Chapter 20 The Surgery of Temporal Lobe Epilepsy II—Surgical Complications and Long-Term Adverse Effects
Of all therapies for epilepsy, the positive effect of surgery can be the most impressive. However, its success should not lead one to ignore the potential downsides, and there are complications and adverse outcomes of temporal lobe surgery, which are often not stressed sufficiently. Like all treatment, the decision of whether or not to undergo surgical therapy depends on a balance between risk and benefit, and it is important that all patients undergoing temporal lobe surgery are given enough information to make an informed decision on both aspects of the risk–benefit equation. The decision to undergo surgery must always be an individual choice, and in similar situations, different individual patients will make different choices. A knowledge of the risks of surgery is essential and should be communicated accurately to the individual. This is particularly important in temporal lobe epilepsy surgery, where the surgery is elective, the seizures that have the best outcome (mild partial seizures without secondary generalization) are often not the cause of great disability themselves, and there are various alternative therapeutic options. On the latter point, it has been recently shown that the introduction of new medications, even in patients with chronic epilepsy unresponsive to previous drugs, has a significant chance of long-term benefit.1,2 Furthermore, medical options increase over time; for instance, at the present time, a series of novel drug therapies in the drug development pipeline carry significant promise.
Operative Complications of Temporal Lobe Surgery
OPERATIVE MORTALITY
The mortality rate of epilepsy surgery is usually quoted to lie between 0.5% and 1.0%. In the Kings/Maudsley series between 1976–2001, there were 451 temporal lobe resections with 2 perioperative deaths (0.44%), due cerebral edema of uncertain cause (1 case) and cerebral hemorrhage 2 weeks after the operation due to anticoagulation for a DVT (1 case).3 In the large series from Montreal, a recent review reported that there were no deaths in 526 operations.4 In the current authors’ analysis of 737 cases of temporal lobe surgery in the literature (see Chapter 19), there were two postoperative deaths (wound infection with osteomyelitis)5 and six late deaths, three seizure related6,7 and in three cases no details were given.5,8 It should not be forgotten, when considering the mortality of surgery, that the death rate in epilepsy (with active seizures) is two to three times increased in people with epilepsy, and the rate of death of patients on surgical waiting lists for epilepsy surgery is about 1 case per 100 per year.9 The death rate of epilepsy after surgery has been found to be reduced, especially if seizures are controlled, in some studies10 but not in others.11 The risk of death due to surgery is clearly dependent on the underlying etiology. Operations for mesial temporal sclerosis or benign tumors, for instance, have very low mortality, whereas there is a greater risk for operations on vascular lesions and particularly arteriovenous malformations (AVMs). In two large series of surgically treated AVM, although at all cerebral sites and with any presentation, the operative mortality was 11%.12,13
OTHER OPERATIVE MORBIDITY
Infection following epilepsy surgery occurs in most series at a rate below 1%, although is higher where intracranial recordings are carried out. In one series of 122 implantation procedures, operative complications included the need for repeated surgery for additional electrode placement (5.7%); wound infection (2.4%); cerebrospinal fluid leak (1.6%); and subdural hematoma, symptomatic pneumocephalus, bone flap osteomyelitis, and strip electrode fracture requiring operative retrieval (one patient [0.8%] each). There were four cases of transient neurological deficit (3.3%) and no permanent deficit or death associated with invasive monitoring.14 In Olivier’s series of 560 patients, meningitis was reported once, subdural abscess twice, and scalp infections five times. Hemorrhage along the track of the recording electrodes is another hazard, with a risk usually quoted at 1%.14
Neurological Complications of Temporal Lobe Surgery
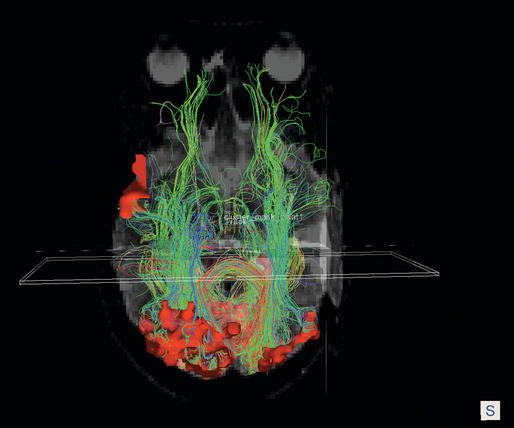
Neurological complications following temporal lobe surgery are well summarized by Polkey (2004) (Table 20-1).15 A visual field loss—usually an homonymous superior quadrantanopia—is a common sequel of an extensive temporal lobectomy, due to damage to Meyers loop of the geniculocalcarine tract (see Figure 20-1). How common this complication is following more restricted operations is unclear but is certainly underestimated. Manji and Plant assessed 24 patients following temporal lobe surgery for epilepsy using sensitive methods and found a field deficit in 13 (54%).16 A proportion of these deficits might have been missed on more routine evaluation. In this series, details of the extent of resection were not given, but the surgery was carried out by experienced epilepsy surgeons using orthodox means.
