7 The role of clinical reasoning in the differential diagnosis and management of chronic pelvic pain
Evidence-based practice – Where did it come from? Where is it going?
Understanding pain: What do we need to know?
It’s about more than pain – Integrated systems for optimal health
The Integrated Systems Model for disability and pain: A framework for understanding the whole person and their problem
Introduction
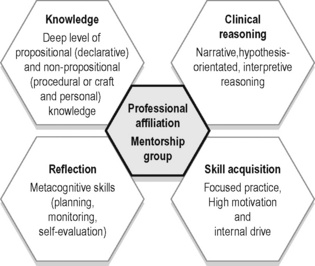
1. Propositional, theoretical or scientific knowledge (Higgs & Titchen 1995), also known as declarative knowledge (Jensen et al. 2007);
2. Non-propositional or professional craft knowledge (knowing how to do something) (Higgs & Titchen 1995) or procedural (Jensen et al. 2007). Non-propositional knowledge also includes personal knowledge or knowing oneself as a person and in relationship with others.
Propositional, or declarative, knowledge refers to the content knowledge that one’s profession is based on and includes factual information derived from formal research trials. In addition, this category includes theoretical knowledge developed from existing empirical protocols and principles, derived from dialogue with professionals in the same discipline, and logic (Higgs 2004).
Most practitioners continue to take post-graduate courses or attend professional conferences to improve their knowledge pertaining to clinical theory and research (propositional) as well as their technical skills (non-propositional or craft); however, Rivett & Jones (2004) note that there is a tendency in both courses and conferences to neglect an essential component of daily clinical practice – clinical reasoning. How should the practitioner integrate into clinical practice the newly learned scientific and theoretical knowledge? Who is it appropriate for and when is the new skill appropriate to use? Clinical practice is, and always will be, a blend of science and ‘art’ with a healthy dose of logic and reasoning. Clinical expertise comes from reasoning, reflection, skill acquisition and the continual life-long pursuit of knowledge (propositional (declarative) and non-propositional (procedural and personal)) (Figure 7.1) (Jensen et al. 2007). This takes time, discipline and often mentorship and professional affiliation with both individuals and groups.
Evidence-based practice: Where did it come from? Where is it going?
The term ‘evidence-based’ was first used in 1990 by David Eddy and ‘evidence-based medicine’ by Guyatt et al. in 1992. The methodologies used to determine ‘best evidence’ were largely established by the Canadian McMaster University research group led by David Sackett and Gordon Guyatt. Professor Archie Cochrane, a Scottish epidemiologist, has been credited with increasing the acceptance of the principles behind evidence-based practice (Cochrane 1972). Cochrane’s work was honoured through the naming of centres of evidence-based medical research, Cochrane Centers, and an international organization, the Cochrane Collaboration. Since the early 1990s there has been an explosion of research evidence, and accessibility to this evidence has been facilitated for those involved in research or formal study through easy internet access to full-text articles in indexed journals. Unfortunately, access to full-text articles is still limited, or expensive, for clinicians not affiliated with research centres or universities.
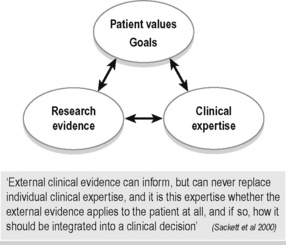
Evidence-based practice (EBP) embraces all disciplines of health care (not just medicine) and has become synonymous with best practice, but what does the term really mean? To some, it appears that EBP means that a clinician can only use assessment tests and treatment techniques/protocols that have been validated through the scientific process with high-ranking studies as valued by the ‘levels of evidence’. This is difficult to adhere to for many reasons, one being that there is not enough evidence at this time. Indeed, could there ever be enough scientific evidence for every situation met in clinical practice? Sackett and colleagues define EBP as ‘the integration of best research evidence, with clinical expertise and patient values’ (Sackett et al. 2000) (Figure 7.2). They note that:
Understanding pain: What do we need to know?
Understanding the neurophysiology of pain mechanisms is essential knowledge for treating patients with pelvic pain. Since the proposal of the gate control theory of pain by Melzack and Wall in 1965, significant advances in pain research and therapy have occurred. It is not our intent to provide an in-depth coverage of this topic here, but instead to highlight key features and establish a common language to be used throughout this chapter. See Chapter 3 for a full discussion of pain mechanisms in general, and as these relate to chronic pelvic pain.
What causes pain? Searching for the pain driver
It is now well recognized that the pathoanatomical model is limited in several ways. Pelvic pain commonly exists in the absence of any findings on diagnostic tests (X-ray, CT scan, blood tests, nerve conduction tests, etc.), and damaged tissues can be identified in people who experience no pain (Nachemson 1999, Waddell 2004). Tissues heal and yet the pain experience persists. Furthermore, a focus on only treating ‘the painful tissue’ neglects to consider that other systems or structures, which may be dysfunctional but painfree, could be the underlying cause of excessive mechanical stresses on the painful structures, or the cause of decreased blood flow or nutritional supply. In order to resolve the pain, the painfree but impaired structures or systems need to be treated for long-term resolution. Identification of what tissue hurts does not provide insight as to why it hurts. Finally, significant developments in neuroscience have changed our understanding of what pain is, and have required us to reframe and change our thinking.
We now understand that at any time in one patient there are many ‘pain drivers’ that do not exist solely in the peripheral tissues. Rather than looking for one source of pain, we need to consider that multiple mechanisms are at play in the experience of pain in all our patients. These mechanisms can be broadly separated into peripherally mediated (nociception and peripheral neurogenic pain) or centrally mediated (related to processing in the central nervous system (CNS)) (Butler 2000), and will be discussed in more detail later in this section.
Classifying pain
Timelines and mechanism of injury
Patients are commonly classified according to the timeline or duration of their pain experience, and the cause or mechanism of their injury. In general, problems are considered to be acute if they are within the first 6 weeks to 3 months (depending on the type of tissue injured) after an initiating incident (Brukner & Khan 2002, Magee et al. 2007). Tissue injury results in a known sequence of events aimed at protecting and repairing the damaged structures. These stages of tissue healing occur in three overlapping stages that have been given multiple names but refer to the same processes:
The term chronic is often used to indicate the persistence of pain beyond the normal timeline for tissue healing (Bonica 1953, Merskey & Bogduk 1994), as opposed to a stage of the tissue-healing process. In the Classification of Chronic Pain (Merskey & Bogduk 1994) published by the International Association for the Study of Pain, it is noted that the normal time of healing ‘may be less than one month, or more often, more than six months. With nonmalignant pain, three months is the most convenient point of division between acute and chronic pain, but for research purposes six months will often be preferred.’ Chronic pain is also further outlined as ‘a persistent pain that is not amenable, as a rule to treatments based upon specific remedies, or to the routine methods of pain control such as non-narcotic analgesics’ (Merskey & Bogduk 1994).
More recently, the term persistent low back pain has emerged in the literature, to indicate pain that continues past the expected timeframe for tissue healing. Others are suggesting that acute episodes of low back pain would be better termed recurrent episodes in a chronic problem as the underlying mechanisms contributing to recurrent low back pain are likely to be different from a first-time traumatic episode of low back pain, and recurrence of pain after an acute episode is a common problem (Pengel et al. 2003).
Thus, although the mechanism of onset and timeframes related to the pain experience are important to know, we must take care that this information does not lead us to assume that certain timelines necessitate certain pain mechanisms. Acute pain can be largely driven by central mechanisms. Persistent pain can also be largely driven by peripheral mechanisms. That is, persistent or chronic pain states may have central components, but these are not necessarily the dominant mechanism for every patient simply because the pain experience has persisted for a long period of time. While evidence supports that ‘the relationship between pain and the state of the tissues becomes less predictable as pain persists (Moseley 2007), we need to remember that the pain experience is uniquely individual. Regardless of whether the pain is a newly occurring event or a persistent experience, it is a multidimensional experience, and thus any person presenting with pain should be evaluated with a framework in mind that allows for the consideration of all these factors. As Butler (2000, p. 53) notes:
Classification by pain mechanisms
So what are the different biological mechanisms that drive the pain experience? Pain mechanisms can be further categorized (Gifford 1998, Butler 2000) as they relate to:
• Contextual factors of the immediate circumstance (i.e. how dangerous is this sensation in the light of environmental and internal factors?); as well as
• Past experiences and personal knowledge that collectively contribute to the individual’s beliefs, attitudes, emotions and physical responses.
Input mechanisms as they pertain to pain include all the sensory information reaching the CNS from the body internally and externally. This includes nociceptive pain from tissues including bones, ligaments, tendons, muscles, connective tissue, viscera, etc. (Gifford 1998, Butler 2000, Wright 2002) and peripheral neurogenic pain from neural tissue outside of the CNS. Processing occurs in the dorsal root ganglion and in the CNS. In the brain, an individual’s thoughts and feelings (cognitions + emotions = perception) are integrated and can influence the output mechanisms, which include:
1. Somatic or motor (altered posture, altered motor control);
2. Autonomic (increased sympathetic response for ‘fight or flight’);
3. Neuroendocrine (increased stress, heightened emotions, hormonal changes);
Thinking within the context of stress biology creates a broader framework for understanding pain. Gifford (1998), in proposing the Mature Organism Model (Figure 7.3), notes that
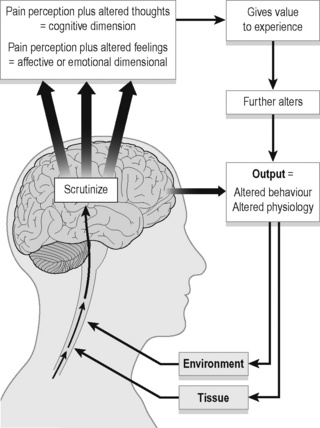
Figure 7.3 • The Mature Organism Model of Gifford (1998).
