The Pineal Gland and Melatonin
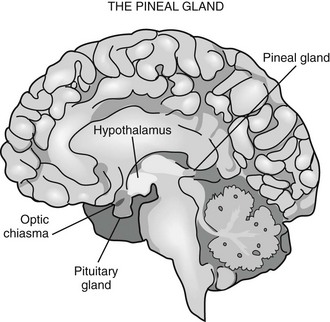
Pineal Gland
Neural Innervation of The Pineal Gland
Phylogenetically, the pineal gland is derived from photoreceptor cells; however, these properties have been lost in humans. The mammalian pineal gland does not have photoreceptor activity but rather receives photosensory information from the neuroretina. This information is relayed through the retinohypothalamic tract to the suprachiasmatic nucleus (SCN) of the hypothalamus, which functions as a circadian oscillator or clock (Fig. 15-1). Fibers from the SCN then descend to the spinal cord, projecting to the superior cervical ganglia, from which postganglionic adrenergic neurons return to innervate the pineal gland. These fibers contain norepinephrine and neuropeptide Y. Through this pathway, melatonin synthesis is controlled and its rhythm entrained (synchronized) to the 24-hour light/dark cycle (see below). It is generated in the SCN by a closed loop of negative feedback of clock gene expression.1
Melatonin
The word melatonin is a derivative of the Greek words melas and tonein, coined because of the property of melatonin to lighten amphibian skin.2 Melatonin is N-acetyl-5-methoxytryptamine, an indolamine derivative of tryptophan. The molecule is extremely well conserved across the phyla and has been identified in all major taxa, including bacteria, unicellular eukaryotes, many plants species, and all animals.3 In the vertebrate, melatonin is primarily secreted by the pineal gland, although a variety of other tissues, including the retina, bone marrow, skin, lymphocytes, and gastrointestinal tract, also synthesize the hormone. Melatonin derived from the gastrointestinal tract can be released into the circulation after ingestion of high dietary tryptophan.4
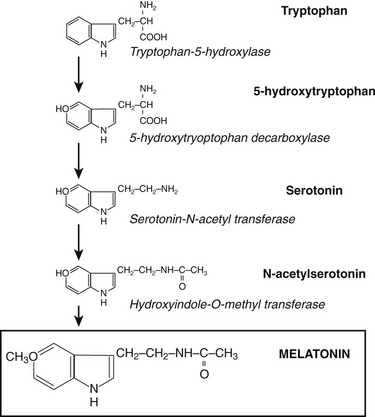
Synthesis and Secretion of Melatonin
Through the neuroretinal pathways described above, the pineal gland becomes a neuroendocrine transducer, transmitting the SCN message into a hormonal code which signals that light or darkness has arrived. Indeed, the main function of the pineal gland is to translate SCN activity into the rhythmic release of melatonin, which in turn helps synchronize several daily and seasonal cycles. The synthesis of melatonin is presented in Fig. 15-2. The hormone is synthesized from the amino acid tryptophan, which is converted into serotonin prior to being processed into melatonin. Melatonin synthesis and secretion are greater during the dark phase compared to the light phase of the cycle. During the light phase of the photoperiod, SCN activity is high, resulting in low norepinephrine levels. Under reduced adrenergic activity, tryptophan is converted into serotonin in a two-step process via the intermediary, 5-hydroxytryptophan. At this point, serotonin does not come into contact with the enzyme responsible for converting it into melatonin. Therefore, plasma levels of melatonin are low during the light phase. However, with the arrival of the dark period, SCN activity becomes quiescent, and noradrenergic activity increases, resulting in activation of β-adrenergic receptors (and to a lesser extent α-adrenergic receptors) on the pinealocyte. The β-adrenergic receptors are coupled to cyclic adenosine monophosphate (cAMP)/protein kinase A signaling pathways that stimulate melatonin synthesis (Fig. 15-3). Stimulation of α-adrenergic receptors potentiates β-adrenergic function, resulting in a cascade that mobilizes calcium ions, phosphatidylinositol, diacylglycerol, and protein kinase C.5 This process requires serotonin to be first converted into N-acetyl serotonin by the enzyme serotonin-N-acetyltransferase, which in turn is converted into melatonin after coming into contact with the enzyme hydroxyindole-O-methyltransferase (HIOMT).6 The longer the dark phase, the longer the duration of melatonin secretion.
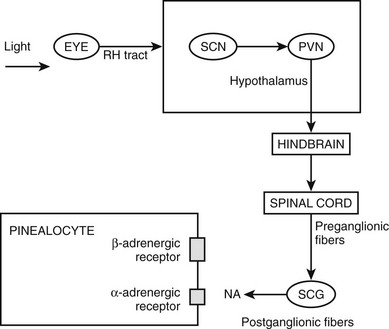
Figure 15-2 Pathways providing light and neural input to the pineal gland. The melatonin rhythm is synchronized to a 24-hour light/dark cycle primarily by the retina and retinohypothalamic projections to the SCN. RH, retinohypothalamic; SCN, suprachiasmatic nucleus; PVN, paraventricular nucleus; SCG, superior cervical ganglion; NA, norepinephrine.
Melatonin is not stored after synthesis but merely diffuses out of the pineal gland into the blood stream and cerebrospinal fluid. In the transition from light to dark, plasma melatonin concentrations increase from 2 to 10 pg/mL to 100 to 200 pg/mL.7 Melatonin levels start to rise during the evening, reach maximum levels in the middle of the night, and start decreasing in the early morning before sunrise. Although the melatonin rhythm is highly responsive to the light cycle, it does persist when people are placed for a few days in a dark room and does not immediately phase shift when the light schedule is altered.7 This indicates that the rhythm is not simply generated by the light/dark cycle but is free running and modulated by endogenous signals probably arising in the SCN. Indeed, the rhythm is abolished by lesioning of the SCN and persists, albeit in modified form, in the blind.8 The day-to-day pattern of melatonin secretion is extremely stable within an individual.9 However, the melatonin rhythm varies widely among individuals, in part owing to genetic determinants.9a In fact, a small number of healthy persons have no detectable melatonin in plasma at any time of day.10
Melatonin has a bi-exponential half-life, with a first distribution T½ of 2 minutes and a second of 20 minutes. The hormone is lipophilic and enters tissues rapidly. Up to 70% of melatonin is bound to albumin in plasma.11 In addition to blood, saliva, and urine, melatonin is also found in cerebrospinal fluid (CSF), the anterior chamber of the eye, and in many reproductive fluids, including semen, amniotic fluid, and breast milk.12 Melatonin levels in plasma, CSF, saliva, and urine become undetectable following removal of the pineal gland, demonstrating that the pineal gland is the main source of melatonin in these compartments.13
Melatonin secretion varies across the life cycle. Secretion of the hormone begins in the fourth month of postnatal life and then increases rapidly, peaking between the ages of 1 and 3. Nocturnal melatonin secretion then starts a marked decline over each decade of life14 (Fig. 15-4). Nocturnal melatonin concentrations can also be affected by drugs that interfere with the transmission of neurotransmitter signals to the pineal gland. These drugs include β-blockers, caffeine, and ethanol.15–17 Nocturnal melatonin secretion is also suppressed by exposure to environmental lighting. Indeed, there is a dose-dependent and spectral-sensitive acute suppressive effect of light on melatonin.18,19 Even low light levels found indoors can suppress nocturnal melatonin production. In addition to light, exercise and postural changes decrease plasma melatonin levels.20,21
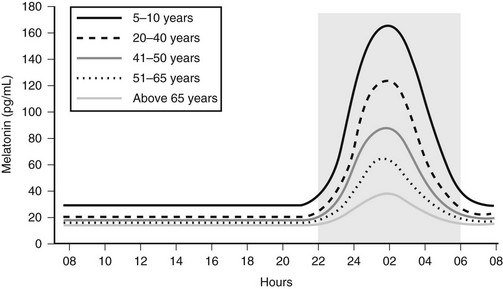
Figure 15-4 Circadian profile of serum melatonin across the life cycle; gray area indicates darkness.