TABLE 20–1 Complication Rates of Epilepsy Surgery Reported at the Palm Desert Conferences of 1987 and 1993
| Complication | 1987 | 1993 |
|---|---|---|
| Transient hemiparesis | 0.7% | 4% |
| Permanent hemiparesis | 0.7% | 2% |
| III nerve palsy | <1% | |
| Complete hemianopia | 0.6% | 3% |
| Speech disturbance | 1.4% | |
| Nonneurological mortality | 0.47% | <1% |
1987 figures from Van Buren 1993; 1993 figures from Pilcher 1993 (cited by Polkey 2004).
Penfield was the first to report a transient hemipareisis in 5% of patients undergoing temporal lobectomy. The rate of hemipareisis depends on etiology and is now lower, between 1 and 3%, in patients following temporal lobe surgery for mesial temporal sclerosis. Hemipareisis is usually due to interference with perforating vessels supplying the internal capsule or the anterior choroidal or even posterior choroidal artery, or in the case of arteriovenous malformations, to hemorrhage or infarction. A 2% rate of mild but permanent hemipareisis due to anterior choroidal artery damage was reported in the recent series by Sindou et al.17 Diencephalic infarction occasionally occurs with hemianopia, ophthalmoplegia, and speech disturbance in addition to hemipareisis or hemiplegia. A higher risk of hemiparesis is conferred in surgery on tumors or vascular malformations, and also in operations on the insula region, due to the vacular palisade overlying the insula. Clearly, the surgical technique and the experience of the surgical team are also important factors, and the rate of vascular complications is generally higher the more inexperienced the surgeon—a major reason for the current recommendation that epilepsy surgery be carried out in appropriate centers.
Cranial nerve palsy has been reported (in less than 1% of cases). The third nerve is the most vulnerable but fourth and sixth nerve palsies also occur. A facial palsy can occur and is usually transient. Facial pain is another complication, possibly more common, which can be persistent and may be due to the section of superficial nerves during the craniotomy.
A range of other complications has been recorded. Complications include distant hemorrhage in the cerebellum, pneumocephalus, hematoma, meningitis (2% in the series of Sindou et al.17), acute hydrocephalus requiring shunt insertion (2% in the series of Sindou et al.17), scalp infections, and wound pain. It has also to be noted that most of the large series report complication rates retrospectively, and most also depend on surgical notes. Almost certainly this will lead to an underestimation of the true complication rate, but the extent of this underestimate is unclear. The published figures, however, should certainly be considered minimum estimates.
Effects of Temporal Lobe Surgery on Memory Function
The effects of bilateral hippocampal and temporal resection are most famously recorded in great detail in the case of HM, whose severe permanent antegrade amnesia has been the subject of intensive study.18 Bilateral resections were then largely abandoned, but in the 1960s to 1980s, most of the work on postoperative patients (after unilateral or bilateral resections) was concerned with psychological theories of memory, which has been of little clinical or practical value. More recent work has taken a more prosaic and pragmatic emphasis on psychometric testing to identify tests that will predict the surgical outcome for memory after unilateral resection.