Adapted from Rivett & Jones (2004). Improving clinical reasoning in manual therapy. In: Jones, M.A., Rivett, D. (Eds.), Clinical reasoning for manual therapists, Churchill Livingstone; and Gifford (1998) Physiotherapy 84:27.
It has been proposed that continued activation of the stress-regulation systems and excessive or prolonged cortisol output has a destructive effect on peripheral tissues such as muscle, bone and nerve tissue, thereby perpetuating a vicious cycle of stress, pain and tissue injury (Melzack 2005).
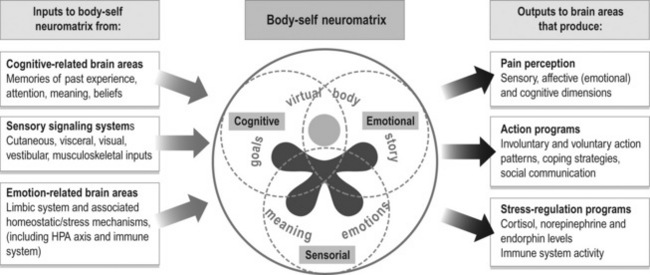
Melzack’s model has four components (2001, 2005):
1. The body-self neuromatrix – an anatomical substrate in the brain of the body-self;
2. Cyclical processing and synthesis of nerve impulses which produces a neurosignature;
3. The flow of neurosignatures is projected back to areas of the brain, the sentient neural hub, which converts them into the flow of awareness;
4. Activation of an action neuromatrix occurs to provide the pattern of movements to bring about the desired goal.
Figure 7.4 is a modification of Melzack’s representation of the body-self neuromatrix to illustrate the sensorial, cognitive and emotional dimensions of pain. Perhaps the best summary for this section highlighting the broad view we need to take when considering pain comes from this leader in the study of pain himself, Ronald Melzack (2001):
Classification and clinical prediction rules: Are we searching for the holy grail?
Given the multidimensional nature of pain, it is not surprising that using pain presentation (location, duration, onset) as the sole means to classify patients and determine best treatment has been ineffective. Fritz and colleagues report that despite over 1000 randomized clinical trials investigating the effectiveness of interventions for the management of low back pain, ‘the evidence remains contradictory and inconclusive’ (Fritz et al. 2007). One key reason believed to contribute to this state of the evidence is the lack of classification of low back pain patients into subgroups, not only for studying treatment efficacy, but also for determining aetiological and prognostic factors (Leboeuf-Yde et al. 1997, Riddle 1998, Gombatto et al. 2007).
Sahrmann in the late 1980s noted:
The classification for lumbopelvic pain has evolved since the pathoanatomically based classification of MacNab (1977) with a variety of patient characteristics proposed for use in creating homogeneous subgroups (McKenzie 1981, Kirkaldy-Willis 1983, Bernard & Kirkaldy-Willis 1987, Coste et al. 1992, Delitto et al. 1995, Sahrmann 2001, O’sullivan 2005, Reeves et al. 2005, Fritz et al. 2007, O’sullivan & Beales 2007) (Table 7.1).
Table 7.1 Multiple proposals for the classification of patients
| Model/system | Description | Diagnostic/classification determinants |
|---|---|---|
| Pathoanatomical (McNab 1977, Kirkaldy-Willis & Hill 1979, Kirkaldy-Willis 1983, Nachemson 1999) | Focuses on structural changes which occur as a consequence of inflammation, infection, metabolic disorders, trauma and/or disease (pathology-based) | Radiological diagnosis, blood work |
| Mechanical diagnosis and therapy (The McKenzie Method) (McKenzie 1981) | Directional preference and centralization or peripheralization of pain with repeated movements. Four subgroups: |
(confirmed/negated by features of the objective examination)
O’sullivan (2005) noted that a limitation of many classification systems is that often only a single dimension (pathoanatomical, psychosocial, neurophysiological, motor control, signs and symptoms, etc.) is used to create subgroups. Classification systems will be most useful in clinical practice if variables across multiple domains are used to create subgroups.
• Presence or absence of identifiable underlying pathology (pathoanatomical, peripheral pain generator models);
• Pain presentation (central, unilateral, with or without radiation of symptoms to the lower extremity) (signs and symptoms models);
• Underlying pain mechanisms/neurophysiology;
• Response of pain to movement (centralization or peripheralization) (signs and symptoms models) (movement impairment models);
• Physical impairments such as loss or increase of mobility, altered motor control, altered posture/spinal alignment, and the relationship of symptom provocation to these impairments (motor control models, signs and symptoms models, movement impairment models);
• Response to specific treatments (manipulation, stabilization exercises, specific exercises, traction); and
• Psychosocial and cognitive features such as fear avoidance, coping strategies and beliefs (biopsychosocial models).
In recent years, the development of clinical prediction rules (CPRs) has emerged as another way to classify patients. CPRs are derived statistically with the aim of identifying the combinations of clinical examination findings that can predict a condition or outcome. Thus, they are proposed to be a useful tool to assist in clinical decision-making by improving the accuracy of diagnosis, prognosis or prediction of response to specific treatment protocols (Beattie & Nelson 2006, Cook 2008, Fritz 2009). Development of CPRs in physiotherapy has mainly focused on the response to treatment protocols (Fritz 2009) in order to identify subgroups of patients most likely to respond to a specific treatment approach. It is important to note that, at this time, CPRs are still in their infancy of development and validation, and are not yet at the appropriate stage to be widely applied in clinical practice (Cook 2008).
It has been suggested that CPRs will best impact physiotherapy practice where there is complexity in the clinical decision-making process, and that ‘an appeal of CPRs is their potential to make [the] subgrouping process more evidence based and less reliant on unfounded theories and tradition’ (Fritz 2009). However, the use of CPRs should be balanced with the knowledge that:
Consider the one domain of underlying pain mechanism as a way to create subgroups.
Butler (2000) notes that:
In their classification of pelvic pain disorders, O’sullivan & Beales (2007) categorize non-specific pelvic pain disorders into two groups: one that has centrally mediated pain, and one that has peripherally mediated pain. Although the group of centrally mediated pain is further classified into those with non-dominant psychosocial factors and those with dominant psychosocial factors, the treatment protocol for the subgroup of centrally mediated pelvic girdle pain is medical management (central nervous system modulation), psychological (cognitive-behavioural therapy), and functional capacity rehabilitation. Specific interventions directed at identified physical impairments in the periphery are not recommended, and yet it is highly unlikely that many patients will have 100% centrally mediated pain. In the authors’ experience, even in patients with a strong contributor of central sensitization to their pain experience, careful assessment often reveals specific meaningful tasks that relate to a consistent reproduction of symptoms. It is reasonable to suggest that even if peripheral mechanisms only contribute 20% to the complete picture, addressing that 20% in addition to the other approaches will provide the greatest chance for the best outcome. Furthermore, it is likely that by addressing the physical impairments, psychosocial variables will also be impacted, further advancing the goals of treating drivers of central sensitization. It is also crucial to recognize that our patients change as a result of their changing life circumstances and our interactions with them (both physical and personal). Thus, during the course of treatment continual re-evaluation is necessary to adapt the treatment programme accordingly. Sticking to a rigid plan based on an initial placement into a subgroup may result in the provision of sub-optimal care.
Finally, in our quest for better classification schemes and science to support and test our clinical approaches, it is important to remember that at the end of the day no matter how detailed and well defined our classification schemes, the person presenting to the clinician is a unique individual with unique life experiences. There will never be one recipe for treatment that is the best fit for all patients. Furthermore, patient values and beliefs are central to the treatment process, and if they do not want to receive what is considered ‘best practice’ from the current evidence, we cannot force it on them. Given the same impairment in the tissues, no two individuals will have exactly the same perception and presentation (experience and behaviour) because ‘how they manifest their pain or illness is shaped in part by who they are’ (Jones & Rivett 2004). A reminder, the highest level of evidence for therapeutic interventions is a systematic review, or meta-analysis, of only randomized, double-blind, placebo-controlled trials which involve a homogeneous patient population and condition. Is this possible in the light of what is known about pain? Do homogeneous populations really exist in clinical practice?
Science can provide us with an abundance of knowledge to challenge, refine, reshape and validate our clinical practice, but it cannot provide all of the information needed in any individual patient encounter; it does not paint the whole picture of the patient. In order to effectively treat patients, therapists need to have well-organized knowledge including propositional (knowledge ratified by research trials), non-propositional (professional craft or ‘knowing how’ knowledge) and personal (knowledge gained from personal experiences (Jones & Rivett 2004).
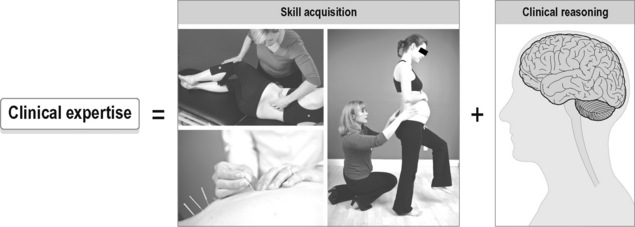
Personal and craft knowledge cannot be learned from RCTs, mechanistic studies, basic physiology studies, or clinical prediction rules. Ultimately, it is the development of clinical expertise that creates optimal patient care. According to Ericsson & Smith (1991) expertise has been defined as ‘having the ability to do the right thing at the right time’.
Clinical expertise has two components: skill acquisition (do the right thing) and clinical reasoning (at the right time) (Figure 7.5). Clinical reasoning skills facilitate the organization and integration of knowledge gained both in and out of the clinic, and the wise application of that knowledge for each individual patient.
