The Peritoneum
Normal Peritoneum
Submesothelial mesenchyme anchors the mesothelium to the underlying tissue. Submesothelial stromal cells are important in the development of deciduosis, endosalpingiosis, endometriosis, and disseminated peritoneal leiomyomatosis. Microscopically, these peritoneal lesions are characterized by müllerian differentiation and are thought to derive from the so-called ‘secondary müllerian system,’ i.e., the pelvic and lower abdominal mesothelium and the underlying mesenchyme of females.1 In fact, the concept of the secondary müllerian system refers to a mechanism by which benign lesions and tumors of müllerian histology might arise from the peritoneum.1
Inflammatory and Reactive Lesions
In adult females, most infections are ascending, as in pelvic inflammatory disease, which results in localized acute peritonitis. Acute diffuse peritonitis, characterized by a serosal fibrinopurulent exudate, is most commonly associated with perforated viscera as in appendicitis or diverticulitis and is usually bacterial or chemical in origin. In addition to the acute inflammatory reaction itself, chronic changes may occur, such as are seen in granulomatous and histiocytic reactions. In some cases, the inflammatory process leads to reactive changes.
Granulomatous Peritonitis
Both, infectious and noninfectious agents can cause granulomatous peritonitis. Among the former, Mycobacterium tuberculosis is the most common, and, less frequently, fungi and parasites. Granulomas are also induced by foreign material including keratin, by vernix caseosa or by meconium, in the form of necrotic pseudoxanthomatous nodules or as a postcautery reaction.2
Tuberculosis
Tuberculous peritonitis is still encountered in the peritoneum, usually in immunosuppressed patients.3 It may also occur as a complication of chronic peritoneal dialysis.4 It may be secondary to tuberculous salpingitis or result from miliary tuberculosis. Clinically, it may manifest nonspecifically as widespread carcinomatosis.5 The presence of ascites, a pelvic mass, and marked elevation of serum levels of CA125 may lead to a false clinical suspicion of ovarian cancer.3,6 The granulomas are characterized by caseous necrosis and Langhans type giant cells; mycobacteria may be demonstrated by acid-fast stains or immunofluorescence techniques.
Surgical Glove Powder
Surgical glove powder, either talc or starch granules, is a common cause of granulomas. At laparotomy, the peritoneal granulomas may simulate carcinomatosis or tuberculosis. Usually the starch granulomas resolve within a few months, leaving no residua or only adhesions; however, some patients develop fibrosing peritonitis. Commonly, starch granulomas exhibit a typical foreign-body reaction and, less frequently, they appear as sarcoid granulomas,7,8 which lack necrosis, or tuberculoid granulomas, with necrosis, that simulate tuberculosis. The polyhedral and translucent starch granules are periodic acid–Schiff (PAS) positive and exhibit the typical Maltese cross under polarized light. Rarely, fat necrosis and rheumatoid-type necrotizing foci are identified as reactions to starch. Talc was once an important cause of granulomatous and fibrosing peritonitis because of its application as a lubricant on surgical gloves; however, its use has been discontinued. Talc is a greater irritant than starch and is poorly absorbed by some patients. Talc granulomas are of the typical foreign-body type. Multinucleated giant cells are numerous and contain pleomorphic crystal spicules readily seen with polarized light.
Cystic Teratoma (Dermoid Cyst) Rupture
Rupture of a mature cystic teratoma (dermoid cyst) is typically associated with widespread peritoneal granulomas and adhesions. The squamous cells, hairs, and sebum trigger a foreign-body reaction. This phenomenon occurs especially when the teratoma is removed by cystectomy during laparoscopic surgery.9 On occasion the reaction may also appear as sclerosing peritonitis and mimics a neoplasm at operation.10
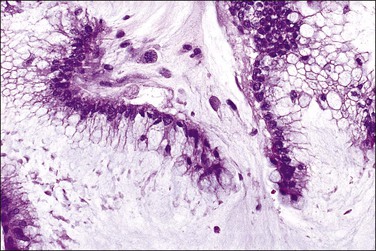
Keratin
Peritoneal foreign-body granulomas to keratin may be found in association with uterine or ovarian endometrioid carcinomas with squamous differentiation, or, less frequently, with squamous cell carcinomas of the cervix or atypical polypoid adenomyomas.11 Uterine examples are thought to result from retrograde transmission of acellular keratinous debris through the fallopian tubes (Figure 31.1). Granulomas have been seen on the serosa of the adnexa, uterus, colon, and appendix. These granulomas are easily misinterpreted as metastatic carcinoma. Follow-up on these patients indicates that cell-free granulomas lack prognostic significance.11
Cauterized Tissue
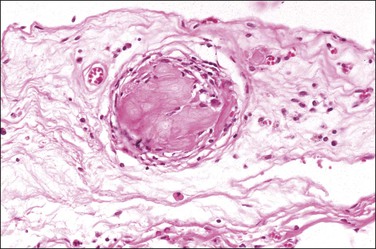
Foreign-body granulomatous reactions to cauterized tissue in pelvic peritoneal and ovarian biopsies are occasionally encountered in patients who have had endometriotic or other lesions treated in the weeks prior to biopsy. The lesions show central eosinophilic, focally refractile, amorphous material (representing the coagulated tissue and carbonaceous debris) palisaded by large numbers of multinucleated foreign-body giant cells and a peripheral lymphocytic infiltrate (Figure 31.2). Lesions tend to hyalinize with age and may persist for many years.
Cesarean Delivery
Complicating cesarean delivery, the amniotic fluid contents may spill into the peritoneal cavity causing a syndrome clinically similar to bowel perforation.12,13 Amniotic fluid contains squamous cells, keratin, and sometimes lanugo hair (vernix caseosa). It may also contain meconium, which itself is composed of bile, pancreatic, and intestinal secretions.14 Grossly, the amniotic fluid contents appear as cheese-like yellow patches limited to the serosal layer of visceral organs.15 Meconium peritonitis caused by bowel perforation in utero can also be a problem in newborn infants. The hallmark of meconium peritonitis is calcification, which presumably results from the action of pancreatic enzymes.
Non-Granulomatous Histiocytic Lesions
Histiocytic infiltrates rather than discrete granulomas are occasionally found in the peritoneum.9 Melanin-rich histiocytes are sometimes found in cases where an ovarian dermoid cyst has ruptured. The spillage contains melanin, which the peritoneal histiocytes phagocytose. Grossly, the peritoneum may appear to be stained black or display small tumor-like nodules on its surface. Distinction of benign peritoneal melanosis from metastatic malignant melanoma is usually straightforward because of the bland nuclear features of the pigmented histiocytes and the absence of mitoses. Appropriate immunohistochemical stains can further indicate that the cells are histiocytes and not atypical melanocytes.16
Occasionally, foci of endometriosis may disclose an abundance of histiocytes filled with ceroid, a wax-like, finely granular, and golden to yellow-brown pigment that is a form of lipofuscin, a lipid-containing residue of lysosomal digestion that is considered an aging or ‘wear and tear’ pigment. Ceroid is believed to be the end result of the breakdown of blood products after removal of iron. These histiocytic foci are sometimes called ‘necrotic pseudoxanthomatous nodules.’2,17,18
Fibrosing Lesions
Sclerosing peritonitis is a reactive process in which a thickened fibrous or myofibromatous stroma develops on the peritoneal serosa. It is often idiopathic,19,20 although in some cases the cause is identified, such as prior peritoneal inflammation or a ruptured ovarian dermoid with spillage of the contents (see previous granulomatous reactions),10,21 chronic dialysis,22–26 or after surgical procedures.
In some cases, the sclerosing peritonitis has been described as part of a syndrome, often in association with a ‘luteinized thecoma of the ovary.’27–32 Clinically, most of the women are young, usually under 30 years of age. Common presenting signs include abdominal enlargement and sometimes small bowel obstruction. Ascites may be present. Even when the patients have a luteinized thecoma, none has endocrine symptoms. A significant number of patients have been exposed to propranolol-type beta-blocking agents or antiepileptics.
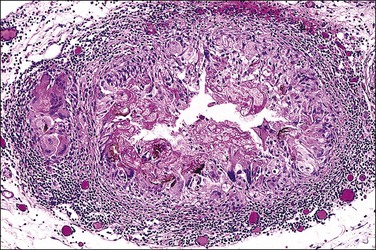
Grossly, opaque to light-brown 1–3 mm granules or nodules appear matted together on the peritoneum or on the serosa of the involved organs. The omentum is usually indurated. Microscopic examination discloses a fibrotic process, with various chronic inflammatory cells (Figure 31.3). There is usually some degree of mesothelial hyperplasia. Deeper tissues are relatively spared. Nodules are composed of moderately cellular fascicles of benign-appearing spindle cells resembling fibroblasts and myofibroblasts that contain occasional mitotic figures. In addition to cytokeratin reactivity, the cells also disclose immunoreactivity for vimentin and smooth muscle actin.27,30
Rarely, single or multiple fibrous nodules ranging up to 6 cm may occur in the gastrointestinal tract or mesentery in adults.32 Microscopically, the lesions are composed of fibroblasts, collagen, and scattered mononuclear inflammatory cells. The fibroblastic cells show variable immunoreactivity for vimentin, CD117, muscle-specific actin, smooth muscle actin, and desmin, with negative staining for CD34 and ALK-1. These nodules have been designated as ‘fibrous pseudotumors.’32
Tumor-Like Lesions
Mesothelial Hyperplasia
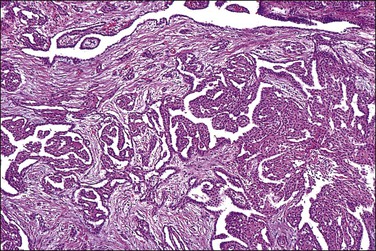
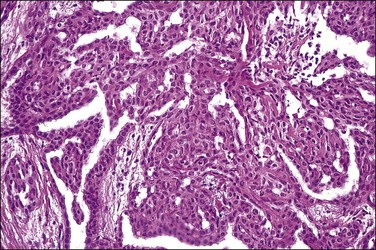
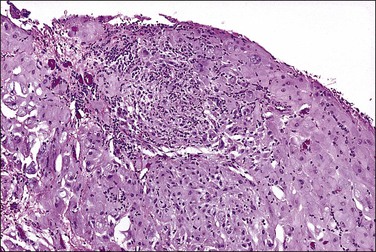
Mesothelial hyperplasia is a common response to inflammation that occurs in any process that leads to irritation of a serosal surface, such as ascites, hernia sacs, endometriosis, pelvic inflammatory disease, or ovarian tumors.33–35 Grossly, the hyperplastic lesions may be seen at operation as multiple small nodules, but more commonly are incidental findings on microscopic examination. Microscopically, the changes range from a mild (Figure 31.4) to a substantial increase in the number of mesothelial cells (Figure 31.5), most of which have transformed from flat and relatively inconspicuous to cuboidal or even columnar. With marked hyperplasia, the mesothelial proliferation appears as sheets, clusters, ribbons, tubules, and sometimes as papillary formations that can be misinterpreted as metastatic adenocarcinoma (Figure 31.6). Psammoma bodies are encountered occasionally and eosinophilic elongated cells resembling rhabdomyoblasts have been described.
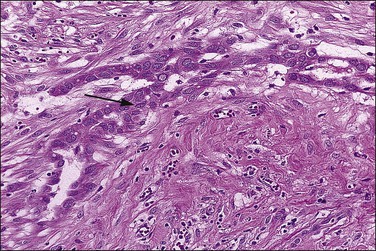
Figure 31.6 Reactive mesothelium. The enlarged mesothelial cells (arrow) that cover a focus of fibrous reaction superficially resemble metastatic adenocarcinoma.
Reactive mesothelial cells tend to be uniform in appearance. With minor degrees of reactivity, the nuclei are small, regular, round, or oval, and exhibit central nucleoli. The cytoplasm is eosinophilic or sometimes vacuolated and contains acid mucin (predominantly hyaluronic acid). With increasing degrees of reactivity, the nuclei enlarge and the chromatin increases. Nucleoli become more apparent and, in the extreme case, may become quite large and prominent (Figure 31.7). Cells may become binucleated or multinucleated. In cytologic preparations the large macronucleoli may be mistaken as evidence for malignancy.