About one-third of patients currently suffer memory decline following temporal lobe resection. About 15% experience a slight improvement in memory; these patients are more likely to be seizure free, and the improvement is probably due to the removal of the adverse effects of seizures on memory.19 A number of factors are now generally accepted to be predictive of memory outcome and are useful considerations when counseling patients about the risk of temporal lobe surgery to memory (Table 20-2):
TABLE 20–2 Factors Influencing Memory Outcome After Unilateral Temporal Lobe Surgery
| Age | Poorer outcome after age of 50 years |
| Gender | Slightly more risk to memory for males |
| Preoperative IQ | Patients with a higher IQ do less well. |
| Preoperative memory deficits | The better the preoperative memory, the more likely are postoperative deficits, and the likely there is to be a negative effect on functional memory outcomes. |
| Dominant vs. nondominant resections | Dominant resections have a generally greater negative effect on functional memory outcome than nondominant resections. |
| Unilateral hippocampal atrophy | The more atrophic the resected hippocampus is, the less effect will the surgery have on memory. |
| Bilateral hippocampal damage | Unilateral resections in the presence of bilateral hippocampal damage carry a significant risk of severe amnesia. |
| Cortical dysplasia | Resections for cortical dysplasia have a poorer memory outcome than resections for MTS. |
| Extent of surgical resection | Large dominant resections probably have a poorer memory outcome than smaller dominant resections, but the evidence is conflicting. |
| Seizure outcome after surgery | There is a better outcome in those patients who attain seizure freedom after surgery. |
| Affect, attention, psychiatric status | These can greatly affect memory function and are often more important functionally than even moderate changes in psychometric test results. |
Predictive models would be helpful in this regard, but although obviously possible to construct, would require standardized methods of assessment and multicenter international collaboration, and none are currently available. Furthermore, there remains in all cases a significant degree of uncertainty about memory outcome. On occasions, patients who have favorable preoperative factors suffer significant memory deficits postsurgery and vice versa.35 Preoperative prediction of memory outcome is not yet totally reliable science, and for this reason patients should be warned that memory is at some risk, even if the preoperative assessment is favorable.
A final complicating factor is the poor correlation between memory changes on psychometric testing and the change in memory function noticed by the patient.36 The reasons for this disjunction are not clear, but memory is greatly influenced by affective factors and the attentional states of individuals, and this may have more functional impact than, say, relatively minor changes in psychometric parameters. The lack of sensitivity of the current psychometric testing instruments to day-to-day experience of memory is, however, a factor that greatly limits their usefulness.
Psychosis after Epilepsy Surgery
In a summary of previous literature in 1992, a mean of 7.6% of patients (range 3.8% to 35.7% in different series) developed de novo psychosis after temporal lobe surgery.37 More recent surveys have in fact shown lower rates, perhaps due to more cautious selection, with de novo psychosis occurring in about 1 to 4% of patients.38–41 At the Maudsley hospital, 11 patients from a recent series of 320 cases of temporal lobectomy for medically intractable epilepsy were found to develop a de novo schizophrenia-like psychosis postoperatively. This developed typically in the first year following the operation and was not related to postoperative-seizure outcome. Patients who became psychotic were more likely to have preoperative bilateral electroencephalogram (EEG) abnormalities, pathologies other than mesial temporal sclerosis, and a smaller amygdala on the unoperated side.41
If psychosis is present preoperatively, there is a risk of an exacerbation of the psychosis. In an early series from the Maudsley Hospital, Taylor reported that 16 of 100 patients were psychotic preoperatively, of whom only four were improved after the operation.42 Leinonen et al. (1994) reported that five of 57 (8.8%) adult patients developed postoperative psychosis after temporal lobectomy.43 Two (3.5%) had preoperative psychosis, and three (5.3%) de novo psychoses. However, in more recent experience, less postoperative psychiatric morbidity has been reported. In a series of 74 patients from Denmark, 11 were psychotic preoperative, and after the operation one patient became nonpsychotic, five were unchanged, and five were improved.44 A series of five patients with psychosis from the Montreal Neurological Institute were reported who underwent temporal lobectomy with seizure remission and without any change in their psychosis.45 These patients, though, were highly selected and able fully to consent to the surgery, which suggests that the psychosis was mild. Certainly, the authors have observed, at an anecdotal level, florid postoperative psychoses developing in patients with relatively mild preoperative psychotic states. It is also commonly stated that both the preoperative and postoperative psychoses of epilepsy respond relatively well to psychotropic therapy, and this is also our anecdotal experience.