It’s about more than pain – Integrated systems for optimal health
While pain is important, it is also recognized that simply relieving a patient’s pain does not necessarily result in a full return to all functional activities. Furthermore, there are subgroups of patients, such as high-level athletes, whose functional goals and measures (race time, power delivery in a stroke for example) are just as, if not more, meaningful to them than the relief of pain. Indeed, there is an increasing market in helping people without pain to optimize performance as well as prevent injury by facilitating strategies for better posture and movement. Pain is not a problem for these people, but an inability to meet their functional goals is. Non-painful impairments are also recognized as a potential contributor to the development of pain, both in sites distal to the impaired area and in the area itself. Furthermore, if we take the broader view that ‘pain is an opinion on the organism’s state of health rather than a mere reflexive response to injury’ (Ramachandran in Doidge 2007), we need to alter our focus and consider what it means to be ‘in health’ and not only what it means to be ‘in pain’. The World Health Assembly has defined health as ‘a state of complete physical, mental, and social well-being and not merely the absence of disease or infirmity’ (WHO Constitution). Speaking at the 1985 annual conference of the American Medical Association (Seattle, USA), Dr. Paul Brenner defined health even more broadly as ‘the full acceptance and appreciation of life’. Restoring health is about more than removing disease; creating optimal strategies for function and performance is about more than removing pain.
What it means to be ‘in health’ is individually defined. Therefore, changing our focus from removing pain to restoring optimal health and optimal strategies for function and performance is intrinsically linked to the patient’s values and goals. Our role as clinicians is to best facilitate and empower patients on their journey to achieve their personal optimal health and function. To do this effectively, we need not only to understand their pain, but also to understand to them as a person. Jones & Rivett (2004) refer to this as ‘understanding both the problem and the person’:
The Integrated Model of Function was developed from anatomical and biomechanical studies of the pelvis, as well as from the clinical experience of treating patients with lumbopelvic pain (Lee DG & Vleeming 1998, 2004, 2007, Lee DG 2004, Lee DG & Lee LJ 2008). From its inception, the Integrated Model of Function focused on the evaluation of the function of the pelvis, and how the pelvis effectively transfers loads across tasks with varying characteristics. The model addresses why the pelvis is painful by identifying the underlying impairments in four specific components: form closure, force closure, motor control and emotions. This is in opposition to pathoanatomical models that seek only to identify pain-generating structures. This model has continued to evolve with the publication of anatomical, biomechanical and neurophysiological research as well as the clinical expertise gained through collaborative efforts worldwide, and remains a useful framework to understand the pelvis in function and in dysfunction.
The Integrated Systems Model for disability and pain (Lee LJ & Lee DG 2011a) evolved from working with the Integrated Model of Function and was first introduced in 2007 as the System-Based Classification for Failed Load Transfer (Lee DG & Lee LJ 2007, Lee DG et al. 2008). We have since recognized that using the word ‘classification’ is limiting for this model because its primary purpose is not to place patients into homogeneous subgroups. In contrast, it is a framework to understand and interpret the unique picture of each individual patient in the clinical context to facilitate decision-making and treatment planning. The model provides a context to organize all the different types of knowledge needed (scientific, theoretical, professional craft, procedural, and personal) and provides for the development and testing of multiple hypotheses as the multidimensional picture of the patient emerges. A multimodal treatment plan can then be designed based on the complete picture of the person and their presenting problem(s).
The Integrated Systems Model for disability and pain allows clinicians to characterize all the components that contribute to what Melzack terms the ‘message that represents the whole body’ as a ‘flow of awareness’ (Melzack 2005). It is an integrated, evidence-based model that considers disability and pain as defined and directed by the patient’s values and goals. The model relates impairments found in systems, underlying pain mechanisms, and the impact of these impairments on their current whole-body strategies for function and performance. Thus the model analyses the patient’s current whole-body strategies, determines the underlying reasons for those strategies, and relates these to current knowledge about the necessary state required in all systems to provide optimal strategies for function and performance and, ultimately, for health. As a systems-based model, it has inherent flexibility to evaluate and integrate new evidence from research and innovative clinical approaches as they emerge. As a patient-centred model, it can continually adapt to changing goals and values of the patient. As the model applies to the whole person, rather than to a specific type of pain presentation or body region, it can be used across pain and disease populations and is not only applied to patients with pelvic pain. In the context of the lumbopelvic–hip (LPH) complex, the Integrated Model of Function fits within, and is encompassed by, The Integrated Systems Model for disability and pain. The Integrated Model of Function provides a way to subgroup patients with failed load transfer (FLT) in the LPH complex, i.e. those with a primary form closure, force closure, motor control or emotional deficits. The broader Integrated Systems Model for disability and pain also allows for subgrouping according to the primary system impairment but includes the role of the rest of the body, multiple system types, and all brain/mind states to the observed FLT in the LPH complex (considers more systems and causes both intrinsic and extrinsic to the pelvis). For example, is the primary impairment causing the FLT intrinsic to the pelvis itself (SIJ laxity → pelvic-driven pelvic pain) or extrinsic to the pelvis (thorax-driven or foot-driven pelvic pain) or due to a negative cognitive or emotional state. The Integrated Systems Model also considers the interaction and contribution of multiple systems (articular, myofascial, neural, visceral, hormonal, neuroendocrine, etc.). Several cases that highlight this approach can be found in the fourth edition of the Pelvic Girdle (Lee DG & Lee LJ 2011a).
The Integrated Systems Model for disability and pain: A framework for understanding the whole person and their problem
Underlying constructs of the model
1. The terms body, function/functioning, disability, impairment and health condition are taken from the International Classification of Functioning, Disability and Health (ICF) definitions (2001, pp. 189–190):
2. A body system is a group of organs/structures with co-ordinated activities, achieving the same general function in the body. A system shares common characteristics, including:
3. In a state of optimal health, an individual will have the option to choose from a wide variety of strategies that provide for optimal function and performance during any meaningful task (movement, activity, or role in a desired context and environment). Determining whether a task is meaningful requires understanding the person and their values and goals.
4. By definition, optimal function and performance occurs in a state of health, and will be a state free from undesired pain experiences. Given the definitions of health above, optimal function and performance is individually defined, and attainable in the presence of any health condition, although it may be influenced by specific features of the health condition.
5. Pain is not the only reason that people become disabled. Disability, or the inability to do what the person wants to do, can exist without pain.
6. Optimal function and performance for any task requires the synergistic, integrated operation of multiple systems in the body. ‘Synergy’ is defined as a ‘combined or cooperative action or force’ (Webster’s New World College Dictionary) and, ‘simply defined, it means that the whole is greater than the sum of its parts’ (Wikipedia). To ‘integrate’ is to ‘form, coordinate, or blend into functioning or unified whole’ (Merriam-Webster’s Online Dictionary). Synergy and integration require that each system, and thus the components of each system down to the cellular level, is functioning, and that the many complex feedback and feedforward mechanisms that control each system are working optimally. Then, the systems must work together to produce desired outputs in the body. Congruence of information received from feedback and sensory systems is also important. Not all the underlying mechanisms that produce the integrated, synergistic operation of body systems are fully understood, although science is continuing to reveal the connectedness and interdependence of body systems. Melzack’s concept of the body-self neuromatrix (see Figure 7.10) highlights this need for synergy and integration.
7. Impairment(s) in any one or combination of systems can give rise to undesired outputs in one or more systems. These outputs include painful states, non-optimal posture and movement (inefficient, loss of desired performance or output), loss of function, overactive and/or sustained stress response, and negative emotional states.
8. Designing and implementing the most effective treatment plan for restoring health depends on identifying the relevant impairments in the key systems that are barriers to healing and that need to be addressed in order to restore function and health. The relevance of each impairment is determined through a clinical reasoning process that uses a combination of different types of reasoning. Each impairment is evaluated in the context of meaningful tasks to determine how much the impairment contributes to the non-optimal strategies for function and performance, and the pain experience. The impairments/systems/regions with high contribution values are called the key ‘driver(s)’ in this model. The term ‘pain driver’ is used to refer to the underlying cause(s) of the pain experience, which could be the pain mechanism itself or a multitude of combined impairments that collectively increase physical and psychological stress and perpetuate the pain experience by exceeding the adaptive/coping mechanisms of specific tissues and the person as a whole. Note that, since the human body is dynamic, i.e. a changing entity, the key drivers for disability and/or pain at different points in time can change. Furthermore, the driver(s) of disability may be different than the driver(s) of pain.
9. The Integrated Systems Model is applicable to disability and/or pain of any duration; i.e., from acute onset to chronic, persistent or recurrent problems.
10. Every person is unique genetically, emotionally, cognitively, culturally and socially; the activities and roles that have meaning for them and their pain experience will be uniquely their own. In this way, the specific combination of impairments and systems that contribute to output experiences will be different for each patient. However, taken together, science and clinical expertise provide us with the necessary information to allow us to identify common patterns and parameters for normal and abnormal functioning of systems, as well as how subgroups of patients with certain common features (determined in research by inclusion and exclusion criteria of the study) respond to different treatment approaches. This information is invaluable and indispensable, and the continued pursuit of furthering our knowledge base (both propositional and non-propositional knowledge) in research and in the clinic creates a continually refined understanding of what allows us to enjoy health. However, knowledge gained from either the clinic or the research lab has limitations. Clinicians must constantly examine their emerging hypotheses for multiple types of bias. While clinical practice guidelines derived from research can be helpful and provide new insight, they may also be inappropriate and incorrect for certain patients. Therefore, caution is always necessary when developing general treatment protocols based on ‘homogeneous populations’ since homogeneous populations are an illusion and do not truly exist outside of research constructs.
11. Each person is a dynamic entity and can change from moment to moment and day to day. Science continues to find more evidence of this. Clinically, this implies that continual reassessment is essential for revising hypotheses about the drivers of the patient’s problem.
