrast agent remained confined to the joint cavity without escape to the surrounding structures, especially the epidural space or posteriorly to the C2 ganglion, which will adversely affects the specificity of the block (Figs. 5-16 and 5-17). The AP view usually demonstrates the bilateral concavity of the joint with the contrast material inside the joint space (Fig. 5-16), and sometimes it shows that the lateral AAJ space may communicate with that of the median AAJ space (Fig. 5-18) and the contralateral AAJ space (Fig. 5-19). After careful negative aspiration, 1.0 mL of a mixture of bupivacaine 0.5% and 10 mg of triamcinolone is injected.27
Technique of Atlanto-Occipital Joint Injections
The positioning and approach are similar to those for AAJ injection. The patient needs to flex his or her head over the neck as much as possible (chin on chest) to open the suboccipital space posteriorly. The AOJ should be accessed from the most superior posterior lateral aspect to avoid the vertebral artery (Figs. 5-20 and 5-21).
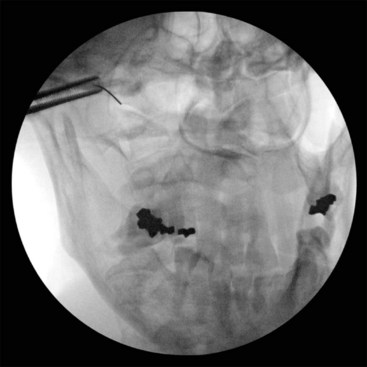
Fig. 5-20 Atlanto-occipital joint injection, anteroposterior view.
(Reproduced with permission from the Ohio Pain and Headache Institute.)
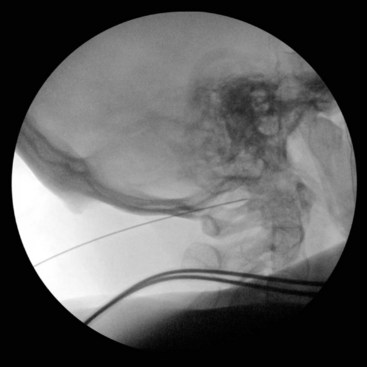
Fig. 5-21 Atlanto-occipital joint injection, lateral view.
(Reproduced with permission from the Ohio Pain and Headache Institute.)
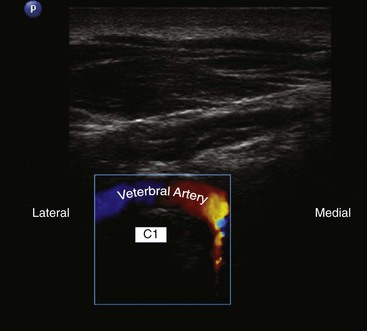
More recently, ultrasound-assisted AOJ injection in conjunction with fluoroscopy was described. With real-time sonography, the vertebral artery is identified as it curves medially behind the C1 body and accordingly can be avoided from the needle path, and then the procedure can be continued with fluoroscopy to confirm intraarticular placement of the needle (Fig. 5-22).30
Efficacy of Atlanto-Axial and Atlanto-Occipital Joint Injections
Narouze and colleagues27 studied 115 patients with cervicogenic headache. Thirty-two patients had a clinical picture suggestive of AAJ pain, and the diagnosis was confirmed in 15 patients with complete abolition of the headache (pain score of 0) after AAJ injection. The prevalence of AAJ pain among patients with cervicogenic headache was 13% (15 of 115 patients). At 1, 3, and 6 months after AAJ intraarticular steroid injection, the mean pain scores dropped from a baseline of 6.8 to 1.9, 3.6, and 3.7 respectively. The authors concluded that intraarticular steroid injection is effective for short-term relief of pain originating from the lateral AAJ.
No data are available to demonstrate the efficacy of AOJ intraarticular steroid injections.
Complications of Atlanto-Axial and Atlanto-Occipital Joint Injections
One possible complication is vertebral artery injection or injury. Injection of a contrast agent should be performed under real-time fluoroscopy, preferably with digital subtraction, if available, before the injection of the local anesthetic because negative aspiration is unreliable.31 Meticulous attention should be paid to avoid intravascular injection because vertebral artery anatomy may be variable. Recently, ultrasound-assisted AAJ injection was reported in an effort to add more safety to the procedure because ultrasonography can identify the relevant soft tissue structures nearby the joint (e.g., vertebral artery and C2 dorsal root ganglion) (Fig. 5-23).30
Spinal cord injury and syringomyelia are potential serious complications if the needle is directed further medially.9
1 Goadsby P. Pathophysiology of cluster headache: a trigeminal autonomic cephalgia. Lancet Neurol. 2002;1:251-257.
2 Salar G, Ori C, Iob I, et al. Percutaneous thermocoagulation for sphenopalatine ganglion neuralgia. Acta Neurochir (Wien). 1987;84:24-28.
3 Sanders M, Zuurmond W. Efficacy of sphenopalatine ganglion blockade in 66 patients suffering from cluster headaches: a 12- to17-month follow-up evaluation. J Neurosurg. 1997;87:876-880.