Affective Disorders after Temporal Lobe Epilepsy Surgery
Depression is common in temporal lobe epilepsy—occurring at lifetime frequencies of up to 30% in population-based studies. After temporal lobe surgery, there is a significant risk of depression, and occasionally this is severe and life threatening. Wrench et al. found 26% of 44 patients to be depressed 1 month after surgery and 30% at 3 months.46 The disturbances of mood were significantly related to adjustment difficulties. Devinski et al.47 and Devinsky/Altshuler et al.48 report figures around 10% at 3 months. At 2 years, moderate or severe depression was reported by 17.6% of patients who were not seizure free. It is general experience that rates are higher in patients who continue to have seizures. It has been reported that depression is more common after right temporal resections,49 but the current consensus now is that there are no striking differences between right and left temporal resections. The most important factor predicting the occurrence of depression is a history of preoperative depression. The suicide rate is elevated after temporal lobectomy, at least partly due to postoperative depression.50
The precipitation of anxiety after temporal lobe surgery is a real and often unrecognized problem. Wrench et al. found that 42% of patients have an anxiety state 1 month postoperatively and 24% 3 months after surgery.46 Patients who are seizure-free have somewhat lower levels of anxiety, but the relationship to seizure outcome does not appear to be a strong one.47 The affective outcome of surgery can be a significant determinant of the patient’s satisfaction with surgery.51
Other Psychiatric and Psychological Adverse Outcomes of Temporal Lobe Epilepsy Surgery
Large resections of brain tissue will inevitably have psychological consequences. The effects of temporal lobe resections on memory have been the focus of attention, and to a lesser extent studies of depression and psychoses, but it is remarkable how little systematic study there has been of other effects. A range of potential organic psychosyndromes has been almost completely ignored, and where information exists it is often anecdotal at best. There are probably several reasons for this highly unsatisfactory situation (Table 20-3), and we consider that this is an area that should be given a high priority for future studies.
TABLE 20–3 The Reasons for the Lack of Study of Psychiatric or Cognitive Consequences of Epilepsy Surgery
| Deficiencies in psychological measurement tools—this is a major reason |
| An extraordinary lack of preoperative vs. postoperative comparisons in individual patients and the small numbers of patients possible in prospective studies |
| The lack of comparative control data in nonoperated patients with epilepsy |
| The focus on the physiological identification of the epileptogenic zone without the ability to assess cognitive function with the same accuracy |
| The inherent bias introduced by the natural optimism of teams carrying out epilepsy surgery |
| The difficulty of long-term study |
The concept that certain areas of the brain are inneloquent (“silent cortex”) is no longer widely held among psychologists and cognitive neurologists, but still dominates thinking in the field of epilepsy surgery and particularly in relation to neocortical resections. Frontal lobe resections for frontal lobe epilepsy is a good example, where the aphorism of Rasmussen that the larger the resection the better the seizure outcome still results in enormous resections, with scant regard of psychological consequences. The most common resection in epilepsy is, of course, of temporal lobe cortex, and this is an area which by no measure can be considered ineloquent; yet there is little psychological study of the consequences on cognitive function other than crude measures of memory. There are complicating factors, not least the effects of epilepsy on normal functioning, but the absence of study is a shocking deficiency. Epilepsy surgery is not usually considered to be psychosurgery, and yet the psychological consequences in some cases result in marked impairment.