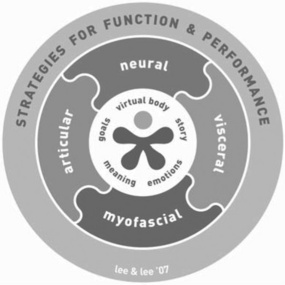
Components of the model: The Clinical Puzzle – A tool for clinical reasoning and developing clinical expertise
The Clinical Puzzle (Figure 7.6) is a graphic that conceptualizes The Integrated Systems Model for disability and pain. It represents the person and their problem(s), and the systems that support optimal strategies for function and performance. The puzzle is used clinically and in teaching as a tool for clinical reasoning and decision-making.
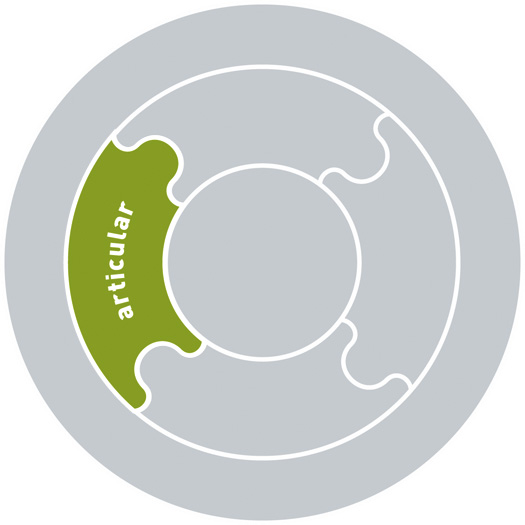
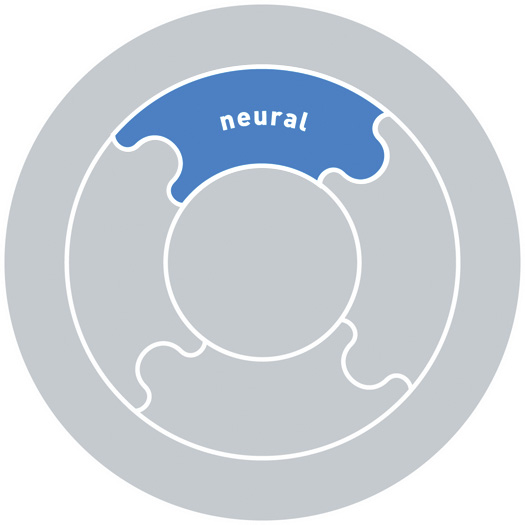
Articular, myofascial, neural, visceral systems
Specific impairments in the articular, myofascial, neural and visceral systems are listed in Box 7.1. The articular system includes the bones and joints (passive structures) in the musculoskeletal system. The myofascial system includes muscle, tendinous and fascial connections, as well as the multiple layers of fascia throughout the body. The neural system includes all components of the central and peripheral nervous system. It also includes the neural drive to muscles, which is reflected in the resting tone and activity or control of the muscle system. The visceral system includes all the viscera of the body.
Box 7.1 The conditions associated with the four systems of the Clinical Puzzle
Articular
• Ligament sprain or tear (grades I–III)
• Labral or intra-articular meniscal tear
• Intervertebral disc strain/tear/herniation/prolapse
• Joint subluxation or dislocation
• Stress fracture, osteitis, periostitis, apophysitis
• Osteochondral/chondral fractures, minor osteochondral injury
• Chondropathy (softening, fibrillation, fissuring, chondromalacia)
• Fibrosis/osteophytosis of the zygapophyseal and intervertebral joints, sacroiliac joint, hip joint
Myofascial
• Intramuscular strain/tear (grades I–III)
• Musculotendinous strain/tear
• Complete or partial tendon rupture or tear
• Tendon pathology – tendon rupture, partial tendon tears, tendinopathy (acute or chronic), paratendinopathy, pantendinopathy
• Skin lacerations/abrasions/puncture wounds
• Muscular or fascial scarring or adhesions
• Loss of fascial integrity of the anterior abdominal wall including:
Neural
• Peripheral nerve trunk or nerve injury (neuropraxia, neurotemesis, axonotemesis)
• Central nervous system injury
• Absence of recruitment, inappropriate timing (early or late) of muscle recruitment
• Inappropriate amount (increased or decreased) of muscle activity (all relative to demands of task)
• Hypertonicity or hypotonicity of muscles at rest
• Sensitization of the peripheral or central nervous system, altered central nervous system processing
If one considers all of the possible combinations of impairments and the associated findings that can lead to disability and/or pain, the pelvis can seem complicated. In reality, when reflective critical thinking and a thorough examination are used, the primary cause and initial treatment plan emerges. The Clinical Puzzle for The Integrated Systems Model is a useful tool for understanding the whole person and their problem(s). It allows for organization of key information gained through the examination process, comparing and contrasting this information to current propositional knowledge and personal knowledge of the clinician, and for reflection and interpretive reasoning of the findings. This facilitates the formation of hypotheses to explain the relationships between physical impairments, pain mechanisms, psychosocial features, disability, health conditions, and the patient’s values and goals. The goal of the clinical reasoning process, facilitated by the puzzle, is to determine which hypothesis provides the ‘most likely and most lovely’ (Kerry et al. 2008) explanation of the patient’s whole experience, from which an integrated multimodal treatment plan is formulated and implemented. As treatment evolves over several sessions, the focus often changes as the patient’s journey towards function and better health occurs.
What follows is a case study of a woman with peripartum pelvic pain. This case will illustrate how clinical reasoning and The Integrated Systems Model is used to establish a prescriptive treatment plan for the management of one clinical puzzle. All of the assessment tests described in the case below are fully described in Chapter 8 of the fourth edition of The Pelvic Girdle (Lee DG & Lee LJ 2011b).
Case study 7.1
Kristi’s story
Strategies for function and performance
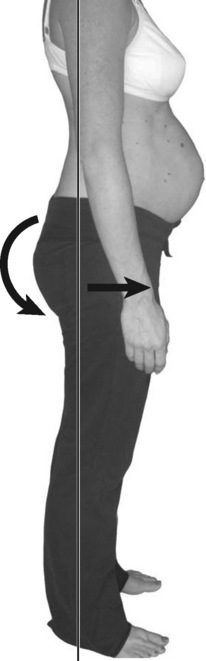
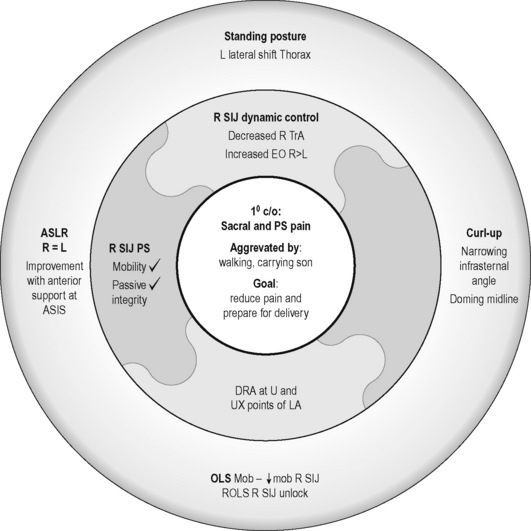
Standing posture
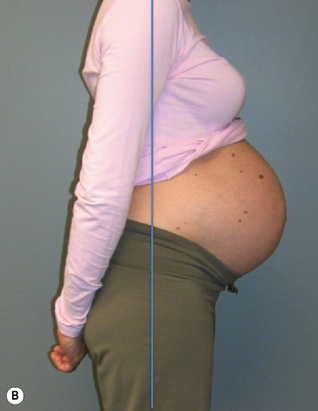
Kristi’s stood with a left lateral shift of her thorax relative to her pelvis (Figure 7.7) and the strategy she used to support her abdomen was non-optimal in that there was excessive use of the external obliques (EOs) bilaterally and insufficient use of transversus abdominis (TrA) (confirmed via ultrasound imaging)![]() . (Note: while we recognize that it is possible that there was a very small activation of TrA that is below the threshold for detection with ultrasound (Hodges et al. 2003a), clinical experience suggests that if no architectural change in TrA is seen on ultrasound imaging, then there is insufficient activation for functional tasks.)
. (Note: while we recognize that it is possible that there was a very small activation of TrA that is below the threshold for detection with ultrasound (Hodges et al. 2003a), clinical experience suggests that if no architectural change in TrA is seen on ultrasound imaging, then there is insufficient activation for functional tasks.)
Active straight leg raise
During the active straight leg raise (ASLR) test (Mens et al. 1999), Kristi did not note any difference in the effort required to lift her right or left leg; however, she did note that the effort to perform this task was reduced when her pelvis was compressed at the anterior aspect at the level of the anterior superior ![]() iliac spines (ASIS).
iliac spines (ASIS).
Articular system analysis
This system requires analysis whenever there is failure to control articular motion during any functional task. Given the location of Kristi’s pain, both the SIJs and pubic symphysis required analysis for mobility and integrity of the passive system restraints. The pubic symphysis was dynamically controlled and pain-free on stress testing, and there was no loss of integrity of the passive system. The mobility of the SIJs was symmetric when tested passively (compared to asymmetric when tested actively; see OLS above) and the passive restraints to articular motion were intact (i.e. no motion of the joint was palpable when the joint was tested in the close-packed position)![]() . These findings indicate that the articular system is not the cause of her lack of motion control during right single leg loading.
. These findings indicate that the articular system is not the cause of her lack of motion control during right single leg loading.