The exuberant and sometimes pseudoinfiltrative growth that mesothelium can show, together with the increased mitotic activity that is frequently observed, may lead to a false impression of primary or metastatic carcinoma, despite the benign cytologic appearance of the cells33 (Figure 31.6). Carcinoma cells generally demonstrate greater nuclear pleomorphism and more conspicuous mitotic activity. However, clusters of mesothelial cells are easily mistaken for metastatic carcinoma. This is true especially when mesothelial cells extensively involve sinusoids in pelvic lymph nodes either as small papillary clusters or as sheets of somewhat discohesive cells.36 Exuberant surface proliferations, sometimes forming sessile or polypoid nodules, can also simulate mesothelioma, a problem also encountered in the walls of hernia sacs. A useful morphologic feature that can help distinguish reactive mesothelial cell aggregates from metastatic carcinoma is their orientation at low-power magnification to one another (often in a line that can be traced for some considerable distance) (Figure 31.6) and their relation to the position of the original peritoneal surface (as demonstrated by the presence of the peritoneal elastic lamina).37
Mesothelial hyperplasia must be distinguished from malignant peritoneal mesothelioma. The presence of necrosis, marked nuclear pleomorphism, and deep infiltration favors malignant mesothelioma.38 Immunostains may help in the differential diagnosis. Strong immunoreactivities for p5339 and epithelial membrane antigen (EMA; nuclear and cytoplasmic, respectively) are characteristic of the cells of malignant mesothelioma but not reactive mesothelial cells; in contrast, hyperplasic mesothelial cells are usually desmin positive.40 Proliferative markers such as Ki-67 may also be helpful (approximately 25% vs 5% labeling index for malignant mesothelioma vs mesothelial hyperplasia, respectively).41 In some cases, however, the distinction between a reactive and malignant mesothelial lesion may be difficult or impossible, particularly in a biopsy samples. An apparently benign mesothelial proliferation occasionally precedes the appearance of a malignant peritoneal mesothelioma.38,42
Mesothelial hyperplasia should also be distinguished from a borderline serous tumor of primary peritoneal origin. Grossly visible tumor, columnar cells with or without cilia, the presence of neutral mucin, and numerous psammoma bodies all favor a serous tumor. Immunohistochemical markers for epithelial differentiation may also be useful in the distinction (see Chapter 25).
Peritoneal Inclusion Cysts
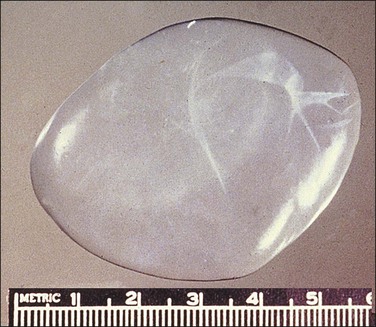
Peritoneal inclusion cysts are unilocular or multilocular mesothelial-lined lesions that occur almost exclusively in women in the reproductive age group. They usually involve the pelvis, although may occur in other abdominal locations, including the omentum and mesentery, and are frequently associated with prior abdominal surgery.43 The origin of peritoneal inclusion cysts remains controversial; some authors consider them reactive lesions that develop in response to injury, whereas others favor their neoplastic nature.
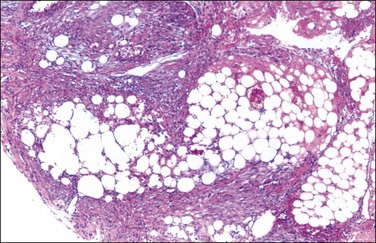
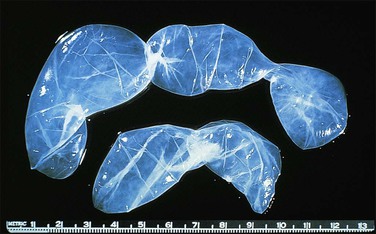
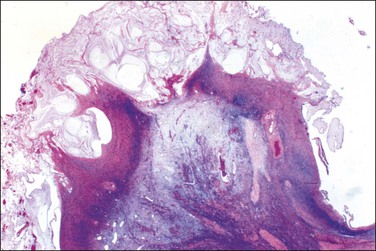
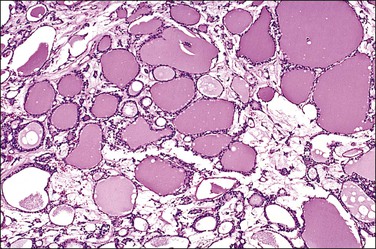
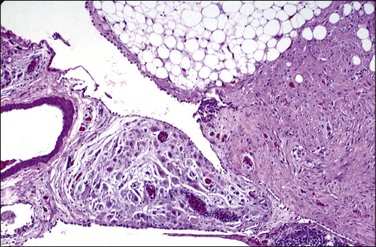
Unilocular peritoneal inclusion cysts are usually incidental findings at laparotomy. Multilocular peritoneal inclusion cysts, also referred to as ‘benign cystic mesotheliomas,’ frequently form large bulky masses (Figures 31.8–31.11) simulating a cystic ovarian tumor. Cysts are thin walled, contain clear proteinaceous fluid, and are lined by a single layer of flat to cuboidal, hobnail-shaped, mesothelial cells (Figure 31.12) with bland nuclear features, although a degree of reactive atypia is occasionally seen. Tubal and squamous metaplasia of the mesothelial lining sometimes occurs. Inflammatory infiltrates, if present at all, are limited to sparse lymphocytic collections. The mesothelial cells are typically immunoreactive for calretinin, and less frequently positive for estrogen (ERs) or progesterone receptors (PRs), or both.44
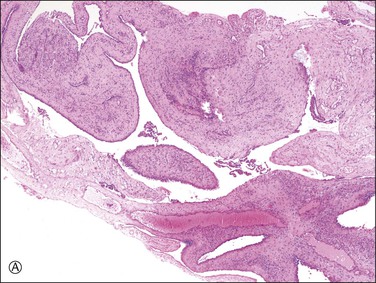
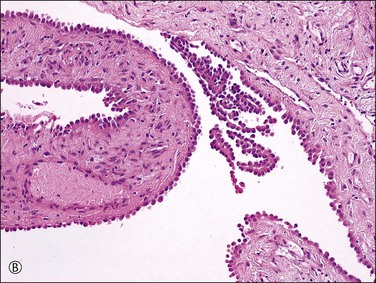
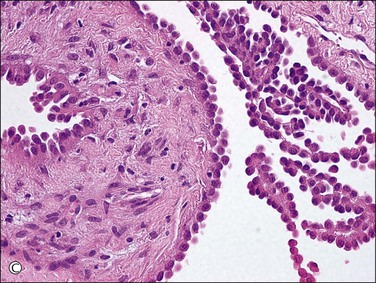
Figure 31.12 (A) Thin-walled mesothelial cysts in peritoneum. (B) The cyst is lined by numerous mesothelial cells. (C) Detail of the mesothelial cells.
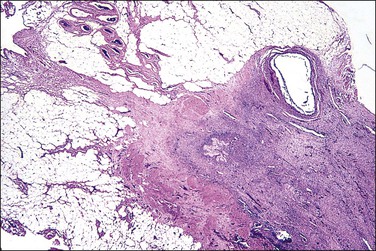
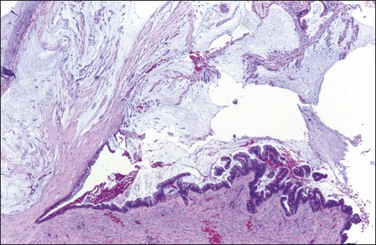
In patients who have had peritonitis, fibrinous adhesions that are superficial to the deeper lining of normal mesothelium may develop and the underlying serosa can be mistaken for invasive serous carcinoma until attention is paid to its regularity and benign histology (Figure 31.13).
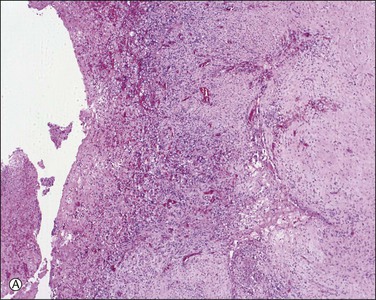
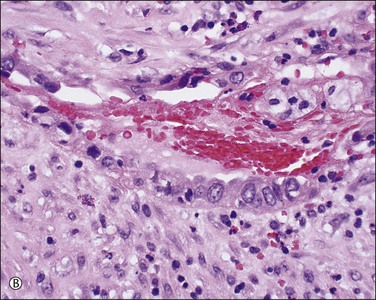
Figure 31.13 (A) Serosa covered by adhesions. It is easy to mistake the normal mesothelium for serous adenocarcinoma due to its location within the peritoneal wall. (B) Detail of mesothelial inclusion.
Peritoneal inclusion cysts are confused with multilocular cystic lymphangiomas, which typically occur in children, more often in boys. Lymphangiomas are almost always localized in the mesentery of the small intestine, mesocolon, omentum, or retroperitoneum. They contain chylous material and, microscopically, show intramural lymphoid aggregates and smooth muscle, which are absent in peritoneal inclusion cysts.
Although no malignant behavior has been reported in peritoneal inclusion cysts, recurrence occurs in approximately one-half of cases from months to several years postoperatively.45 GnRH agonists or tamoxifen have successfully been applied to some patients.44
Ovarian Remnant Syndrome
This condition exists if a patient who has had a ‘total bilateral oophorectomy’ later develops a palpable mass or experiences pelvic pain or other symptoms referable to ovarian tissue that has been left behind (Figures 31.14 and 31.15). This condition is described more fully in Chapter 24.
Supernumerary or Accessory Ovaries
Supernumerary ovaries are ectopic ovaries located at some distance from the eutopic ovary. It is rare but occasional cases have been reported in the peritoneal cavity.46 This condition is described more fully in Chapter 23.
Splenosis
Nodules of splenic tissue, usually less than 1 cm in diameter, are randomly distributed in the peritoneal cavity. The etiology is trauma, most commonly a motor vehicle accident, which has resulted in splenic rupture.47 Splenosis is generally asymptomatic but may cause abdominal or pelvic pain simulating endometriosis, or produce intestinal obstruction due to the development of adhesions. Splenosis may be encountered as an incidental finding or mistakenly interpreted as endometriosis, benign or malignant vascular tumors, or metastatic cancer.48–50
Trophoblastic Implants
Finding disseminated trophoblastic implants in the peritoneum is uncommon (Figure 31.16). They may occur on occasion with peritoneal pregnancy, or following laparoscopic treatment of tubal pregnancy, where the frequency has been estimated at 3.6%.51,52 Viability is suggested by rising human chorionic gonadotropin concentrations following surgery. The condition is best avoided by meticulous inspection of the abdomen after resection of the tubal pregnancy. Microscopically, the implants may show trophoblastic tissue including chorionic villi. Some implants, however, may resemble a placental site nodule.
Infarcted Appendix Epiploica
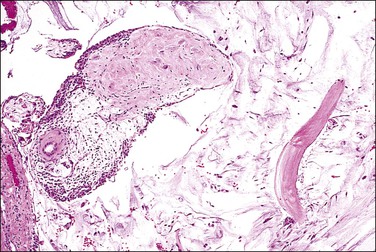
Appendices epiploicae are small polypoid processes of adipose tissue that project from the serosa of the large intestine, especially the transverse and sigmoid colon. Occasionally, they undergo torsion, infarction, and later detachment and can be found lying free within the peritoneal cavity.53,54 Typically in these cases, the center contains hyalinized fibrous tissue and often some residual adipose tissue that is mummified. The outer rim and variable portions of the core may calcify, resulting in a hard tumor-like mass (Figure 31.17).
Mesothelial Neoplasms
Adenomatoid Tumor
Adenomatoid tumors are benign neoplasms of mesothelial origin, encountered most often in the fallopian tubes where frequently they are sieve like or multicystic. In contrast, they are also found subserosally in the uterine corpus near the fallopian tube, where they more usually simulate leiomyomas. They are seldom encountered elsewhere in the peritoneal cavity (see Chapter 21). Clinically, they are asymptomatic, and rarely recur after adequate excision. Grossly, adenomatoid tumors are usually solitary, less than 2 cm in diameter and have a white-gray appearance. Microscopically, multiple small slit-like or ovoid spaces are lined by a single layer of cells. Nuclear atypia is absent or minimal, and mitotic figures are rarely seen.
Well-Differentiated Papillary Mesothelioma
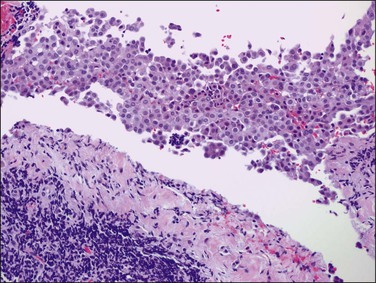
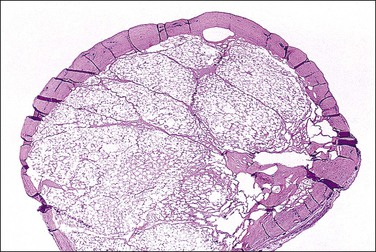
A rare form of peritoneal mesothelioma is the well-differentiated papillary mesothelioma. Most patients are of reproductive age, although an occasional patient has been postmenopausal. Also encountered in males, less common sites include the tunica vaginalis testis, pericardium, and pleura. These tumors are typically asymptomatic and often found incidentally at operation. Grossly, they are usually multiple, broad-based, wart-like excrescences that are polypoid or slightly nodular. Color and texture are similar to ovarian cortical tissue but sometimes firmer. They are generally small, usually measuring less than 2 cm in diameter.55 An occasional tumor is solitary.55
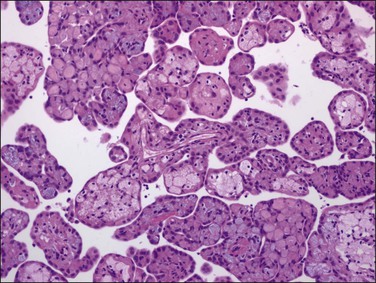
On microscopic examination, the neoplasm consists of relatively thick papillae composed of dense fibrous or hyalinized tissue covered by a single layer of cytologically benign, small flattened to cuboidal cells (Figure 31.18). Nuclei are bland, with a low nuclear grade (Figure 31.19). Mitoses are rare, usually under 1, but may be as high as 3, mitotic figures per 10 HPFs. The diagnosis should be made with caution, as malignant mesotheliomas may have foci that, viewed in isolation, resemble this tumor.56 These lesions can usually be reliably distinguished from serous epithelial tumors, since the architecture of the latter discloses feathery irregular clusters of cells in which the nuclei are far more atypical and higher grade. Psammoma bodies may be encountered in rare cases. These tumors are nearly always benign, but rare tumors have acted aggressively.57,58
Diffuse Malignant Mesothelioma
Peritoneal diffuse malignant mesotheliomas are much less common than their pleural counterparts, accounting for about 10% of all malignant mesotheliomas.59 Only one-third of these tumors occur in middle-aged or postmenopausal women and they must be distinguished from the more prevalent serous adenocarcinomas, including those arising from the peritoneum itself and those metastatic from an ovarian or fallopian tube primary. The survival rate for women with malignant mesothelioma is worse than that for women with serous adenocarcinoma, and the treatment of the two diseases currently differs.
