3 The Role of Priming the Skin for Peels
Introduction
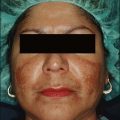
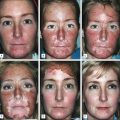
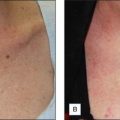
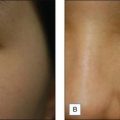
Skin priming can be divided into two phases: (1) pretreatment and (2) preparation. These two phases are differentiated and determined by timing and the agents used. The pretreatment phase consists of topical agents applied in the days or weeks preceding the peel. The preparation phase encompasses those steps taken directly before the peel is performed. These include patient degreasing and cleansing just before and upon arrival at the office. The goal of both phases is to thin the epidermal barrier, enhance uniform active agent penetration, accelerate healing, and reduce postoperative side effects and complications, most importantly postinflammatory hyperpigmentation. See Box 3.1.
Ayres SIII. Superficial chemosurgery in treating aging skin. Archives of Dermatology. 1962;85:578.
Brody HJ, Hailey CW. Medium-depth chemical peeling of the skin: a variation of superficial chemosurgery. Journal of Dermatologic Surgery and Oncology. 1986;12(12):1268-1275.
Chiarello SE, Resnik BI, Resnik SS. The TCA Masque: a new cream formulation used alone and in combination with Jessner’s solution. Dermatologic Surgery. 1996;8:687-690.
Chun EY, Lee JB, Lee KH. Focal trichloroacetic acid peel method for benign pigmented lesions in dark-skinned patients. Dermatologic Surgery. 2004;30(4):512-516. discussion 516
Coleman WPIII, Futrell JM. The glycolic acid trichloroacetic acid peel. Journal of Dermatologic Surgery and Oncology. 1994;20(1):76-80.
Garg VK, Sarkar R, Agarwal R. Comparative evaluation of beneficiary effects of priming agents (2% hydroquinone and 0.025% retinoic acid) in the treatment of melasma with glycolic acid peels. Dermatologic Surgery. 2008;34(8):1032-1040.
Hevia O, Nemeth AJ, Taylor JR. Tretinoin accelerates healing after trichloroacetic acid chemical peel. Archives of Dermatology. 1991;127(5):678-682.
Hung VC, Yu-yun Lee J, Zitelli JA, et al. Topical tretinoin and epithelial wound healing. Archives of Dermatology. 1989;125:65-69.
Koppel RA, Coleman KM, Coleman WPIII. The efficacy of EMLA versus ELA-Max for pain relief in medium-depth chemical peeling: a clinical and histopathologic evaluation. Dermatologic Surgery. 2000;26(1):61-64.
Lee JB, Chung WG, Kwahck H, et al. Focal treatment of acne scars with trichloroacetic acid: chemical reconstruction of skin scars method. Dermatologic Surgery. 2002;28(11):1017-1021. discussion 1021
MacKee GM, Karp FL. The treatment of post-acne scars with phenol. British Journal of Dermatology. 1952;64:456.
Mandy S. Tretinoin in the preoperative and postoperative management of dermabrasion. Journal of the American Academy of Dermatology. 1986;15(4):878-879. 888–889
Matarasso SL, Glogau RG, Markey AC. Wood’s lamp for superficial chemical peels. Journal of the American Academy of Dermatology. 1994;30(6):988-992.
Monheit GD. The Jessner’s + TCA Peel: a medium depth chemical peel. Journal of Dermatologic Surgery and Oncology. 1989;15:924-930.
Moy LS, Howe K, Moy RL. Glycolic acid modulation of collagen production in human skin fibroblast cultures in vitro. Dermatologic Surgery. 1996;22(5):439-441.
Nanda S, Grover C, Reddy BSN. Efficacy of hydroquinone (2%) versus tretinoin (0.025%) as adjunct topical agents for chemical peeling in patients of melasma. Dermatologic Surgery. 2004;30(3):385-389.
Peikert JM, Krywonis NA, Rest EB, et al. The efficacy of various degreasing agents used in trichloroacetic acid peels. Journal of Dermatology and Surgical Oncology. 1994;20(11):724-728.
Resnik SS, Lewis L. The cosmetic uses of trichloroacetic acid peeling in dermatology. Southern Medical Journal. 1973;66(2):225-227.
Rubin MG. The efficacy of a topical lidocaine/prilocaine anesthetic gel in 35% trichloroacetic acid peels. Dermatologic Surgery. 1995;21(3):223-225.
Van Scott EJ, Yu RJ. Alpha hydroxy acids: procedures for use in clinical practice. Cutis; cutaneous medicine for the practitioner. 1989;43(3):222-228.
West TB, Alster TS. Effect of pretreatment on the incidence of hyperpigmentation following cutaneous CO2 laser resurfacing. Dermatologic Surgery. 1999;25(1):15-17.