Melatonin Receptors
In the mammal, there are at least two melatonin receptors, designated MT1 and MT2, which belong to the superfamily of G protein–coupled receptors containing the usual seven transmembrane domains. A third melatonin binding site, MT3, was found to be the enzyme quinone reductase 2.22 These receptors have distinct structures, chromosomal localization, and pharmacologic properties.23 The MT1 and MT2 receptors are differentially expressed across the nervous system. The highest density is found in the SCN, followed by the anterior pituitary and the retina.24 Studies have shown that the MT1 receptor in the SCN allows melatonin to inhibit firing of SCN neurons during the nighttime.24 The SCN MT2 receptor possibly mediates the effect of melatonin on SCN circadian rhythms.24 Both receptors are co-localized within the SCN and are coupled to multiple signaling pathways, with suppression of cyclic AMP production through a Gi-dependent process being the most common.23 The receptors can also stimulate phospholipase C, affecting various ion channels, as well as stimulating the mitogen-activated protein kinase cascade and the estrogen-dependent signaling cascade.25 Melatonin is a small, lipophilic compound which easily crosses membranes. In this regard, melatonin has been shown to bind to specific nuclear receptors of the retinoic acid receptor family26,27 and to calmodulin. Melatonin receptors have also been identified in the heart, kidney, liver, and many other peripheral tissues.28,29
The MT1 and MT2 receptors have different profiles for receptor desensitization. Exposure of the MT1 receptor to supraphysiologic concentrations of melatonin causes an increase in MT1 receptor density, with a concurrent decrease in affinity and functional sensitivity.24 In contrast, exposure of the MT2 receptor to physiologic concentrations of melatonin can induce a concentration and time-dependent receptor desensitization and internalization.30 The interplay between desensitization of the MT1 and MT2 receptors to daytime and nighttime physiologic concentrations of melatonin may promote changes in melatonin receptor function throughout the human circadian cycle. With the SCN ultimately controlling the production of melatonin, a feedback loop is created that may use melatonin as the regulatory control of SCN firing and phase-shifting functions.31 Desensitization of MT1 and MT2 receptors needs to be taken into account when administering melatonin to patients.
Melatonin Metabolism
Melatonin in the circulation is mainly inactivated by the liver’s cytochrome P450-specific mono-oxygenase, which first hydroxylates position C6 and then conjugates the product to a sulfate excreted into urine and feces as 6-sulfatooxymelatonin.32 The metabolism in nonhepatic tissue is different and is mainly through deacetylation. Melatonin can also be nonenzymatically metabolized in all cells and also extracellularly by free radicals.
Actions of Endogenous and Exogenous Melatonin
Seasonal variations in day length have reciprocal effects on melatonin secretion and can thus differentially affect plasma melatonin levels, depending on the location of the human population. For example, individuals living at high northern latitudes have a lengthening of melatonin secretion during winter nights.33 This may help explain how changes in the seasons affect reproductive vitality. In the laboratory setting, artificial shorting or lengthening of “night” will also adjust the duration of melatonin secretion.34
Circadian rhythms are biological processes that have a 24-hour periodicity even in the absence of external cues. Melatonin is involved in regulating several circadian cycles, including core body temperature and the sleep/wake cycle. The temporal organization of human circadian rhythms has been assigned the following terms: “biological day,” “biological night,” “biological dawn,” and “biological dusk.”35 During this 24-hour cycle, rising melatonin levels are associated with decreasing core body temperature, cortisol levels, and alertness. As melatonin levels wane, core body temperature, REM sleep propensity, and cortisol levels increase. It remains unclear whether there is a causal relationship between melatonin and these changes in the sleep/wake cycle. However, exogenously administered melatonin can phase shift body temperature, sleep timing, and the endogenous melatonin cycle.32 The ability of melatonin to phase shift circadian cycles has been exploited in the treatment of jet lag and shift-work disorders (see below).
Sleep
The evening rise in melatonin precedes bedtime by about 2 hours and suggests a causal relationship. Indeed, the peak in plasma melatonin occurs when alertness is at its nadir. However, the sleep/wake cycle is regulated by both homeostatic and circadian processes and is only partially influenced by melatonin. The Smith-Magenis syndrome illustrates the association between melatonin and the sleep/wake cycle. The manifestations of this disorder include excessive daytime sleepiness and insomnia at night. Treatment of these patients with a β-adrenergic blocker decreases daytime melatonin levels and diminishes daytime sleepiness.36 Administration of physiologic and pharmacologic doses of melatonin to normal sleepers induces soporific symptoms. However, evidence of a direct relationship between endogenous melatonin and insomnia has been mixed.37,38 The number of reports that have measured plasma melatonin concentrations in sleep disorders is surprisingly low, considering its use as sleep-promoting agent for insomnia. Sleep disturbances have been described in some but not all pinealectomized patients, and administration of melatonin has sometimes been found to be efficacious in this setting.39
Although the exact role of endogenous melatonin in regulating human sleep remains to be fully elucidated, exogenous melatonin administration (0.1 to 0.3 mg) resulting in physiologic levels of melatonin will promote the onset and maintenance of sleep.32 Both a 1997 and a 2005 meta-analysis showed that melatonin administration decreases sleep latency, increases sleep efficiency, and increases total sleep duration in patients with primary insomnia.40,41 However, a meta-analysis published in 2006 which included patients with secondary causes of insomnia produced less clear-cut results.42
The sleep-promoting effect of melatonin has been linked to the inhibition of neuronal activity through activation of SCN MT1 receptors.43 A synthetic melatonin agonist, Ramelteon, which selectively activates MT1 and MT2 melatonin receptors, has been approved for the treatment of insomnia. Ramelteon shows 10-fold higher binding affinity for the MT1 compared to the MT2 receptor and 17 times higher affinity than melatonin for either receptor. The drug shows no affinity for a large number of other G protein–coupled receptors, enzymes, and neurotransmitter channels. In addition to Ramelteon, there are several other melatonin agonists under development.24
Jet Lag
Jet lag is a syndrome associated with transmeridian travel resulting in disturbances to circadian rhythms. Manifestations of jet lag include problems sleeping, malaise, fatigue, and reduced performance. A similar syndrome may result from shift work and rarely from the transition to daylight savings time. Eastbound travel is generally associated with worse symptoms than westbound travel. The severity of jet lag is also proportional to the number of time zones crossed. Left untreated, the relationship between endogenous rhythms and environmental cues will come back into synchrony over several days to a week. The ability of melatonin to speed up the process of resynchronization accounts for its efficacy in the treatment of jet lag. Phase shifting of the neuronal firing rhythms has been linked to activation of the MT2 receptor at dusk, although MT1 may also be involved.44
For air travelers crossing five or more time zones going in the eastward direction, melatonin has been found to be effective in treating jet lag when administered close to the target bedtime at the travel destination.45 Doses from 0.5 to 5 mg are found to be effective. Treatment should begin the first evening upon arrival to the destination. Side effects of melatonin in this setting include daytime sleepiness, especially with the higher doses, dizziness, headaches, and loss of appetite.45 In addition to melatonin administration, proper nutrition, going to sleep according to the new time zone, exercising, and maximizing light exposure when awake can help alleviate jet lag symptoms.