Neural system analysis
When Kristi was given a verbal cue intended to isolate a co-contraction of the deep system (TrA, pelvic floor) from the superficial muscle system![]() , an asymmetrical response to the bilateral cue occurred between the left and right TrA; the left TrA responded (and was isolated from the superficial muscles) whereas the right did not respond at all. This finding was confirmed via ultrasound imaging. In addition, an asymmetrical response occurred between the left and the right IO; the right IO responded whereas the left did not. These findings support the hypothesis that Kristi’s current pattern of deep and superficial abdominal muscle recruitment was providing insufficient support to the anterior pelvis during the ASLR and other tasks. This indicates that the neural system needs to be trained in order to restore optimal load transfer through the right side of the pelvis. However, given that 66% of women present with a diastasis of the rectus abdominis in the third trimester (Boissonault & Blaschak 1988), the myofascial system warrants examination (and follow-up) in every pregnant woman attending for treatment (Lee DG 2011). One of the mechanisms by which TrA provides support to the lumbopelvis is via increasing fascial tension (Hodges et al. 2003b); if there are impairments in the myofascial system of the abdominal wall (midline abdominal fascia and linea alba), TrA may not be able to provide support to the pelvis regardless of whether optimal control by the neural system is restored. Thus, further tests are required to determine if Kristi’s inability to transfer load through the right side of her pelvis is primarily driven by a neural impairment, or a combination of both a neural and a myofascial impairment.
, an asymmetrical response to the bilateral cue occurred between the left and right TrA; the left TrA responded (and was isolated from the superficial muscles) whereas the right did not respond at all. This finding was confirmed via ultrasound imaging. In addition, an asymmetrical response occurred between the left and the right IO; the right IO responded whereas the left did not. These findings support the hypothesis that Kristi’s current pattern of deep and superficial abdominal muscle recruitment was providing insufficient support to the anterior pelvis during the ASLR and other tasks. This indicates that the neural system needs to be trained in order to restore optimal load transfer through the right side of the pelvis. However, given that 66% of women present with a diastasis of the rectus abdominis in the third trimester (Boissonault & Blaschak 1988), the myofascial system warrants examination (and follow-up) in every pregnant woman attending for treatment (Lee DG 2011). One of the mechanisms by which TrA provides support to the lumbopelvis is via increasing fascial tension (Hodges et al. 2003b); if there are impairments in the myofascial system of the abdominal wall (midline abdominal fascia and linea alba), TrA may not be able to provide support to the pelvis regardless of whether optimal control by the neural system is restored. Thus, further tests are required to determine if Kristi’s inability to transfer load through the right side of her pelvis is primarily driven by a neural impairment, or a combination of both a neural and a myofascial impairment.
Myofascial system analysis
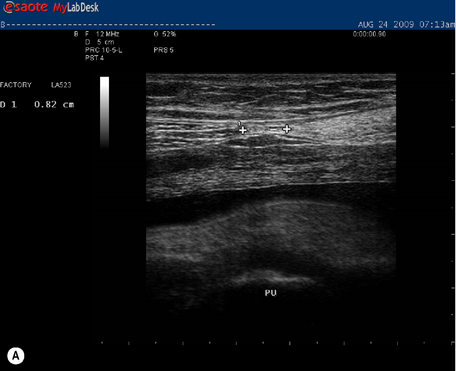
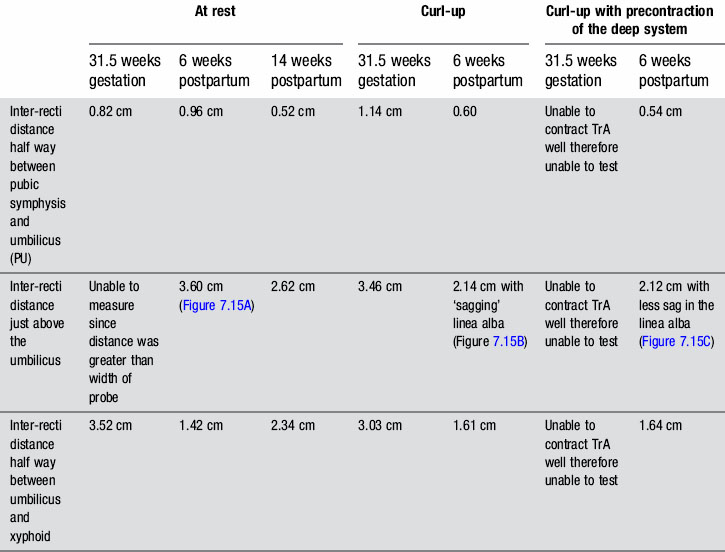
In individuals less than 45 years of age, the distance between the left and right rectus abdominis (i.e. the inter-recti distance) is considered to be ‘normal’ if it is no greater than 1 cm at a midway point between the pubic symphysis and the umbilicus (PU point), 2.7 cm just above the umbilicus (U point) and 0.9 cm at a midway point between the umbilicus and the xyphoid (UX point) (Rath et al. 1996). At 31.5 weeks gestation, Kristi was within these normal limits at the PU point (0.82 cm) and greater than this at both the U point (>width of probe at rest and 3.46 cm during a curl-up) and UX point (3.52 cm at rest and 3.03 cm during a curl-up) (Table 7.2, Figure 7.8 ![]() ). It was again noted that Kristi was using a non-optimal strategy for the curl-up task (insufficient and asymmetric activation of TrA and excessive activation of the EO). Palpation of the linea alba between the left and right rectus abdominis suggested it was intact; however, the ability of linea alba to transfer forces could not be assessed at this time since Kristi was unable to contract TrA optimally (neural impairment, see above). That is, since Kristi was unable to recruit an optimal isolated contraction of the deep system, the impact of a precontraction of the deep system on the inter-recti distance and the shape of the linea alba could not be assessed at this time.
). It was again noted that Kristi was using a non-optimal strategy for the curl-up task (insufficient and asymmetric activation of TrA and excessive activation of the EO). Palpation of the linea alba between the left and right rectus abdominis suggested it was intact; however, the ability of linea alba to transfer forces could not be assessed at this time since Kristi was unable to contract TrA optimally (neural impairment, see above). That is, since Kristi was unable to recruit an optimal isolated contraction of the deep system, the impact of a precontraction of the deep system on the inter-recti distance and the shape of the linea alba could not be assessed at this time.
Table 7.2 Inter-recti distance measured via ultrasound imaging both at rest and during a short head/neck curl-up task at 31.5 weeks gestation and 6 weeks postpartum. The rest measures are also provided at 14 weeks postpartum
Clinical impression derived from hypothesis development, reflection and interpretive reasoning
From this initial assessment, the primary hypothesis was that Kristi’s pain experience was primarily peripherally mediated and, according to The Integrated Systems Model the impairment that needed to be addressed first was in the neural system (see Kristi’s clinical puzzle, Figure 7.9). The passive restraints of the pubic symphysis and the right and left sacroiliac joints were intact (no articular impairment). The neural system assessment revealed deficits in the activation of both the deep and superficial systems; asymmetric responses were noted in both the TrAs and the internal obliques (IOs) in response to a verbal cue intended to isolate a symmetric response of the deep system. While the inter-recti distances at both the U and UX points of the linea alba (myofascial tests) were wider than normal values according to Rath et al. (1996), there appeared to be sufficient tension in this midline structure to effectively force close and control motion of the joints of the pelvis if the deep system (i.e. TrA and PF) was functioning optimally. This hypothesis would have to be tested after the neural system deficits were addressed.
The findings from this examination were explained to Kristi and a treatment session followed to teach her a better strategy for supporting her abdomen and for transferring loads through her trunk and pelvis![]() . This involved releasing (relaxing) the left and right EOs and then ‘waking up’ the right TrA and facilitating its co-contraction with the other muscles of the deep system. She was able to feel a ‘lightening of load’ on her pubic symphysis with this better strategy and was also able to stand on her right leg without losing control of the right side of her pelvis. Kristi was encouraged to practice this co-activation of the deep system (pelvic floor and TrA) frequently (three sets of ten contractions held for 10 seconds) over the remaining weeks of her pregnancy and a post-delivery home visit was advised (Lee LJ & Lee DG 2011b).
. This involved releasing (relaxing) the left and right EOs and then ‘waking up’ the right TrA and facilitating its co-contraction with the other muscles of the deep system. She was able to feel a ‘lightening of load’ on her pubic symphysis with this better strategy and was also able to stand on her right leg without losing control of the right side of her pelvis. Kristi was encouraged to practice this co-activation of the deep system (pelvic floor and TrA) frequently (three sets of ten contractions held for 10 seconds) over the remaining weeks of her pregnancy and a post-delivery home visit was advised (Lee LJ & Lee DG 2011b).
Two days postpartum
Kristi had an uneventful home delivery and incurred a small tear in her perineum requiring two stitches in the ‘muscular layer’ and one stitch in her skin. She reported that, during the final weeks of her pregnancy, she was able to control her pelvic pain when she engaged her deep system. Currently, her pelvic pain had recurred and additionally she was now experiencing pain in her perineum and occasional urinary incontinence.
Strategies for function and performance, myofascial and neural system analysis
Standing posture
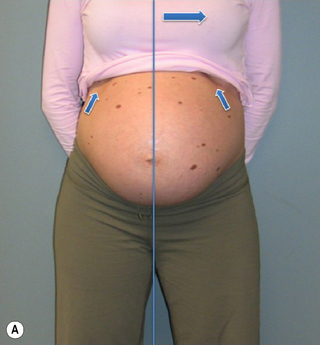
Kristi’s standing posture was quite different from that noted at 31.5 weeks gestation (Figure 7.10). She stood with an anterior pelvic sway and a posterior pelvic tilt, a flattened lumbar lordosis, and increased extension of the upper lumbar spine and lower thorax.