Clinical manifestations usually are nonspecific and include ascites, abdominal discomfort, digestive disturbances, and weight loss. Ascites is present in most cases, and cytologic examination of the ascitic fluid may be diagnostic in some cases. The diagnosis, however, usually requires laparotomy or laparoscopy and biopsy. While most malignant mesotheliomas are highly aggressive, some peritoneal malignant mesotheliomas pursue a more indolent course.60 It is generally stated in the literature that asbestos exposure is uncommon in women with peritoneal mesothelioma. In one (2003) population-based study of peritoneal malignant mesotheliomas, 29% of 96 men had asbestos-related jobs whereas none of 113 women had occupational or environmental risk factors.61 In fact, men with peritoneal mesotheliomas typically have had a heavier burden and more prolonged exposure to asbestos than men with pleural mesotheliomas. Most males with peritoneal malignant mesotheliomas reported in the literature survived less than 2 years after diagnosis, although there have been occasional long-term survivors. A study of peritoneal malignant mesotheliomas in women,60 however, found that 40% of the patients survived longer than 4 years. The histopathologic subtype (see later) is of prognostic significance, as biphasic peritoneal malignant mesotheliomas are associated with a much shorter survival than pure epithelial tumors62 and deciduoid mesotheliomas are usually rapidly fatal.63,64 Increasing nuclear and nucleolar size has been shown to correlate with shorter survival in epithelial tumors.62 Also, p16 loss independently correlates with increased risk of death according to one study,65 while another failed to identify any morphologic features that differentiated those cases with a highly aggressive course from indolent ones.66 Two studies have identified a number of favorable prognostic factors including an age less than 60 years, low nuclear grade, low mitotic index, minimal residual disease after cytoreduction, and lack of deep invasion.67,68
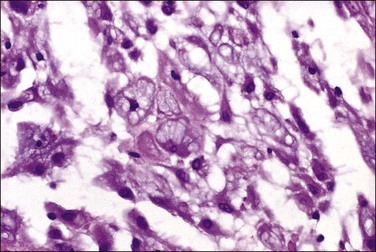
Pathology
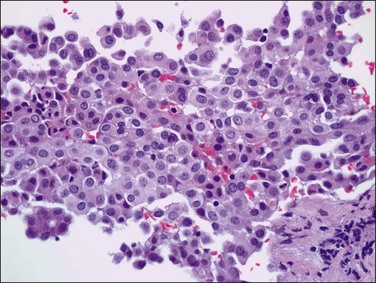
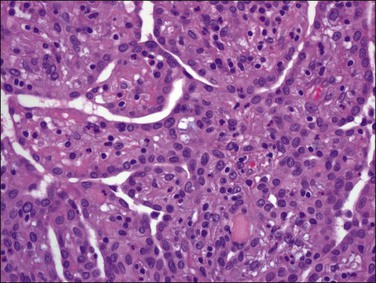
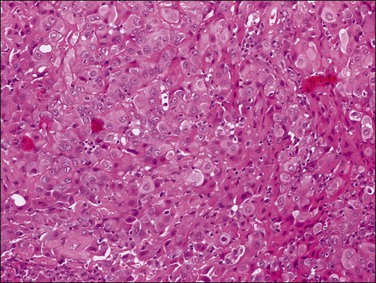
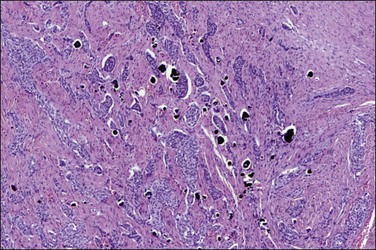
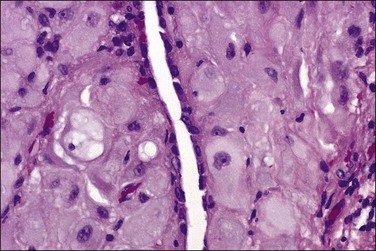
Tumors may extensively involve and diffusely thicken the peritoneum and the serosa of the various abdominal and pelvic organs and typically consist of multiple nodules measuring less than 1.5 cm in greatest dimension. Some tumors incite a striking desmoplastic reaction. On microscopic examination, most tumors have only an epithelial component, which usually has a tubulopapillary to focally solid pattern. The epithelial variant of malignant mesothelioma has polygonal or cuboidal cells with moderately abundant eosinophilic cytoplasm (Figures 31.20 and 31.21). The tumor cells usually resemble mesothelial cells, with a more or less constant nuclear : cytoplasmic ratio and only mild to moderate nuclear atypia (Figures 31.22–31.24); in some cases, however, the nuclei become larger and more bizarre as the cytoplasmic volume increases.69 Mitotic figures usually are present but are not numerous. In rare cases, the cytoplasm is abundant, amphophilic, and glassy, mimicking an exuberant ectopic decidual reaction (so-called ‘deciduoid mesothelioma’) (Figure 31.25).56,70 Psammoma bodies are found in approximately one-third of cases (Figure 31.26), but are usually less common than in serous tumors. Unlike pleural mesotheliomas, sarcomatoid or fibrous variants are extremely rare.56,60,68,71 Intra-abdominal lymph nodes may be involved.
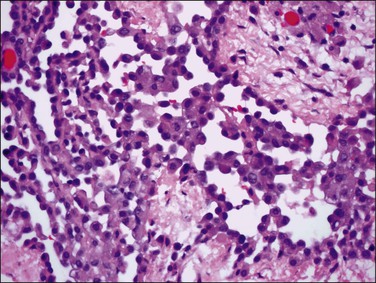
Figure 31.22 Diffuse malignant mesothelioma. Individual cells have central oval–round uniform nuclei and abundant eosinophilic cytoplasm.
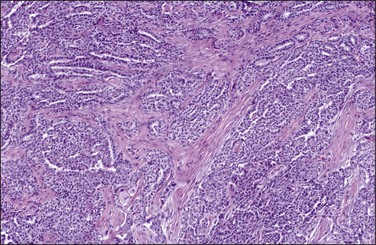
Figure 31.23 Diffuse malignant mesothelioma in which the regular-sized cells are arranged in glandular and tubular structures.
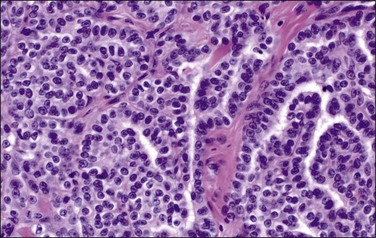
Figure 31.24 Diffuse malignant mesothelioma in which the cells display a more or less constant nuclear : cytoplasmic ratio and only mild to moderate nuclear atypia.
Differential Diagnosis
The diagnosis that results in substantially differing treatment plans is the high-grade serous adenocarcinoma. Psammocarcinoma and low-grade serous carcinoma with abundant psammoma bodies are readily distinguished from mesothelioma, in which psammoma bodies are few when they are present. Mesothelial cells tend to be uniform, polygonal, and have moderate to extensive amounts of eosinophilic cytoplasm. Adenocarcinomas, in contrast, tend to have columnar cells, occasional cells with bizarre nuclear features, and variable numbers of psammoma bodies. Complicating the distinction, it is now recognized that malignant mesotheliomas can on rare occasion arise within the ovary72 (Table 31.1).
Table 31.1
Malignant Mesothelioma versus Serous Carcinoma: Differential Diagnosis
| Feature | Malignant Mesothelioma | Serous Carcinoma |
| Clinical | ||
| History of asbestos exposure | Often positive | None |
| Diffuse peritoneal tumor mass | Yes | Usually dominant ovarian |
| Responsive to therapy | Rapidly fatal and unresponsive | Some respond |
| Histologic | ||
| Sarcomatoid and adenomatoid foci | Present | Absent |
| Columnar cells | Rare | Numerous |
| Psammoma bodies | Rare | Often present |
| Nuclei | Round | Oval or elongate |
| Mucins (scanty) | Cytoplasmic acid mucin | Apical neutral mucin |
| Ultrastructural | ||
| Microvilli | Abundant | Usually sparse |
| Cilia | Never numerous | Often numerous |
| Intracytoplasmic lumina | Common | Uncommon |
| Apical ‘snouts’ | Rare | Common |
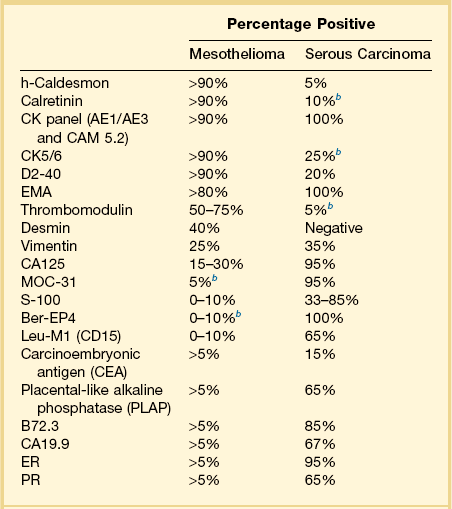
Immunohistochemistry is important in establishing the proper diagnosis.73–76 Differential immunoreactivities for mesothelioma and serous carcinoma are shown in Table 31.2. Calretinin, a 29 kDa calcium-binding protein, is present in nearly all epithelial mesotheliomas (Figure 31.27), but rarely in adenocarcinomas.77 Both mesothelioma and adenocarcinoma are immunoreactive for low molecular weight cytokeratins. Cytokeratin (CK) 5/6 is usually expressed by mesotheliomas, but seldom by adenocarcinomas. Vimentin and desmin are commonly detected in mesotheliomas. Once, it was believed that vimentin expression favored a diagnosis of mesothelioma, but it is now known that this intermediate filament is seen in normal and neoplastic cells of both epithelial and mesenchymal origin. Moreover, keratins and vimentin are commonly coexpressed by adenocarcinomas of müllerian type. CA125, an antigen initially identified in cell lines of ovarian serous adenocarcinomas, can be expressed by mesotheliomas. Thus, no single immunohistochemical stain is diagnostic in the separation of peritoneal malignant mesothelioma from adenocarcinoma, and a panel of antibodies should be interpreted in conjunction with the H&E and mucin stains.
Miscellaneous Primary Tumors
Intra-Abdominal Desmoplastic, Small Round Cell Tumor
Desmoplastic small, round cell tumor is the descriptive designation for a rare, undifferentiated, and highly aggressive tumor that, with few exceptions, involves the peritoneal serosa78 and contiguous organs such as the kidney and ovary.79 It usually appears during adolescence and early adulthood, with a mean age of 25 years, but occasionally may be found in older women.80,81 It is far more common in men than in women. The prognosis is poor.
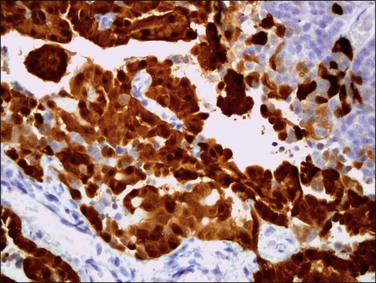
Grossly, most tumors are bulky abdominal masses that have spread diffusely over the peritoneal surface with prominent involvement of the tunica vaginalis or the ovaries, mimicking a primary testicular or ovarian tumor.82 The characteristic microscopic pattern is nests of ‘small, blue cells’ embedded in a desmoplastic fibrous stroma (Figure 31.28). The tumor cells are uniform with scanty cytoplasm and indistinct cell borders (Figure 31.29). About one-third of tumors exhibit a wider range of morphologic features, principally as spindle-shaped cells with epithelioid to focally sarcomatoid arrangements. Mitotic figures are numerous and foci of necrosis are usually present. Invasion of vascular spaces is common but lymph node involvement is rare.
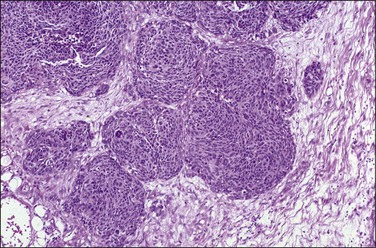
Figure 31.28 Desmoplastic small, round cell tumor. Nests of ‘small, blue cells’ embedded in a desmoplastic fibrous stroma.
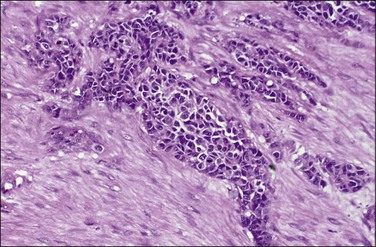
Figure 31.29 Desmoplastic small, round cell tumor. The tumor cells are uniform with scanty cytoplasm and indistinct cell borders.