EFFECTS OF TEMPORAL LOBECTOMY ON PSYCHOSEXUAL FUNCTION
Sexual function was first noted to be affected in temporal lobe epilepsy by Gastaut and Collomb, and hyposexuality is a common complaint among patients.52 Following temporal lobe surgery, however, there can be marked changes in sexual function. At an anecdotal level, a common change is diminution of libido and sometimes also male erectile dysfunction. More commonly described in the literature, but less common in practice, is the occurrence of sudden hypersexuality following temporal lobe surgery, which can on occasions result in serious social disturbance.53 An extreme example is the Klüver-Bucy syndrome, which is a consequence of bilateral temporal lobectomy in animals and in humans, comprising of hypersexuality, visual agnosia, strong oral tendencies, overeating, and hypermetamorphosis (defined by Klüver and Bucy as the “excessive tendency to take notice of and to attend and react to every visual stimulus,” probably equating to environmental dependency syndrome in current terminology).54 Cases are also occasionally recorded following unilateral temporal lobectomy.
EFFECT OF TEMPORAL LOBECTOMY ON EMOTIONAL COLORING AND EMOTIONAL CAPACITY
At an anecdotal level, temporal lobectomy can have a marked effect on emotional responses. It is not uncommon to hear a patient describe some degree of flattening of affect and lack of emotional coloring, and occasionally these effects can be severe. Malmgren and colleagues have defined two disorders-the Astheno-Emotional Disorder and the Emotional-Motivational Blunting Disorder-which have similar traits—in what is probably the best longitudinal study of the psychiatric consequences of temporal lobectomy. Evidence was found of this change in almost half of 53 temporal lobectomy patients in the first year after temporal lobe surgery (and a similar proportion of patients undergoing extratemporal, mainly frontal resections).55 At an anecdotal level, the experience of the authors of this chapter supports this finding. Testing emotionality is difficult, and the lack of psychological instruments has impeded research in this area. One study, however, has shown marked changes in the interpretation of fearful expressions following left anterior temporal lobectomy, which was attributed by the authors to the removal of amygdala.56 Emotional disturbance occurred in 38.9% of 90 patients after temporal lobectomy in another series (11.1% new onset emotional disturbance).57
EFFECTS OF TEMPORAL LOBE SURGERY ON THE DYSPHORIC SYNDROME
Blumer and his colleagues have defined an “interictal dysphoric syndrome” which is a frequently observable trait in temporal lobe epilepsy. It comprises a constellation of symptoms and is defined by the presence of at least three of the following: depression, anergia, irritability, pain, insomnia, euphoric mode, fear, and anxiety. In Blumer’s series of 44 patients undergoing temporal lobectomy, 39% experienced an exacerbation of these symptoms or a de novo dysphoric state following surgery.53
OTHER POSTOPERATIVE PSYCHIATRIC CHANGES
A range of other psychiatric complications has been reported after epilepsy surgery, but almost all at an anecdotal level, and the extent of this problem is not well studied. In one retrospective chart review of 325 anterior temporal lobectomy and 125 extratemporal cases, seven patients were found to have developed undifferentiated somatoform disorder after anterior temporal lobectomy, one patient pain and body dysmorphia, one patient another pain disorder, and one patient body dysmorphia alone, but none was found after extratemporal surgeries. Nine of these 10 cases followed a right anterior temporal lobectomy.57 Lipson et al. reported a case who selectively and strikingly lost emotional attachments to family members after right temporal lobectomy.58 Mayanagi et al. reported a series of 100 temporal lobectomies, of whom nine patients developed various psychiatric symptoms developed after surgery; four of these were classified as “neurotic” and five “psychotic.”59 Patients with psychosis had delusions of various types as a core symptom, combined with other symptoms such as anxiety, irritability, aggression, and depressive state. In two patients with psychosis who had episodes of delusions in the interictal phase before surgery, the symptoms were exacerbated and extremely resistant to therapy.
THE CONCEPT OF REDUCED CEREBRAL RESERVE
Another worrying prospect is that temporal lobe resection will reduce “cerebral reserve” and thus render the patient to earlier or more severe psychological or psychiatric symptoms over time. Certainly, again at an anecdotal level, the authors have observed a number of patients who have, years after a temporal lobectomy, undergone cognitive decline. A rather typical syndrome seems to be of increasing vagueness, circumstantiality, loss of social skills, and a loss of cognitive sharpness. The issue of “cerebral reserve” has been addressed by a number of authors recently but there is a really striking lack of long-term studies in this area, and this is a deficiency that should be addressed.60,61 There is the related concern that as the person ages, the reserve capacity of the brain will diminish and so the adverse effects of the resection will become more obvious. It is partly for this reason that temporal lobectomy is largely reserved for young patients.