Similar to individuals with jet lag, shift workers often have symptoms of fatigue, sleep disturbances, and gastrointestinal problems.46 Studies have observed marked variability in circadian melatonin profile in these workers.47 When administered at the desired bedtime during a night shift, melatonin seems to improve sleep and increase daytime alertness.46
Disorders in the sleep/wake cycle, core body temperature, and cortisol secretion are very common in blind people with no significant light perception.48 Many blind individuals have abnormal melatonin or 6-sulfatoxymelatonin circadian profiles.49,50 In these settings, melatonin has proven effective in phase-shifting the circadian clock and in so doing, stabilizing sleep onset and sometimes improving quality and duration of sleep.49,51
Reproduction
In seasonal-breeding mammals, melatonin is involved in regulating the breeding cycle.52 The effects of melatonin on the reproductive system are generally antigonadotropic. Melatonin inhibits secretion of the gonadotropin hormones LH and FSH. This inhibitory affect is most likely due to inhibition of GnRH from the hypothalamus.53 For seasonal-breeding animals, seasonal changes in day length and thus melatonin secretion will up-regulate or down-regulate the gonadal axis. For example, during the shorter period of daylight that accompanies the nonbreeding season, melatonin levels are increased and down-regulate the gonadal axis. As the breeding season approaches and length of daylight increases, melatonin levels fall, allowing rejuvenation of the gonads.
Humans are not seasonal breeders, but melatonin may mediate the moderate seasonal fluctuations observed in human reproductive function.3 The increased conception rate seen in northern latitudes during the summer season has been reported to be caused by changes in gonadotropin induced by changes in melatonin secretion. Melatonin levels have been reported elevated in male infertility54 and in men with hypogonadotropic hypogonadism.55,56 Moreover, elevated melatonin levels have been reported in women with stress- and exercise-induced amenorrhea and in men with infertility.57 Exogenous melatonin administration has been shown to suppress LH levels in men and women and to reduce sperm motility in men.58
Melatonin has also been implicated in sexual maturation, where it may have inhibitory action on the onset of puberty. It has been proposed that a decline of melatonin below a threshold value may be a signal that activates GnRH pulsation in early puberty.3 Circumstantial evidence that melatonin is involved in sexual maturation includes the observation that children with precocious puberty have lower plasma melatonin levels, whereas children with delayed puberty exhibit higher nocturnal melatonin concentrations. However, a causal relationship between the pineal gland and human reproductive function is far from clear.
Temperature
Body temperature and plasma melatonin levels have a reciprocal profile; the 24-hour temperature nadir correlates with peak plasma melatonin levels.59 It has been estimated that approximately half of the nighttime decline in core temperature is induced by melatonin.60 Supporting a causal relationship between melatonin and body temperature are studies showing that exogenously administered melatonin will decrease body temperature in humans.61
Blood Pressure
There is circumstantial evidence that melatonin may be involved in the regulation of blood pressure. During the night, humans have lower blood pressure, heart rate, and cardiac output which are all associated with increases in plasma melatonin levels. There is an increased risk of myocardial infarction and stroke in the early morning when melatonin levels are low. Moreover, patients with coronary heart disease have lower melatonin levels and higher norepinephrine levels compared to persons without cardiac disease. It is known that the nocturnal surge in melatonin is blunted in patients with hypertension, especially nocturnal hypertension.62 To this end, studies have shown that melatonin (~2 mg) can lower nighttime blood pressure.63
Immune System and Cancer
In animal models, melatonin has immunoenhancing properties and can reverse the immunosuppressive effects of acute stress, cancer drug therapy, and viral infections.64 Surgical and functional pinealectomy has been shown to reduce thymic and splenic weights in mice, rats, and hamsters corresponding to a reduction in B and T cells.65 Pinealectomy alters the activity of thymic enzymes and in the newborn rodent is associated with disorganization of thymic structures. In the rodent, neonatal pinealectomy also impairs immune function. Conversely, the administration of melatonin to rodents increases thymic and splenic weight, provides protection against the catabolic effects of dexamethasone on body weight and atrophy of the thymus and adrenal glands, and enhances immune responses to certain infectious agents.66 Melatonin administration increases the number of natural killer (NK) cells and monocytes in bone marrow and increases helper T-cell activity and interleukin-2 (IL-2) production in human lymphocytes.66 In general, these observations show that melatonin’s stimulatory effects on the immune system are best observed in states of immunosuppression. The role of pineal-derived and immune cell–derived melatonin on human immunity is not clear.
Melatonin is also reported to have oncostatic actions.3 Pinealectomized rats and hamsters have accelerated growth in a variety of transplanted cancers. Although chronic melatonin administration has oncostatic effects in breast and ovarian cancer in rodent models, high-dose melatonin did not alter survival in human melanoma patients.67 However, co-administration of melatonin and interferon gamma improved tumor regression in patients with metastatic renal cell carcinoma.68 Melatonin has also been shown to protect the host from negative effects of IL-2 while synergizing with the anticancer action of the cytokine.69 Much more information is needed to prove that melatonin will serve as a useful oncostatic medication in humans.
Antioxidant and Anti-Aging
At supraphysiologic concentrations, melatonin has antioxidant properties. Melatonin’s free-radical scavenging properties are due in part to its ability to modulate catalase, superoxide dismutase, and glutathione peroxidase. Compared to two well-known scavengers, glutathione and mannitol, melatonin is 4 times and 14 times more effective, respectively.70 The small molecule is both lipophilic and hydrophilic and easily enters the cytosol and nucleus, thus making it a potentially efficient antioxidant.71 However, the question still remains whether in physiologic concentrations, melatonin is an efficient free-radical scavenger. In a meta-analysis of three trials of melatonin treatment for the cognitive impairment associated with dementia, no evidence of benefit was seen.72
Psychiatric Disorders
Altered melatonin rhythms and levels have been observed in depression, mania, schizophrenia, anorexia nervosa, and other psychiatric disorders.73 However, there is no compelling evidence that melatonin contributes to the symptomatology associated with these psychiatric disorders.
Lesions of the Pineal Gland and Pineal Region
Pineal Gland Cysts
A pineal cyst is a benign lesion lined by normal pineal parenchymal and glial cells. It is not uncommon for the endocrinologist to order a magnetic resonance imaging (MRI) scan of the brain during the evaluation of a pituitary disorder and also find an incidental pineal cyst. Indeed, the asymptomatic pineal cyst is a common neuroimaging finding seen on approximately 4% of MRI scans and observed in up to 40% of routine autopsies.74 The main importance in identifying a pineal cyst is to distinguish it from cystic tumors that also occur in this region and require treatment.
On computerized tomography (CT) images, pineal cysts are hypodense; occasionally there is evidence of hemorrhage. Cyst walls may or may not show contrast enhancement, and calcifications within the wall are found in about half of cases. On MRI images, the pineal cyst is round and smooth without intracystic trabeculations. It has low signal intensity on both T1WI and T2WI.75 Occasionally the imaging appearance of a benign pineal cyst is indistinguishable from small cystic neoplasms. For example, similar to pineal cysts, pineocytomas may be isointense with respect to CSF, but the latter usually have intratumoral trabeculations.
Most pineal cysts are small, and patients are asymptomatic. Some cysts spontaneously resolve. One study observed that in 32 patients with pineal cysts, 75% remained stable over time,16% decreased in size or resolved, whereas only 8% increased in size.76 Small, asymptomatic cysts require no intervention.
Rarely, a pineal cyst becomes symptomatic.77 Symptomatic cysts tend to be larger than their asymptomatic counterparts. Although symptomatic pineal cysts have been reported in patients from 7 to 69 years of age, most occur be between 21 and 30 years of age. There is a 3:1 female predominance. Patients generally present with headaches as a result of acute hydrocephalus. A high degree of clinical suspicion is needed, because headache may be the only symptom. Headache may have either a prolonged intermittent or acute course.
Tumors in The Pineal Gland Region
Meningiomas constitute about 8% of lesions in the pineal gland region, and pineal meningiomas account for less than 1% of all meningiomas.78 They tend to occur in middle age and have a female predominance. Patients with meningiomas in the pineal region usually present with headaches and the other signs related to acute hydrocephalus.