Virtually all tumors are CK positive (CK monoclonal antibodies CAM 5.2, AE1/AE3), but lack CK20 expression, indicative that they are not of large intestinal origin.66,83,84 Roughly four-fifths are also reactive with antibodies to EMA, neuron-specific enolase, desmin (with paranuclear dot-like reactivity) (Figure 31.30), and vimentin, suggestive that there is both epithelial and mesenchymal (divergent) differentiation. Between two-fifths and two-thirds of tumors express Ber-EP4, CD57 (Leu-7), CD15 (Leu-M1), and CA125, suggestive that the tumor is not mesothelial in origin. Wilms’ tumor (WT1) protein is detected immunohistochemically in 90% of cases.85 Most other common immunohistochemical stains lack reactivity in most cases. Electron microscopic examination shows the tumor cells have mesenchymal–fibroblastic features.
Desmoplastic small round cell tumor exhibits a reciprocal translocation t(11;22)(p13;q12), resulting in fusion of the EWS1 gene on chromosome 22 and the Wilms’ tumor suppressor gene (WT1) on chromosome 11, which appears to be unique for this tumor.86 The translocation results in a loss of three specific amino acids87 and appears to have an oncogenic effect. Other translocation patterns have also been described.86,88 The fusion protein that is produced seems to function as a potent activator of transcription, suggesting that the Wilms’ tumor gene gains function as a result of the fusion. Thus, the fusion gene seems to function as a dominant oncogene in this disease.89
Solitary Fibrous Tumor of Peritoneum (‘Fibrous Mesothelioma’)
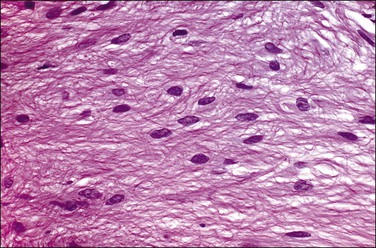
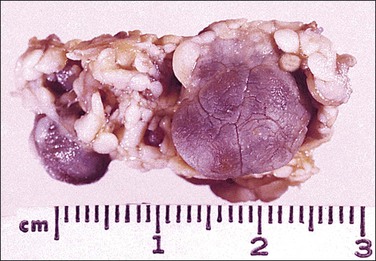
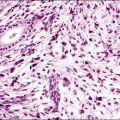
Solitary fibrous tumors, previously called ‘fibrous mesotheliomas,’ are primitive tumors composed of fibroblasts and primitive mesenchymal cells that can manifest multidirectional differentiation. They are rare tumors found most often in the pleura,90 but do occur occasionally in the peritoneum.91,92 Most patients remain well after tumor excision, although occasional neoplasms have acted aggressively.
Grossly, the tumors vary in size from 1 cm to over 20 cm in diameter (Figure 31.31). They are usually solitary and appear encapsulated by fibrous tissue. Microscopically, tumors are composed of spindle cells in a markedly collagenized stroma, often with abundant blood vessels, in a hemangiopericytoma-like pattern (Figure 31.32). Tumor cells may have fascicular, cord-like, and irregular arrangements and are found interspersed in strands in between thick collagen bundles. Nuclei are often vesicular and the nucleoli inconspicuous. Mitoses are rare.
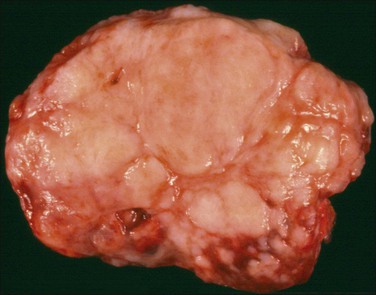
Figure 31.31 Solitary benign fibrous mesothelioma (‘solitary fibrous tumor’). The cut surface appears multinodular and fleshy.
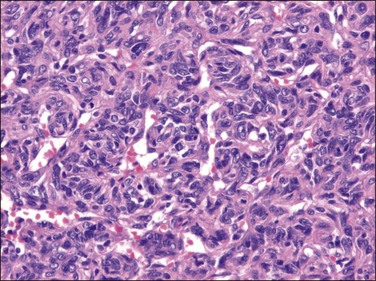
Figure 31.32 Solitary benign fibrous mesothelioma (‘solitary fibrous tumor’). The tumor is composed of spindle cells arranged in a hemangiopericytoma-like pattern.
Tumor cells are reactive for vimentin but not for CK. CD34, a sialylated transmembrane glycoprotein found initially in endothelial cells and myeloid progenitor cells, is usually demonstrable.90 CD31, a platelet endothelial cell adhesion molecule, is not. In contrast, desmoplastic mesotheliomas, tumors in the differential diagnosis, are reactive for CK but not for CD34.93
Inflammatory Myofibroblastic Tumor
This lesion has also been referred to as inflammatory pseudotumor94 or plasma cell granuloma.95 Most tumors arise in the lung, mesentery, omentum, or retroperitoneum. The abdominal lesions are usually found in the mesentery of patients younger than 20 years of age who present with a mass, fever, weight loss, anemia, thrombocytosis, and polyclonal hypergammaglobulinemia. Microscopic examination reveals myofibroblastic spindle cells, mature plasma cells, and small lymphocytes. The spindle cells often show positive cytoplasmic immunoreactivity for ALK-1, with associated chromosomal translocations detected in approximately 50% of cases. Inflammatory myofibroblastic tumors are regarded as neoplasms of low-grade or intermediate biologic behavior, which can be associated with favorable outcome, but have a tendency for local recurrence and a low risk of distant metastasis. ALK-negative tumors are more likely to be associated with metastases.96
Other Tumors
A variety of tumors arise rarely in the peritoneum. Their histogenesis is not always certain. Of the less rare tumors, adenosarcomas are often associated with endometriosis.97 Carcinosarcomas have also been described,98 as have the stromal sarcomas99 and rhabdomyosarcomas.100 Pure epithelial tumors, such as clear cell adenocarcinoma, have been described, also in association with endometriosis.97 Any tumor usually associated with an endometrial origin could well have arisen in extrauterine endometriosis. This subject is discussed more fully in Chapter 22.
Metastatic Tumors
Pseudomyxoma Peritonei
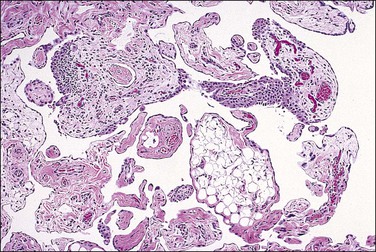
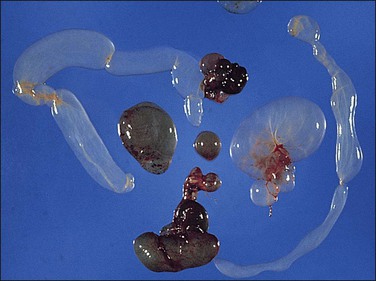
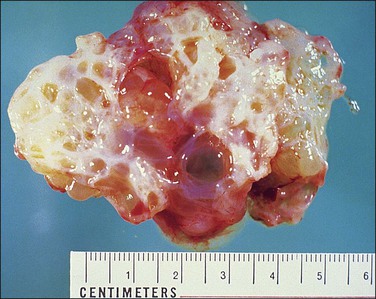
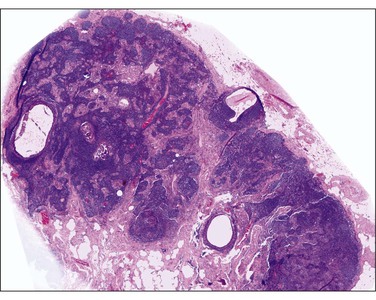
Pseudomyxoma peritonei is a clinical term that refers to the accumulation of jelly-like mucus in the pelvis or abdominal cavity (‘gelatinous ascites’) resulting from peritoneal spread of a low-grade mucinous tumor, usually of the appendix and less commonly of other intestinal locations.101 Unilateral or bilateral ovarian involvement is common in these cases (Figure 31.33). The ovarian and appendiceal tumors may present simultaneously or metachronously.
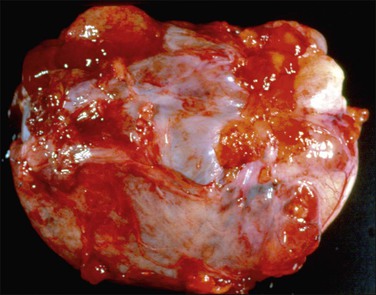
Figure 31.33 Mucinous cystic ovarian tumor associated with pseudomyxoma peritonei and a similar appendiceal tumor. Note the presence of mucin deposits on the surface of the cyst. (Reproduced with permission from Prat J. Pathology of the ovary. Philadelphia: Saunders; 2004. p. 83–109.)
Pathology
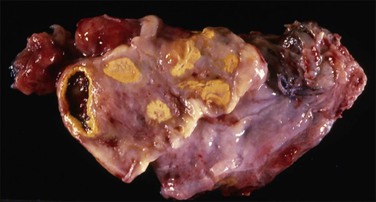
Pseudomyxoma peritonei is a disease of MUC2-expressing goblet cells, which secrete voluminous quantities of mucin in a ratio of mucin : cells exceeding 10 : 1.102 During the operation, it is critical for the surgeon to inspect and remove the appendix. Usually, the appendix will be enlarged (Figure 31.34) or adherent to the omentum, but in some cases it appears grossly normal and the primary tumor is found only after a thorough histologic evaluation (Figure 31.35).
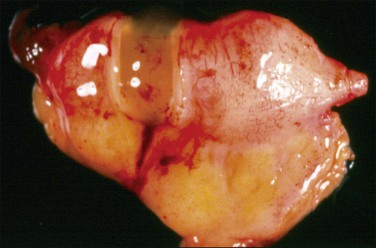
Figure 31.34 Mucinous appendiceal tumor (mucocele) associated with pseudomyxoma peritonei and bilateral ovarian mucinous tumors. (Reproduced with permission from Prat J. Pathology of the ovary. Philadelphia: Saunders; 2004. p. 83–109.)
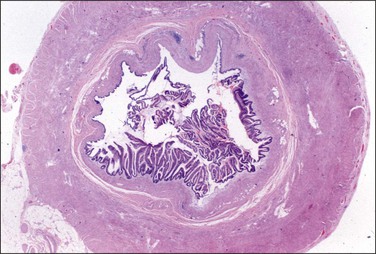
Figure 31.35 Mucinous appendiceal tumor associated with pseudomyxoma peritonei and bilateral ovarian mucinous tumors. (Reproduced with permission from Prat J. Ovarian tumors of borderline malignancy (tumors of low malignant potential): a critical appraisal. Adv Anat Pathol 1999;6:247–74.)
The mucinous deposits may have several histologic appearances.103–106 The mucus may be acellular (‘mucinous ascites’) or may contain mucinous epithelial cells (Figure 31.36). The mucinous material often contains inflammatory cells, mesothelial cells, and, if present for some time, may display capillaries and fibroblasts indicating that organization has occurred. If epithelial cells are present, the degree of nuclear atypia (variously described as low grade or high grade, or alternatively as benign, borderline, or malignant) should be indicated in the report, as well as whether the mucin dissects into tissues with a fibrous response or is merely on the surface. The finding of occasional mitoses or lack of cytoplasmic mucin suggests that the tumor is of at least borderline malignancy. Alternatively, the presence of cribriform pattern or signet-ring cells warrant a diagnosis of adenocarcinoma.103
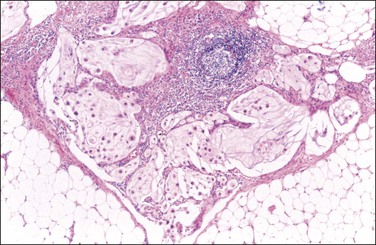
Figure 31.36 Pseudomyxoma peritonei associated with mucinous tumors of the appendix and ovaries. Note the presence of tumor cells floating in pools of mucin dissecting through the fat. (Reproduced with permission from Prat J. Pathology of the ovary. Philadelphia: Saunders; 2004. p. 83–109.)
Patients in whom the tumor appears benign (Figures 31.37–31.39) or borderline (peritoneal ‘adenomucinosis’)103 usually have a more favorable clinical course than those in whom the tumor appears histologically malignant (peritoneal carcinomatosis).107,108 Nevertheless, the former tumors may lead to significant morbidity and mortality (10 year survival rate of 45%)105 and their designation as low-grade mucinous carcinomas has recently been proposed.103
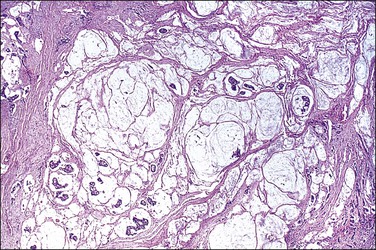
Figure 31.37 Pseudomyxoma peritonei. Multiple clusters and tumor cells are present in the mucinous material.
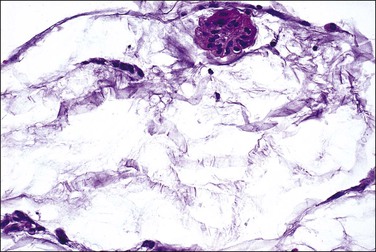
Figure 31.38 Pseudomyxoma peritonei, high magnification. Clusters of tumor cells are present in the mucin.