1. Luciano AL, Shorvon SD. Results of treatment changes in patients with apparently drug-resistant chronic epilepsy. Ann Neurol. 2007;62(4):375-381.
2. Callaghan BC, Anand K, Hesdorffer D, Hauser WA, French JA. Likelihood of seizure remission in an adult population with refractory epilepsy. Ann Neurol. 2007;62(4):382-389.
3. Polkey CE. Complications of epilepsy surgery. In: Shorvon SD, Perucca E, Fish D, Dodson E, editors. Treatment of Epilepsy. 2nd. Oxford: Blackwell Science; 2004:849-860.
4. Pilcher WH, Rusyniak WG. Complications of epilepsy surgery. Neurosurg Clin N Am. 1993;4:311-325.
5. Jensen I, Vaernet K. Temporal lobe epilepsy. Follow-up investigation of 74 temporal lobe resected patients. Acta Neurochirurgica. 1977;37:173-200.
6. Falconer M, Serafetinides EA. A follow-up study of surgery in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 1963;26:154-165.
7. Theodore WH, Sato S, Kufta C, et al. Temporal lobectomy for uncontrolled seizures: the role of positron emission tomography. Ann Neurol. 1992;32:789-794.
8. Penfield W, Paine K. Results of surgical therapy for focal epileptic seizures. Can Med Assoc. 1955;73:515-521.
9. Sperling MR, Feldman H, Kinman J, Liporace JD, O’Connor MJ. Seizure control and mortality in epilepsy. Ann Neurol. 1999;46(1):45-50.
10. Sperling MR, Harris A, Nei M, Liporace JD, O’Connor MJ. Mortality after epilepsy surgery. Epilepsia. 2005;46(Suppl 11):49-53.
11. Stavem K, Guldvog B. Long-term survival after epilepsy surgery compared with matched epilepsy controls and the general population. Epilepsy Res. 2005;63(1):67-75.
12. Albert P. Personal experience in the treatment of 178 cases of arteriovenous malformations of the brain. Acta Neurochir (Wien). 1982;61:207-226.
13. Abad JM, Alvarez F, Manrique M, Garcia-Blazquez M. Cerebral arteriovenous malformations. Comparative results of surgical vs conservative treatment in 112 cases. J Neurosurg Sci. 1983;27:203-210.
14. Johnston JMJr, Mangano FT, Ojemann JG, et al. Complications of invasive subdural electrode monitoring at St. Louis Children’s Hospital, 1994-2005. J Neurosurg. 2006;105:343-347.
15. Polkey CE. Complications of epilepsy surgery. In: Shorvon SD, Dreifuss F, Fish D, Thomas D, editors. Treatment of Epilepsy. Oxford: Blackwell Science; 1996:780-793.
16. Manji H, Plant GT. Epilepsy surgery, visual fields, and driving: a study of the visual field criteria for driving in patients after temporal lobe epilepsy surgery with a comparison of Goldmann and Esterman perimetry. J Neurol Neurosurg Psychiatry. 2000;68:80-82.
17. Sindou M, Guenot M, Isnard J, et al. Temporo-mesial epilepsy surgery: outcome and complications in 100 consecutive adult patients. Acta Neurochir (Wien). 2006;148:39-45.
18. Baxendale S. Amnesia in temporal lobectomy patients: historical perspective and review. Seizure. 1998;7:15-24.
19. Vaz SA. Nonverbal memory functioning following right anterior temporal lobectomy: a meta-analytic review. Seizure. 2004;13:446-452.
20. Alpherts WC, Vermeulen J, van Rijen PC, et al. Dutch Collaborative Epilepsy Surgery Program. Verbal memory decline after temporal epilepsy surgery? A 6-year multiple assessments follow-up study. Neurology. 2006;67:626-631.