One leg standing, active straight leg raise and curl-up tasks
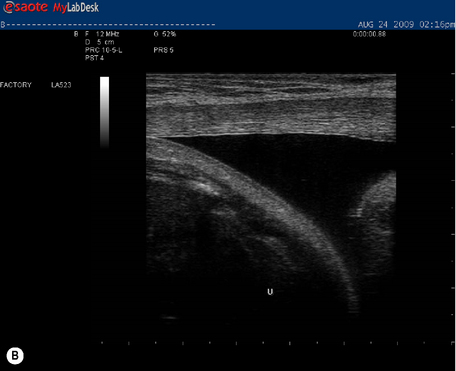
Kristi was unable to control motion of either the left or right side of her pelvis during single leg loading; both legs were difficult to lift and the effort to do so was reduced when her pelvis was compressed anteriorly during the ASLR task. Significant doming of the midline of her abdomen was noted during a ![]() curl-up task (Figure 7.11). There was minimal palpable tension in the linea alba during the curl-up task when no attempt was made to precontract the transversus abdominis (myofascial system analysis). There was a significant diastasis of the rectus abdominis (DRA) from the U to UX points (>3 finger widths).
curl-up task (Figure 7.11). There was minimal palpable tension in the linea alba during the curl-up task when no attempt was made to precontract the transversus abdominis (myofascial system analysis). There was a significant diastasis of the rectus abdominis (DRA) from the U to UX points (>3 finger widths).
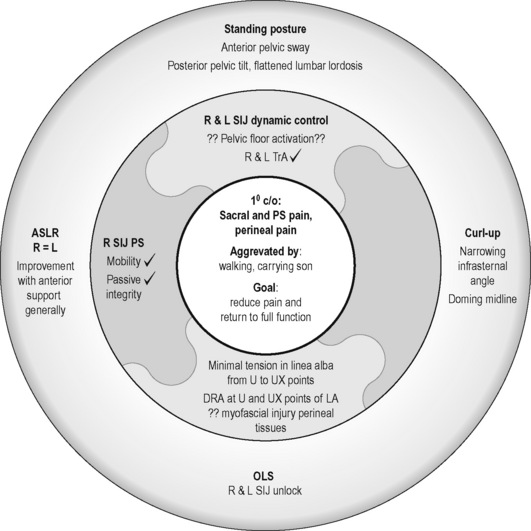
See Figure 7.12 for Kristi’s current clinical puzzle, reflect on the findings, and make a hypothesis as to what her management should be prior to reading the next section.
Clinical reasoning and early postpartum management
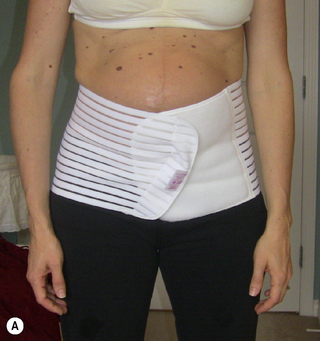
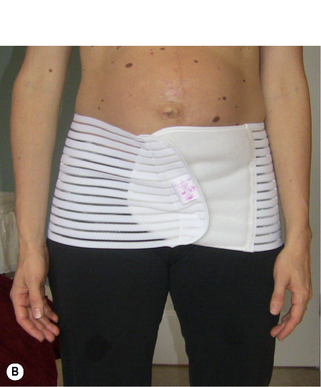
At this very early postpartum stage, Kristi was doing quite well. She had sustained some trauma to her pelvic floor as a consequence of her vaginal delivery and this would need to be further assessed once her wounds had healed. In the meantime, she was given postural advice regarding her standing, sitting and nursing positions and advised to wear an abdominal binder to help support her pelvis as healing occurred (Figure 7.13). The binder would also help to ‘remind her brain’ as to the way her lower abdomen should be supported by the deep muscle system. She was also advised to apply ice to her perineum and to slowly increase the activation of her pelvic floor as her pain subsided. A subsequent in-office visit was recommended at 6 weeks postpartum.
Six weeks postpartum
Kristi reported that she was now pain-free and no longer incontinent and was keen to return to her kickboxing class. She was managing all her homecare responsibilities with ease and was eager to ‘get fit’ once again. However, even though she was asymptomatic, several things of potential concern were noted on her follow-up examination.
Strategies for function and performance
Standing posture
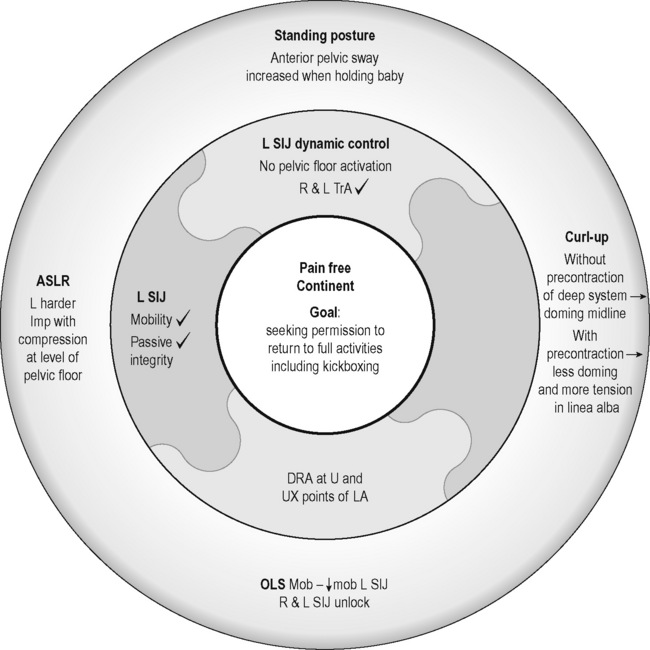
Kristi was able to find a much better standing posture (Figure. 7.14A) although she still had a tendency to sway her pelvis anteriorly especially when she carried her newborn (Figure 7.14B). A better strategy for standing was reviewed (Figure 7.14C).
Articular and neural system analysis
The passive mobility of the SIJs was symmetric and the integrity of the passive system restraints was intact, therefore the articular system was not hypothesized to be the cause of the unlocking noted during single leg loading. With respect to the deep muscle system, an isolated, symmetric response of the TrAs occurred with a cue to contract the pelvic floor; however, no response of the pelvic floor was seen via ultrasound imaging (both perineal and transabdominal approach) in response to any verbal cue![]() (Lee DG & Lee LJ 2011b). Video 12A shows an optimal response of the pelvic floor to a cue to contract; note the cranioventral lift of the anorectal angle towards the neck of the bladder. This contrasts significantly with the lift seen in Video 12B, which contains perineal ultrasound imaging of her attempt to recruit her pelvic floor.
(Lee DG & Lee LJ 2011b). Video 12A shows an optimal response of the pelvic floor to a cue to contract; note the cranioventral lift of the anorectal angle towards the neck of the bladder. This contrasts significantly with the lift seen in Video 12B, which contains perineal ultrasound imaging of her attempt to recruit her pelvic floor.
Curl-up task and myofascial system analysis
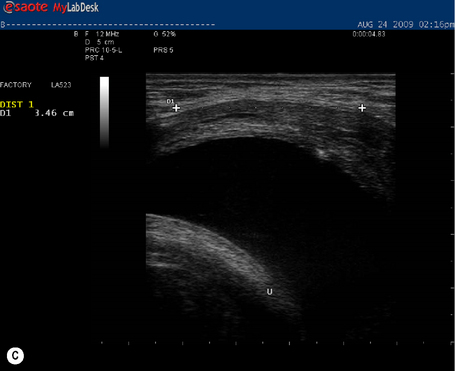
During a curl-up task, doming of the midline of the abdomen occurred and minimal tension was palpable in the linea alba, particularly just above the umbilicus![]() ; however, the infrasternal angle did not narrow (i.e. there didn’t appear to be excessive activation of the EOs during this task). At rest, the inter-recti distance was within normal limits at the PU point (0.96 cm), and still wider than normal at both the U (3.60 cm) and UX (1.42 cm) points (Table 7.2, Figure 7.15A). Just above the umbilicus, this distance narrowed to 2.14 cm during a curl up, which is within normal limits; however, the strategy that produced this narrowing was non-optimal in that insufficient tension was generated in the linea alba (note the sagging of the linea alba in Figure 7.15B). When Kristi activated the TrAs prior to doing the curl-up, the inter-recti distance at the U point was 2.12 cm (essentially no change); however, the strategy was better in that there was no doming of the midline abdomen and more tension could be seen, and felt, in the linea alba (Figure 7.15C, Video 13). Kristi also noted that it took less effort to curl when she activated the TrAs first. Coldron et al. (2008) have measured the inter-recti distance from 1 day to 1 year postpartum and note that the distance decreases markedly from day 1 to 8 weeks, and that without any intervention (e.g. exercise training or other physiotherapy) there was no further closure at the end of the first year. Whether training has any effect on the inter-recti distance has not been studied nor do we definitively know if closure is necessary for restoration of function in all women.
; however, the infrasternal angle did not narrow (i.e. there didn’t appear to be excessive activation of the EOs during this task). At rest, the inter-recti distance was within normal limits at the PU point (0.96 cm), and still wider than normal at both the U (3.60 cm) and UX (1.42 cm) points (Table 7.2, Figure 7.15A). Just above the umbilicus, this distance narrowed to 2.14 cm during a curl up, which is within normal limits; however, the strategy that produced this narrowing was non-optimal in that insufficient tension was generated in the linea alba (note the sagging of the linea alba in Figure 7.15B). When Kristi activated the TrAs prior to doing the curl-up, the inter-recti distance at the U point was 2.12 cm (essentially no change); however, the strategy was better in that there was no doming of the midline abdomen and more tension could be seen, and felt, in the linea alba (Figure 7.15C, Video 13). Kristi also noted that it took less effort to curl when she activated the TrAs first. Coldron et al. (2008) have measured the inter-recti distance from 1 day to 1 year postpartum and note that the distance decreases markedly from day 1 to 8 weeks, and that without any intervention (e.g. exercise training or other physiotherapy) there was no further closure at the end of the first year. Whether training has any effect on the inter-recti distance has not been studied nor do we definitively know if closure is necessary for restoration of function in all women.