The secondary ovarian tumors are commonly bilateral and typically show surface involvement (Figure 31.40) and/or the presence of pools of mucin dissecting through the ovarian stroma (pseudomyxoma ovarii) (Figure 31.41). These features are in contrast to those of ovarian borderline tumors of intestinal type, which are usually unilateral and only occasionally associated with pseudomyxoma peritonei.109–112
Pathogenesis
The origin of pseudomyxoma peritonei has been a matter of debate.106 Historically, most of these tumors were thought to be ovarian in origin, especially when the associated ovarian tumor was of large size or had the appearance of a mucinous borderline tumor. Over two decades ago112 opinions began to shift toward the appendix as the site of origin in most cases. Based on genetic analyses, most cases are now believed to be of appendiceal origin,113 and more specifically from goblet cells expressing MUC2.114 A recent review has gone so far as to state, ‘pseudomyxoma peritonei almost never results from a ruptured primary ovarian neoplasm, but often produces secondary borderline-like ovarian tumors’109 (Figure 31.42). In an exceptional case, however, pseudomyxoma peritonei can arise from the rupture of a mucinous tumor of intestinal type that has arisen in ovarian teratomas.
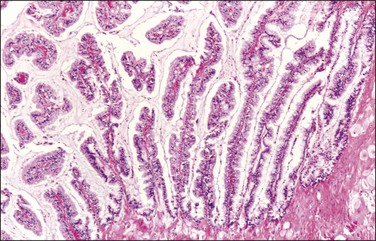
Figure 31.42 Mucinous ovarian tumor associated with pseudomyxoma peritonei and a similar appendiceal tumor. The tumor resembles a mucinous borderline tumor of the ovary. (Reproduced with permission from Cuatrecasas et al.)
Specific CK panels have been employed to distinguish ovarian mucinous tumors from gastrointestinal mucinous tumors. Tumors of müllerian origin are usually reactive for CK7 but not CK20, whereas tumors of lower intestinal origin have findings that generally are reversed, i.e., CK20 reactivity but generally not for CK7.102,115–118 Most cases of pseudomyxoma peritonei show reaction patterns consistent with an appendiceal origin.111,119
Molecular genetic studies in synchronous ovarian and appendiceal tumors associated with pseudomyxoma peritonei have revealed a concordance of K-ras mutational pattern in both tumors in each patient (Figure 31.43).113 These findings suggest their clonal nature and supports that, in the light of the clinicopathologic data, the appendix is the most likely origin.113
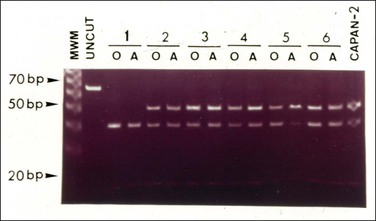
Figure 31.43 Mucinous ovarian tumors associated with mucinous tumors of the appendix and pseudomyxoma peritonei. Mutational pattern studied by RFLP-PCR. MWM, molecular weight marker, 10 bp DNA Ladder (Life Technologies Inc., Gaithersburg, MD); UNCUT, undigested 65 bp DNA amplified PCT product. Lane 15, CAPAN 2: positive control (CAPAN 2 cell line). Cases 1–6 are underlined. The mutational band (52 bp) for codon samples in five of the six cases (cases 2–6). The 40 bp fragment represents the normal allele. (Reproduced with permission from Cuatrecasas et al.)
Prognosis and Treatment
Patients with pseudomyxoma peritonei containing epithelial cells that are benign or borderline appearing usually have a protracted clinical course. The 5 and 10 year survival rates are 75% and 68%, respectively. In contrast, when the epithelial cells of the pseudomyxoma peritonei appear malignant (peritoneal carcinomatosis), the clinical course is more aggressive and approximately 90% of patients die within 3 years. Cytoreductive surgery at initial presentation and repeated palliative debulking, mucolytic agents, chemotherapy and/or radiotherapy have done relatively little to modify the natural history of this disease.
Gliomatosis Peritonei
Gliomatosis peritonei is a rare condition in which peritoneal implants composed largely or exclusively of fully mature glial tissue are found in the abdominal cavity, usually in association with a solid ovarian teratoma,120–122 which can be mature or immature.123 Tears in the capsule of the ovarian tumor have been identified, suggesting a mechanism by which the gliomatous tissue leaks into the abdominal cavity (Figure 31.44). Nevertheless, a molecular genetic study has suggested that glial implants may also arise by metaplasia of pluripotent peritoneal stem cells.124
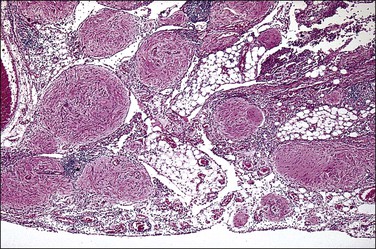
Figure 31.44 Gliomatosis peritonei. Multiple nodules of mature glial tissue are implanted within omental adipose tissue.
At the time of laparotomy, either when the ovarian tumor is discovered or subsequently, implants in the peritoneum are found to be composed of glial tissue only (Figures 31.44 and 31.45). Occasionally, other teratomatous elements are identified. Microscopic implants should be graded separately from the ovarian tumor, and this will determine whether subsequent therapy is needed. Usually, the implants are grade 0 or 1 (Figure 31.45). In some cases, they are grade 2 or 3. Although most patients with this condition do well, recurrences have been recorded125 as well as subsequent malignant transformation.126 This condition is described more fully in the section on ovarian teratomas in Chapter 29. Gliomatosis peritonei has also been reported in a patient with a ventriculoperitoneal shunt.127
Strumosis Peritonei
Strumosis peritonei is a rare condition in which nodules found singly or throughout the omentum are composed largely of well-differentiated thyroid tissue. The lesion most likely represents a metastatic or implanted form of malignant struma ovarii. Most cases occur in association with a solid ovarian teratoma or a struma ovarii. Nodules may be several millimeters to centimeters in size and grossly, on cut section, resemble colloid (Figure 31.46). Microscopically, the thyroid tissue may resemble a macrofollicular adenoma (Figure 31.47). Like gliomatosis peritonei, a defective capsule has been found in most cases with ‘implants’ from the ovary, suggesting a mechanism of spread into the abdominal cavity. While some patients with the condition do well, recurrence is unpredictable and some patients have had a clinically more aggressive course. This condition is described more fully on ovarian teratomas in Chapter 29.
Lesions of the Secondary Müllerian System
Lesions of the secondary müllerian system include those containing endometrioid, serous, and mucinous epithelium, simulating normal or neoplastic endometrial, tubal, and endocervical epithelium. Proliferation of the subjacent mesenchyme may accompany epithelial differentiation of the mesothelium or may give rise to a variety of pure mesenchymal lesions composed of endometrial stromal-type cells, decidua, or smooth muscle. Microscopically, these peritoneal lesions exhibit müllerian differentiation and share an origin from the so-called secondary müllerian system (Figure 31.48).1 The müllerian potential of this layer is consistent with its close embryonic relation to the müllerian ducts, which arise by invagination of the celomic epithelium. The origin of many of these lesions, however, is not known with certainty, and other proposed histogenetic mechanisms are discussed in Chapter 22.
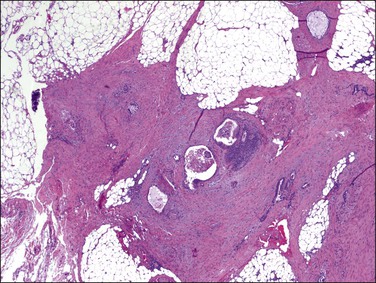
Figure 31.48 Müllerianosis. Numerous irregular müllerian glands surrounded by a prominent myofibroblastic reaction are seen in the omentum. The lesion resembles metastatic adenocarcinoma. The patient had bilateral mucinous müllerian (endocervical-type) borderline tumors of the ovary.
Endometriosis
Endometriosis is a disease principally involving the peritoneal cavity (see Chapter 22).
Endosalpingiosis
Endosalpingiosis is the presence of benign glands lined by tubal-type epithelium involving the peritoneum, subperitoneal tissues, ovarian surface, and retroperitoneal lymph nodes. This disorder occurs almost exclusively in females, typically during their reproductive years, although occasional cases have been described in postmenopausal women. Endosalpingiosis is almost always an incidental finding at laparotomy and some cases accompany endometriosis in laparoscopic biopsies of women being investigated for infertility.128
An origin from the secondary müllerian system is favored by most investigators, but the association of endosalpingiosis with chronic salpingitis implicates implantation of sloughed tubal epithelium as a possible histogenetic mechanism in some cases.129 Similarly, its association with serous borderline tumors suggests that some foci of endosalpingiosis may represent tumor implants that have undergone maturation.130,131
At the time of second-look laparotomy, endosalpingiosis in the absence of residual tumor does not justify additional treatment.132
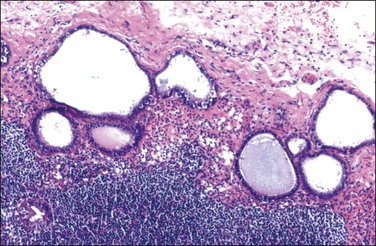
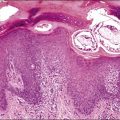
Endosalpingiosis, if seen macroscopically, may appear as a focal granularity, with a single to few tiny bumps or cysts. Microscopically, it appears as multiple, simple glands, lined by a single layer of tubal-like epithelium (Figure 31.49) exhibiting pale ciliated cells, secretory cells, and intercalated or ‘peg’ cells; i.e., the three cell types of the normal epithelium of the fallopian tube. Nuclei are basally situated, mitotic activity is absent, and there is no nuclear atypia. Psammoma bodies are often present within the glandular lumens or in the surrounding stroma. If the epithelium is destroyed, the psammoma bodies may be found free in the stroma. The glands are surrounded by PAS-positive basement membranes and show PAS-positive, diastase-resistant material in their lumens. Immunoreactions for estrogen and progesterone receptors are usually positive.
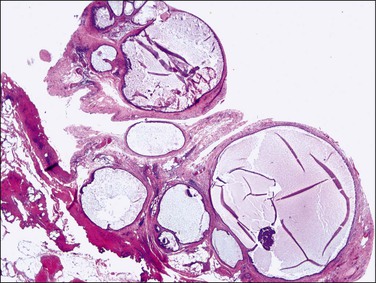
Figure 31.49 Endosalpingiosis. Numerous cystic glands lined by tubal epithelium appear in the serosa of the bladder.
The glands of endosalpingiosis may show irregular contours, crowding, and intraluminal stromal papillae. Occasionally, the process may be so extensive as to mimic a neoplasia and the glands may be larger, exhibiting architectural complexity; however, the cells lack significant nuclear atypia (Figure 31.50).
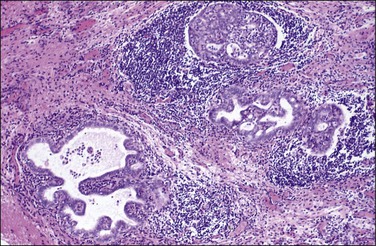
Figure 31.50 Florid endosalpingiosis mimicking adenocarcinoma. The serous glands show architectural complexity but the cells lack significant nuclear atypia.
The term atypical endosalpingiosis refers to lesions in which there is cellular stratification, including cellular buds, cribriform patterns, and varying degrees of cellular atypia, occurring in the absence of a serous borderline tumor. Histologically, such lesions merge with peritoneal serous borderline tumors (see Chapter 25).133 Not uncommonly, the serous foci exhibit more epithelial proliferation than would be expected in benign lesions. The criteria used for assessing whether the excessive growth reflects neoplasms of borderline malignancy are identical to those for serous tumors in general and are summarized in Table 31.3. Rare extraovarian borderline and malignant serous tumors have been shown to arise from endosalpingiosis.65,134
Table 31.3
Features Distinguishing Endosalpingiosis from Serous Borderline Tumor of the Peritoneum
| Endosalpingiosis | Serous Borderline Tumor (Primary and Implanted) | |
| Location | Surface and subperitoneal | Surface and subperitoneal |
| Architecture | Simple round or oval gland | Simple glands, often with focal or complex papillary epithelial tufts, detached cell clusters, and psammoma bodies |
| Cytology | No atypia | Mild-to-severe atypia and often cellular stratification |
| Stromal reaction | None to focal hyalinization | None to exuberant desmoplasia |
Intranodal Glands of Müllerian Type (Müllerian Inclusion Cysts)
Benign-appearing glands of müllerian type or ‘müllerian inclusion cysts’ may be found in pelvic and para-aortic lymph nodes of females,135,136 and less frequently in inguinal and femoral lymph nodes.131 These glands are almost always incidental microscopic findings in lymph nodes removed in cases of pelvic carcinoma; therefore, their reported frequency varies from 2% to 41%, depending upon the number of lymph nodes removed and the extent of the microscopic sampling.131 These patients often have endosalpingiosis of the peritoneum, acute and chronic salpingitis,136 or coexistent ovarian serous tumors, which may be benign, borderline tumors, or carcinomas.137
Müllerian inclusion cysts are not grossly identifiable as such. The glands are usually located in the periphery of the node, most commonly within the capsule or between cortical lymphoid follicles. Histologically, the glands are identical to those of endosalpingiosis and exhibit a single layer of müllerian-type epithelium, generally with prominent cilia. Cells are cytologically bland and cuboidal to columnar. Nuclear contours are regular, the chromatin is even, and nucleoli are rarely seen. Mitoses are not identified. Intraglandular or periglandular psammoma bodies are commonly found. In rare cases, the cells can show nuclear atypia and stratification; the latter can produce an intraglandular cribriform pattern. These cases of atypical intranodal endosalpingiosis may occasionally be the site of origin of serous tumors.138–140 In fact, müllerian inclusion cysts are more common in women with serous borderline tumors and low-grade serous carcinomas than in patients with high-grade serous carcinomas.131
Intranodal inclusion cysts lined by benign endometrioid epithelium, mucinous epithelium of endocervical or goblet cell type, or metaplastic squamous epithelium have also been reported1,141,142 (Figures 31.51 and 31.52).