Pineal meningiomas usually present with dural attachment, which can compromise venous flow. Less commonly, the tumor is not attached to dura. Peripheral calcification is commonly observed on CT imaging. On MRI, meningiomas have well-defined margins, and attachments to the falx can be appreciated on sagittal images.75 Cerebral angiography should be performed to outline the relationship between tumor and the surrounding vasculature. The main therapy is surgical resection.
Pineal Parenchymal Tumors
Approximately 10% to 28% of pineal-region tumors are of pineal parenchymal origin.79 Pineal parenchymal tumors occur throughout the life cycle but are 10 times more common in children than in adults.80 These tumors originate from parenchymal cells showing varying degrees of differentiation, from primitive parenchymal cells through tumors consisting of well-differentiated pinealocytes. Some experts classify this histologic continuum into three types of pineal cell neoplasms: (1) pineocytoma (most differentiated), (2) pineal parenchymal tumors of intermediate differentiation, and (3) pineoblastoma (least differentiated).81 The less differentiated tumors are more aggressive, more apt to metastasize, and are associated with a worse prognosis. Adults are more likely to present with pineocytoma, whereas children are more likely to develop pineoblastoma.82
Pineocytoma
Pineocytomas are slow-growing lesions containing mature cells that are histologically almost indistinguishable from normal pineal parenchyma. Pineocytomas correspond histologically to World Health Organization (WHO) grade II. These tumors can occur in any age but tend to affect young adults, with a peak incidence at age 30 to 35.83 Pineocytomas often have calcifications that are peripherally displaced. These well-circumscribed lesions account for 45% of pineal parenchymal tumors.84 They usually do not seed the CSF.
Microscopically, pineocytomas are composed of sheets of non-pleomorphic, mature-appearing cells containing pseudorosettes arranged in lobules with occasional or absent mitotic figures.85 Necrosis is absent. The pseudorosettes are separated by fibrovascular septa. The absence of rosettes (i.e., lack of neuronal differentiation) is associated with a poorer prognosis. The cells almost always stain positive for neuron-specific enolase, retinal S-antigen and rhodopsin and sometimes for neurofilament protein, chromogranin A, β-tubulin III and αB crystalline.85 Staining for neuron-specific enolase can be used to distinguish pineocytomas and pineoblastomas from astrocytic tumors.
Pineoblastoma
The pineoblastoma is a neoplasm composed of poorly differentiated, primitive cells with significant potential for leptomeningeal and extracranial spread. This tumor is a variant of primitive neuroectodermal tumors and is similar to medulloblastoma and retinoblastinoma.85 The tumors correspond histologically to WHO grade IV.81 Pineoblastomas usually occur in the first 2 decades of life. They constitute approximately 45% of pineal parenchymal tumors.86 The 5-year prognosis is approximately 58% or less.
Microscopically, pineoblastomas are highly cellular tumors composed of irregular sheets of pleomorphic, small, undifferentiated cells without lobular architecture. Necrosis is common. Pineoblastomas lack the pseudorosettes found in pineocytomas but can have Homer-Wright or Flexner-Wintersteiner rosettes, which are markers of retinoblastic differentiation.85 This tumor is indistinguishable from medulloblastoma. Pineoblastomas express neuronal and photosensory markers similar to pineocytomas, but staining is more variable.87
Germ Cell Tumors
Germ cell tumors account for approximately 30% to 85% of tumors in the pineal region.82 Males are twice as likely as females to develop germ cell lesions in the pineal region, with evidence of an even higher male-to-female ratio for nongerminomatous germ cell tumors. Germ cell tumors account for approximately 1% to 2% of intracranial tumors in adults and 10% in children. For unclear reasons, pineal germ cell tumors have a relatively higher incidence in Japan and East Asia than in the West. Most patients with germ cell tumors are between 10 and 30 years of age, with a peak age of presentation in the second decade.
Similar to germ cell tumors in the periphery, central nervous system germ cell tumors occur in midline locations and most commonly occur in the pineal region and in the hypothalamic-neurohypophyseal region. In several series, approximately 15% of germ cell tumors were located in both the pineal and neurohypophyseal regions.88 These bifocal lesions are often brought to attention with the onset of diabetes insipidus. Imaging typically shows a small pineal-region mass and thickening of the infundibulum.
Germ cell tumors are derived from embryonal cells and are generally classified into six types: germinoma, teratoma, mixed germ cell tumors, embryonal carcinoma, choriocarcinoma, and yolk sack tumor. The exact incidence of the specific types of germ cell tumors is difficult to determine, but approximately a third are germinomas, a third are teratomas, and a fifth are mixed germ cell lesions, with the remainder subtypes accounting for the rest. Mixed germ cell tumors have multiple subtypes; the prognosis is determined by the most malignant cell type.89 Teratomas have the capacity to differentiate from ectoderm, mesoderm, or endoderm, and mature tumors do not metastasize. Yolk sack tumors, choriocarcinoma, and embryonal cell tumors are the least common of the germ cell tumors and are generally aggressive. Germ cell tumors frequently metastasize and disseminate into the CSF. Most nongerminomatous germ cell tumors and some germinomas are associated with tumor markers in CSF. Beta human chorionic gonadotropin (β-hCG) is elevated in choriocarcinomas, whereas α-fetoprotein (AFP) is elevated in yolk sac and embryonic tumors.90 High elevation of these markers is diagnostic of nongerminomatous germ cell tumors, and the degree of elevation correlates with aggressiveness of the tumor.91 Although markers can be elevated in blood, the CSF compartment is a much more sensitive indicator of disease. Germinomas are much less likely than nongerminomatous tumors to be associated with high elevations in CSF, AFP, or β-hCG.
Clinical Presentation for All Tumors in the Pineal Region
Pineal regional tumors share a clinical presentation because all tumors in this region cause symptoms by compression or invasion of local surrounding structures. This often results in blockage of CSF flow at the level of the third ventricle or aqueduct, resulting in hydrocephalus. The most common symptoms are headaches, visual abnormalities, nausea, vomiting, and difficulty walking (Table 15-1).
Table 15-1
Symptoms and Signs Associated With Lesions in the Pineal Region
Symptoms
Headaches
Vision abnormalities
Nausea and vomiting
Impaired ambulation
Signs
Papilledema
Ataxia
Loss of upward gaze
Tremor
Altered pupillary reflexes
The most common presenting signs seen in patients with pineal body lesions are papilledema, ataxia, loss of upward gaze, tremor, altered papillary reflexes, and hyperactive deep tendon reflexes (see Table 15-1). Owing to pressure placed on the pretectal area of the brain, many patients present with abnormalities in vertical gaze, especially upward gaze, nystagmus, and impaired convergence and divergence. This is known as Parinaud’s Syndrome (Table 15-2). Patients may also have leptomeningeal symptoms, which are common in pineoblastomas and germ cell tumors.