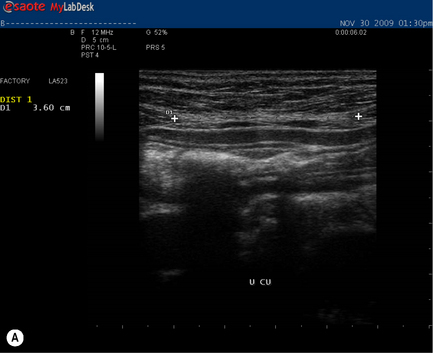
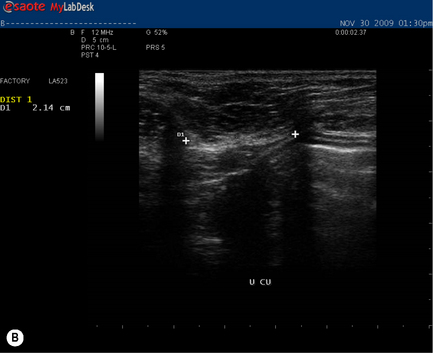
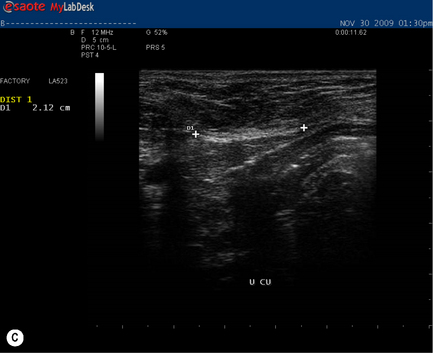
Figure 7.15 • Ultrasound images of the linea alba and response during a curl-up task. (A) This is the inter-recti distance at the U point at rest – 3.60 cm which is wider than normal values according to Rath et al. (1996) (2.70 cm). (B) During a curl-up with no precontraction of the deep system, Kristi’s strategy resulted in narrowing of the inter-recti distance (2.14 cm); however, note the ‘sagging’, which is reflective of minimal, if any, tension in the linea alba (C) When the deep system was activated prior to the curl-up task, the inter-recti distance at the U point also narrowed; however, the strategy chosen to curl-up was better in that the linea alba was tensed and sagged less
See Figure 7.16 for Kristi’s current clinical puzzle and reflect on the findings to determine what her management should be prior to reading the next section. Would you allow her to return to kickboxing at this time?
Clinical reasoning and management
Kristi was surprised to discover that her ‘Kegel’ exercises were not effectively producing a contraction of her pelvic floor. Bump et al. (1991) reported that 50% of women cannot effectively activate the pelvic floor in response to a verbal cue and either an internal or ultrasound imaging examination is needed to ensure that a response is occurring. Although she was not experiencing incontinence of urine, stool or gas, the OLS and ASLR tests as well as the results of her neural system evaluation support a specific training programme to ‘wake up’ and integrate her pelvic floor with the other muscles of the deep system. It is possible that this deficit was responsible for the ‘unlocking’ of both sides of her pelvis in single leg loading tasks and while she was disappointed at not being able to attend her kickboxing class immediately, she did understand the reasons why it was necessary to address the neural deficit and train her deep muscle system before increasing the loads through her pelvis.
Kristi was given the Pelvic Floor Educator™ (www.neenhealth.com) (Figure 7.17), which is a useful biofeedback tool for training the pelvic floor. She was instructed on how to use this tool to ensure proper activation of her pelvic floor and once she was able to do this, she was advised to integrate the contraction with her TrA cue such that the deep muscle system was trained together.
Twelve weeks postpartum
From her assessment (which included an intravaginal examination), Johanne noted the following:
1. Hyperaesthesia (pain) in the deep posterolateral vaginal wall;
2. A low cervix (and uterus) (0.5 cm above the hymen) which was likely interfering with the trajectory of lift from the pelvic floor contraction;
3. Hypertonicity of the levator ani with decreased ability to relax; and
4. Restricted mobility of the right obturator nerve and the nerves of both the pelvic and coccygeal plexus.
Fourteen weeks postpartum
Strategies for function and performance
Curl-up task and myofascial system analysis
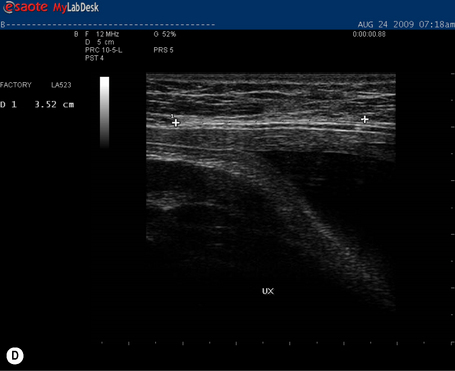
During a curl-up task, there was no doming of the midline of the abdomen and lovely tension was now palpable in the linea alba. The infrasternal angle, however, was now widening suggesting an imbalance of activation between the IOs and EOs during this task (with net vector from internal oblique)![]() . This was a change in strategy for this task from that used in the final trimester of her pregnancy. At rest, the inter-recti distance at the PU point was now 0.52 cm (reduced from 0.96 cm at 6/52 postpartum), at the U point was 2.62 cm (reduced from 3.60 cm at 6/52 postpartum) and at the UX point was 2.34 cm (an increase from 1.42 cm at 6/52 postpartum) (see Table 7.2). The widening of the upper portion of the linea alba is reflective of the non-optimal strategy she was using to do her curl-up exercises.
. This was a change in strategy for this task from that used in the final trimester of her pregnancy. At rest, the inter-recti distance at the PU point was now 0.52 cm (reduced from 0.96 cm at 6/52 postpartum), at the U point was 2.62 cm (reduced from 3.60 cm at 6/52 postpartum) and at the UX point was 2.34 cm (an increase from 1.42 cm at 6/52 postpartum) (see Table 7.2). The widening of the upper portion of the linea alba is reflective of the non-optimal strategy she was using to do her curl-up exercises.
Neural system analysis
An isolated, symmetric response of the TrAs continued to occur with a cue to contract the pelvic floor and a small cranioventral lift could now be seen via ultrasound imaging of the pelvic floor (perineal approach)![]() . Her pelvic organs appeared to be well supported when she performed a valsalva manoeuvre.
. Her pelvic organs appeared to be well supported when she performed a valsalva manoeuvre.
Clinical reasoning and management
The widening of the upper portion of the linea alba can be explained by the over-activation of the IOs (or underactivation of the EOs) during the multiple curl-ups Kristi was doing to ‘get back in shape’. She was advised on how to correct this strategy (Video 15) to prevent further widening of the linea alba and perhaps to facilitate its closure. The treatment provided by Johanne Sabourin for Kristi’s pelvic floor had certainly ‘released and woken up’ the levator ani, yet more improvement was needed if effective load transfer and pelvic organ support were to be ensured throughout her lifetime. Kristi was advised to continue with the training programme provided and to work on integrating these new strategies into her activities of daily living and other physical activities (i.e. kickboxing) (Lee LJ 2011). She was also advised to return for a follow-up examination in 6 months time.
Summary
According to the definition of Sackett et al. (2000), we believe that The Integrated Systems Model is an evidence-based approach in that it considers the patient’s values (thoughts, feelings, expectations) and integrates the practitioner’s expertise (clinical reasoning and skills) and the available research evidence into decision-making for appropriate therapeutic interventions. In our experience, there are no recipes, prediction rules or guidelines for patients presenting with chronic pelvic disability with or without pain and it is likely that a multimodel approach will always be more effective for long-term success. While temporary improvement in function and/or pain may be gained by using one component of the therapeutic intervention (release or align or connect or move), it is the long-term solution that is sought by the patient. We strive to empower our patients to understand what is driving their disability or pain experience, to be aware of the contexts or situations that facilitate their poor strategies and to learn how they can change those strategies and move towards ones that are more optimal for their bodies and health (Empower through Knowledge, Movement and Awareness). In this way, we hope that they can Move better, Feel better, and Be better! More information on The Integrated Systems Model approach can be found in the fourth edition of The Pelvic Girdle and online at www.discoverphysio.ca.
This chapter has illustrated the myriad of factors the clinician may need to take into account when assessing and developing a treatment plan for the patient with CPP. The next chapter describes the multidisciplinary and multispeciality approach to the management of CPP, from a pain specialist (Chapter 8.1) and from a physiotherapeutic perspective (Chapter 8.2).
Beattie P., Nelson R. Clinical prediction rules: what are they and what do they tell us?. Aust. J. Physiother.. 2006;52(3):157.
Bernard T.N., Kirkaldy-Willis W.H. Recognizing specific characteristics of nonspecific low back pain. Clin. Orthop.. 1987;217:266.
Boissonault J.S., Blaschak M.J. Incidence of diastasis recti abdominis during the childbearing year. Phys. Ther.. 1988;68(7):1082.
Bonica J.J. The management of pain. Philadelphia: Lea & Febiger; 1953.
Brukner P., Khan K. Clinical sports medicine, second ed. Sydney, Australia: McGraw-Hill; 2007.
Bump R.C., Hurt G.W., Fantl J.A., et al. Assessment of Kegal pelvic muscle exercise performance after brief verbal instruction. Am. J. Obstet. Gynecol.. 1991;165:322.
Butler D.S. The sensitive nervous system. Adelaide, Australia: NOI Group Publications; 2000.
Cochrane A.L. Effectiveness and Efficiency: Random Reflections on Health Services. Nuffield Provincial Hospitals Trust, London. Reprinted in 1989 in association with the BMJ; Reprinted in 1999 for Nuffield Trust by the Royal Society of Medicine Press, London. 1972.
Coldron Y., Stokes M.J., Newham D.J., et al. Postpartum characteristics of rectus abdominis on ultrasound imaging. Man. Ther.. 2008;13:112.
Cook C. Potential pitfalls of clinical prediction rules. J. Man. Manip. Ther.. 2008;16(2):69.
Coste J., Paolaggi J.B., Spira A. Classification of nonspecific low back pain, I: psychological involvement in low back pain. Spine. 1992;17:1028.