Differential Diagnosis
In most cases, the distinction between glandular inclusions and metastatic adenocarcinoma is not difficult. However, if an ovarian serous borderline tumor is present differential diagnosis may be difficult or even impossible. The capsular location of the glands, the presence of ciliated cells, the lack of severe nuclear atypia and mitotic activity, and the absence of a fibroblastic stromal reaction favor a benign diagnosis, i.e., endosalpingiosis. However, as indicated above, serous borderline tumors or low-grade serous carcinomas may occasionally originate within pelvic or para-aortic lymph nodes.138–140 This diagnosis is suggested in cases in which the intranodal tumor merges with foci of atypical endosalpingiosis.
Endocervicosis
Benign glands of endocervical type involving the peritoneum have been described as ‘endocervicosis.’ Nearly all reported cases involve the peritoneum overlying the posterior uterine serosa, cul-de-sac, vaginal apex, outer wall of the uterine cervix, and the urinary bladder.143,144 Microscopically, the glands are located predominantly within the smooth muscle of the muscularis propria and mimic invasive well-differentiated adenocarcinoma. The presence of mild epithelial atypia and reactive periglandular stroma contributes to this misdiagnosis. However, the absence of a mucosal-based tumor and severe nuclear atypia facilitate the diagnosis of endocervicosis.143,145
Deciduosis
If seen grossly, the nodules are tan to pale brown, slightly gelatinous, and rarely more than several millimeters. Occasionally, the lesions may reach several centimeters in size and consist of multiple, tiny, soft nodules separated by thin, white, rubbery septa.146
The decidual reaction most often appears as solid clusters of cohesive, decidualized cells with sharp cell borders (Figure 31.53). Cytoplasm may be abundant and glass like or may show some degrees of degeneration (Figure 31.54). Some cells have a clear vacuolated cytoplasm that superficially resembles the soap bubble-like physaliferous cell of chordoma (Figure 31.54). Some nodules may also show transitions between a more solid and a more myxoid pattern of decidual reaction (Figure 31.55).
Disseminated Peritoneal Leiomyomatosis
Disseminated peritoneal leiomyomatosis is a rare condition characterized by widespread nodules of benign smooth muscle on the peritoneal surface of the pelvis and abdomen in women of reproductive age (see Chapter 19). Most patients have uterine leiomyomas at the time of diagnosis.147–149 The most common presentation is as an incidental finding at the time of caesarean section. The intraoperative appearance is so alarming that frozen section examination is often requested to rule out peritoneal carcinomatosis. The peritoneal myomatous nodules develop on a background of an altered hormonal milieu, such as with pregnancy, oral contraceptive use,150 hormonal therapy, or steroid-producing ovarian tumors.151 While this lesion is often classified as a tumor-like condition, since it commonly regresses, the tumors in some cases persist, suggesting a neoplastic process. On rare occasions, some have shown malignant transformation.152–154
Pathology
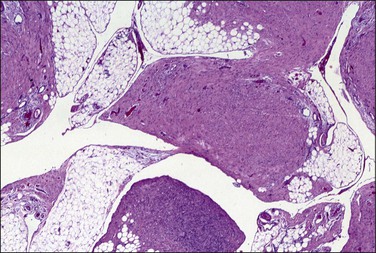
Grossly, multiple gray-white nodules that may be granular to several millimeters in diameter are found covering the peritoneal surfaces of the pelvis, pelvic organs, intestines, and omentum. Microscopically, the nodules consist of smooth muscle arranged like leiomyomas (Figure 31.56). Cells usually lack atypia and mitotic activity, and only rarely have features of malignancy. In many cases, the tumor is admixed with stromal cells resembling decidua (Figure 31.57). Smooth muscle cells are markedly reactive for desmin and muscle-specific antigens, CD10, ER, and PR, but without reactivity for keratin. Although the etiology of disseminated peritoneal leiomyomatosis is unknown, this immunohistochemical profile has been used to argue in support of the hypothesis that the condition arises from multicentric differentiation of submesothelial stem cells. Ultrastructural examination discloses myofilamentous bundles with focal electron-dense bodies typical of smooth muscle cell differentiation.
Pathogenesis
Progesterone stimulation appears to be critical in the development of these tumors in nearly all cases. They are almost always strongly reactive for PRs and usually reactive for ERs although with less intensity.155 In postpartum women, ER reactivity in decidualized nodules is either weak or absent.156 Such an immunohistochemical profile might be expected, since ovarian and placentally derived progesterone is critical in transforming endometrial stromal cells into decidua during pregnancy and since foci of decidual cells are found with the smooth muscle cells.
Regression has been documented in cases where excess hormonal stimulation has been removed, e.g., with the cessation of oral contraceptives, oophorectomy, or following childbirth.151 Based on the earlier observations, the general view has been that disseminated peritoneal leiomyomas are metaplastic in origin and hormonally responsive. Yet, rather than this lesion being polyclonal, one study in which 42 leiomyomatous lesions from four patients were studied found that the leiomyomas were monoclonal, which strongly suggests that the lesions are neoplastic and from the same precursor lesion.157
Other conditions also give rise to one or more peritoneal leiomyomas. Uterine subserosal leiomyomas may become detached and implant elsewhere on the peritoneum (parasitic leiomyomas). Leiomyomas arising in the deep retroperitoneal–abdominal soft tissue can involve the peritoneum.158,159 Like uterine leiomyomas, they can be hormonally reactive, which is unlike leiomyomas of deep somatic soft tissue, e.g., extremities, which are hormonally unreactive.160
Disseminated peritoneal leiomyomatosis may regress spontaneously or after therapy with GnRH agonist. Five cases of malignant form have been reported.152
References
1. Lauchlan, SC. The secondary mullerian system revisited. Int J Gynecol Pathol. 1994; 13:73–79.
2. Clement, PB. Reactive tumor-like lesions of the peritoneum. Am J Clin Pathol. 1995; 103:673–676.
3. Koc, S, Beydilli, G, Tulunay, G, et al. Peritoneal tuberculosis mimicking advanced ovarian cancer: a retrospective review of 22 cases. Gynecol Oncol. 2006; 103:565–569.
4. Abraham, G, Mathews, M, Sekar, L, et al. Tuberculous peritonitis in a cohort of continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 2001; 21(Suppl):S202–S204.
5. Groutz, A, Carmon, E, Gat, A. Peritoneal tuberculosis versus advanced ovarian cancer: a diagnostic dilemma. Obstet Gynecol. 1998; 91(5 Pt 2):868.
6. Piura, B, Rabinovich, A, Leron, E, et al. Peritoneal tuberculosis—an uncommon disease that may deceive the gynecologist. Eur J Obstet Gynecol Reprod Biol. 2003; 110:230–234.
7. Bernaciak, J, Spina, JC, Curros, ML, et al. Case report: peritoneal sarcoidosis in an unusual location. Semin Respir Crit Care Med. 2002; 23:597–600.
8. Bourdillon, L, Lanier-Gachon, E, Stankovic, K, et al. Lofgren syndrome and peritoneal involvement by sarcoidosis—case report. Chest. 2007; 132:310–312.
9. Rosen, DMB, Lam, AM, Carlton, MA, Cario, GM. The safety of laparoscopic treatment for ovarian dermoid tumours. Aust N Z J Obstet Gynaecol. 1998; 38:77–79.
10. Reich, O, Kometter, R, Pickel, H. Chronic sclerosing peritonitis after spontaneous rupture of a cystic teratoma: a pitfall in surgical staging of ovarian tumours. Geburt Frauenheil. 1999; 59:94–95.
11. Wu, TI, Chang, TC, Hsueh, S, Lai, CH. Ovarian endometrioid carcinoma with diffuse pigmented peritoneal keratin granulomas: a case report and review of the literature. Int J Gynecol Cancer. 2006; 16:426–429.
12. Davis, JR, Miller, HS, Feng, JD. Vernix caseosa peritonitis: report of two cases with antenatal onset. Am J Clin Pathol. 1998; 109:320–323.
13. Tawfik, O, Prather, J, Bhatia, P, et al. Vernix caseosa peritonitis as a rare complication of cesarean section. A case report. J Reprod Med. 1998; 43:547–550.
14. George, E, Leyser, S, Zimmer, HL, et al. Vernix caseosa peritonitis. An infrequent complication of cesarean section with distinctive histopathologic features. Am J Clin Pathol. 1995; 103:681–684.
15. Mahmoud, A, Silapaswan, S, Lin, K, Penney, D. Vernix caseosa: an unusual cause of post-cesarean section peritonitis. Am Surg. 1997; 63:382–385.
16. Jaworski, RC, Boadle, R, Greg, J, Cocks, P. Peritoneal ‘melanosis’ associated with a ruptured ovarian dermoid cyst: report of a case with electron-probe energy dispersive x-ray analysis. Int J Gynecol Pathol. 2001; 20:386–389.
17. Carey, M, Kirk, ME. Necrotic pseudoxanthomatous nodules of the omentum and peritoneum–a peculiar reaction to endometriotic cyst contents. Obstet Gynecol. 1993; 82(4 Part 2):650–652.
18. Seidman, JD, Oberer, S, Bitterman, P, Aisner, SC. Pathogenesis of pseudoxanthomatous salpingiosis. Mod Pathol. 1993; 6:53–55.
19. Dehner, LP, Coffin, CM. Idiopathic fibrosclerotic disorders and other inflammatory pseudotumors. Semin Diagn Pathol. 1998; 15:161–173.
20. Frigerio, L, Taccagni, GL, Mariani, A, et al. Idiopathic sclerosing peritonitis associated with florid mesothelial hyperplasia, ovarian fibromatosis, and endometriosis: a new disorder of abdominal mass. Am J Obstet Gynecol. 1997; 176:721–722.
21. Stenram, U. Sclerosing peritonitis in a case of benign cystic ovarian teratoma. A case report. APMIS. 1997; 105:414–416.
22. Afthentopoulos, IE, Passadakis, P, Oreopoulos, DG. Sclerosing peritonitis in continuous ambulatory peritoneal dialysis patients: one center’s experience and review of the literature. Adv Ren Replace Ther. 1998; 5:157–167.
23. Cancarini, GC, Sandrini, M, Vizzardi, V, et al. Clinical aspects of peritoneal sclerosis. J Nephrol. 2001; 14(Suppl):S39–S47.
24. Di Paolo, N, Garosi, G. Peritoneal sclerosis. J Nephrol. 1999; 12:347–361.
25. Garosi, G, Di Paolo, N, Sacchi, G, Gaggiotti, E. Sclerosing peritonitis: a nosological entity. Perit Dial Int. 2005; 25(Suppl):S110.
26. Krediet, RT, Zweers, MM, van Westrhenen, R, et al. What can we do to preserve the peritoneum? Perit Dial Int. 2003; 23(Suppl):S14–S19.
27. Clement, PB, Young, RH, Hanna, W, Scully, RE. Sclerosing peritonitis associated with luteinized thecomas of the ovary. A clinicopathological analysis of six cases. Am J Surg Pathol. 1994; 18:1–13.
28. Iwasa, Y, Minamiguchi, S, Konishi, I, et al. Sclerosing peritonitis associated with luteinized thecoma of the ovary. Pathol Int. 1996; 46:510–514.
29. Nishida, T, Ushijima, K, Watanabe, J, et al. Sclerosing peritonitis associated with luteinized thecoma of the ovary. Gynecol Oncol. 1999; 73:167–169.
30. Spiegel, GW, Swiger, FK. Luteinized thecoma with sclerosing peritonitis presenting as an acute abdomen. Gynecol Oncol. 1996; 61:275–281.
31. Werness, BA. Luteinized thecoma with sclerosing peritonitis. Arch Pathol Lab Med. 1996; 120:303–306.
32. Yantiss, RK, Nielsen, GP, Lauwers, GY, et al. Reactive nodular fibrous pseudotumor of the gastrointestinal tract and mesentery. Am J Surg Pathol. 2003; 27:532–540.
33. Clement, PB, Young, RH. Florid mesothelial hyperplasia associated with ovarian tumors—a potential source of error in tumor diagnosis and staging. Int J Gynecol Pathol. 1993; 12:51–58.
34. Kerner, H, Gaton, E, Czernobilsky, B. Unusual ovarian, tubal and pelvic mesothelial inclusions in patients with endometriosis. Histopathology. 1981; 5:277–282.
35. Rosai, J, Dehner, LP. Nodular mesothelial hyperplasia in hernia sacs. A benign reactive condition stimulating a neoplastic process. Cancer. 1975; 35:165–175.