Table 15-2
Symptoms
Difficulty looking up
Double vision
Blurred vision
Oscillopsia
Signs
Vertical gaze abnormalities, especially upgaze
Convergence retraction nystagmus
Lid retraction (Collier’s “tucked lid” sign)
Impaired convergence and divergence
Excessive convergence tone
Bilateral ptosis
Pupillary abnormalities (large with light-near dissociation)
Because 10% to 15% of germinomas are simultaneously located in both the pineal and suprasellar region, patients with bifocal tumors usually present with evidence of hypothalamic and/or pituitary dysfunction, including diabetes insipidus. An interesting phenomenon has been observed where diabetes insipidus has been found in patients for whom the tumor is restricted to the pineal region. It has been posited that the presence of diabetes insipidus in patients with pineal germinomas indicates that germinomatous tissue is also present on the floor of the third ventricle despite negative neuroradiographic findings.92 Patients with bifocal disease may also present with precocious puberty with or without elevations in LH and/or β-hCG.92
Imaging
Magnetic Resonance Imaging
On MRI, pineal neoplasms are often lobulated, solid tumors.93 Generally, pineoblastomas are isointense to gray matter on T2WI (weighted image) and may also show brain edema or invasion in the surrounding brain parenchyma.94 Pineocytomas, with a higher degree of cytoplasm, have relatively higher signal intensity on T2WI.95
The solid components of germ cell tumors range from iso- to hypointense, relative to the gray matter on T1-weighted image (T1WI), and mixed iso- and hyperintense onT2-weighted image (T2WI).75 Germinomas are usually mildly hypointense on T1WI and mildly hyperintense on T2WI and may be isointense on both pulse sequence.96
Teratomas of the pineal are extremely heterogeneous, contain multiple cystic regions, and often have dense calcification.97 These tumors show high intensity on both T1WI and T2WI.
Computer Axial Tomography
On computer axial tomography (CAT; or computed tomography [CT]) scan, pineocytomas are typically isodense and enhance homogeneously with contrast.75 Cysts and calcifications are present in approximately 50% of pineocytomas, with peripheral calcifications more suggestive of pineocytoma than germinoma.98,99 Pineoblastomas are hyperintense on CT and are not generally calcified.98 Calcifications can be identified in two thirds of pineal gliomas.
Staging Workup and Histologic Diagnosis
After imaging and CSF analysis, histologic diagnosis is required for optimal management of pineal-region tumors. Biopsy is usually necessary because histology predicts tumor behavior and will also determine the therapeutic approaches. Either stereotactic or open biopsy can be completed.100,101 Although the stereotactic approach has minimal morbidity, an open procedure allows for greater diagnostic accuracy, since more tissue is procured. The exception to the biopsy requirement are nongerminomatous germ cells tumors where their presence is assumed when imaging studies are consistent with the diagnosis and when CSF levels of AFP and/or β-hCG are elevated.
Although approximately a third of pineal and germ cell tumors may be cured by surgery alone, it is generally recommended that biopsy for histologic determination be completed before gross total resection is attempted.101 This recommendation is based on the fact that many tumors arising in this area are sensitive to both radiation therapy (RT) and chemotherapy, and under certain circumstances these treatments may be preferred over surgery. For example, over 90% of germinomas are cured with RT alone, and the procedure is associated with lower morbidity and mortality compared to surgery.102
General Treatment Remarks
Tumors of the pineal region are not common. As a result, there is a paucity of well-designed, prospective trials of sufficient sample size to inform the clinician of the best treatment modalities. Indeed, there is no unanimity concerning treatment for this group of tumors. However, it is clear that the variables that most significantly influence survival are extent of disease at presentation, tumor histology, degree of cellular differentiation, and residual disease following therapy.103
In addition to the ability of surgery to help determine tumor histology and to debulk or remove the lesion, another role for surgery is to treat the frequent occurrence of hydrocephalus. This can be accomplished with placement of a ventriculoperitoneal (VP) shunt or a third ventriculostomy.101 An endoscopic third ventriculostomy can be completed when the diagnostic biopsy is performed. This procedure is preferred over VP shunts, which can be complicated by infection, injury, and malfunction.
Craniospinal radiation is often employed to treat local and disseminated disease, but it can also be used prophylactically when histology predicts aggressive behavior. For example, prophylactic postoperative craniospinal radiation is frequently recommended for pineoblastomas and germ cell tumors where leptomeningeal spread is more likely to occur compared to pineocytomas.104 The best overall cure rates have been reported in series where craniospinal radiation has been utilized. However, the use of prophylactic radiation (and all forms of radiation) must be balanced with its potential for inducing cognitive deficits in the young, as well as neuroendocrine dysfunction in all patients. Arguments remain regarding optimal dosing and volume of treatment (lesion versus brain versus craniospinal) when radiation is employed.
There is a growing role for multimodality strategies combining radiation, chemotherapy, and surgery to treat aggressive tumors.82
Specific Treatments
Pineocytoma: Pineocytomas do not metastasize, and therefore surgery is first-line treatment.104a For most pineocytomas, complete surgical resection is often possible and provides excellent long-term, recurrence-free survival; no further treatment is required. The 5-year survival rate is greater than 86%. Based on this data, adjuvant radiotherapy is not typically recommended after gross total resection of a pineocytoma. Many advocate adjuvant RT for only those pineocytomas which lack neuronal differentiation and which behave more like pineoblastomas.
The role of stereotactic radiosurgery to replace surgery as first-line treatment of pineocytomas is being studied; however, experience remains limited.105–107 The focused beam minimizes radiation exposure to the surrounding CNS. This form of therapy avoids the risks of general anesthesia and craniotomy. In the largest series of 30 pineal tumors treated with stereotactic radiation, all patients with pineocytomas remained disease-free.106 More studies are needed to decide whether stereotactic radiotherapy, like surgery, can be considered first-line therapy for pineocytomas.
Pineoblastoma: The aggressive nature of pineoblastoma and many intermediate pineal parenchymal tumors requires a combination of several treatment modalities. Generally, surgery is performed first to debulk or for total gross resection. The benefits of aggressive surgical resection among pineoblastoma and the other more malignant tumors are not clear. Some but not all studies show that the degree of tumor removal correlates with improved outcome.82 Regardless of the aggressiveness of the surgical procedure, patients with pineoblastomas will require additional therapy following resection.
Radiation is always employed following surgery to treat pineoblastoma. In a study of pineoblastoma patients drawn from the Brain Tumor Registry of Japan, cranial irradiation (>40 Gy) and total gross resection were associated with improved survival compared to surgery with lower doses of radiation or treatment with only surgery.103 In the uncommon situation when a pineoblastoma is discovered before evidence of metastasis, prophylactic craniospinal radiation can be employed; however, as stated above, this must be weighed against the side effects of radiation.
The most aggressive pineal tumors are not generally effectively treated with surgery and radiation. Therefore, various chemotherapy regiments have been attempted.108–110 Unfortunately, most studies have shown that chemotherapy alone is not effective compared to radiation alone or radiation with chemotherapy. This is especially disappointing, since infants can be treated with chemotherapy without incurring significant cognitive impairment, whereas radiation is contraindicated. Recent studies have employed high-dose chemotherapy supported by autologous hematopoietic stem cell transplantation, showing improved treatment outcomes.111
Germinomas: Germinomas are extremely sensitive to radiation and 5-year survival is greater than 90%.112 However, there is still uncertainty concerning the optimal dose and volume of radiation. Given the potential for radiation to induce significant cognitive impairment in children, there are multiple small studies being conducted examining the efficacy of either reducing the dose of radiation or eliminating it altogether by substituting chemotherapy in its place,82 but a study that employed only chemotherapy had a relapse rate that was considerably higher than studies utilizing only radiation therapy.113 This has prompted studies to employ combination therapy (chemo and radiation), where patients who respond to chemotherapy receive reduced-dose radiation therapy. This approach appears to be more promising.82
Teratomas: Surgery is first-line treatment for the mature teratomas, and a relatively favorable outcome is observed. In contrast, immature teratomas have a less favorable prognosis, with survival rates ranging between 50% and 70%.114 Both radiation and chemotherapy, alone or combination, have been used in this setting.115
Nongerminomatous Germ Cell Tumors: Similar to the pineoblastoma, nongerminomatous germ cells tumors are extremely aggressive, requiring multiple treatment modalities. These tumors are markedly less sensitive to radiation compared to germinomas. Generally, nongerminomatous germ cells tumors are diagnosed by evidence of high CSF tumor markers and then treated with radiation and/or chemotherapy. Surgery is performed when there is residual mass. This has been dubbed the “second look” strategy.82 In this setting of residual tumor, surgery is both diagnostic and therapeutic.