21. Stroup E, Langfitt J, Berg M, et al. Predicting verbal memory decline following anterior temporal lobectomy (ATL). Neurology. 2003;60:1266-1273.
22. Davies KG, Bell BD, Bush AJ, Wyler AR. Prediction of verbal memory loss in individuals after anterior temporal lobectomy. Epilepsia. 1998;39:820-828.
23. Baxendale SA. Neuropsychologic outcomes after epilepsy surgery in adults. In: Schachter S, Holmes G, Kasteleijn-Nolst Trenité D, editors. Behavioural Aspects of Epilepsy. New York: Demos Medical Publishing; 2008:311-317.
24. Ferguson SM, McSweeny AJ, Rayport M. Memory function after temporal lobectomy for seizure control: a comparative neuropsychiatric and neuropsychological study. Int Rev Neurobiol. 2006;76:65-86.
25. Lineweaver TT, Morris HH, Naugle RI, et al. Evaluating the contributions of state-of-the-art assessment techniques to predicting memory outcome after unilateral anterior temporal lobectomy. Epilepsia. 2006;47(11):1895-1903.
26. LoGalbo A, Sawrie S, Roth DL, et al. Verbal memory outcome in patients with normal preoperative verbal memory and left mesial temporal sclerosis. Epilepsy Behav. 2005;6(3):337-341.
27. Bjørnaes H, Stabell KE, Røste GK, Bakke SJ. Changes in verbal and nonverbal memory following anterior temporal lobe surgery for refractory seizures: effects of sex and laterality. Epilepsy Behav. 2005;6(1):71-84.
28. Martin RC, Kretzmer T, Palmer C, et al. Risk to verbal memory following anterior temporal lobectomy in patients with severe left-sided hippocampal sclerosis. Arch Neurol. 2002;59:1895-1901.
29. Cukiert A, Sousa A, Machado E, et al. Results of surgery in patients with bilateral independent temporal lobe spiking (BITLS) with normal MRI or bilateral mesial temporal sclerosis (MTS) investigated with bilateral subdural grids. Arq Neuropsiquiatr. 2000;58:1009-1013.
30. Helmstaedter C, Reuber M, Elger CC. Interaction of cognitive aging and memory deficits related to epilepsy surgery. Ann Neurol. 2002;52:89-94.
31. Griffin S, Tranel D. Age of seizure onset, functional reorganization, and neuropsychological outcome in temporal lobectomy. J Clin Exp Neuropsychol. 2007;29:13-24.
32. Bengtson M, Martin R, Sawrie S, et al. Gender, memory, and hippocampal volumes: relationships in temporal lobe epilepsy. Epilepsy Behav. 2000;1:112-119.
33. Graydon FJ, Nunn JA, Polkey CE, Morris RG. Neuropsychological outcome and the extent of resection in the unilateral temporal lobectomy. Epilepsy Behav. 2001;2:140-151.
34. Gleissner U, Helmstaedter C, Schramm J, Elger CE. Memory outcome after selective amygdalohippocampectomy: a study in 140 patients with temporal lobe epilepsy. Epilepsia. 2002;43:87-95.
35. Kapur N, Prevett M. Unexpected amnesia: are there lessons to be learned from cases of amnesia following unilateral temporal lobe surgery? Brain. 2003;126:2573-2585.
36. Sawrie SM, Martin RC, Kuzniecky R, et al. Subjective versus objective memory change after temporal lobe epilepsy surgery. Neurology. 1999;53:1511-1517.
37. Trimble MR. Behaviour changes following temporal lobectomy, with special reference to psychosis. J Neurol Neurosurg Psychiatry. 1992;55:89-91.
38. Manchanda R, Miller H, McLachlan RS. Post-ictal psychosis after right temporal lobectomy. J Neurol Neurosurg Psychiatry. 1993;56:277-279.
39. Christodoulou C, Koutroumanidis M, Hennessy MJ, Elwes RD, Polkey CE, Toone BK. Postictal psychosis after temporal lobectomy. Neurology. 2002;59(9):1432-1435.