36. Clement, PB, Young, RH, Oliva, E, et al. Hyperplastic mesothelial cells within abdominal lymph nodes: mimic of metastatic ovarian carcinoma and serous borderline tumor—a report of two cases associated with ovarian neoplasms. Mod Pathol. 1996; 9:879–886.
37. Knudsen, PJ. The peritoneal elastic lamina. J Anat. 1991; 177:41–46.
38. Churg, A, Cagle, PT, Roggli, VL. Tumors of the serosal membranes. Atlas of tumor pathology, ser IV. Washington, DC: Armed Forces Institute of Pathology; 2006.
39. Kafiri, G, Thomas, DM, Shepherd, NA, et al. p53 expression is common in malignant mesotheliomas. Histopathology. 1992; 21:331–334.
40. Henderson, DW, Shilkin, KB, Whitaker, D. Reactive mesothelial hyperplasia vs. mesothelioma, including mesothelioma in situ. Am J Clin Pathol. 1998; 110:397–404.
41. Taheri, ZM, Mehrafza, M, Mohammadi, F, et al. The diagnostic value of Ki-67 and repp 86 in distinguishing between benign and malignant mesothelial proliferations. Arch Pathol Lab Med. 2008; 132:694–697.
42. Padmanabhan, V, Mount, SL, Eltabbakh, GH. Peritoneal atypical mesothelial proliferation with progression to invasive mesothelioma: a case report and review of the literature. Pathology. 2003; 35:260–263.
43. Lamovec, J, Sinkovec, J. Multilocular peritoneal inclusion cyst (multicystic mesothelioma) with hyaline globules. Histopathology. 1996; 28:466–469.
44. Sawh, RN, Malpica, A, Deavers, MT, et al. Benign cystic mesothelioma of the peritoneum: a clinicopathologic study of 17 cases and immunohistochemical analysis of estrogen and progesterone receptor status. Hum Pathol. 2003; 34:369–374.
45. Ross, MJ, Welch, WR, Scully, RE. Multilocular peritoneal inclusion cysts (so-called cystic mesotheliomas). Cancer. 1989; 64:1336–1346.
46. Kuga, T, Esato, K, Takeda, K, et al. A supernumerary ovary of the omentum with cystic change: report of two cases and review of the literature. Pathol Int. 1999; 49:566–570.
47. Sarraf, KM, Abdalla, M, Al-Omari, O, Sarraf, MG. Diagnostic difficulties of pelvic splenosis: case report. Ultrasound Obstet Gynecol. 2006; 27:220–221.
48. Lim, C, McIlroy, K, Briggs, G, Tan, L. Splenosis mimicking lymphoma. Pathology. 2007; 39:183–185.
49. Peitsidis, P, Akrivos, T, Vecchini, G, et al. Splenosis of the peritoneal cavity resembling an adnexal tumor: case report. Clin Exp Obstet Gynecol. 2007; 34:120–122.
50. Vydianath, B, Gurumurthy, M, Crocker, J. Solitary ovarian splenosis. J Clin Pathol. 2005; 58:1224–1225.
51. Rehbock, J, Dimpfl, T, Assemi, C. Disseminated peritoneal trophoblastic implants after surgery of tubal pregnancies—a typical complication of the laparoscopic technique? Geburt Frauenheil. 1997; 57:155–157.
52. Tsutsumi, O, Ando, K, Momoeda, M. Ruptured isthmal pregnancy following laparoscopic salpingostomy in the ipsilateral tube. Int J Gynecol Obstet. 1997; 57:187–189.
53. Ghosh, P, Strong, C, Naugler, W, et al. Peritoneal mice implicated in intestinal obstruction—report of a case and review of the literature. J Clin Gastroenterol. 2006; 40:427–430.
54. Vuong, PN, Guyot, H, Moulin, G, et al. Pseudotumoral organization of a twisted epiploic fringe or ‘hard-boiled egg’ in the peritoneal cavity. Arch Pathol Lab Med. 1990; 114:531–533.
55. Goldblum, J, Hart, WR. Localized and diffuse mesotheliomas of the genital tract and peritoneum in women—a clinicopathologic study of nineteen true mesothelial neoplasms, other than adenomatoid tumors, multicystic mesotheliomas, and localized fibrous tumors. Am J Surg Pathol. 1995; 19:1124–1137.
56. Baker, PM, Clement, PB, Young, RH. Malignant peritoneal mesothelioma in women – a study of 75 cases with emphasis on their morphologic spectrum and differential diagnosis. Am J Clin Pathol. 2005; 123:724–737.
57. Butnor, KJ, Sporn, TA, Hammar, SP, Roggli, VL. Well-differentiated papillary mesothelioma. Am J Surg Pathol. 2001; 25:1304–1309.
58. Hoekstra, AV, Riben, MW, Frumovitz, M, et al. Well-differentiated papillary mesothelioma of the peritoneum: a pathological analysis and review of the literature. Gynecol Oncol. 2005; 98:161–167.
59. Davidson, B, Risberg, B, Berner, A, et al. The biological differences between ovarian serous carcinoma and diffuse peritoneal malignant mesothelioma. Semin Diagn Pathol. 2006; 23:35–43.
60. Kerrigan, SAJ, Turnnir, RT, Clement, PB, et al. Diffuse malignant epithelial mesotheliomas of the peritoneum in women – a clinicopathologic study of 25 patients. Cancer. 2002; 94:378–385.
61. Hemminki, K, Li, XJ. Time, trends and occupational risk factors for peritoneal mesothelioma in Sweden. J Occup Environ Med. 2003; 45:451–455.
62. Cerruto, CA, Brun, EA, Chang, D, Sugarbaker, PH. Prognostic significance of histomorphologic parameters in diffuse malignant peritoneal mesothelioma. Arch Pathol Lab Med. 2006; 130:1654–1661.
63. Shia, J, Erlandson, RA, Klimstra, DS. Deciduoid mesothelioma: a report of 5 cases and literature review. Ultrastruct Pathol. 2002; 26:355–363.
64. Ordóñez, NG. Deciduoid mesothelioma: report of 21 cases with review of the literature. Mod Pathol. 2012; 25:1481–1495.
65. Carrick, KS, Milvenan, JS, Albores-Saavedra, J. Serous tumor of low malignant potential arising in inguinal endosalpingiosis. Int J Gynecol Pathol. 2003; 22:412–415.
66. Ordonez, NG. Desmoplastic small round cell tumor: II: an ultrastructural and immunohistochemical study with emphasis on new immunohistochemical markers. Am J Surg Pathol. 1998; 22:1314–1327.
67. Feldman, AL, Libutti, SK, Pingpank, JF, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol. 2003; 15(21):4560–4567.
68. Nonaka, D, Kusamura, S, Baratti, D, et al. Diffuse malignant mesothelioma of the peritoneum—a clinicopathologic study of 35 patients treated locoregionally at a single institution. Cancer. 2005; 104:2181–2188.
69. McCaughey, WT, Colby, TV, Battifora, H, et al. Diagnosis of diffuse malignant mesothelioma: experience of a US/Canadian Mesothelioma Panel. Mod Pathol. 1991; 4:342–353.
70. Shanks, JH, Harris, M, Banerjee, SS, et al. Mesotheliomas with deciduoid morphology—a morphologic spectrum and a variant not confined to young females. Am J Surg Pathol. 2000; 24:285–294.
71. Borczuk, AC, Taub, RN, Hesdorffer, M, et al. P16 loss and mitotic activity predict poor survival in patients with peritoneal malignant mesothelioma. Clin Cancer Res. 2005; 11:3303–3308.
72. Clement, PB, Young, RH, Scully, RE. Malignant mesotheliomas presenting as ovarian masses—a report of nine cases, including two primary ovarian mesotheliomas. Am J Surg Pathol. 1996; 20:1067–1080.
73. Attanoos, RL, Webb, R, Dojcinov, SD, Gibbs, AR. Value of mesothelial and epithelial antibodies in distinguishing diffuse peritoneal mesothelioma in females from serous papillary carcinoma of the ovary and peritoneum. Histopathology. 2002; 40:237–244.
74. Comin, CE, Saieva, C, Messerini, L. h-Caldesmon, calretinin, estrogen receptor, and Ber-EP4: a useful combination of immunohistochemical markers for differentiating epithelioid peritoneal mesothelioma from serous papillary carcinoma of the ovary. Am J Surg Pathol. 2007; 31:1139–1148.
75. Gown, AM. Uses of antibody panels in the analysis of metastatic carcinomas of unknown primary. Acta Histochem Cytochem. 1999; 32:153–159.
76. Miller, RT, Immunocytochemistry of epithelial tumors 1999. ASCP National Meeting. American Society of Clinical Pathology: New Orleans, LA, 1999:1–47.
77. Ordonez, NG. Value of calretinin immunostaining in differentiating epithelial mesothelioma from lung adenocarcinoma. Mod Pathol. 1998; 11:929–933.
78. Lae, ME, Roche, PC, Jin, L, et al. Desmoplastic small round cell tumor—a clinicopathologic, immunohistochemical, and molecular study of 32 tumors. Am J Surg Pathol. 2002; 26:823–835.
79. Young, RH, Eichhorn, JH, Dickersin, GR, Scully, RE. Ovarian involvement by the intra-abdominal desmoplastic small round cell tumor with divergent differentiation: a report of three cases. Hum Pathol. 1992; 23:454–464.
80. Fukunaga, M, Endo, Y, Takaki, K, et al. Postmenopausal intra-abdominal desmoplastic small cell tumor. Pathol Int. 1996; 46:281–285.
81. Wolf, AN, Ladanyi, M, Paull, G, et al. The expanding clinical spectrum of desmoplastic small round-cell tumor: a report of two cases with molecular confirmation. Hum Pathol. 1999; 30:430–435.
82. Prat, J, Matias-Guiu, X, Algaba, F. Desmoplastic small round-cell tumor. Am J Surg Pathol. 1992; 16:306–307.
83. Gerald, WL, Ladanyi, M, de Alava, E, et al. Clinical, pathologic, and molecular spectrum of tumors associated with t(11;22)(p13;q12): desmoplastic small round-cell tumor and its variants. J Clin Oncol. 1998; 16:3028–3036.
84. Ordonez, NG. Desmoplastic small round cell tumor: I: a histopathologic study of 39 cases with emphasis on unusual histological patterns. Am J Surg Pathol. 1998; 22:1303–1313.
85. Barnoud, R, Delattre, O, Peoc’h, M, et al. Desmoplastic small round cell tumor: RT-PCR analysis and immunohistochemical detection of the Wilm’s tumor gene WT1. Pathol Res Pract. 1998; 194:693–700.
86. Ordi, J, de Alava, E, Torne, A, et al. Intraabdominal desmoplastic small round cell tumor with EWS/ERG fusion transcript. Am J Surg Pathol. 1998; 22:1026–1032.
87. Kim, J, Lee, K, Pelletier, J. The desmoplastic small round cell tumor t(11;22) translocation produces EWS/WT1 isoforms with differing oncogenic properties. Oncogene. 1998; 16:1973–1979.
88. Shimizu, Y, Mitsui, T, Kawakami, T, et al. Novel breakpoints of the EWS gene and the WT1 gene in a desmoplastic small round cell tumor. Cancer Genet Cytogenet. 1998; 106:156–158.
89. Benjamin, LE, Fredericks, WJ, Barr, FG, Rauscher, FJ, 3rd. Fusion of the EWS1 and WT1 genes as a result of the t(11;22)(p13;q12) translocation in desmoplastic small round cell tumors. Med Pediatr Oncol. 1996; 27:434–439.
90. Hanau, CA, Miettinen, M. Solitary fibrous tumor: histological and immunohistochemical spectrum of benign and malignant variants presenting at different sites. Hum Pathol. 1995; 26:440–449.
91. Fukunaga, M, Naganuma, H, Ushigome, S, et al. Malignant solitary fibrous tumour of the peritoneum. Histopathology. 1996; 28:463–466.
92. Fukunaga, M, Naganuma, H, Nikaido, T, et al. Extrapleural solitary fibrous tumor: a report of seven cases. Mod Pathol. 1997; 10:443–450.
93. Flint, A, Weiss, SW. CD-34 and keratin expression distinguishes solitary fibrous tumor (fibrous mesothelioma) of pleura from desmoplastic mesothelioma. Hum Pathol. 1995; 26:428–431.
94. Day, DL, Sane, S, Dehner, LP. Inflammatory pseudotumor of the mesentery and small intestine. Pediatr Radiol. 1986; 16:210–215.
95. Pettinato, G, Manivel, JC, De Rosa, N, et al. Inflammatory myofibroblastic tumor (plasma cell granuloma). Clinicopathologic study of 20 cases with immunohistochemical and ultrastructural observations. Am J Clin Pathol. 1990; 94:538–546.
96. Coffin, CM, Hornick, JL, Fletcher, CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007; 31:509–520.
97. Stern, RC, Dash, R, Bentley, RC, et al. Malignancy in endometriosis: frequency and comparison of ovarian and extraovarian types. Int J Gynecol Pathol. 2001; 20:133–139.
98. Shen, DH, Khoo, US, Xue, WC, et al. Primary peritoneal malignant mixed mullerian tumors—a clinicopathologic, immunohistochemical, and genetic study. Cancer. 2001; 91:1052–1060.
99. Chang, KL, Crabtree, GS, Limtan, SK, et al. Primary extrauterine endometrial stromal neoplasms—a clinicopathologic study of 20 cases and a review of the literature. Int J Gynecol Pathol. 1993; 12:282–296.