Craniospinal radiation is almost uniformly recommended for patients with evidence of disseminated disease at the time of diagnosis; however, it is rarely curative as a single treatment modality. Recent results suggest that chemotherapy can improve the overall duration and rate of survival when used in conjunction with craniospinal radiotherapy as part of initial treatment, with survival rates of up to 60%.116 Similar to pineoblastoma, the rationale for chemotherapy is to improve survival in those patients with disseminated disease or reduce consequences from radiation in patients with localized disease. Several platinum-based regiments have been employed with varying degrees of success.117,118
Although all nongerminomatous germ cell tumors are partially sensitive to radiation and chemotherapy, the relative roles of surgery, radiotherapy, and chemotherapy in the management of such lesions remain controversial. There is no general consensus concerning the dose or volume of irradiation or of a particular chemotherapy regimen needed for any form of germ cell tumor. Nongerminomatous germ cell tumors, including mixed germ cell tumors and embryonal cell carcinomas or tumors that have been termed yolk sac tumors, have a poorer prognosis, with reported survival rates ranging between 40% and 70%.92 The efficacy of prophylactic craniospinal radiation in patients without disseminated disease at the time of diagnosis remains unclear, but it is generally administered.
Trilateral Retinoblastoma: Uncommonly, patients with hereditary retinoblastoma develop a midline neuroblastic tumor referred to as a trilateral retinoblastoma.119 Approximately three fourths of reported cases arise in the pineal gland. The mean age at diagnosis is 31 months. Despite aggressive therapy, this is ultimately a fatal disease. The average survival from diagnosis is 6 to 11 months.
References
1. Reppert, SM, Weaver, DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676.
2. Lerner, AB, Case, JD, Takahashi, Y. Isolation of melatonin, a pineal factor that lightens melanocytes. J Am Chem Soc. 1958;80:2057–2058.
3. Pandi-Perumal, SR, Srinivassan, V, Maestroni, GJM, et al. Melatonin: Nature’s most versatile biological signal? FEBS J. 2006;273:2813–2836.
4. Bubenik, GA. Gastrointestinal melatonin: Localization, function, and clinical relevance. Dig Dis Sci. 2002;47:2336–2348.
5. Sugden, D. Melatonin biosynthesis in the mammalian pineal gland. Experientia. 1989;45:922–931.
6. Hardeland, R, Pandi-Perumal, SR, Cardinali, DP. Melatonin. Int J Biochem Cell Biol. 2006;38:313–316.
7. Lynch, JH, Jimerson, DC, Ozaki, Y, et al. Entrainment of rhythmic melatonin secretion in man to a 12-hour phase shift in the light/dark cycle. Life Sci. 1978;23:1557.
8. Klein, DC, Moore, RY. Pineal N-acetyltransferase and hydroxyindole-O-methyltransferase: control by the retino-hypothalamic tract and the suprachiasmatic nucleus. Brain Res. 1979;174:245–262.
9. Arendt, J. Melatonin. Clin Endocrinol. 1988;29:205–229.
9a. Bergiannaki, J-D, Paparrigopoulos, TJ, Syrengela, M, et al. Low and high melatonin excretors among healthy individuals. J Pineal Res. 1995;18:159–164.
10. Arendt, J. Mammalian pineal rhythms. Pineal Res Rev. 1985;3:161–213.
11. Cardinali, DP, Lynch, HJ, Wurtman, RJ. Binding of melatonin to human and rat plasma proteins. Endocrinology. 1972;91:1213–1218.
12. Martin XD, Malina HZ, Brennan MC, et al: The ciliary body: The third organ found to synthesize indole amines in humans, Eur J Ophthalmol 2:76–72, 1992.
13. Nelson, RJ, Drazen, DL. Melatonin mediates seasonal adjustments in immune function. Reprod Nutr Devel. 1999;39:383–398.
14. Karasek, M, Winczyk, K. Melatonin in humans. J Physiol and Pharmacol. 2006;57(5):19–39.
15. Mayeda, A, Mannon, S, Hofstetter, J, et al. Effects of indirect light and propranolol on melatonin levels in normal human subjects. Psychiatry Res. 1998;81:9.
16. Wright, KP, Jr., Badia, P, Myers, BL, et al. Caffeine and light effects on nighttime melatonin and temperature levels in sleep-deprived humans. Brain Res. 1997;747:78.
17. Ekman, AC, Leppaluoto, J, Huttunen, P, et al. Ethanol inhibits melatonin secretion in healthy volunteers in a dose-dependent randomized double blind cross-over study. J Clin Endocrinol Metab. 1993;77:780.
18. Brainard, GC, Hanifin, JP, Greeson, JM, et al. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412.
19. McIntyre, IM, Normal, TR, Burrows, GD. Human melatonin suppression by light is intensity dependent. J Pineal Res. 1989;6:151–156.
20. Buxton, OM, L’Hermite-Baleriaux, M, Hirshfeld, U, et al. Acute and delayed effects of exercise on human melatonin secretion. J Biol Rhythms. 1997;12:568–574.
21. Kräuchi, K, Cajochen, C, Wirz-Justice, A. A relationship between heat loss and sleepiness: Effects of postural change and melatonin administration. J Appl Physiol. 1997;83:134–139.
22. Nosjean, O, Ferro, M, Coge, F, et al. Identification of the melatonin binding site MT3 as the quinine reductase 2. J Biol Chem. 2000;275:31311–31317.
23. Dubocovich, ML, Markowska, M, et al. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front Biosci. 2003;8:d1093–d1108.
24. Dubocovich, ML. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Medicine. 2007;8:S34–S42.
25. Sanchez-Barcelo, EJ, Cos, S, Mediavilla, D, et al. Melatonin-estrogen interactions in breast cancer. J Pineal Res. 2005;38:217–222.
26. Benitez-King, G. Melatonin as a cytoskeletal modulator: Implications for cell physiology and disease. J Pineal Res. 2006;40:1–9.
27. Carlberg, C. Gene regulation by melatonin. Ann YY Acad Sci. 2000;917:387–396.
28. Ekmekcioglu, C. Melatonin receptors in humans: biological role and clinical relevance. Biomed Pharmacother. 2006;60:97–108.
29. Poon, AMS, Mak, ASY, Luk, HT. Melatonin and 2[125]iodomelatonin binding sites in the human colon. Endocrinol Res. 1996;22:77–94.
30. Gerdin, MJ, Masana, MI, Dubocovich, ML. Melotonin-mediated regulation of human MT(1) melatonin receptors expressed in mammalian cells. Biochem Pharmacol. 2004;67:2023–2030.
31. Hastings, MH, Reddy, AB, Maywood, ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661.
32. Macchi, MM, Bruce, JN. Human pineal physiology and functional significance of melatonin. Frontiers in Neuroendocrinol. 2004;25:177–195.
33. Lacoste, L, Wetterberg, L. Individual variations of rhythms in morning and evening types with special emphasis on seasonal differences. In: Wetterberg L, ed. Light and Biological Rhythms in Man. New York: Pergamon Press; 1993:287–304.
34. Vondrasová-Jel.’ková, D, Hájek, H, Illnerová, H. Adjustment of the human melatonin and cortisol rhythms to shortening of the natural summer photoperiod. Brain Res. 1999;816:249–253.
35. Wehr, TA, Aeschback, D, Duncan, WC, Jr. Evidence for a biological dawn and dusk in the human circadian timing system. J Physiol. 2001;535:937–951.
36. De Leersnyder, H, de Blois, MC, Bresson, JL, et al. Inversion of the circadian melatonin rhythm in Smith-Magenis syndrome. Rev Neurol (Paris). 2003;159(6):S21–S26.