40. Koch-Stoecker S. Personality disorders as predictors of severe postsurgical psychiatric complications in epilepsy patients undergoing temporal lobe resections. Epilepsy Behav. 2002;3(6):526-531.
41. Shaw P, Mellers J, Henderson M, et al. Schizophrenia-like psychosis arising de novo following a temporal lobectomy: timing and risk factors. J Neurol Neurosurg Psychiatry. 2004;75:1003-1008.
42. Taylor DC. Mental state and temporal lobe epilepsy. A correlative account of 100 patients treated surgically. Epilepsia. 1972;13:727-765.
43. Leinonen E, Tuunainen A, Lepola U. Postoperative psychoses in epileptic patients after temporal lobectomy. Acta Neurol Scand. 1994;90(6):394-399.
44. Jensen I, Larsen JK. Psychoses in drug-resistant temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 1979;42(10):948-954.
45. Reutens DC, Savard G, Andermann F, Dubeau F, Olivier A. Results of surgical treatment in temporal lobe epilepsy with chronic psychosis. Brain. 1997;120(Pt 11):1929-1936.
46. Wrench J, Wilson SJ, Bladin PF. Mood disturbance before and after seizure surgery: a comparison of temporal and extratemporal resections. Epilepsia. 2004;45:534-543.
47. Devinsky O, Barr WB, Vickrey BG, et al. Changes in depression and anxiety after resective surgery for epilepsy. Neurology. 2005;65:1744-1749.
48. Altshuler L, Rausch R, Delrahim S, et al. Temporal lobe epilepsy, temporal lobectomy, and major depression. J Neuropsychiatry Clin Neurosci. 1999;11:436-443.
49. Quigg M, Broshek DK, Heidal-Schiltz S, et al. Depression in intractable partial epilepsy varies by laterality of focus and surgery. Epilepsia. 2003;44:419-424.
50. Hillemacher T, Kraus T, Stefan H, Kerling F. Suicidal attempts and aggressive behaviours after temporal lobectomy in epilepsy. Eur J Neurol. 2007;14:e10.
51. Wilson SJ, Bladin PF, Saling MM, Pattison PE. Characterizing psychosocial outcome trajectories following seizure surgery. Epilepsy Behav. 2005;6:570-580.
52. Gastaut H, Collomb H. Sexual behavior in psychomotor epileptics. Ann Med Psychol (Paris). 1954;112(2):657-696.
53. Blumer D, Wakhlu S, Davies K, Hermann B. Psychiatric outcome of temporal lobectomy for epilepsy: incidence and treatment of psychiatric complications. Epilepsia. 1998;39:478-486.
54. Danek A. “Hypermetamorphosis.” Heinrich Neumann’s (1814-1884) legacy. Nervenarzt. 2007;78:342-346.
55. Malmgren K. Psychiatric outcomes after epilepsy surgery in adults. In: Schachter S, Holmes G, Kasteleijn-Nolst Trenité D, editors. Behavioural Aspects of Epilepsy. New York: Demos Medical Publishing; 2008:319-325.
56. Dulay MF, York MK, Soety EM, et al. Memory, emotional and vocational impairments before and after anterior temporal lobectomy for complex partial seizures. Epilepsia. 2006;47:1922-1930.
57. Naga AA, Devinsky O, Barr WB. Somatoform disorders after temporal lobectomy. Cogn Behav Neurol. 2004;17:57-61.
58. Lipson SE, Sacks O, Devinsky O. Selective emotional detachment from family after right temporal lobectomy. Epilepsy Behav. 2003;4:340-342.
59. Mayanagi Y, Watanabe E, Nagahori Y, Nankai M. Psychiatric and neuropsychological problems in epilepsy surgery: analysis of 100 cases that underwent surgery. Epilepsia. 2001;42(Suppl 6):19-23.
60. Helmstaedter C, Reuber M, Elger CC. Interaction of cognitive aging and memory deficits related to epilepsy surgery. Ann Neurol. 2002;52:89-94.
61. Pai MC, Tsai JJ. Is cognitive reserve applicable to epilepsy? The effect of educational level on the cognitive decline after onset of epilepsy. Epilepsia. 2005;46(Suppl 1):7-10.