Delitto A., Erhard R.E., Bowling R.W. A treatment-based classification approach to low back syndrome: identifying and staging patients for conservative treatment. Phys. Ther.. 1995;75:470.
Doidge N. The brain that changes itself. Stories of personal triumph from the frontiers of brain science. New York: Penguin Books; 2007.
Ericsson K.A., Smith. Towards a general theory of expertise: prospects and limits. New York: Cambridge University Press; 1991.
Fisher J.P., Hassan D.T., O’Connor N. Minerva. BMJ. 1995;310:70.
Fritz J.M. Clinical prediction rules in physical therapy: coming of age? J. Orthop. Sports Phys. Ther.. 2009;39(3):159.
Fritz J.M., Cleland J.A., Childs J.D. Subgrouping patients with low back pain: evolution of a classification approach to physical therapy. J. Orthop. Sports Phys. Ther.. 2007;37(6):290.
Gifford L. Pain, the tissues and the nervous system: a conceptual model. Physiotherapy. 1998;84(1):27.
Gombatto S.P., Collins D.R., Sahrmann S.A., Engsberg J.R., Van Dillen L.R. Patterns of lumbar region movement during trunk lateral bending in 2 subgroups of people with low back pain. Phys. Ther.. 2007;87(4):441.
Guyatt G., Cairns J., Churchill D., et al. Evidence-based medicine. A new approach to teaching the practice of medicine. [Evidence-Based Medicine Working Group]. J. Am. Med. Assoc.. 1992;268:2420.
Higgs J. Educational theory and principles related to learning clinical reasoning. In: Jones M.A., Rivett D.A., editors. Clinical reasoning for manual therapists. Edinburgh: Elsevier, 2004.
Higgs J., Titchen A. Propositional, professional and personal knowledge in clinical reasoning. In: Higgs J., Jones M., editors. Clinical reasoning in the health professions. second ed. Oxford: Butterworth-Heinemann; 1995:129.
Hodges P.W., Kaigle Holm A., et al. Intervertebral stiffness of the spine is increased by evoked contraction of transversus abdominis and the diaphragm: in vivo porcine studies. Spine. 2003;28(23):2594.
Hodges P.W., Pengel L.H.M., Herbert R.D., Gandevia S.C. Measurement of muscle contraction with ultrasound imaging. Muscle Nerve. 2003;27:682.
Jensen G.M., Gwyer J., Hack L.M., Shepard K.F. Expertise in Physical Therapy Practice, second ed. Saunders; 2007.
Jones M.A., Rivett D. Introduction to clinical reasoning. In: Jones M.A., Rivett D.A., editors. Clinical reasoning for manual therapists. Edinburgh: Elsevier, 2004. p. 3
Kerry R., Maddocks M., Mumford S. Philosophy of science and physiotherapy: an insight into practice. Physiother. Theory Pract.. 2008;24(6):1.
Kirkaldy-Willis W.H., editor. Managing low back pain. New York: Churchill Livingstone, 1983.
Kirkaldy-Willis W.H., Hill R.J. A more precise diagnosis for low back pain. Spine. 1979;4:102.
Kirkaldy-Willis W.H., Wedge J.H., Yong-Hing K., et al. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine. 1978;3:319.
Laslett M., Williams W. The reliability of selected pain provocation tests for sacroiliac joint pathology. Spine. 1994;19(11):1243.
Laslett M., Aprill C.H., McDonald B., et al. Diagnosis of sacroiliac joint pain: validity of individual provocation tests and composites of tests. Man. Ther.. 2005;10:207.
Leboeuf-Yde C., Lauritsen J.M., Lauritzen T. Why has the search for causes of low back pain largely been inconclusive? Spine. 1997;22(8):877.
Lee D.G. The pelvic girdle, third ed. Edinburgh: Churchill Livingstone; 2004.
Lee D.G. Pregnancy and its potential complications. Ch. 6. Lee D.G., editor. The Pelvic Girdle, fourth ed, Edinburgh: Churchill Livingstone, 2011. (at press)
Lee D.G., Lee L.J. Bridging the gap: the role of the pelvic floor in musculoskeletal and urogynecological function. Proceedings of the World Physical Therapy Conference. Canada: Vancouver; 2007.
Lee D.G., Lee L.J. Integrated, multimodal approach to the treatment of pelvic girdle pain and dysfunction. In: Magee D.J., Zachazewski J.E., Quillen W.S., editors. Pathology and intervention in musculoskeletal rehabilitation. Saunders, Elsevier; 2008:473.
Lee D.G., Lee L.J. Clinical reasoning, treatment planning and case reports. Ch. 9. Lee D.G., editor. The Pelvic Girdle, fourth ed, Edinburgh: Churchill Livingstone, 2011. (at press)
Lee D.G., Lee L.J. Techniques and tools for assessing the lumbopelvic-hip complex. Ch. 8. Lee D.G., editor. The Pelvic Girdle, fourth ed, Edinburgh: Churchill Livingstone, 2011. (at press)
Lee D.G., Vleeming A. Impaired load transfer through the pelvic girdle – a new model of altered neutral zone function. Proceedings from the 3rd interdisciplinary world congress on low back and pelvic pain. Austria: Vienna; 1998.
Lee D.G., Vleeming A. The management of pelvic joint pain and dysfunction. In: Boyling J.D., Jull G., editors. Grieve’s modern manual therapy. The vertebral column. third ed. Churchill Livingstone, Elsevier; 2004:495.
Lee D.G., Vleeming A. An integrated therapeutic approach to the treatment of pelvic girdle pain. In: Vleeming A., Mooney V., Stoeckart R., editors. Movement, stability & lumbopelvic pain. second ed. Elsevier; 2007:621.
Lee D.G., Lee L.J., McLaughlin L.M. Stability, continence and breathing: The role of fascia following pregnancy and delivery. J. Bodyw. Mov. Ther.. 2008;12:333.
Lee L.J. Training new strategies for posture and movement. Ch. 12. Lee D.G., editor. The Pelvic Girdle, fourth ed, Edinburgh: Churchill Livingstone, 2011.
Lee L.J., Lee D.G. Clinical practice – the reality for clinicians. Ch. 7. Lee D.G., editor. The Pelvic Girdle, fourth ed, Edinburgh: Churchill Livingstone, 2011.
Lee L.J., Lee D.G. Tools and techniques for ‘waking up’ and coordinating the deep and superficial muscle systems. Ch. 11. Lee D.G., editor. The Pelvic Girdle, fourth ed, Edinburgh: Churchill Livingstone, 2011.
MacNab I. Backache. Baltimore: Williams & Wilkins; 1977.
Magee D.J., Zachazewski J.E., Quillen W.S. Scientific foundations and principles of practice in musculoskeletal rehabilitation. St Louis: Saunders Elsevier; 2007.
McKenzie R.A. The lumbar spine: mechanical diagnosis and therapy. New Zealand, Wellington: Spinal Publications; 1981.
Melzack R. Pain and the neuromatrix in the brain. J. Dent. Educ.. 2001;65(12):1378.
Melzack R. Evolution of the neuromatrix theory of pain. The Prithvi Raj Lecture: Presented at the third World Congress of World Institute of Pain, Barcelona 2004. Pain Pract.. 2005;5(2):85.
Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150:971.
Mens J.M.A., Vleeming A., Snijders C.J., Stam H.J., Ginai A.Z. The active straight leg raising test and mobility of the pelvic joints. Eur. Spine J.. 1999;8:468.
Merskey H., Bogduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms, second ed. Seattle, USA: IASP Press; 1994. Prepared by the Task Force on Taxonomy of the International Association for the Study of Pain
Moseley G.L. Reconceptualising pain according to modern pain science. Phy. Ther. Rev.. 2007;12:169.
Nachemson A. Back pain; delimiting the problem in the next millennium. Int. J. Law Psychiatry. 1999;22(5–6):473.
O’sullivan P. Diagnosis and classification of chronic low back pain disorders: maladaptive movement and motor control impairments as underlying mechanism. Man. Ther.. 2005;10(4):242.
O’sullivan P., Beales D. Diagnosis and classification of pelvic girdle pain disorders – Part 1: a mechanism based approach within a biopsychosocial framework. Man. Ther.. 2007;12:86.
Pengel L.H.M., Herbert R.D., Maher C.G., Refshauge K.M. Acute low back pain: systematic review of its prognosis. Br. Med. J.. 2003:323-327.
Rath A.M., Attali P., Dumas J.L., et al. The abdominal linea alba: an anatomo-radiologic and biomechanical study. Surg. Radiol. Anat.. 1996;18:281.
Reeves N.P., Cholewicki J., Milner T.E. Muscle reflex classification of low-back pain. J. Electromyogr. Kinesiol.. 2005;15(1):53.
Riddle D.L. Classification and Low Back Pain: A Review of the Literature and Critical Analysis of Selected Systems. Phys. Ther.. 1998;78:708.
Rivett D.A., Jones M.A. Improving clinical reasoning in manual therapy. In: Jones M.A., Rivett D., editors. Clinical reasoning for manual therapists. Edinburgh: Elsevier; 2004:403.
Sackett D.L., Straus S., Richardson W.S., Rosenberg, Haynes R.B. Evidence-based medicine. How to practice & teach EBM. New York: Elsevier Science; 2000.
Sahrmann S.A. Diagnosis by the physical therapist: a prerequisite for treatment. Phys. Ther.. 1988;68(11):1703.
Sahrmann S. Diagnosis and treatment of movement impaired syndromes. St Louis: Mosby; 2001.
Waddell G. The back pain revolution, second ed. Edinburgh: Churchill Livingstone; 2004.
Wright A. Neurophysiology of pain and pain modulation. In: Strong J., Unruh A.M., Wright A., Baxter G.D., editors. Pain, a textbook for therapists. Edinburgh: Churchill Livingstone, 2002.