37. Riemann, D, Klein, T, Rodenbeck, A, et al. Nocturnal cortisol and melatonin secretion in primary insomnia. Psychiatry Res. 2002;113:17–27.
38. Lavie, P. Melatonin: Role in gating nocturnal rise in sleep propensity. J Biol Rhythms. 1997;12:657–665.
39. Ates, O, Cayli, S, Gurses, I, et al. Effect of pinealectomy and melatonin replacement on morphological and biochemical recovery after traumatic brain injury. Int J Dev Neurosci. 2006;24(6):357–363.
40. Zhdanova, IV, Wurtman, RJ. Efficacy of melatonin as a sleep-promoting agent. J Biol Rhythms. 1997;12:644.
41. Brzezinski, A, Vangel, MG, Wurtman, RJ, et al. Effects of exogenous melatonin on sleep: A meta-analysis. Sleep Med Rev. 2005;9:41.
42. Buscemi, N, Vandermeer, B, Hooton, N, et al. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: Meta-analysis. BMJ. 2006;332:385.
43. Liu, C, Weaver, DR, Jin, X, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102.
44. Dubocovich, M, Hudson, RL, Sumaya, IC, et al. Effect of MT melatonin receptor deletion on melatonin-mediated phase shift of circadian rhythms in the C57BL/6 mouse. J Pineal Res. 2005;39:113–120.
45. Herxheimer A, Petrie KH: Melatonin for the prevention and treatment of jet lag. Cochrane Database Syst Rev:CD001520, 2002.
46. Arendt, J, Deacon, S. Treatment of circadian rhythm disorders—Melatonin. Chronobiol Int. 1997;14:185–204.
47. Wiebel, L, Spiegel, K, Gfonfier, C, et al. Twenty-four-hour melatonin and core body temperature rhythms: Their adaption in night workers. Am J Physiol. 1997;272:R948–R954.
48. Skene, DJ, Lockley, SW, Arendt, J. Melatonin in circadian sleep disorders in the blind. Biol Signals Recept. 1999;8:90–95.
49. Lewy, AJ, Newsom, DA. Different types of melatonin circadian secretory rhythms in some blind people. J Clin Endocrinol Metab. 1983;56:1103–1107.
50. Lockley, SW, Skene, DJ, Arendt, T, et al. Relationship between melatonin rhythms and visual loss in the blind. J Clin Endocrinol Metab. 1997;82:3763–3770.
51. Lockley, SW, Skene, DJ, James, K, et al. Melatonin administration can entrain the free-running circadian system in blind subjects. J Endocrinol. 2000;164:R1–R6.
52. Goldman, BD, Elliott, JA. Photoperiodism and seasonality in hamsters: Role of the pineal gland. In: Stetson MH, ed. Processing of Environmental Information in Vertebrates. New York: Springer Verlag; 1988:203–218.
53. Roy, D, Belsham, DD. Melatonin receptor activation regulates GnRH gene expression and secretion in GT1–7 GnRH neurons: Signal transduction mechanisms. J Biol Chem. 2001;277:251–258.
54. Karasek, M, Pawlikowski, M, Nowakowska-Jankiewicz, B, et al. Circadian variations in plasma melatonin, FSH, LH, prolactin and testosterone levels in infertile men. J Pineal Res. 1990;9:149–157.
55. Puig-Domingo, M, Webb, SM, Serrano, J, et al. Melatonin-related hypogonadotropic hypogonadism. N Engl J Med. 1992;327:1356–1359.
56. Luboshitzky, R, Lavi, S, Thuma, I, et al. Increased nocturnal melatonin secretion in male patients with hypogonadotropic hypogonadism and delayed puberty. J Clin Endocrinol Metab. 1995;80:2144–2148.
57. Berga, SL, Yen, SSC. Amplification of nocturnal melatonin secretion in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1988;66:242–244.
58. Voordouw, BCG, Euser, R, Verdonk, RER, et al. Melatonin and melatonin-progestin combinations alter pituitary-ovarian function in women and can inhibit ovulation. J Clin Endocrinol Metab. 1992;74:10817.
59. Akerstedt, T, Froberg, JE, Friberg, W, et al. Melatonin excretion, body temperature and subjective arousal during 64 hours of sleep deprivation. Psychoneuroendocrinology. 1979;4:219.
60. Cagnacci, A, Elliot, JA, Yen, SS. Melatonin: A major regulator of the circadian rhythm of core body temperature in humans. J Clin Endocrinol Metab. 1992;75:447–452.
61. Deacon, S, English, J, Arendt, J. Acute phase-shifting effects of melatonin associated with suppression of core body temperature in humans. Neurosci Lett. 1994;178:32–34.
62. Jones, M, Garfinkel, D, Zisapel, N, et al. Impaired nocturnal melatonin secretion in non-dipper hypertensive patients. Blood Press. 2003;12:19.
63. Grossman, E, Laudon, M, Yalcin, R, et al. Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am J Med. 2006;119:898.
64. Conti, A. Oncology in neuroimmunology. What progress has been made? In: Conti A, Maestroni GJM, Cann SMM, et al, eds. Neuroimmunomodulation: Perspectives at the New Millennium. New York: The New York Academy of Sciences; 2000:68–83.
65. Carrillo-Vico, A, Guerrero, JM, Lardone, PJ, et al. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27(2):189–200.
66. Maestroni, GJM. The immunoendocrine role of melatonin. J Pineal Res. 1993;14:1–10.
67. Robinson, WA. Melatonin in the treatment of the human malignant metastatic melanoma. In: Proceedings of the NATO workshop. The Pineal Gland and its hormones: Fundamentals and Clinical Perspectives. Italy: Erice; 1994.
68. Neri, B, Fiorelli, C, Moroni, F, et al. Modulation of human lymphoblastoid interferon activity by melatonin in metastatic renal cell carcinoma. A phase II study. Cancer. 1994;73:3015–3019.
69. Lissoni, P. Efficacy of melatonin in immunotherapy. In: Bartsch C, Bartsch H, Blask DE, et al, eds. The Pineal Gland and Cancer: Neuroendocrine Mechanisms in Malignancy. Berlin: Springer-Verlag; 2001:465–475.
70. Tan, DX, Chen, LD, Peoggeler, B, et al. A potent, endogenous hydroxyradical scavenger. Endocrine J. 1993;1:57–60.
71. Reiter, RJ. Oxidative damage in the central nervous system: protection by melatonin. Progr Neurobiol. 1998;56:359–384.
72. Jansen SL, Forbes DA, Duncan V, et al: Melatonin for cognitive impairment. Cochrane Database Syst Rev :CD003802, 2006.
73. Brown, GM. Melatonin in psychiatric and sleep disorders: Therapeutic implications. CNS Drugs. 1995;3:209–226.
74. Michielsen, G, Benoit, Y, Baert, E, et al. Symptomatic pineal cysts: Clinical manifestations and management. Acta Neurochir (Wien). 2002;144:233–242.
75. Korogi, Y, Takahashi, M, Ushio, Y. MRI of pineal region tumors. J Neuro-Oncology. 2001;54:251–261.
76. Barboriak, DP, Lee, L, Provenzale, JM. Serial MR imaging of pineal cysts: Implications for natural history and follow-up. AJR Am J Roentgenol. 2001;176:737.
77. Patel, AJ, Fuller, GN, Wildrick, DM, et al. Pineal cyst apoplexy: Case report and review of the literature. Neurosurgery. 2005;57:1066.
78. Konovalov, AN, Spallone, A, Pitzkhelauri, DI. Meningioma of the pineal region: A surgical series of 10 cases. J Neurosurgery. 1996;85:586.