100. Kaplan, AM, Creager, AJ, Livasy, CA, et al. Intra-abdominal embryonal rhabdomyosarcoma in an adult. Gynecol Oncol. 1999; 74:282–285.
101. Fox, H. Pseudomyxoma peritonei. Br J Obstet Gynaecol. 1996; 103:197–198.
102. Guerrieri, C, Franlund, B, Fristedt, S, et al. Mucinous tumors of the vermiform appendix and ovary, and pseudomyxoma peritonei: histogenetic implications of cytokeratin 7 expression. Hum Pathol. 1997; 28:1039–1045.
103. Bradley, RF, Stewart, JH, Russell, GB, et al. Pseudomyxoma peritonei of appendiceal origin: a clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol. 2006; 30:551–559.
104. Jackson, SL, Fleming, RA, Loggie, BW, Geisinger, KR. Gelatinous ascites: a cytohistologic study of pseudomyxoma peritonei in 67 patients. Mod Pathol. 2001; 14:664–671.
105. Misdraji, J, Yantiss, RK, Graeme-Cook, FM, et al. Appendiceal mucinous neoplasms—a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003; 27:1089–1103.
106. Young, RH. Pseudomyxoma peritonei and selected other aspects of the spread of appendiceal neoplasms. Semin Diagn Pathol. 2004; 21:134–150.
107. Lee, KR, Scully, RE. Mucinous tumors of the ovary. A clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with ‘pseudomyxoma peritonei. Am J Surg Pathol. 2000; 24:1447–1464.
108. Ronnett, BM, Yan, H, Kurman, RJ, et al. Patients with pseudomyxoma peritonei associated with disseminated peritoneal adenomucinosis have a significantly more favorable prognosis than patients with peritoneal mucinous carcinomatosis. Cancer. 2001; 92:85–91.
109. Hart, WR. Mucinous tumors of the ovary: a review. Int J Gynecol Pathol. 2005; 24:4–25.
110. Prayson, RA, Hart, WR, Petras, RE. Pseudomyxoma peritonei. A clinicopathologic study of 19 cases with emphasis on site of origin and nature of associated ovarian tumors. Am J Surg Pathol. 1994; 18:591–603.
111. Ronnett, BM, Kurman, RJ, Zahn, CM, et al. Pseudomyxoma peritonei in women: a clinicopathologic analysis of 30 cases with emphasis on site of origin, prognosis, and relationship to ovarian mucinous tumors of low malignant potential. Hum Pathol. 1995; 26:509–524.
112. Young, RH, Gilks, CB, Scully, RE. Mucinous tumors of the appendix associated with mucinous tumors of the ovary and pseudomyxoma peritonei. A clinicopathological analysis of 22 cases supporting an origin in the appendix. Am J Surg Pathol. 1991; 15:415–429.
113. Cuatrecasas, M, Matias-Guiu, X, Prat, J. Synchronous mucinous tumors of the appendix and the ovary associated with pseudomyxoma peritonei: a clinicopathologic study of six cases with comparative analysis of c-Ki-ras mutations. Am J Surg Pathol. 1996; 20:739–746.
114. O’Connell, JT, Tomlinson, JS, Roberts, AA, et al. Pseudomyxoma peritonei is a disease of MUC2-expressing goblet cells. Am J Pathol. 2002; 161:551–564.
115. Loy, TS, Calaluce, RD, Keeney, GL. Cytokeratin immunostaining in differentiating primary ovarian carcinoma from metastatic colonic adenocarcinoma. Mod Pathol. 1996; 9:1040–1044.
116. Ronnett, BM, Kurman, RJ, Shmookler, BM, et al. The morphologic spectrum of ovarian metastases of appendiceal adenocarcinomas: a clinicopathologic and immunohistochemical analysis of tumors often misinterpreted as primary ovarian tumors or metastatic tumors from other gastrointestinal sites. Am J Surg Pathol. 1997; 21:1144–1155.
117. Ronnett, BM, Shmookler, B, Diener-West, M, et al. Immunohistochemical evidence supporting the appendiceal origin of pseudomyxoma peritonei in women. Int J Gynecol Pathol. 1997; 16:1–9.
118. Vang, R, Gown, AM, Barry, TS, et al. Cytokeratins 7 and 20 in primary and secondary mucinous tumors of the ovary: analysis of coordinate immunohistochemical expression profiles and staining distribution in 179 cases. Am J Surg Pathol. 2006; 30:1130–1139.
119. Ronnett, BM, Zahn, CM, Kurman, RJ, et al. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis: a clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to ‘pseudomyxoma peritonei. Am J Surg Pathol. 1995; 19:1390–1408.
120. Gocht, A, Lohler, J, Scheidel, P, Stegner, H-E. Gliomatosis peritonei combined with mature ovarian teratoma. Pathol Res Pract. 1995; 191:1029–1035.
121. Hamada, Y, Tanano, A, Sato, M, et al. Ovarian teratoma with gliomatosis peritonei: report of two cases. Surg Today. 1998; 28:223–226.
122. Nanda, S, Kalra, B, Arora, B, Singh, S. Massive mature solid teratoma of the ovary with gliomatosis peritonei. Aust N Z J Obstet Gynaecol. 1998; 38:329–331.
123. Schmidt, D, Kommoss, F. Teratoma of the ovary. Clinical and pathological differences between mature and immature teratomas. Pathologe. 2007; 28:203–208.
124. Ferguson, AW, Katabuchi, H, Ronnett, BM, Cho, KR. Glial implants in gliomatosis peritonei arise from normal tissue, not from the associated teratoma. Am J Pathol. 2001; 159:51–55.
125. Calder, CJ, Light, AM, Rollason, TP. Immature ovarian teratoma with mature peritoneal metastatic deposits showing glial, epithelial, and endometrioid differentiation—a case report and review of the literature. Int J Gynecol Pathol. 1994; 13:279–282.
126. Dadmanesh, F, Miller, DM, Swenerton, KD, Clement, PB. Gliomatosis peritonei with malignant transformation. Mod Pathol. 1997; 10:597–601.
127. Hill, DA, Dehner, LP, White, FV, Langer, JC. Gliomatosis peritonei as a complication of a ventriculoperitoneal shunt: case report and review of the literature. J Pediatr Surg. 2000; 35:497–499.
128. Jansen, RP, Russell, P. Nonpigmented endometriosis: clinical, laparoscopic, and pathologic definition. Am J Obstet Gynecol. 1986; 155:1154–1159.
129. Zinsser, KR, Wheeler, JE. Endosalpingiosis in the omentum. A study of autopsy and surgical material. Am J Surg Pathol. 1982; 6:109–117.
130. McCaughey, WTE, Kirk, ME, Lester, W, et al. Peritoneal epithelial lesions associated with proliferative serous tumours of the ovary. Histopathology. 1984; 8:195–208.
131. Moore, WF, Bentley, RC, Berchuck, A, Robboy, SJ. Some mullerian inclusion cysts in lymph nodes may sometimes be metastases from serous borderline tumors of the ovary. Am J Surg Pathol. 2000; 24:710–718.
132. Copeland, LJ, Silva, EG, Gershenson, DM, et al. The significance of müllerian inclusions found at second-look laparotomy in patients with epithelial ovarian neoplasms. Obstet Gynecol. 1988; 71:763–770.
133. Bell, DA, Scully, RE. Serous borderline tumors of the peritoneum. Am J Surg Pathol. 1990; 14:230–239.
134. McCoubrey, A, Houghton, O, McCallion, K, et al. Serous adenocarcinoma of the sigmoid mesentery arising in cystic endosalpingiosis. J Clin Pathol. 2005; 58:1221–1223.
135. Horn, LC, Bilek, K. Frequency and histogenesis of pelvic retroperitoneal lymph node inclusions of the female genital tract. An immunohistochemical study of 34 cases. Pathol Res Pract. 1995; 191:991–996.
136. Kheir, SM, Mann, WJ, Wilkerson, JA. Glandular inclusions in lymph nodes. The problem of extensive involvement and relationship to salpingitis. Am J Surg Pathol. 1981; 5:353–359.
137. Prade, M, Spatz, A, Bentley, R, et al. Borderline and malignant serous tumor arising in pelvic lymph nodes: evidence of origin in benign glandular inclusions. Int J Gynecol Pathol. 1995; 14:87–91.
138. Djordjevic, B, Malpica, A. Lymph node involvement in ovarian serous tumors of low malignant potential: with lymph node involvement: a clinicopathologic study of thirty-six cases. Am J Surg Pathol. 2010; 34:1–9.
139. Djordjevic, B, Clement-Kruzel, S, Atkinson, NE, Malpica, A. Nodal endosalpingiosis in ovarian serous tumors of low malignant potential with lymph node involvement: a case for a precursor lesion. Am J Surg Pathol. 2010; 34:1442–1448.
140. Djordjevic, B, Malpica, A. Ovarian serous tumors of low malignant potential with nodal low-grade serous carcinoma. Am J Surg Pathol. 2012; 36:955–963.
141. Lauchlan, SC. The secondary mullerian system. Obstet Gynecol Surv. 1972; 27:133–146.
142. Mills, SE. Decidua and squamous metaplasia in abdominopelvic lymph nodes. Int J Gynecol Pathol. 1983; 2:209–215.
143. Clement, PB, Young, RH. Endocervicosis of the urinary bladder. A report of six cases of a benign mullerian lesion that may mimic adenocarcinoma. Am J Surg Pathol. 1992; 16:533–542.
144. Nazeer, T, Ro, JY, Tornos, C, et al. Endocervical type glands in urinary bladder: a clinicopathologic study of six cases. Hum Pathol. 1996; 27:816–820.
145. Young, RH, Clement, PB. Mullerianosis of the urinary bladder. Mod Pathol. 1996; 9:731–737.
146. Begin, LR. Florid soft-tissue decidual reaction—a potential mimic of neoplasia. Am J Surg Pathol. 1997; 21:348–353.
147. Altinok, G, Usubutun, A, Kucukali, T, et al. Disseminated peritoneal leiomyomatosis—a benign entity mimicking carcinomatosis. Arch Gynecol Obstet. 2000; 264:54–55.
148. Heinig, J, Neff, A, Cirkel, U, Klockenbusch, W. Recurrent leiomyomatosis peritonealis disseminata after hysterectomy and bilateral salpingo-oophorectomy during combined hormone replacement therapy. Eur J Obstet Gynecol Reprod Biol. 2003; 111:216–218.
149. Langenberg, R, Wojdat, R, Volz-Koster, S, Volz, J. Disseminated intraperitoneal leiomyomatosis—case report of a rare differential diagnosis of metastasizing ovarian carcinoma. Geburt Frauenheil. 2005; 65:1074–1076.
150. Scharlau, LL, Scharlau, J, Mathuis, C, Schremmer, CN. Diffuse peritoneal leiomyomatosis: a case report. Geburt Frauenheil. 2000; 60:225–228.
151. Hardman, IWJ, Majmudar, B. Leiomyomatosis peritonealis disseminata: clinicopathologic analysis of five cases. South Med J. 1996; 89:291–294.
152. Bekkers, RLM, Willemsen, WNP, Schijf, CPT, et al. Leiomyomatosis peritonealis disseminata: does malignant transformation occur? A literature review. Gynecol Oncol. 1999; 75:158–163.
153. Fulcher, AS, Szucs, RA. Leiomyomatosis peritonealis disseminata complicated by sarcomatous transformation and ovarian torsion: presentation of two cases and review of the literature. Abdom Imaging. 1998; 23:640–644.
154. Herrero, J, Kamali, P, Kirschbaum, M. Leiomyomatosis peritonealis disseminata associated with endometriosis: a case report and literature review. Eur J Obstet Gynecol Reprod Biol. 1998; 76:189–191.
155. Butnor, KJ, Burchette, JL, Robboy, SJ. Progesterone receptor activity in leiomyomatosis peritonealis disseminata. Int J Gynecol Pathol. 1999; 18:259–264.
156. Buttner, A, Bassler, R, Theele, C. Pregnancy-associated ectopic decidua (deciduosis) of the greater omentum. An analysis of 60 biopsies with cases of fibrosing deciduosis and leiomyomatosis peritonealis disseminata. Pathol Res Pract. 1993; 189:352–359.
157. Quade, BJ, McLachlin, CM, Soto-Wright, V, et al. Disseminated peritoneal leiomyomatosis: clonality analysis by X chromosome inactivation and cytogenetics of a clinically benign smooth muscle proliferation. Am J Pathol. 1997; 150:2153–2166.
158. Billings, SD, Folpe, AL, Weiss, SW. Do leiomyomas of deep soft tissue exist? An analysis of highly differentiated smooth muscle tumors of deep soft tissue supporting two distinct subtypes. Am J Surg Pathol. 2001; 25:1134–1142.
159. Paal, E, Miettinen, M. Retroperitoneal leiomyomas: a clinicopathologic and immunohistochemical study of 56 cases with a comparison to retroperitoneal leiomyosarcomas. Am J Surg Pathol. 2001; 25:1355–1363.
160. Billings, SD, Folpe, AL, Weiss, SW. Do leiomyomas of deep soft tissue exist? An analysis of highly differentiated smooth muscle tumors of deep soft tissue supporting two distinct subtypes. Am J Surg Pathol. 2001; 25:1134–1142.