4 Sluder G. The role of the sphenopalatine ganglion in nasal headache. NY State J Med. 1908;27:8-13.
5 Prasanna A, Murthy PS. Sphenopalatine ganglion block under vision using rigid nasal sinuscope. Reg Anesth. 1993;18:139-140.
6 Ruskin SL. Techniques of sphenopalatine therapy. Eye Ear Nose Throat Mon. 1951;30:28-31.
7 Vallejo R, Benyamin R, Yousuf N, et al. Computed tomography-enhanced sphenopalatine ganglion blockade. Pain Pract. 2007;7(1):44-46.
8 Narouze S, Kapural L, Casanova J, et al. Sphenopalatine ganglion radiofrequency ablation for the management of chronic cluster headache. Headache. 2009;49:571-577.
9 Narouze S. Complications of head and neck procedures. Tech Reg Anesth Pain Manag. 2007;11:171-177.
10 Konen A. Unexpected effects due to radiofrequency thermocoagulation of the sphenopalatine ganglion: two case reports. Pain Digest. 2000;10:30-33.
11 Bayer E, Racz GB, Miles D, et al. Sphenopalatine ganglion pulsed radiofrequency treatment in 30 patients suffering from chronic face and head pain. Pain Pract. 2005;5:223-227.
12 Narouze SN. Role of sphenopalatine ganglion neuroablation in the management of cluster headache. Curr Pain Headache Rep. 2010;14:160-163.
13 Young RF, Vermulen S, Posewitz A. Gamma knife radiosurgery for the treatment of trigeminal neuralgia. Stereotact Funct Neurosurg. 1998;70(suppl 1):192-199.
14 Belber CJ, Rak RA. Balloon compression rhizolysis in the surgical management of trigeminal neuralgia. Neurosurgery. 1987;20:908-913.
15 Hakanson S. Trigeminal neuralgia treated by the injection of glycerol into the trigeminal cistern. Neurosurgery. 1981;9:638-646.
16 Lopez BC, Hamlyn PJ, Zakrzewska JM. Systematic review of ablative neurosurgical techniques for the treatment of trigeminal neuralgia. Neurosurgery. 2004;54:973-982. discussion 982-983
17 Erdine S, Ozyalcin NS, Cimen A, et al. Comparison of pulsed radiofrequency with conventional radiofrequency in the treatment of idiopathic trigeminal neuralgia. Eur J Pain. 2007;11:309-313.
18 Spatz AL, Zakrzewska JM, Kay EJ. Decision analysis of medical and surgical treatments for trigeminal neuralgia: how patient evaluations of benefits and risks affect the utility of treatment decisions. Pain. 2007;131:302-310.
19 Tatli M, Satici O, Kanpolat Y, et al. Various surgical modalities for trigeminal neuralgia: Literature study of respective long-term outcomes. Acta Neurochir (Wien). 2008;150:243-255.
20 Murphy T. Somatic blockade of head and neck. In: Cousins M, Bridenbaugh P, editors. Clinical anesthesia and management of pain. ed 3. Philadelphia: Lippincott-Raven; 1998:489-514.
21 Kanpolat Y, Savas A, Bekar A, et al. Percutaneous controlled radiofrequency trigeminal rhizotomy for the treatment of idiopathic trigeminal neuralgia: 25-year experience with 1,600 patients. Neurosurgery. 2001;48:524-532. discussion 532-534
22 Wu CY, Meng FG, Xu SJ, et al. Selective percutaneous radiofrequency thermocoagulation in the treatment of trigeminal neuralgia: report on 1860 cases. Chin Med J. 2004;117:467-470.
23 Zakrzewska JM, Jassmin S, Bulman JS. A prospective, longitudinal study on patients with trigeminal neuralgia who underwent radiofrequency thermocoagulation of the Gasserian ganglion. Pain. 1999;79:51-58.
24 Aprill C, Axinn MJ, Bogduk N. Occipital headaches stemming from the lateral atlanto-axial (C1-2) joint. Cephalalgia. 2002;22(1):15-22.
25 Bogduk N. The neck and headache. Neurol Clin. 2004;22(1):151-171.
26 Narouze SN, Casanova J. The efficacy of lateral atlanto-axial intra-articular steroid injection in the management of cervicogenic headache. Anesthesiology. 2004;101(suppl A):A1005.
27 Narouze SN, Casanova J, Mekhail N. The longitudinal effectiveness of lateral atlanto-axial intra-articular steroid injection in the management of cervicogenic headache. Pain Med. 2007;8:184-188.
28 Narouze SN, Gutenberg L. Radiofrequency denervation of the lateral atlantoaxial joint for the treatment of cervicogenic headache. Reg Anesth Pain Med. 2007;32(suppl A):A-8.
29 Ghanayem AJ, Leventhal M, Bohlman HH. Osteoarthrosis of the atlantoaxial joints: long term follow up after treatment with arthrodesis. J Bone Joint Surg (Am). 1996;78:1300-1307.
30 Narouze S. Ultrasonography in pain medicine: future directions. Tech Reg Anesth Pain Manage. 2009;13(3):198-202.
31 Edlow BL, Wainger BJ, Frosch MP, et al. Posterior circulation stroke after C1-C2 intraarticular facet steroid injection: evidence for diffuse microvascular injury. Anesthesiology. 2010;112:1532-1535.