79. Lantos, PL, VandenBerg, SR, Dleihues, P, Tumors of the nervous system. Greenfield’s neuropathology, 6th ed.. Graham, DI, Lantos, PL, eds. Greenfield’s neuropathology, Vol 2. London: Arnold, 1997:677–682.
80. Mena, H, Nakazato, Y, Jouvet, A, et al. Pineocytoma. In: Kleihues P, Cavenee WK, eds. Pathology and Genetics: Tumors of the Nervous System. Lyon: International Agency for Research on Cancer; 2000:118.
81. Mena, H, Nakazato, Y, Jouvet, A, et al. Pineoblastoma. In: Kleihues P, Cavenee WK, eds. Pathology and Genetics: Tumors of the Nervous System. Lyon: International Agency for Research on Cancer; 2000:116.
82. Blakeley, JO, Grossman, SA. Management of pineal region tumors. Curr Treat Options Oncol. 2006;7:505–516.
83. Nomura, K. Epidemiology of germ cell tumors in Asia of pineal region tumor. J Neuro-Oncology. 2001;54:211–217.
84. Konovalov, AN, Pitskhelauri, DI. Principles of treatment of the pineal region tumors. Surg Neurol. 2003;59:250–268.
85. Hirato, J, Nakazato, Y. Pathology of pineal region tumors. J Neuro-Oncol. 2001;54:239–249.
86. Brain Tumor Registry of Japan, Vol 9, 1969–1990. The Committee of Brain Tumor Registry of Japan, Tokyo, 1996.
87. Mena, H, Rushing, EJ, Ribas, JL, et al. Tumors of pineal parenchymal cells: A correlation of histological features, including nucleolar organizer regions, with survival in 35 cases. Hum Pathol. 1995;26:20–30.
88. Jennings, MT, Gelman, R, Hochberg, F. Intracranial germ-cell tumors: Natural history and pathogenesis. J Neurosurg. 1985;63:155–167.
89. Brandes, AA, Pasetto, LM, Monfardini, S. The treatment of cranial germ cell tumours. Cancer Treat Rev. 2000;26:23–242.
90. Matsutani, M. Clinical management of primary central nervous system germ cell tumors. Semin Oncol. 2004;31:676–683.
91. Choi, JU, Kim, DS, Chung, SS, et al. Treatment of germ cell tumors in the pineal region. Childs Nerv Syst. 1998;14:41–48.
92. Packer, RJ, Cohen, BH, Coney, K. Intracranial Germ Cell Tumors. The Oncologist. 2000;5:312–320.
93. Lambrinides, K, Reichert, M. MR imaging of pineoblastomas. Radiol Technol. 1994;66:106.
94. Nakamura, M, Saeki, N, Iwadate, Y, et al. Neuroadiological characteristics of pineocytoma and pineoblastoma. Neuroradiology. 2000;42:509.
95. Ganti, SR, Hilal, SK, Stein, BM, et al. CT of the pineal region tumors. AJR Am J Roentgenol. 1986;146:451.
96. Sumida, M, Uozumi, T, Kiya, K, et al. MRI of intracranial germ cell tumors. Neuroradiology. 1995;37:32–37.
97. Satoh, H, Uozumi, T, Kiya, K, Juris, K, et al. MRI of pineal region tumors: Relationship between tumors and adjacent structures. Neuroradiology. 1995;37:624–630.
98. Chiechi, MV, Smirniotopoulos, JG, Mena, H. Pineal parenchymal tumors: CT and MR features. J Comput Assist Tomogr. 1995;19:509.
99. Vaquero, J, Ramiro, J, Martinez, R, et al. Clinicopathological experience with pineocytomas: Report of five surgically treated cases. Neurosurgery. 1990;27:612.
100. Little, KM, Friedman, AH, Fukushima, T. Surgical approaches to pineal region tumors. J Neuro-Oncology. 2001;54:287–299.
101. Bruce, JN, Ogden, AT. Surgical strategies for treating patients with pineal region tumors. J Neuro-Oncology. 2004;69:221–236.
102. Endo, H, Kumabe, T, Jokura, H, et al. Stereotactic radiosurgery followed by whole ventricular irradiation for primary intracranial germinoma of the pineal region. Minim Invasive Neurosurg. 2005;48:186–190.
103. Lee, JYK, Wakabayashi, T, Yoshida, J. Management and survival of pineoblastoma: An analysis of 34 adults from the Brain Tumor Registry of Japan. Neurol Med Chir (Tokyo). 2005;45:132–142.
104. Hasegawa, T, Kondziolka, D, hadjipanayis, CG, et al. The role of radiosurgery for the treatment of pineal parenchymal tumors. Neurosurgery. 2002;51:880–889.
104a. Bruce, JN, Ogden, AT. Surgical strategies for treating patients with pineal region tumors. J Neurooncol. 2004;69:221–236.
105. Dempsey, PK, Lunsford, LD. Streotactic radiosurgery for pineal region tumors. Neurosurg Clin N Am. 1992;3:245.
106. Kobayashi, T, Kida, Y, Mori, Y. Stereotactic gamma radiosurgery for pineal and related tumors. J Neuro-Oncology. 2001;54:301.
107. Kondziolka, D, Hadjipanayis, CG, Flickinger, JC, et al. The role of radiosurgery for the treatment of pineal parenchymal tumors. Neurosurgery. 2002;51:880.
108. Jackson, ASN, Plowman, PN. Pineal parenchymal tumors. I. Pineocytoma: A tumor responsive to platinum-based chemotherapy. Clin Oncol. 2004;16:238–243.
109. Lutterbach, J, Fauchon, F, Schild, SE, et al. Malignant pineal parenchymal tumors in adult patients: patterns of care and prognostic factors. Neurosurgery. 2002;51:44–56.
110. Fauchon, F, Jouvet, A, Paquis, P, et al. Parenchymal pineal tumors: A clinicopathological study of 76 cases. Int J Radiat Oncol Biol Phys. 2000;46:959–968.
111. Gururangan, S, McLaughlin, C, Quinn, J, et al. High-dose chemotherapy with autologous stem-cell rescue in children and adults with newly diagnosed pineoblastomas. J Clin Oncol. 2003;21:2187.
112. Hussain, SA, Ma, YT, Cullen, MH. Management of metastatic germ cell tumors. Expert Rev Anticancer Ther. 2008;8(5):771–784.
113. Balmaceda, C, Heller, G, Rosenblum, M, et al. Chemotherapy without irradiation: A novel approach for newly-diagnosed central nervous system (CNS) germ-cell tumors (GCT), Results of an international cooperative trial. J Clin Oncol. 1994;14:2908–2915.
114. Gobel, U, Schneider, DT, Calaminus, G, et al. Germ cell tumors in childhood and adolescence. Ann Oncol. 2000;11:263–271.
115. Garre, ML, El-Hossainy, MO, Fonelli, P, et al. Is chemotherapy effective therapy for intracranial immature teratoma? A case report. Cancer. 1996;77:97–982.
116. Baranzelli, MC, Patte, C, Bouffet, E, et al. An attempt to treat pediatric intracranial alphaFP and beta HCG secreting germ cell tumors with chemotherapy alone. SFOP experience with 18 cases. Societe Francaise d’Oncologic Pediatrique. J Neuro-Oncology. 1998;37:229–239.
117. Buckner, JC, Peethambaram, PP, Smithson, WA, et al. Phase II trial of primary chemotherapy followed by reduced-dose radiation for CNS germ cell tumors. J Clin Oncol. 1999;17:933–940.
118. Kretschmar, C, Kleinberg, L, Greenberg, M, et al. Pre-radiation chemotherapy with response-based radiation therapy in children with central nervous system germ cell tumors: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2006.
119. Marcus, DM, Brooks, SE, Leff, G, et al. Trilateral retinoblastoma: Insights into histogenesis and management. Surv Ophthalmol. 1998;43:59.